To the Editor
Autoreactivity could partly underlie the dysregulated adaptive immunity and chronic inflammation in nasal polyps. This hypothesis is supported by 3 main considerations: the first is the chronic relapsing course of the disease, as observed also in autoimmune diseases. The second is the local production of immunoglobulins with high total and specific IgE levels and increased IgA and IgG levels.1,2 Together with the increased expression of recombination activating genes in nasal polyp tissue,3 which implies local receptor revision, it could be hypothesized that the locally produced antibodies are autoreactive. The third consideration is the frequent chronic colonization with Staphylococcus aureus in chronic rhinosinusitis with nasal polyps (CRSwNP). These microorganisms release enterotoxins that can act as immune-activating superantigens.4
Several antibodies have been detected in patients with nasal polyps in the past. The group of Zambetti et al5 performed an autologous serum skin test (ASST) and found histamine-releasing factors in the circulation of patients with nasal polyps.5 Furthermore, antinuclear antibodies have been detected in nasal polyp tissue, namely, anti–double-stranded DNA (dsDNA) IgG.6 Our aim was to further explore the role of these antibodies in CRSwNP and to expand the search for autoreactivity with several tests. Methods are described in this article’s On-line Repository at www.jacionline.org.
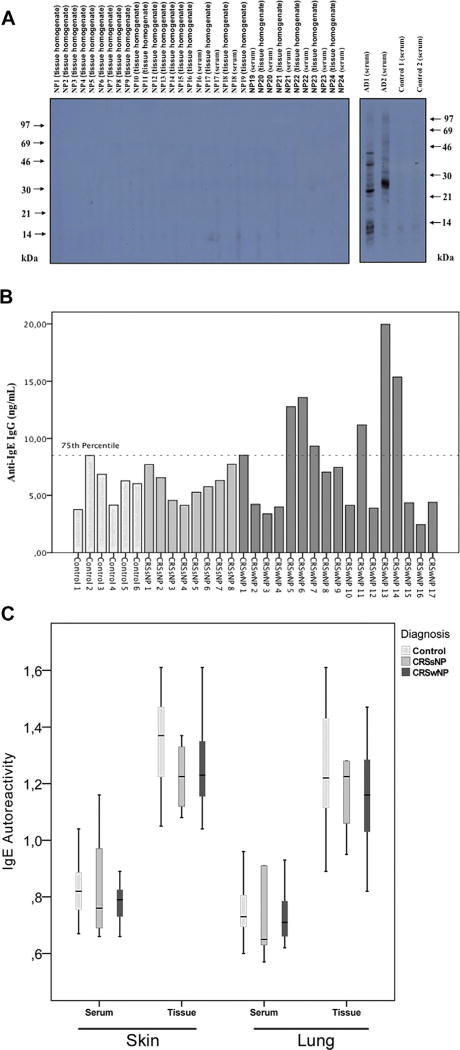
Autoreactive IgE and IgG autoreactive to IgE, high-affinity receptor for the Fc region of IgE (FcεRI), or nuclear components were analyzed. Our study confirmed the presence of autoreactive anti-dsDNA IgG (Fig 1), although we found no evidence for IgE autoreactivity, nor did we detect significantly elevated local or systemic IgG antibodies against IgE or FcεRI (Fig 2, A–C).
FIG 1.
The detection of local and serum anti-dsDNA IgG. Data are expressed in Box-Whisker plots presenting the results of local and systemic anti-dsDNA IgG in patients with nasal polyps and healthy subjects. Significance (P) values after Mann-Whitney U test are represented by * when P < .05.
FIG 2.
The detection of autoreactive IgE to the human epithelial cell line A431 (A), of local anti-IgE IgG (B) and of autoreactive IgE to skin and lung epithelium (C). A, Positive (atopic dermatitis [AD]) and negative controls are shown in this western blot. Molecular weights (kDa) are indicated at the left and right margins. B, Each subject’s anti-IgE IgG value is presented in the bar graph. The 75th percentile (8.991 ng/mL) is represented by the dotted line. C, Median values, 25th and 75th percentiles are presented by Box-Whisker plots.
The ASST is an in vivo test that is currently used to assess autoreactivity in chronic idiopathic urticaria. Baseline characteristics of patients included in the ASST are depicted in Table E1 in this article’s Online Repository at www.jacionline.org. None of our patients with CRSwNP had a positive ASST result, nor was there a reaction to the negative control, although there was a wheal and flare in the positive control for all subjects. The high negative predictive value for the presence of systemic anti-IgE or anti-FcεRI IgG excludes circulating autoantibodies to these autoantigens. Our results are in contrast to those of the group of Zambetti et al,5 who found a positive ASST result in 55% and 8%, respectively, of the nasal polyp and control groups.5 It is remarkable that different studies report quite divergent ASST results, with positive results in adult patients with chronic urticaria ranging between 4% and 76%. The immense variation in results could be a consequence of differences in patient selection, methodology, and test interpretation.7 In the present study, the methodology was performed in accordance with the guidelines written by a panel of experts of the European Academy of Allergy and Clinical Immunity and the Global Allergy and Asthma European Network.7 The Global Allergy and Asthma European Network panel previously reported that the clinical relevance of the ASST is proven only for chronic urticaria. This aspect is in line with our results.
Antinuclear IgG autoantibodies against dsDNA were significantly (P = .010) elevated in homogenates of CRSwNP tissue (31.20 IU/mL [12.34–84,09 IU/mL]) as compared with control tissue (7.70 IU/mL [5.46–24.05 IU/mL]). In contrast, anti-dsDNA IgG was not significantly elevated in any of the serum samples (Fig 1), suggesting that the production of anti-dsDNA IgG occurs locally.
Anti-dsDNA IgG levels in nasal polyps were then correlated with clinical and demographical parameters which can be found in Table E2. Correlations can be found in Table E3 in this article’s Online Repository at www.jacionline.org. We found a significant inverse correlation with IFN-γ in the population with CRSwNP (P = .013; ρ = −0.607). Furthermore, levels of autoantibodies were higher in patients with aspirin intolerance. There was a trend toward higher anti-dsDNA autoantibodies in patients with CRSwNP with the highest IgE and eosinophil cationic protein levels. The median value in the female and the older population was nonsignificantly higher. We could not link the anti-dsDNA IgG levels to the presence of staphylococcal enterotoxin specific IgE. Anti-dsDNA IgG levels were equally elevated in both patients with asthma with CRSwNP and patients without asthma with CRSwNP and anti-dsDNA IgG could also not be linked with IL-17 levels.
The exact role of autoreactive anti-dsDNA IgG is unclear. Whether autoreactivity is simply an epiphenomenon as a result of chronic inflammation or a contributor to the perpetuation of the disease remains uncertain.
Longitudinal studies are needed because the major challenge will be to separate cause from effect. Furthermore, other causes of anti-dsDNA IgG production should be excluded. For example, transient anti-dsDNA can be related to infections with, for example, pneumococcus, EBV, HIV, polyomavirus toxoplasmose, and rubella.8–11
A limitation of this study includes that the average age of onset for nasal polyps is higher than the average age for control patients who require a septoplasty or conchotomy. This could influence anti-dsDNA levels as the prevalence of autoreactive antibodies increases with age.12
Although during our quest anti-dsDNA IgG was the only significantly detectable autoreactive antibody, the search for other autoantibodies in CRSwNP should be continued. In particular, a larger study detecting anti-IgE IgG in nasal polyps should be performed as we observed a trend of increasing values in patients with CRSwNP (7.05 ng/mL [4.07–11.97 ng/mL]) compared with homogenates of patients with chronic rhinosinusitis without NPs (6.04 ng/mL [4.75–7.43 ng/mL]) and control subjects (6.6 ng/mL [4.07–7.25 ng/mL]). Furthermore, these autoantibodies have been found upregulated in asthma as well.13 Patients with nasal polyps exhibiting higher levels of anti-IgE IgG do not have specific characteristics, such as high recurrence rates or aspirin-exacerbated respiratory disease.
To conclude, we extensively tested CRSwNP for autoimmune factors. No IgE autoreactivity to epithelial antigens, or anti-IgE IgG, was detected, although IgG autoantibodies against dsDNA were upregulated. Our results confirm the presence of anti-dsDNA IgG as reported previously by Tan et al,6 although we could not detect IgG to IgE/FcεRI in the systemic circulation of patients with nasal polyps. However, our knowledge about the significance of anti-dsDNA is incomplete. Further research is needed to establish whether autoreactivity is truly involved in the pathogenesis of nasal polyps.
Acknowledgments
R. Valenta declares a grant from the Austrian Science Fund and grants/grants pending as well as providing consultancy to Biomay AG, Thermofisher, and Fresenius Medical Care. I. Mittermann declares a grant from the Austrian Science Fund. R. P. Schleimer has received research support from the National Institutes of Health; has received consultancy fees from Intersect ENT, GlaxoSmithKline, Allakos, Aurasense, Merck, BioMarck, Sanofi, AstraZeneca/MedImmune, and Genentech; and has stock/stock options in Allakos, Aurasense, and BioMarck. B. K. Tan declares grants from the National Institutes of Health. P. Gevaert declares a grant from Fonds Wetenschappelijk Onderzoek and providing consultancy to and receiving travel support from various sources.
Footnotes
Disclosure of potential conflict of interest: E. de Schryver declares being employed at Ghent University Hospital. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy. 2007;37:1840–7. doi: 10.1111/j.1365-2222.2007.02838.x. [DOI] [PubMed] [Google Scholar]
- 2.Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013;131:1075–83. 1083.e1–7. doi: 10.1016/j.jaci.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gevaert P, Nouri-Aria KT, Wu H, Harper CE, Takhar P, Fear DJ, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013;68:55–63. doi: 10.1111/all.12054. [DOI] [PubMed] [Google Scholar]
- 4.Bachert C, Zhang N, van Zele T, Gevaert P, Patou J, van Cauwenberge P. Staphylococcus aureus enterotoxins as immune stimulants in chronic rhinosinusitis. Clin Allergy Immunol. 2007;20:163–75. [PubMed] [Google Scholar]
- 5.Zambetti G, Ciofalo A, Soldo P, Fusconi M, Romeo R, Greco A, et al. Autologous serum skin test reactivity and basophil histamine release test in patients with nasal polyposis: preliminary results. Int J Immunopathol Pharmacol. 2010;23:641–7. doi: 10.1177/039463201002300228. [DOI] [PubMed] [Google Scholar]
- 6.Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:1198–206.e1. doi: 10.1016/j.jaci.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konstantinou GN, Asero R, Maurer M, Sabroe RA, Schmid-Grendelmeier P, Grattan CE. EAACI/GA(2)LEN task force consensus report: the autologous serum skin test in urticaria. Allergy. 2009;64:1256–68. doi: 10.1111/j.1398-9995.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 8.Fredriksen K, Skogsholm A, Flaegstad T, Traavik T, Rekvig OP. Antibodies to dsDNA are produced during primary BK virus infection in man, indicating that anti-dsDNA antibodies may be related to virus replication in vivo. Scand J Immunol. 1993;38:401–6. doi: 10.1111/j.1365-3083.1993.tb01744.x. [DOI] [PubMed] [Google Scholar]
- 9.Hansen KE, Arnason J, Bridges AJ. Autoantibodies and common viral illnesses. Semin Arthritis Rheum. 1998;27:263–71. doi: 10.1016/s0049-0172(98)80047-4. [DOI] [PubMed] [Google Scholar]
- 10.Poole BD, Scofield RH, Harley JB, James JA. Epstein-Barr virus and molecular mimicry in systemic lupus erythematosus. Autoimmunity. 2006;39:63–70. doi: 10.1080/08916930500484849. [DOI] [PubMed] [Google Scholar]
- 11.Blank M, Barzilai O, Shoenfeld Y. Molecular mimicry and auto-immunity. Clin Rev Allergy Immunol. 2007;32:111–8. doi: 10.1007/BF02686087. [DOI] [PubMed] [Google Scholar]
- 12.Manoussakis MN, Tzioufas AG, Silis MP, Pange PJ, Goudevenos J, Moutsopoulos HM. High prevalence of anti-cardiolipin and other autoantibodies in a healthy elderly population. Clin Exp Immunol. 1987;69:557–65. [PMC free article] [PubMed] [Google Scholar]
- 13.Chan YC, Ramadani F, Santos AF, Pillai P, Ohm-Laursen L, Harper CE, et al. “Auto-anti-IgE“: naturally occurring IgG anti-IgE antibodies may inhibit allergen-induced basophil activation. J Allergy Clin Immunol. 2014;134:1394–401.e4. doi: 10.1016/j.jaci.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]