Abstract
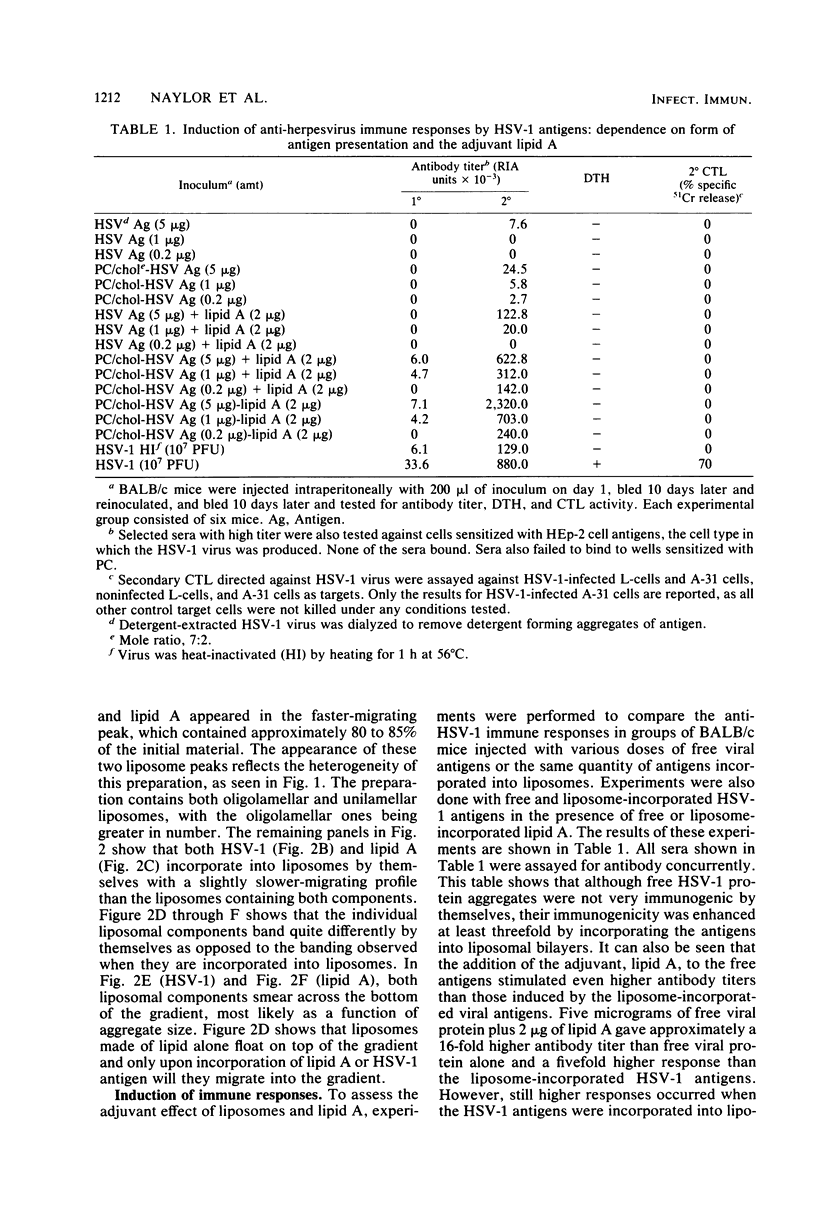
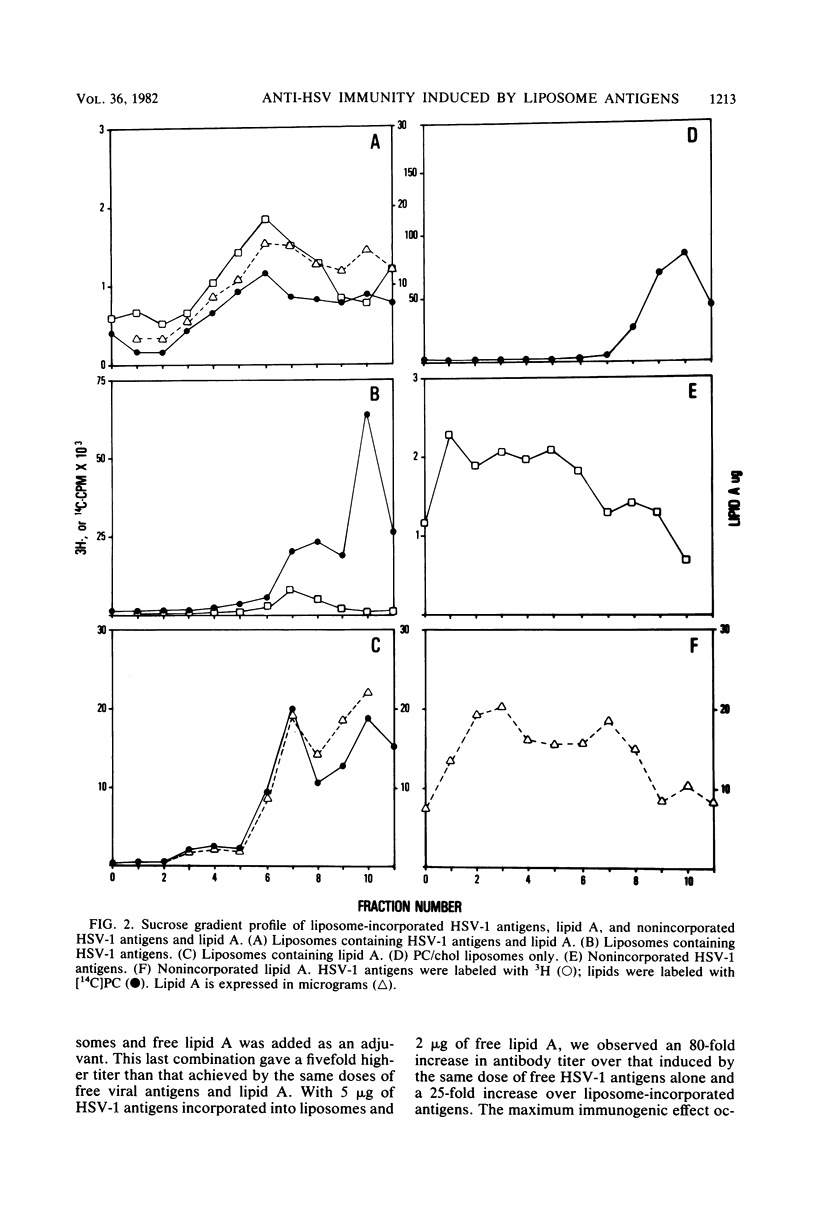
To establish the requirements for a potential subunit vaccine against herpes simplex virus (HSV), we analyzed the effects on immunogenicity of incorporating detergent-extracted glycoprotein-enriched HSV type 1 (HSV-1) antigens into liposomes alone or with the adjuvant lipid A. Incorporating HSV-1 antigens into liposomes enhanced their immunogenicity for antibody production as detected by radioimmunoassay. Antibody levels to free and liposome-bound antigens were enhanced by administering lipid A as an adjuvant. The maximum immunogenic effect was obtained by incorporating lipid A into liposomes containing the HSV-1 proteins. Such liposomes induced secondary antibody responses higher than those engendered by virus infection. Whereas infectious virus induced cell-mediated immunity detectable by the delayed-type hypersensitivity reactions and cytotoxic T lymphocyte production, none of the liposome preparations induced cell-mediated immunity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry L. J. Bacterial toxins. CRC Crit Rev Toxicol. 1977 Nov;5(3):239–318. doi: 10.3109/10408447709082601. [DOI] [PubMed] [Google Scholar]
- Bone D. R., Courtney R. J. A temperature-sensitive mutant of herpes simplex virus type 1 defective in the synthesis of the major capsid polypeptide. J Gen Virol. 1974 Jul;24(1):17–27. doi: 10.1099/0022-1317-24-1-17. [DOI] [PubMed] [Google Scholar]
- CYNKIN M. A., ASHWELL G. Estimation of 3-deoxy sugars by means of the malonaldehyde-thiobarbituric acid reaction. Nature. 1960 Apr 9;186:155–156. doi: 10.1038/186155a0. [DOI] [PubMed] [Google Scholar]
- Chedid L., Carelli C., Audibert F. Recent developments concerning muramyl dipeptide, a synthetic immunoregulating molecule. J Reticuloendothel Soc. 1979 Dec;26(Suppl):631–641. [PubMed] [Google Scholar]
- Crowle A. J. Delayed hypersensitivity in the mouse. Adv Immunol. 1975;20:197–264. doi: 10.1016/s0065-2776(08)60209-6. [DOI] [PubMed] [Google Scholar]
- Dancey G. F., Yasuda T., Kinsky S. C. Effect of liposomal model membrane composition on immunogenicity. J Immunol. 1978 Apr;120(4):1109–1113. [PubMed] [Google Scholar]
- Dancey G. F., Yasuda T., Kinsky S. C. Enhancement of liposomal model membrane immunogenicity by incorporation of lipid A. J Immunol. 1977 Dec;119(6):1868–1873. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hale A. H., McGee M. P. A study of the inability of subcellular fractions to elicit primary anti-H-2 cytotoxic T lymphocytes. Cell Immunol. 1981 Mar 1;58(2):277–285. doi: 10.1016/0008-8749(81)90221-5. [DOI] [PubMed] [Google Scholar]
- Huang L., Pagano R. E. Interaction of phospholipid vesicles with cultured mammalial cells. I. Characteristics of uptake. J Cell Biol. 1975 Oct;67(1):38–48. doi: 10.1083/jcb.67.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramp W. J., Six H. R., Drake S., Kasel J. A. Liposomal enhancement of the immunogenicity of adenovirus type 5 hexon and fiber vaccines. Infect Immun. 1979 Aug;25(2):771–773. doi: 10.1128/iai.25.2.771-773.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawman M. J., Naylor P. T., Huang L., Courtney R. J., Rouse B. T. Cell-mediated immunity to herpes simplex virus: induction of cytotoxic T lymphocyte responses by viral antigens incorporated into liposomes. J Immunol. 1981 Jan;126(1):304–308. [PubMed] [Google Scholar]
- Lawman M. J., Rouse B. T., Courtney R. J., Walker R. D. Cell-mediated immunity against herpes simplex induction of cytotoxic T lymphocytes. Infect Immun. 1980 Jan;27(1):133–139. doi: 10.1128/iai.27.1.133-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morein B., Helenius A., Simons K., Pettersson R., Käriäinen L., Schirrmacher V. Effective subunit vaccines against an enveloped animal virus. Nature. 1978 Dec 14;276(5689):715–718. doi: 10.1038/276715a0. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Mechanisms of recovery from Herpesvirus infections -a review. Can J Comp Med. 1978 Oct;42(4):414–427. [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Lawman M. J. Induction of cytotoxic T lymphocytes against herpes simplex virus type i: role of accessory cells and amplifying factor. J Immunol. 1980 May;124(5):2341–2346. [PubMed] [Google Scholar]
- Sanchez Y., Ionescu-Matiu I., Dreesman G. R., Kramp W., Six H. R., Hollinger F. B., Melnick J. L. Humoral and cellular immunity to hepatitis B virus-derived antigens: comparative activity of Freund complete adjuvant alum, and liposomes. Infect Immun. 1980 Dec;30(3):728–733. doi: 10.1128/iai.30.3.728-733.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid D. S., Larsen H. S., Rouse B. T. The role of accessory cells and T cell-growth factor in induction of cytotoxic T lymphocytes against herpes simplex virus antigens. Immunology. 1981 Dec;44(4):755–763. [PMC free article] [PubMed] [Google Scholar]
- Schuster B. G., Neidig M., Alving B. M., Alving C. R. Production of antibodies against phosphocholine, phosphatidylcholine, sphingomyelin, and lipid A by injection of liposomes containing lipid A. J Immunol. 1979 Mar;122(3):900–905. [PubMed] [Google Scholar]
- Skelly R. R., Munkenbeck P., Morrison D. C. Stimulation of T-independent antibody responses by hapten-lipopolysaccharides without repeating polymeric structure. Infect Immun. 1979 Feb;23(2):287–293. doi: 10.1128/iai.23.2.287-293.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrigiani G. Quantitative estimation of antibody in the immunoglobulin classes of the mouse. II. Thymic dependence of the different classes. J Immunol. 1972 Jan;108(1):161–164. [PubMed] [Google Scholar]
- Wagner H., Hardt C., Heeg K., Pfizenmaier K., Solbach W., Bartlett R., Stockinger H., Röllinghoff M. T-T cell interactions during cytotoxic T lymphocyte (CTL) responses: T cell derived helper factor (Interleukin 2) as a probe to analyze CTL responsiveness and thymic maturation of CTL progenitors. Immunol Rev. 1980;51:215–255. doi: 10.1111/j.1600-065x.1980.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Yasuda T., Dancey G. F., Kinsky S. C. Immunogenic properties of liposomal model membranes in mice. J Immunol. 1977 Dec;119(6):1863–1867. [PubMed] [Google Scholar]
- Yasuda T., Dancey G. F., Kinsky S. C. Immunogenicity of liposomal model membranes in mice: dependence on phospholipid composition. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1234–1236. doi: 10.1073/pnas.74.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Dalrymple J. M., Artenstein M. S. Analysis of parameters affecting the solid phase radioimmunoassay quantitation of antibody to meningococcal antigens. J Immunol. 1976 Nov;117(5 PT2):1788–1798. [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R. Liposomes in immunology: multilamellar phosphatidylcholine liposomes as a simple, biodegradable and harmless adjuvant without any immunogenic activity of its own. Immunol Commun. 1980;9(3):243–256. doi: 10.3109/08820138009065997. [DOI] [PubMed] [Google Scholar]