Abstract
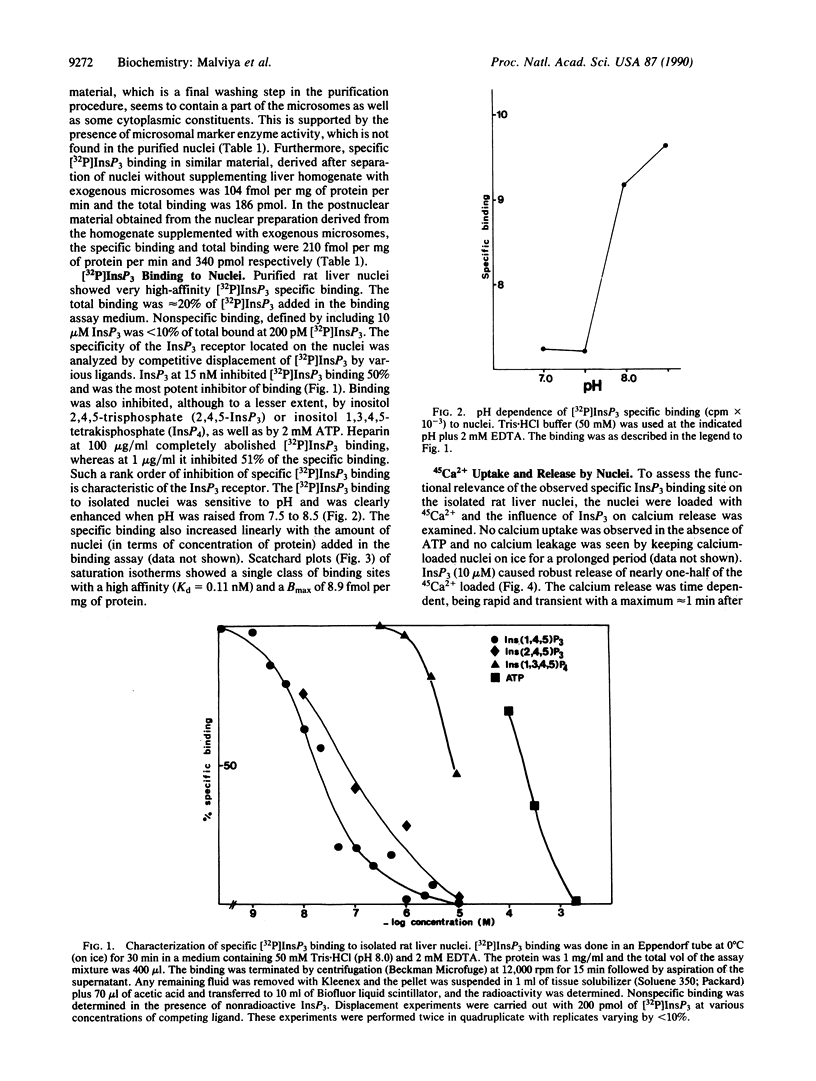
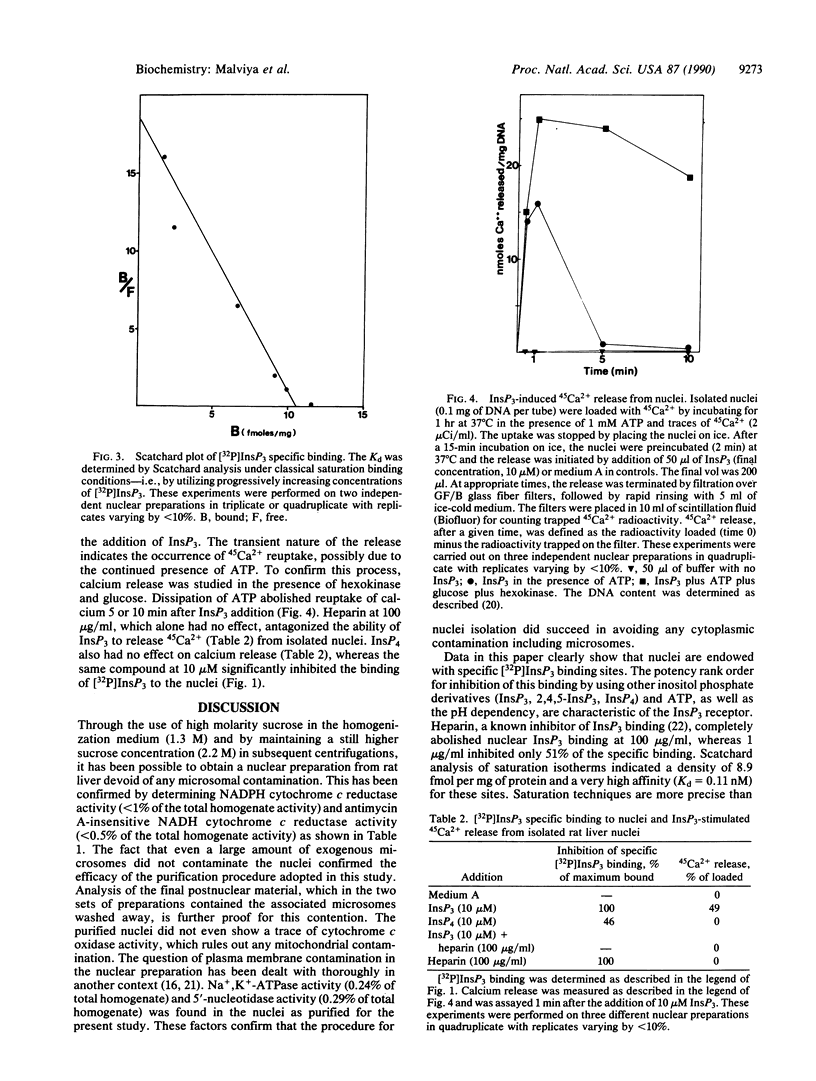
It is well known that inositol 1,4,5-trisphosphate binding and release of calcium are mediated by the same protein. Several reports have indicated the location of the inositol 1,4,5-trisphosphate receptor in organelles other than endoplasmic reticulum. Immunocytochemical studies on the subcellular localization of 1,4,5-trisphosphate receptor in the Purkinje cells from two laboratories have given contradictory results regarding the nuclear location of this receptor. In this paper, a high-affinity inositol 1,4,5-[32P]trisphosphate binding site (Kd = 0.11 nM) on nuclei isolated from rat liver and devoid of any microsomal, mitochondrial, or plasma membrane constituents is documented. Furthermore, we present data demonstrating that inositol 1,4,5-trisphosphate is capable of releasing 45Ca2+ from the intact isolated liver nuclei. A rapid and transient release of calcium that was taken up by nuclei in the presence of ATP is observed. The role of inositol 1,4,5-trisphosphate in the coupling between cytoplasmic second messengers and nuclear events activated during signal transduction is postulated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Morgan A., O'Sullivan A. J., Moreton R. B., Berridge M. J., Mata A. M., Colyer J., Lee A. G., East J. M. Distribution of two distinct Ca2+-ATPase-like proteins and their relationships to the agonist-sensitive calcium store in adrenal chromaffin cells. Nature. 1989 Nov 2;342(6245):72–74. doi: 10.1038/342072a0. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Chilvers E. R., Willcocks A. L., Nahorski S. R. Heterogeneity of [3H]inositol 1,4,5-trisphosphate binding sites in adrenal-cortical membranes. Characterization and validation of a radioreceptor assay. Biochem J. 1990 Jan 15;265(2):421–427. doi: 10.1042/bj2650421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Snyder S. H. Calcium flux mediated by purified inositol 1,4,5-trisphosphate receptor in reconstituted lipid vesicles is allosterically regulated by adenine nucleotides. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2147–2151. doi: 10.1073/pnas.87.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Supattapone S., Snyder S. H. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989 Nov 2;342(6245):87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989 Nov 2;342(6245):32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Guillemette G., Balla T., Baukal A. J., Catt K. J. Characterization of inositol 1,4,5-trisphosphate receptors and calcium mobilization in a hepatic plasma membrane fraction. J Biol Chem. 1988 Apr 5;263(10):4541–4548. [PubMed] [Google Scholar]
- Hernández-Cruz A., Sala F., Adams P. R. Subcellular calcium transients visualized by confocal microscopy in a voltage-clamped vertebrate neuron. Science. 1990 Feb 16;247(4944):858–862. doi: 10.1126/science.2154851. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Maeda N., Niinobe M., Mikoshiba K. A cerebellar Purkinje cell marker P400 protein is an inositol 1,4,5-trisphosphate (InsP3) receptor protein. Purification and characterization of InsP3 receptor complex. EMBO J. 1990 Jan;9(1):61–67. doi: 10.1002/j.1460-2075.1990.tb08080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malviya A. N., Rendon A., Aunis D. Interaction of antimycin with cytochrome b-561. A study in secretory granules and in plasma membrane isolated from chromaffin cells of bovine adrenal medulla. FEBS Lett. 1983 Aug 22;160(1-2):153–158. doi: 10.1016/0014-5793(83)80956-9. [DOI] [PubMed] [Google Scholar]
- Masmoudi A., Labourdette G., Mersel M., Huang F. L., Huang K. P., Vincendon G., Malviya A. N. Protein kinase C located in rat liver nuclei. Partial purification and biochemical and immunochemical characterization. J Biol Chem. 1989 Jan 15;264(2):1172–1179. [PubMed] [Google Scholar]
- Mauger J. P., Claret M., Pietri F., Hilly M. Hormonal regulation of inositol 1,4,5-trisphosphate receptor in rat liver. J Biol Chem. 1989 May 25;264(15):8821–8826. [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J., Cohn J., Malter H. Ion channels in the nuclear envelope. Nature. 1990 Feb 22;343(6260):764–767. doi: 10.1038/343764a0. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Transient calcium release induced by successive increments of inositol 1,4,5-trisphosphate. Proc Natl Acad Sci U S A. 1990 May;87(10):3841–3845. doi: 10.1073/pnas.87.10.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989 Nov;12(11):459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Nicotera P., McConkey D. J., Jones D. P., Orrenius S. ATP stimulates Ca2+ uptake and increases the free Ca2+ concentration in isolated rat liver nuclei. Proc Natl Acad Sci U S A. 1989 Jan;86(2):453–457. doi: 10.1073/pnas.86.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Palade P., Dettbarn C., Volpe P., Alderson B., Otero A. S. Direct inhibition of inositol-1,4,5-trisphosphate-induced Ca2+ release from brain microsomes by K+ channel blockers. Mol Pharmacol. 1989 Oct;36(4):664–672. [PubMed] [Google Scholar]
- Ross C. A., Meldolesi J., Milner T. A., Satoh T., Supattapone S., Snyder S. H. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature. 1989 Jun 8;339(6224):468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- Rossier M. F., Capponi A. M., Vallotton M. B. The inositol 1,4,5-trisphosphate-binding site in adrenal cortical cells is distinct from the endoplasmic reticulum. J Biol Chem. 1989 Aug 25;264(24):14078–14084. [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Supattapone S., Wilson V. S., Snyder S. H. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem. 1987 Sep 5;262(25):12132–12136. [PubMed] [Google Scholar]



