Abstract
AIM
To evaluate the effect of initial stent position on transjugular intrahepatic portosystemic shunt (TIPS).
METHODS
We studied 425 patients from January 2004 to January 2015 with refractory ascites or variceal bleeding who required TIPS placement. Patients were randomly divided into group A (stent in hepatic vein, n = 57), group B (stent extended to junction of hepatic vein and inferior vena cava, n = 136), group C (stent in left branch of portal vein, n = 83) and group D (stent in main portal vein, n = 149). Primary unassisted patency was compared using Kaplan-Meier analysis, and incidence of recurrence of bleeding, ascites and hepatic encephalopathy (HE) were analyzed.
RESULTS
The mean primary unassisted patency rate in group B tended to be higher than in group A at 3, 6 and 12 mo (P = 0.001, 0.000 and 0.005), and in group D it tended to be lower than in group C at 3, 6 and 12 mo (P = 0.012, 0.000 and 0.028). The median shunt primary patency time for group A was shorter than for group B (5.2 mo vs 9.1 mo, 95%CI: 4.3-5.6, P = 0.013, log-rank test), while for group C it was longer than for group D (8.3 mo vs 6.9 mo, 95%CI: 6.3-7.6, P = 0.025, log-rank test). Recurrence of bleeding and ascites in group A was higher than in group B at 3 mo (P = 0.014 and 0.020), 6 mo (P = 0.014 and 0.019) and 12 mo (P = 0.024 and 0.034. Recurrence in group D was higher than in group C at 3 mo (P = 0.035 and 0.035), 6 mo (P = 0.038 and 0.022) and 12 mo (P = 0.017 and 0.009). The incidence of HE was not significantly different among any of the groups (P = 0.965).
CONCLUSION
The initial stent position can markedly affect stent patency, which potentially influences the risk of recurrent symptoms associated with shunt stenosis or occlusion.
Keywords: Transjugular intrahepatic portosystemic shunt, Liver cirrhosis, Stent position, Portal hypertension
Core tip: We studied a large cohort of patients with cirrhosis who underwent transjugular intrahepatic portosystemic shunt for recurrent variceal bleeding and ascites. Initial stent position at both the distal and proximal endpoints can markedly affect stent patency, which potentially influences the risk of recurrent symptoms associated with shunt stenosis or occlusion.
INTRODUCTION
Transjugular intrahepatic portosystemic shunt (TIPS) is currently used for the treatment of complications of portal hypertension[1]. The establishment of TIPS has been widely accepted as an alternative to surgery in the management of complications from portal hypertension, such as variceal bleeding, refractory ascites, Budd-Chiari syndrome, hepatorenal syndrome, hepatic hydrothorax and even hepatopulmonary syndrome[2]. After TIPS was introduced as an alternative treatment for complications related to portal hypertension, it has become progressively recognized as an effective therapeutic option in a growing number of clinical situations[3,4].
Despite its efficacy in preventing such syndromes, however, TIPS is prone to shunt stenosis or occlusion leading to shunt failure, and approximately half of all patients with TIPS require shunt revision during follow-up[5], making close surveillance and frequent costly revisions mandatory[6]. Recently, the use of a new generation of covered stents has overcome the problem of shunt dysfunction with significant improvement in TIPS patency and clinical efficacy[7]. Many experimental and clinical studies[8] have been performed with the use of covered stent grafts to improve the long-term patency of TIPS. The best results have been achieved with the use of stent grafts covered with polytetrafluoroethylene (PTFE), as reported by Nishimine et al[9]; and these positive results were confirmed by Haskal[10] and Jung et al[11]. Even with these new stents, however, post-TIPS shunt obstruction and a high clinical symptom recurrence rate remain problematic.
The purpose of this study was to evaluate the effect of initial stent position on primary shunt patency and time to recurrence of TIPS-related symptoms of ascites or variceal bleeding.
MATERIALS AND METHODS
Patient information
We retrospectively enrolled 1950 patients referred to us on an intention-to-treat basis who underwent a TIPS procedure at our institution between January 2004 and January 2015. The Ethic Institutional Review Board Committee approved the study protocol and all patients agreed to treatment by written consent. We reviewed the medical records and medical images for 436 patients to gather information regarding the underlying etiology, clinical presentation, age, sex, and severity of cirrhosis. Four hundred and twenty-five patients successfully underwent TIPS, and the demographic data were compared between the groups. Age, sex, etiology and Child-Pugh score are shown in Table 1, and there were no significant differences among the groups.
Table 1.
Demographic characteristics of the patients
| Parameter |
Group |
P value | |||
| A | B | C | D | ||
| n | 57 | 136 | 83 | 149 | |
| Male/female | 24/33 | 64/72 | 42/41 | 70/79 | 0.806 |
| Age in yr | 39.35 ± 19.56 | 36.97 ± 15.79 | 34.33 ± 12.35 | 37.69 ± 16.69 | 0.959 |
| Etiology, viral/not viral | 52/5 | 125/11 | 78/5 | 136/13 | 0.806 |
| Child-Pugh class, A/B/C/D | 6/44/7 | 18/99/19 | 8/62/13 | 16/109/24 | 0.968 |
Study design
This study was a randomized, single-center, open-label trial that compared the effect of primary stent position on primary shunt patency at different stent ends, leading to different clinical manifestations. The patients were randomly divided into four groups: A (stent in hepatic vein, n = 57), B [stent extended to junction of hepatic vein and inferior vena cava (IVC), n = 136], C (stent in left branch of portal vein, n = 83) and D (stent in main portal vein, n = 149), according to the initial stent position in the distal inflow and proximal outflow ends. The inclusion criteria were portal-hypertension-related complications of recurrent variceal bleeding (n = 309) after a session of variceal sclerotherapy or refractory ascites (n = 116) or both (n = 78) that required TIPS placement. The exclusion criteria were as follows: variceal bleeding as an emergency indication, portal vein thrombosis, present history of hepatic encephalopathy (HE), severe right-sided heart failure, severe liver failure (bilirubin > 4 mg/dL), polycystic liver disease, dilated biliary ducts, age > 75 years, bilirubin level > 5 mg/dL, creatinine level > 3 mg/dL, Child-Pugh score > 11, hepatic carcinoma, sepsis, spontaneous bacterial peritonitis, and patients who underwent liver transplantation.
TIPS procedure
TIPS was carried out under standard local anesthesia. All of the patients were evaluated and followed up by the same medical team using a prospective protocol diagnostic work-up and surveillance strategy. Before the operation, the patients’ medical histories were taken, and after TIPS, the four groups were followed up according to the same protocol.
TIPS was performed through a transjugular approach, as described previously[12]. After mesenteric artery angiography was performed, the right hepatic vein was reached using a TIPS set (RUPS-100; Cook Inc., Bloomington, IL, United States), and the left or right branch of the portal vein was punctured under the guidance of digital subtraction angiography in both the posterior anterior and lateral positions. When the branch of the portal vein was punctured and confirmed by portoangiography, a 7-8-mm balloon (Cook Inc.) dilated the hepatic tract. A 7-8-mm covered stent (Fluency; Bard, Voisins le Bretonneux, France) was used for TIPS creation and two bare 10-mm stents were used inside the Bard stent. Portosystemic gradient (PSG) and right atrial pressure were measured before and after TIPS.
The entire length of the intrahepatic tract should be covered by the stent graft. The stent was positioned as described in the study design for each group (Figure 1).
Figure 1.
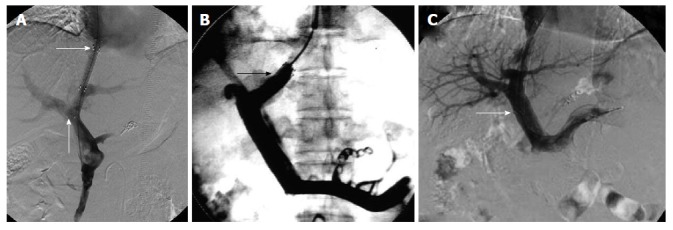
Stent was positioned as described in the study design for each group. A: Ideal position of the stent endpoint in the TIPS procedure. The transverse arrow indicates the proximal end of the stent at the junction of the hepatic vein and IVC. No angle was formed between the stent and left branch of the portal vein. The proximal end of the stent was located at the junction of the hepatic vein and IVC and did not cause stenosis or occlusion, and did not affect liver transplantation. The arrow pointing up indicates the distal end of the stent located at the left branch of the portal vein. The stent was in parallel to the direction of blood flow, therefore, it was not easy to cause stent stenosis or occlusion; B: The proximal end of the stent in the hepatic vein in the TIPS procedure. The arrow indicates the proximal end of the stent in the hepatic vein. The stent endpoint caused stenosis or occlusion due to blood flow shear force and vascular intimal hyperplasia; C: Distal end of the stent in the main hepatic vein in the TIPS procedure. The arrow indicates the distal end of the stent in the main hepatic vein in the transjugular intrahepatic portosystemic shunt procedure. The distal end of the stent was prone to stimulate the blood vessel wall and interfered with the portal vein blood flow. The stent endpoint caused stenosis or occlusion due to blood flow shear force and vascular intimal hyperplasia. TIPS: Transjugular intrahepatic portosystemic shunt; IVC: Inferior vena cava.
The shunts were dilated to full nominal diameter to reach a target PSG of < 12 mmHg and gastroesophageal collateral vessels observed during the TIPS procedure were embolized with coils (Cook Inc.). A covered stent (Viatorr; W.L. Gore and Associates, Flagstaff, AZ, United States) was not used because they were not approved by the State Food and Drug Administration in the Chinese Mainland for the patients included in this study. Subsequent direct portography was performed to evaluate whether the portal venous system was completely patent. After the TIPS procedure, intravenous heparin (4000 U/d; Chase Sun Pharma Co. Ltd, Tianjing, China) was given for 3 d and then oral warfarin (2.5 mg/d; Orion Pharma Co. Ltd., Orionintie, Finland) was prescribed to achieve 2 of international normalized ratio.
Follow-up
Baseline duplex sonography was performed on the day after TIPS creation. Subsequent shunt velocities were compared to this baseline result during follow-up. After TIPS, patients were placed into a routine follow-up protocol identical for each group. They were seen as outpatients 1 mo after the procedure and then every 3 mo, or whenever needed. Each consultation included a clinical examination, blood chemistry, and assessment of HE. Ultrasonography was performed at 1 and 4 wk after TIPS and then at 3 and 6 mo, and at 6-mo intervals thereafter, or in case of recurrent bleeding or ascites.
The primary endpoint of the study was primary unassisted patency, which was determined from the review of interventional radiology clinic records. Primary unassisted patency rate, the first stenosis or occlusion time was compared.
Shunt dysfunction that needed shunt revision during TIPS venography, or significant recurrent symptoms were used as endpoints for the loss of primary unassisted patency. TIPS angiography was performed in patients with recurrent symptoms of suspected shunt dysfunction. TIPS revision was performed when a hemodynamically significant shunt stenosis (> 50%) was present with recurrent variceal bleeding, recurrent or gradually worsening ascites, and PSG ≥ 15 mmHg unless grade III /IV encephalopathy (West Haven Criteria) was present. Patients lost to follow-up were censored at the time of the last known imaging of the shunt (duplex ultrasonography or shunt venography).
Statistical analysis
Results are expressed as mean ± SD. Primary patency and the first stenosis or occlusion time were calculated using the Kaplan-Meier method, and the resultant curves were compared by means of the log-rank test. Logistic regression analysis was then performed for the variables. The differences between the groups were compared using one-way analysis of variance followed by least significant difference t tests. Differences were considered significant at P < 0.05. The statistical analyses were performed with SPSS version 20.0 (SPSS, Chicago, IL, United States).
RESULTS
We created a shunt between the hepatic vein, or the IVC and the portal vein, with successful deployment of the stent graft, and no patients had stents extending into the right atrium at the time of TIPS procedure. Among 436 patients, 425 (97.5%) had technically successful TIPS, and no patient died within 30 d after TIPS, with an early survival rate of 100%.
Before TIPS placement, the mean right atrial pressure in the four groups was 2.81 ± 1.58 mmHg in group A, 2.87 ± 1.58 mmHg in group B, 2.79 ± 1.45 mmHg in group C and 2.80 ± 1.44 mmHg in group D. After TIPS placement, the mean right atrial pressure was 2.98 ± 1.11 mmHg in group A (P = 0.335), 3.01 ± 1.11 mmHg in group B (P = 0.235), 3.03 ± 1.03 mmHg in group C (P = 0.149) and 2.95 ± 1.04 mmHg in group D (P = 0.101). There were no significant differences before and after TIPS placement (P > 0.05). After TIPS placement, the mean PSG value decreased from 31.08 ± 8.11 to 13.81 ± 4.50 mmHg in group A (P = 0.014), 33.73 ± 7.77 to 15.0 ± 4.32 mmHg in group B (P = 0.009), 32.69 ± 7.55 to 14.57 ± 4.12 mmHg in group C (P = 0.015) and 32.65 ± 7.26 to 14.34 ± 3.84 mmHg in group D (P = 0.012). There were significant differences before and after TIPS placement (P < 0.05) (Table 2).
Table 2.
Mean pressure of the right atrium and portosystemic gradient value before and after transjugular intrahepatic portosystemic shunt placement
| Group |
Pressure of the right atrium in mmHg |
P value |
PSG in mmHg |
P value | ||
| Before TIPS | After TIPS | Before TIPS | After TIPS | |||
| A | 2.81 ± 1.58 | 2.98 ± 1.11 | 0.335 | 31.08 ± 8.11 | 13.81 ± 4.50 | 0.014 |
| B | 2.87 ± 1.58 | 3.01 ± 1.11 | 0.235 | 33.73 ± 7.77 | 15.00 ± 4.32 | 0.009 |
| C | 2.79 ± 1.45 | 3.03 ± 1.03 | 0.149 | 32.69 ± 7.55 | 14.57 ± 4.12 | 0.015 |
| D | 2.80 ± 1.44 | 2.95 ± 1.04 | 0.101 | 32.65 ± 7.26 | 14.34 ± 3.84 | 0.012 |
TIPS: Transjugular intrahepatic portosystemic shunt; PSG: Portosystemic gradient.
In group A (Table 3), 74 patients showed stent stenosis or occlusion of the outflow endpoint via venography. Forty-four patients showed recurrent variceal bleeding, 43 showed ascites, and 10 showed both recurrent variceal bleeding and ascites. Of all the patients who showed stent dysfunction, 51 patients underwent balloon dilation, and in 23 the stent was replaced and extended to the IVC. Nine patients manifested HE: seven were grade I and two were grade II. After drug treatment, the symptoms disappeared in patients with grade I or II HE.
Table 3.
Clinical characteristics of the patients
| Characteristic | Group A | Group B | P value | Group C | Group D | P value | |
| Unassisted | 3 mo | 75.4% | 92.6% | 0.001 | 88.0% | 73.8% | 0.012 |
| 6 mo | 57.9% | 89.2% | 0.000 | 86.0% | 66.4% | 0.000 | |
| Patency rate | 12 mo | 54.4% | 75.0% | 0.005 | 74.7% | 60.4% | 0.028 |
| Median primary patency in mo | 5.2 | 9.1 | 0.013 | 8.3 | 6.9 | 0.025 | |
| Mean primary patency | 4.98 | 15.01 | 0.006 | 13.28 | 8.20 | 0.009 | |
| Recurrence | 3 mo | 15.8% | 5.1% | 0.014 | 6.0% | 15.4 | 0.035 |
| of bleeding in mo | 6 mo | 28.1% | 13.2% | 0.014 | 9.6% | 20.1% | 0.038 |
| 12 mo | 33.3% | 18.4% | 0.024 | 13.3% | 26.8% | 0.017 | |
| Median recurrent bleeding in mo | 5.2 | 7.4 | 0.016 | 13.61 | 7.47 | 0.011 | |
| Mean recurrent bleeding in mo | 4.21 | 6.93 | 0.023 | 8.7 | 6.3 | 0.018 | |
| Recurrence | 3 mo | 17.5% | 6.6% | 0.020 | 5.9% | 16.4% | 0.035 |
| of ascites in mo | 6 mo | 22.8% | 10.3% | 0.019 | 10.5% | 25.5% | 0.022 |
| 12 mo | 35.1% | 20.6% | 0.034 | 19.3% | 35.6% | 0.009 | |
| Mean recurrent ascites in mo | 6.11 | 11.45 | 0.011 | 14.26 | 7.19 | 0.005 | |
| Median recurrent ascites in mo | 5.9 | 10.4 | 0.007 | 9.1 | 6.8 | 0.009 | |
| Stent dysfunction times | 74 | 53 | 0.037 | 50 | 117 | 0.021 | |
| Hepatic encephalopathy cases | 9 | 22 | 13 | 22 | 0.965 |
In group B, 53 patients showed stent stenosis or occlusion of the outflow endpoint via venography. A total of 29 patients showed recurrent variceal bleeding, 24 showed ascites, and 19 showed both variceal bleeding and ascites. Of all the patients who showed stent dysfunction, 41 underwent balloon dilation, and in 12 the stent was replaced and extended to the IVC. Twenty-two patients had HE: 13 were grade I, 6 were grade II, and 3 were grade III. After drug treatment, the symptoms disappeared in patients with grade I or II HE, and in patients with grade III HE, the symptoms disappeared after implantation of shunt-reducing stents.
In group C, 50 patients showed stent stenosis or occlusion via venography, 24 showed recurrent variceal bleeding, 27 showed ascites, and 9 showed both variceal bleeding and ascites. Among the patients who showed stent dysfunction, 38 underwent balloon dilation and 12 underwent stent replacement. Thirteen patients had HE: 8 were grade I and 5 were grade II. After drug treatment, the symptoms disappeared in patients with grade I or II HE.
In group D, 117 patients showed stent stenosis or occlusion via venography, 93 showed recurrent variceal bleeding, 114 showed ascites, and 30 showed both variceal bleeding and ascites. Among the patients who showed stent dysfunction, 89 underwent balloon dilation and 28 underwent stent replacement. Twenty-two patients had HE: 14 were grade I, 6 were grade II, and 2 were grade III. After drug treatment, the symptoms disappeared in patients with grade I or II HE, and in patients of grade III HE, the symptoms disappeared after implantation of shunt-reducing stents. There was a significant difference in stent dysfunction times between groups C and D (P = 0.021).
The unassisted patency rates for groups A and B were 75.4% vs 92.6% (3 mo, P = 0.001), 57.9% vs 89.2% (6 mo, P = 0.000), and 54.4% vs 75.0% (12 mo, P = 0.005), respectively, and these differences were significant (P < 0.05). The primary unassisted patency rates of groups C and D were 88.0% vs 73.8% (3 mo, P = 0.012), 86.0% vs 66.4% (6 mo, P = 0.000), and 74.7% vs 60.4% (12 mo, P = 0.028), respectively, and these differences were significant (P < 0.05).
As for the stent stenosis or occlusion time, in group A, the first symptoms were seen at 3.6 and 6.7 mo later, but the first symptoms were 5.4 and 7.4 mo later in group B. The mean shunt primary patency time was 4.98 mo in group A and 15.01 mo in group B (P = 0.006). The median shunt primary patency time was 5.2 mo in group A and 9.1 mo in group B (95%CI: 4.3-5.6) (P = 0.013, log-rank test). There was a significant difference in stent dysfunction times between groups A and B (P = 0.037). As for the stent stenosis or occlusion time, in group C, the first symptoms were seen at 3.6 and 6.7 mo later, but the first symptoms were seen at 5.4 and 7.4 mo later in group D. The mean shunt primary patency time was 13.28 mo in group C and 8.20 mo in group D (P = 0.009). The median shunt primary patency time was 8.3 mo in group C and 6.9 mo in group D (95%CI: 6.3-7.6) (P = 0.025, log-rank test) (Figure 2A).
Figure 2.
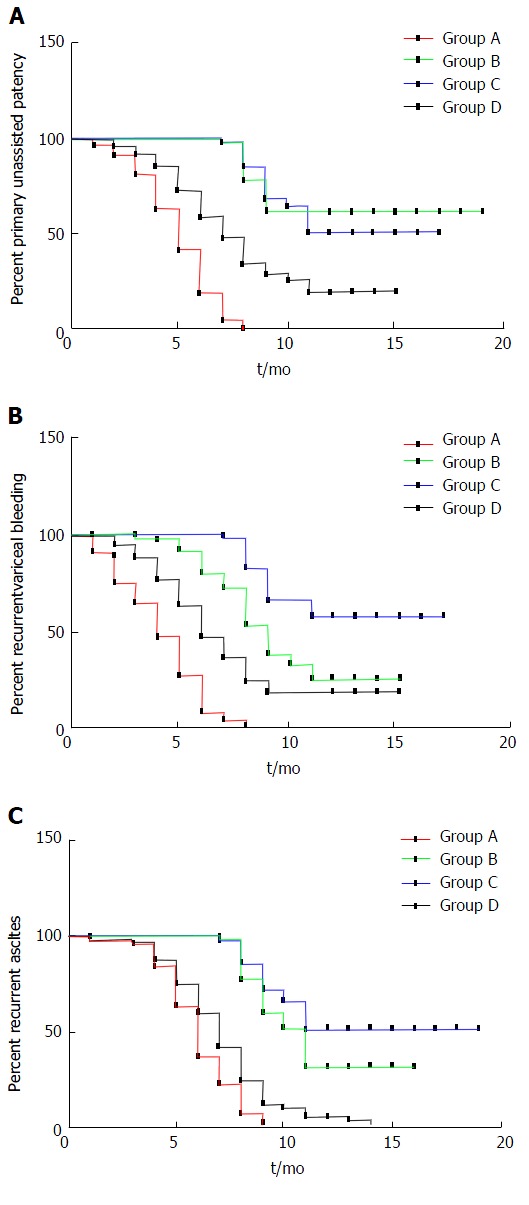
Primary unassisted patency (A), recurrent variceal bleeding (B) and recurrent ascites (C) in four groups of patients with transjugular intrahepatic portosystemic shunt. A: The median shunt primary patency time was 5.2 mo in group A and 9.1 mo in group B (95%CI: 4.3-5.6, P = 0.013, log-rank test). The median shunt primary patency time was 8.3 mo in group C and 6.9 mo in group D (95%CI: 6.3-7.6, P = 0.025, log-rank test); B: The median time to recurrence of bleeding was 5.2 mo in group A and 7.4 mo in group B (95%CI: 3.2-8.5, P = 0.016, log-rank test). The median time to recurrence of bleeding was 8.7 mo in group C and 6.3 mo in group D (95%CI: 3.2-8.5, P = 0.011, log-rank test); C: The median time to recurrence of ascites was 5.9 mo in group A and 10.4 mo in group B (95%CI: 6.5-9.2, P = 0.007, log-rank test). The median time to recurrence of ascites was 9.1 mo in group C and 6.8 mo in group D (95%CI: 6.5-9.2, P = 0.009, log-rank test).
Recurrent bleeding and ascites in group A were higher than in group B at 3 mo (15.8% vs 5.1%, P = 0.014; 17.5% vs 6.6%, P = 0.020), 6 mo (28.1% vs 13.2%, P = 0.014; 22.8% vs 10.3%, P = 0.019), and 12 mo (33.3% vs 18.4%, P = 0.024; 35.1% vs 20.6%, P = 0.034). The mean time to recurrent bleeding time was 4.21 mo in group A and 6.93 mo in group B (P = 0.023). The median time to recurrent bleeding was 5.2 mo in group A and 7.4 mo in group B (95%CI: 3.2-8.5) (P = 0.016, log-rank test). The mean time to recurrence of ascites was 6.11 mo in group A and 11.45 mo in group B (P = 0.011). The median time to recurrence of ascites was 5.9 mo in group A and 10.4 mo in group B (95%CI: 6.5-9.2) (P = 0.007, log-rank test) (Figure 2B and 2C).
The recurrence of bleeding and ascites in group D were higher than in group C at 3 mo (15.4% vs 6.0%, P = 0.035; 16.4% vs 5.9%, P = 0.035), 6 mo (20.1% vs 9.6%, P = 0.038; 25.5% vs 10.5%, P = 0.022), and 12 mo (26.8% vs 13.3%, P = 0.017; 35.6% vs 19.3%, P = 0.009). The mean time to recurrent bleeding was 13.61 mo in group C and 7.47 mo in group D (P = 0.018). The median time to recurrent bleeding was 8.7 mo in group C and 6.3 mo in group D (95%CI: 3.2-8.5) (P = 0.011, log-rank test). The mean time to recurrence of ascites was 14.26 mo in group C and 7.19 mo in group D (P = 0.005). The median time to recurrence of ascites was 9.1 mo in group C and 6.8 mo in group D (95%CI: 6.5-9.2) (P = 0.009, log-rank test).
In all the patients among the four groups, the incidence of HE did not differ significantly (P = 0.965).
DISCUSSION
It was believed previously that the shear force of blood flow at the end of the stent, and fibrotic healing response to the injury of shunt creation leads to parenchymal stenosis, resulted in stenosis and occlusion due to the pseudointimal hyperplasia of the shunt end[13]. A previous study has suggested that the end of the stent positioned in the hepatic vein within 2 cm of the junction of hepatic vein and IVC improves the primary patency of TIPS[14] when deployed with bare metallic stent. The other factors of tract angle influence the primary patency of the TIPS[12], such as portal vein to the parenchymal tract and hepatic vein to the parenchymal tract.
Andring et al[15] have suggested that whether the end of the stent position in relation to the hepatic vein IVC confluence or other factors of tract angle, such as portal vein to parenchymal tract and hepatic vein to parenchymal tract, have little effect on the primary patency rate, which leads to recurrence of symptoms and related mortality following TIPS.
PTFE-covered stent grafts increase the patency of the stent for the TIPS procedure[16,17]. However, it is reported that TIPS stent dysfunction and related complications remain problems that disturb the preferred clinical outcomes[18,19]. It is recommended that the outflow of the PTFE-covered stent is connected to the junction of the hepatic vein and IVC, and the inflow to the main portal vein[10,20].
The standard of position of the stent graft in the hepatic vein in TIPS creation is based on the study by Clark et al[21], in which the bare-metal stents used led to the suggestion. They suggested that the hepatic venous end of the bare metal stent was positioned within 2 cm of the junction of the hepatic vein and IVC was superior primary patency during TIPS creation.
The dilemma of initial stent position during TIPS placement can have several clinical implications[22]. Andring et al[15] have shown that the hepatic venous end of TIPS stent graft position in relation to the hepatic vein and IVC junction has little effect on the primary patency rate following TIPS. Similarly, other factors such as whether access to the portal vein of the stent involved the inflow end also has no significant effect on primary patency, which leads to recurrence of symptoms and TIPS-related mortality. Others believe that the initial stent position within the outflow end of the TIPS stent graft is an important determinant of primary shunt patency, and have suggested that adequate stent coverage of the hepatic venous outflow affects stent patency[23,24]. For patients in whom the caudal end of the stent was not parallel to the vascular wall of the portal vein, chronic injury to the portal vein intima caused by the end of the stent graft can be responsible for the stenosis or occlusion of the portal vein[14].
Our center has been engaged in TIPS treatment since 1993, from the outset of using bare stents to stent grafts after 2004. Placement of the stent in the left branch of the portal vein decreases the risk of HE, and highly angulated and/or tortuous parenchymal tracts, affects shunt patency by creating areas of altered shear stress with potentially accelerated pseudointimal hyperplasia[25,26]. During the TIPS procedure, we punctured as far as possible to the left branch of the portal vein and the stent was straight, avoiding the sheer force of the blood flow caused by the stent. In our study, all 425 patients had the left branch of the portal vein punctured, in an attempt to minimize the occurrence of HE.
In this study, we investigated the problem of initial stent position at the time of TIPS creation and predicted stent patency. As reported previously[27], we did not compare long-term outcomes among the four groups because patients who were later found to have TIPS shunt terminating in the hepatic vein or main portal vein underwent TIPS revision with placement of an additional stent to extend the outflow to the IVC, and/or dilated balloon.
As seen in our study, the primary unassisted patency rate in group B tended to be higher than in group A, and the median unassisted patency time was shorter in group A than in group B. We confirmed that the initial end of the stent position within the outflow of the TIPS shunt is an important determinant of shunt patency. The stenosis or occlusion sites in the cases with shunt dysfunction correlated well with their initial stent position, and we suggest that an adequate stent should be extended to the junction of the hepatic vein and IVC.
It is believed that, in patients who are potential liver transplantation candidates, the outflow position of the initial stent must be chosen with care as to avoid where it will interfere with placement of the suprahepatic clamp[28,29]. However, for orthotopic liver transplantation, the lack of liver tissue attachment to the stent, which allows an easier stent-graft removal, and the need to cover the IVC does not seem to be a contraindication in patients awaiting liver transplantation[30]. For piggy-back liver transplantation, stent placement at the junction of the hepatic vein and IVC does not influence the suprahepatic clamp[31]. So, we suggest that an adequate stent should be extended to the junction of the hepatic vein and IVC, and it should not influence liver transplantation.
Acceptance of the PTFE-covered stent (Viatorr) in the Chinese marketplace means that it will be widely deployed in TIPS placement. The inflow endpoint of the stent is the main portal vein[32]. The bare part of the stent is stiff and may cause endothelial injuries, with the subsequent development of thrombosis. A modification of the uncovered portion of the stent graft would probably be necessary to avoid portal vein stenosis.
In our study, the primary unassisted patency rate in group C tended to be higher than in group D, and the median unassisted patency time was longer in group C than group D. Our experience was consistent with the hypothesis that the end of the stent leads to chronic injury to the portal vein intima that is responsible for portal vein stenosis or occlusion. The portal blood flow is remodeled by the inflow position after stent placement, which produces a vortex and turbulence, and the shear force and uneven flow cause endometrial damage, thrombosis, intimal hyperplasia and stenosis[12,33,34]. Thus, we suggest that if improvement is needed at the front end of the stent, one should not enter the main portal vein in order to reduce the possibility of stenosis or occlusion.
The incidence of HE reported in the literature varies widely[3]. However, in our study, HE occurred at the same rate at the first year after TIPS creation. We speculate that the prevalence of HE was equal in the inpatients treated with an 8-mm stent, and shunt dysfunction needed immediate revision during TIPS venography for stent patency. We recommend the use of 8-mm stent grafts in most patients.
Our study had some limitations. First, it was a retrospective, single-center study, although there was a wide range of patient populations. We now anticipate a multicenter study. Second, we have yet to apply the PTFE-covered stent (Viatorr) stent, which has not been used in this capacity, but it is expected that some suggestions will be provided based on previous experience. Third, the specification of balloon and stent was deficient, which may have resulted in errors.
In conclusion, the initial stent position within the outflow and inflow of the TIPS creation is an important determinant of shunt primary patency. We suggest that the initial stent position of the outflow should be extended to the junction of the hepatic vein and IVC, and the inflow to the left branch of the portal vein.
ACKNOWLEDGMENTS
We thank all the patients who were involved in this study and our colleagues in the Department of Medical Records for their contributions to the data collection.
COMMENTS
Background
Transjugular intrahepatic portosystemic shunt (TIPS) is currently used for the treatment of complications of portal hypertension. Despite its efficacy in preventing syndromes, TIPS is prone to shunt stenosis or occlusion leading to shunt failure, and about 50% of patients with TIPS require shunt revision, which makes close surveillance and frequent costly revisions mandatory. Even with new stents or stent grafts covered with polytetrafluoroethylene (PTFE), post-TIPS shunt obstruction and a high rate of symptom recurrence remain problems. The purpose of this study was to evaluate the effect of selected technical factors of the primary stent position on primary shunt patency, and time to recurrence of symptoms in TIPS with a stent graft to avoid the need for repeat interventions.
Research frontiers
Previous studies based on TIPS created with bare metallic stents have suggested that the positioning of the hepatic venous end of the stent within 2 cm of the hepatic vein/inferior vena cava (IVC) junction improves the primary patency of TIPS. It is recommended that the outflow of the PTFE-covered stent (Viatorr) is placed at the junction of the hepatic vein and IVC, and the inflow at the main portal vein. These results help to explain the inflow of the stent to the portal vein, outflow of the stent to the hepatic vein and IVC, by retrospective analysis of a large patient sample and long-term case review. The results of this study also provide useful clinical suggestions.
Innovations and breakthroughs
In this study, the initial position of the stent inflow at the junction of the hepatic vein and IVC prolongs the median primary unassisted patency rate and reduces the incidence of recurrent bleeding and ascites. These results are in agreement with previous reports. However, in this study, the initial position of the stent outflow at the left branch of the portal vein also prolonged the median primary unassisted patency rate and reduced the incidence of recurrent bleeding and ascites. This emphasizes that the initial stent position within the outflow and inflow of the TIPS is an important determinant of shunt patency, and suggests that the initial stent position of the outflow should be extended to the junction of the hepatic vein and IVC, and the inflow to the left branch of the portal vein.
Applications
The initial stent position within the outflow and inflow of the TIPS is an important determinant of shunt patency. This study suggests that the initial stent position of the outflow should be extended to the junction of hepatic vein and IVC, and the inflow to the left branch of the portal vein.
Terminology
TIPS is currently used for the treatment of complications of portal hypertension by establishing a shunt between the intrahepatic portal vein and vena cava to relieve portal hypertension.
Peer-review
The author reported 425 patients with refractory ascites or variceal bleeding treated with TIPS placement. To date this size of cohort study have never been reported and is essential to be published. Their results demonstrated that the initial stent position influences stent patency, and the risk of recurrent symptoms associated with shunt stenosis or occlusion.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of Air Force General Hospital of PLA, Beijing, China.
Informed consent statement: Patients were not required to give informed consent because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: The authors declare that there is no conflict of interest related to this study.
Data sharing statement: No additional data are available.
Peer-review started: March 10, 2017
First decision: March 30, 2017
Article in press: May 19, 2017
P- Reviewer: Cao GW, Kaimakliotis P, Wasserberg N S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Li D
References
- 1.García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 2.Ascha M, Abuqayyas S, Hanouneh I, Alkukhun L, Sands M, Dweik RA, Tonelli AR. Predictors of mortality after transjugular portosystemic shunt. World J Hepatol. 2016;8:520–529. doi: 10.4254/wjh.v8.i11.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nardelli S, Gioia S, Pasquale C, Pentassuglio I, Farcomeni A, Merli M, Salvatori FM, Nikolli L, Torrisi S, Greco F, et al. Cognitive Impairment Predicts The Occurrence Of Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt. Am J Gastroenterol. 2016;111:523–528. doi: 10.1038/ajg.2016.29. [DOI] [PubMed] [Google Scholar]
- 4.Rössle M. TIPS: 25 years later. J Hepatol. 2013;59:1081–1093. doi: 10.1016/j.jhep.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Rösch J, Keller FS. Transjugular intrahepatic portosystemic shunt: present status, comparison with endoscopic therapy and shunt surgery, and future prospectives. World J Surg. 2001;25:337–345; discussion 345-346. doi: 10.1007/s002680020380. [DOI] [PubMed] [Google Scholar]
- 6.Rossi P, Salvatori FM, Fanelli F, Bezzi M, Rossi M, Marcelli G, Pepino D, Riggio O, Passariello R. Polytetrafluoroethylene-covered nitinol stent-graft for transjugular intrahepatic portosystemic shunt creation: 3-year experience. Radiology. 2004;231:820–830. doi: 10.1148/radiol.2313030349. [DOI] [PubMed] [Google Scholar]
- 7.Bercu ZL, Fischman AM, Kim E, Nowakowski FS, Patel RS, Schiano TD, Chang CY, Lookstein RA. TIPS for refractory ascites: a 6-year single-center experience with expanded polytetrafluoroethylene-covered stent-grafts. AJR Am J Roentgenol. 2015;204:654–661. doi: 10.2214/AJR.14.12885. [DOI] [PubMed] [Google Scholar]
- 8.Weber CN, Nadolski GJ, White SB, Clark TW, Mondschein JI, Stavropoulos SW, Shlansky-Goldberg RD, Trerotola SO, Soulen MC. Long-Term Patency and Clinical Analysis of Expanded Polytetrafluoroethylene-Covered Transjugular Intrahepatic Portosystemic Shunt Stent Grafts. J Vasc Interv Radiol. 2015;26:1257–1265; quiz 1265. doi: 10.1016/j.jvir.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Nishimine K, Saxon RR, Kichikawa K, Mendel-Hartvig J, Timmermans HA, Shim HJ, Uchida BT, Barton RE, Keller FS, Rösch J. Improved transjugular intrahepatic portosystemic shunt patency with PTFE-covered stent-grafts: experimental results in swine. Radiology. 1995;196:341–347. doi: 10.1148/radiology.196.2.7617843. [DOI] [PubMed] [Google Scholar]
- 10.Haskal ZJ. Improved patency of transjugular intrahepatic portosystemic shunts in humans: creation and revision with PTFE stent-grafts. Radiology. 1999;213:759–766. doi: 10.1148/radiology.213.3.r99dc28759. [DOI] [PubMed] [Google Scholar]
- 11.Jung HS, Kalva SP, Greenfield AJ, Waltman AC, Walker TG, Athanasoulis CA, Wicky ST. TIPS: comparison of shunt patency and clinical outcomes between bare stents and expanded polytetrafluoroethylene stent-grafts. J Vasc Interv Radiol. 2009;20:180–185. doi: 10.1016/j.jvir.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Bai M, He CY, Qi XS, Yin ZX, Wang JH, Guo WG, Niu J, Xia JL, Zhang ZL, Larson AC, et al. Shunting branch of portal vein and stent position predict survival after transjugular intrahepatic portosystemic shunt. World J Gastroenterol. 2014;20:774–785. doi: 10.3748/wjg.v20.i3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo TS, Oh JH, Park YK, Song HY, Park SJ, Yuk SH. Efficacy of a dexamethasone-eluting nitinol stent on the inhibition of pseudo-intimal hyperplasia in a transjugular intrahepatic portosystemic shunt: an experimental study in a swine model. Korean J Radiol. 2005;6:241–247. doi: 10.3348/kjr.2005.6.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cura M, Cura A, Suri R, El-Merhi F, Lopera J, Kroma G. Causes of TIPS dysfunction. AJR Am J Roentgenol. 2008;191:1751–1757. doi: 10.2214/AJR.07.3534. [DOI] [PubMed] [Google Scholar]
- 15.Andring B, Kalva SP, Sutphin P, Srinivasa R, Anene A, Burrell M, Xi Y, Pillai AK. Effect of technical parameters on transjugular intrahepatic portosystemic shunts utilizing stent grafts. World J Gastroenterol. 2015;21:8110–8117. doi: 10.3748/wjg.v21.i26.8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Z, Han G, Wu Q, Ye X, Jin Z, Yin Z, Qi X, Bai M, Wu K, Fan D. Patency and clinical outcomes of transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stents versus bare stents: a meta-analysis. J Gastroenterol Hepatol. 2010;25:1718–1725. doi: 10.1111/j.1440-1746.2010.06400.x. [DOI] [PubMed] [Google Scholar]
- 17.Gandini R, Konda D, Simonetti G. Transjugular intrahepatic portosystemic shunt patency and clinical outcome in patients with Budd-Chiari syndrome: covered versus uncovered stents. Radiology. 2006;241:298–305. doi: 10.1148/radiol.2411050347. [DOI] [PubMed] [Google Scholar]
- 18.Parvinian A, Gaba RC. Outcomes of TIPS for Treatment of Gastroesophageal Variceal Hemorrhage. Semin Intervent Radiol. 2014;31:252–257. doi: 10.1055/s-0034-1382793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owen JM, Gaba RC. Transjugular Intrahepatic Portosystemic Shunt Dysfunction: Concordance of Clinical Findings, Doppler Ultrasound Examination, and Shunt Venography. J Clin Imaging Sci. 2016;6:29. doi: 10.4103/2156-7514.186510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saad N, Darcy M, Saad W. Portal anatomic variants relevant to transjugular intrahepatic portosystemic shunt. Tech Vasc Interv Radiol. 2008;11:203–207. doi: 10.1053/j.tvir.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Clark TW, Agarwal R, Haskal ZJ, Stavropoulos SW. The effect of initial shunt outflow position on patency of transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2004;15:147–152. doi: 10.1097/01.rvi.0000109401.52762.56. [DOI] [PubMed] [Google Scholar]
- 22.Fillinger MF, Kerns DB, Bruch D, Reinitz ER, Schwartz RA. Does the end-to-end venous anastomosis offer a functional advantage over the end-to-side venous anastomosis in high-output arteriovenous grafts? J Vasc Surg. 1990;12:676–688; discussion 688-690. doi: 10.1067/mva.1990.24914. [DOI] [PubMed] [Google Scholar]
- 23.Tripathi D, Redhead D. Transjugular intrahepatic portosystemic stent-shunt: technical factors and new developments. Eur J Gastroenterol Hepatol. 2006;18:1127–1133. doi: 10.1097/01.meg.0000236871.78280.a7. [DOI] [PubMed] [Google Scholar]
- 24.Maleux G, Nevens F, Wilmer A, Heye S, Verslype C, Thijs M, Wilms G. Early and long-term clinical and radiological follow-up results of expanded-polytetrafluoroethylene-covered stent-grafts for transjugular intrahepatic portosystemic shunt procedures. Eur Radiol. 2004;14:1842–1850. doi: 10.1007/s00330-004-2359-4. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Xiao T, Chen W, Long Q, Li R, Fang D, Wang R. Outcomes of transjugular intrahepatic portosystemic shunt through the left branch vs. the right branch of the portal vein in advanced cirrhosis: a randomized trial. Liver Int. 2009;29:1101–1109. doi: 10.1111/j.1478-3231.2009.02016.x. [DOI] [PubMed] [Google Scholar]
- 26.Farsad K, Kaufman JA. Novel Image Guidance Techniques for Portal Vein Targeting During Transjugular Intrahepatic Portosystemic Shunt Creation. Tech Vasc Interv Radiol. 2016;19:10–20. doi: 10.1053/j.tvir.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Barrio J, Ripoll C, Bañares R, Echenagusia A, Catalina MV, Camúñez F, Simó G, Santos L. Comparison of transjugular intrahepatic portosystemic shunt dysfunction in PTFE-covered stent-grafts versus bare stents. Eur J Radiol. 2005;55:120–124. doi: 10.1016/j.ejrad.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Chui AK, Rao AR, Shi LW, Ong J, Waugh RC, Verran DJ, Shun A, Sheil AG. Liver transplantation in patients with transjugular intrahepatic portosystemic shunts. Transplant Proc. 2000;32:2204–2205. doi: 10.1016/s0041-1345(00)01635-3. [DOI] [PubMed] [Google Scholar]
- 29.Bonnel AR, Bunchorntavakul C, Rajender Reddy K. Transjugular intrahepatic portosystemic shunts in liver transplant recipients. Liver Transpl. 2014;20:130–139. doi: 10.1002/lt.23775. [DOI] [PubMed] [Google Scholar]
- 30.Patel NH, Patel J, Behrens G, Savo A. Transjugular intrahepatic portosystemic shunts in liver transplant recipients: technical considerations and review of the literature. Semin Intervent Radiol. 2005;22:329–333. doi: 10.1055/s-2005-925559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurusamy KS, Pamecha V, Davidson BR. Piggy-back graft for liver transplantation. Cochrane Database Syst Rev. 2011;(1):CD008258. doi: 10.1002/14651858.CD008258.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Otal P, Smayra T, Bureau C, Peron JM, Chabbert V, Chemla P, Joffre F, Vinel JP, Rousseau H. Preliminary results of a new expanded-polytetrafluoroethylene-covered stent-graft for transjugular intrahepatic portosystemic shunt procedures. AJR Am J Roentgenol. 2002;178:141–147. doi: 10.2214/ajr.178.1.1780141. [DOI] [PubMed] [Google Scholar]
- 33.Gallego C, Velasco M, Marcuello P, Tejedor D, De Campo L, Friera A. Congenital and acquired anomalies of the portal venous system. Radiographics. 2002;22:141–159. doi: 10.1148/radiographics.22.1.g02ja08141. [DOI] [PubMed] [Google Scholar]
- 34.Saad WE, Davies MG, Lee DE, Patel NC, Sahler LG, Sasson T, Kitanososno T, Waldman DL. Transjugular intrahepatic portosystemic shunt in a living donor left lateral segment liver transplant recipient: technical considerations. J Vasc Interv Radiol. 2005;16:873–877. doi: 10.1097/01.RVI.0000157776.47580.F7. [DOI] [PubMed] [Google Scholar]


