ABSTRACT
Resistance to carbapenem antibiotics through the production of New Delhi metallo-β-lactamase-1 (NDM-1) constitutes an emerging challenge in the treatment of bacterial infections. To monitor the possible source of the spread of these organisms in Dhaka, Bangladesh, we conducted a comparative analysis of wastewater samples from hospital-adjacent areas (HAR) and from community areas (COM), as well as public tap water samples, for the occurrence and characteristics of NDM-1-producing bacteria. Of 72 HAR samples tested, 51 (71%) samples were positive for NDM-1-producing bacteria, as evidenced by phenotypic tests and the presence of the blaNDM-1 gene, compared to 5 of 41 (12.1%) samples from COM samples (P < 0.001). All tap water samples were negative for NDM-1-producing bacteria. Klebsiella pneumoniae (44%) was the predominant bacterial species among blaNDM-1-positive isolates, followed by Escherichia coli (29%), Acinetobacter spp. (15%), and Enterobacter spp. (9%). These bacteria were also positive for one or more other antibiotic resistance genes, including blaCTX-M-1 (80%), blaCTX-M-15 (63%), blaTEM (76%), blaSHV (33%), blaCMY-2 (16%), blaOXA-48-like (2%), blaOXA-1 (53%), and blaOXA-47-like (60%) genes. Around 40% of the isolates contained a qnr gene, while 50% had 16S rRNA methylase genes. The majority of isolates hosted multiple plasmids, and plasmids of 30 to 50 MDa carrying blaNDM-1 were self-transmissible. Our results highlight a number of issues related to the characteristics and source of spread of multidrug-resistant bacteria as a potential public health threat. In view of the existing practice of discharging untreated liquid waste into the environment, hospitals in Dhaka city contribute to the potential dissemination of NDM-1-producing bacteria into the community.
IMPORTANCE Infections caused by carbapenemase-producing Enterobacteriaceae are extremely difficult to manage due to their marked resistance to a wide range of antibiotics. NDM-1 is the most recently described carbapenemase, and the blaNDM-1 gene, which encodes NDM-1, is located on self-transmissible plasmids that also carry a considerable number of other antibiotic resistance genes. The present study shows a high prevalence of NDM-1-producing organisms in the wastewater samples from hospital-adjacent areas as a potential source for the spread of these organisms to community areas in Dhaka, Bangladesh. The study also examines the characteristics of the isolates and their potential to horizontally transmit the resistance determinants. The significance of our research is in identifying the mode of spread of multiple-antibiotic-resistant organisms, which will allow the development of containment measures, leading to broader impacts in reducing their spread to the community.
KEYWORDS: NDM-1, wastewater, tap water, hospital, Dhaka city
INTRODUCTION
Multidrug-resistant (MDR) bacterial pathogens are a major problem in health care settings. Infections caused by carbapenemase-producing Enterobacteriaceae are extremely difficult to manage due to their marked resistance to a wide range of antibiotics. New Delhi metallo-β-lactamase (NDM) is the most recently described carbapenemase, and the rapid spread of NDM-1-producing bacteria constitutes a major global public health threat (1). The presence of NDM-1-producing organisms has been detected in clinical specimens (2–4), as well as in the aquatic environment of areas where bacterial infections are widespread. Contamination of surface water or drinking water with NDM-1-producing bacteria has been reported (5, 6). The source of spread of MDR bacteria, in particular, the spread of the genetic determinants of NDM-1 and the NDM-1-producing organisms and their significance as a public health threat, needs to be adequately investigated. The potential for contamination of drinking water with NDM-1-producing bacteria in Dhaka, Bangladesh, is real given that the water supply may be contaminated with sewage-derived bacteria (7).
The NDM-1 gene is located on self-transmissible plasmids that carry a considerable number of other antibiotic resistance genes (8). The widespread use of antibiotics in humans and in the food chain and their spillover into the environment accelerate the development, selection, and/or horizontal transfer of antibiotic resistance plasmids in a given bacterial population (9). It is conceivable that the primary source of these bacteria is hospitals and other health care settings where severe cases of bacterial infections are presented, and the volume of antibiotic use is high (10, 11). Therefore, wastewater from hospital settings is likely to contain MDR bacteria, unless these are treated adequately before discharge. The present study was primarily designed to determine the prevalences of NDM-1-producing organisms and MDR bacteria in the wastewater samples from hospital-adjacent areas and from community areas in Dhaka, Bangladesh, as well as to characterize the isolates for their potential to spread and transmit the resistance determinants.
RESULTS
High prevalence of NDM-1-producing bacteria in hospital wastewaters.
Of the 113 wastewater samples, 29 (26%) samples were found to be positive for the blaNDM-1 gene encoding NDM-1 when tested directly by PCR. All 29 NDM-1-positive samples were collected from wastewater in hospital-adjacent areas, and NDM-1-positive bacterial colonies were isolated from all 29 samples.
Culture of PCR-negative samples on ChromID Carba (bioMérieux, Marcy l'Etoile, France) agar plates showed bacterial growth in some samples, and by subsequent PCR, we detected an additional 28 samples that contained blaNDM-1. In the case of tap water samples, although 16 of 113 (14%) samples showed bacterial growth on ChromID Carba agar plates, all were found to be negative for blaNDM-1 by a subsequent PCR test. In total, we confirmed that 56 of 113 (49.5%) wastewater samples harbored blaNDM-1 gene-positive bacteria. Of these 56 samples, 51 (91%) samples were collected from hospital-adjacent areas, and the remaining 5 (9%) samples were from community areas. Thus, significantly more samples of hospital wastewater contained blaNDM-1-positive bacteria than wastewater from the community (Table 1). From the 56 blaNDM-1-positive samples, we collected a total of 104 bacterial isolates, each with independent characteristics, based on their cultural or antibiotic susceptibility or plasmid contents. Besides, 108 (96%) of 113 wastewater samples were found to contain bacteria showing a carbapenem resistance phenotype on MacConkey agar plates containing meropenem and in ChromID Carba agar, irrespective of their blaNDM-1 gene content.
TABLE 1.
Relative prevalence of NDM-1-producing organisms in wastewater samples from HAR and COM in Dhaka
| NDM-1 status (n) | No. (%) of samples collected |
P value | |
|---|---|---|---|
| HAR (n = 72) | COM (n = 41) | ||
| NDM-1 positive (56) | 51 (71) | 5 (12) | <0.001 |
| NDM-1 negative (57) | 21 (29) | 36 (88) | <0.001 |
Production of MBL by blaNDM-1 gene-positive isolates.
All 104 isolates showed a reduction in imipenem MIC of ≥3-fold in the presence of EDTA and thus were detected as positive for metallo-β-lactamase (MBL) production, according to the manufacturer's interpretation criteria (bioMérieux).
NDM-1-producing bacterial isolates comprise diverse species with multiple antibiotic resistance phenotypes.
We analyzed all 104 NDM-1-producing isolates by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) and identified the organisms at the species level. Klebsiella pneumoniae was found to be the most prevalent (44% [n = 46]), followed by Escherichia coli (29% [n = 30]), Acinetobacter spp. (15% [n = 16]), Enterobacter spp. (9% [n = 9]), Citrobacter freundii (2% [n = 2]), and Providencia rettgeri (1% [n = 1]). Among Acinetobacter spp., A. baumannii (7.6% [n = 8]) was the predominant species, followed by A. pittii (3.8% [n = 4]) and A. baylyi (1% [n = 1]). Three isolates that could not be identified by MALDI-TOF MS were detected as Acinetobacter spp. by 16S rRNA gene sequencing. Enterobacter aerogenes (5.7% [n = 6]) was the prevalent species among Enterobacter spp., followed by E. cloacae (2% [n = 2]) and E. asburiae (1% [n = 1]). All isolates were resistant to piperacillin-tazobactam, ceftazidime, cefotaxime, and imipenem. The majority of the isolates were resistant to amikacin (86%), gentamicin (91%), aztreonam (89%), and ciprofloxacin (88.9%). Only 19% of the isolates were resistant to minocycline, and 4.8% were resistant to colistin. All isolates were susceptible to tigecycline (Table 2). Although there was a high rate of resistance among the isolates, the proportion of isolates that were resistant to various antibiotics varied slightly from species to species. E. coli was less resistant to aminoglycosides (amikacin and gentamicin) than other species, while a small proportion of both E. coli and Klebsiella pneumoniae isolates were resistant to colistin. Enterobacter spp. and Acinetobacter spp. were less resistant to ciprofloxacin and aztreonam, respectively, than were E. coli and Klebsiella pneumoniae (Table 2).
TABLE 2.
Antibiotic susceptibilities of NDM-1-producing bacterial isolates obtained from wastewater samples in Dhaka
| Antibiotic |
E. coli (n = 30) |
Klebsiella pneumoniae (n = 46) |
Acinetobacter spp. (n = 16) |
Enterobacter spp. (n = 9) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (mg/liter) | MIC90 (mg/liter) | % resistant | MIC50 (mg/liter) | MIC90 (mg/liter) | % resistant | MIC50 (mg/liter) | MIC90 (mg/liter) | % resistant | MIC50 (mg/liter) | MIC90 (mg/liter) | % resistant | |
| Piperacillin-tazobactam | >256 | >256 | 100 | >256 | >256 | 100 | >256 | >256 | 100 | >256 | >256 | 100 |
| Minocycline | 3 | 32 | 13 | 4 | 48 | 24 | 1.5 | 96 | 12.5 | 4 | 32 | 22 |
| Tigecycline | 0.38 | 1 | 0 | 0.75 | 2.0 | 0 | 0.38 | 2.0 | 0 | 0.75 | 1.5 | 0 |
| Ciprofloxacin | >32 | >32 | 100 | >32 | >32 | 89 | >32 | >32 | 100 | 3 | >32 | 40 |
| Imipenem | >32 | >32 | 96 | >32 | >32 | 100 | >32 | >32 | 100 | >32 | >32 | 100 |
| Colistin | 1.5 | 4 | 7 | 1.5 | 4 | 4 | 1.5 | 4 | 0 | 1.5 | 4 | 0 |
| Cefotaxime | >256 | >256 | 100 | >256 | >256 | 100 | >256 | >256 | 100 | >256 | >256 | 100 |
| Ceftazidime | >256 | >256 | 100 | >256 | >256 | 100 | >256 | >256 | 100 | >256 | >256 | 100 |
| Amikacin | >256 | >256 | 77 | >256 | >256 | 91 | >256 | >256 | 81 | >256 | >256 | 100 |
| Gentamicin | >256 | >256 | 77 | >256 | >256 | 98 | >256 | >256 | 94 | >256 | >256 | 100 |
| Aztreonam | >256 | >256 | 100 | >256 | >256 | 96 | >256 | >256 | 56 | >256 | >256 | 89 |
NDM-1-producing bacteria carried diverse additional antibiotic resistance genes.
The presence of various antibiotic resistance determinants in the 104 NDM-1-producing bacterial isolates was detected by PCR (Table 3). Eighty-one percent (n = 84) of the isolates were positive for blaCTX-M, and almost all of these were positive for blaCTX-M-1 (98% [n = 83]) and blaTEM (95% [n = 80]). Two isolates were positive for blaOXA-48-like, representing the copresence of class B and class D carbapenemases. blaCTX-M-15 was present in 66 isolates (63%), and blaCMY-2 was present in 17 isolates (16%). None of the colistin-resistant isolates were positive for the MCR-1 gene.
TABLE 3.
Prevalences of different antibiotic resistance genes in NDM-1-producing organisms obtained from wastewater samples in Dhaka
| NDM-1-producing bacterial species (no. of isolates) | % isolates positive for antibiotic resistance gene |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| blaTEM | blaSHV | blaCTX-M conserved group | blaCTX-M-15 | blaCMY-2 | blaOXA-48 | blaOXA-1 | blaOXA-47 |
qnr genes |
rmtB | rmtC | armA | |||
| qnrA | qnrB | qnrS | ||||||||||||
| E. coli (30) | 87 | 3 | 90 | 73 | 43 | 3 | 53 | 60 | 0 | 13 | 3 | 23 | 3 | 37 |
| K. pneumoniae (46) | 85 | 61 | 93 | 74 | 2 | 0 | 65 | 74 | 0 | 24 | 41 | 4 | 0 | 46 |
| Acinetobacter spp. (3) | 67 | 33 | 67 | 67 | 0 | 0 | 33 | 33 | 0 | 0 | 67 | 0 | 0 | 67 |
| A baumannii (8) | 25 | 13 | 25 | 25 | 0 | 0 | 25 | 25 | 0 | 13 | 0 | 0 | 0 | 0 |
| A pittii (4) | 50 | 0 | 50 | 0 | 0 | 0 | 50 | 50 | 0 | 25 | 25 | 0 | 0 | 0 |
| A baylyi (1) | 100 | 100 | 100 | 100 | 0 | 0 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| E. asburiae (1) | 100 | 0 | 100 | 100 | 0 | 0 | 100 | 100 | 0 | 100 | 0 | 0 | 0 | 100 |
| E. cloacae (2) | 100 | 0 | 50 | 50 | 0 | 0 | 50 | 50 | 0 | 50 | 0 | 0 | 0 | 50 |
| E aerogenes (6) | 33 | 50 | 50 | 17 | 0 | 0 | 33 | 33 | 0 | 0 | 0 | 0 | 0 | 83 |
| C. freundii (2) | 100 | 0 | 100 | 100 | 100 | 50 | 0 | 50 | 0 | 50 | 0 | 0 | 0 | 0 |
| P. rettgeri (1) | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| Total (104) | 77 | 34 | 81 | 63 | 16 | 2 | 54 | 61 | 0 | 19 | 22 | 9 | 1 | 40 |
Genetic determinant of NDM-1 production was horizontally transmissible.
All NDM-1-positive isolates carried multiple plasmids ranging in size from 1.4 to 140 MDa, and the distribution of plasmids among isolates was heterogeneous. All NDM-1-producing isolates were tested for mobility of the NDM-1 determinants by conjugation with E. coli J53, and transconjugants containing NDM-1 plasmid were obtained from 28 (27%) isolates. Plasmids of different sizes ranging from 30 MDa to 50 MDa were found to be transmissible via conjugation. All transconjugants carrying the plasmids were found to be positive for the blaNDM-1 gene by PCR and were resistant to imipenem.
Genetic fingerprints suggest transmission of NDM-1-producing bacteria between HAR and COM environments.
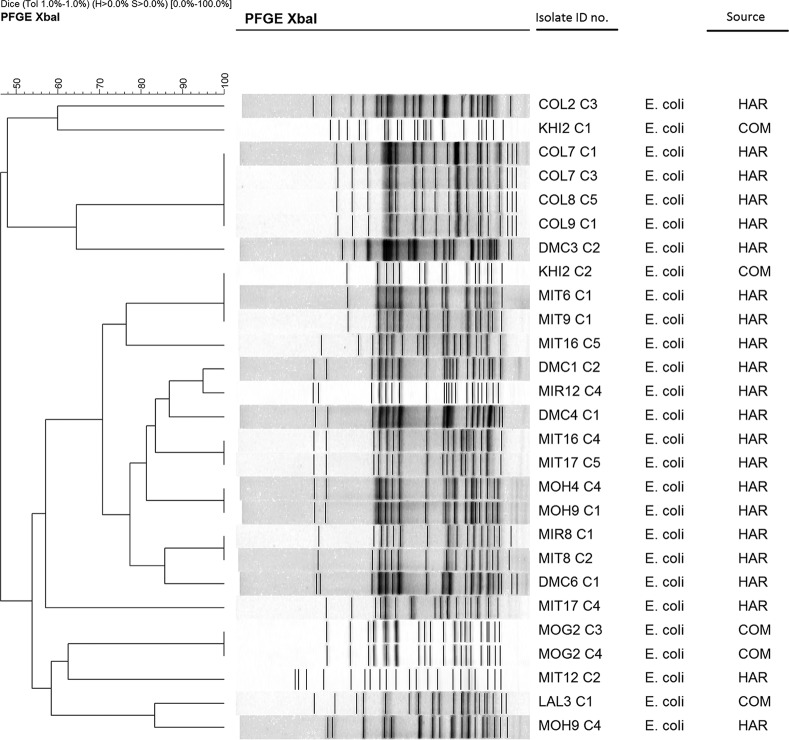
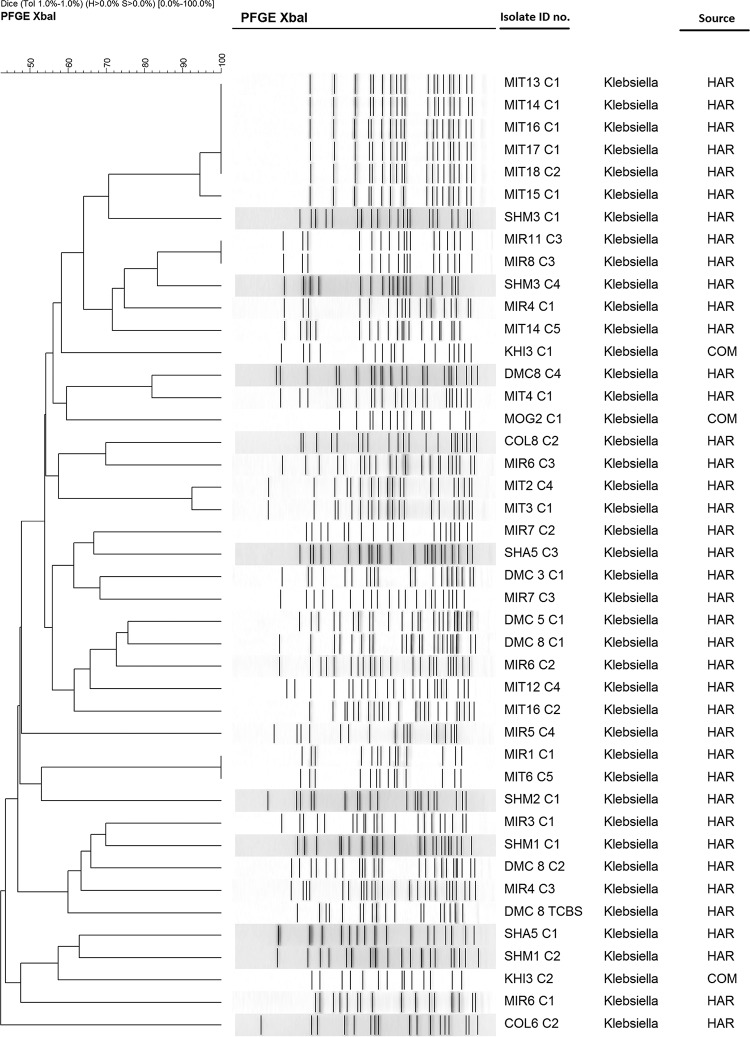
Pulsed-field gel electrophoresis (PFGE) of the XbaI-digested chromosomal DNA of the NDM-1-producing E. coli and Klebsiella pneumoniae isolates revealed 16 to 20 reproducible DNA fragments ranging from 20 to 1,135 bp in size. Banding patterns were interpreted according to established criteria (12). We detected 18 different PFGE patterns among the 27 E. coli isolates and 37 different patterns among the 43 isolates of K. pneumoniae. For both E. coli and K. pneumoniae, several isolates from different locations showed identical PFGE patterns, indicating dissemination of the same clone between the locations (Fig. 1 and 2).
FIG 1.
Dendrogram generated by BioNumerics software, showing distances calculated by the Dice similarity index of PFGE XbaI profiles for NDM-1-producing E. coli strains. The degree of similarity (%) is shown on the scale. HAR, hospital-adjacent areas; COM, community areas; ID, identification.
FIG 2.
Dendrogram generated by BioNumerics software, showing distances calculated by the Dice similarity index of PFGE XbaI profiles for NDM-1-producing Klebsiella pneumoniae strains. The degree of similarity (%) is shown on the scale. HAR, hospital-adjacent areas; COM, community areas.
DISCUSSION
We predict that the results presented here will have significant implications for preventing the spread of NDM-1-producing bacteria in the environment, particularly in a developing country setting. Our results also highlight a number of issues related to the characteristics, genetic determinants, and spread of multiple-drug-resistant bacteria as a potential public health threat. Although around 50% of wastewater samples were positive for NDM-1-producing bacteria, 96% of the samples caused bacterial growth on carbapenem-selective plates, suggesting that the additional 46% of samples contained carbapenem-resistant organisms, but the mechanism of resistance was different. Furthermore, antibiotic susceptibility testing of NDM-1-producing isolates revealed that all isolates were resistant to multiple classes of antibiotics, and a majority of isolates were resistant to all classes of antibiotics except for tigecycline.
Notably, although we did not find any NDM-1-producing bacteria in tap water samples, 16 tap water samples (14% [n = 113]) yielded bacterial growth on carbapenem-selective plates, indicating that these samples also contained carbapenem-resistant organisms with one or more different mechanisms of resistance. The tap water samples included in this study were meant to examine the possibility of transmission of NDM-1-producing organisms through the household water supply. In our previous study, we found that around 63% of tap water samples from Dhaka city were contaminated with E. coli, and a significant number of E. coli isolates (36%) were resistant to multiple classes of antibiotics, including the extended-spectrum β-lactamases (26%) (7). Around 85% of the supply water in Dhaka city is extracted from the underground, while the remaining 15% comes from surface water sources (13). There is no clear information on how supply water in Dhaka city becomes contaminated, but the poor infrastructure of water supply lines is one of the most important reasons (14). This can be further explained by the fact that water at the point of extraction is of good quality, but water at the point of use is mostly contaminated (14).
Identification of organisms by MALDI-TOF MS analysis revealed that Klebsiella pneumoniae, E. coli, and Acinetobacter baumannii were the predominant species producing NDM-1. These three species are associated with a majority of clinical cases or incidents of carbapenem-resistant or NDM-1-producing bacteria worldwide, including in Bangladesh (1). In our previous studies, we found that Klebsiella pneumoniae and E. coli were the most common enteric Gram-negative organisms that possess the blaNDM-1 gene (2, 15). In the present study, we identified a number of rarely reported species as NDM-1 producers. Most of these organisms have not been previously reported as NDM-1 producers from Bangladesh, such as Enterobacter asburiae, Providencia rettgeri, and Acinetobacter baylyi. These findings suggest that the gene encoding NDM-1 is spreading to nonconventional organisms, and its distribution is more widespread in the environment than was previously appreciated. The versatility and self-transmissible nature of the plasmid harboring the genetic determinants of NDM-1 are the most likely causes of its widespread distribution among microbiota (2, 15). These assumptions are supported by the results of our laboratory experiments of conjugative transfer of the plasmid. Successful conjugants were produced in 27% of NDM-1-producing isolates, suggesting that horizontal transmission of NDM-1 plasmid may be the main driving force for widespread occurrence of NDM-1-producing organisms in the environment. In agreement with this notion, we found that NDM-1-producing E. coli and K. pneumoniae isolates belonged to diverse clones based on their PFGE profiles.
The present study suggests that in Dhaka, hospitals are acting as an important source of NDM-1-producing bacteria, and untreated hospital waste is an important source of these bacteria. The implications of our findings are likely to be significant from a public health standpoint. A significantly higher number of samples collected from hospital-adjacent areas contained NDM-1-producing bacteria than samples from community areas. It is likely that hospital liquid wastes are directly discharged into the municipal sewage lines. Although this study was focused primarily on samples collected in Dhaka city, a similar situation may exist in any developing country setting where there is a high prevalence of MDR organisms among patients due to unregulated use of antibiotics, lack of adequate sanitation and hygiene, and poor waste management policies. This study also highlights the need for constant monitoring of hospital wastewater for antibiotic-resistant bacteria and the urgent need to place efficient wastewater treatment plants in health care settings as part of biosecurity programs. More work should be done to understand the spread of MDR bacteria, including the NDM-1-producing ones in the environment and the resultant health implications.
MATERIALS AND METHODS
Types of sample and collection sites.
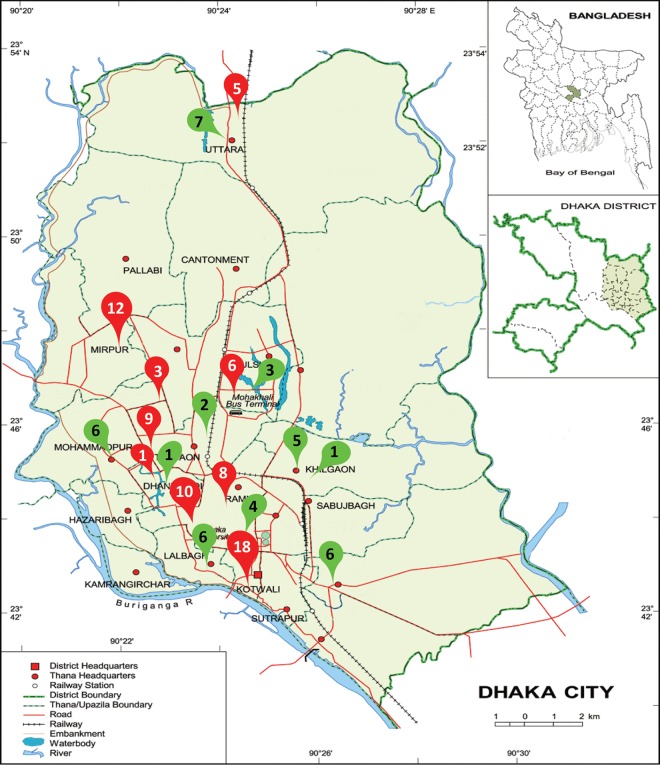
The study site was the Dhaka metropolitan area. We collected wastewater samples from an open drainage system and tap water supplied by the municipal authority. These samples were collected during May to July 2012, mostly from two types of localities in Dhaka city, hospital-adjacent areas (HAR) and community areas (COM). Samples were obtained from wastewater drains within 3 m of boundaries of the large public/private hospitals or clinics, as well as from similar types of drains across the community/residential areas where there were no hospitals or clinics nearby. We collected tap water samples from the same areas where we collected wastewater samples. Samples were collected from the point-of-use taps that are publicly accessible and where people use the water daily. We collected 113 wastewater samples (72 from HAR and 41 from COM) and an equal number of tap water samples from a total of 17 areas within the Dhaka metropolitan area, which covers an area of approximately 10 km2. Of these 17 locations, 9 locations were selected based on the presence of hospitals (Fig. 3).
FIG 3.
Sample collection sites in the map of Dhaka city. Inset, location of Dhaka district within map of Bangladesh and location of Dhaka city within Dhaka district. The red pointers indicate the locations adjacent to hospitals (HAR), and the green pointers indicate the locations in community areas (COM). Numbers within pointers denote the number of wastewater samples collected from each area. An equal number of tap water samples was collected from each area. Map template from World Map (http://www.worldmap1.com/map/Dhaka-map).
Sample collection and processing.
For the collection of wastewater samples, we used the procedure described by Walsh and colleagues (5). We collected an approximately 100-μl wastewater sample by dipping a sterile cotton swab in the running wastewater drain (Fig. 4). For tap water, we collected 40 ml of water from the tap according to the standard procedure described previously (5). The flow of sample processing and analysis is shown in a process chart in Fig. 5.
FIG 4.
Sampling sites. Photos of wastewater drains and a public tap water point.
FIG 5.
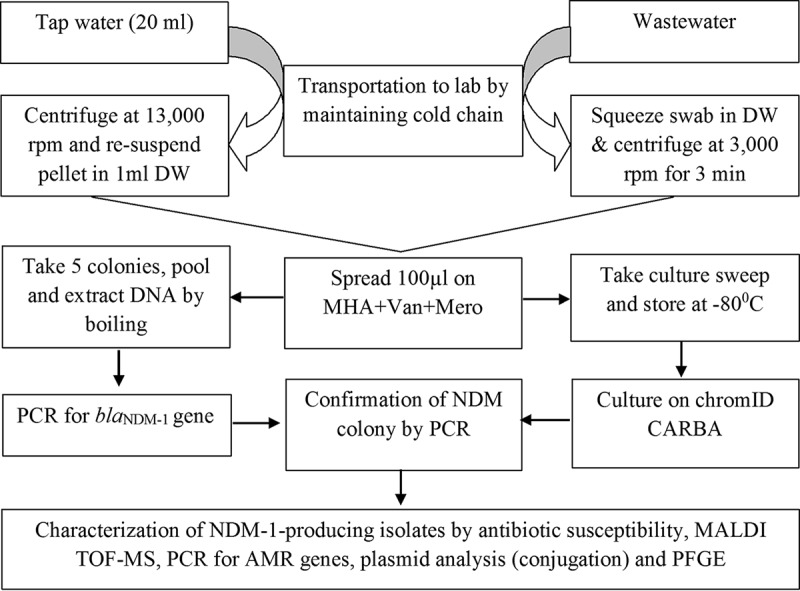
Flow diagram of methodology used in the study. DW, distilled water; Van, vancomycin; Mero, meropenem; AMR, antibiotic resistance.
Detection of NDM-1-producing isolates by direct PCR.
All samples were tested for the NDM-1 gene by PCR, according to the procedure described earlier (2). Concentrated wastewater and tap water samples were directly used as the templates in PCR.
Initial screening of the NDM-1-producing bacteria by culture methods.
We plated 50 μl of each sample concentrate onto Mueller-Hinton agar (MHA) plates (Becton Dickinson, Oxford, UK) containing 100 mg/liter vancomycin and 0.5 mg/liter meropenem and incubated at 37°C. We randomly selected 5 isolated colonies from MHA plates and subcultured these on MacConkey agar medium containing 0.5 mg/liter meropenem. We tested each of these colonies for the NDM-1 gene by PCR, according to the procedure described earlier. Colonies obtained from MHA plates derived from NDM-1 gene-negative samples were not subcultured into MacConkey agar, and instead, a culture sweep from these plates was stored at −70°C. Later on, we inoculated these culture sweeps on a ChromID Carba plate, a chromogenic culture medium selective for carbapenem-resistant Enterobacteriaceae, and incubated them at 37°C for 18 to 24 h. We randomly picked up to 5 colonies/plate and subcultured these on MacConkey agar containing 0.5 mg/liter meropenem. Finally, we tested these colonies for the NDM-1 gene by PCR (Fig. 5).
Detection of bacterial species by MALDI-TOF MS analysis.
The primary identification was confirmed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) using the Biotyper system (Bruker Daltonics, Bremen, Germany). In brief, an individual colony from an overnight subculture plate was transferred to a selected position on an US IVD 48 spot target (target). The target was then air-dried, and US IVD HCCA portioned (matrix) was added. The standard solvent in the matrix solution extracts proteins from the microorganisms. When dried matrix crystallizes, the inoculated target was analyzed on the MALDI Biotyper system. The analytical data were processed with the Bruker Biotyper software (version V4·0·0·1_4613-5627; Bruker Daltonics). A log(score) value of ≥2.00 was considered an excellent probability for test organism identification at the species level, and an organism identification was considered low confidence if log(score) was between 1.70 and <2.00. Identifications were performed in duplicate, according to the manufacturer's instructions. Isolates that could not be identified using MALDI-TOF MS were analyzed by 16S rRNA gene sequencing for identification.
Determination of MICs.
All NDM-1-positive isolates were tested for MICs against piperacillin-tazobactam, imipenem, cefotaxime, ceftazidime, aztreonam, ciprofloxacin, gentamicin, amikacin, minocycline, and tigecycline using Etest, according to the manufacturer's instructions (bioMérieux, Marcy l'Etoile, France). The MIC for colistin was determined by the broth microdilution method (16). The MICs were read in the intersection point of inhibitory eclipse, according to the manufacturer's recommendation. CLSI breakpoints were used to categorize the isolates into susceptible and resistant strains (17).
Determination of MBL producers by MBL Etest.
The blaNDM-1-producing isolates were tested for metallo-β-lactamase (MBL) by Etest (bioMérieux, Marcy l'Etoile, France). MBL Etest strips with imipenem (IP) (4 to 256 μg/ml) and IPI (imipenem-EDTA) (1 to 64 μg/ml) were applied on Mueller-Hinton agar and were incubated for 16 to 20 h at 37°C. A reduction in the IP MIC of ≥3-fold in the presence of EDTA was interpreted as being suggestive of MBL production. Equally, the presence of a “phantom” zone between the two gradient sections or deformation of the IP ellipses was also indicative of the presence of MBL. The strips were read according to the manufacturer's recommendation.
Detection of antibiotic resistance genes by PCR assays.
NDM-1-producing isolates were tested for various antibiotic resistance genes by PCR, including blaTEM, blaSHV, blaOXA-1, blaOXA-47-like, blaOXA-48-like, blaCTX-M conserved group, blaCTX-M-1 group, blaCTX-M-15, blaCMY-2, 16S RNA methylase-encoding genes (rmtC, rmtB, and armA), and qnr genes (qnrA, qnrB, and qnrS), according to procedures described earlier (15). Colistin-resistant isolates were tested for the MCR-1 gene by PCR, according to the protocol described by Liu and colleagues (18).
Plasmid profile analysis and conjugation assays.
Plasmid DNA was prepared according to the rapid alkaline lysis method and separated by horizontal electrophoresis in 0.7% agarose gels in Tris-borate-EDTA (TBE) buffer at room temperature at 100 V (50 mA) for 4 h (19). The molecular weights of unknown plasmids were determined by comparing their band patterns with those obtained from plasmids used as standards. Plasmids present in E. coli PDK-9 (140, 105, 2.7, and 2.1 MDa), R1 (62 MDa), RP4 (36 MDa), Sa (23 MDa), and V-517 (35.8, 4.8, 3.7, 3.4, 3.1, 2.0, 1.8, and 1.4 MDa) strains were used as molecular weight standards (20). Conjugation was carried out by both broth mating and filter mating assays at 30°C using NDM-1-producing isolates as a donor and sodium azide-resistant E. coli J53 as a recipient. Transconjugant selection was performed on MacConkey agar plates containing meropenem (0.5 mg/liter) and sodium azide (100 mg/ml). Transconjugants were tested for the NDM-1 gene, as described above, by PCR. The MICs of antibiotics against all transconjugants were determined as described above. Plasmid analysis of the transconjugants was carried out as described previously (18).
Genetic fingerprinting by PFGE.
Genomic DNA was prepared in agarose blocks and digested with the restriction enzyme XbaI (New England BioLabs). DNA fragments were separated by PFGE on a CHEF MAPPER apparatus (Bio-Rad), according to the PulseNet program developed for E. coli (21). Analysis of TIFF images was carried out with the BioNumerics software (Applied Maths) based on Dice coefficient and unweighted pair group method using average linkages (UPGMA) to generate dendrograms with 1.0% tolerance values.
ACKNOWLEDGMENTS
This research study was funded by icddr,b's core donors and the Global Health Equity Scholars (GHES) fellowship program of the Fogarty International Center (FIC) of National Institutes of Health (NIH), USA (subaward 60160040-105415-A).
icddr,b acknowledges with gratitude the commitment of GHES and FIC of NIH to its research efforts. icddr,b also gratefully acknowledges the following donors who provide unrestricted support: the Government of the People's Republic of Bangladesh, Global Affairs Canada (GAC), Swedish International Development Cooperation Agency (Sida), and the Department for International Development (UK Aid).
We declare no competing interests.
REFERENCES
- 1.Berrazeg M, Diene S, Medjahed L, Parola P, Drissi M, Raoult D, Rolain J. 2014. New Delhi Metallo-beta-lactamase around the world: an eReview using Google Maps. Euro Surveill 19:pii=20809 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20809. [DOI] [PubMed] [Google Scholar]
- 2.Islam MA, Talukdar PK, Hoque A, Huq M, Nabi A, Ahmed D, Talukder KA, Pietroni MA, Hays JP, Cravioto A, Endtz HP. 2012. Emergence of multidrug-resistant NDM-1-producing Gram-negative bacteria in Bangladesh. Eur J Clin Microbiol Infect Dis 31:2593–2600. doi: 10.1007/s10096-012-1601-2. [DOI] [PubMed] [Google Scholar]
- 3.Islam MA, Nabi A, Rahman M, Islam M, Ahmed D, Faruque AS, Hossain A, van Belkum A, Endtz HP. 2014. Prevalence of faecal carriage of NDM-1-producing bacteria among patients with diarrhoea in Bangladesh. J Med Microbiol 63:620–622. doi: 10.1099/jmm.0.064527-0. [DOI] [PubMed] [Google Scholar]
- 4.Perry JD, Naqvi SH, Mirza IA, Alizai SA, Hussain A, Ghirardi S, Orenga S, Wilkinson K, Woodford N, Zhang J, Livermore DM, Abbasi SA, Raza MW. 2011. Prevalence of faecal carriage of Enterobacteriaceae with NDM-1 carbapenemase at military hospitals in Pakistan, and evaluation of two chromogenic media. J Antimicrob Chemother 66:2288–2294. doi: 10.1093/jac/dkr299. [DOI] [PubMed] [Google Scholar]
- 5.Walsh TR, Weeks J, Livermore DM, Toleman MA. 2011. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis 11:355–362. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 6.Toleman MA, Bugert JJ, Nizam SA. 2015. Extensively drug-resistant New Delhi metallo-β-lactamase-encoding bacteria in the environment, Dhaka, Bangladesh, 2012. Emerg Infect Dis 21:1027–1030. doi: 10.3201/eid2106.141578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talukdar PK, Rahman M, Rahman M, Nabi A, Islam Z, Hoque MM, Endtz HP, Islam MA. 2013. Antimicrobial resistance, virulence factors and genetic diversity of Escherichia coli isolates from household water supply in Dhaka, Bangladesh. PLoS One 8:e61090. doi: 10.1371/journal.pone.0061090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh TR, Toleman MA. 2011. The new medical challenge: why NDM-1? Why Indian? Expert Rev Anti Infect Ther 9:137–141. doi: 10.1586/eri.10.159. [DOI] [PubMed] [Google Scholar]
- 9.Andersson DI, Hughes D. 2014. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12:465–478. doi: 10.1038/nrmicro3270. [DOI] [PubMed] [Google Scholar]
- 10.Picão RC, Cardoso JP, Campana EH, Nicoletti AG, Petrolini FV, Assis DM, Juliano L, Gales AC. 2013. The route of antimicrobial resistance from the hospital effluent to the environment: focus on the occurrence of KPC-producing Aeromonas spp. and Enterobacteriaceae in sewage. Diagn Microbiol Infect Dis 76:80–85. doi: 10.1016/j.diagmicrobio.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Goic-Barisic I, Hrenovic J, Kovacic A, Musić MŠ. 2016. Emergence of oxacillinases in environmental carbapenem-resistant Acinetobacter baumannii associated with clinical isolates. Microb Drug Resist 22:559–563. doi: 10.1089/mdr.2015.0275. [DOI] [PubMed] [Google Scholar]
- 12.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahidul MK, Mehadee H, Siunjukta A. 2014. Incidence of multiple potentially pathogenic bacteria in tap water from different restaurants in Dhaka city, Bangladesh. Int Food Res J 21:131–134. [Google Scholar]
- 14.Mahbub KR, Nahar A, Ahmed MM, Chakraborty A. 2011. Quality analysis of Dhaka WASA drinking water: detection and biochemical characterization of the isolates. J Environ Sci Nat Res 4:41–49. [Google Scholar]
- 15.Islam MA, Huq M, Nabi A, Talukdar PK, Ahmed D, Talukder KA, Cravioto A, Endtz HP. 2013. Occurrence and characterization of multidrug-resistant New Delhi metallo-β-lactamase-1-producing bacteria isolated between 2003 and 2010 in Bangladesh. J Med Microbiol 62:62–68. doi: 10.1099/jmm.0.048066-0. [DOI] [PubMed] [Google Scholar]
- 16.European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2016. Recommendations for MIC determination of colistin (polymyxin E) as recommended by the joint CLSI-EUCAST Polymyxin Breakpoints Working Group. European Committee on Antimicrobial Susceptibility Testing, Växjö, Sweden: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf. [Google Scholar]
- 17.CLSI. 2009. Performance standards for antimicrobial disk susceptibility testing, M100-S19, vol 29, no 3 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 18.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 19.Kado CI, Liu ST. 1981. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol 145:1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talukder KA, Islam MA, Dutta DK, Hassan F, Safa A, Nair GB, Sack DA. 2002. Phenotypic and genotypic characterization of serologically atypical strains of Shigella flexneri type 4 isolated in Dhaka, Bangladesh. J Clin Microbiol 40:2490–2497. doi: 10.1128/JCM.40.7.2490-2497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Barrett TJ. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis 3:59–67. doi: 10.1089/fpd.2006.3.59. [DOI] [PubMed] [Google Scholar]