Abstract
Background
Radiation-related lymphopenia has been associated with poor patient outcome. Our aim was to identify predictors of lymphopenia after palliative radiotherapy, with a focus on dose-volume parameters.
Patients and methods
To retrospectively assess patients with various cancers who had undergone palliative radiotherapy, we delineated three organs at risk: the volume enclosed by the body surface contour (body A), the volume left after excluding air, pleural effusion, ascites, bile, urine, and intestinal content (body B), and the volume of the bone marrow (BM). We then noted the absolute volume of the three organs at risk that had received 5-30 Gy, and assessed the predictive value for post-treatment lymphopenia of grade 3 or higher (LP3+).
Results
Of 54 patients, 23 (43%) developed LP3+. Univariate logistic regression analysis showed that body A V5, body A V10, body B V5, body B V10, the number of fractions, and splenic irradiation were significant predictors of LP3+ (p < 0.05). By multivariate analysis, body A V5, body A V10, body B V5, body B V10, and the number of fractions retained significance (p < 0.05). BM dose-volume parameters did not predict lymphopenia.
Conclusions
Higher body A and body B dose-volume parameters and a larger number of fractions may be predictors of severe lymphopenia after palliative radiotherapy.
Key words: palliative radiotherapy, radiation-related lymphopenia, dose-volume parameters
Introduction
The important role of lymphocytes in the immune response to cancer1 is evidenced by the better survival of lung-, colorectal-, and breast cancer-, and glioblastoma patients whose cancer tissues manifest lymphocyte infiltration.2-5 Survival tends to be poor in cancer- and lymphoma patients with lymphopenia before undergoing treatment6-10, and treatment-related lymphopenia is associated with a poor outcome in patients subjected to curative chemoradiotherapy for pancreatic-, lung-, cervical-, and nasopharyngeal cancer and malignant glioma.11-18
The irradiation of circulating peripheral blood may elicit radiation-related lymphopenia.19,20 Although studies to evaluate the effect of irradiation on lymphocytes showed that radiation-related lymphopenia was associated with organ-specific (lung14 and brain21) dose-volume parameters, dosimetric predictors applicable at various treatment sites remained to be identified.
Radiation-related lymphopenia has been studied mainly in patients who had received curative treatment11-18; there are few reports on patients subjected to palliative radiotherapy (RT). Because lymphocytes are highly radiosensitive, exposure to even low doses of radiation can lead to a decrease in the number of peripheral blood lymphocytes.22,23 Consequently, even low radiation doses delivered by palliative RT can lead to lymphopenia affecting the immune system and the treatment outcome.
Focusing on dose-volume parameters, we attempted to identify predictors of lymphopenia after palliative RT. We used organs at risk based on body surface contour to evaluate their predictive value.
Patients and methods
Patients
This retrospective study was approved by the institutional review board of Kumamoto University Hospital (No. 1171). The study was carried out according to the Declaration of Helsinki. Our inclusion criteria were as follows: patients treated with palliative RT between October 2010 and June 2013 at the Kumamoto University Hospital; the availability of laboratory data acquired within 2 weeks prior to the start of RT; and of two or more laboratory data obtained within one month after the start of RT, the latest data recorded at least 2 weeks after the start of RT. The exclusion criteria were hematologic tumor; chemotherapy, molecular targeted therapy, interferon treatment, or radiotherapy delivered from one month before to one month after the start of RT; or grade 2 or higher lymphopenia based on the Common Terminology Criteria for Adverse Events (CTCAE) v 4.0 at the start of RT. Patient-, tumor-, and treatment data were obtained from medical charts.
Laboratory data
The study endpoints were (1) the absolute lymphocyte count at nadir, defined as the lowest value recorded within one month after the start of RT, (2) the lymphocyte count ratio, obtained by dividing the nadir absolute lymphocyte count by the pre-RT absolute lymphocyte count, and (3) lymphopenia of grade 3 or higher (LP3+, absolute lymphocyte count < 500 × 106/L) determined by CTCAE v 4.0, with the highest grade within one month after the start of RT recorded for analysis.
Dose-volume parameters
All patients underwent CT simulation in the supine position, and three-dimensional treatment planning. For this study, we delineated organs at risk on planning CT images with commercially available software (Velocity AI, Velocity Medical System, Atlanta, GA, USA). To evaluate the effect of radiation on peripheral blood lymphocytes, one radiation oncologist delineated the volume of two organs at risk where body A is the volume enclosed by the body surface contour, and body B is the volume left after excluding air, pleural effusion, ascites, bile, urine, and intestinal content (Figure 1). For body A, the body surface contour was obtained first by using threshold-based segmentation and then by manual correction. To obtain body B, we excluded volumes whose irradiation would not contribute to the reduction of lymphocytes; lung tissue was included and the trachea and bronchi were excluded. For bone marrow (BM), all bones were delineated by threshold-based segmentation and manual correction (Figure 1); intervertebral disks and costal-, thyroid-, cricoid-, and tracheal cartilage were excluded. The distal half of the femur and humerus were also excluded from BM because they contain little proliferating bone marrow.24 The absolute volumes of the three organs at risk receiving 5-, 10-, 20-, and 30 Gy (V5, V10, V20, and V30) were recorded.
Figure 1.
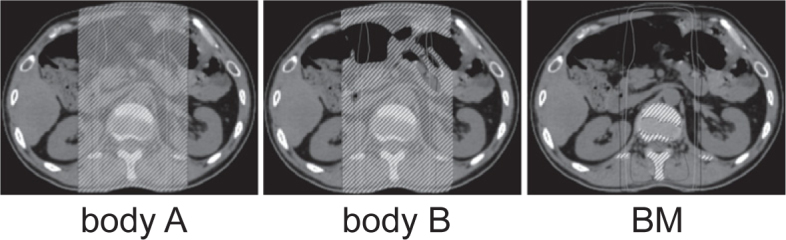
Delineation of the three organs at risk. Body A is the volume enclosed by the body surface contour. Body B excludes air, pleural effusion, ascites, bile, urine, and the intestinal content. BM = bone marrow.
Statistical analysis
Data were summarized by using descriptive statistics (frequency, percentage, median, range). The correlation between the dose-volume parameters and the nadir lymphocyte count was evaluated with the Spearman correlation coefficient. For univariate and multivariate logistic regression analysis, the age, interval from tumor diagnosis, the pre-RT absolute lymphocyte count, total radiation dose, number of fractions, total monitor units, total irradiation time, and all dose-volume parameters were the continuous variables. The categorical variables included the gender, previous RT, previous chemotherapy, concurrent steroid use, bone metastasis, brain metastasis, splenic irradiation, and thymic irradiation. Variables that were significant in univariate analysis were included in multivariate analysis. The overall survival, calculated from the start of RT, was estimated with the KaplanMeier method; differences determined with the log-rank test. Values of p < 0.05 were considered statistically significant. All statistical analyses were performed with SPSS software, version 23 (IBM SPSS, Armonk, NY, USA).
Results
Patients
We included 54 patients whose solid tumors were treated with palliative RT. The patient and treatment characteristics are summarized in Table 1. As we excluded patients with lymphopenia of grade 2 or higher (absolute lymphocyte count < 800 × 106/L), all included patients had a pre-RT absolute lymphocyte count > 800 × 106/L. The median follow-up period from the start of RT was 4.5 months (range 0−42.0 months).
Table 1.
Patient and treatment characteristics (n = 54)
| Characteristic | No. of patients | % |
|---|---|---|
| Patient characteristics | ||
| Male gender | 33 | 61 |
| Age (years) | ||
| Median | 69 | |
| Range | 39-86 | |
| Primary tumor | ||
| Lung | 14 | 26 |
| Gastrointestinal | 9 | 17 |
| Skin | 4 | 7 |
| Liver | 3 | 6 |
| Uterus | 3 | 6 |
| Othersa | 21 | 39 |
| Previous radiotherapy | 15 | 28 |
| Previous chemotherapy | 26 | 48 |
| Concurrent steroid use | 23 | 43 |
| Bone metastasis | 27 | 50 |
| Brain metastasis | 11 | 20 |
| Interval from tumor diagnosis to radiotherapy (months) | ||
| Median | 13 | |
| Range | 0-168 | |
| Pre-radiotherapy absolute lymphocyte count (× 106/L) | ||
| Median | 1356 | |
| Range | 844-3468 | |
| Treatment characteristics | ||
| Total radiation dose (Gy) | ||
| Median | 30 | |
| Range | 16-50 | |
| Number of fractions | ||
| Median | 10 | |
| Range | 4-25 | |
| Fraction size (Gy) | ||
| Median | 3 | |
| Range | 2-5 | |
| Total monitor units for all fractions | ||
| Median | 4433 | |
| Range | 1896–13890 | |
| Total irradiation time for all fractions (minutes) | ||
| Median | 7.5 | |
| Range | 3.2-37.7 | |
| Treatment site | ||
| Head and neck | 14 | 26 |
| Chest | 24 | 44 |
| Abdomen | 10 | 19 |
| Pelvis | 11 | 20 |
| Limb | 1 | 2 |
| Splenic irradiationb | 9 | 17 |
| Thymic irradiationc | 15 | 28 |
Others include head and neck (3 patients), breast (3 patients), mediastinal (3 patients), urogenita (8 patients), and soft tissue (4 patients) tumors;
Yes, if any part of the spleen was covered by the 5 Gy idodose line;
Yes, if any part of the thymus was covered by the 5 Gy idodose line.
Laboratory data
The median pre-RT absolute lymphocyte count was 1356 × 106/L (range 844-3468 × 106/L), and the median nadir post-RT absolute lymphocyte count was 536 × 106/L (range 131-1653 × 106/L). The median lymphocyte count ratio, obtained by dividing the nadir-by the pre-RT absolute lymphocyte count, was 0.410 (range 0.108-0.983). Of the 54 patients, 12 (22%), 19 (35%), 20 (37%), and 3 (6%) patients had post-treatment lymphopenia of grade 1, 2, 3, and 4, respectively; a total of 23 (43%) patients developed LP3+.
Dose-volume parameters
The median (range) V5, V10, V20, and V30 for body A were 2.880 (0.399-8.976), 2.519 (0.344-6.596), 1.629 (0.000-4.255), and 0.660 (0.000-3.365) × 103 mL, respectively. These values were 2.704 (0.3928.143), 2.384 (0.341-5.959), 1.597 (0.000-3.919), and 0.645 (0.000-3.326) × 103 mL for body B and 0.348 (0.000-0.974), 0.258 (0.000-0.909), 0.174 (0.0000.737), and 0.076 (0.000-0.724) × 103 mL for BM.
Correlation between the dose-volume parameters and the nadir lymphocyte count
There was a negative correlation between body dose-volume parameters (V5, V10, and V30 for body A and body B) and the nadir lymphocyte count (p < 0.05, Table 2). Higher body A and body B dose-volume parameters were correlated with a lower post-RT lymphocyte count. There was no significant correlation between BM dose-volume parameters and the nadir lymphocyte count (Table 2). We observed a strong correlation between body A V5 and body B V5 (Speaman’s rho = 0.992, p < 0.001), between body A V10 and body B V10 (Speaman’s rho = 0.992, p < 0.001), between body A V20 and body B V20 (Speaman’s rho = 0.997, p < 0.001), and between body A V30 and body B V30 (Speaman’s rho = 0.999, p < 0.001).
Table 2.
Spearman correlation coefficients between the dose-volume parameters and the nadir lymphocyte count
| Nadir lymphocyte count | ||||
|---|---|---|---|---|
| Absolute lymphocyte count at nadir | Lymphocyte count ratioa | |||
| Variable | Spearman’s rho | p-value | Spearman’s rho | p-value |
| Body A V5 | -0.265 | 0.053 | -0.350 | 0.010 |
| Body A V10 | -0.283 | 0.038 | -0.367 | 0.006 |
| Body A V20 | -0.231 | 0.093 | -0.255 | 0.063 |
| Body A V30 | -0.305 | 0.025 | -0.281 | 0.039 |
| Body B V5 | -0.274 | 0.045 | -0.347 | 0.010 |
| Body B V10 | -0.280 | 0.040 | -0.352 | 0.009 |
| Body B V20 | -0.236 | 0.086 | -0.260 | 0.057 |
| Body B V30 | -0.302 | 0.027 | -0.280 | 0.041 |
| BM V5 | -0.152 | 0.27 | -0.210 | 0.13 |
| BM V10 | -0.161 | 0.25 | -0.207 | 0.13 |
| BM V20 | -0.135 | 0.33 | -0.161 | 0.25 |
| BM V30 | -0.168 | 0.23 | -0.185 | 0.18 |
BM = bone marrow;
The lymphocyte count ratio was calculated by dividing the nadir absolute lymphocyte count by the pre-radiotherapy absolute lymphocyte count.
Predictors of severe treatment-related lymphopenia
Univariate logistic regression analysis showed that body A V5, body A V10, body B V5, body B V10, the number of fractions, and splenic irradiation were significant predictors of LP3+ (p < 0.05, Table 3). For multivariate analysis, we took into account factors with p < 0.05 by univariate analysis (number of fractions and splenic irradiation) to test the independent significance of dose-volume parameters (Table 4). We found that body A V5, body A V10, body B V5, body B V10, and the number of fractions retained significance (p < 0.05). Higher body A and body B dose-volume parameters and a larger number of fractions were predictive of LP3+.
Table 3.
Univariate logistic regression analysis for lymphopenia of grade 3 or higher
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Patient characteristics | |||
| Male vs. female | 1.39 | 0.46–4.22 | 0.55 |
| Age (per 1 year increase) | 0.99 | 0.94–1.03 | 0.53 |
| Previous radiotherapy (yes vs. no) | 1.02 | 0.30–3.47 | 0.98 |
| Previous chemotherapy (yes vs. no) | 0.53 | 0.18–1.58 | 0.26 |
| Concurrent steroid use (yes vs. no) | 1.07 | 0.36–3.17 | 0.91 |
| Bone metastasis (yes vs. no) | 0.86 | 0.29–2.53 | 0.78 |
| Brain metastasis (yes vs. no) | 0.72 | 0.18–2.84 | 0.64 |
| Interval from tumor diagnosis to radiotherapy (per 1 month increase) | 1.00 | 0.99–1.02 | 0.81 |
| Pre-radiotherapy absolute lymphocyte count (per increase of 1 × 106/l) | 0.99 | 0.99–1.00 | 0.23 |
| Treatment characteristics | |||
| Total radiation dose (per 1-Gy increase) | 1.06 | 0.99–1.14 | 0.11 |
| Number of fractions (per 1-fraction increase) | 1.18 | 1.01–1.38 | 0.036 |
| Total monitor units over the entire treatment | |||
| course (per increase of 100 monitor units) | 1.01 | 0.99–1.03 | 0.29 |
| Total irradiation time over the entire treatment course (per 1-minute increase) | 0.99 | 0.92–1.08 | 0.91 |
| Splenic irradiation (yes vs. no) a | 6.34 | 1.18–34.24 | 0.032 |
| Thymic irradiation (yes vs. no) b | 0.58 | 0.17–2.03 | 0.39 |
| Body A V5 (per 1 × 103 mL increase) | 1.55 | 1.06–2.26 | 0.025 |
| Body A V10 (per 1 × 103 mL increase) | 1.60 | 1.04–2.45 | 0.032 |
| Body A V20 (per 1 × 103 mL increase) | 1.60 | 0.96–2.68 | 0.074 |
| Body A V30 (per 1 × 103 mL increase) | 1.87 | 0.95–3.68 | 0.069 |
| Body B V5 (per 1 × 103 mL increase) | 1.58 | 1.05–2.38 | 0.027 |
| Body B V10 (per 1 × 103 mL increase) | 1.63 | 1.04–2.56 | 0.035 |
| Body B V20 (per 1 × 103 mL increase) | 1.59 | 0.94–2.72 | 0.087 |
| Body B V30 (per 1 × 103 mL increase) | 1.84 | 0.93–3.67 | 0.082 |
| BM V5 (per 1 × 103 mL increase) | 3.62 | 0.37–35.36 | 0.27 |
| BM V10 (per 1 × 103 mL increase) | 2.88 | 0.27–31.10 | 0.38 |
| BM V20 (per 1 × 103 mL increase) | 1.78 | 0.15–20.92 | 0.65 |
| BM V30 (per 1 × 103 mL increase) | 4.73 | 0.19–115.78 | 0.34 |
BM = bone marrow; OR = odds ratio; CI = confidence interval
Yes, if any part of the spleen was covered by the 5 Gy idodose line
Yes, if any part of the thymus was covered by the 5 Gy idodose line.
Table 4.
Multivariate logistic regression analysis for lymphopenia of grade 3 or higher
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Body A V5 (per 1 × 103 mL increase) | 1.58 | 1.03–2.42 | 0.036 |
| Number of fractions (per 1-fraction increase) | 1.26 | 1.03–1.53 | 0.027 |
| Splenic irradiation (yes vs. no) a | 5.12 | 0.74–35.28 | 0.098 |
| Body A V10 (per 1 × 103 mL increase) | 1.68 | 1.04–2.70 | 0.034 |
| Number of fractions (per 1-fraction increase) | 1.26 | 1.03–1.55 | 0.026 |
| Splenic irradiation (yes vs. no) a | 5.25 | 0.77–35.76 | 0.090 |
| Body B V5 (per 1 × 103 mL increase) | 1.63 | 1.03–2.57 | 0.038 |
| Number of fractions (per 1-fraction increase) | 1.25 | 1.03–1.53 | 0.028 |
| Splenic irradiation (yes vs. no) a | 5.46 | 0.79–37.53 | 0.085 |
| Body B V10 (per 1 × 103 mL increase) | 1.72 | 1.04–2.87 | 0.036 |
| Number of fractions (per 1-fraction increase) | 1.26 | 1.03–1.55 | 0.027 |
| Splenic irradiation (yes vs. no) a | 5.59 | 0.82–37.99 | 0.078 |
CI = confidence interval; OR = odds ratio
Yes, if any part of the spleen was covered by the 5 Gy idodose line; Factors with p < 0.05 by univariate analysis (number of fractions and splenic irradiation) were taken into account to test the indep
Relationship between radiation-related lymphopenia and overall survival
The median survival for all patients was 6.3 months (95% confidence interval: 4.1–8.5 months). The overall survival based on the grade of radiation-related lymphopenia is shown in Figure 2. There was no statistically significant difference in the overall survival of patients with LP3+ and the other grades of lymphopenia (p = 0.79).
Figure 2.
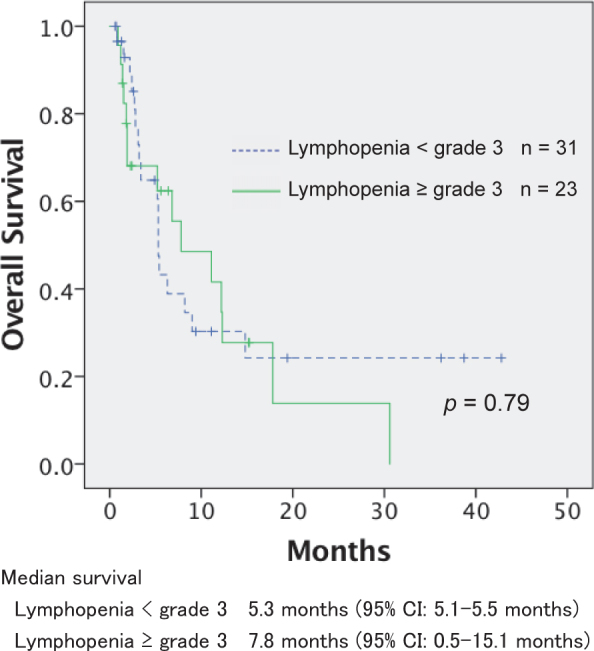
Overall survival according to the grade of radiationrelated lymphopenia.
CI = confidence interval.
Discussion
We found that body A V5, body A V10, body B V5, body B V10, and the number of fractions were significant predictors of severe radiation-related lymphopenia. Higher body A and body B dose-volume parameters and a larger number of fractions were predictive of LP3+. In contrast, irradiation to lymphoid organs such as the bone marrow, spleen, and thymus were not predictive of radiation-related lymphopenia.
Others19,20 suggested that the irradiation of circulating peripheral blood may lead to the development of radiation-related lymphopenia. This hypothesis is supported by findings that lymphopenia was observed after the delivery of RT to various body parts that did, or did not, include lymphoid organs.19,25 The irradiation of extracorporeal blood can lead to long-lasting lymphopenia.26 Tang et al.14 found that in lung cancer patients, higher lung V5 to V10 values were associated with a lower lymphocyte nadir, and Huang et al.21 reported that in patients with high-grade glioma, higher brain volume receiving 25 Gy was a significant predictor of acute severe lymphopenia during RT and concurrent temozolomide. We document that the body dose-volume parameters we applied are useful predictors of lymphopenia in patients exposed to RT at different sites including the head and neck, the chest, abdomen, and the pelvis.
Our univariate and multivariate logistic regression analysis showed that irradiation to the bone marrow, spleen, and thymus was not a consistently significant predictor. Lymphoid organs such as the thymus, bone marrow, and spleen are central components of the mammalian immune system; lymphocytes are developed in these organs.27 While splenic irradiation was a significant predictor of LP3+ by univariate logistic regression analysis, it lost its significance upon multivariate analysis. Because the 95% confidence interval was wide in our multivariate analysis (Table 4), the predictive value of splenic irradiation should be examined in large patient populations. We detected no significant association between lymphopenia and bone marrow irradiation although Sini et al.28 reported that the exposure of bone marrow to radiation played a significant role. They found that higher BM V40 was associated with higher risk of acute Grade3 or late Grade2 lymphopenia in prostate cancer patients treated with whole-pelvis RT. Because information on the role of lymphoid organs in radiation-related lymphopenia is limited, additional studies are warranted.
The number of fractions was a significant predictor of severe radiation-related lymphopenia. This finding agrees with earlier observations.20,29 MacLennan et al.29 analyzed the consequences of prophylactic cranial irradiation in children with leukemia. In their prospective study, the total radiation dose was constant (24 Gy) and the number of fractions was determined by the participating centers. They found that the level of radiation-related lymphopenia induced by that total dose depended on the number of fractions into which it was divided. The mean lymphocyte count of patients examined 3 months after receiving this dose in 5-, 12-, and 20 fractions was 1.84-, 1.12-, and 0.64 × 109/L, respectively. Yovino et al.20 analyzed a model that calculated the radiation dose received by circulating lymphocytes; they found that as the number of fractions increased, the percentage of blood receiving > 0.5 Gy increased rapidly. We also found that the number of fractions was a significant predictor of radiation-related lymphopenia, however, in our study the total radiation dose was not a significant predictor. Because lymphocytes are highly radiosensitive22,23, their number killed by one fraction may not be strongly associated with the dose per fraction. A larger dose per fraction might be relatively less effective in killing lymphocytes than a small dose.
We observed a strong correlation between body A and body B dose-volume parameters when the volume was equal (e.g. body A and body B exposed to 5 Gy). It is easier to obtain the volume of body A than body B because body A is based on the body surface contour that can be acquired by auto-segmentation using commercially available software tools. Body A dose-volume parameters may be a convenient tool for predicting radiation-related lymphopenia.
Our data showed that there was no significant difference in the overall survival of patients with LP3+ and other grades of lymphopenia. Although lymphopenia related to curative chemoradiotherapy has been shown to be associated with poor patient outcomes11-18, its prognostic value for palliative RT remains to be determined.
Our study has some limitations. The study population was small and some useful predictors of lymphopenia may have gone undetected. Also, as our study was retrospective, laboratory data were acquired at different points after the start of RT. Consequently, the true nadir lymphocyte count may not have been evaluated in some patients.
In summary, we identified body A and body B dose-volume parameters were useful new predictors of radiation-related lymphopenia. These body dose-volume parameters were acquired by delineating the body surface and they may be convenient for predicting radiation-related lymphopenia. As the parameters are not organ-specific, they are applicable at various treatment sites. Although their predictive value for radiation-related lymphopenia should be examined in groups of patients with different diseases, our findings may help to elucidate the mechanisms underlying the elicitation of radiation-related lymphopenia in patients treated with palliative RT.
Footnotes
Disclosure: No potential conflicts of interest were disclosed.
References
- 1.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y. et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007;7:4. [PMC free article] [PubMed] [Google Scholar]
- 4.Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R. et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin Cancer Res. 2011;17:4296–4308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- 5.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A. et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123:2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Scodan R, Massard C, Mouret-Fourme E, Guinebretierre JM, Cohen-Solal C, De Lalande B. et al. Brain metastases from breast carcinoma: Validation of the radiation therapy oncology group recursive partitioning analysis classification and proposition of a new prognostic score. Int J Radiat Oncol Biol Phys. 2007;69:839–845. doi: 10.1016/j.ijrobp.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Fumagalli LA, Vinke J, Hoff W, Ypma E, Brivio F, Nespoli A. Lymphocyte counts independently predict overall survival in advanced cancer patients: A biomarker for IL-2 immunotherapy. J Immunother. 2003;26:394–402. doi: 10.1097/00002371-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 8.De Giorgi U, Rihawi K, Aieta M, Lo Re G, Sava T, Masini C. et al. Lymphopenia and clinical outcome of elderly patients treated with sunitinib for metastatic renal cell cancer. J Geriatr Oncol. 2014;5:156–163. doi: 10.1016/j.jgo.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Jin Y, Ye X, He C, Zhang B, Zhang Y. Pretreatment neutrophil-to-lymphocyte ratio as predictor of survival for patients with metastatic nasopharyngeal carcinoma. Head Neck. 2015;37:69–75. doi: 10.1002/hed.23565. [DOI] [PubMed] [Google Scholar]
- 10.Feng J, Wang Z, Guo X, Chen Y, Cheng Y, Tang Y. Prognostic significance of absolute lymphocyte count at diagnosis of diffuse large B-cell lymphoma: A meta-analysis. Int J Hematol. 2012;95:143–148. doi: 10.1007/s12185-011-0993-6. [DOI] [PubMed] [Google Scholar]
- 11.Balmanoukian A, Ye X, Herman J, Laheru D, Grossman SA. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest. 2012;30:571–576. doi: 10.3109/07357907.2012.700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T. et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2015;38:259–265. doi: 10.1097/COC.0b013e3182940ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wild AT, Herman JM, Dholakia AS, Moningi S, Lu Y, Rosati LM. et al. Lymphocyte-sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2016;94:571–579. doi: 10.1016/j.ijrobp.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang C, Liao Z, Gomez D, Levy L, Zhuang Y, Gebremichael RA. et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014;89:1084–1091. doi: 10.1016/j.ijrobp.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Wu ES, Oduyebo T, Cobb LP, Cholakian D, Kong X, Fader AN. et al. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol. 2016;140:76–82. doi: 10.1016/j.ygyno.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S. et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendez JS, Govindan A, Leong J, Gao F, Huang J, Campian JL. Association between treatment-related lymphopenia and overall survival in elderly patients with newly diagnosed glioblastoma. J Neurooncol. 2016;127:329–335. doi: 10.1007/s11060-015-2037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho O, Oh YT, Chun M, Noh OK, Hoe JS, Kim H. Minimum absolute lymphocyte count during radiotherapy as a new prognostic factor for nasopharyngeal cancer. Head Neck. 2016;38(Suppl 1):E1061–E1067. doi: 10.1002/hed.24158. [DOI] [PubMed] [Google Scholar]
- 19.Raben M, Walach N, Galili U, Schlesinger M. The effect of radiation therapy on lymphocyte subpopulations in cancer patients. Cancer. 1976;37:1417–1421. doi: 10.1002/1097-0142(197603)37:3<1417::aid-cncr2820370324>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 20.Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: Modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31:140–144. doi: 10.3109/07357907.2012.762780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, DeWees TA, Badiyan SN, Speirs CK, Mullen OF, Fergus S. et al. Clinical and dosimetric predictors of acute severe lymphopenia during radiation therapy and concurrent temozolomide for high-grade glioma. Int J Radiat Oncol Biol Phys. 2015;92:1000–1007. doi: 10.1016/j.ijrobp.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Trowell OA. The sensitivity of lymphocytes to ionising radiation. J Pathol Bacteriol. 1952;64:687–704. doi: 10.1002/path.1700640403. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res. 1990;123:224–227. [PubMed] [Google Scholar]
- 24.Campbell BA, Callahan J, Bressel M, Simoens N, Everitt S, Hofman MS. et al. Distribution atlas of proliferating bone marrow in non-small cell lung cancer patients measured by FLT-PET/CT imaging, with potential applicability in radiation therapy planning. Int J Radiat Oncol Biol Phys. 2015;92:1035–1043. doi: 10.1016/j.ijrobp.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 25.Campbell AC, Hersey P, MacLennan IC, Kay HE, Pike MC. Immunosuppressive consequences of radiotherapy and chemotherapy in patients with acute lymphoblastic leukaemia. Br Med J. 1973;2:385–388. doi: 10.1136/bmj.2.5863.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weeke E. The development of lymphopenia in uremic patients undergoing extracorporeal irradiation of the blood with portable beta units. Radiat Res. 1973;56:554–559. [PubMed] [Google Scholar]
- 27.Boehm T, Bleul CC. The evolutionary history of lymphoid organs. Nat Immunol. 2007;8:131–135. doi: 10.1038/ni1435. [DOI] [PubMed] [Google Scholar]
- 28.Sini C, Fiorino C, Perna L, Noris Chiorda B, Deantoni CL, Bianchi M. et al. Dose-volume effects for pelvic bone marrow in predicting hematological toxicity in prostate cancer radiotherapy with pelvic node irradiation. Radiother Oncol. 2016;118:179–184. doi: 10.1016/j.radonc.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 29.MacLennan IC, Kay HE. Analysis of treatment in childhood leukemia. IV. The critical association between dose fractionation and immunosuppression induced by cranial irradiation. Cancer. 1978;41:108–111. doi: 10.1002/1097-0142(197801)41:1<108::aid-cncr2820410116>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]


