Abstract
The following is a special report on alkylphosphocholine analogs as targeted imaging and therapy agents for cancer, and their potential role in diagnosis and treatment in glioblastoma and brain metastases. These novel cancer-targeting agents display impressive tumor avidity with low background in the normal brain, and multimodal diagnostic imaging and therapy capabilities. The use of these agents may significantly improve diagnosis, treatment and post-treatment follow-up in patients with brain malignancies
KEYWORDS : cancer imaging, cancer therapy, contrast agents, fluorescence-guided surgery, glioblastoma multiforme, multimodality imaging, personalized medicine, theranostics
Practice points.
Glioblastoma is the most prevalent primary brain cancer with more than 10,000 cases a year in the USA.
Ten to thirty percent of cancer patients with systemic malignancies develop metastatic brain cancers, which comprise the majority of all brain cancers.
Advanced imaging of brain malignancies, improving surgical resections, more effective therapies and post-treatment distinctions between progression and pseudo-progression are clinical challenges in the management of brain malignancies.
Alkylphosphocholine (APC) analogs are a special class of ‘diapeutic’ (diagnostic imaging and therapy) agents that display impressive broad-spectrum avidity for malignancies and low background in normal brain.
The multimodal imaging and therapy capabilities, and identical mechanism of uptake and retention of our APC analogs may address unmet clinical needs in brain malignancies by providing synergism between diagnostic imaging, therapy and post-therapy follow-up.
Positron emission tomography APC analog can be used for localizing and imaging cancer throughout the body in surgical planning and clinical follow-up.
Fluorescent APC analog can be used for intraoperative cancer visualization and to improve resections.
Cancer-targeted radiotherapeutic APC analogs can be used to increase radiotherapy efficacy and decrease off-target toxicities.
Together, this suite of diapeutic analogs can have transformative implications on the management of brain malignancies, addressing limitations in diagnostic imaging, treatment and follow-up.
‘Theranostics’ are defined as diagnostic tests that inform treatment choice and clinical outcome. To improve patient outcomes, theranostics would ideally be used in therapy decision-making to predict treatment efficacy and response, and change clinical management by avoiding unnecessary risks and associated costs of ineffective care [1,2]. Optimizing therapeutic efficacy and safety is especially warranted in treating primary brain tumors or brain metastases, where tumor cells are infiltrating or adjacent to vital functional brain tissue, and deleterious side effects carry significant morbidity and mortality. Therefore, theranostics can have transformative implications in managing brain tumor patients. In this report, we highlight alkylphosphocholine (APC) analogs as a special theranostics type: the ‘diapeutic’ (diagnostic imaging and therapy) agent. APC analogs have a cancer-selective retention mechanism mediated by an identical cancer-targeting chemical backbone, exhibit broad specificity for tumor cells in multimodal imaging and therapy, and could predict, inform and monitor therapy outcomes. We discuss how this versatility may ultimately transform clinical management of primary and metastatic brain tumors.
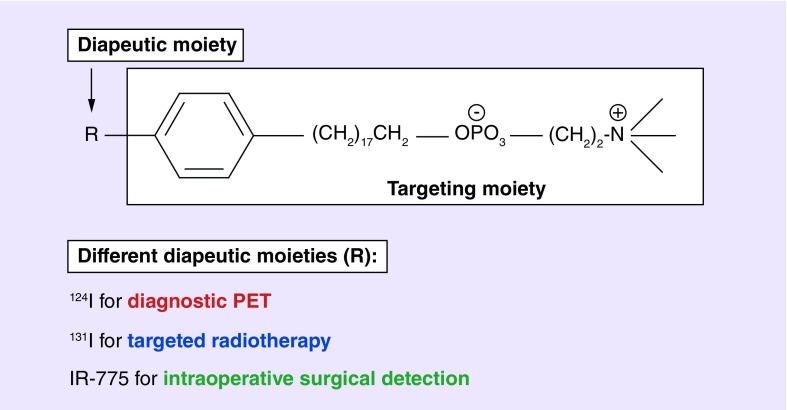
124I-CLR1404, 131I-CLR1404 and the near-infrared fluorescent analog CLR1502 are related novel small molecule, cancer-targeting APC analogs. These APC analogs share a common targeting moiety that is selectively retained by tumor cells of many different histologies linked to a functional moiety fashioned for diagnostic imaging or therapy (Figure 1) [3,4]. The lead compound CLR1404 was selected from over 30 radioiodinated phospholipid ether analogs from four different structural classes including an APC class [5]. CLR1404 exhibited the highest tumor signal to background ratio in rodent flank xenograft models and excellent in vivo stability against deiodination due to iodine's position on the aromatic ring [5]. APC derivatives localize to cellular and intracellular membranes via uptake into cholesterol-rich plasma membrane lipid raft domains overexpressed in cancer cells [3,6]. The relative deficiency of phospholipid catabolizing enzymes in tumor versus normal cells results in prolonged tumor retention, in contrast to APC clearance by normal tissues. Remarkably, substitution of iodine with a large optically active functional group (e.g., bodipy, IR-775) does not alter APC's tumor selectivity. APCs are being further evaluated in multiple early phase human clinical imaging and radiotherapy trials, after extensive testing and demonstration of prolonged selective retention in over 60 in vivo rodent and human cancers and cancer stem cell models [3,7,8]. Two radioinodinated CLR1404 analogs have recently undergone five multi-institutional human imaging and therapy clinical trials,124I-CLR1404 for positron emission tomography (PET) imaging and 131I-CLR1404 for therapy and single photon emission computed tomography imaging. Preliminary data from a multi-institutional Phase II trial for glioblastoma (GBM) imaging with 124I-CLR1404 is currently being evaluated [7,8]. The near-infrared fluorescent analog CLR1502 is also nearing clinical trial testing.
Figure 1. . Alkylphosphocholine analogs are composed of a targeting moiety and a diapeutic moiety.
The diapeutic moiety can be substituted with 124I for diagnostic PET imaging, 131I for targeted radiotherapy, and a near-infrared dye (IR-775) for intraoperative surgical detection. PET: Positron emission tomography.
While theranostic strategies often rely on technologies and techniques that are radically different to inform clinical decisions, the identical tumor-targeting backbone of all three APC analogs confers a shared mechanism of uptake and retention. Due to their identical uptake and retention mechanisms, CLR1404 derivatives have similar pharmacological half-life, tissue biodistribution and cancer-selective retention characteristics. This commonality ensures that the synergistic use of these agents reliably and specifically targets the same cancer cells in diagnosis and staging, treatment planning and dosimetry and potentially subsequent clinical follow-up and monitoring of treatment response. To illustrate the potential advantage of diapeutic agents, consider a patient with a brain malignancy: 124I-CLR1404 is administered initially for whole body or global assessment of cancer lesion(s) and staging. Fluorescent analog CLR1502 would then be used locally for optical cancer visualization to optimize surgical resection, and illuminate residual unresectable tumor. Postoperative uptake of APC PET imaging tracer can be used to calculate personalized dosimetry for the targeted radiotherapy agent 131I-CLR1404. Follow-up APC PET imaging may further inform patient management through possible detection of tumor recurrence. As this example demonstrates, due to the identical cancer-targeting mechanism of diapeutic APCs, this suite of APC analogs can be used at multiple clinical phases, potentially maximizing effectiveness and simultaneously minimizing complications during surgery and radiotherapy on a patient-to-patient basis.
Diapeutic APCs have four primary advantages over current standard of care for brain cancer diagnostic imaging and therapy, which include: targeting of metastatic malignancies in addition to primary brain malignancies; impressive PET signal to background in the brain; long retention profiles that permit evaluation of postsurgical outcomes; and patient-specific staging and dosimetry information for the next phases of clinical care [9]. Currently, GBM is the most prevalent primary brain cancer with approximately 20,000 diagnosed per year in the USA, and 10–30% of patients with systemic malignancies develop brain metastases, with 200,000 cases per year in the USA [10–12]. Primary and metastatic tumors in the brain can be detected and treated with APC analogs due to their observed broad-spectrum selective uptake by many kinds of cancer histologies, with potential benefit for a significant number of cancer patients [3,13].
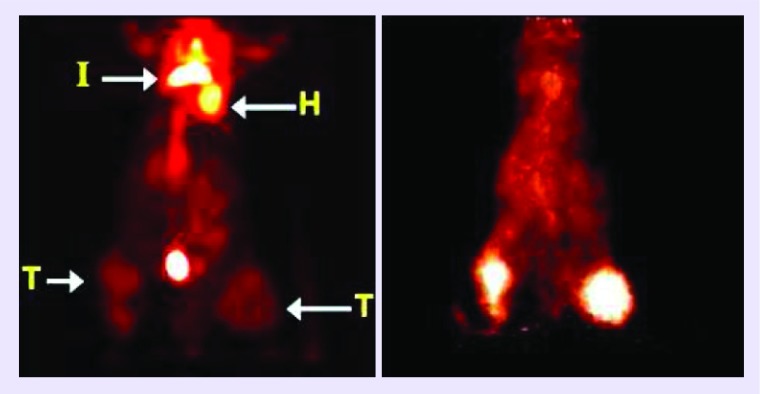
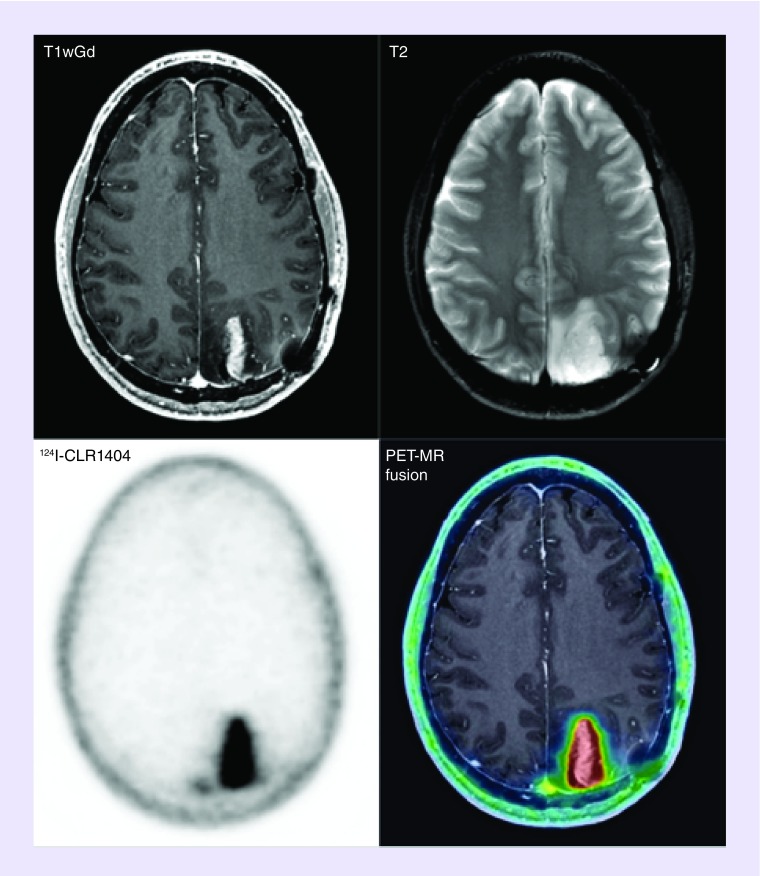
PET with fludeoxyglucose (18F-FDG), a positron-emitting glucose analog, is used widely in solid tumor imaging for the purpose of diagnosing, staging, restaging and monitoring therapy response in most cancers with the notable exception of primary and metastatic lesions to the brain [14–16]. In the context of brain lesions, 18F-FDG currently suffers from low sensitivity and specificity due to its high physiologic uptake in normal brain tissue, low uptake in necrotic lesions and propensity for false-positive inflammation [17,18]. Importantly, 124I-CLR1404 has shown impressive resolution on brain cancer PET-imaging in rodents and humans due to its selectivity and retention in multiple tumor histologies including GBMs and metastatic brain malignancies. APC analogs show high sensitivity and specificity for tumor detection and low uptake in background tissues and inflammatory lesions (Figure 2) [3]. 124I-CLR1404 PET imaging of a malignant glioma patient demonstrated probable recurrence with very high signal to background that was surgically verified (Figure 3) [3]. Lastly, the diapeutic paradigm and long retention in cancers permit 124I-CLR1404 imaging to provide critical information for biopsy or surgery, personalized, tailored dosimetry for the targeted radiotherapy compound 131I-CLR1404 and follow-up monitoring.
Figure 2. . MicroPET projection view images of a mouse with human PC3 tumors (T) in the right flank and pseudo metastasis in the left tibia (T), a carrageenan-induced inflammatory lesion (I) located between the scapulae, and heart (H).
Left image was acquired 1 h after administration of 18F-FDG. Right image was obtained 24 h after administering 124I-CLR1404 to the same mouse, 24 h after the FDG scan.
Reprinted with permission from [3] © AAAS (Supplementary Material). 18F-FDG: PET with fludeoxyglucose.
Figure 3. . Malignant glioma was resected 2 years before this axial T1-contrast MR and positron emission tomography imaging that shows likely recurrence in a patient.
Reprinted with permission from [3] © AAAS (Supplementary Material).
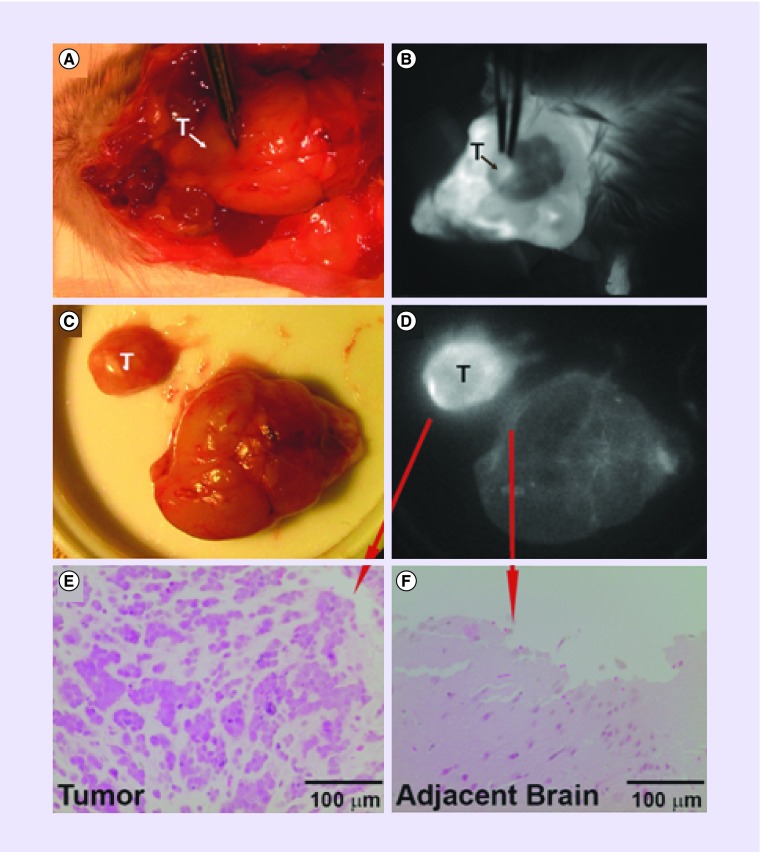
Diapeutic APCs may also improve resection of brain tumors. The goal of maximal safe resection of brain tumors is often difficult due to tumor proximity to normal brain tissue, or infiltration into normal brain parenchyma [19]. Patients with gross total resection for GBMs and low-grade gliomas have longer median survival than patients with subtotal resection outcomes [20–22]. Recently, the use of optical agents for intraoperative imaging has shown promise in surgical oncology to improve resection outcomes, and thus patient survival. A randomized Phase III clinical trial in Europe demonstrated that patients who received fluorescence-guided surgery with 5-aminolevulinic acid followed by radiotherapy had improved resection outcomes, and progression-free survival by 6 months without a decline in performance status compared with those that received conventional surgery under white light followed by radiotherapy [23,24]. Real-time tumor visualization may achieve additional clinical benefits including more efficient surgeries, decreased operative times, decreased anesthesia exposure and reduced complications due to avoidance of normal structures. The near-infrared fluorescent APC analog CLR1502 showed robust tumor fluorescence in orthotopic GBM xenografts derived from human cancer stem cells [13] (Figure 4 & Supplementary Material). The high tumor fluorescence of CLR1502 may result from active targeting and penetration and lower near-infrared range background characteristics. This agent has also been used as a fluorescent tumor label in preclinical colon cancer, breast cancer and prostate cancer models in addition to GBM models, and shows promise as an intraoperative surgical adjunct [25–27]. Furthermore, postresection fluorescence signal from CLR1502 and APC PET uptake signal can provide information about the extent of resection.
Figure 4. . CLR1502 fluorescence imaging of a patient-derived glioblastoma stem cell orthotropic xenograft.
(A & B) White light and fluorescence imaging of glioblastoma stem cell xenograft and (C & D) after resection. (E & F) Verification of tumor and normal brain by histology.
Reprinted with permission from [13] © Wolters Kluwer Health, Inc.
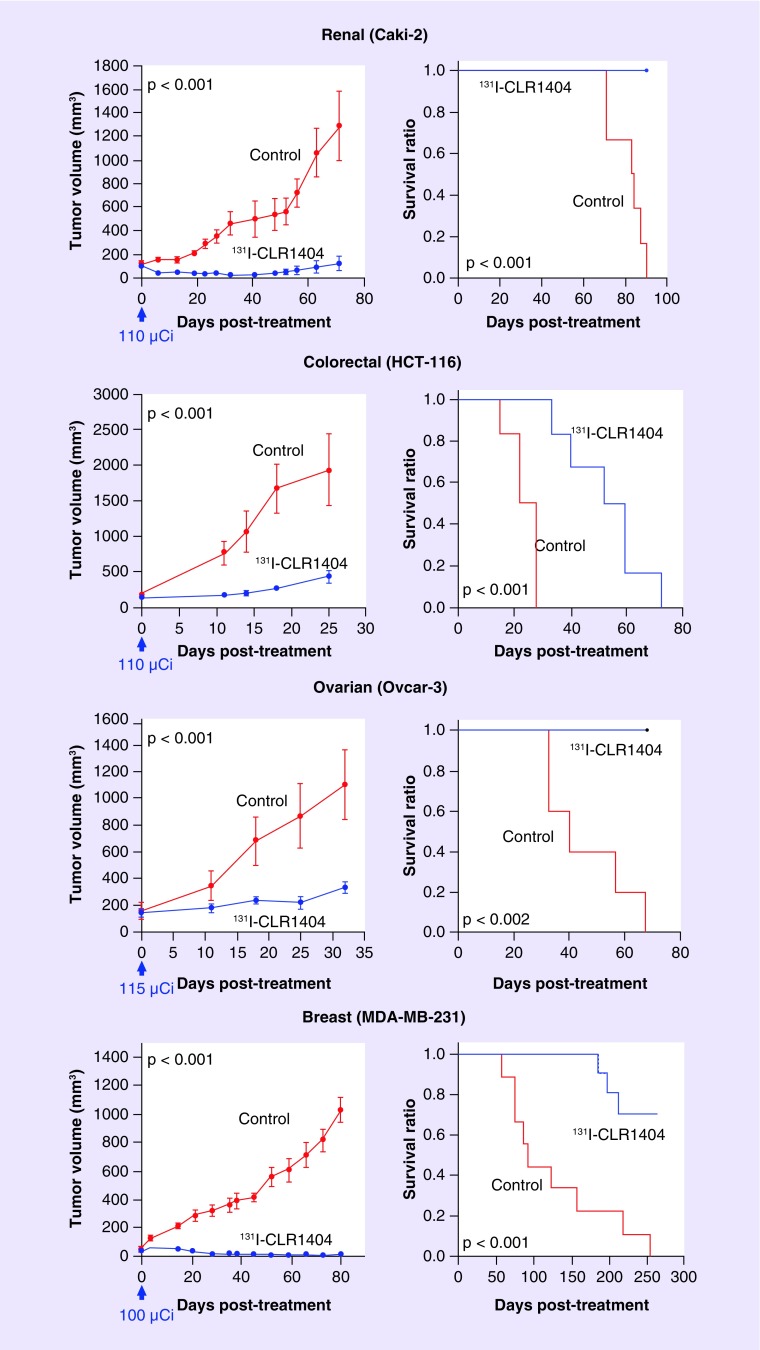
In addition, the targeted radiotherapeutic analog 131I-CLR1404 may be a safe and more effective alternative to current radiotherapy regimens in targeting tumor cells that are not resectable during initial treatment. Preclinical work with human cancer cell lines that frequently metastasize to the brain demonstrated that a single, nonoptimized dose of 100–115 µCi of 131I-CLR1404 significantly improved survival in mice with subcutaneously implanted flank xenografts (Figure 5). Furthermore, glioblastoma stem cells are resistant to radiation therapy and chemotherapy with temozolomide, and may account for tumor recurrence despite current therapies [28]. Importantly, 131I-CLR1404 targets brain cancer progenitor cells in addition to bulk tumor cells, while avoiding normal brain tissues, which may improve radiotherapy outcomes [3]. Optimizing 131I-CLR1404 dosing on patient-specific basis, minimizing off-target toxicities and monitoring radiotherapy or chemotherapy outcomes can be achieved using 124I-CLR1404 PET imaging in the proposed diapeutic paradigm.
Figure 5. . Tumor growth and animal survival after 131I-CLR1404 therapy.
Nude mice harboring human tumor xenografts (n = 6 renal, 6 colorectal, 5 ovarian and 9 breast) were administered a single, nonoptimized 131I-CLR1404 dose (arrow). Control animals were administered a mass-equivalent CLR1404 dose (n = 6).
Reprinted with permission from [3] © AAAS.
At last, diapeutic APCs are proposed for use in clinical follow-up imaging to assess treatment efficacy or disease progression. Approximately 20% of patients treated with chemoradiotherapy show post-treatment-related enhancement on MRI also known as pseudoprogression, frequently difficult to distinguish from tumor progression or recurrence [29,30]. Much research in tumor imaging technologies and strategies is being pursued to address this clinical challenge to distinguish between pseudoprogression and true tumor progression [31]. Several amino-acid tracers such as 18F-fluorothymidine and 18F-fluoro-glutamine undergoing clinical assessment have variable signal sensitivity and specificity based on tumor metabolism [16,29,32]. In contrast, 124I-CLR1404 is being evaluated as a possible agent that may detect tumor progression due to its favorable selectivity and retention profile, and lack of uptake in areas of inflammation or necrosis [3].
Conclusion
In summary, the diapeutic utility of APC analogs, which rely on the identical APC cancer specific mechanism of uptake and retention, synergizes each step in patient management from diagnostic imaging to post-treatment monitoring to a degree that is not seen with other theranostic strategies using different distinct modalities or mechanisms. Diapeutic APC analogs are a class of cancer-targeted agents with broad-spectrum cancer targeting and multimodal diagnostic imaging and therapy capabilities that may significantly advance care of patients with brain malignancies.
Synthesis and testing of APC analogs with other capabilities may increase the diagnostic and therapeutic arsenal for this targeting platform. The use of APC agents as diapeutics will be advanced by studies on dosimetry with the PET agent, microdosimetry studies of targeted radiotherapeutics and studies on therapeutic effects. Notably, regulatory approval bodies (e.g., US FDA) do not have a current framework for evaluating the planned clinical combination trials with multiple APC agents that are needed to evaluate the diapeutic synergism of these agents or for evaluating optical detection technologies. While we are able to visualize high-grade gliomas and metastatic lesions that have disrupted barriers, visualization of lower grade gliomas has not been adequately studied. Studies are underway to determine and characterize the blood–brain barrier permeability of APC analogs [33]. Despite the need for further work, the promise of this diapeutic paradigm has already started to materialize with 124I-CLR1404 as a diagnostic agent for cancer diagnosis, staging and dosimetry of therapeutic analogs, its companion 131I-CLR1404 as a therapeutic agent for targeted radiation therapy and CLR1502 as intraoperative fluorescent agent to aid surgical resection. Introduction of next-generation APC analogs will increase the complement of diapeutic APC analogs in imaging and therapy to potentially advance the clinical management of brain cancer patients.
Supplementary Material
Footnotes
Financial & competing interests disclosure
JP Weichert is founder of Cellectar Biosciences, Inc., which holds the licensing rights to the described APC analogs. Cellectar Biosciences, Inc. provided the APC analogs used for this research. The authors grant permission to publish this information or an appropriate summary thereof with the abstract. This work was supported in part by NIH grants R01CA158800 (JP Weichert, LT Hall, JS Kuo), R01NS75995 (JS Kuo), T32 GM008692 (RR Zhang), UL1RR025011 and P30CA014520 (UW Carbone Cancer Center), Headrush Brain Tumor Research Professorship (JS Kuo) and Roger Loff Memorial Fund (JS Kuo). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Hegi ME, Diserens A-C, Godard S, et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin. Cancer Res. 2004;10(6):1871–1874. doi: 10.1158/1078-0432.ccr-03-0384. [DOI] [PubMed] [Google Scholar]
- 2.Nicolaides NC, O'Shannessy DJ, Albone E, Grasso L. Co-development of diagnostic vectors to support targeted therapies and theranostics: essential tools in personalized cancer therapy. Front. Oncol. 2014;4:141. doi: 10.3389/fonc.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weichert JP, Clark PA, Kandela IK, et al. Alkylphosphocholine analogs for broad-spectrum cancer imaging and therapy. Sci. Transl. Med. 2014;6(240):240ra275. doi: 10.1126/scitranslmed.3007646. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Highlights the preclinical and human clinical studies performed with the radioiodinated APC analogs 124I-CLR1404 and 131I-CLR1404.
- 4.Weichert JP, Van Dort ME, Groziak MP, Counsell RE. Radioiodination via isotope exchange in pivalic acid. Int. J. Rad. Appl. Instrum. A. 1986;37(8):907–913. [Google Scholar]
- 5.Pinchuk AN, Rampy MA, Longino MA, et al. Synthesis and structure−activity relationship effects on the tumor avidity of radioiodinated phospholipid ether analogues. J. Med. Chem. 2006;49(7):2155–2165. doi: 10.1021/jm050252g. [DOI] [PubMed] [Google Scholar]; •• Highlights studies performed to assess the structure–activity relationships of alkylphosphocholines and related compound classes in rodent models.
- 6.Van Blitterswijk WJ, Verheij M. Anticancer mechanisms and clinical application of alkylphospholipids. Biochim. Biophys. Acta. 2013;1831(3):663–674. doi: 10.1016/j.bbalip.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Lubner SJ, Mullvain J, Perlman S, et al. A Phase 1, multi-center, open-label, dose-escalation study of 131I-CLR1404 in subjects with relapsed or refractory advanced solid malignancies. Cancer Invest. 2015;33(10):483–489. doi: 10.3109/07357907.2015.1081691. [DOI] [PubMed] [Google Scholar]
- 8.Grudzinski JJ, Titz B, Kozak K, et al. A Phase 1 study of 131I-CLR1404 in patients with relapsed or refractory advanced solid tumors: dosimetry, biodistribution, pharmacokinetics, and safety. PLoS ONE. 2014;9(11):e111652. doi: 10.1371/journal.pone.0111652. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Highlights the pharmacokinetics and biodistribution of 131I-CLR1404 in human patients with advanced solid tumors.
- 9.Ricci PE, Dungan DH. Imaging of low- and intermediate-grade gliomas. Semin. Radiat. Oncol. 2001;11(2):103–112. doi: 10.1053/srao.2001.21420. [DOI] [PubMed] [Google Scholar]
- 10.Johnson J, Young B. Demographics of brain metastasis. Neurosurg. Clin. N. Am. 1996;7(3):337–344. [PubMed] [Google Scholar]
- 11.Graus F, Walker RW, Allen JC. Brain metastases in children. J. Pediatr. 1983;103(4):558–561. doi: 10.1016/s0022-3476(83)80583-6. [DOI] [PubMed] [Google Scholar]
- 12.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl. 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanson KI, Clark PA, Zhang RR, et al. Fluorescent cancer-selective alkylphosphocholine analogs for intraoperative glioma detection. Neurosurgery. 2015;76(2):115–124. doi: 10.1227/NEU.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Highlights the work performed using orthotopic glioblastoma models with the near-infrared fluorescent analog CLR1502.
- 14.Nimsky C, Ganslandt O, Cerny S, Hastreiter P, Greiner G, Fahlbusch R. Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery. 2000;47(5):1070–1080. doi: 10.1097/00006123-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Boellaard R, O'Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur. J. Nucl. Med. Mol. Imaging. 2010;37(1):181–200. doi: 10.1007/s00259-009-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Cloughesy T, Kamdar N, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J. Nucl. Med. 2005;46(6):945–952. [PubMed] [Google Scholar]
- 17.Heiss W-D, Raab P, Lanfermann H. Multimodality assessment of brain tumors and tumor recurrence. J. Nucl. Med. 2011;52(10):1585–1600. doi: 10.2967/jnumed.110.084210. [DOI] [PubMed] [Google Scholar]
- 18.Chao ST, Suh JH, Raja S, Lee S-Y, Barnett G. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int. J. Cancer. 2001;96(3):191–197. doi: 10.1002/ijc.1016. [DOI] [PubMed] [Google Scholar]
- 19.Preusser M, De Ribaupierre S, Wöhrer A, et al. Current concepts and management of glioblastoma. Ann. Neurol. 2011;70(1):9–21. doi: 10.1002/ana.22425. [DOI] [PubMed] [Google Scholar]
- 20.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J. Neurosurg. 2001;95(2):190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 21.McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63(4):700–708. doi: 10.1227/01.NEU.0000325729.41085.73. [DOI] [PubMed] [Google Scholar]
- 22.McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J. Neurosurg. 2009;110(1):156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 23.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre Phase III trial. Lancet Oncol. 2006;7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 24.Van den Bent MJ, Stupp R, Mason W, Mirimanoff RO, Lacombe D, Gorlia T. Impact of extent of resection on overall survival in newly-diagnosed glioblastoma after chemo-irradiation with temozolomide: further analysis of EORTC study 26981. EJC Suppl. 2005;3(2):134. [Google Scholar]
- 25.Korb ML, Warram JM, Grudzinski J, Weichert J, Jeffery J, Rosenthal EL. Breast cancer imaging using the near-infrared fluorescent agent, CLR1502. Mol. Imaging. 2014 doi: 10.2310/7290.2014.00040. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Deming DA, Maher ME, Leystra AA, et al. Phospholipid ether analogs for the detection of colorectal tumors. PLoS One. 2014;9(10):e109668. doi: 10.1371/journal.pone.0109668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hafeez BB, Zhong W, Weichert J, Dreckschmidt NE, Jamal MS, Verma AK. Genetic ablation of PKC epsilon inhibits prostate cancer development and metastasis in transgenic mouse model of prostate adenocarcinoma. Cancer Res. 2011;71(6):2318–2327. doi: 10.1158/0008-5472.CAN-10-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 29.Brandsma D, Stalpers L, Taal W, Sminia P, Van Den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 30.Taal W, Brandsma D, De Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113(2):405–410. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 31.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in Neuro-Oncology Working Group. J. Clin. Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 32.Venneti S, Dunphy MP, Zhang H, et al. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo . Sci. Transl. Med. 2015;7(274):274ra217–274ra217. doi: 10.1126/scitranslmed.aaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark PA, Al-Ahmad AJ, Qian T, et al. Analysis of cancer-targeting alkylphosphocholine analogue permeability characteristics using a human induced pluripotent stem cell blood–brain barrier model. Mol. Pharmaceut. 2016 doi: 10.1021/acs.molpharmaceut.6b00441. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.