Abstract
Malignant glioma, the most common malignant brain tumor in adults, is difficult to treat due to its aggressive invasive nature. Enzyme/prodrug suicide gene therapy based on the herpes simplex virus thymidine kinase (HSVtk)/ganciclovir (GCV) system is an efficient strategy for treating malignant gliomas. In the present study, we evaluated treatment with multilineage-differentiating stress-enduring (Muse) cells, which are endogenous non-tumorigenic pluripotent-like stem cells that are easily collectable from the bone marrow as SSEA-3+ cells, as carriers of the HSVtk gene. Human Muse cells showed potent migratory activity toward glioma cells both in vitro and in vivo. HSVtk gene-transduced Muse cells (Muse-tk cells) at a cell number of only 1/32 that of U87 human glioma cells completely eradicated U87 gliomas in nude mouse brains, showing a robust in vivo bystander effect. Pre-existing intracranial U87 gliomas in nude mouse brains injected intratumorally with Muse-tk cells followed by intraperitoneal GCV administration were significantly reduced in size within 2 weeks, and 4 of 10 treated mice survived over 200 days. These findings suggest that intratumoral Muse-tk cell injection followed by systemic GCV administration is safe and effective and that allogeneic Muse-tk cell-medicated suicide gene therapy for malignant glioma is clinically feasible.
Keywords: bystander effect, gene therapy, glioma, herpes simplex virus thymidine kinase, migration, stem cells
Introduction
Malignant glioma is the most common lethal intracranial tumor, characterized by uncontrolled cellular proliferation, diffuse infiltration, and fierce resistance to apoptosis.1 Survival or maintenance of a baseline quality of life for the patients has improved over the last decade due to multidisciplinary approaches that involve maximal surgical resection using image guidance interventions concomitant with adjuvant radiochemotherapy.2, 3, 4, 5, 6, 7, 8 Nevertheless, clinical trials indicate a median progression-free survival from diagnosis of 7.1 to 10.7 months and a median overall survival from diagnosis of 14.6 to 20.5 months2, 5, 6, 9, 10 in patients with glioblastoma multiforme (GBM), the most malignant phenotype among the gliomas. The limited therapeutic effects are mainly due to incomplete tumor resection and local recurrence because GBM has a highly invasive nature into the surrounding eloquent brain tissues.11 Furthermore, it is extremely difficult to eradicate residual tumor cells by post-operative radiochemotherapy due to dose-limiting local or systemic toxicities and ineffective delivery of the drugs across the blood-brain barrier.12, 13 Therefore, an alternative tumor-selective treatment is strongly desired.
Because malignant gliomas rarely metastasize outside the central nervous system and the majority of recurrence occurs in the proximity of the resection site, local gene therapy is considered strategically suitable. One of the first and most widely used local gene therapies is the herpes simplex virus-thymidine kinase (HSVtk)/ganciclovir (GCV) system. Prodrug GCV is systemically non-toxic and readily crosses the blood-brain barrier, leading to tumor cell death by incorporation of phosphorylated GCV into replicating cells. The phosphorylated GCV is also able to pass through gap junctions from the HSVtk-transduced tumor cells to adjacent HSVtk-non-transduced cells and kill neighboring dividing tumor cells. This interesting property of the HSVtk/GCV systems is called the “bystander effect,” which can be defined as the death of unmodified tumor cells adjacent to genetically modified cells.14 Clinical studies of retrovirus-mediated HSVtk/GCV gene therapy have been performed to evaluate the bystander effect. Although clinical safety was demonstrated, the therapeutic benefits were not strong enough because of the limited distribution of viral vectors throughout the invasive tumor.15 For that reason, stem-cell-based gene therapies using neural stem cells (NSCs) and mesenchymal stem cells (MSCs) have been applied due to their unique tumor-tropic activity toward solid and invasive tumor cells.16, 17 The enzyme/prodrug systems, including the HSVtk/GCV system, using NSCs and MSCs have been extensively studied.18, 19, 20, 21, 22, 23, 24 Adult NSCs, however, are not easily obtainable, and fetal NSCs, which are associated with ethical problems, are reported to be tumorigenic.25 MSCs are collectable from easily accessible sources, such as the bone marrow. The effectiveness of MSC-HSVtk/GCV therapy, however, is not stable because of the heterogeneous population.26
Multilineage-differentiating stress-enduring (Muse) cells are endogenous pluripotent-like stem cells that can be efficiently isolated from adult mesenchymal tissues, such as the bone marrow, adipose tissue, and dermis, as well as from commercially obtained cultured fibroblasts, as cells positive for the pluripotent surface maker, stage-specific embryonic antigen-3 (SSEA-3).27, 28, 29 They comprise ∼0.03% of the mononucleated fraction of the bone marrow, and thus ∼50 mL of bone marrow aspirate yields 1 million Muse cells after 2 days of culture.30 Muse cells comprise several percent of the total population of commercially available fibroblasts, demonstrating their feasibility, applicability, and convenience for practical use.27, 28, 29 Because these cells normally reside in adult tissue, they are non-tumorigenic and their telomerase expression level is nearly the same as that in somatic cells.27, 28, 29 Muse cells exhibit self-renewal and pluripotency without introducing exogenous genes and, unlike embryonic stem cells and induced pluripotent stem cells, they do not form teratomas in vivo. Furthermore, when human Muse cells are injected intravenously into partial hepatectomy and skeletal muscle degeneration models in immunodeficient mice, Muse cells efficiently migrate to and integrate into damaged lesions of the respective tissues.27, 29 The easy accessibility, moderate proliferation rate, non-tumorigenicity, and in vivo migratory capability of Muse cells make them a highly attractive vehicle for stem-cell-based suicide gene therapy.
Here, we present the first human Muse-cell-mediated enzyme/prodrug strategy for the treatment of GBM using established human cultured fibroblast-derived Muse cells expressing HSVtk (Muse-tk cells). Using a nude mouse intracranial tumor model with human GBM cells, we investigated (1) the potency of the bystander effect between Muse-tk and human GBM cells, (2) the tumoricidal efficacy of intratumoral Muse-tk cell injection and systemic GCV, (3) the in vivo migratory activity of Muse-tk cells to the tumor cells, and (4) the fate of Muse-tk cells in the brain after GCV treatment. The results of this preclinical study suggest that Muse cell-based glioma suicide gene therapy is safe and effective and therefore feasible to advance to clinical trials.
Results
In Vitro Sensitivity of Muse-tk Cells to GCV
HSVtk-IRES2-EGFP labeled SSEA-3+ normal human dermal fibroblasts (http://www.lonza.com) were used in the present study (Muse-tk cells). To examine the sensitivity of Muse-tk cells to GCV, Muse-tk cells or naive Muse cells were cultured in medium containing GCV ranging from 0.001 to 300 μg/mL. The viability of the Muse-tk cells, evaluated using a tetrazolium-based colorimetric (MTT) assay, significantly decreased in the medium containing ≥0.01 μg/mL concentrations of GCV, with 3 μg/mL GCV killing all of the Muse-tk cells (Figure S1). In contrast, no significant toxicity was observed in naive Muse cells, even at 3 μg/mL GCV (Figure S1).
In Vitro Bystander Effect between Muse-tk and Glioma Cells
Human GBM cells (U87 and U251) and Muse-tk cells were co-cultured at various ratios in medium with or without GCV. The proliferation activity of U87 and U251 cells in medium containing 2 μg/mL GCV was inhibited only when they were co-cultured with Muse-tk cells (Figures 1A and 1B). The inhibition of U87 and U251 cell growth was significant when the Muse-tk cell:tumor cell (M/T) ratio was more than 1:64 (Figures 1A and 1B). The proliferation of U87 and U251 cells was not inhibited in the medium containing 2 μg/mL GCV when Muse-tk cells were not present (Figures 1A and 1B, as shown in M/T[0:1]). The real-time bystander effect between Muse-tk and U87 cells observed under a culture microscope is shown in Figure 1C and Movie S1 (M/T ratio 1:1). Some Muse-tk cells were still alive at 96 hr (3 days) after GCV treatment because cell division rate of Muse-tk cells was much slower than that of tumor cells.
Figure 1.
In Vitro Bystander Effect between Muse-tk and GBM Cells
(A and B) Proliferation activity of U87 (A) and U251 (B) human GBM cells in medium containing 2 μg/mL GCV was significantly inhibited when the tumor cells were co-cultured with Muse-tk cells at an M/T ratio as low as 1:64 (mean ± SD). No proliferation inhibition of tumor cells was observed when the cells were not co-cultured with Muse-tk cells (M/T ratio of 0:1). (C) Real-time bystander effect between Muse-tk and U87 cells observed under a culture microscope is shown.
In Vivo Bystander Effect between Muse-tk and U87-luc Cells in the Nude Mouse Intracranial Tumor Model
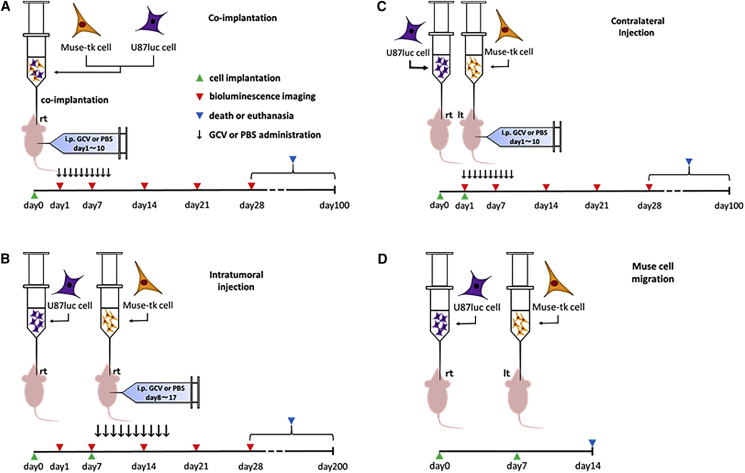
96 female BALB/c slc nu/nu mice were divided into six groups. Bioware Ultra Cell Line U-87 MG-luciferase2 cells (U87-luc cells) that express luciferase and emit bioluminescence signals upon intraperitoneal injection of D-luciferin were used for the intracranial tumor model. U87-luc cells (1 × 105 cells), mixed with Muse-tk cells at M/T ratios of 1:4, 1:8, 1:16, 1:32, 1:64, and 0:1, were intracranially inoculated (n = 16/group; Figure 2A; co-implantation). Half of the mice in each group (n = 8) were intraperitoneally injected with GCV (50 mg/kg body weight) in 200 μL of PBS (5 mg/mL) twice daily (100 mg/kg) for 10 consecutive days, and the other half was injected with PBS. Tumor growth was monitored every 7 days by bioluminescent imaging with an IVIS200 imaging system (Caliper Life Sciences).
Figure 2.
Experimental Protocols
(A) In vivo bystander effect experiment. (B) Tumor mass reduction by intratumoral injection of Muse-tk cells, followed by GCV administration. (C) Tumor mass reduction by Muse-tk cell injection in the hemisphere contralateral to the tumor. (D) In vivo Muse-tk cell migration to the tumor after intracranial injection into the contralateral hemisphere.
The bioluminescent signal intensity, indicative of the tumor volume, gradually increased in all of the mice implanted with a mixture of Muse-tk and U87-luc cells with PBS injection (PBS groups, dashed lines in Figure 3A). When GCV was administered, however, the bioluminescent signal intensity was significantly reduced compared with PBS groups on day 14 after co-implantation at M/T ratios of 1:4, 1:8, and 1:16, and this significant reduction was maintained on day 28 (GCV groups, solid lines in Figure 3A; Table S1A). Although GCV was administered, the M/T(1:64) group showed no reduction of tumor volume on day 14 compared to the PBS groups and continued to grow on day 28 (Figure 3A).
Figure 3.
In Vivo Bystander Effect between Muse-tk and U87-luc Cells in the Nude Mouse Intracranial Tumor Model
Nude mice were intracranially implanted with U87-luc cells (1 × 105 cells/mouse) mixed with Muse-tk cells in various proportions (M/T ratio = 1:4, 1:8, 1:16, 1:32, 1:64, and 0:1) and half of the mice in each group were intraperitoneally injected with GCV (M/T ratio/GCV) or PBS (M/T ratio/PBS) (n = 8/group). (A) The bioluminescent signal intensity, indicative of the tumor volume, gradually increased in the mice not treated with GCV (PBS groups, dashed lines). When GCV was administered, the bioluminescent signal intensity was significantly reduced compared with that in the PBS groups at 14 days after implantation and thereafter in the mice implanted with a mixture of Muse-tk and U87-luc cells at M/T ratios ≥1:32, but not at an M/T ratio of 1:64 (GCV groups, solid lines, *p < 0.05, **p < 0.01). (B) Representative bioluminescent images (M/T ratios of 1:32 and 0:1). (C) Representative H&E images. (D) Survival time was also examined in the same animals used for the bioluminescent imaging experiment. All the mice not treated with GCV died from the tumor within 60 days after tumor implantation (PBS groups, dashed lines). Administration of GCV (GCV groups, solid lines) significantly prolonged survival time in the mice implanted with a mixture of Muse-tk and U87-luc cells at M/T ratios ≥1:32 compared with the corresponding PBS groups (**p < 0.01), but not at a ratio of 1:64.
Survival time was also examined in the same animals used for the bioluminescent imaging for up to 100 days after tumor inoculation. All the mice treated with PBS died from tumors within 60 days after tumor inoculation (PBS groups, dashed lines in Figure 3B). When GCV was administered (GCV groups, solid lines in Figure 3B), survival time was significantly prolonged in the mice with M/T(1:4) to M/T(1:32) compared with the corresponding PBS groups (Table S1A), but not in the mice with M/T(1:64).
Representative bioluminescent images of the mice implanted with M/T(1:32) and M/T(0:1) (U87-luc cells alone) treated with GCV or PBS are shown in Figure 3C, revealing a substantial reduction in tumor size only in M/T(1:32)/GCV, whereas the other three groups showed a rapid increase in tumor volume at days 14 and 28. Representative H&E-stained sections obtained at the time of death from mice implanted with M/T(1:32) and M/T(0:1) (U87-luc cells alone) treated with GCV or PBS are shown in Figure 3D. The mice implanted with M/T(1:32)/GCV were killed on day 100 at the end of the survival experiment. Although faint, small bioluminescent signals were still detected on day 28 in the M/T(1:32)/GCV group (Figure 3C), no tumor mass was observed in the histologic analysis at day 100 (Figure 3D), suggesting that the bystander effect of Muse-tk cells could sustainably kill the U87-luc cells until the GBM cells become histologically undetectable.
Tumor Mass Reduction Effect on Pre-existing U87-luc Cells by Intratumoral Injection of Muse-tk Cells Followed by Intraperitoneal Injection of GCV
Because the co-implantation experiments indicated a strong inhibition of tumor mass growth, we evaluated the tumor suppressive effect of Muse-tk cells on pre-existing U87-luc brain tumors (Figure 2B). 7 days after U87-luc cell implantation, half of the mice were treated with intratumoral injection of Muse-tk cells (1 × 105), which was the same number as U87-luc cells, and the other half was treated with just PBS alone (day 7) (Figure 2B; intratumoral injection). The mice were then intraperitoneally injected with GCV (50 mg/kg) or PBS twice daily (100 mg/kg/day) for 10 consecutive days from day 8 to day 17 (Figure 2B). Altogether, there were four groups (n = 10/group): intratumoral injection of Muse-tk cells followed by intraperitoneal GCV (Muse-tk/GCV) or PBS (Muse-tk/PBS) and intratumoral injection of PBS followed by intraperitoneal GCV (PBS/GCV) or PBS (PBS/PBS).
The bioluminescence intensity gradually increased in the control mice (Muse-tk/PBS, PBS/GCV, and PBS/PBS), suggesting gradual tumor growth (Figure 4A; Table S1B). On the other hand, the bioluminescence intensity in the Muse-tk/GCV mice decreased and was significantly lower than that in the three control groups on day 28 (*p < 0.05, Figure 4A; Table S1B). All the control mice (Muse-tk/PBS, PBS/GCV, and PBS/PBS) died from the tumor within 60 days after tumor implantation (Figure 4B). The Muse-tk/GCV mice survived significantly longer than the other three groups, and 4 of 10 mice in this group survived longer than 200 days (Figure 4B; Table S1B). Representative bioluminescent images and H&E-stained sections from mice in each group are shown in Figure 4C, demonstrating a remarkable reduction of the tumor mass at day 28 in the Muse-tk/GCV group compared to the other three groups. Histologic examination at day 200 revealed no visible tumors in three of the four mice (Figure 4D). Therefore, similar to the co-implantation model, the bystander effect of Muse-tk cells could sustainably kill U87-luc cells, making the GBM cells histologically undetectable, even though Muse-tk cells were administered after establishing the GBM cell tumor.
Figure 4.
Tumor Mass of Pre-existing Brain Tumor Was Reduced by Intratumoral Injection of Muse-tk Cells Followed by GCV Administration
On day 7 after U87-luc cell implantation (1 × 105 cells, n = 40), half of the mice were injected intratumorally with Muse-tk cells (1 × 105 cells) and the other half were injected with PBS. The mice were then intraperitoneally injected with GCV or PBS for 10 days from day 8. (A) Bioluminescent signal intensity, indicative of the tumor volume, became gradually stronger in mice injected with PBS (PBS/GCV and PBS/PBS groups) or Muse-tk cells, but not in mice injected with GCV (Muse-tk/PBS group). Bioluminescence signal intensity in mice intratumorally injected with Muse-tk cells and intraperitoneal GCV (Muse-tk/GCV group) decreased and was significantly weaker on day 28 than that in the other three control groups (*p < 0.05). (B) Representative bioluminescent images. (C) Representative H&E images. (D) Survival time was examined in the same animals used for the bioluminescent imaging experiment. All U87-luc bearing mice in the PBS/GCV, PBS/PBS, and Muse-tk/PBS groups died from the tumor within 60 days after tumor implantation, whereas survival of mice in the Muse-tk/GCV group was significantly longer than that in the other three groups (***p < 0.001).
Muse-tk Cells Implanted in the Contralateral Intact Hemisphere Reduced Tumor Mass after GCV Administration
As mentioned above, we observed a potent bystander effect-mediated pre-existing tumor mass reduction by intratumoral injection of Muse-tk cells followed by intraperitoneal GCV administration. The failure of clinical trials of viral-mediated HSVtk/GCV gene therapy has been mainly attributed to the limited distribution of the viral vector throughout the invasive glioblastoma,15 and an advantage of the use of genetically modified stem cells is their inherent migratory activity toward tumors.16 Therefore, we examined how the injected Muse-tk cells travel and the strength of the bystander effect-mediated tumor mass reduction efficacy following the intracranial injection of Muse-tk cells at a distant site from the tumor inoculation. The day after U87-luc inoculation (1 × 105, day 1), mice were implanted with Muse-tk cells (1 × 105) into the equivalent location of the contralateral intact hemisphere as the tumor inoculation and then GCV or PBS was intraperitoneally injected for 10 consecutive days from day 1 (Figure 2C; contralateral injection). The bioluminescent signal intensity in the GCV group on day 28 was significantly lower than that in the PBS group (Figure 5A; Table S1C) and survival of the GCV group was also significantly prolonged (Figure 5B; Table S1C).
Figure 5.
Muse-tk Cells Implanted in the Contralateral Hemisphere Reduced Tumor Mass after GCV Administration
Muse-tk cells (1 × 105) were injected in the left hemisphere of the brain 1 day after U87-luc tumor implantation in the right hemisphere (n = 11). Of the 11 animals, five were injected intraperitoneally with GCV (GCV group) and the other six were injected with PBS (PBS group) for 10 days from the day of Muse-tk injection. The bioluminescent signal intensity, indicative of the tumor volume, measured on day 28 after tumor inoculation was significantly weaker in the GCV group than in the PBS group (A, *p < 0.05) and the survival was significantly longer in the GCV group than in the PBS group (B, *p < 0.05).
In Vivo and In Vitro Migration of Muse-tk Cells toward Glioma Cells
The mechanism underlying the tumor mass reduction by the contralateral Muse-tk cell injection followed by GCV administration is considered to be the bystander effect generated between the tumor and Muse-tk cells that migrated from the contralateral brain by crossing the corpus callosum into the ipsilateral side. To confirm the presence of Muse-tk cells around the inoculated tumor, Muse-tk cells (5 × 105) labeled with Qtracker 525 were implanted in the brain hemisphere contralateral to the tumor site and the mice were killed 7 days later (Figure 2D). When U87-luc cells (shown as red cells in Figure 6A, top) were implanted, numerous Qtracker 525-labeled green fluorescence-positive cells (Muse-tk cells) were observed around the tumor site and also in the corpus callosum, suggesting that the Muse-tk cells migrated from the contralateral injection site to the tumor site. No such Muse-tk cell migration was observed in the mice injected with PBS (Figure 6A, bottom).
Figure 6.
In Vivo and In Vitro Migration of Muse-tk Cells toward GBM Cells
(A and B) Muse-tk cells (green cells) implanted in the left hemisphere (A, top, left panel) migrated toward U87-luc GBM cells (red cells) (A, top right panel) traveling through the corpus callosum (A, top, middle panel), but did not migrate toward a PBS-injected site (B, top right). (C) The in vitro migratory capacity of Muse-tk cells toward brain tumor cells was also examined using a Matrigel invasion assay with conditioned medium of U87 and U251 human GBM cells in the lower chamber. Only Muse-tk cells, and not non-Muse-tk cells, migrated to the lower chamber filled with the conditioned medium of U87 and U251 cells (mean number of migrating cells ± SD, triplicate, **p < 0.01). (D) Representative microphotographs.
The in vitro migratory capacity of Muse-tk cells toward brain tumor cells was also examined using a Matrigel invasion assay and conditioned medium (CM) of U87 and U251 cells in the lower chamber.31 Only Muse-tk cells, and not non-Muse-tk cells (fibroblasts other than Muse cells, namely SSEA-3− cells, which were introduced with tk), migrated to the lower chamber filled with CM of U87 and U251 cells (Figures 6B and 6C). Neither Muse-tk cells nor non-Muse-tk cells migrated toward the lower chamber filled with unconditioned medium (data not shown).
Eradication of the Implanted Muse-tk Cells in the Mouse Brain by GCV Administration
To verify whether implanted human Muse-tk cells in the mouse brain are eradicated by GCV, we conducted real-time PCR for detection of the human-specific Alu sequence. No Alu sequence was detected in the group of mice injected with intracranial Muse-tk cells and subsequent intraperitoneal GCV administration at day 100 (Muse-tk/GCV), whereas the Alu sequence was detected in the mice injected with Muse-tk cells but no GCV administration at day 3 (Muse-tk/PBS, positive control; Figure 7). This finding indicates that the human-specific Alu sequence was under the detection limit in Muse-tk cells implanted in the mouse brain after GCV administration.
Figure 7.
Eradication of the Implanted Muse-tk Cells by GCV Administration
(A) Experimental protocol for detecting the Alu sequence found in human genetic DNA in a mouse brain implanted with Muse-tk cells and treated with GCV. (B) No Alu sequence was detected after GCV administration (mean ± SD).
Discussion
Complete surgical removal of gliomas is almost impossible because of their potent invasive nature into surrounding eloquent brain tissues. Residual tumor cells are usually resistant to standard radiochemotherapy, and most patients experience tumor regrowth after a certain period. Because gliomas rarely metastasize to elsewhere in the body, local tumor therapy against those residual tumor cells is expected to improve patient prognosis. Local application with biodegradable 1,3-bis (2-chloroethyl)-1-nitrosourea wafers (Gliadel wafers) was developed in an attempt to deliver concentrated chemotherapeutic agents to the postoperative residual tumor without systemic toxicity. Implantation of Gliadel wafers at the time of tumor removal prolongs the survival of GBM patients somewhat but not drastically.8 This might be because the spread of the drug from the Gliadel wafers to the surrounding brain tissue is limited to within the range of millimeters.32
As an alternative local therapy for residual tumor cells after surgical removal, we previously tested genetically engineered NSCs with HSVtk/GCV because of their active migratory activity toward tumor cells. Rat brain tumors were successfully treated through the bystander effect by an intratumoral injection of NSCs transduced with the HSVtk gene (NSC-tk cells) followed by systemic GCV administration.20 Tumor volume reduction and prolonged survival of rats were observed even when NSC-tk cells were injected at intracranial sites distant from the tumor.33 Although NSCs are an efficient vehicle for HSVtk, they are associated with ethical and practical problems. A very limited amount of NSCs normally reside deep in the adult brain and are therefore not easily accessible on a clinical scale. The fetal brain is another potential source, but ethical issues and tumorigenicity limit their use.25 Bone marrow cells or bone-marrow MSCs transduced with the HSVtk gene also demonstrate the bystander effect.18 The cells, however, comprise heterogeneous populations, and thus their composition and characteristics are altered by multiple factors, such as quality of serum, handling of cells, passage number, and timing of the use.26 For this reason, their migratory capacity toward tumors and potency of the bystander effect dramatically fluctuates.
Undifferentiated stem cells generally exhibit the potential to migrate to glioma cells, and can thus be utilized as a vehicle of suicide genes for local tumor therapy.34 We would like to emphasize that Muse cells have several advantages over other stem cells, including NSCs and MSCs. (1) They are collectable from practical sources, such as the bone marrow, adipose tissue, and dermis, and thus pose no ethical problems. (2) They are non-tumorigenic and their karyotype remains normal after several passages and they do not form teratomas in immunodeficient mice.27, 29, 35, 36 The expression level of telomerase, an indicator of tumorigenic activity, is similar to that of somatic cells, suggesting that Muse cells have a low risk of tumorigenicity.29, 36 (3) The doubling time is ∼1.3 days/cell division, nearly equal to that of fibroblasts, and thus use of these cells is clinically feasible. Finally, (4) Muse cells are homogeneous in terms of marker expression and basic characteristics. For safety reasons, well-characterized “cell products” are ideal for first-in-human clinical trials. Muse-tk cells demonstrated a preference for migrating toward inoculated gliomas and a bystander effect. They do not remain in the brain tissue after GCV administration for up to 100 days, suggesting their safety. On the basis of these results, Muse-tk cells are considered to be practical candidates for application to stem cell-based suicide gene therapy for gliomas.
In vitro co-culture experiments demonstrated a potent bystander effect between Muse-tk cells and two representative human GBM cell lines (U87 and U251). The bystander effect is potent up to M/T ratios of 1:64 for U87 and 1:16 for U251 (Figure 1). These observations are similar to our previous data with murine NSC-tk cells.37 In vivo co-implantation experiments in nude mice demonstrated a bystander effect between Muse-tk and U87-luc cells, which was similarly potent to that observed in the in vitro study and in our previous study using murine NSC-tk cells.37 When GCV was administered, tumor size, evaluated by bioluminescent imaging, was significantly smaller and survival time was significantly prolonged compared with that in the PBS group at M/T ratios as low as 1:32 (Figure 3). These results indicate that when highly purified and well-characterized Muse cells are used as the cellular vector, the bystander effect between tumor and Muse-tk cells is sufficiently potent for clinical use. These data are useful for making a clinical protocol to estimate how many treatment cells are needed for a certain target tumor.
To simulate a clinical trial, intracranial U87-luc tumors (1 × 105) were established in nude mice, and after 7 days, the tumors were treated by intratumoral injection of the same number (1 × 105) of Muse-tk cells, followed by systemic GCV administration for 10 days. Because the doubling time of U87-luc cells is ∼2 days, the M/T ratio at the time of GCV administration on day 7 was estimated to be ∼1/10. Significant tumor mass reduction and prolonged survival were achieved (Figures 4A and 4B). Of the 10 treated mice, 4 survived more than 200 days and no evidence of tumor recurrence was observed in three mice at this time point, which is considered to be equivalent to a clinically “cured” state. In the previous similar study,20 the intracranial C6 tumor (1 × 105) in Sprague-Dawley (SD) rats was treated by intratumoral injection of SD rat-derived NSC-tk cells (2 × 106) on day 7, followed by 10 days GCV administration. Significant tumor mass reduction and prolonged survival were also achieved, and 6 of 9 rats survived more than 100 days. Another similar study using SD rat-derived MSC-tk also showed prolonged survival more than 100 days in 2 of 6 rats.18 A limitation of those studies was that only established cell lines were used. Patient-derived GBM xenograft cells, rather than immortalized cell lines, which have been shown to preserve tumor phenotype, should be also tested to obtain more clinically relevant outcomes.
In the contralateral injection model, Muse-tk cells were implanted in the hemisphere contralateral to the injection site of the implantation of the same number of U87-luc tumor cells (1 × 105) in the nude mouse brain on day 1, followed by systemic GCV administration for 10 days. Marginal, but significant, tumor mass reduction and prolonged survival were also achieved in the contralateral model (Figures 5A and 5B), indirectly suggesting Muse-tk cell migration toward the tumor site. We also observed numerous migrated Muse-tk cells at the tumor site as well as some in the corpus callosum using immunochemical techniques, providing direct evidence for migrating cells, though not quantitatively. Muse-tk cell distribution in the brain after implantation should be more precisely analyzed using in vivo imaging system to find the best timing for GCV administration.
Unlike regenerative therapies, therapeutic Muse cells do not need to survive for a long period in stem cell-based tumor treatment strategies. Most Muse-tk cells are killed by GCV and also may not be able to survive for a longer period possibly due to immune rejection in the host animals. We demonstrated by qPCR of the human Alu sequence that the signal for Muse-tk cells in the brain after GCV administration was under the detection level, even in nude mice. These results suggest that intracranial Muse-tk cell injection is safe in terms of its own tumorigenicity in the present protocol.
In clinical trials, allogeneic Muse cells prepared in advance will be applied to the surface of the resection cavity and then GCV will be systemically administered after a certain period, e.g., 7 days later. Another application will be intratumoral Muse-tk cell injection for small deep-seated tumors, followed by GCV administration, as in the present animal experiment. Because this treatment strategy theoretically has no systemic adverse effects, intratumoral administration of Muse-tk cells can be repeated at certain intervals until the satisfied effect is achieved. For human trials, it is important to noninvasively visualize the therapeutic Muse-tk cell distribution in the brain. A highly promising noninvasive method to visualize the presence of HSVtk gene-expressing cells in the brain is positron emission tomographic scans using radioactive nucleic acid analogs, such as 9-(4-18F-fluoro-3-[hydroxymethyl]butyl)guanine, as the reporter.38, 39 In clinical trials, these strategies could be used to visualize the distribution of the treatment cells and decide the timing of the GCV administration as well as follow the fate of the injected Muse cells.
Materials and Methods
Preparation of Human Muse-tk Cells
For lentivirus production, pMD2G, pCMV-deltaR8.74, and pWPXL-HSVtk-IRES2-EGFP were transfected into LentiX-293T packaging cells (Takara Bio) using Lipofectamine 2000 (Thermo Fisher Scientific). 3 days after transfection, the viral supernatant was collected, centrifuged, and filtered through a 0.45-μm filter. For human Muse-tk cell sorting, HSVtk-IRES2- EGFP-labeled normal human dermal fibroblasts were incubated with rat anti-SSEA-3 immunoglobulin M (IgM) antibody (1:1,000; BioLegend), detected by allophycocyanin-conjugated anti-rat IgM (1:100; Jackson ImmunoResearch) in the antibody diluents and sorted by Special Order Research Products FACSAriaII (Becton Dickinson, http://www.bd.com) as described previously.27 We also collected ∼5% of cells with the lowest SSEA-3 expression as non-Muse-tk cells. Muse-tk cells and non-Muse-tk cells as a control were used in the following studies.
In Vitro Sensitivity of Muse-tk Cells to GCV
Muse-tk cells or naive Muse cells were seeded in a 96-well cell-culture plate at a density of 103 cells per well. α-minimal essential medium (MEM) containing 10% fetal bovine serum (FBS) and various concentrations (0.001–300 μg/mL) of GCV (Wako Pure Chemical Industries) was added, and the cells were incubated for 7 days. The number of living cells was determined by MTT assay and percent viability was expressed as the percentage absorbance of each GCV concentration to 0.001 μg/mL GCV concentration.
In Vitro Bystander Effect between Muse-tk and Glioma Cells
Human glioma cells (U87 and U251) were obtained from the American Type Culture Collection (ATCC). To determine the lowest number of Muse-tk cells that could provide an effective anti-tumor effect in combination with GCV, 5 × 103 human glioma cells were co-cultured with various numbers of Muse-tk cells at M/T ratios of 1:1, 1:2, 1:4, 1:8, 1:16, 1:32, and 1:64 seeded in a 96-well cell-culture plate in α-MEM containing 10% FBS with or without 2 μg/mL GCV for 7 days. Muse-tk cells alone (M/T[1:0], positive control) or tumor cells alone (0:1, negative control) were also cultured. The medium was changed every 2 days. The number of living cells was determined by MTT assay on day 7, and percent viability was expressed as the percentage absorbance of GCV+/GCV− for each M/T ratio.
The real-time bystander effect was also observed with a culture microscope (BioStation IM, Nikon). Cells were labeled using CellTracker probes (Molecular Probes) following the manufacturer’s instructions. Muse-tk cells (1 × 105) labeled with CellTracker Green CMFDA (C7025) and an equal number of U87 cells labeled with CellTracker Blue CMAC (C2110) were incubated in 2 mL of DMEM containing 10% FBS at 37°C under 5% CO2 for 12 hr to confirm that the cells attached and grew on 35-mm glass-based dishes (Iwaki). After the 12-hr incubation, the medium was replaced with 2 mL of fresh medium with or without 2 μg/mL GCV. After additional replacement of 4 mL of fresh medium with or without 2 μg/mL GCV at 34 hr, the dishes were plated on an incubator/microscope to collect time-lapse images from 36 to 96 hr after GCV addition. Frames were taken at 30-min intervals.
In Vivo Bystander Effect between Muse-tk and U87-luc Cells in the Nude Mouse Intracranial Tumor Model
Experiments were conducted in accordance with the guidelines approved by the Animal Care Committee at the Hamamatsu University School of Medicine Animal Care Facility. 96 female BALB/c slc nu/nu mice (17–22 g, 6–8 weeks old, Nippon SLC) were subcutaneously injected with an anesthetic mixture comprising 0.75 mg/kg medetomidine (Nippon Zenyaku Kogyo), 4.0 mg/kg midazolam (Astellas Pharma), and 5.0 mg/kg butorphanol (Meiji Seika Pharma). A sagittal incision was made, and a burr hole was placed 0.2 mm posterior and 2 mm lateral to bregma. A 23G needle was inserted at the depth of 4.5 mm from the brain surface, left in position for 1 min, and then withdrawn to the depth of 3.5 mm where the cells were infused. Bioware Ultra Cell Line U-87 MG-luciferase2 cells (U87-luc, Caliper Life Sciences; 1 × 105 cells/mouse) were mixed with Muse-tk cells at M/T ratios of 1:4, 1:8, 1:16, 1:32, and 1:64 in 5 μL of PBS and inoculated into the right brain of nude mice at a rate of 2 × 104 cells/min using a Hamilton syringe using stereotaxic guidance (n = 16 for each group). U87-luc cells alone (1 × 105 cells) were also inoculated as a control (M/T ratio of 0:1, n = 16). The mice were intraperitoneally injected with GCV (50 mg/kg body weight in 200 μL of PBS; 5 mg/mL) twice daily (100 mg/kg) or PBS only from day 1 after tumor implantation for consecutive 10 days (Figure 2A).
Altogether, there were 12 groups (n = 8/group): intracranial implantation of M/T ratios of 1:4, 1:8, 1:16, 1:32, 1:64, and 0:1 with or without intraperitoneal GCV injection. To monitor the bioluminescence signals of U87-luc cells, XenoLight Rediject D-luciferin (Summit Pharmaceuticals International) at 150 mg/kg body weight was intraperitoneally injected, followed by subcutaneous injection of the anesthetic mixture, and then the mice were placed on a stage inside the camera box of the IVIS200 imaging system coupled with a cooled CCD camera (Caliper Life Sciences) 25 min after D-luciferin injection. The detected light emitted from U87-luc cells was digitized and electronically displayed as a pseudo color overlay onto a grayscale image of the animal. Images and measurements of luminescent signals were consecutively acquired for each animal on days 1, 14, and 28, and then analyzed with Living Image software version 3.0 (Caliper Life Sciences) and quantified as photons per second. Survival of all 96 mice used for bioluminescence imaging was investigated. The animals were killed if they presented symptoms such as severe paresis and/or ataxia or when their body weight decreased to less than 80%. Surviving mice were killed on day 100 and their brains were obtained for histologic examination with H&E.
Tumor Mass Reduction Effect on Pre-existing Brain Tumor by Intratumoral Injection of Muse-tk Cells Followed by GCV Administration
40 female BALB/c slc nu/nu mice were intracranially implanted with U87-luc cells (1 × 105) on day 0, as previously described. 7 days after U87-luc cell implantation, half of the mice were treated with intratumoral injection of Muse-tk cells (1 × 105) and the other half were treated with PBS alone (day 7; Figure 2B). The mice were then intraperitoneally injected with GCV (50 mg/kg) twice daily (100 mg/kg/day) or PBS from day 8 for 10 consecutive days. Altogether, there were four groups (n = 10/group): intratumoral injection of Muse-tk cells followed by intraperitoneal GCV (Muse-tk/GCV) or PBS (Muse-tk/PBS) and intratumoral injection of PBS followed by intraperitoneal GCV (PBS/GCV) or PBS (PBS/PBS). To monitor bioluminescence signals of U87-luc cells, the IVIS200 imaging system was used as previously described. Images and measurements of luminescent signals were acquired on days 1, 7, and 28. Survival of all 40 mice used for bioluminescence imaging was monitored. Histologic examination with H&E was also performed in the tumor-bearing mice and surviving mice.
Muse-tk Cells Implanted in the Contralateral Hemisphere Reduce Tumor Mass after GCV Administration
A total of 16 female BALB/c slc nu/nu mice were used. U87-luc cells (1 × 105) in 5 μL of PBS were implanted into the right hemisphere of the brain (0.2 mm posterior, 2 mm right, and 3.5 mm deep), and then Muse-tk cells (1 × 105) in 5 μL of PBS were implanted into the left hemisphere (0.2 mm posterior, 2 mm left, and 3.5 mm deep) 1 day after tumor implantation (day 1; Figure 2C). Five mice died on the day of the surgery, probably due to the stress of bilateral brain surgery. The remaining 11 mice were intraperitoneally injected with 50 mg/kg GCV twice daily (100 mg/kg/day, n = 5) or PBS (n = 6) from day 1 for 10 consecutive days. To monitor the bioluminescence signals of U87-luc cells, the IVIS200 imaging system was used as previously described. Images and measurements of luminescent signals were acquired on days 1, 14, and 28. Survival of all 11 mice was monitored.
In Vivo Migration of Muse-tk Cells toward Glioma Cells
Adult female BALB/c slc nu/nu mice were used. U87-luc cells (1 × 105) in 5 μL of PBS or PBS alone as a control were implanted in the right hemisphere on day 0 as previously described. Muse-tk cells were labeled using Qtracker probes (Molecular Probes) following the manufacturer’s instructions. Muse-tk cells (5 × 105) labeled with Qtracker 525 Cell Labeling Kit (Q25049) in 5 μL of PBS were implanted in the left hemisphere on day 7. The mice were killed by cardiac perfusion with PBS, followed by 2% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB) on day 14 after U87-luc implantation (7 days after Muse-tk cells transplantation; Figure 2D).
The brains were harvested, immersed in 2% PFA in BP at 4°C for 4 hr, transferred to 10%, 15%, and 20% sucrose in PBS, embedded in an optimum cutting temperature compound (OCT; Sakura Finetek Japan) on dry ice, sliced in 20-μm-thick sections with a cryostat, and mounted on MAS-coated glass slides (Matsunami Glass). The cryostat sections were examined under fluorescence microscopy. For immunohistochemical analysis, the tissue sections were fixed by 2% PFA in PB for 15 min and washed three times with PBS containing 0.05% Tween 20. The tissue sections were then incubated in 5% donkey normal serum in PBS containing 0.2% Triton X-100 for 60 min at room temperature to block non-specific binding sites. The rabbit polyclonal anti-firefly luciferase antibody (Abcam, 1:500) was applied for 60 min at room temperature. After five washes with PBS containing 0.1% donkey normal serum and 0.05% Tween 20, the slides were incubated with the secondary antibody, Alexa Fluor 594 conjugated AffiniPure donkey anti-rabbit IgG (H+L) (Jackson ImmunoResearch, 1:500) for 60 min at room temperature. The tissues were mounted using ProLong Diamond Antifade Mountant with 4’,6-diamidino-2-phenylindole (Molecular Probes).
In Vitro Migratory Capacity of Muse-tk Cells toward Glioma Cells
In vitro migration of Muse-tk cells toward glioma cells was examined using the 24-well Matrigel Invasion Chamber (BD Biosciences Discovery Labware), which contained an 8-μm pore size polyethylene terephthalate membrane treated with Matrigel Basement Membrane Matrix in the insert. First, 0.5 mL of DMEM was added to the lower part of the inserts and the bottom of the wells and allowed to rehydrate for 2 hr at 37°C in a 5% CO2 humidified atmosphere. The DMEM was then carefully removed without disturbing the layer of Matrigel Matrix on the membrane. Muse-tk cells and non-Muse-tk cells as a control were washed twice in PBS and resuspended to 1 × 105 cells/mL. Cell suspension (0.5 mL; 5 × 104 cells) was added to the upper insert. The lower chamber was filled with 0.75 mL of glioma cell (U87 and U251) CM. The CM was obtained by collecting, centrifuging, and filtering medium from U87 or U251 clones (1 × 106), which were cultured in 10 mL of DMEM without FBS for 48 hr. Following incubation of the Matrigel Invasion Chambers for 24 hr at 37°C in 5% CO2, the noninvading cells and Matrigel Matrix were removed from the upper surface of the membrane in the inserts with a cotton swab. Cells migrating to the lower surface of the membrane were stained with the Diff-Quick kit (International Reagents) by sequentially transferring the inserts to air dry. The number of cells that had migrated was counted in four high-power fields per membrane at 200x magnification. All experiments were conducted in triplicate, and the results are expressed as mean number of cells migrating per field ± SD.
Eradication of the Implanted Muse-tk Cells by GCV Administration
Ten adult female BALB/c slc nu/nu mice were divided into three groups. The first group (n = 4), as a positive control, was injected intracranially with Muse-tk cells (1 × 104) with subsequent intraperitoneal PBS administration, and then killed 3 days after the Muse-tk cell injection (Muse-tk/PBS). The second group (n = 3), as the experimental group, was intracranially injected with Muse-tk cells (1 × 105), followed by intraperitoneal GCV administration for 10 days, and then killed 100 days after Muse-tk cell injection (Muse-tk/GCV). The last group (n = 3), as a negative control, was intracranially injected with PBS and killed 100 days after PBS injection (PBS). The brains were removed and analyzed by real-time PCR assay for detection of human genomic DNA.
Real-Time PCR Assay for Detection of the Alu Sequence
Genomic DNA was collected from the brain on day 3 or day 100 after Muse-tk cell injection using the REDExtract-N-Amp Tissue PCR kit (Sigma-Aldrich) according to the manufacturer’s protocol. The PCR reactions were performed in a volume of 20 μL containing 10 μL of TaqMan Universal Master Mix II with UNG (Applied Biosystems), 900 nM forward and reverse primers, 250 nM TaqMan probe, and 100 ng of target template. PCR reactions were incubated at 50°C for 2 min and at 95°C for 10 min, followed by 50 cycles at 95°C for 15 s and 60°C for 1 min. Standard curves were generated by serially diluted human genomic DNA extracted from normal human dermal fibroblasts, which was mixed with mouse genomic DNA obtained from the mouse brain. The following PCR primers were used:
Sense; 5′-CATGGTGAAACCCCGTCTCTA-3′
Antisense; 5′-GGGTTCAAGCGATTCTCCTG-3′
TaqMan probe; 5′-FAM-ATTAGCCGGGCGTGGTGGCG-TAMRA-3′.
Statistical Analysis
All statistical data are presented as mean ± SD. The statistical significance of differences was determined by unpaired Student’s t test or repeated-measures ANOVA, followed by Fisher’s least significant difference post hoc analysis. The statistical analysis of survival data was performed using the log rank test based on the Kaplan-Meier analysis, followed by the Bonferroni method for pairwise multiple comparison tests. Descriptive statistics were performed with EZR on R commander version 1.27. A p value of less than 0.05 was considered to be statistically significant.
Author Contributions
T.Y. contributed to conception of the work, data collection and analysis, and writing of the article. S.W. provided Muse cells and contributed to data collection. H.K., S.K., and T.S. contributed to conception of the work. M.D. and H.N. contributed to supervision and revision of the manuscript.
Acknowledgments
This work is supported in part by Japan Society for the Promotion of Science grants 25293306, 25462252, and 16K10753 to H.N. and 16K10752 to T.S.
Footnotes
Supplemental Information includes one figure, one table, and one movie and can be found with this article online at http://dx.doi.org/10.1016/j.omto.2017.06.001.
Supplemental Information
Real-time bystander effect between Muse-tk and U87 cells observed under a culture microscope (GCV: 2 μg/ml, Muse-tk:U87 ratio of 1:1).
References
- 1.Furnari F.B., Fenton T., Bachoo R.M., Mukasa A., Stommel J.M., Stegh A., Hahn W.C., Ligon K.L., Louis D.N., Brennan C. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 2.Chinot O.L., Wick W., Mason W., Henriksson R., Saran F., Nishikawa R., Carpentier A.F., Hoang-Xuan K., Kavan P., Cernea D. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 3.Sanai N., Polley M.Y., McDermott M.W., Parsa A.T., Berger M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 4.Stummer W., Pichlmeier U., Meinel T., Wiestler O.D., Zanella F., Reulen H.J., ALA-Glioma Study Group Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups. National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R., Taillibert S., Kanner A.A., Kesari S., Steinberg D.M., Toms S.A., Taylor L.P., Lieberman F., Silvani A., Fink K.L. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314:2535–2543. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 7.Van Meir E.G., Hadjipanayis C.G., Norden A.D., Shu H.K., Wen P.Y., Olson J.J. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J. Clin. 2010;60:166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westphal M., Hilt D.C., Bortey E., Delavault P., Olivares R., Warnke P.C., Whittle I.R., Jääskeläinen J., Ram Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duntze J., Litré C.F., Eap C., Théret E., Debreuve A., Jovenin N., Lechapt-Zalcman E., Metellus P., Colin P., Guillamo J.S. Implanted carmustine wafers followed by concomitant radiochemotherapy to treat newly diagnosed malignant gliomas: prospective, observational, multicenter study on 92 cases. Ann. Surg. Oncol. 2013;20:2065–2072. doi: 10.1245/s10434-012-2764-x. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert M.R., Dignam J.J., Armstrong T.S., Wefel J.S., Blumenthal D.T., Vogelbaum M.A., Colman H., Chakravarti A., Pugh S., Won M. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orringer D., Lau D., Khatri S., Zamora-Berridi G.J., Zhang K., Wu C., Chaudhary N., Sagher O. Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J. Neurosurg. 2012;117:851–859. doi: 10.3171/2012.8.JNS12234. [DOI] [PubMed] [Google Scholar]
- 12.Oberoi R.K., Parrish K.E., Sio T.T., Mittapalli R.K., Elmquist W.F., Sarkaria J.N. Strategies to improve delivery of anticancer drugs across the blood-brain barrier to treat glioblastoma. Neuro-oncol. 2016;18:27–36. doi: 10.1093/neuonc/nov164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paw I., Carpenter R.C., Watabe K., Debinski W., Lo H.W. Mechanisms regulating glioma invasion. Cancer Lett. 2015;362:1–7. doi: 10.1016/j.canlet.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Culver K.W., Ram Z., Wallbridge S., Ishii H., Oldfield E.H., Blaese R.M. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256:1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 15.Rainov N.G. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum. Gene Ther. 2000;11:2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 16.Aboody K.S., Brown A., Rainov N.G., Bower K.A., Liu S., Yang W., Small J.E., Herrlinger U., Ourednik V., Black P.M. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc. Natl. Acad. Sci. USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehtesham M., Kabos P., Gutierrez M.A., Chung N.H., Griffith T.S., Black K.L., Yu J.S. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;62:7170–7174. [PubMed] [Google Scholar]
- 18.Amano S., Li S., Gu C., Gao Y., Koizumi S., Yamamoto S., Terakawa S., Namba H. Use of genetically engineered bone marrow-derived mesenchymal stem cells for glioma gene therapy. Int J Oncol. 2009;35:1265–1270. doi: 10.3892/ijo_00000443. [DOI] [PubMed] [Google Scholar]
- 19.Bak X.Y., Lam D.H., Yang J., Ye K., Wei E.L., Lim S.K., Wang S. Human embryonic stem cell-derived mesenchymal stem cells as cellular delivery vehicles for prodrug gene therapy of glioblastoma. Hum Gene Ther. 2011;22:1365–1377. doi: 10.1089/hum.2010.212. [DOI] [PubMed] [Google Scholar]
- 20.Li S., Tokuyama T., Yamamoto J., Koide M., Yokota N., Namba H. Bystander effect-mediated gene therapy of gliomas using genetically engineered neural stem cells. Cancer Gene Ther. 2005;12:600–607. doi: 10.1038/sj.cgt.7700826. [DOI] [PubMed] [Google Scholar]
- 21.Matuskova M., Hlubinova K., Pastorakova A., Hunakova L., Altanerova V., Altaner C., Kucerova L. HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Lett. 2010;290:58–67. doi: 10.1016/j.canlet.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 22.Miletic H., Fischer Y., Litwak S., Giroglou T., Waerzeggers Y., Winkeler A., Li H., Himmelreich U., Lange C., Stenzel W. Bystander killing of malignant glioma by bone marrow-derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol Ther. 2007;15:1373–1381. doi: 10.1038/sj.mt.6300155. [DOI] [PubMed] [Google Scholar]
- 23.Mori K., Iwata J., Miyazaki M., Osada H., Tange Y., Yamamoto T., Aiko Y., Tamura M., Shiroishi T. Bystander killing effect of tymidine kinase gene-transduced adult bone marrow stromal cells with ganciclovir on malignant glioma cells. Neurol Med Chir (Tokyo). 2010;50:545–553. doi: 10.2176/nmc.50.545. [DOI] [PubMed] [Google Scholar]
- 24.Uchibori R., Okada T., Ito T., Urabe M., Mizukami H., Kume A., Ozawa K. Retroviral vector-producing mesenchymal stem cells for targeted suicide cancer gene therapy. J Gene Med. 2009;11:373–381. doi: 10.1002/jgm.1313. [DOI] [PubMed] [Google Scholar]
- 25.Amariglio N., Hirshberg A., Scheithauer B.W., Cohen Y., Loewenthal R., Trakhtenbrot L., Paz N., Koren-Michowitz M., Waldman D., Leider-Trejo L. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandhaanam S.D., Pathalam G., Dorairaj S., Savariar V. Mesenchymal stem cells (MSC): identification, proliferation and differentiation. PeerJ PrePrints. 2013;1:e148v1. [Google Scholar]
- 27.Kuroda Y., Kitada M., Wakao S., Nishikawa K., Tanimura Y., Makinoshima H., Goda M., Akashi H., Inutsuka A., Niwa A. Unique multipotent cells in adult human mesenchymal cell populations. Proc. Natl. Acad. Sci. USA. 2010;107:8639–8643. doi: 10.1073/pnas.0911647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuroda Y., Wakao S., Kitada M., Murakami T., Nojima M., Dezawa M. Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat. Protoc. 2013;8:1391–1415. doi: 10.1038/nprot.2013.076. [DOI] [PubMed] [Google Scholar]
- 29.Wakao S., Kitada M., Kuroda Y., Shigemoto T., Matsuse D., Akashi H., Tanimura Y., Tsuchiyama K., Kikuchi T., Goda M. Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc. Natl. Acad. Sci. USA. 2011;108:9875–9880. doi: 10.1073/pnas.1100816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dezawa M. Muse cells provide the pluripotency of mesenchymal stem cells: direct contribution of muse cells to tissue regeneration. Cell Transplant. 2016;25:849–861. doi: 10.3727/096368916X690881. [DOI] [PubMed] [Google Scholar]
- 31.Koizumi S., Gu C., Amano S., Yamamoto S., Ihara H., Tokuyama T., Namba H. Migration of mouse-induced pluripotent stem cells to glioma-conditioned medium is mediated by tumor-associated specific growth factors. Oncol. Lett. 2011;2:283–288. doi: 10.3892/ol.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleming A.B., Saltzman W.M. Pharmacokinetics of the carmustine implant. Clin. Pharmacokinet. 2002;41:403–419. doi: 10.2165/00003088-200241060-00002. [DOI] [PubMed] [Google Scholar]
- 33.Li S., Gao Y., Tokuyama T., Yamamoto J., Yokota N., Yamamoto S., Terakawa S., Kitagawa M., Namba H. Genetically engineered neural stem cells migrate and suppress glioma cell growth at distant intracranial sites. Cancer Lett. 2007;251:220–227. doi: 10.1016/j.canlet.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 34.Augello A., Kurth T.B., De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur. Cell. Mater. 2010;20:121–133. doi: 10.22203/ecm.v020a11. [DOI] [PubMed] [Google Scholar]
- 35.Gimeno M.L., Fuertes F., Barcala Tabarrozzi A.E., Attorressi A.I., Cucchiani R., Corrales L., Oliveira T.C., Sogayar M.C., Labriola L., Dewey R.A. Pluripotent nontumorigenic adipose tissue-derived Muse cells have immunomodulatory capacity mediated by transforming growth factor-β1. Stem Cells Transl. Med. 2017;6:161–173. doi: 10.5966/sctm.2016-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogura F., Wakao S., Kuroda Y., Tsuchiyama K., Bagheri M., Heneidi S., Chazenbalk G., Aiba S., Dezawa M. Human adipose tissue possesses a unique population of pluripotent stem cells with nontumorigenic and low telomerase activities: potential implications in regenerative medicine. Stem Cells Dev. 2014;23:717–728. doi: 10.1089/scd.2013.0473. [DOI] [PubMed] [Google Scholar]
- 37.Li S., Tokuyama T., Yamamoto J., Koide M., Yokota N., Namba H. Potent bystander effect in suicide gene therapy using neural stem cells transduced with herpes simplex virus thymidine kinase gene. Oncology. 2005;69:503–508. doi: 10.1159/000091032. [DOI] [PubMed] [Google Scholar]
- 38.Hung S.C., Deng W.P., Yang W.K., Liu R.S., Lee C.C., Su T.C., Lin R.J., Yang D.M., Chang C.W., Chen W.H. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin. Cancer Res. 2005;11:7749–7756. doi: 10.1158/1078-0432.CCR-05-0876. [DOI] [PubMed] [Google Scholar]
- 39.Yaghoubi S., Barrio J.R., Dahlbom M., Iyer M., Namavari M., Satyamurthy N., Goldman R., Herschman H.R., Phelps M.E., Gambhir S.S. Human pharmacokinetic and dosimetry studies of [(18)F]FHBG: a reporter probe for imaging herpes simplex virus type-1 thymidine kinase reporter gene expression. J. Nucl. Med. 2001;42:1225–1234. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Real-time bystander effect between Muse-tk and U87 cells observed under a culture microscope (GCV: 2 μg/ml, Muse-tk:U87 ratio of 1:1).