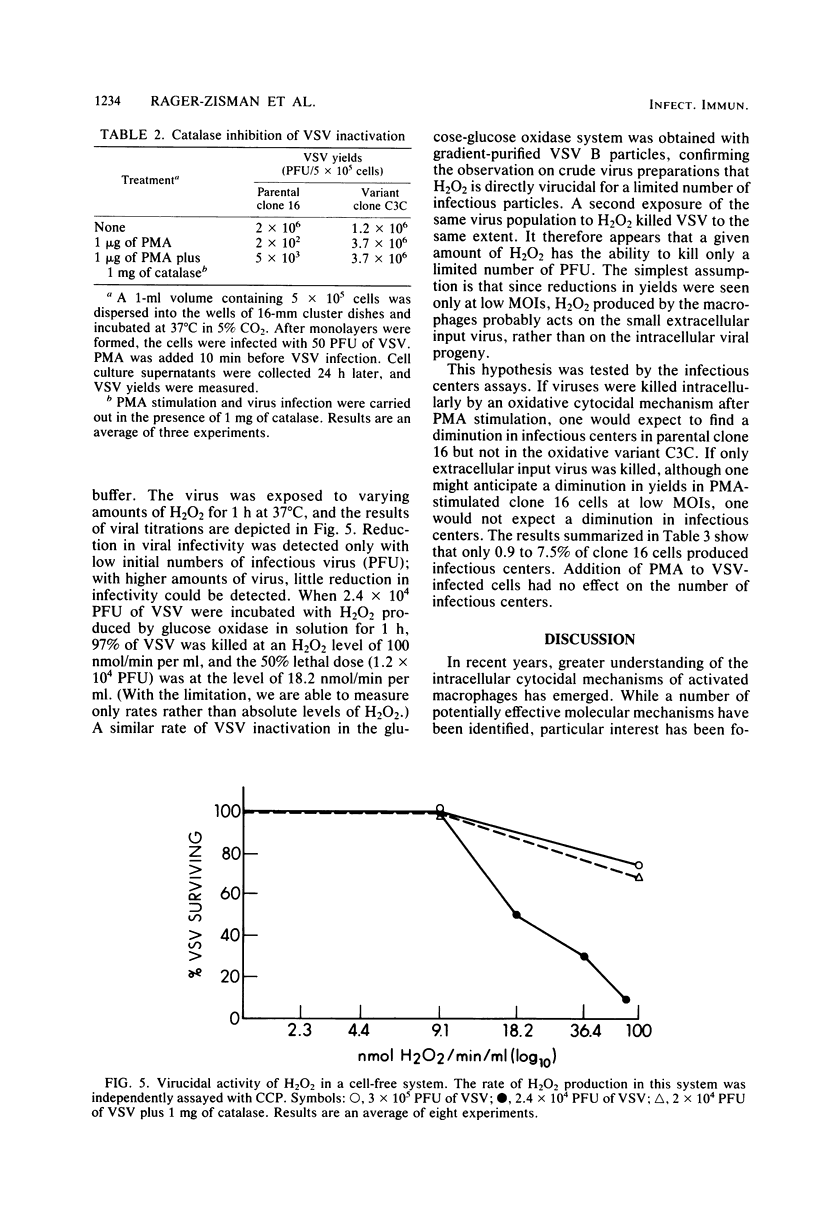
Abstract
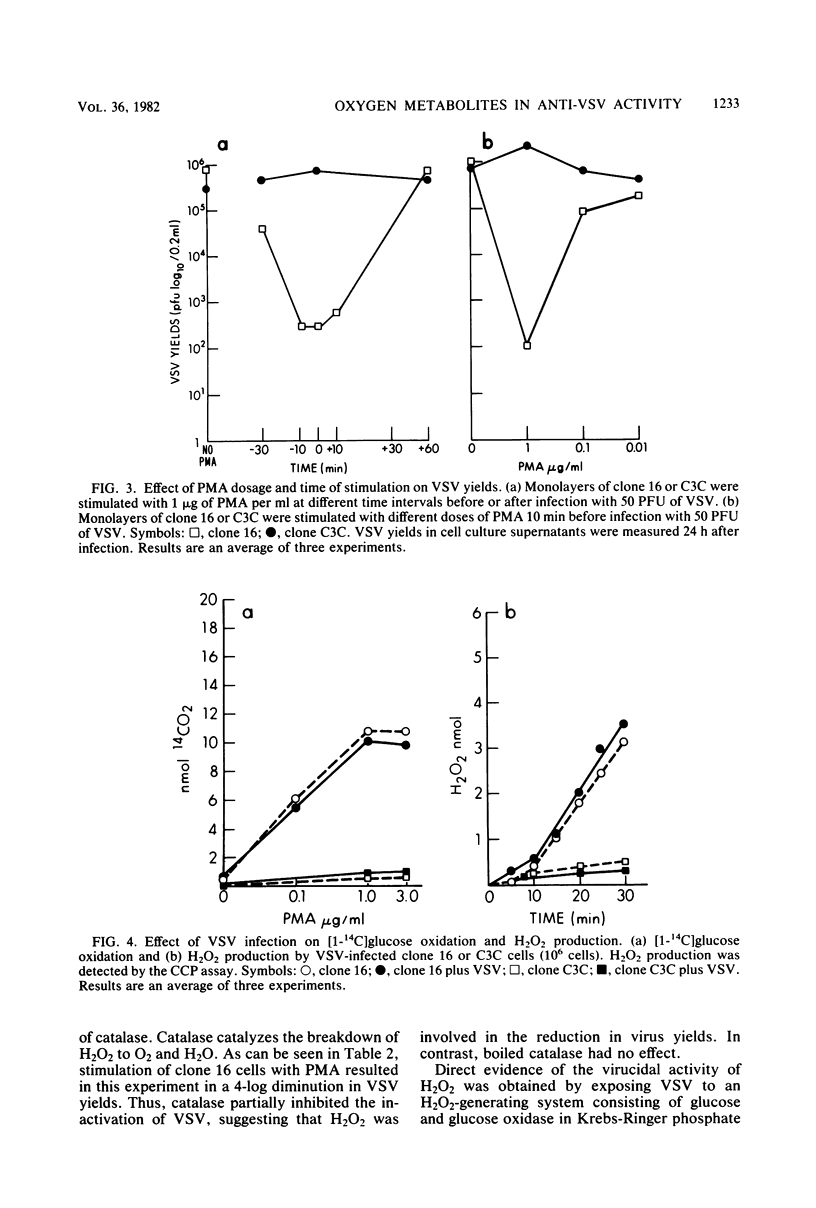
The role of oxygen metabolites in mediating virucidal activity was studied in two cloned macrophage-like cell lines. The parental cell line, J774.16, upon appropriate stimulation with either phorbol myristate acetate (PMA) or aggregated immunoglobulin, is induced to oxidize glucose via the hexose monophosphate shunt and produce O2- and H2O2. A variant derived from it, clone C3C, is defective in oxidative metabolism and cannot be stimulated to produce O2- or H2O2. Significant differences in yields of vesicular stomatitis virus (VSV) between stimulated clone 16 cells and unstimulated cells could be obtained only when low multiplicities were used for infection. Under the same conditions, PMA stimulation of the variant clone C3C produced no reduction in yields. The effect of PMA on virus yields in clone 16 was short-lived and dose dependent. PMA stimulation of either cell line had no effect on the number of infectious centers, suggesting that the antiviral effect was likely to be an extracellular, rather than an intracellular, one. Using glucose oxidase plus aglucose to generate H2O2 in solution, we observed that H2O2 alone is capable of killing limited amounts of VSV. The inactivation of VSV, both by H2O2 in solution and by activated clone 16 cells, could be inhibited by catalase. We conclude that intracellular resistance to VSV is primarily mediated through nonoxidative mechanisms, since activated macrophages can kill only a limited number of infectious virus particles extracellularly by means of secreted H2O2.
Full text
PDF


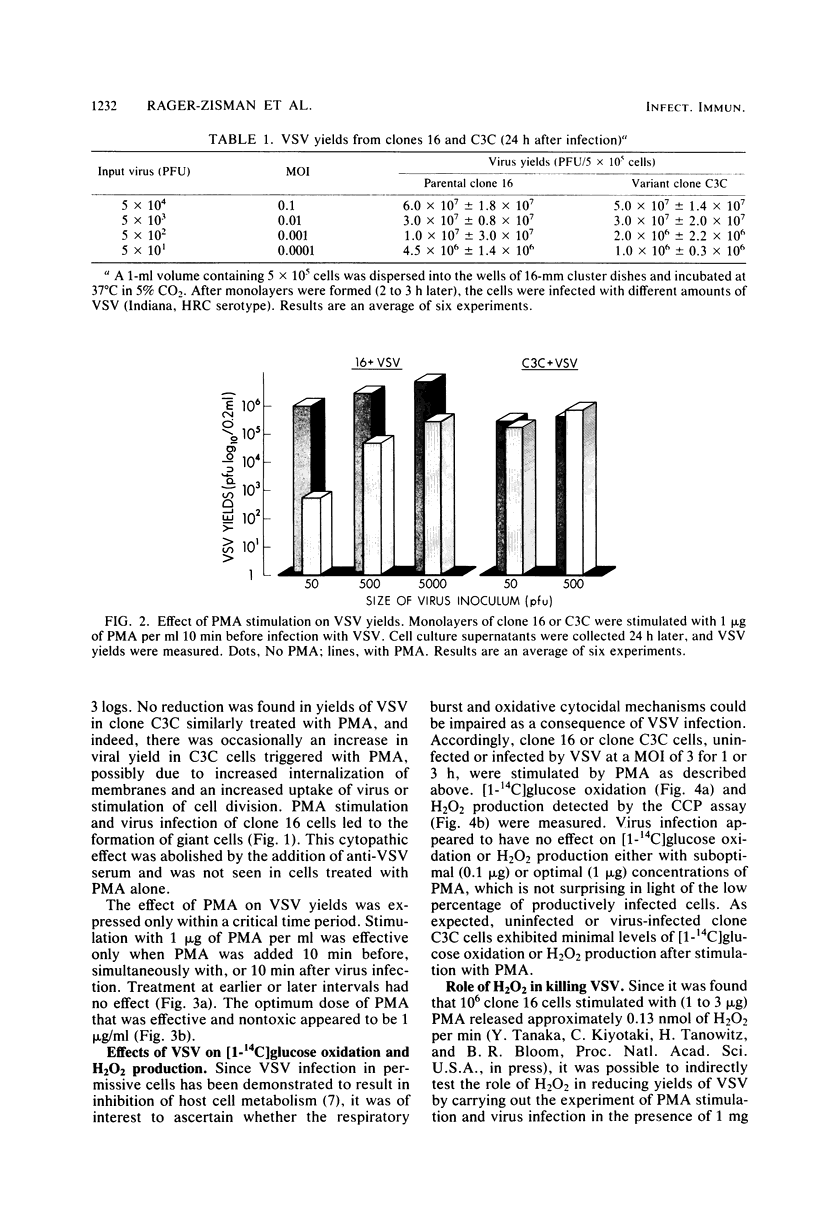



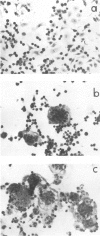
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Belding M. E., Klebanoff S. J., Ray C. G. Peroxidase-mediated virucidal systems. Science. 1970 Jan 9;167(3915):195–196. doi: 10.1126/science.167.3915.195. [DOI] [PubMed] [Google Scholar]
- Damiani G., Kiyotaki C., Soeller W., Sasada M., Peisach J., Bloom B. R. Macrophage variants in oxygen metabolism. J Exp Med. 1980 Oct 1;152(4):808–822. doi: 10.1084/jem.152.4.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. B., Willingham M. C., Pastan I. alpha 2-macroglobulin adsorbed to colloidal gold: a new probe in the study of receptor-mediated endocytosis. J Cell Biol. 1981 Apr;89(1):29–34. doi: 10.1083/jcb.89.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow L. A. Transfer of interferon-producing macrophages: new approach to viral chemotherapy. Science. 1970 Nov 20;170(3960):854–856. doi: 10.1126/science.170.3960.854. [DOI] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr Oxygen metabolism and the microbicidal activity of macrophages. Fed Proc. 1978 Nov;37(13):2759–2764. [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. S., Morahan P. S. Activated macrophages mediate interferon-independent inhibition of herpes simplex virus. Cell Immunol. 1981 Feb;58(1):72–84. doi: 10.1016/0008-8749(81)90150-7. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D. G., Baehner R. L. Disorders of phagocytic cell function. Prog Hematol. 1971;7(0):235–254. [PubMed] [Google Scholar]
- Nowakowski M., Feldman J. D., Kano S., Bloom B. R. The production of vesicular stomatitis virus by antigen- or mitogen-stimulated lymphocytes and continuous lymphoblastoid lines. J Exp Med. 1973 Apr 1;137(4):1042–1059. doi: 10.1084/jem.137.4.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. The role of antibody and host cells in the resistance of mice against infection by coxsackie B-3 virus. J Gen Virol. 1973 Jun;19(3):329–338. doi: 10.1099/0022-1317-19-3-329. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Merigan T. C. A useful quantitative semimicromethod for viral plaque assay. Proc Soc Exp Biol Med. 1973 Apr;142(4):1174–1179. doi: 10.3181/00379727-142-37202. [DOI] [PubMed] [Google Scholar]
- Roos D., Weening R. S. Defects in the oxidative killing of microorganisms by phagocytic leukocytes. Ciba Found Symp. 1978 Jun 6;(65):225–262. [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. Macrophages and viral immunity. Semin Hematol. 1970 Apr;7(2):185–214. [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]
- Zisman B., Wheelock E. F., Allison A. C. Role of macrophages and antibody in resistance of mice against yellow fever virus. J Immunol. 1971 Jul;107(1):236–243. [PubMed] [Google Scholar]