Abstract
Purpose
Calcium nephrolithiasis is associated with an increased risk of osteoporosis and fracture. Hypercalciuria has been assumed to be pathogenic for bone loss in kidney stone formers, although this association was shown in small crosssectional studies. We explored the association of urine calcium with bone mineral density in kidney stone formers.
Materials and Methods
We retrospectively studied bone mineral density in kidney stone formers. Excluded were subjects with hypercalcemia, chronic bowel disease, primary hyperparathyroidism, distal renal tubular acidosis or endogenous creatinine clearance less than 40 ml per minute. We included 250 males and 182 females subdivided into 145 who were estrogen treated and postmenopausal, and 37 who were nonestrogen treated and postmenopausal. We assessed the association of lumbar spine and femoral neck bone mineral density with 24-hour urine calcium on random and restricted diets, and while fasting using univariable and multivariable models adjusting for body mass index, urine sodium and sulfate.
Results
On multivariable analysis no significant association was found between urine calcium on a random or a restricted diet, or during fasting conditions and femoral neck or lumbar spine bone mineral density in men and estrogen treated women. In estrogen untreated women lumbar spine bone mineral density inversely correlated with urine calcium on the restricted diet (r = −0.38, p = 0.04 and adjusted r = −0.45, p = 0.02) and in the fasting state (r = −0.42, p = 0.05).
Conclusions
Unlike in previous small cross-sectional studies we found no significant relationship between urine calcium and bone mineral density in a large group of calcium kidney stone formers. However, a significant inverse relationship was found in estrogen untreated kidney stone formers only. This study suggests that mechanism(s) other than hypercalciuria explain the lower bone mineral density and the higher fracture risk in patients who are kidney stone formers. It also highlights the role of estrogen on bone integrity.
Keywords: kidney calculi, estrogens, urine, calcium, bone density
Nephrolithiasis is a major health and economic burden in the United States and worldwide.1 Several population based epidemiological studies have established a high prevalence of both axial and appendicular skeletal fractures among kidney stone formers.2,3 However, in a recent large cohort of 9,856 postmenopausal women stone formation was not associated with either BMD or skeletal fractures after adjusting for covariates that are associated with both osteoporosis and kidney stones.4 Major limitations of some of these population based cohort studies have been the reliance on self-report of stone incidence, lack of availability of stone composition, dietary composition, fluid intake, calcium and vitamin D supplementations, drugs known to influence the risk of kidney stone disease and most importantly urinary biochemical profiles.5–7
Furthermore, most of these population based studies did not adjust for covariates influencing the development of osteoporosis such as ethnicity, general health and use of bone protective agents.2,3 Nevertheless, hypercalciuria and a negative calcium balance, which are common in patients with idiopathic hypercalciuria,8 have been assumed to be pathogenic for bone loss in kidney stone formers. This association was shown in several cross-sectional studies in a small number of kidney stone formers.9–11
We performed a multivariable analysis including urinary sodium and sulfate as surrogate markers for salt and animal protein intake, respectively, which have both been shown to increase urinary calcium excretion5 and increase the risk of bone disease.11 We also included BMI in the multivariable analysis due to its positive correlation with BMD.12
To date the underlying pathophysiological mechanisms of bone loss and higher fracture risk in stone formers has not been fully elucidated. In view of the limitations of past studies we examined the relationship between urinary calcium excretion and BMD in a larger population of male and female untreated kidney stone formers on a random diet, those following a restricted dietary regimen limiting salt intake and those in the fasting state to cover all physiological states of urinary calcium excretion.
MATERIALS AND METHODS
Study Participants
The data on calcium stone forming subjects were obtained retrospectively from the Kidney Stone Registry at our institution between 1980 and 2015. Included in study were subjects 21 years old or older with calcium nephrolithiasis who underwent BMD measurement. Excluded were individuals with creatinine clearance less than 40 ml per minute, renal tubular acidosis, chronic diarrhea, primary hyperparathyroidism, chronic urinary tract infection, uric acid stone formers, cystinuria, patients taking any anti-osteoporotic medication, calcium and vitamin D supplements, and patients in poor general health. A total of 3,390 patient records were screened. Based on these criteria data were obtained on 432 patients (250 males and 182 females) who were evaluated in our clinic.
Of the 182 female subjects 80 were considered postmenopausal due to natural cessation of menses or to surgical intervention. Of the 80 postmenopausal women 43 were on HRT at the time of evaluation. Postmenopausal women not on HRT were classified as nonestrogen exposed whereas premenopausal women and postmenopausal women taking HRT were classified as estrogen exposed. Subject data were obtained at the initial stone visit in which participants were instructed to hold all medications known to influence urinary kidney stone profiles, such as alkali treatment and thiazide diuretics, for 1 week prior to the clinic visit.
This study was approved by the institutional review board at University of Texas Southwestern Medical Center at Dallas and all participants provided written informed consent.
Data Collection and Measurements
Initial stone visits typically included 2, 24-hour urine samples obtained while the subjects were maintained on an ad lib diet (random 24-hour urine). For data analysis purposes the 2, 24-hour urine samples were averaged. Following the initial visit a single 24-hour urine sample was obtained while following a low sodium diet (approximately 100 mEq sodium per day) for 7 days (restricted urine). All 24-hour urine samples were analyzed for total volume, creatinine, calcium, sodium and SO4.
A fasting 2-hour urine sample was obtained after the patients fasted overnight for total volume, calcium and creatinine (fasting urine). At the end of the 2-hour fasting collection a venous serum sample was obtained for calcium and creatinine.
Postmenopausal status was defined as participants who had lacked a menstrual cycle in the past 12 months due to natural or surgical causes after excluding other causes of menstrual cycle cessation, such as pregnancy, breastfeeding and other medical conditions/treatments. HRT was defined as the current use of estrogen or an estrogen containing agent, such as estrogen pills/combination pills or estrogen patches/combination patches.
Analytical Procedures
BMD was measured by trained certified DXA technologists. The LS and FN were measured either by dual photon absorptiometry (Lunar Radiation, Madison, Wisconsin) or by DXA (Hologic, Bedford, Massachusetts). At the time of conversion from dual photon absorptiometry to DXA the instruments were cross calibrated. To account for differences in instruments Z-scores are reported. The instruments were calibrated daily using a phantom for the lumbar spine. Yearly precision measurements were performed as recommended by ISCD (International Society for Clinical Densitometry).
Urine calcium was measured by atomic absorption spectroscopy while urine creatinine was measured using the picric acid method. Urinary sodium was analyzed by flame photometry and urinary SO4 was measured by ion chromatography.
Statistical Analyses
Results are presented as the mean ± SD unless otherwise specified. The relationship between bone mineral density and urinary calcium variables were analyzed with the Spearman correlation coefficient. The Spearman partial correlation was used to adjust for BMI, urine sodium and urine sulfate. Two-sided p <0.05 was considered statistically significant. In females the interaction between estrogen status and urinary calcium was assessed by linear regression analysis using log transformed urinary calcium. Statistical analysis was performed with SAS®, version 9.4.
RESULTS
Kidney Stone Former Characteristics
The demographic and biochemical characteristics of stone formers who did vs did not undergo DXA testing were similar in our stone registry. A total of 250 males and 182 females were included in analysis. Race and ethnic distribution was similar in males and females (table 1). In the male cohort mean age was 48 ± 13 years and mean BMI was 29.0 ± 5.3 kg/m2. Mean age in the estrogen treated and estrogen untreated cohorts was 41 ± 10 and 57 ± 10 years, and mean BMI was 26.8 ± 5.9 and 28.9 ± 6.1 kg/m2, respectively.
Table 1.
Study cohort demographics, bone mineral density and urinary parameters
| Female | |||
|---|---|---|---|
|
|
|||
| Male | Estrogen | No Estrogen | |
| No. pts | 250 | 145 | 37 |
| No. race: | |||
| Native American | 2 | 1 | 0 |
| Asian | 8 | 3 | 0 |
| African American | 6 | 3 | 0 |
| Hispanic | 11 | 6 | 1 |
| Caucasian | 223 | 132 | 36 |
| Mean ± SD age | 48.3 ± 12.7 | 41.1 ± 10.2 | 56.7 ± 10.2 |
| Mean ± SD BMI (kg/m2) | 29.0 ± 5.3 | 26.8 ± 5.9 | 28.9 ± 6.1 |
| Mean ± SD Z-score: | |||
| Lumbar spine | −0.3 ± 1.6 | −0.2 ± 1.1 | 0.0 ± 1.8 |
| Femoral neck | −0.2 ± 1.1 | −0.2 ± 1.0 | −0.2 ± 1.0 |
| Mean ± SD fasting urinary Ca/Cr (mg/mg) | 0.08 ± 0.04 | 0.09 ± 0.05 | 0.12 ± 0.05 |
| Mean ± SD random diet urinary values: | |||
| Calcium (mg/day) | 269 ± 124 | 225 ± 118 | 245 ± 124 |
| Sodium (mEq/day) | 209 ± 81 | 157 ± 62 | 151 ± 70 |
| SO4 (mEq/day) | 43.2 ± 15.8 | 32.6 ± 15.6 | 31.7 ± 13.0 |
| Mean ± SD restricted diet urinary values: | |||
| Calcium (mg/day) | 200 ± 105 | 167 ± 83 | 209 ± 93 |
| Sodium (mEq/day) | 132 ± 75 | 104 ± 53 | 109 ± 59 |
| SO4 (mEq/day) | 43.6 ± 18.3 | 31.7 ± 14.8 | 32.1 ± 15.5 |
In the male cohort the mean LS Z-score was −0.3 ± 1.6 and the mean FN Z-score was −0.2 ± 1.0. In females the estrogen treated and estrogen untreated cohorts had a mean LS Z-score of −0.2 ± 1.1 and 0.0 ± 1.8, and a mean FN Z-score of −0.2 ± 1.0 and −0.2 ± 1.0, respectively. Of calcium stone formers 14% of males, 11% of estrogen treated females and 14% of estrogen untreated females had a Z-score of less than −2.0, which is below the expected range for age and gender. Of calcium stone formers 58% of males, 40% of estrogen treated females and 62% of estrogen untreated females were considered hypercalciuric on a random diet with urinary calcium greater than 250 mg/day or on a restricted diet with urinary calcium greater than 200 mg per day.
Bone Mineral Density Z-Score Relationship to Urinary Calcium
Male Cohort
On the univariable (unadjusted) and multivariable (adjusted) analyses in the male cohort 24-hour urinary calcium while on the random and restricted diets showed no relationship with LS Z-scores (table 2 and supplementary figure, http://jurology.com/). There was also no relationship of either model between fasting Ca/Cr and lumbar spine Z-scores.
Table 2.
Correlations between urinary calcium and bone mineral density in male calcium stone formers during random and restricted diets, and fasting
| Unadjusted (Spearman test) |
Adjusted* | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Z-Score | No. Pts | ρ | p Value | Partial ρ | p Value |
| L2-L4: | |||||
| Random urine calcium | 246 | 0.02 | 0.74 | −0.09 | 0.17 |
| Restricted urine calcium | 222 | 0.02 | 0.76 | −0.08 | 0.26 |
| Fasting Ca/Cr† | 215 | −0.01 | 0.86 | −0.03 | 0.71 |
| Femoral neck: | |||||
| Random urine calcium | 245 | 0.20 | 0.002 | 0.03 | 0.66 |
| Restricted urine calcium | 223 | 0.13 | 0.06 | 0.01 | 0.87 |
| Fasting Ca/Cr† | 215 | 0.02 | 0.80 | 0.002 | 0.98 |
Random 24-hour and restricted urinary calcium regression adjusted for BMI, and urinary sodium and SO4.
Adjusted for BMI.
For the FN Z-scores the only significant relationship was with random diet 24-hour urinary calcium in the univariable model. This relationship was not found after multivariable adjustment, that is after adjusting for BMI, urine sodium and urine sulfate. The unadjusted and adjusted relationships between 24-hour urinary calcium on a restricted diet were nonsignificant after multivariable adjustment.
Female Cohorts
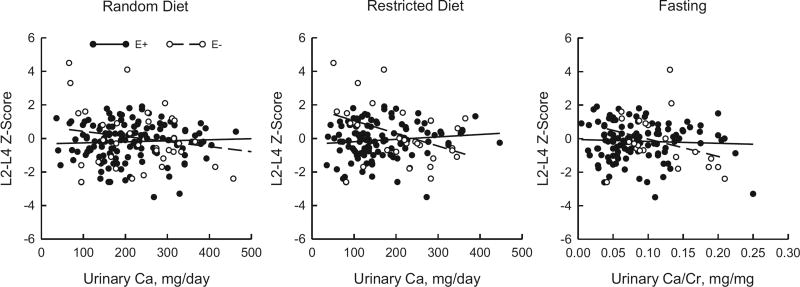
On regression analysis of urine calcium on the LS Z-score a significant interaction between estrogen status and urine calcium on the random and restricted diets was observed. This indicated different slopes between estrogen treated and estrogen untreated females (see figure).
Regression analysis in female cohorts revealed significant interactions between estrogen status and urinary calcium (Ca) for random (p = 0.01) and restricted (p <0.001) diet calcium but not for fasting Ca/Cr (p = 0.30).
In the estrogen treated cohort no significant relationship was found between 24-hour urinary calcium and the random or the restricted diet, or the LS Z-score in the unadjusted or adjusted models (table 3). Similarly, fasting Ca/Cr showed no significant relationship with the LS Z-score whether adjusted or unadjusted. However, in the estrogen treated group 24-hour urinary calcium on the random or restricted diet showed significant relationships with the FN Z-score in the unadjusted model.
Table 3.
Correlations between urinary calcium and bone mineral density in female calcium stone formers by menopausal status and estrogen use during random and restricted diets, and fasting
| Estrogen Unadjusted* |
Estrogen Adjusted† | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Z-Score | No. Pts | ρ | p Value | Partial ρ | p Value |
| Estrogen | |||||
| L2-L4: | |||||
| Random urine calcium | 139 | 0.05 | 0.59 | −0.12 | 0.16 |
| Restricted urine calcium | 121 | 0.10 | 0.29 | −0.02 | 0.82 |
| Fasting Ca/Cr | 117 | 0.02 | 0.84 | 0.02 | 0.83 |
| Femoral neck: | |||||
| Random urine calcium | 141 | 0.27 | 0.001 | 0.13 | 0.13 |
| Restricted urine calcium | 123 | 0.25 | 0.006 | 0.09 | 0.31 |
| Fasting Ca/Cr | 118 | 0.01 | 0.90 | 0.02 | 0.82 |
| No estrogen | |||||
| L2-L4: | |||||
| Random urine calcium | 34 | −0.17 | 0.34 | −0.31 | 0.09 |
| Restricted urine calcium | 29 | −0.38 | 0.04 | −0.45 | 0.02 |
| Fasting Ca/Cr | 22 | −0.42 | 0.05 | −0.40 | 0.07 |
| Femoral neck: | |||||
| Random urine calcium | 35 | 0.01 | 0.96 | −0.08 | 0.67 |
| Restricted urine calcium | 30 | −0.04 | 0.83 | −0.21 | 0.29 |
| Fasting Ca/Cr | 23 | −0.27 | 0.21 | −0.32 | 0.15 |
Spearman test.
Random and restricted 24-hour urinary calcium regressions adjusted for BMI, sodium and SO4. and fasting urinary Ca/Cr correlations adjusted for BMI.
These associations were nonsignificant in the adjusted model. In the estrogen untreated cohort a significant relationship was found between 24-hour restricted diet calcium and the LS Z-score (see figure). The significance of this relationship persisted after adjusting for confounding variables, including BMI, sodium and sulfate. Fasting Ca/Cr vs the LS Z-score was significant in the unadjusted model but nonsignificant in the adjusted model (p = 0.07). No significant relationships were detected between urinary calcium and FN Z-scores in the estrogen untreated cohort.
In all multivariable models examined BMI was the strongest covariate accounting for the association of urine calcium with BMD.
DISCUSSION
To our knowledge this is the first study to examine the association of urinary calcium and BMD in a large number of calcium stone formers while accounting for gender, menopausal state and estrogen status. Following multiple adjustments we found no significant association between urinary calcium and BMD at either the LS or the FN in men or in estrogen treated women (tables 1 and 2).
Estrogen deficiency inhibits intestinal calcium absorption and directly enhances bone resorption.13 These effects may be accentuated when dietary calcium intake is restricted. In nonestrogen treated postmenopausal women there was a significant inverse association between urinary calcium and BMD at the LS. The association between 24-hour urinary calcium on a restricted diet and the LS BMD was statistically significant (p = 0.02). However, this relationship was not significant on random diet 24-hour urinary calcium (p = 0.09) or fasting urinary calcium (p = 0.07, table 2).
The finding in the LS was expected since estrogen deficiency is known to mainly affect trabecular-rich bone, which is the main constituent of the vertebral spine. This observation suggests that increased bone resorption may account for such a relationship in this subset of females with kidney stones.
Our data contrast with findings in several previous cross-sectional studies in which an association between urinary calcium and BMD was observed in kidney stone formers.11,14–17 The explanation for the differences between those studies and our current data might be driven by sample size, by the inclusion of both genders and estrogen status, and also by publications prior to the adoption of T-scores and Z-scores by WHO.14,15,17–19
However, our data are consistent with a recent analysis of 1,500 elderly men in MrOS (Osteoporosis fractures in Men), which showed a lack of relationship between hypercalciuria and osteoporosis.20 In that cohort men with osteoporosis more often had a history of fractures or kidney stones. However, among more than 300 men with both BMD and 24-hour urine calcium no relationship was found between urinary calcium and osteoporosis. The results of our study suggest that urinary calcium may be a marker but not a cause of bone loss in this population, as previously conceived.14,15,17–19
The mechanism(s) by which bone loss develops in patients with kidney stones is yet to be fully explained. Osteoporosis is a heterogeneous and complex bone disorder characterized by an imbalance in bone resorption and bone formation as well as deterioration of the microarchitectural integrity of bone. Bone histomorphometric analyses performed in kidney stone formers have shown impaired bone formation as a characteristic finding.21,22 This would not be the expected finding if hypercalciuria alone has a sole pathogenetic role in the development of bone loss in this population.
Idiopathic hypercalciuria is a heterogeneous disorder which could result from increased synthesis of calcitriol or increased sensitivity to calcitriol, up-regulating its own receptor but influencing the target organs differently. There is evidence of increased intestinal absorption of calcium in this population.23,24 In vitro organ culture incorporating 3H-proline into collagenase digested protein in fetal rat calvaria showed an inhibitory role of calcitriol in collagen synthesis as well as in osteoblastic cell proliferation.25 The inhibitory levels of calcitriol was shown to be exerted at 10−10 M, which is not different from the level of these vitamin D metabolites attained in hypercalciuric stone formers.26–28
These results are in line with our previous study in a group of healthy eugonadal men with idiopathic osteoporosis who had impaired bone formation concomitant with calcitriol levels and hypercalciuria.29 In these subjects hypercalciuria did not appear to be due to increased skeletal calcium loss but rather related to enhanced intestinal calcium absorption caused by increased calcitriol levels. This phenotype is similar to that of hypercalciuric kidney stone formers.
The novelty of the current study is its investigation of a substantial number of kidney stone formers, considering different gender groups, menopausal status and use of estrogen. However, there are certain limitations as well. The observational design limited an understanding of the causality in relationships. We could not present fracture data but bone density has been used as a proxy for bone strength and in a large number of studies it has been shown to be a predictor of fracture risk.30 An additional limitation is the lack of data on dietary calcium intake and calciotriopic hormones. Finally, the small sample size of the estrogen untreated group may have reduced the power to detect significant relationships between BMD and urine calcium excretion.
CONCLUSIONS
The results of this study suggest that urinary calcium may be only a marker of but not pathogenetic for the development of bone loss in this population. Future investigations must be directed toward understanding whether the coexistence of hypercalciuria in this population augments bone formation defects.
Acknowledgments
Crystal Aguirre and Tamara Crowe assisted with preparation and review of the manuscript.
Supported by NIH (National Institutes of Health) Grants R01 DK081423 and RO1 DK081423-06, ONR Award N00014-11-1-0203-03 and the Charles YC Pak Foundation.
Abbreviations and Acronyms
- BMD
bone mineral density
- BMI
body mass index
- Ca/Cr
calcium-to-creatinine ratio
- DXA
dual energy x-ray absorptiometry
- FN
femoral neck
- HRT
hormone replacement therapy
- LS
lumbar spine
Footnotes
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
References
- 1.Pearle MS, Calhoun EA, Curhan GC, et al. Urologic Diseases in America project: urolithiasis. J Urol. 2005;173:848. doi: 10.1097/01.ju.0000152082.14384.d7. [DOI] [PubMed] [Google Scholar]
- 2.Denburg MR, Leonard MB, Haynes K, et al. Risk of fracture in urolithiasis: a population-based cohort study using the Health Improvement Network. Clin J Am Soc Nephrol. 2014;9:2133. doi: 10.2215/CJN.04340514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melton LJ, Crowson CS, Khosla S, et al. Fracture risk among patients with urolithiasis: a population-based cohort study. Kidney Int. 1998;53:459. doi: 10.1046/j.1523-1755.1998.00779.x. [DOI] [PubMed] [Google Scholar]
- 4.Carbone LD, Hovey KM, Andrews CA, et al. Urinary tract stones and osteoporosis: findings from the Women’s Health Initiative. J Bone Miner Res. 2015;30:2096. doi: 10.1002/jbmr.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 6.Meschi T, Maggiore U, Fiaccadori E, et al. The effect of fruits and vegetables on urinary stone risk factors. Kidney Int. 2004;66:2402. doi: 10.1111/j.1523-1755.2004.66029.x. [DOI] [PubMed] [Google Scholar]
- 7.Candelas G, Martinez-Lopez JA, Rosario MP, et al. Calcium supplementation and kidney stone risk in osteoporosis: a systematic literature review. Clin Exp Rheumatol. 2012;30:954. [PubMed] [Google Scholar]
- 8.Coe FL, Favus MJ, Crockett T, et al. Effects of low-calcium diet on urine calcium excretion, parathyroid function and serum 1,25(OH)2D3 levels in patients with idiopathic hypercalciuria and in normal subjects. Am J Med. 1982;72:25. doi: 10.1016/0002-9343(82)90567-8. [DOI] [PubMed] [Google Scholar]
- 9.Letavernier E, Traxer O, Daudon M, et al. Determinants of osteopenia in male renal-stone-disease patients with idiopathic hypercalciuria. Clin J Am Soc Nephrol. 2011;6:1149. doi: 10.2215/CJN.10191110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asplin JR, Donahue S, Kinder J, et al. Urine calcium excretion predicts bone loss in idiopathic hypercalciuria. Kidney Int. 2006;70:1463. doi: 10.1038/sj.ki.5001778. [DOI] [PubMed] [Google Scholar]
- 11.Pietschmann F, Breslau NA, Pak CYC. Reduced vertebral bone-density in hypercalciuric nephrolithiasis. J Bone Miner Res. 1992;7:1383. doi: 10.1002/jbmr.5650071205. [DOI] [PubMed] [Google Scholar]
- 12.Wang MC, Bachrach LK, Van Loan M, et al. The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone. 2005;37:474. doi: 10.1016/j.bone.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher JC, Riggs BL and DeLuca HF. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J Clin Endocrinol Metab. 1980;51:1359. doi: 10.1210/jcem-51-6-1359. [DOI] [PubMed] [Google Scholar]
- 14.Pacifici R, Rothstein M, Rifas L, et al. Increased monocyte interleukin-1 activity and decreased vertebral bone-density in patients with fasting idiopathic hypercalciuria. J Clin Endocrinol Metab. 1990;71:138. doi: 10.1210/jcem-71-1-138. [DOI] [PubMed] [Google Scholar]
- 15.Trinchieri A, Nespoli R, Ostini F, et al. A study of dietary calcium and other nutrients in idiopathic renal calcium stone formers with low bone mineral content. J Urol. 1998;159:654. [PubMed] [Google Scholar]
- 16.Pasch A, Frey FJ, Eisenberger U, et al. PTH and 1.25 vitamin D response to a low-calcium diet is associated with bone mineral density in renal stone formers. Nephrol Dial Transplant. 2008;23:2563. doi: 10.1093/ndt/gfn091. [DOI] [PubMed] [Google Scholar]
- 17.da Silva AMM, dos Reis LM, Pereira RC, et al. Bone involvement in idiopathic hypercalciuria. Clin Nephrol. 2002;57:183. doi: 10.5414/cnp57183. [DOI] [PubMed] [Google Scholar]
- 18.Lawoyin S, Sismilich S, Browne R, et al. Bonemineral content in patients with calcium urolithiasis. Metabolism. 1979;28:1250. doi: 10.1016/0026-0495(79)90139-2. [DOI] [PubMed] [Google Scholar]
- 19.Ghazali A, Fuentes V, Desaint C, et al. Low bone mineral density and peripheral blood monocyte activation profile in calcium stone formers with idiopathic hypercalciuria. J Clin Endocrinol Metab. 1997;82:32. doi: 10.1210/jcem.82.1.3649. [DOI] [PubMed] [Google Scholar]
- 20.Fink HA, Litwack-Harrison S, Taylor BC, et al. Clinical utility of routine laboratory testing to identify possible secondary causes in older men with osteoporosis: the Osteoporotic Fractures in Men (MrOS) Study. Osteoporos Int. 2016;27:331. doi: 10.1007/s00198-015-3356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bordier P, Ryckewart A, Gueris J, et al. On the pathogenesis of so-called idiopathic hypercalciuria. Am J Med. 1977;63:398. doi: 10.1016/0002-9343(77)90278-9. [DOI] [PubMed] [Google Scholar]
- 22.Heller HJ, Zerwekh JE, Gottschalk FA, et al. Reduced bone formation and relatively increased bone resorption in absorptive hypercalciuria. Kidney Int. 2007;71:808. doi: 10.1038/sj.ki.5002181. [DOI] [PubMed] [Google Scholar]
- 23.Breslau NA, Preminger GM, Adams BV, et al. Use of ketoconazole to probe the pathogenetic importance of 1,25-dihydroxyvitamin D in absorptive hypercalciuria. J Clin Endocrinol Metab. 1992;75:1446. doi: 10.1210/jcem.75.6.1464646. [DOI] [PubMed] [Google Scholar]
- 24.Zerwekh JE, Pak CY, Kaplan RA, et al. Pathogenetic role of 1 alpha,25-dihydroxyvitamin D in sarcoidosis and absorptive hypercalciuria: different response to prednisolone therapy. J Clin Endocrinol Metab. 1980;51:381. doi: 10.1210/jcem-51-2-381. [DOI] [PubMed] [Google Scholar]
- 25.Raisz LG, Kream BE, Smith MD, et al. Comparison of the effects of vitamin D metabolites on collagen synthesis and resportion of fetal rat bone in organ culture. Calcif Tissue Int. 1980;32:135. doi: 10.1007/BF02408532. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan RA, Haussler MR, Deftos LJ, et al. Role of 1alpha,25-dihydroxyvitamin-D in mediation of intestinal hyperabsorption of calcium in primary hyperparathyroidism and absorptive hypercalciuria. J Clin Invest. 1977;59:756. doi: 10.1172/JCI108696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broadus AE, Insogna KL, Lang R, et al. Evidence for disordered control of 1,25-dihydroxyvitamin-D production in absorptive hypercalciuria. N Engl J Med. 1984;311:73. doi: 10.1056/NEJM198407123110201. [DOI] [PubMed] [Google Scholar]
- 28.Favus MJ, Karnauskas AJ, Parks JH, et al. Peripheral blood monocyte vitamin D receptor levels are elevated in patients with idiopathic hypercalciuria. J Clin Endocrinol Metab. 2004;89:4937. doi: 10.1210/jc.2004-0412. [DOI] [PubMed] [Google Scholar]
- 29.Zerwekh JE, Sakhaee K, Breslau NA, et al. Impaired bone-formation in male idiopathic osteoporosis—further reduction in the presence of concomitant hypercalciuria. Osteoporos Int. 1992;2:128. doi: 10.1007/BF01623819. [DOI] [PubMed] [Google Scholar]
- 30.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]