ABSTRACT
The susceptibility of the Iberian ibex (Capra pyrenaica) to Mycoplasma conjunctivae ocular infection and the changes in their interaction over time were studied in terms of clinical outcome, molecular detection, and IgG immune response in a captive population that underwent a severe infectious keratoconjunctivitis (IKC) outbreak. Mycoplasma conjunctivae was detected in the Iberian ibex, coinciding with the IKC outbreak. Its prevalence had a decreasing trend in 2013 that was consistent with the clinical resolution (August, 35.4%; September, 8.7%; November, 4.3%). Infections without clinical outcome were, however, still detected in the last handling in November. Sequencing and cluster analyses of the M. conjunctivae strains found 1 year later in the ibex population confirmed the persistence of the same strain lineage that caused the IKC outbreak but with a high prevalence (75.3%) of mostly asymptomatic infections and with lower DNA load of M. conjunctivae in the eyes (mean quantitative PCR [qPCR] cycle threshold [CT], 36.1 versus 20.3 in severe IKC). Significant age-related differences of M. conjunctivae prevalence were observed only under IKC epizootic conditions. No substantial effect of systemic IgG on M. conjunctivae DNA in the eye was evidenced with a linear mixed-models selection, which indicated that systemic IgG does not necessarily drive the resolution of M. conjunctivae infection and does not explain the epidemiological changes observed. The results show how both epidemiological scenarios, i.e., severe IKC outbreak and mostly asymptomatic infections, can consecutively occur by entailing mycoplasma persistence.
IMPORTANCE Mycoplasma infections are reported in a wide range of epidemiological scenarios that involve severe disease to asymptomatic infections. This study allows a better understanding of the transition between two different Mycoplasma conjunctivae epidemiological scenarios described in wild host populations and highlights the ability of M. conjunctivae to adapt, persist, and establish diverse interactions with its hosts. The proportion of asymptomatic and clinical M. conjunctivae infections in a host population may not be regarded only in response to intrinsic host species traits (i.e., susceptibility) but also to a specific host-pathogen interaction, which in turn influences the infection dynamics. Both epidemic infectious keratoconjunctivitis and a high prevalence of asymptomatic M. conjunctivae infections may occur in the same host population, depending on the circulation of M. conjunctivae, its maintenance, and the progression of the host-pathogen interactions.
KEYWORDS: asymptomatic infection, Capra pyrenaica, Iberian ibex, infectious keratoconjunctivitis, host-pathogen interactions, infection persistence, molecular epidemiology, virulence
INTRODUCTION
Mycoplasma spp. are small bacteria without a cell wall that have a strict parasitic life in association with their hosts, either as commensals or pathogens (1). Mycoplasma has several singular mechanisms for host adaptation and survival (2–4), which includes one of the highest nucleotide substitution rates among bacteria that provides chances for novel interactions with its hosts (5, 6). Mycoplasma infections can therefore involve diverse epidemiological scenarios, resulting either in the development of severe disease or in asymptomatic carriers that may or maybe not further develop clinical symptoms (1, 7). To properly assess host-mycoplasma interaction dynamics, a longitudinal sampling design is required. Unfortunately, such sampling conditions are usually unfeasible in wild host species.
Infectious keratoconjunctivitis (IKC) is a contagious ocular disease caused by Mycoplasma conjunctivae that affects small domestic ruminants and, more importantly, wild Caprinae, in which mortality can reach 30% (8). Despite being a long-known disease of wild mountain ungulates (9), several aspects of IKC epidemiology in natural systems are not fully understood, and apparent differences in susceptibility are associated with host species and its functional roles in alpine multihost systems (10–13). Clinical stages of IKC may evolve from conjunctivitis to several degrees of keratoconjunctivitis, with clinical recovery as the predominant outcome of the disease (14, 15). Mycoplasma conjunctivae may still persist in the eyes up to 6 months beyond the disappearance of clinical signs (14, 15).
Whereas endemic and subclinical infections of M. conjunctivae are common among small domestic ruminants, mainly in sheep (16), subclinical infections in wild mountain ungulates are reported less often and/or occur at a lower prevalence (11, 12). The local fading out of clinical disease (IKC) and the more severe clinical signs typically exhibited by wild hosts have led some to propose that M. conjunctivae cannot be maintained in wild host populations (10, 17–19). However, diverse epidemiological scenarios have been described in wild Caprinae based on field records of IKC, including its apparently endemic occurrence (12, 20). The recurrent detection of M. conjunctivae strain clusters in wild host populations also suggests that it may eventually persist in natural systems (20).
Mycoplasma conjunctivae infection elicits a strong immune IgG response, as described for IKC outbreaks in wild Caprinae (21), and may be an important component of the host immune response. Nevertheless, field observations suggest that acquired immunity does not prevent subsequent IKC episodes (8). Therefore, the maintenance of specific IgG may be crucial to avoid M. conjunctivae persistence in the host population.
Susceptibility of the Iberian ibex (Capra pyrenaica) to M. conjunctivae infection has been reported to be associated with a few sporadic IKC cases in massifs from Spain but, to our knowledge, no IKC outbreaks have been described (22, 23). This medium-size Caprinae is a species endemic to the Iberian Peninsula and is adapted to rocky mountain ecosystems. It inhabits the Mediterranean mountain ranges of the Iberian Peninsula, where it is the most abundant mountain ungulate, with over 50,000 ibexes (24).
In this study, an IKC outbreak in the Iberian ibex is described, taking advantage of the opportunity to perform individual sequential sampling in a captive population of 60 individuals. The objectives of this study were to (i) assess Iberian ibex susceptibility to M. conjunctivae infection in captive and free-ranging animals and describe the dynamics of the IKC outbreak, (ii) assess the presence of M. conjunctivae in the ibex population 1 year later, (iii) evaluate the influences of sex and age on M. conjunctivae infection, and (iv) investigate the relationships between clinical signs, molecular detection, and IgG immune response against M. conjunctivae and possible changes over time.
RESULTS
Clinical follow-up and course of the IKC outbreak.
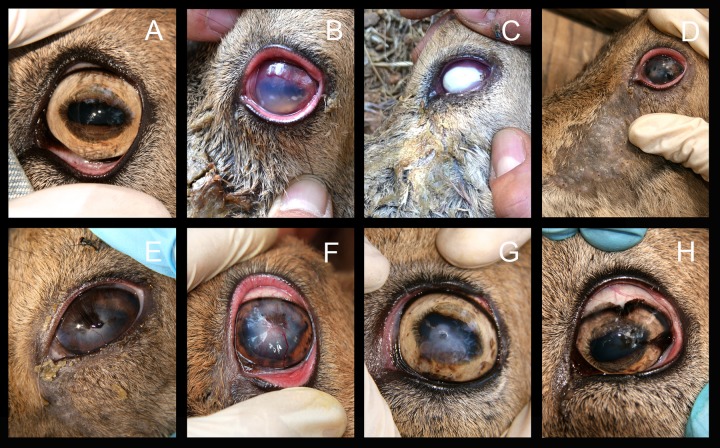
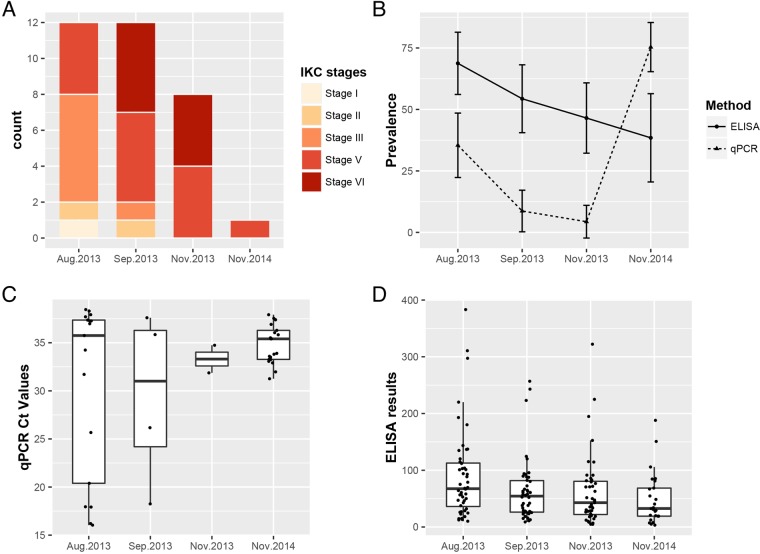
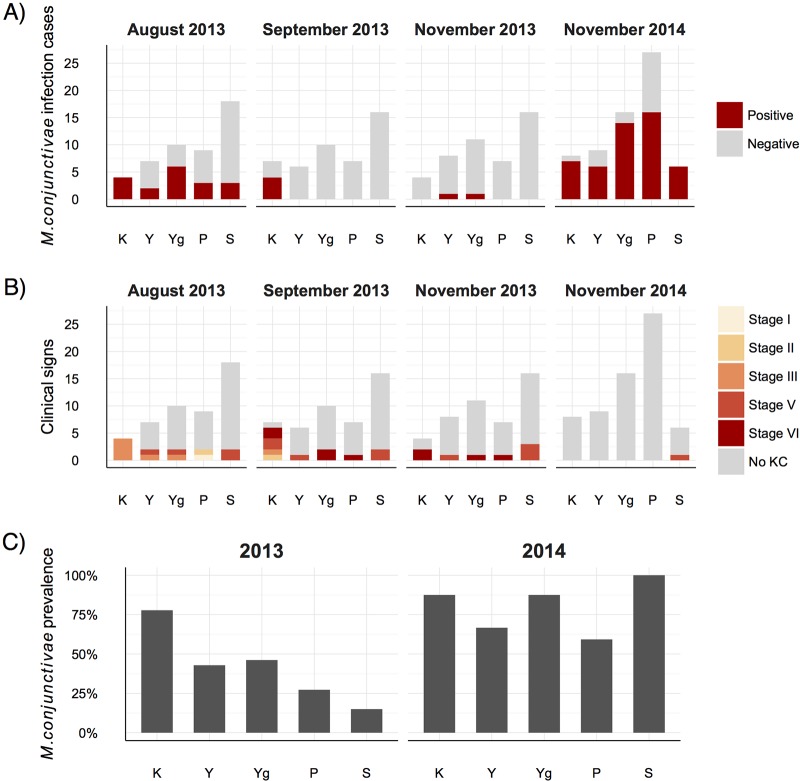
Ocular clinical signs were not observed in ibexes sampled in 2010 and 2011. The first evidence of ocular disease appeared on 1 July 2013, when two Iberian ibexes with severe ocular clinical signs were captured by hand due to visual impairment and total blindness. Both ibexes suffered from bilateral keratoconjunctivitis with widespread corneal opacity and abundant neovascularization in the peripheral cornea, accompanied by severe mucopurulent effusion, corresponding to IKC stage III (Fig. 1). From the first handling in August 2013 to November 2013, the ocular clinical signs progressed from predominantly severe clinical signs (IKC stages II and III) to chronic and healing stages V and VI (Fig. 1 and 2A). Clinical signs were bilateral mainly in severe IKC stages II (1/2) and III (7/9) and less common in initial IKC stage I (0/1) and healing stages V (2/14) and VI (3/9). Bilateral infections were different between eyes only in two cases, with one showing IKC stages V and VI and the other one showing IKC stages II and III. Among age categories, younger ibexes and especially kids had a higher proportion of severe clinical signs (IKC stages II and III) than older animals (Fig. 3). No IKC stage IV (severe IKC signs) or direct mortality was attributable to M. conjunctivae infection during the IKC outbreak. Only one ibex showed unilateral IKC stage V in November 2014. Although ocular clinical signs were observed in free-ranging ibexes from the Sierra Nevada Natural Space (SNNS) in 2013, they were not present in any of the 17 ibexes captured and sampled outside the enclosure.
FIG 1.
Ocular lesions in Iberian ibex corresponding to IKC stages of disease severity and resolution observed during the natural course of an IKC outbreak. (A) Normal eye appearance of Iberian Ibex. (B) IKC stage II with moderate corneal opacity, neovascularization of the peripheral cornea, and ocular discharge. (C) IKC stage III with severe corneal opacity, neovascularization of the peripheral cornea, and ocular discharge. (D) Chronic IKC stage III with infraorbital alopecia. (E to G) Different IKC stage V corresponding to healing process with neovascularization of the vertex of the cornea, slight central corneal opacity, and evidences of a central ulcer in F and G. (H) IKC stage VI, pigmentation of the cornea.
FIG 2.
Clinical, serological, and molecular trends of M. conjunctivae infection in the Iberian ibex population. Results showed handlings that correspond with two epidemiological scenarios, epizootic IKC disease in 2013 and mostly asymptomatic infections in 2014. (A) Number of IKC cases by clinical stage. (B) Prevalence of M. conjunctivae-seropositive ibexes assessed by an indirect ELISA and prevalence of M. conjunctivae detection in eye swabs by qPCR. Ninety-five percent confidence intervals are represented by error bars. (C) Distribution of CT values of the M. conjunctivae-specific qPCR. Note that box plot of CT values from November 2013 is built with only two observations. (D) Absorbance relative values of the indirect ELISA that recognize IgG specific for M. conjunctivae.
FIG 3.
(A to C) Mycoplasma conjunctivae infection cases (A), clinical signs (KC) (B), and prevalence (C) shown by age class (K, kids; Y, yearlings; Yg, young; P, prime age; S, senescence) in the Iberian ibex population in two different epidemiological scenarios, an IKC outbreak in 2013 and mostly asymptomatic infections in 2014. Higher M. conjunctivae prevalence and more severe clinical signs are observed in kids only during the IKC outbreak.
Mycoplasma conjunctivae qPCR detection and sequencing.
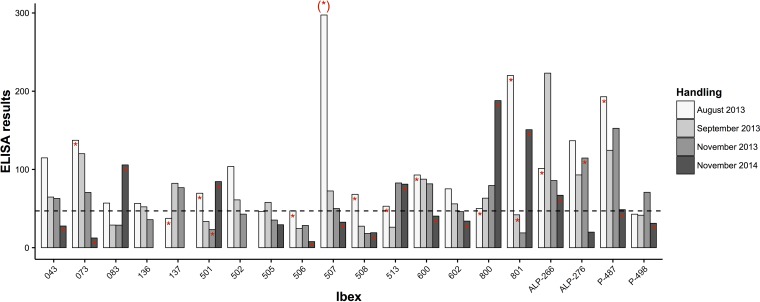
Mycoplasma conjunctivae was first detected in ibexes showing IKC in July 2013. The prevalence showed a decreasing trend from August 2013 until November 2013, in concordance with the course of the clinical signs (Table 1 and Fig. 2A and B). Two ibexes that were quantitative PCR (qPCR) positive for M. conjunctivae and/or showed IKC in August 2013 were qPCR negative by September 2013, and they turned qPCR positive in November 2013 (Fig. 4, ibexes 501 and ALP-276). The prevalence of M. conjunctivae rose again in November 2014 to significantly higher values than those of all 2013 handlings (August, χ2 = 17.1, df = 1, P < 0.001; September, χ2 = 46.5, df = 1, P < 0.001; November, χ2 = 53.1, df = 1, P < 0.001), in the absence of clinical signs in most of the ibexes (Table 1 and Fig. 2B). One out of the 17 free-ranging Iberian ibexes sampled was positive for M. conjunctivae by the qPCR (Table 1).
TABLE 1.
Summary of individuals sampled, ocular clinical signs (KC), and results of specific qPCR and IgG antibodies against M. conjunctivae
| Yr of sampling | Mo of sampling | No. of KC positives/no. sampleda | qPCR |
ELISA |
No. of positive ibexes per technique/no. of total positive ibexes |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. of positives/no. sampled | Prevalence (% [95% CI]) | No. of positives/no. sampled | Prevalence (% [95% CI]) | qPCR+/KCa | ELISA+/KCa | ELISA+/qPCR+ | |||
| Captive ibexes | |||||||||
| 2010 | November | 0/64 | 0/64 | 0 (0–5.7) | 0/0 | ||||
| 2011 | November | 0/51 | 0/51 | 0 (0–7.0) | 0/0 | ||||
| 2013 | July | 2/2 | 2/2 | 1/2 | 2/2 | 1/2 | 1/2 | ||
| August | 12/48 | 17/48 | 35.4 (23.4–49.6) | 33/48 | 68.7 (54.7–80.0) | 6/12 | 10/12 | 14/17 | |
| September | 12/46 | 4/46 | 8.7 (3.4–20.3) | 25/46 | 54.3 (40.2–67.8) | 4/12 | 5/12 | 1/4 | |
| November | 8/46 | 2/46 | 4.3 (1.2–14.5) | 20/43 | 46.5 (32.5–61.1) | 1/8 | 2/8 | 1/2 | |
| 2014 | November | 1/69 | 52/69 | 75.3 (64.0–84.0) | 10/26 | 38.5 (23.4–59.3) | 1/1 | 0/1 | 13/21 |
| Free-ranging ibexes | |||||||||
| 2013–2014 | October–April | 0/14 | 1/17 | 5.9 (0.3–27.0) | 1/13 | 7.8 (0.4–33.3) | 1/17 | 0/1 | |
Ocular clinical signs of current inflammation (IKC stages from I to III) and healing (IKC stage V) or past infections (IKC stages VI).
FIG 4.
Bar graph showing individual trends of the ELISA results from a random selection of ibexes that were sampled in most of the handlings in 2013 during the IKC outbreak and in 2014 with mostly asymptomatic infections. Numbers on the x axis are the individual ibex reference, and the ELISA results on the y axis correspond to the relative percentage from the positive reference standard. The detection of M. conjunctivae in eye swabs is shown with a red asterisk in the top of the bars, and the cutoff for seropositivity is shown with a horizontal dashed line. The asterisk in parentheses above the bar for ibex 507 indicates that it corresponds to a previous sampling in July 2013.
Asymptomatic infections were detected in 11 out of 17 (64.7%) qPCR-positive ibexes in August 2013, 0 out of four ibexes (0.0%) in September 2013, and one out of two ibexes (50.0%) in November 2013. Fifty-one out of the 52 (98.1%) qPCR-positive ibexes in November 2014 were asymptomatic, as was the free-ranging positive ibex. With regard to the whole population, asymptomatic infections were higher in August 2013 (22.9%) during the IKC outbreak and in November 2014 (73.9%) than in September 2013 (0.0%) and November 2013 (2.2%).
Among the M. conjunctivae-infected ibexes, qPCR-positive eyes were 100% bilateral in July 2013 (2/2), 47.1% in August 2013 (8/17), 50.0% in September 2013 (2/4), 0% in November 2013 (0/2), and 46.1% in November 2014 (24/52). Infection of both eyes was associated with severe IKC signs (stages II and III) in 2013 (10 out of 12 bilateral infections) but not in 2014, when no ibexes showed severe IKC signs.
Only two ibex kids with severe IKC were qPCR positive in two consecutive samplings in 2013, which implies that were probably infected for at least 7 weeks. The prevalence of M. conjunctivae was inversely related to age class during the outbreak of IKC in 2013 (χ2 = 19.24, df = 4, P < 0.001) but not in 2014 (χ2 = 7.73, df = 4, P = 0.102) (Fig. 3).
The detection of M. conjunctivae by qPCR was consistent in IKC stages with evidence of current inflammation (IKC stage I, 1/1; IKC stage II, 1/2; and IKC stage III, 8/9), but not in healing IKC stage V (4/14) or with evidences of past inflammatory processes in IKC stage VI (0/9). The mean threshold cycle (CT) values of the qPCR were significantly lower in the severe IKC stages II and III (20.3) than in IKC resolution stage V (mean CT, 35.1; P = 0.045) and asymptomatic ibexes (mean CT, 36.1; P < 0.001). The only IKC stage I observed had a high CT value (38.3; see Fig. 5). The mean CT values of the qPCR-positive samples increased progressively in the consecutive handlings from September 2013 onwards, in accordance with the fading away of the clinical signs throughout the outbreak of 2013 and 2014 (Fig. 2C).
FIG 5.
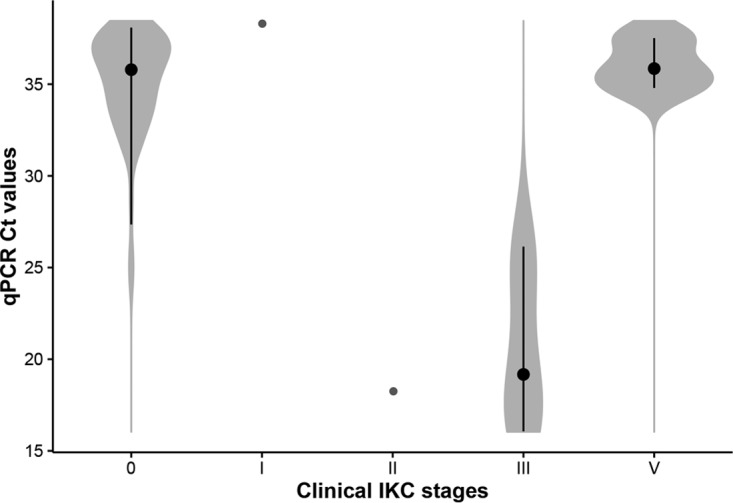
Violin plot showing the distribution of the CT values in eye swabs in IKC clinical stages that were qPCR positive. CT values were significantly lower in severe IKC stages (II and III) than initial (I) and healing stages (V) and asymptomatic infections (0). Black bars show the interquartile range, and black dots indicate the median of the CT values for each group.
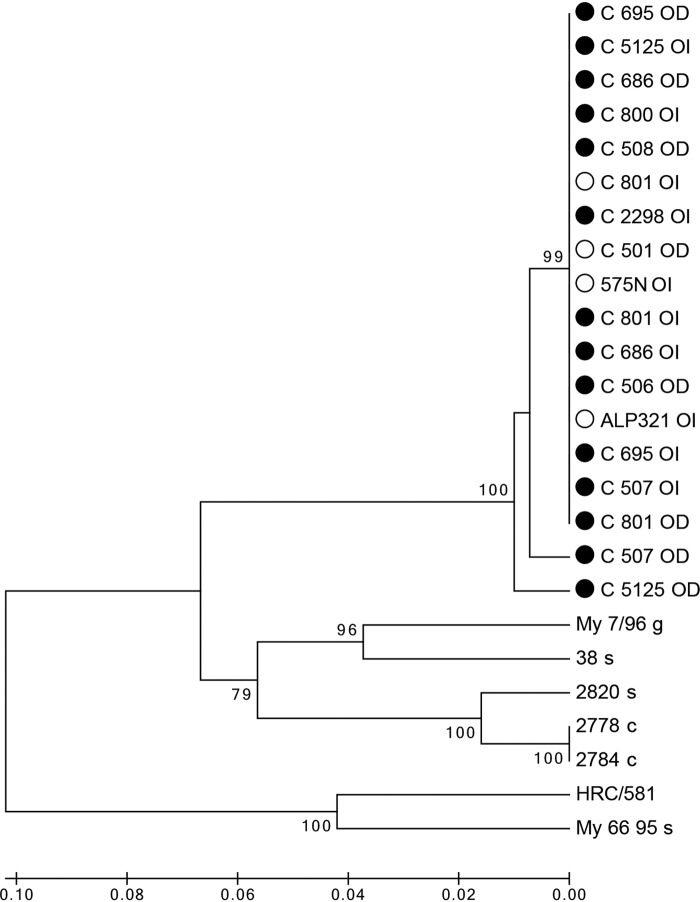
Eighteen PCR amplicons of approximately 700 bp that corresponded to the variable domain of the M. conjunctivae lppS gene were sequenced from nine ibexes sampled in 2013 (two ibexes from July, C_507 and C_686; five ibexes from August, C_506, C_508, C_800, C_801, and C_695; and two ibexes from September, C_2298 and C_5125) and four ibexes sampled in November 2014 (C_501, C_801, 575N, and ALP321). The M. conjunctivae strains causing the IKC outbreak in 2013 and those associated with asymptomatic infections from 2014 belonged to the same cluster, which was clearly separated from the rest of the sequences included in the tree, suggesting a phylogenetic relationship and a common origin (Fig. 6).
FIG 6.
Cluster analysis of the M. conjunctivae strains detected in this study based on the variable domain of the lppS gene. Name of sequence indicates the individual reference followed by right (OD) or left (OI) eye from which M. conjunctivae was detected. The year of sampling is shown with a black or white dot for 2013 or 2014, respectively. For comparison, sequences from isolates in other hosts are included: HRC/581 type strain, sequences obtained in sheep infections in eastern Swiss Alps (38 s and 2820 s) and Croatia (My 66 95), from a goat in Croatia (My 7/96), and from chamois in the Austrian Alps (2778 c and 2784 c). A separate clade of the ibex strains, all with a common origin, is observed in the tree.
ELISA for M. conjunctivae antibodies.
The cutoff determined by the Youden index resulted in a sensitivity of 93.9% and a specificity of 84.3% for the enzyme-linked immunosorbent assay (ELISA). The seroprevalence was higher in the first handling (August 2013) and decreased steadily across all the consecutive samplings (Table 1 and Fig. 2B). Among ibexes that were negative to the ELISA in August 2013, 33% (4/12) turned positive in September 2013. Among ibexes that were negative to the ELISA in September 2013, 33% (4/12) turned positive in November 2013. Both the mean and individual ELISA values showed a similar decreasing trend throughout the 2013 and 2014 handlings (Fig. 2D and 4).
The number of qPCR-positive ibexes that also had a positive result to the ELISA decreased across sampling periods. In August 2013 82.4% (14/17) of the qPCR-positive ibexes showed evidence of a systemic IgG response. In September, however, this proportion decreased to 25% (1/4), slightly rising in November 2013 (50% [1/2]) and 2014 (61.9% [9/21]). The ELISA value of each individual declined steadily after the first M. conjunctivae detection in the eye swabs in most of the ibexes (Fig. 4). The ELISA values were not clearly related to the presence or the severity of the clinical signs, and three ibexes with severe IKC did not seroconvert during or after the M. conjunctivae infection (e.g., Fig. 4, ibex 506).
None of the selected linear mixed models included the optical density of the ELISA as an explanatory fixed effect (Table 2), which indicated that no substantial variability of the CT values was explained by the ELISA results (proxy for IgG). The best model included only the age of the ibexes, which explained 68.35% of the observed variability of CT values (Akaike weight by age [wiage] = 0.80, F = 17.36). A small proportion of the CT response was due to the ibex random term (17.05%) (Table 2).
TABLE 2.
Linear mixed-model selection performed to assess the effect of ELISA results (proxy for IgG response) on CT values obtained by a M. conjunctivae-specific qPCR (proxy for M. conjunctivae loads) in eye swabs of Iberian ibexesa
| Biological model | K | AICc | Δi | wi | Marginal R2 | Conditional R2 |
|---|---|---|---|---|---|---|
| Age | 5 | 38.47 | 0.00 | 0.80 | 0.61856109 | 0.68359573 |
| Age + yr | 6 | 43.26 | 4.79 | 0.07 | 0.61088941 | 0.67602488 |
| Age + ELISA | 6 | 48.54 | 10.07 | 0.01 | 0.61074212 | 0.70032295 |
| Yr | 4 | 49.86 | 11.39 | 0.00 | 0.16534722 | 0.29751655 |
| Mo | 3 | 50.08 | 11.61 | 0.00 | 0.00000000 | 0.00652695 |
| ELISA | 4 | 57.87 | 19.40 | 0.00 | 0.03221345 | 0.03221350 |
| ELISA + yr | 5 | 58.69 | 20.22 | 0.00 | 0.15863676 | 0.28898391 |
| Age × ELISA | 8 | 67.30 | 28.83 | 0.00 | 0.58927052 | 0.60548979 |
Nested models for the random terms were fitted with the individual ibex and the year (corresponding to two epidemiological scenarios) as random effects. K, number of parameters; AICc, Akaike information criterion corrected for small sample sizes; Δi, difference of AICc with respect to the best model; wi, Akaike weight; marginal R2, observed variability in the response variable explained by the fixed factors; conditional R2, observed variability in the response variable explained by both the fixed and random factors. The model with substantial support is in bold.
DISCUSSION
Iberian ibex susceptibility to M. conjunctivae infection has been clearly confirmed by this study, which can suffer from IKC outbreaks but also can maintain M. conjunctivae at high prevalence with mostly asymptomatic infections. Both epidemiological situations (i.e., IKC outbreak and endemic asymptomatic infections) have been described in other domestic (16, 25) and wild free-ranging (12, 20, 26, 27) hosts. The results of the present study show how both epidemiological situations can occur consecutively in a host population, depending on M. conjunctivae circulation, its maintenance in the population, and the progression of the host-pathogen interactions.
Infectious keratoconjunctivitis outbreak.
There are few reports of IKC in Iberian ibex, and we are not aware of any prior descriptions of an IKC outbreak. The consistent detection of M. conjunctivae in severe IKC stages (II and III) and the correlation of CT values with IKC stages confirm that it was the primary agent of the outbreak. Ibexes that showed IKC stages II and III in which M. conjunctivae was not detected were seropositive and most probably correspond to false-negative results of the detection method (sampling/qPCR), or, less probably, were caused by other infectious agents (28, 29). The absence of M. conjunctivae in advanced and healing IKC stages V and VI agrees with resolution of the infection and with previous research (30). Since M. conjunctivae was not detected in the captive ibex population before 2013 and was also confirmed in free-ranging ibexes from the surrounding SNNS (clinical signs compatible with IKC were also observed; data not shown), M. conjunctivae was probably introduced along with asymptomatic carriers or was transported by eye-frequenting insects from outside the enclosure (31). Previous research in the Iberian ibex population from SNNS failed to detect M. conjunctivae (32), which suggests a likely recent introduction of this Mycoplasma in the SNNS ibex population.
Clinical signs during the IKC outbreak in 2013 were as severe as those reported in other IKC outbreaks in wild Caprinae (27, 33) and in naive domestic sheep flocks (25, 34). According to the progression of clinical signs and the molecular and serological results, the IKC outbreak probably started before July 2013, and clinical resolution (no severe IKC stage II or III) was observed by November 2013. However, both seroconversion and M. conjunctivae detection in the last 2013 handling indicate that there was still transmission of M. conjunctivae among ibexes almost 5 months after identification of the first IKC cases.
Asymptomatic postepizootic M. conjunctivae persistence.
The sequences obtained from asymptomatic infections in 2014 were very similar to the sequences from the IKC epizootic in 2013, indicating that the same M. conjunctivae lineage persisted after the initial disease outbreak. Whether M. conjunctivae persisted throughout the whole study period in the captive population or was reintroduced is not known, but persistence in host populations through singular mechanisms of host immune evasion is a common feature of mycoplasma infections (2, 35, 36). It is unlikely that a rare event, such as the introduction of similar M. conjunctivae strains from outside the double-fence enclosure, occurred over 2 consecutive years. On the other hand, the known clinical outcome of the introduction of M. conjunctivae in a host population does not correspond with the epidemiological scenario found in 2014 (25, 37). The persistence of M. conjunctivae has also been recently suggested in free-ranging wild host populations (20).
Within-host persistence of M. conjunctivae after clinical recovery has been reported from 3 to 6 months in sheep (15) and at least up to 2 months in Alpine ibex (Capra ibex) (14). In the Iberian ibex, the maximum period of infection demonstrated in this study was 7 weeks in 2013. Given that mycoplasma virulence and clinical signs are positively related with transmission in ocular diseases (38), the high M. conjunctivae prevalence detected in 2014 was presumably achieved by chronic persistence in the eyes (15). Altogether, this suggests that M. conjunctivae probably became endemic in the Iberian ibex population with apparently low health implications, as typically reported in domestic sheep flocks (16, 39).
Age and sex effect.
The higher prevalence of M. conjunctivae in younger Iberian ibexes during the IKC outbreak concurs with previous studies that reported higher M. conjunctivae prevalences in yearling ibexes (12) and lambs (39). A specific role of lambs for M. conjunctivae persistence in sheep flocks has also been suggested based on this higher prevalence and milder clinical signs (15, 40). However, there were no age class differences in M. conjunctivae in the ibexes in 2014, suggesting that this potential age factor may be dynamic and dependent on the established interaction between the hosts and the circulating mycoplasma strains. In contrast with previous reports of IKC epizootics in sheep and chamois (27, 40–42), more severe clinical signs and higher mycoplasma DNA loads were exhibited by kids than adults in this study. It suggests that in this case, ocular tissue damage was apparently directly caused by M. conjunctivae and was probably less influenced by the adverse effects of exacerbated immune responses (2, 20, 43). Captive conditions may have had an influence on these results and on the lack of female-biased IKC described in disease outbreaks (26, 33).
Immune IgG response.
Strong immune reactions against M. conjunctivae, as described in natural IKC outbreaks, were observed in the ibexes (Fig. 4). However, M. conjunctivae detection was not always consistent with a subsequent increase in IgG in sera, as was previously reported (37, 44), and this suggests that a systemic involvement of the immune system is not always triggered by the ocular infection. Moreover, mixed models indicated that systemic antibodies had a limited role in recovery from infection, which is not rare in mycoplasmas (45–48). Acquired immunity may be therefore based on the local production of IgG, other immunoglobulins, and/or local immune mechanisms that occur in mucosal and local infections (45, 49).
Mycoplasmas typically cause chronic and persistent infections, and systemic IgG titers can be maintained stably in sera for long time periods through continuous stimulation of the immune system (47). Systemic IgG levels in the Ibexes were, however, short-lived, which agrees with some previous reports of single natural infections (50, 51). These results further suggest that systemic IgG does not play an important role for acquired immunity against M. conjunctivae.
Since the immune responses of the ibexes were assessed by an antigen-based diagnostic technique, changes in the antigenic profile of M. conjunctivae during the infection can explain part of the individual variability observed (35). Although the indirect ELISA may not be a good diagnostic tool for M. conjunctivae infection in case studies, it has been demonstrated to be effective for M. conjunctivae surveillance in Iberian ibex populations. Further research is needed to elucidate the immune mechanisms developed by ruminant hosts to face and overcome M. conjunctivae infection.
Host-pathogen interactions.
Two different epidemiological scenarios were found in the ibex population, suggesting different Mycoplasma-host interactions if we consider the prevalence, the M. conjunctivae DNA abundance, and the clinical outcome of the infection. The clearance of M. conjunctivae from the eyes in most of the Iberian ibexes that suffered from severe IKC (2013 outbreak) indicated that the infection triggered a host reaction directed toward the elimination of ocular infections. The subsequent higher prevalence of asymptomatic infections in 2014, however, suggests a longer low-DNA M. conjunctivae (proxy for bacterial load) persistence in the eyes, owing to changes in the host-M. conjunctivae interaction. Similar to these results, low mycoplasma loads have been associated with minimal direct damage to the epithelium and with persistent infections (52), and a correlation between disease severity and M. conjunctivae loads has also been reported in free-ranging chamois and Alpine ibexes independently of the infecting strain (11, 12). The transition between the two scenarios observed is consistent with transmission/recovery trade-offs of pathogen virulence, in which gained virulence favors persistence by reducing the upregulation of the immune response of the host with a lower replication rate (loads) and tissue damage (53, 54).
Evidence of one past ocular inflammatory process in 2014 (IKC stage V) in at least one Iberian ibex, however, suggests that the infection may still be pathogenic if influenced by other factors (12, 16, 55).
Conclusions.
This study allows a better understanding of the transition between two different M. conjunctivae epidemiological scenarios described and highlights the ability of M. conjunctivae to establish diverse interactions with its hosts. The outcome of the M. conjunctivae infection can be varied and more dependent on a specific host-mycoplasma interaction rather than intrinsic host species traits (i.e., susceptibility). Protective immune reactions against M. conjunctivae are not based on a systemic IgG response, which is probably not important to prevent repeated infections in the Iberian ibex.
Infectious diseases have been identified as potential threats for wild Iberian ibex populations (24). The surveillance of M. conjunctivae infection and the potential impact in free-ranging Iberian ibex populations may therefore be considered in future studies on the management of this species. The potential role of the Iberian ibex as a reservoir host for other susceptible species should be also considered in animal health policies.
MATERIALS AND METHODS
Study area and sampling procedure.
This study was performed in a stock reservoir of 60 captive Iberian ibexes located in Dílar (37°03′N, 03°33′W [WGS84 coordinate system]), within the Sierra Nevada Natural Space (SNNS) (southern Spain). The double-fence enclosure was built in 1993 to preserve the genetic diversity of the Iberian ibex population of SNNS from the massive die-offs that occurred because of sarcoptic mange (Sarcoptes scabiei) in the late 1980s (56). This enclosure includes 30 ha of Mediterranean forest composed of pine woods (Pinus spp.) and Mediterranean shrubs. Herd size can vary in about 15% over a year, and the herd is handled yearly for health surveillance. The captive population was historically free of ocular disease until summer/autumn 2013, when an IKC outbreak occurred.
A longitudinal sampling was performed on the captive ibex population, which was sampled yearly between 2010 and 2014, except for 2012, when no samples were collected, and 2013, when the outbreak took place and up to three handlings were carried out (Table 1). For each handling, ocular swabs were taken of each eye between the third eyelid and the palpebral conjunctiva with sterile cotton swabs. Blood samples were also collected from jugular veins from 2013 onwards. From October 2013 to April 2014, ocular swabs and blood samples from 17 free-ranging ibexes captured in the SNNS were also collected (Table 1). Captures were performed for other purposes by means of tele-anesthesia (combination of 3 mg/kg of body weight xylazine and 3 mg/kg ketamine) (57).
Blood samples were placed in sterile tubes, allowed to clot at room temperature, and centrifuged at 1,500 × g to obtain the sera. Ocular swabs and the resulting serum samples were stored frozen at −20°C within 24 h from sample collection. Sex determination was made by visual inspection of genitalia, and age was determined by counting the annual horn segments (58). The ibexes were classified into five different age categories according to social segregation and aging process: kids, less than 6 months; yearlings, from 6 months to 2 years; young, from 2 to 3 years; prime age, from 3 to 7 years; and senescence from 8 years onwards (11, 24, 59).
The handling procedures of the ibexes were designed to minimize stress and health risks for subjects in accordance with the current guidelines for ethical use of animals in research (60) and the European (2010/63/EU) and Spanish (R.D. 53/2013) legislations. This study complied with all Andalusian, Spanish, and European legal requirements and guidelines regarding animal welfare, was approved by the Ethics on Animal Welfare Committee of the Universidad de Jaén, and was authorized by the Dirección General de Producción Agrícola y Ganadera of the Consejería de Agricultura, Pesca y Medio Ambiente of the Junta de Andalucía.
Clinical signs.
Ocular clinical signs were macroscopically classified into seven categories based on severity and resolution course of the ocular disease (61) (see Fig. 1): 0, asymptomatic (no apparent clinical signs); I, mild signs (hyperemia of the conjunctiva and moderate ocular discharge without evident corneal damage or inflammation); II, moderate signs (ocular discharge, moderate corneal opacity, and neovascularization of peripheral cornea); III, severe signs (ocular discharge, widespread and severe corneal opacity, and neovascularization of peripheral and central cornea); IV, very severe signs (clinical signs of stage III might be present along with staphyloma and/or corneal perforation); V, evidence of clinical resolution (slight central opacity of the cornea; concentric pigmentation of the cornea and central neovascularization might be still present); VI, clinical resolution without current inflammatory features (invading pigmentation from the limbus to the vertex of the cornea).
Mycoplasma conjunctivae qPCR detection and sequencing.
For M. conjunctivae detection, eye swabs were placed with 0.5 ml of lysis buffer (100 mM Tris-HCl [pH 8.5], 0.05% Tween 20, 0.24 mg/ml proteinase K) into sterile tubes and were mixed with a vortex. Lysis of the cells was performed at 60°C for 60 min, followed by inactivation of the proteinase K at 97°C for 15 min.
Lysates were directly used as the test sample in a M. conjunctivae-specific qPCR (62). Each reaction mixture consisted of 2.5 μl of the sample, 900 nM LPPS-TM-L (5′-CAGCTGGTGTAGCACTTTTTGC-3′) and LPPS-TM-R (5′-TTAACACCTATGCTCTCGTCTTTGA-3′) primers, 300 nM LPPS-TM-FT probe (5′-TGCTTCGACTACCAAATATGATGGTGATCCTCT-3′; 6-carboxyfluorescein [6-FAM] 5′ reporter dye and 6-carboxytetramethylrhodamine [TAMRA] quencher 3′), 12.5 μl of 2× TaqMan universal PCR master mix (Applied Biosystems, Warrington, UK), an exogenous internal positive control (IPC; Applied Biosystems), and water up to 25 μl of volume. The cycle threshold was set at 0.05, and the PCR cycle when the sample fluorescence crossed the threshold was recorded as the CT value, which inversely corresponds to the initial amount of target DNA in the test sample. All samples were analyzed in duplicate, including positive and negative controls, and positive-sample reactions were repeated if standard deviation between the replicates was more than one CT.
For molecular epidemiological studies, a nested PCR that targets the 3′ end of the gene coding for the serine-rich part of the membrane protein LppS of M. conjunctivae was performed on randomly selected samples with low CT values by the qPCR. The reactions were performed according to Belloy et al. (17), with minor modifications of the primers, using the Serstart3 (5′-TTTAGTAGACTCCACTTCACC-3′) and LppTA2 (5′-TTTGATCTCTCCACCTTCAGC-3′) primers for the first PCR and Serstart2 (5′-CACTATACTTAACAGATAGTCC-3′) and LppTA (5′-GGCACTAATAGTGCGTAATTC-3′) primers in a nested reaction if the first amplification was not enough.
For DNA sequence analyses, all products from the nested PCR were purified with the High Pure PCR product purification kit (Roche Diagnostics, Rotkreuz, Switzerland). The sequencing of the amplicons was performed using the Ser_start2, Ser_start0 (5′-ATACTCAAAGTGGAAATAATGGAA-3′), and Ser_end0 (5′-GCAACAACAATAGTAAGAGCAG-3′) primers using the BigDye Terminator cycle sequencing kit (Applied Biosystems, Foster City, CA, USA).
The resulting sequences were trimmed to obtain the variable part of the lppS gene corresponding to nucleotide positions 3935 to 5035 of lppS of the M. conjunctivae type strain HRC/581 (accession no. AJ318939). Alignment and editing of the sequence data were performed with the BioEdit software. A cluster analysis tree of the sequences was inferred using the unweighted pair group method using average linkages (UPGMA) with 1,000 bootstrap replications, performed with the MEGA 7 software (63). DNA sequence data from the type strain HRC/581 (GenBank accession no. LT174654) and from six strains more from different hosts and geographical placements (2880 s, accession no. LT174667; 38 s, accession LT174670; My 66 95 s, accession no. LT174668; My 7/96 g, accession no. LT174669; 2778 c, accession no. LT174666; and 2784 c, accession no. LT174671) were included in the tree for comparison.
ELISA for M. conjunctivae antibodies.
The humoral immune response against the M. conjunctivae infection was assessed in sera with an indirect ELISA based on the Tween 20 membrane proteins extract of the M. conjunctivae strain HRC/581T (64). This ELISA showed good sensitivity and specificity for M. conjunctivae antibodies in sheep and has also been validated for alpine chamois (Rupicapra rupicapra) (10, 15). Cross-board titrations were performed to determine the serum sample dilution, the concentration of Tween 20 extracts, and the conjugated antibodies to obtain an optimal signal/noise ratio. All the samples were analyzed in duplicate, and all serum samples from the same ibex were included in the same ELISA plate to have the same analytical conditions for each individual. Serum from a captive-born ibex negative for the M. conjunctivae-specific qPCR in eye swabs that never had ocular disease was used as the negative reference standard. On the other hand, serum from an ibex that showed severe IKC during the outbreak and with M. conjunctivae infection confirmed by qPCR was used as the positive reference standard.
For ELISA development, Nunc A/S MaxiSorp microtiter plates (Nunc, Roskilde, Denmark) were coated with the Tween 20 extracts at an optical concentration of 5 μg/ml, according to standard methods (65). Briefly, serum samples were applied to the plates diluted 1:50 with a 5× ELISA diluent solution (eBioscience, San Diego, CA, USA) and incubated for 90 min at room temperature. Then, the wells were washed, and a monoclonal anti-sheep/goat IgG antibody conjugated to horseradish peroxidase (Sigma, St. Louis, MO, USA) diluted 1:15,000 was added and incubated for 90 min at room temperature. Subsequently, the wells were washed, and the chromogen solution 3,3′,5,5′-tetramethylbenzidine (Sigma) was added and incubated for 15 min according to the producers' instructions, when 0.16 M sulfuric acid was applied to stop the reaction. The values of the samples were calculated as the mean optical density of the duplicates measured at 405 nm (OD450) and were expressed as the percentage of the positive reference standard, with the negative reference standard value as zero, according to accepted methods (65).
The optimal cutoff point was obtained with the Youden index, which is the point at which maximum sensitivity and specificity are achieved based on values of both diseased and healthy ibexes (66). Serum samples from Iberian ibexes from two populations in Catalonia (Northeast Spain), where ocular disease has not been described, were used as negative controls for the cutoff determination (n = 71). On the other hand, serum samples from ibexes from the reported outbreak that exhibited ocular disease and were positive with the qPCR were used as positive controls for the cutoff determination (n = 33).
Statistical analyses.
Data were analyzed separately for 2013 (epidemic IKC) and 2014 (nonepidemic IKC). Mycoplasma conjunctivae infection was treated as a categorical variable, with two levels (positive and negative), in which ibexes were considered positive if the qPCR result was positive in any of the three samplings from 2013. Differences in M. conjunctivae prevalence related to age class and sex were assessed with Fisher's exact test.
To assess the importance of the IgG humoral response in the M. conjunctivae clearance, we fit a set of linear mixed-effects models (LMM) in which M. conjunctivae abundance in the eye (CT values of the qPCR) was explained by the fixed effects of IgG concentration (relative ELISA OD450), age class, and their two-way interactions. Since at least two blood samplings (one each year) were taken from each ibex, the ibex was considered a random term in the LMM, using a repeated measure-fixed block design. Then, the best random structure was selected according to Zuur et al. (67). For the purpose of this analysis and in order to increase the sample size per category, the young, prime age, and senescence categories were merged. Because of the short-term persistence of the IgG antibodies, only ibexes that were positive with the qPCR were considered. The ELISA results were square root transformed to reduce residual variability and the effects of outliers (68). A theoretic information approach based on Akaike's information criterion corrected for small sample sizes (AICc) was then used to select the best model (69). Briefly, competing models were ranked according to the difference between their Akaike scores and the score of the model with the lowest AICc. The models that substantially explain the observed variability have Akaike differences (∆i) of <2 units in comparison with the model with the lowest AICc. Then, the Akaike weight (wi) was calculated to know the relative probability that a given model is the best of those being compared. For the best models, the lack of residual patterns was checked, and to obtain a general measure of goodness of fit, the marginal (variance explained by the fixed terms) and conditional (by the fixed and random terms) variances were also calculated, according to the protocol reported by Nakagawa and Schielzeth (70).
All the statistical analyses were performed with the R software (71), setting α at 0.05 when appropriate. The linear mixed models were fitted with the R package “lme4” (72). The R package “OpticalCutpoints” was used to determine the cutoff point of the indirect ELISA and the derived estimates of the sensitivity and specificity with a 95% confidence interval (CI) (73).
Accession number(s).
The partial lppS gene sequences from the ibexes were deposited in GenBank under the following accession numbers (OI, left eye; OD, right eye): LT629278 (C_507 OI), LT629279 (C_507 OD), LT629280 (C_686 OI), LT629281 (C_686 OD), LT629282 (C_506 OD), LT629283 (C_508 OD), LT629284 (695 OI), LT629285 (C_695 OD), LT629286 (C_800 OI), LT629287 (C_2298 OI), LT629288 (C_5125 OI), LT629289 (C_5125 OD), LT629290 (C_801 OI), LT629291 (C_801 OD), LT629292 (575N OI), LT629293 (C_501 OD), LT629294 (C_801 OI), and LT629295 (ALP321 OI).
ACKNOWLEDGMENTS
We thank the National Park Service and all who have helped in the fieldwork, especially Isidro Puga, José López, Elias Martínez, Manuela Fernández, Antonio José Rodríguez, Apolo Sánchez, Isaac Pimentel, Debora Forte, Antonio Falbo, and Gael Aleix. We also acknowledge the invaluable technical assistance of Amandine Ruffieux, from the Institute of Veterinary Bacteriology, University of Bern.
This study was funded by the research projects CGL2009-11631 and CGL2012-40043-C02-02 of the Spanish MICINN. X. Fernández-Aguilar was supported by the FI-DGR program from the Government of Catalonia. E. Serrano was supported by the postdoctoral program (grant SFRH/BPD/96637/2013) of the Fundação para a Ciência e a Tecnologia, Portugal, the University of Aveiro (Department of Biology), and FCT/MEC through financial support to CESAM RU (UID/AMB/50017) and, where applicable, cofinanced by the FEDER, within the PT2020 partnership agreement.
REFERENCES
- 1.Citti C, Blanchard A. 2013. Mycoplasmas and their host: emerging and re-emerging minimal pathogens. Trends Microbiol 21:196–203. doi: 10.1016/j.tim.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Rosengarten R, Citti C, Glew M, Lischewski A, Droesse M, Much P, Winner F, Brank M, Spergser J. 2000. Host-pathogen interactions in mycoplasma pathogenesis: virulence and survival strategies of minimalist prokaryotes. Int J Med Microbiol 290:15–25. doi: 10.1016/S1438-4221(00)80099-5. [DOI] [PubMed] [Google Scholar]
- 3.Robinson MW, Buchtmann KA, Jenkins C, Tacchi JL, Raymond BB, To J, Roy Chowdhury P, Woolley LK, Labbate M, Turnbull L, Whitchurch CB, Padula MP, Djordjevic SP. 2013. MHJ_0125 is an M42 glutamyl aminopeptidase that moonlights as a multifunctional adhesin on the surface of Mycoplasma hyopneumoniae. Open Biol 3:130017. doi: 10.1098/rsob.130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Citti C, Nouvel L-X, Baranowski E. 2010. Phase and antigenic variation in mycoplasmas. Future Microbiol 5:1073–1085. doi: 10.2217/fmb.10.71. [DOI] [PubMed] [Google Scholar]
- 5.Delaney NF, Balenger S, Bonneaud C, Marx CJ, Hill GE, Ferguson-Noel N, Tsai P, Rodrigo A, Edwards SV. 2012. Ultrafast evolution and loss of CRISPRs following a host shift in a novel wildlife pathogen, Mycoplasma gallisepticum. PLoS Genet 8:e1002511. doi: 10.1371/journal.pgen.1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woese CR, Stackebrandt E, Ludwig W. 1985. What are mycoplasmas: the relationship of tempo and mode in bacterial evolution. J Mol Evol 21:305–316. doi: 10.1007/BF02115648. [DOI] [PubMed] [Google Scholar]
- 7.Osnas EE, Hurtado PJ, Dobson AP. 2015. Evolution of pathogen virulence across space during an epidemic. Am Nat 185:332–342. doi: 10.1086/679734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giacometti M, Janovsky M, Belloy L, Frey J. 2002. Infectious keratoconjunctivitis of ibex, chamois and other Caprinae. Rev Sci Tech 21:335–345. doi: 10.20506/rst.21.2.1338. [DOI] [PubMed] [Google Scholar]
- 9.Gauthier D. 1991. La Kerato-conjonctivite infectieuse du chamois. Étude épidémiologique dans le Départament de la Savoie 1983–1990; PhD thesis Université Claude Bernard, Lyon, France. [Google Scholar]
- 10.Giacometti M, Janovsky M, Jenny H, Nicolet J, Belloy L, Goldschmidt-Clermont E, Frey J. 2002. Mycoplasma conjunctivae infection is not maintained in Alpine chamois in Eastern Switzerland. J Wildl Dis 38:297–304. doi: 10.7589/0090-3558-38.2.297. [DOI] [PubMed] [Google Scholar]
- 11.Ryser-Degiorgis MP, Bischof DF, Marreros N, Willisch C, Signer C, Filli F, Brosi G, Frey J, Vilei EM. 2009. Detection of Mycoplasma conjunctivae in the eyes of healthy, free-ranging Alpine ibex: possible involvement of Alpine ibex as carriers for the main causing agent of infectious keratoconjunctivitis in wild Caprinae. Vet Microbiol 134:368–374. doi: 10.1016/j.vetmic.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Mavrot F, Vilei EM, Marreros N, Signer C, Frey J, Ryser-Degiorgis M-P. 2012. Occurrence, quantification, and genotyping of Mycoplasma conjunctivae in wild Caprinae with and without infectious keratoconjunctivitis. J Wildl Dis 48:619–631. doi: 10.7589/0090-3558-48.3.619. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann L, Jambresic S, Giacometti M, Frey J. 2008. Specificity of Mycoplasma conjunctivae strains for alpine chamois Rupicapra r. rupicapra. Wildlife Biol 14:118–124. doi: 10.2981/0909-6396(2008)14[118:SOMCSF]2.0.CO;2. [DOI] [Google Scholar]
- 14.Giacometti M, Nicolet J, Frey J, Krawinkler M, Meier W, Welle M, Johansson KE, Degiorgis MP. 1998. Susceptibility of alpine ibex to conjunctivitis caused by inoculation of a sheep-strain of Mycoplasma conjunctivae. Vet Microbiol 61:279–288. doi: 10.1016/S0378-1135(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 15.Janovsky M, Frey J, Nicolet J, Belloy L, Goldschmidt-Clermont E, Giacometti M. 2001. Mycoplasma conjunctivae infection is self-maintained in the Swiss domestic sheep population. Vet Microbiol 83:11–22. doi: 10.1016/S0378-1135(01)00407-2. [DOI] [PubMed] [Google Scholar]
- 16.Fernández-Aguilar X, Cabezón O, Marco I, Mentaberre G, Frey J, Lavín S, López-Olvera JR. 2013. Mycoplasma conjunctivae in domestic small ruminants from high mountain habitats in northern Spain. BMC Vet Res 9:253. doi: 10.1186/1746-6148-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belloy L, Janovsky M, Vilei EM, Pilo P, Giacometti M, Frey J. 2003. Molecular epidemiology of Mycoplasma conjunctivae in Caprinae: transmission across species in natural outbreaks. Appl Environ Microbiol 69:1913–1919. doi: 10.1128/AEM.69.4.1913-1919.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deutz A, Spergser J, Frei J, Rosengarten R, Köfer J. 2004. Mycoplasma conjunctivae in two sheep farms—clinics, diagnostics and epidemiology. Wien Tierarztl Monatsschr 91:152–157. [Google Scholar]
- 19.Giacometti M. 2012. Infectious keratoconjunctivitis in Caprinae, p 373–377. In Gavier-Wid́en D, Duff JP, Meredith A (ed), Infectious diseases of wild mammals and birds in Europe. Wiley-Blackwell, West Sussex, United Kingdom. [Google Scholar]
- 20.Gelormini G, Gauthier D, Vilei EM, Crampe J-P, Frey J, Ryser-Degiorgis M-P. 2017. Infectious keratoconjunctivitis in wild Caprinae: merging field observations and molecular analyses sheds light on factors shaping outbreak dynamics. BMC Vet Res 13:67–85. doi: 10.1186/s12917-017-0972-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Degiorgis M-P, Abdo E-M, Nicolet JN, Frey J, Mayer D, Giacometti M. 2000. Immune responses to Mycoplasma conjunctivae in alpine ibex, alpine chamois, and domestic sheep in Switzerland. J Wildl Dis 36:265–271. doi: 10.7589/0090-3558-36.2.265. [DOI] [PubMed] [Google Scholar]
- 22.Arnal MC, Revilla M, Corrales JC, Martínez D, Sánchez A, Herrero J, Contreras A, Fernández de Luco D. 2009. Queratoconjuntivitis infecciosa por M. conjunctivae en sarrio (Rupicapra p. pyrenaica) y en cabra montés (Capra pyrenaica) en Aragón, p 61. XXI Reunión de la Sociedad Española de Anatomía Patológica Veterinaria; Lugo, Spain, 24 to 26 June 2009. [Google Scholar]
- 23.Cubero MJ, González M, León L. 2002. Enfermedades infecciosas de las poblaciones de cabra montés, p 197–254. In Pérez-Jiḿenez JM. (ed), Distribución, genética y estatus sanitario de las poblaciones andaluzas de cabra montés. Junta de Andalucía, Granada, Spain. [Google Scholar]
- 24.Acevedo P, Cassinello J. 2009. Biology, ecology and status of Iberian ibex Capra pyrenaica: a critical review and research prospectus. Mam Rev 39:17–32. doi: 10.1111/j.1365-2907.2008.00138.x. [DOI] [Google Scholar]
- 25.Naglić T, Hajsig D, Frey J, Seol B, Busch K, Lojkić M. 2000. Epidemiological and microbiological study of an outbreak of infectious keratoconjunctivitis in sheep. Vet Rec 147:72–75. doi: 10.1136/vr.147.3.72. [DOI] [PubMed] [Google Scholar]
- 26.Degiorgis M-P, Frey J, Nicolet J, Abdo EM, Fatzer R, Schlatter Y, Reist S, Janovsky M, Giacometti M. 2000. An outbreak of infectious keratoconjunctivitis in Alpine chamois (Rupicapra r. rupicapra) in Simmental-Gruyères, Switzerland. Schweiz Arch Tierheilkd 142:520–527. [Google Scholar]
- 27.Arnal MC, Herrero J, de la Fe C, Revilla M, Prada C, Martínez-Durán D, Gómez-Martín A, Fernández-Arberas O, Amores J, Contreras A, García-Serrano A, de Luco DF. 2013. Dynamics of an infectious keratoconjunctivitis outbreak by Mycoplasma conjunctivae on Pyrenean chamois Rupicapra p. pyrenaica. PLoS One 8:e61887. doi: 10.1371/journal.pone.0061887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodríguez JL, Poveda JB, Rodríguez F, Espinosa de los Monteros A, Ramírez AS, Fernández SA. 1996. Ovine infectious keratoconjunctivitis caused by Mycoplasma agalactiae. Small Rumin Res 22:93–96. doi: 10.1016/0921-4488(96)00857-7. [DOI] [Google Scholar]
- 29.Andrews A, Goddard P, Wilsmore A, Dagnell G. 1987. A chlamydial keratoconjunctivitis in a British sheep flock. Vet Rec 120:238–239. doi: 10.1136/vr.120.10.238. [DOI] [PubMed] [Google Scholar]
- 30.Surman PG. 1973. Mycoplasma aetiology of keratoconjunctivitis (“pink-eye”) in domestic ruminants. Aust J Exp Biol Med Sci 51:589–607. doi: 10.1038/icb.1973.56. [DOI] [PubMed] [Google Scholar]
- 31.Degiorgis M-P, Obrecht E, Ryser A, Giacometti M. 1999. The possible role of eye-frequenting flies in the transmission of Mycoplasma conjunctivae. Mitt Schweiz Entomol Ges 72:189–194. [Google Scholar]
- 32.González-Candela M, Cubero-Pablo MJ, Martín-Atance P, León-Vizcaíno L. 2006. Potential pathogens carried by Spanish ibex (Capra pyrenaica hispanica) in southern Spain. J Wildl Dis 42:325–334. doi: 10.7589/0090-3558-42.2.325. [DOI] [PubMed] [Google Scholar]
- 33.Tschopp R, Frey J, Zimmermann L, Giacometti M. 2005. Outbreaks of infectious keratoconjunctivitis in alpine chamois and ibex in Switzerland between 2001 and 2003. Vet Rec 157:13–18. doi: 10.1136/vr.157.1.13. [DOI] [PubMed] [Google Scholar]
- 34.Motha MXJ, Frey J, Hansen MF, Jamaludin R, Tham KM. 2003. Detection of Mycoplasma conjunctivae in sheep affected with conjunctivitis and infectious keratoconjunctivitis. N Z Vet J 51:186–190. doi: 10.1080/00480169.2003.36362. [DOI] [PubMed] [Google Scholar]
- 35.Baranowski E, Bergonier D, Sagné E, Hygonenq M-C, Ronsin P, Berthelot X, Citti C. 2014. Experimental infections with Mycoplasma agalactiae identify key factors involved in host-colonization. PLoS One 9:e93970. doi: 10.1371/journal.pone.0093970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arfi Y, Minder L, Di Primo C, Le Roy A, Ebel C, Coquet L, Claverol S, Vashee S, Jores J, Blanchard A, Sirand-Pugnet P. 2016. MIB-MIP is a mycoplasma system that captures and cleaves immunoglobulin G. Proc Natl Acad Sci U S A 113:5406–5411. doi: 10.1073/pnas.1600546113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baas EJ, Trotter SL, Franklin RM, Barile MF. 1977. Epidemic caprine keratoconjunctivitis: recovery of Mycoplasma conjunctivae and its possible role in pathogenesis. Infect Immun 18:806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams PD, Dobson AP, Dhondt KV, Hawley DM, Dhondt AA. 2014. Evidence of trade-offs shaping virulence evolution in an emerging wildlife pathogen. J Evol Biol 27:1271–1278. doi: 10.1111/jeb.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernández-Aguilar X, Rossi L, Cabezón O, Giorgino A, Victoriano LLopis I, Frey J, López-Olvera JR. Infectious keratoconjunctivitis and occurrence of Mycoplasma conjunctivae and Chlamydiaceae in small domestic ruminants from the Central Karakoram, Pakistan. Vet Rec, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosie BD. 2007. Infectious keratoconjunctivitis, p 342–345. In Aitken ID. (ed), Diseases of sheep. Blackwell Publishing, Oxford, United Kingdom. [Google Scholar]
- 41.Crampe J-P. 2008. Keratoconjonctivite de l'Isard. L'épizootie de 2007–2008 en vallée de Cauterets: modalités et conséquences démographiques; Parc National Les Pyrénées, Tarbes, France. [Google Scholar]
- 42.Jones G, Foggie A, Sutherland A, Harker DB. 1976. Mycoplasmas and ovine keratoconjunctivitis. Vet Rec 99:137–141. doi: 10.1136/vr.99.8.137. [DOI] [PubMed] [Google Scholar]
- 43.Rottem S, Naot Y. 1998. Subversion and exploitation of host cells by mycoplasmas. Trends Microbiol 6:436–440. doi: 10.1016/S0966-842X(98)01358-4. [DOI] [PubMed] [Google Scholar]
- 44.Trotter SL, Franklin RM, Baas EJ, Barile MF. 1977. Epidemic caprine keratoconjunctivitis: experimentally induced disease with a pure culture of Mycoplasma conjunctivae. Infect Immun 18:816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simecka JW, Ross SE, Cassell GH, Davis JK. 1993. Interactions of mycoplasmas with B cells: antibody production and nonspecific effects. Clin Infect Dis 17:S176–S182. doi: 10.1093/clinids/17.Supplement_1.S176. [DOI] [PubMed] [Google Scholar]
- 46.Noormohammadi AH, Jones JE, Underwood G, Whithear KG. 2002. Poor systemic antibody response after vaccination of commercial broiler breeders with Mycoplasma gallisepticum vaccine ts-11 not associated with susceptibility to challenge. Avian Dis 46:623–628. doi: 10.1637/0005-2086(2002)046[0623:PSARAV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 47.Schieck E, Liljander A, Hamsten C, Gicheru N, Scacchia M, Sacchini F, Heller M, Schnee C, Sterner-Kock A, Hlinak A, Naessens J, Poole J, Persson A, Jores J. 2014. High antibody titres against predicted Mycoplasma surface proteins do not prevent sequestration in infected lung tissue in the course of experimental contagious bovine pleuropneumonia. Vet Microbiol 172:285–293. doi: 10.1016/j.vetmic.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 48.Gourlay R, Howard C. 1979. Human and animal mycoplasmas, p 50–95. In Tully JG, Whitcomb RF (ed), The mycoplasma, vol II: human and animal mycoplasmas. Academic Press, London, United Kingdom. [Google Scholar]
- 49.Avakian AP, Ley DH. 1993. Protective immune response to Mycoplasma gallisepticum demonstrated in respiratory-tract washings from M. gallisepticum-infected chickens. Avian Dis 37:697–705. doi: 10.2307/1592017. [DOI] [PubMed] [Google Scholar]
- 50.Lambert M. 1987. Contagious agalactia of sheep and goats. Rev Sci Tech Off Int Epiz 6:699–711. [DOI] [PubMed] [Google Scholar]
- 51.Waites KB, Balish MF, Atkinson TP. 2009. New insights into the pathogenesis and detection of Mycoplasma pneumoniae infections. Future Microbiol 3:635–648. doi: 10.2217/17460913.3.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGowin CL, Annan RS, Quayle AJ, Greene SJ, Ma L, Mancuso MM, Adegboye D, Martin DH. 2012. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun 80:3842–3849. doi: 10.1128/IAI.00819-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alizon S. 2008. Transmission-recovery trade-offs to study parasite evolution. Am Nat 172:E113–E121. doi: 10.1086/589892. [DOI] [PubMed] [Google Scholar]
- 54.Frank SA, Schmid-Hempel P. 2008. Mechanisms of pathogenesis and the evolution of parasite virulence. J Evol Biol 21:396–404. doi: 10.1111/j.1420-9101.2007.01480.x. [DOI] [PubMed] [Google Scholar]
- 55.Mavrot F, Zimmermann F, Vilei EM, Ryser-Degiorgis MP. 2012. Is the development of infectious keratoconjunctivitis in Alpine ibex and Alpine chamois influenced by topographic features? Eur J Wildl Res 58:869–874. doi: 10.1007/s10344-012-0651-1. [DOI] [Google Scholar]
- 56.León-Vizcaíno L, Ruíz de Ybáñez MR, Cubero MJ, Ortíz JM, Espinosa J, Pérez L, Simón MA, Alonso F. 1999. Sarcoptic mange in Spanish ibex from Spain. J Wildl Dis 35:647–659. doi: 10.7589/0090-3558-35.4.647. [DOI] [PubMed] [Google Scholar]
- 57.Casas-Díaz E, Marco I, López-Olvera JR, Mentaberre G, Lavín S. 2011. Comparison of xylazine-ketamine and medetomidine-ketamine anaesthesia in the Iberian ibex (Capra pyrenaica). Eur J Wildl Res 57:887–893. doi: 10.1007/s10344-011-0500-7. [DOI] [Google Scholar]
- 58.Fandos P. 1991. La cabra montés (Capra pyrenaica) en el Parque Natural de las Sierras de Cazorla, Segura y las Villas. ICONA-CSIC, Madrid, Spain. [Google Scholar]
- 59.Gaillard J-M, Festa-Bianchet M, Yoccoz NG, Loison A, Toïgo C. 2000. Temporal variation in fitness components and population dynamics of large herbivores. Annu Rev Ecol Evol Syst 31:367–393. doi: 10.1146/annurev.ecolsys.31.1.367. [DOI] [Google Scholar]
- 60.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2010. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mayer D, Degiorgis MP, Meier W, Nicolet J, Giacometti M. 1997. Lesions associated with infectious keratoconjunctivitis in alpine ibex. J Wildl Dis 33:413–419. doi: 10.7589/0090-3558-33.3.413. [DOI] [PubMed] [Google Scholar]
- 62.Vilei EM, Bonvin-Klotz L, Zimmermann L, Ryser-Degiorgis M-P, Giacometti M, Frey J. 2007. Validation and diagnostic efficacy of a TaqMan real-time PCR for the detection of Mycoplasma conjunctivae in the eyes of infected Caprinae. J Microbiol Methods 70:384–386. doi: 10.1016/j.mimet.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 63.Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Belloy L, Giacometti M, Abdo EM, Nicolet J, Krawinkler M, Janovsky M, Bruderer U, Frey JF. 2001. Detection of specific Mycoplasma conjunctivae antibodies in the sera of sheep with infectious keratoconjunctivitis. Vet Res 32:155–164. doi: 10.1051/vetres:2001118. [DOI] [PubMed] [Google Scholar]
- 65.Nicolet J, Martel JL. 1996. ELISA in large animals, p 105–113. In Tully JG, Razing S (ed), Molecular and diagnostic procedures in mycoplasmology, vol II. Academic Press, San Diego, CA. [Google Scholar]
- 66.Yin J, Tian L. 2014. Joint inference about sensitivity and specificity at the optimal cut-off point associated with Youden index. Comput Stat Data Anal 77:1–13. doi: 10.1016/j.csda.2014.01.021. [DOI] [Google Scholar]
- 67.Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. Springer-Verlag, New York, NY. [Google Scholar]
- 68.Zuur AF, Ieno EN, Smith GM. 2007. Analyzing ecological data. Springer, New York, NY. [Google Scholar]
- 69.Johnson JB, Omland KS. 2004. Model selection in ecology and evolution. Trends Ecol Evol 19:101–108. doi: 10.1016/j.tree.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 70.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. doi: 10.1111/j.2041-210x.2012.00261.x. [DOI] [Google Scholar]
- 71.R Development Core Team. 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org. [Google Scholar]
- 72.Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Grothendieck G. 2016. Package ‘lme4′: linear mixed-effects models using ‘Eigen’ and S4. R package version 1.1-12. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 73.López-Ratón M, Rodríguez-Álvarez MX, Cadarso-Suárez C, Gude-Sampedro F. 2014. OptimalCutpoints: an R package for selecting optimal cutpoints in diagnostic tests. J Stat Softw 62(8). doi: 10.18637/jss.v061.i08. [DOI] [Google Scholar]