Significance
Stroke is an age-related disease that disproportionately affects women. Although experimental studies have identified several hormonal and genetic factors underlying these differences, little is known about how reproductive experience influences risk. This study examined the role of pregnancy and parturition on neurovascular function and behavior in both normal female mice and in females exposed to stroke. We found that reproductive experience increases systemic metabolic risk and results in significant behavioral deficits that are associated with CNS immunosuppression. After stroke, however, multiparous females exhibited smaller infarct volumes, attenuated inflammatory responses, enhanced angiogenesis, and improved behavioral recovery. Although the precise mechanisms underlying this paradoxical finding remain unknown, parity was associated with higher VEGF and improved postischemic vascular remodeling.
Keywords: sex differences, multiparity, microglia, ischemic stroke, microchimerism
Abstract
Females show a varying degree of ischemic sensitivity throughout their lifespan, which is not fully explained by hormonal or genetic factors. Epidemiological data suggest that sex-specific life experiences such as pregnancy increase stroke risk. This work evaluated the role of parity on stroke outcome. Age-matched virgin (i.e., nulliparous) and multiparous mice were subjected to 60 min of reversible middle cerebral artery occlusion and evaluated for infarct volume, behavioral recovery, and inflammation. Using an established mating paradigm, fetal microchimeric cells present in maternal mice were also tracked after parturition and stroke. Parity was associated with sedentary behavior, weight gain, and higher triglyceride and cholesterol levels. The multiparous brain exhibited features of immune suppression, with dampened baseline microglial activity. After acute stroke, multiparous mice had smaller infarcts, less glial activation, and less behavioral impairment in the critical recovery window of 72 h. Behavioral recovery was significantly better in multiparous females compared with nulliparous mice 1 mo after stroke. This recovery was accompanied by an increase in poststroke angiogenesis that was correlated with improved performance on sensorimotor and cognitive tests. Multiparous mice had higher levels of VEGF, both at baseline and after stroke. GFP+ fetal cells were detected in the blood and migrated to areas of tissue injury where they adopted endothelial morphology 30 d after injury. Reproductive experience has profound and complex effects on neurovascular health and disease. Inclusion of female mice with reproductive experience in preclinical studies may better reflect the life-long patterning of ischemic stroke risk in women.
Nearly 800,000 people in the United States experience a new or recurrent stroke each year, and 55,000 more women than men are affected by stroke (1). Stroke is a sexually dimorphic disease impacted by genetics, hormones, and the environment (2). According to CDC data, 85% of women in the United States have given birth by age 40, whereas the number of lifetime pregnancies per woman varies by race and socioeconomic status. Therefore, child-bearing women represent a significant proportion of the female population, including those at risk for stroke. Moreover, this suggests that a large proportion of the elderly female population—who is at highest risk for stroke—may be differentially at risk. Epidemiological data suggest that increasing parity is associated with higher risk of cardiovascular disease (CVD) and stroke and late-life vascular comorbidities, including carotid atherosclerosis (3, 4). However, recent reports suggest that parity has a significant protective effect against CVD mortality (5). The effect of parity on outcome following ischemic stroke is yet unresolved.
Pregnancy induces profound acute and long-term physiological changes in the body that influence future vascular health through hormonally mediated changes in circulation, vascular tissue structure, coagulation, and the pathological state of preeclampsia. The mechanisms underlying the neurovascular changes associated with parity are initiated during the perigestational period, but the duration of these physiological changes is unknown. The risk of stroke is highest in the 2 d prior to delivery and 1 d postpartum (6, 7) and the risk remains elevated for at least 12 wk after delivery (8, 9). However, the effect of reproductive experience on stroke risk later in life has not been well studied and has never been modeled in the laboratory.
Critical window events at the fetal–placental interface during gestation include the bidirectional trafficking of cells between the mother and fetus, known as fetal “microchimerism.” The increased immunosuppressive state during pregnancy facilitates the transfer and survival of microchimeric cells (MCs) in the mother (10, 11), which have stem cell-like properties and multipotent potential, and which incorporate into the maternal bone marrow niche where they can persist for decades. These rare cells respond to sterile injuries and participate in regenerative recovery processes in other disease models (12), but whether these cells migrate to the ischemic brain is unknown. The effect of parity on neuroinflammation, cerebral perfusion, ischemic outcome, and functional recovery has not been investigated in preclinical models.
Results
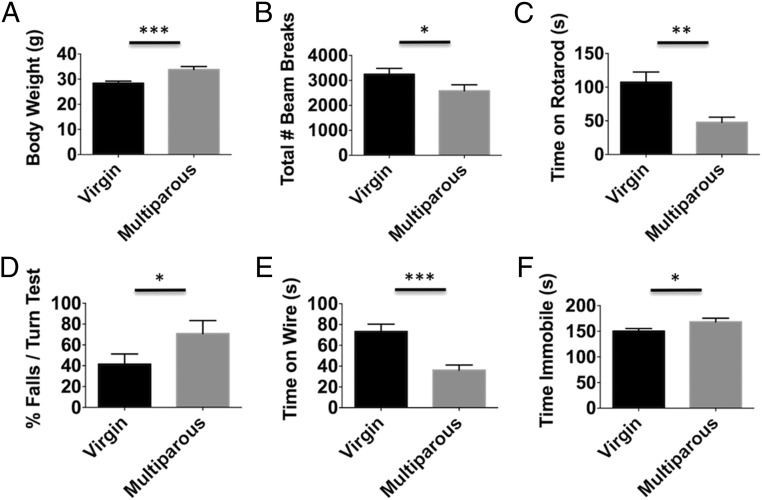
Behavioral assessment of locomotor activity, motor coordination, forelimb strength, and depressive phenotypes in nulliparous and multiparous mice was performed. Multiparous mice weighed significantly more than their virgin (i.e., nulliparous) counterparts (Fig. 1A; P ≤ 0.001). The number of total beam breaks in an open field was significantly decreased in multiparous mice (Fig. 1B; P ≤ 0.05). Parity significantly decreased time spent on an accelerating rotarod (Fig. 1C; P ≤ 0.01). We tested the possibility that rotarod performance was confounded by deficits in balance. Indeed, the percentage of falls in a 180° static rod turning test was significantly greater in multiparous mice (Fig. 1D; P ≤ 0.05). Forelimb grip strength, as determined by the ability to hang on a wire cage, was also significantly impaired in multiparous mice (Fig. 1E; P ≤ 0.001). Moreover, the duration of time multiparous mice spent immobile in a tail suspension test was significantly greater than in nulliparous controls (Fig. 1F; P ≤ 0.05). These findings indicate that parity results in significant baseline behavioral deficits.
Fig. 1.
Reproductive experience negatively affects behavioral performance in females. The body weight of nulliparous (virgin) and multiparous females was compared 3–4 mo following the last pregnancy (A) (n = 16–18 per group). The total number of beam breaks in 20 min in an open field apparatus were quantified (B). Time spent on an accelerating rotarod device before falling was measured (C) (n = 12 per group). The ability to turn 180° on a static bar test was assessed by taking the percentage of the falls over three trials for each subject. These results show multiparous females had a significantly greater percentage of falls in this turning test compared with age-matched virgins (D) (n = 12 per group). In a wire hang test, the latency to fall was measured (E) (n = 12 per group). To determine whether reproductive experience alters depressive behavior, the time spent immobile in a tail suspension test was quantified (F) (n = 20 per group). Error bars show mean SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
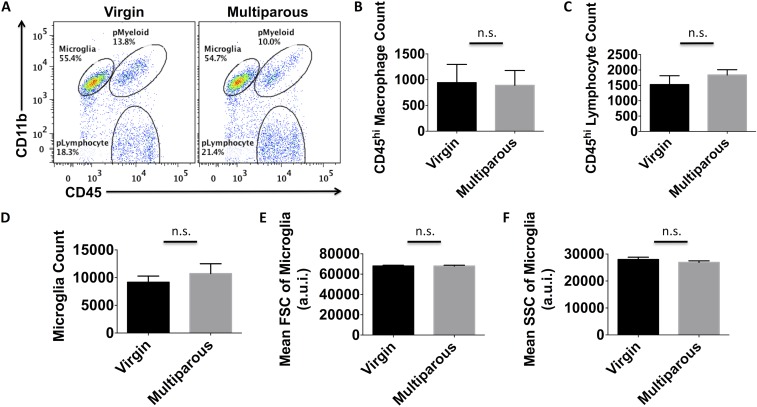
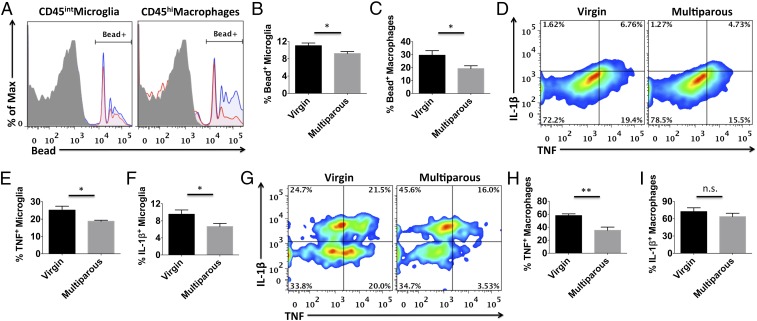
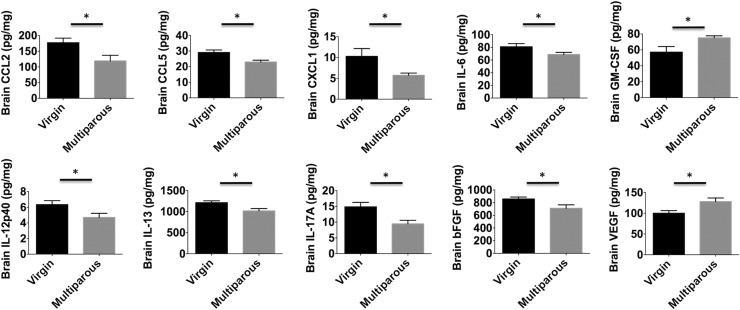
We next examined microglia activity in naïve mice using flow cytometry. No overt changes in the number of brain-resident microglia, perivascular macrophages, or lymphocytes were found between multiparous and nulliparous mice (Fig. S1 A–D). No changes in microglia cell size (forward scatter) or granularity (side scatter) were found based on light scatter properties (Fig. S1 E and F). However, striking functional differences were seen. Microglia and perivascular macrophages from multiparous mice exhibited significantly less TNF and IL-1β production compared with nulliparous controls (Fig. 2 D–H; P ≤ 0.05 and P ≤ 0.01, respectively). Whereas microglia represented one homogeneous population with respect to cytokine expression, perivascular macrophage populations could be divided into mutually exclusive TNF- and IL-1β–expressing subsets. Interestingly, a significant proportion of perivascular macrophages shifted from TNF+IL-1β− to TNF−IL-1β+ expression status with reproductive experience (Fig. 2G). Because proinflammatory cytokine production is associated with phagocytic activity, we then examined the potential for these cells to engulf fluorescent beads. Interestingly, microglia and perivascular macrophages from multiparous mice displayed significantly reduced phagocytic potential (Fig. 2 A–C; P ≤ 0.05). To test whether inflammatory factors present in the maternal brain could partially explain this alteration in microglia function, concentrations of several known cytokines were determined using ELISA. Out of a panel of 25 analytes, 9 were significantly altered with reproductive experience. We found significantly lower brain concentrations of cytokines (IL-6, IL-12p40, IL-13, and IL-17A) and chemokines (CCL2, CCL5, and KC) (Fig. 3A; P ≤ 0.05). Although the concentration of basic fibroblast growth factor (bFGF) was lower in multiparous mice, the proangiogenic factor, vascular endothelial growth factor (VEGF), was significantly higher (Fig. 3A). These data suggest that parity is associated with attenuated proinflammatory cytokine levels and suppressed microglia/perivascular macrophage activity in the female brain, features of enhanced immunosuppression.
Fig. S1.
No overt difference in microglia/macrophage counts between virgin and multiparous brains. Representative dot plot shows the relative density of CD45intCD11b+ microglia, CD45hiCD11b+ myeloid cells, and CD45hiCD11b− lymphocytes in the normal brain of virgin and multiparous females (A). The absolute number of perivascular macrophages (CD45hiCD11b+Ly6C+), lymphocytes (CD45hiCD11b−), and microglia (CD45intCD11b+Ly6C−) per hemisphere were quantified (B–D, respectively). To assess physical properties of microglia activation, the relative cell size [forward light scatter intensity (FSC)] and granularity [side light scatter intensity; (SSC)] was evaluated (E and F, respectively). For all experiments, n = 5 per group. Error bars show mean SEM. a.u.i., arbitrary unit of intensity; n.s., not significant.
Fig. 2.
Microglia and perivascular macrophages from multiparous females have suppressed immune function. Representative histograms depict the relative intensity of fluorescent beads engulfed by phagocytic microglia and perivascular macrophages from nulliparous virgins (blue) and multiparous (red) female brains (A). These percentages were then quantified as shown (B and C, respectively). Cell-specific fluorescence minus one (FMO) controls were used to determine positive gating (shaded gray). Representative smoothened dot plots show the relative baseline production of tumor necrosis factor (TNF) and interleukin-1 beta (IL-1β) cytokines by microglia and perivascular macrophages (D and G, respectively). These percentages were quantified as shown (E, F, H, and I). For all experiments, n = 5 per group. Error bars show mean SEM. n.s., not significant. *P < 0.05; **P < 0.01.
Fig. 3.
Reproductive experience suppresses global cytokine production in the maternal brain. Protein concentrations of several chemokines (RANTES, KC, CCL2), cytokines (IL-6, 12p40, IL-13, IL-17A), and growth factors (bFGF, GM-CSF, VEGF) are diminished or altered in the healthy maternal brain compared with their virgin counterparts. Total levels of the angiogenic factor, VEGF, were significantly elevated in the brain of multiparous mice. For all experiments, n = 6–7 per group. Error bars show mean SEM. *P < 0.05.
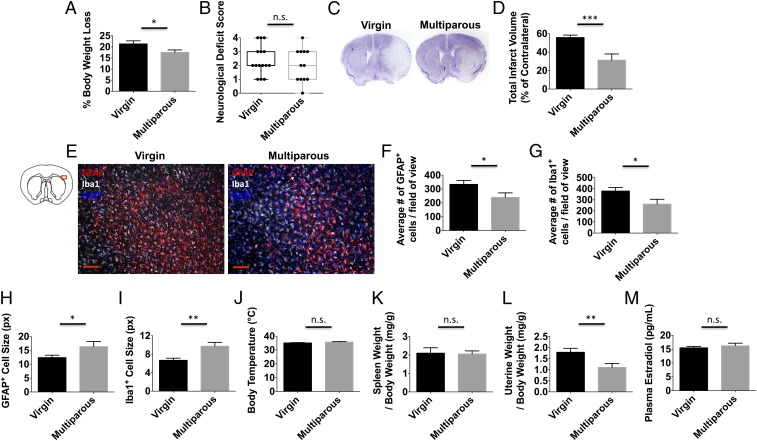
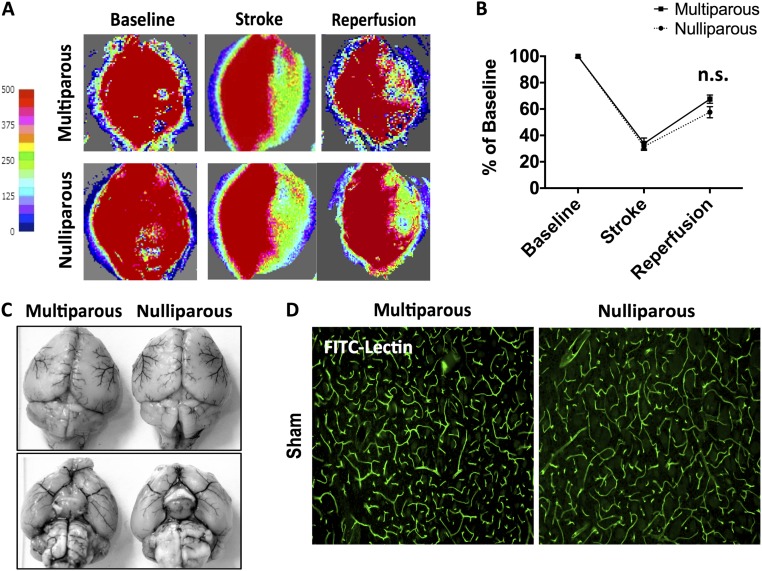
To examine the effect of parity on acute outcomes after stroke, we subjected multiparous and virgin mice to 60 min of middle cerebral artery occlusion followed by 72 h of reperfusion. Although there was a trend for improved neurological deficit scores (NDSs) in multiparous mice, no statistical difference was found (Fig. 4B). However, hemispheric infarct volumes were significantly smaller in multiparous mice compared with nulliparous controls (Fig. 4 C and D; P ≤ 0.001). These findings could not be attributed to differences in vascular anatomy or perfusion rate (Fig. S2 A–D). The percentage of body weight loss over 72 h was also significantly less in multiparous mice (Fig. 4A; P ≤ 0.05). Consistent with these results, we also found that multiparous mice showed a significant attenuation in astrocyte and microglia cell numbers in the ischemic cortex compared with nulliparous controls (Fig. 4 E–G; P ≤ 0.05). Average glial cell size was significantly larger in the multiparous brain (Fig. 4 H and I; P ≤ 0.05 and P ≤ 0.01, respectively). Despite differences in neuronal injury, no changes in body temperature regulation or stress-induced splenic atrophy were observed (Fig. 4 J and K). Although significant uterine growth was seen in multiparous mice (Fig. 4L; P ≤ 0.01), plasma concentrations of estradiol were similar and fell within well-established perimenopausal levels (Fig. 4M). These results suggest that parity provides a moderate level of acute protection following ischemic stroke.
Fig. 4.
Reproductive experience attenuates brain injury following ischemic stroke. Virgin and multiparous mice were subject to sham or stroke surgical conditions and evaluated at 72 h to determine differences in histopathology and physiology. The percentage of body weight loss after stroke was smaller for multiparous females (A) (n = 12–15 per group). Clinical assessment shows that neurological deficit scores at 72 h approached but did not achieve significance between virgin and multiparous mice (B) (n = 12–15 per group). Total hemispheric infarct volume measurements were determined using cresyl violet-stained brain sections. Representative virgin and multiparous cresyl violet-stained ischemic brains (C) and quantification of infarct volumes (D) are shown (n = 10–12 per group). Representative immunohistochemistry of DAPI+ nucleated GFAP+ astrocytes and Iba1+ microglia/macrophages in the ischemic cortex (E). (Scale bar, 100 μm.) The average number of astrocytes (F) and microglia/macrophages (G) counted in the ischemic cortex is shown (n = 10–12 per group). The average cell size was quantified for astrocytes and microglia/macrophages (H and I, respectively), which were larger in multiparous mice. No differences in core body temperature (degrees Celsius) or normalized spleen weight were found between groups after stroke (J and K, respectively; n = 12–14 per group). Quantification of uterine weights (L) and plasma estradiol concentrations (M) at 72 h is shown (n = 12–14 per group). Error bars show mean SEM. DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; Iba1, ionized calcium binding adaptor molecule 1; n.s., not significant; px, pixel. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. S2.
Baseline comparison of cerebral blood flow and vascular anatomy. Representative images of Laser speckle perfusion depict cerebral blood flow at baseline, during MCAO, and after reperfusion (A). Quantification of CBF flux demonstrated similar reductions in both groups (B) (n = 4 per group). There were no differences in large vessel anatomy, including the circle of Willis (C). The cortical microvasculature was assessed by i.v. injection of FITC-lectin using immunohistochemistry. Representative images showed no comparable differences in the staining pattern of FITC-lectin–labeled blood vessels (green) between nulliparous and multiparous mice (D). Error bars show mean SEM. (Magnification: A, 5×; D, 10×.) CBF, cerebral blood flow; FITC, fluorescein isothiocyanate; n.s., not significant.
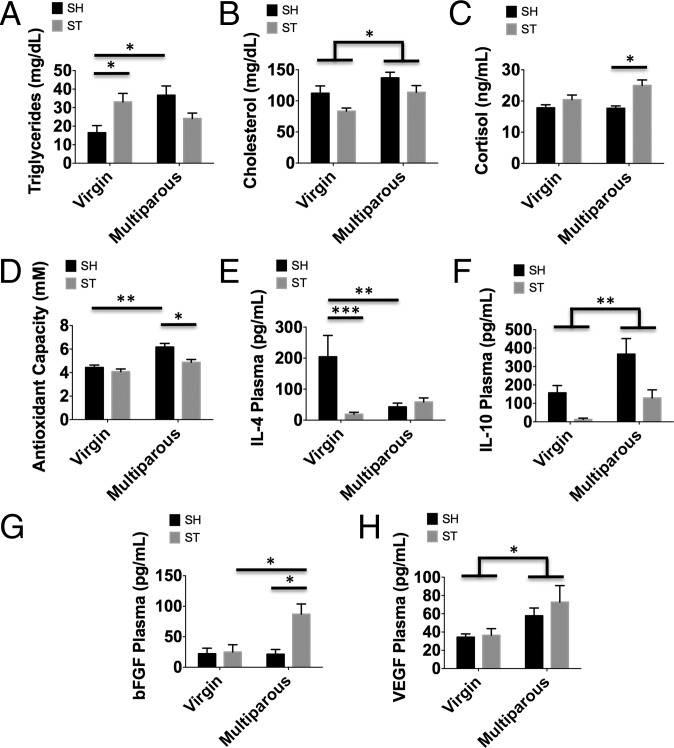
We next evaluated several important physiological and plasma markers associated with vascular risk. Total cholesterol and triglyceride levels were significantly higher in multiparous mice; however, after stroke there are differential changes in each (Fig. 5 A and B; P ≤ 0.05). A significant effect of parity [F(1,18) = 7.986; P ≤ 0.05] and stroke [F(1,18) = 7.102; P ≤ 0.05] was found in cholesterol levels. Antioxidant capacity is significantly increased with reproductive experience (Fig. 5D; P ≤ 0.01). Following stroke, cortisol concentrations were higher and antioxidant capacity was decreased in multiparous females compared with nulliparous virgins (Fig. 5 C and D; P ≤ 0.05). Plasma concentrations of the antiinflammatory cytokine IL-4 were significantly decreased in nulliparous females after stroke, whereas no change was seen in the multiparous cohort (Fig. 5E; P ≤ 0.001). IL-10 increased with reproductive experience, but were significantly attenuated in both mice after stroke (Fig. 5F; P ≤ 0.05). A significant effect of parity was seen in VEGF [F(1,13)=5.188; P ≤ 0.05] and bFGF concentrations (Fig. 5 G and H; P ≤ 0.05). Taken together, these data imply that the protective effects of reproductive experience on stroke outcome may involve alterations in inflammatory- and growth factor-signaling pathways, specifically for the proangiogenic factor, VEGF.
Fig. 5.
Complex effects of reproductive experience on cardiovascular risk biomarkers in sham and stroke-affected females. Virgin and multiparous mice were subject to sham or stroke surgical conditions and plasma was obtained to assess risk-associated biomarker status at 72 h after ischemia. Plasma triglyceride concentrations were measured using ELISA (A) (n = 6–11 per group). Quantification of total cholesterol (B), plasma cortisol levels (C), and antioxidant activity (D) is shown (n = 6–11 per group for each experiment). The data show significant effects of reproductive experience and ischemic stroke on biomarker levels. Comparison of circulating concentrations of antiinflammatory cytokines IL-4 (E) and IL-10 (F) and the growth factors bFGF (G) and VEGF (H) are shown (n = 7 per group). Group effects and multiple comparisons were compared using two-way ANOVA with post hoc Tukey test. Error bars show mean SEM. SH, sham; ST, stroke. *P < 0.05; **P < 0.01; ***P < 0.001.
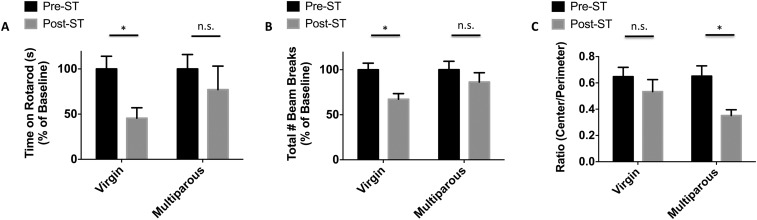
As the NDS is a relatively crude measure of behavioral deficits that has limited sensitivity after day 1, additional behavioral testing was performed at acute endpoints. Multiparous mice had a significantly increased latency to fall while on an accelerating rotarod at 72 h (Fig. S3A; P ≤ 0.05), indicative of improved motor performance. Multiparous mice had less change in locomotor activity (compared as a percent of change from their own baseline) after stroke, relative to nulliparous controls, which showed significant behavioral deficits after injury (Fig. S3B; P ≤ 0.05). Absolute scores after stroke were not significantly different between groups in either the rotarod (47.6 ± 12 and 26.9 ± 11) or open field testing (1,940 ± 198 and 2,185 ± 265). Multiparous mice spent more time in the perimeter after stroke, indicating anxiety-like behavior (Fig. S3C; P ≤ 0.05).
Fig. S3.
The effect of multiparity on acute behavioral outcomes in two independent cohorts of stroke-injured females. The latency to fall on an accelerated rotarod device was measured before and after stroke (A). Quantitative assessment of spontaneous locomotor activity was determined by quantification of beam breaks in an open field apparatus (B). Over the course of 20 min in the open field test, multiparous mice spent significantly less time in the exposed center quadrant of the field after stroke, compared with their age-matched virgin counterparts (C). For all experiments, n = 10–12 per group. Multiple comparisons were performed using two-way ANOVA with post hoc Tukey test. Error bars show mean SEM. n.s., not significant; ST, stroke. *P < 0.05.
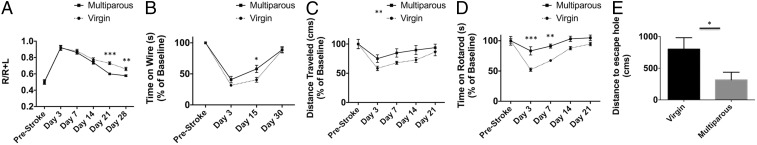
Two separate cohorts of mice were then evaluated over the span of 30 d to monitor long-term functional recovery. Chronic behavioral assessment demonstrated significantly higher impairment in virgin mice after stroke compared with parous females. Virgin females had significantly delayed recovery in sensorimotor function at day 21, as evidenced by an increased asymmetric preference during corner testing (Fig. 6A; day 28 = P ≤ 0.01). Similarly, virgin mice exhibited delayed recovery in forelimb grip strength at day 15 compared with multiparous mice; however, both groups recovered to near baseline value by day 30 (Fig. 6B; P ≤ 0.05). An effect of reproductive experience on the total distance traveled in an open field after stroke was determined by two-way ANOVA group analysis [Fig. 6C; F(1,85) = 8.696; P ≤ 0.01]. Consistent with these findings, virgin females displayed significantly delayed recovery in motor balance and coordination at 1 wk compared with multiparous females, as assessed by rotarod test (Fig. 6D; P ≤ 0.01). Next we examined short-term memory retention at day 30 after stroke using a Barnes maze test in an additional cohort of mice. Multiparous mice traveled shorter distances to locate the escape hole compared with virgin mice (Fig. 6E; P = 0.044). These data show that multiparous females have fewer chronic behavioral impairments after stroke compared with virgin females. Interestingly, correlational analysis revealed that although the number of pregnancies per mouse was not significantly associated with open field performance on day 3 (P = 0.26), there was a significant association by day 7 (P = 0.02). A similar trend was seen in the hang wire, with no association with the number of pregnancies and hang duration early after stroke on day 3 (P = 0.55), but improved performance with increasing pregnancy number by day 7 (P = 0.05). Taken together, these results indicate that reproductive experience facilitates faster recovery in sensorimotor and cognitive deficits after stroke.
Fig. 6.
Multiparous females exhibit fewer sensorimotor and cognitive deficits after stroke. Corner testing revealed significant asymmetry early after stroke in both groups, with markedly delayed recovery in virgin mice seen at days 21 and 28 (A). A mild impairment in forelimb strength was seen in virgin female mice at day 15 after stroke relative to their multiparous counterparts (B). Distance traveled in an open field apparatus is shown and a group effect was found (C). Time spent on an accelerating rotarod was recorded and demonstrated significantly more motor impairment at days 3 and 7 after stroke in virgins relative to multiparous females (D). Following a training period, memory retention was evaluated at day 30 using the Barnes maze test. Compared with virgins, multiparous mice traveled significantly shorter distances to locate the escape hole (E). Data are representative of two independent studies. For all experiments, n = 9–10 per group. Group effects were determined by two-way ANOVA with repeated measures and multiple comparisons were performed using post hoc Tukey test. Error bars show mean SEM. L, left; R, right. *P < 0.05; **P < 0.01; ***P < 0.001.
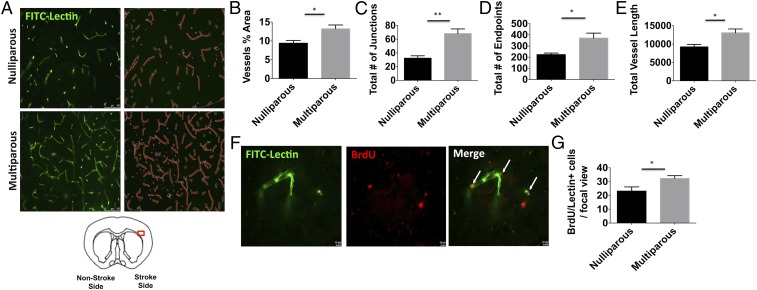
To determine the cellular correlates of recovery, mice were evaluated 30 d after stroke to monitor cellular proliferation. Functional blood vessels were assessed by intravascular injection of FITC-labeled lectin and proliferation was determined by BrdU incorporation. Quantitative measurements of poststroke vascular features were performed on FITC-labeled vessels in the ischemic cortex using software analysis (Fig. 7A). The percentage of area occupied by vessels was significantly greater in multiparous females at day 30 (Fig. 7B; P = 0.018). As well, the total number of vessel junctions and endpoints was comparably higher in multiparous females (Fig. 7 C and D; P = 0.001 and P = 0.013, respectively). Total vessel length was also higher in the ischemic cortex of multiparous females at day 30 after stroke (Fig. 7E; P = 0.015). BrdU/FITC-lectin colabeling was performed to evaluate angiogenesis in the ischemic cortical penumbra (Fig. 7F). Significantly higher numbers of BrdU-labeled newborn cells were found in the postischemic brain of multiparous mice compared with their virgin counterparts (Fig. 7G; P ≤ 0.05). Spearman analysis revealed a positive correlation between behavioral performance in the open field and on rotarod tests at day 3 and the number of BrdU- and FITC-lectin+ cells at day 30, suggesting that motor function in the acute phase after stroke is predictive of the delayed revascularization events at more chronic time points in recovery (Table 1). This positive correlation was demonstrated for spontaneous locomotor activity up to 21 d after stroke. Together these results suggest that reproductive experience stimulates cell proliferation and vascular repair in the postischemic cortex and is directly associated with motor recovery.
Fig. 7.
Reproductive experience enhances revascularization after stroke. Poststroke angiogenesis was assessed at 30 d in animals treated with BrdU and i.v. FITC-lectin injection. Representative microphotographs show FITC-labeled vessels in the ischemic cortex of each group (A). (Magnification: 20×.) AngioTool software was used to make quantitative measurements and perform analyses as illustrated (in red). The percent area containing vessels was quantified (B). The total number of junctions (C) and endpoints (D) is shown. Total vessel length was increased in multiparous females after stroke (E). Representative images of the ischemic penumbra (63× magnification) reveal colabeling of FITC-lectin-stained (green) functional blood vessels with BrdU+ (red) and DAPI+ (blue) nuclei as further highlighted by the white arrows (F). (Scale bar, 10 μm.) Quantification of colabeled BrdU+ cells is shown (G, n = 5 per group). Error bars show mean SEM. *P < 0.05; **P < 0.01.
Table 1.
Correlation between behavioral performance and BrdU+ and lectin+ vessels at day 30 after stroke
| Open field | Rotarod | |||||||
| Lectin | BrdU | Lectin | BrdU | |||||
| Day | ρ | P value | ρ | P value | ρ | P value | ρ | P value |
| 3 | 0.85 | 0.0037* | 0.753 | 0.0191* | 0.767 | 0.0159* | 0.745 | 0.0213* |
| 7 | 0.733 | 0.0246* | 0.82 | 0.0068* | 0.283 | 0.46 | 0.192 | 0.6198 |
| 14 | 0.966 | <0.0001* | 0.77 | 0.0152* | 0.1 | 0.798 | 0.092 | 0.8138 |
| 21 | 0.483 | 0.1875 | 0.652 | 0.0567 | 0.05 | 0.8984 | −0.008 | 0.983 |
A Spearman correlation analysis on the variable of BrdU/lectin and behavioral performance in open field and rotarod tests on different days was performed. Behavioral performance in both tests at day 3 positively correlated with the number of BrdU+ and lectin+ vessels in the ischemic brain of females at day 30. BrdU, bromodeoxyuridine; ρ, correlation coefficient. *P < 0.05.
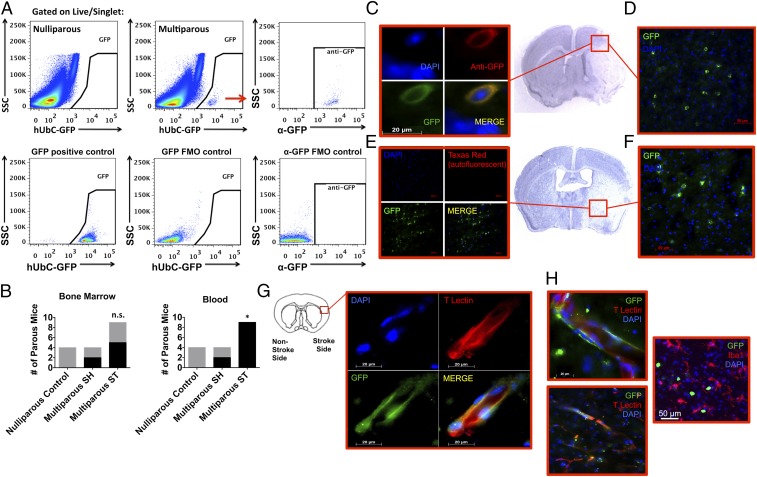
We then investigated whether fetal microchimeric cells are responsive to ischemic stimuli and migrate to areas of brain injury. Using a mating scheme designed to enable tracking of GFP+/− fetal cells in (wild type) maternal mice we subjected mice to stroke and examined acute and long-term survival. The frequency of GFP+ fetal cells in maternal circulation was significantly increased 24 h following stroke (Fig. 8B; P ≤ 0.05), cells that are likely bone marrow derived. In those females in which fetal microchimerism was detected, frequencies of GFP+ fetal cells in the blood ranged from 0.007% to 0.008% in sham to 0.001–0.267% after stroke, whereas frequencies ranged between 0.001% and 0.002% in the bone marrow for both groups. Fetal cells were confirmed using anti-GFP antibody and negative selection based on autofluorescence (Fig. 8 A, C, and E). Immunohistochemistry revealed the presence of GFP+ fetal cells throughout the anterior/posterior axis of the ischemic hemisphere at 72 h, when brain inflammation reaches its height (Fig. 8 E and F). Although sparsely distributed and in too low number to quantify, these cells had diverse phenotypes and were found in clusters mostly confined within the infarcted region. On the working assumption that fetal cells have stem cell potential, we examined the presence of these cells at 30 d after stroke when neurogenic and angiogenic recovery processes are more active. Interestingly, a small number of GFP+ fetal cells within the ischemic region adopted endothelial features, had vessel morphology, and lectin expression, whereas others had more rounded morphology (Fig. 8 G and H). These data suggest that fetal cells are responsive to ischemic stroke in parous females and can mobilize and migrate to areas of tissue injury where they may contribute to the angiogenic response.
Fig. 8.
Fetal microchimeric cells were found in the maternal brain after ischemic injury. Low-frequency GFP+ fetal microchimeric cells were seen in maternal blood, bone marrow, and brain after ischemic stroke. Representative dot plots demonstrate the presence of GFP-positive fetal cells in maternal, but not nulliparous, bone marrow (A). The colabeling of endogenous GFP expression with anti-GFP+ fetal cells is consistent with the staining pattern found in transgenic GFP-expressing mice [A; positive and cell-specific fluorescence minus one (FMO) controls]. The red arrow refers to the GFP-positive cells in the Top Middle plot which were subsequently evaluated by SSC vs. anti-GFP in the Top Right plot. As shown in B, the frequency of parous females in which circulating GFP+ fetal cells (of total live CD45+) were detected was significantly increased at 24 h after stroke (SH = 0.007–0.008% vs. ST = 0.001–0.267%), whereas no change was found for those detected in bone marrow (SH = 0.002% vs. ST = 0.001%). Representative immunohistochemistry reveals clustering of GFP+ fetal cells in affected cortical (D) and striatal (F) brain regions across the anterior/posterior axis. (Magnification: D and F, 40×.) Validation of GFP specificity was proven using an anti-GFP antibody (C) and by the absence of autofluorescence (E). (Magnification: C, 100×; E, 20×.) Fetal cells persisted in the maternal brain for up to 30 d after stroke (G and H). At this later timepoint, GFP+ fetal cells adopted endothelial properties as evidenced by elongated morphology and tomato lectin staining (G and H). Our data shows that some fetal cells exhibit amoeboid morphology, but did not express markers associated with microglia/macrophages (H). Error bars show mean SEM. (Magnification: G, 100×; H, Top, 100×; H, Bottom and Right, 63×.) FM, fetal microchimerism; n.s., not significant; SSC, side light scatter. *P < 0.05.
Discussion
In this work, we demonstrated that parity negatively impacts baseline behavioral performance, CNS immune function, and biomarkers associated with cardiovascular health. Despite these changes, parity conferred both an acute and long-term protective advantage against ischemic brain injury, resulting in smaller infarct volumes, increased VEGF levels, greater revascularization, and fewer behavioral deficits after stroke. Our results suggest that pregnancy-associated changes in immunity and events at the fetal–placental interface have long-term consequences in the healthy and injured maternal brain.
Our understanding of parity-related changes in both the brain’s normal function and its response to injury is poor, largely due to the lack of experimental modeling. To date, studies have been focused on understanding the effects of parity (e.g., diet, obesity, inflammation) on the long-term brain health of offspring rather than on the mother herself. It has been posited that perturbations in brain inflammation disrupt satiety signals in the hypothalamus, contributing to the high incidence of maternal obesity (13). Our data suggest that inflammation and microglia/macrophage activity are chronically suppressed in the brain of multiparous mice, consistent with the systemic immune suppression induced by pregnancy seen by others (14). Although parous and nonparous mice had similar estrogen levels, protection could still be mediated by other sex hormones that are increased at the time of pregnancy such as progesterone and human chorionic gonadotropin (hCG) (15, 16). A growing literature now supports a prominent role for fetal-derived hCG in promoting immune tolerance during pregnancy and transplantation (17). It is possible that these hormones prime the maternal immune system to respond less vigorously to injury-driven danger signals.
As with immune tolerance, many other physiological features of the maternal environment adapt to accommodate and support in utero life. High systemic levels of growth factors and antiinflammatory cytokines have been found in pregnant women and may underlie the reported enhancement of liver and muscle regeneration during pregnancy (18, 19). To this end, we also demonstrated higher growth factor expression and greater antioxidant defenses in naïve multiparous mice. Parabiosis of pregnant mice with nonpregnant female controls confers a regenerative benefit in virgin muscle, and emerging data suggest reproductive experience bestows lifelong benefits (20). Whereas nulliparous mice exhibited sharp reductions in systemic IL-4 and IL-10 concentrations, bFGF and VEGF concentrations remained relatively elevated in multiparous mice following stroke. Our results are consistent with prior work that showed hippocampal excitotoxicity-induced cell damage was lower in multiparous females following kainic acid injection compared with virgins (21). In our study, reproductive experience initiated a protective response to sterile brain injury, mitigating both neuronal injury and glial activation.
Reproductive experience, and to some extent parity, has been shown to improve learning and memory performance in mothers long after parturition (22). These cognitive advantages persist throughout the lifespan. We found that parity enhanced sensorimotor and cognitive function at chronic time points after stroke. Whereas our study assesses ischemic stroke outcomes in multiparous animals and highlights the lasting effects of pregnancy on the maternal immune system, another group has examined stroke outcomes in pregnant females. Spencer (23) reported that neuronal injury was greater in pregnant rats compared with nonpregnant females following global cerebral ischemia. Stroke risk is highest for women during pregnancy and the early postpartum period compared with any other time in her life (24). In contrast, immune-mediated disease and multiple sclerosis symptoms are attenuated during pregnancy (25) and pregnancy-associated febrile responses to immune challenges are also significantly suppressed. Corticosterone is one candidate hormone that potentially mediates these effects as it is altered during pregnancy, is neuroprotective in models of cerebral ischemia (26), and increases myelopoiesis in the bone marrow, augmenting the number of granulocytes in peripheral blood (27). However, cortisol prevents the release of proinflammatory cytokines and up-regulates antiinflammatory cytokines such as IL-4 and IL-10, promoting immune suppression. We found significant increases in cortisol concentration in multiparous females after stroke, which paralleled increases in IL-4 and IL-10. The maternal hypothalamic–pituitary–adrenal (HPA) axis undergoes significant activation during pregnancy, but how long these effects are sustained and whether enhanced glucocorticoid production is responsible for attenuating neuronal death and glial activation in multiparous females following stroke is not known.
Major effects of pregnancy on cerebral endothelial and microcirculation have been described, including increased vascular permeability (28). VEGF is not only increased in circulating blood during pregnancy, but is also up-regulated in cerebral veins (29). Our findings that reproductive experience enhanced vascularization after ischemic injury suggests that VEGF is an important mediator of parity-induced protection. Multiparous females demonstrated greater proliferation and branching of vessels after stroke. No overt differences are seen in perfusion or in the vasculature at baseline, but it seems likely that poststroke angiogenic repair processes are simply not as robust, or perhaps are even impaired, in virgin female mice. We also found a direct correlation between early motor performance and angiogenesis. Animals with fewer motor deficits had the most robust angiogenesis at chronic time points. Although behavioral performance in motor tasks was worse in multiparous females at baseline, these mice were less affected by stroke compared with their virgin counterparts, which displayed significant deficits in functional recovery. It is important to note that the improved behavioral recovery in multiparous females may simply reflect the smaller infarct seen in these mice rather than an enhanced delayed angiogenic response. Behavioral deficits and recovery are known to be related to the location and severity of the initial stroke injury (30, 31). However, the near complete preservation of sensorimotor and cognitive function in the multiparous mice is nonetheless impressive, given that the striatal infarct was prominent in both groups. In addition, scores on the corner and rotarod tests were similar in both groups at acute time points (72 h), suggesting that some of the benefit is due to improved recovery rather than acute neuroprotection. The beneficial effect of parity also was “dose dependent” in that females with the highest number of pregnancies (which varied from four to six litters per mouse) had improved behavioral recovery at day 7, an association that was not seen at day 3, suggesting a neurorestorative effect rather than a neuroprotective effect.
Angiogenesis and vascular integrity have profound implications on neuroinflammation and brain repair following ischemia. Increased leakiness of the blood–brain barrier may facilitate the entry of the maternal systemic factors, including growth factors (e.g., VEGF, placental growth factor), hormones (e.g., estrogen, prolactin), Th2-related cytokines (e.g., IL-4, IL-10), and fetal cells into the maternal brain. Microglial exposure to these factors could explain the microglia and macrophage suppressive phenotype seen in our study. Microglia/macrophages from multiparous females exhibit comparably suppressed homeostatic immune functions, producing lower levels of cytokines and displaying a lower potential to phagocytize material. Glial cell size was increased in the multiparous brain after stroke, suggesting that parity can chronically alter cellular cytoarchitecture and prime microglia toward an M2-like activation state following injury. Whereas depressive behavior and deficits in hippocampal neurogenesis are often associated with systemic immunosuppression in the postpartum period (32), it is well established that antiinflammatory environments and M2-like polarization of activated microglia generally favor positive outcomes following ischemic stroke (33). Consistent with this understanding, others have found that peritoneal macrophages from primiparous females exhibited less of a PMA-induced oxidative burst than nulliparous macrophages (34). The shift toward greater immune tolerance during pregnancy, which is further enhanced with increasing parity, likely underlies these cellular differences in inflammatory function.
Cellular interactions between the mother and fetus at the placental interface also have vast implications for long-term health. During pregnancy, there is a bidirectional trafficking of cells across the placental barrier between the mother and her fetus. Whereas the presence of cells of fetal origin are highest within the mother’s blood stream during gestation at embryonic days, e18 and e19, the cells can persist at lower frequencies for over 27 y after parturition (35, 36). Stroke can induce recruitment of progenitor cells for neovascularization from the bone marrow in rodents and previous studies have shown that human umbilical cord blood and amniotic stem cells are neuroprotective when introduced to rodents after middle cerebral artery occlusion (MCAO) (37, 38). The majority of fetal microchimerism studies have examined the presence and to a lesser extent, the function, of MCs. Although studies indicate the maternal brain has the lowest frequency of fetal microchimerism of all organs (39, 40), MCs have been found in the CNS in both healthy and diseased humans and mice (41). Indeed, the rarity of these cells precluded our efforts to perform quantification and more comprehensive phenotypic analysis. To date, only two brain injury models have examined fetal MCs, one in a model of Parkinson’s disease induced before pregnancy, and the other after local NMDA injection (42, 43). Both studies found the injured brains had increased numbers of fetal cells that later developed a neuronal morphology. We examined an early time point at 72 h to capture the peak of poststroke brain inflammation, with the highest trafficking and infiltration of peripheral leukocytes and progenitor cells into areas of ischemic injury (44). Studies have documented that fetal cells primarily populate the bone marrow (which is activated by stroke via the sympathetic nervous system), and that these cells can mobilize into circulation and enter target tissues in various animal models of cardiac, hepatic, renal, epidermal, and brain injury (45–47). Kara et al. showed that MCs in a mouse model of myocardial infarction selectively home to the injured myocardium and differentiate into a variety of reparative cell types that were functional in vitro (48, 49). Thus, the parous female bone marrow may house an endogenous population of stem cells of fetal origin that can potentially provide regenerative support. The functional contribution of fetal cells to recovery in the injured maternal brain is not known and could be minimal, given their sparse, sporadic distribution. Whether these cells contribute to the proangiogenic environment in the maternal brain warrants further investigation.
In conclusion, we demonstrated that parity has paradoxical effects in health and disease, mirroring the clinical literature. Multiparous mice exhibit increased body weights, elevated triglyceride and cholesterol levels, significant immune suppression, greater sedentary behavior, and muscle fatigue. Whereas these attributes are generally associated with higher metabovascular risk, multiparous females demonstrated a surprising resistance to ischemic brain injury and improved behavioral recovery at chronic time points after stroke. Parity-associated neuroprotection may have evolved out of necessity, ensuring the survival of young offspring. Increased VEGF in multiparous animals appears to lead to an environment that is conducive to poststroke angiogenesis and repair after injury. These studies could help us understand the etiology and pathology of stroke risk and recovery in females.
Materials and Methods
Mice/Animals.
Young adult female mice (C57BL/6J; 12 wk) were pair housed with male (Harlan Laboratories) or female partners in a pathogen-free facility (light cycle 12/12 h light/dark). The number of litters ranged from four to six (Avg = 5.08 ± 0.28) with the average total number of pups birthed of 40.3 ± 3.9. Pups were weaned after 21 d. Nulliparous females did not have litters. All mice were subject to surgery and killed at 12 mo of age, 3–4 mo following the last pregnancy. Young adult C57BL/6J wild type (WT) and C57BL/6-tg(UBC-GFP)30Scha mice were purchased from The Jackson Laboratory. To generate fetal microchimeric mice, male GFP+/+ mice were mated with virgin female WT mice to yield GFP+/− offspring (50). All procedures were performed in accordance with NIH Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Connecticut Health Center and the University of Texas Health Science Center at Houston. All efforts were made to reduce animal suffering. All analysis was performed blinded to surgical conditions and parity.
Ischemic Stroke Model.
Cerebral ischemia was induced by 60 min of reversible unilateral MCAO (25.3- to 39.2-g mice) under isoflurane anesthesia as previously described (51). Rectal temperatures were maintained at ∼37 °C during surgery and ischemia with an automated temperature control feedback system. Due to weight adjusted variation in vessel diameter, 0.21 mm and 0.23 mm silicone-coated sutures were used in mice weighing <30 g and >30 g, respectively, to achieve equivalent occlusion, as described previously (52). Sham-operated animals underwent the same surgical procedure, but the suture was not advanced into the middle cerebral artery. Two independent experiments were performed and the pooled data are shown. Cerebral blood flow was assessed by laser speckle perfusion imaging and vascular anatomy was examined by India ink staining as described in SI Materials and Methods.
Clinical Assessment.
Body weight was measured 3 d before surgery, immediately before surgery, and monitored daily thereafter. Rectal temperatures were recorded during ischemia and immediately before killing. Neurological deficits were assessed by Bederson score from 0 (no deficit) to 4 (severe deficit) with minor modifications (53).
Bromodeoxyuridine Injections.
Bromodeoxyuridine (BrdU) (Sigma-Aldrich) was freshly prepared daily and administered intraperitoneally at 50 mg/kg/d at days 3–7 after stroke to identify newborn cells.
FITC-Lectin Staining.
To examine the cortical microvasculature, fluorescein (FITC)-labeled Lycopersicon esculentum lectin (Vector Labs) was diluted 1:1 with saline, and 200 μL of the solution was injected via the saphenous vein 5 min before killing (54).
Terminal Histopathology.
All animals were killed with avertin overdose (i.p). Transcardial perfusion was performed with cold PBS followed by 4% paraformaldehyde; the brain was fixed for 24 h and placed in cryoprotectant (30% sucrose). The brains were cut into 40-μm free-floating sections on a freezing microtome and every eighth slice was stained by cresyl violet staining to evaluate ischemic cell damage. Infarct volume was analyzed using computer software (Sigma scan Pro5) as previously described (55, 56).
Immunohistochemistry.
Immunohistochemical (IHC) staining of fixed-frozen sections (40-μm thickness) was performed as described previously (57). Detailed methods are described in SI Materials and Methods. Images were acquired with a Zeiss Axiovert 200 M microscope using a X-Cite 120Q fluorescence illumination system (Lumen Dynamics Group Inc.) and Zeiss image acquisition software (Zeiss LSM 510). For analysis of glial activation at 72 h, brain slices were taken at the same distance from bregma (0.5 mm anterior to bregma) to ensure comparison of similar structures. Two 20× fields per animal (n = 10–12 animals per group) were analyzed in the cortical penumbra area of the infarct. The total number of cells was averaged across the two fields of view for each animal and the average number of cells per field of view was used for statistical analysis as described previously (58). DAPI-counterstained Iba1+ and GFAP+ cells were counted using ImageJ software (NIH) by a blinded investigator.
For BrdU/FITC-lectin colabeling, brains were cut into 30-μm sections and blood vessels were costained with rat anti-BrdU (1:100; Abcam), followed by anti-rat secondary conjugated antibody and DAPI. Newborn cells in the infarct cortex were identified by colabeling with BrdU and DAPI. To perform quantification of BrdU+ cells, four coronal brain slices per animal were stained from ∼1.1 mm bregma, 0.8 mm bregma, 0.5 mm bregma and −0.1 mm bregma as described previously (59). AngioTool software (National Cancer Institute) was used to quantify total vessel length, the number of vascular endpoints and junctions, and the percentage of area vascularized from microscopic images collected from brain sections adjacent to BrdU-quantified sections (60).
Behavioral Testing.
All surgical cohorts were tested on each behavioral task twice, 3 d before surgery to establish a baseline reference and again on the day of killing at a fixed time in the morning. All behavioral testing equipment was cleaned with 70% ethanol between animals. Naïve mice were tested in the following order: open field, static rod, rotarod, wirehang, and tail suspension test. For all 72-h surgical cohorts, mice were tested 3 d before surgery and 3 d after reperfusion before killing in the following order: neurological scoring, open field, rotarod, and wire hang test. Two independent cohorts of mice (n = 10 per group) were evaluated across the span of 30 d after stroke to assess long-term recovery. In the first cohort, mice were tested at days −3, 3, 7, 14, 21, and 28 with the corner test and days −3, 3, 15, and 30 on the wire hang test. In the second cohort of mice, mice were tested at days −3, 3, 7, 14, and 21 on the open field and rotarod test. Barnes maze testing was performed on day 30, 4 h after training. Data are presented as both percentage of baseline and as an absolute score. Detailed methods are described in SI Materials and Methods.
Tissue Processing for Flow Cytometry.
Mice were killed and blood was drawn by external cardiac puncture with heparinized needles. Before organ harvest, mice were transcardially perfused with 60 mL of cold, sterile PBS. Femurs were isolated and bone marrow was extracted with a flush of 10 mL of RPMI using a 21-gauge needle. Red blood cell lysis was achieved through three consecutive 10-min incubations with Tris-ammonium chloride. Brain hemispheres (right side) were processed as described (61). Phagocytosis was evaluated using fluorescent latex beads and intracellular staining was performed to assess cytokine production and endogenous GFP expression as described in SI Materials and Methods.
Enzyme-Linked Immunoabsorbant Assay (ELISA).
Brain and plasma cytokine concentrations were determined by ELISA (Bio-Plex Pro Mouse Cytokine Assay, Bio-Rad Laboratories) as previously reported (62). Briefly, 25 µL of plasma and 150 µg of whole cell lysate brain protein were aliquoted in each well. Samples were assayed per manufacturer’s instructions using a Luminex 200 magnetic bead array platform. Detailed methods are described in SI Materials and Methods.
Statistical Analyses.
Data from individual experiments are presented as mean ± SEM and statistically evaluated by Student’s t test (for comparison between two experimental groups) or two-way ANOVA with Tukey’s post hoc test for group effects and multiple comparisons (GraphPad Prism Software). Behavioral analysis of stroke groups was performed using repeated measures two-way ANOVA followed by Tukey or Bonferroni post hoc test. Spearman correlation analysis was performed for the variables of BrdU/lectin and behavioral performance on different days and behavioral performance and number of pregnancies. The incidence of fetal microchimerism in maternal mice was evaluated using the χ2 test. The neurological deficit scores, being ordinal in nature, were analyzed using the Mann–Whitney u test. Significance was set at P ≤ 0.05.
SI Materials and Methods
Cerebral Blood Flow Assessment with Laser Speckle.
Laser Speckle Perfusion imaging of the brain was performed using moorFLPI Full-Field Laser Perfusion Imager (Moor Instruments) as described previously (63). In brief, two identical rectangular regions of interest were selected on each of the two hemispheres. The imaging was set up at a display rate of 25 Hz, a time constant of 1 s, and a camera exposure time of 4 ms. A 2-min baseline CBF Flux was recorded before induction of ischemia. MCAO surgery was performed and live image and flux recordings were made throughout the intraischemic and postreperfusion periods. Data were expressed as percent change in mean CBF flux of 2 min of preischemic period, 50 min of intraischemic period, and 2 min of postreperfusion period.
Vascular Anatomy.
Both nulliparous and multiparous mice (n = 3 per group) were anesthetized by avertin injection and perfused via the left ventricle with cold phosphate-buffered heparinized saline and 4% paraformaldehyde followed by injection of 2 mL of India ink and elemental iron (1% FeSO4, 20% India ink in PBS). The mice were decapitated and the brains were harvested with the circle of Willis intact. The brains were placed in paraformaldehyde overnight at 4 °C and large vessel anatomy was examined (64).
IHC.
Brain slices were mounted onto gelatin-coated slides, air dried, and blocked in 0.1 M phosphate buffer (PB) with 0.3% Triton X-100 (Sigma) and 10% donkey serum [Pyrrolo[1,2-b][1,2,5]benzothiadiazepines (PBTDS)] for 1 h. Primary antibody [rabbit anti-GFP antibody (Abcam; 1:100); anti-Iba1 (Wako; 1:250); anti-GFAP (Dako; 1:500); and tomato lectin (Vector; 1:100)] was added overnight. After washes, the sections were incubated with secondary antibody (1:1,000) and 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI, 1:1,000; Invitrogen). Secondary antibody [Alexa-Fluor 594 donkey anti-rabbit IgG (Invitrogen); Alexa-Fluor 488 donkey anti-rabbit IgG (Invitrogen)] was removed with three consecutive washes in PBTDS, 0.1 M PB, and 0.05 M PB, respectively. GFP+ fetal cells were confirmed by positive selection using endogenous GFP and anti-GFP and negative selection using autofluorescent channels.
Open Field.
Mice were placed in an open field chamber (15 × 15 inches) equipped with 16 infrared beam emitting LEDs. Beam breaks were registered with the PAS Open Field system (San Diego Instruments). The total number of beam breaks or distance traveled (in centimeters) during a 20-min session was analyzed as a measure of spontaneous locomotor activity.
Static Rod.
Balance and coordination was assessed using a wooden rod of 60 cm in length and 2.8 cm in diameter (as in ref. 65) with minor modification. The number of falls and successful turns was tallied for each trial and the percentage was calculated.
Rotarod.
Mice were placed on a rotating rod accelerating from two rotations per minute (rpm) to 20 rpm, over a span of 5 min (66, 67). Subjects were given four trials on the rotarod on day −3 and +3 of testing. The latency of the subject to fall was recorded for each trial (in seconds), and the average latency was used for further analysis.
Tail Suspension.
The tail suspension test (TST) was performed as described previously (68). Mice were suspended 60 cm above a table and recorded for 6 min using a digital video camera (JVC Everio, Victor Company).
Hanging Wire.
The hanging wire tests both limb strength and balance after stroke (55, 69) and was performed as in ref. 70 with slight modification. The mouse was placed on the center of a wire cage top (18 inches × 9 inches) that was slowly inverted. The average time to fall from three trials was taken.
Corner Test.
To assess sensorimotor abnormalities and asymmetry, animals were evaluated using the corner test as in ref. 59. Twenty trials were performed for each mouse and the total number of right turns was counted. Although normal mice do not display a turning preference, after ischemia, mice will exhibit a turning preference to their nonimpaired side. Data are presented as total right turns/(total right + left turns).
Barnes Maze.
Mice were tested for spatial memory acquisition and retention on Barnes maze 120-cm diameter with 20 holes along the perimeter. The escape chamber remained at a stable position, and the walls around the arena were marked with visual cues. During the initial phase, the animal was guided to the escape hole using a transparent Plexiglas cylinder. Subsequently, each mouse was individually tested for two consecutive trials of 3 min each. The latency to first reach the target hole and distance traveled before entering the escape chamber was recorded and analyzed using the EthoVision video tracking software (Noldus).
Tissue Processing for Flow Cytometry.
Cells were washed and blocked with mouse Fc Block (clone 93, eBioscience) before staining with primary antibody-conjugated flourophores: CD45-eF450 (clone 30-F11), CD11b-APCeF780 (clone M1/70), CD3e-APC (clone 17A2), and Ly6C-PerCP-Cy5.5 (clone HK1.4). All antibodies were purchased from eBioscience. For live/dead discrimination, a fixable viability dye, carboxylic acid succinimidyl ester (CASE-AF350, Invitrogen), was diluted at 1:300 from a working stock of 0.3 mg/mL. Cells were briefly fixed in 2% paraformaldehyde (PFA). Data were acquired on a LSRII using FACsDiva 6.0 (BD Biosciences) and analyzed using FlowJo (Tree Star). A gating strategy was designed as described (61). Data represent the mean of individual values in each group.
Phagocytosis Assay.
Fluorescent latex beads [Fluoresbrite Yellow Green (YG) carboxylate microspheres; 1-μm diameter; Polysciences] were added to freshly isolated microglia in a final dilution of 1:100 as described (61).
Intracellular Cytokine Production.
For intracellular cytokine staining, a stock solution of brefeldin A (Sigma) was prepared at 20 mg/mL in DMSO and diluted with PBS to obtain a working solution of 0.5 mg/mL. Mice were killed 12 h after i.v. injection of brefeldin A (250 μL). Leukocytes were collected as described above, and 1 μL of GolgiPlug containing brefeldin A (BD Biosciences) was added to 800 μL complete RPMI. Cells were incubated and processed as described (61). Cells were resuspended in an intracellular antibody mixture containing TNF-PE-Cy7 (clone MP6-XT22, eBioscience) and IL-1β–FITC (clone NJTEN3, eBioscience), and then fixed (71).
Intracellular staining with anti-GFP–AF647 was performed as above without in vitro brefeldin A incubation. Cell populations were gated in the following order: leukocyte, singlet, live, autofluorescence, and anti-GFP+. Bone marrow cells from GFP+/+ and wild-type mice were used for FMO controls to determine gating for endogenous GFP and each conjugated antibody. Blood cells from wild-type female mice were used as a negative control, whereas those from GFP+/− female mice were used as a positive control for GFP fetal cells. To eliminate the possibility of carryover, a blank sample containing PBS alone was run until no events could be detected before an additional sample was run on the cytometer.
Enzyme-Linked Immunoabsorbant Assay.
Plasma biomarkers were measured using a triglyceride colorimetric assay kit (10010303), a cholesterol fluorometric assay kit (10007640), and an antioxidant assay kit (709001) purchased from Cayman Chemical. Plasma concentrations of cortisol (K7430-100, Biovision) and estradiol (BQ180S; BQ kits) were assayed according to manufacturer’s instructions.
Acknowledgments
We gratefully acknowledge Brett Friedler and Dr. Diego Morales for technical assistance. This work was supported by National Institutes of Health Grants R21 NS090422-01A1 and R01NSO55215 (to L.D.M.) and F31 NS083244-01A1 (to R.M.R.) and American Heart Association Grants 15SDG23250025 (to V.R.V.) and 14POST20380612 (to R.V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607002114/-/DCSupplemental.
References
- 1.Mozaffarian D, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics: 2015 update: A report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Ritzel RM, Capozzi LA, McCullough LD. Sex, stroke, and inflammation: The potential for estrogen-mediated immunoprotection in stroke. Horm Behav. 2013;63:238–253. doi: 10.1016/j.yhbeh.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphries KH, et al. Parity and carotid artery atherosclerosis in elderly women: The Rotterdam Study. Stroke. 2001;32:2259–2264. doi: 10.1161/hs1001.097224. [DOI] [PubMed] [Google Scholar]
- 4.Skilton MR, Sérusclat A, Begg LM, Moulin P, Bonnet F. Parity and carotid atherosclerosis in men and women: Insights into the roles of childbearing and child-rearing. Stroke. 2009;40:1152–1157. doi: 10.1161/STROKEAHA.108.535807. [DOI] [PubMed] [Google Scholar]
- 5.Lv H, Wu H, Yin J, Qian J, Ge J. Parity and cardiovascular disease mortality: A dose-response meta-analysis of cohort studies. Sci Rep. 2015;5:13411. doi: 10.1038/srep13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaigobin C, Silver FL. Stroke and pregnancy. Stroke. 2000;31:2948–2951. doi: 10.1161/01.str.31.12.2948. [DOI] [PubMed] [Google Scholar]
- 7.Kittner SJ, et al. Pregnancy and the risk of stroke. N Engl J Med. 1996;335:768–774. doi: 10.1056/NEJM199609123351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamel H, et al. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med. 2014;370:1307–1315. doi: 10.1056/NEJMoa1311485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdul Sultan A, et al. Impact of risk factors on the timing of first postpartum venous thromboembolism: A population-based cohort study from England. Blood. 2014;124:2872–2880. doi: 10.1182/blood-2014-05-572834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeanty C, Derderian SC, Mackenzie TC. Maternal-fetal cellular trafficking: Clinical implications and consequences. Curr Opin Pediatr. 2014;26:377–382. doi: 10.1097/MOP.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutta P, Burlingham WJ. Microchimerism: Tolerance vs. sensitization. Curr Opin Organ Transplant. 2011;16:359–365. doi: 10.1097/MOT.0b013e3283484b57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmood U, O’Donoghue K. Microchimeric fetal cells play a role in maternal wound healing after pregnancy. Chimerism. 2014;5:40–52. doi: 10.4161/chim.28746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebholz SL, et al. Multiparity leads to obesity and inflammation in mothers and obesity in male offspring. Am J Physiol Endocrinol Metab. 2012;302:E449–E457. doi: 10.1152/ajpendo.00487.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Racicot K, Kwon JY, Aldo P, Silasi M, Mor G. Understanding the complexity of the immune system during pregnancy. Am J Reprod Immunol. 2014;72:107–116. doi: 10.1111/aji.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patas K, Engler JB, Friese MA, Gold SM. Pregnancy and multiple sclerosis: Feto-maternal immune cross talk and its implications for disease activity. J Reprod Immunol. 2013;97:140–146. doi: 10.1016/j.jri.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Lissauer D, et al. Progesterone promotes maternal-fetal tolerance by reducing human maternal T-cell polyfunctionality and inducing a specific cytokine profile. Eur J Immunol. 2015;45:2858–2872. doi: 10.1002/eji.201445404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schumacher A, et al. Human chorionic gonadotropin as a central regulator of pregnancy immune tolerance. J Immunol. 2013;190:2650–2658. doi: 10.4049/jimmunol.1202698. [DOI] [PubMed] [Google Scholar]
- 18.Falick Michaeli T, et al. The rejuvenating effect of pregnancy on muscle regeneration. Aging Cell. 2015;14:698–700. doi: 10.1111/acel.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gielchinsky Y, et al. Pregnancy restores the regenerative capacity of the aged liver via activation of an mTORC1-controlled hyperplasia/hypertrophy switch. Genes Dev. 2010;24:543–548. doi: 10.1101/gad.563110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinsley CH, Franssen RA, Meyer EA. Reproductive experience may positively adjust the trajectory of senescence. Curr Top Behav Neurosci. 2012;10:317–345. doi: 10.1007/7854_2011_123. [DOI] [PubMed] [Google Scholar]
- 21.Franssen RA, et al. Reproductive experience facilitates recovery from kainic acid-induced neural insult in female Long-Evans rats. Brain Res. 2012;1454:80–89. doi: 10.1016/j.brainres.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 22.Li R, et al. Early reproductive experiences in females make differences in cognitive function later in life. J Alzheimers Dis. 2013;34:589–594. doi: 10.3233/JAD-122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spencer SJ, Galic MA, Tsutsui M, Pittman QJ, Mouihate A. Effects of global cerebral ischemia in the pregnant rat. Stroke. 2008;39:975–982. doi: 10.1161/STROKEAHA.107.497016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bashiri A, et al. Maternal and neonatal outcome following cerebrovascular accidents during pregnancy. J Matern Fetal Neonatal Med. 2007;20:241–247. doi: 10.1080/14767050601135030. [DOI] [PubMed] [Google Scholar]
- 25.Schwendimann RN, Alekseeva N. Gender issues in multiple sclerosis. Int Rev Neurobiol. 2007;79:377–392. doi: 10.1016/S0074-7742(07)79017-7. [DOI] [PubMed] [Google Scholar]
- 26.Tuor UI, Del Bigio MR. Protection against hypoxic-ischemic damage with corticosterone and dexamethasone: Inhibition of effect by a glucocorticoid antagonist RU38486. Brain Res. 1996;743:258–262. doi: 10.1016/s0006-8993(96)01054-2. [DOI] [PubMed] [Google Scholar]
- 27.Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol. 2004;148:106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Cipolla MJ. The adaptation of the cerebral circulation to pregnancy: Mechanisms and consequences. J Cereb Blood Flow Metab. 2013;33:465–478. doi: 10.1038/jcbfm.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreurs MP, Houston EM, May V, Cipolla MJ. The adaptation of the blood-brain barrier to vascular endothelial growth factor and placental growth factor during pregnancy. FASEB J. 2012;26:355–362. doi: 10.1096/fj.11-191916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–2065, discussion 2066. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- 31.Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Exp Transl Stroke Med. 2010;2:13. doi: 10.1186/2040-7378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pawluski JL, Galea LA. Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience. 2007;149:53–67. doi: 10.1016/j.neuroscience.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 33.Siniscalchi A, et al. Anti-inflammatory strategies in stroke: A potential therapeutic target. Curr Vasc Pharmacol. 2016;14:98–105. doi: 10.2174/1570161113666150923111329. [DOI] [PubMed] [Google Scholar]
- 34.Carvalho-Freitas MI, et al. Reproductive experience modifies dopaminergic function, serum levels of prolactin, and macrophage activity in female rats. Life Sci. 2007;81:128–136. doi: 10.1016/j.lfs.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 35.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93:705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujiki Y, Johnson KL, Tighiouart H, Peter I, Bianchi DW. Fetomaternal trafficking in the mouse increases as delivery approaches and is highest in the maternal lung. Biol Reprod. 2008;79:841–848. doi: 10.1095/biolreprod.108.068973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan T, et al. Neurorestorative therapy of stroke in type 2 diabetes mellitus rats treated with human umbilical cord blood cells. Stroke. 2015;46:2599–2606. doi: 10.1161/STROKEAHA.115.009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elias M, et al. Stroke therapy: The potential of amniotic fluid-derived stem cells. Future Neurol. 2015;10:321–326. doi: 10.2217/FNL.15.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujiki Y, Johnson KL, Peter I, Tighiouart H, Bianchi DW. Fetal cells in the pregnant mouse are diverse and express a variety of progenitor and differentiated cell markers. Biol Reprod. 2009;81:26–32. doi: 10.1095/biolreprod.108.074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujiki Y, et al. Quantification of green fluorescent protein by in vivo imaging, PCR, and flow cytometry: Comparison of transgenic strains and relevance for fetal cell microchimerism. Cytometry A. 2008;73:11–118. doi: 10.1002/cyto.a.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan WF, et al. Male microchimerism in the human female brain. PLoS One. 2012;7:e45592. doi: 10.1371/journal.pone.0045592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan XW, et al. Fetal microchimerism in the maternal mouse brain: A novel population of fetal progenitor or stem cells able to cross the blood-brain barrier? Stem Cells. 2005;23:1443–1452. doi: 10.1634/stemcells.2004-0169. [DOI] [PubMed] [Google Scholar]
- 43.Zeng XX, et al. Pregnancy-associated progenitor cells differentiate and mature into neurons in the maternal brain. Stem Cells Dev. 2010;19:1819–1830. doi: 10.1089/scd.2010.0046. [DOI] [PubMed] [Google Scholar]
- 44.Gelderblom M, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 45.Florim GM, et al. Fetal microchimerism in kidney biopsies of lupus nephritis patients may be associated with a beneficial effect. Arthritis Res Ther. 2015;17:101. doi: 10.1186/s13075-015-0615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seppanen E, et al. Distant mesenchymal progenitors contribute to skin wound healing and produce collagen: Evidence from a murine fetal microchimerism model. PLoS One. 2013;8:e62662. doi: 10.1371/journal.pone.0062662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy E, et al. Biphasic recruitment of microchimeric fetal mesenchymal cells in fibrosis following acute kidney injury. Kidney Int. 2014;85:600–610. doi: 10.1038/ki.2013.459. [DOI] [PubMed] [Google Scholar]
- 48.Kara RJ, et al. A mouse model for fetal maternal stem cell transfer during ischemic cardiac injury. Clin Transl Sci. 2012;5:321–328. doi: 10.1111/j.1752-8062.2012.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kara RJ, et al. Fetal cells traffic to injured maternal myocardium and undergo cardiac differentiation. Circ Res. 2012;110:82–93. doi: 10.1161/CIRCRESAHA.111.249037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, et al. Fetal cells in mother rats contribute to the remodeling of liver and kidney after injury. Biochem Biophys Res Commun. 2004;325:961–967. doi: 10.1016/j.bbrc.2004.10.105. [DOI] [PubMed] [Google Scholar]
- 51.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: Male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 52.Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29:792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bederson JB, et al. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 54.Venna VR, Li J, Hammond MD, Mancini NS, McCullough LD. Chronic metformin treatment improves post-stroke angiogenesis and recovery after experimental stroke. Eur J Neurosci. 2014;39:2129–2138. doi: 10.1111/ejn.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X, et al. Chronic behavioral testing after focal ischemia in the mouse: Functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Liu F, Schafer DP, McCullough LD. TTC, fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manwani B, et al. Functional recovery in aging mice after experimental stroke. Brain Behav Immun. 2011;25:1689–1700. doi: 10.1016/j.bbi.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu F, Akella P, Benashski SE, Xu Y, McCullough LD. Expression of Na-K-Cl cotransporter and edema formation are age dependent after ischemic stroke. Exp Neurol. 2010;224:356–361. doi: 10.1016/j.expneurol.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venna VR, Xu Y, Doran SJ, Patrizz A, McCullough LD. Social interaction plays a critical role in neurogenesis and recovery after stroke. Transl Psychiatry. 2014;4:e351. doi: 10.1038/tp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zudaire E, Gambardella L, Kurcz C, Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS One. 2011;6:e27385. doi: 10.1371/journal.pone.0027385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ritzel RM, et al. Functional differences between microglia and monocytes after ischemic stroke. J Neuroinflammation. 2015;12:106. doi: 10.1186/s12974-015-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ritzel RM, et al. Age- and location-related changes in microglial function. Neurobiol Aging. 2015;36:2153–2163. doi: 10.1016/j.neurobiolaging.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 63.Manwani B, et al. Perfusion of ischemic brain in young and aged animals: A laser speckle flowmetry study. Stroke. 2014;45:571–578. doi: 10.1161/STROKEAHA.113.002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manwani B, et al. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J Cereb Blood Flow Metab. 2015;35:221–229. doi: 10.1038/jcbfm.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deacon RM. Measuring motor coordination in mice. J Vis Exp. 2013;(75):e2609. doi: 10.3791/2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bouët V, et al. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol. 2007;203:555–567. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 67.Truong DT, Venna VR, McCullough LD, Fitch RH. Deficits in auditory, cognitive, and motor processing following reversible middle cerebral artery occlusion in mice. Exp Neurol. 2012;238:114–121. doi: 10.1016/j.expneurol.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 68.Verma R, Friedler BD, Harris NM, McCullough LD. Pair housing reverses post-stroke depressive behavior in mice. Behav Brain Res. 2014;269:155–163. doi: 10.1016/j.bbr.2014.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abe T, et al. The neuroprotective effect of prostaglandin E2 EP1 receptor inhibition has a wide therapeutic window, is sustained in time and is not sexually dimorphic. J Cereb Blood Flow Metab. 2009;29:66–72. doi: 10.1038/jcbfm.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hattori K, et al. Cognitive deficits after focal cerebral ischemia in mice. Stroke. 2000;31:1939–1944. doi: 10.1161/01.str.31.8.1939. [DOI] [PubMed] [Google Scholar]
- 71.Yin Y, Mitson-Salazar A, Prussin C. Detection of intracellular cytokines by flow cytometry. Curr Protoc Immunol. 2015;110:Unit 6.24. doi: 10.1002/0471142735.im0624s110. [DOI] [PubMed] [Google Scholar]