Abstract
The temporal and spatial regulation of cytokinesis requires an interaction between the anaphase mitotic spindle and the cell cortex. However, the relative roles of the spindle asters or the central spindle bundle are not clear in mammalian cells. The central spindle normally serves as a platform to localize key regulators of cell cleavage, including passenger proteins. Using time-lapse and immunofluorescence analysis, we have addressed the consequences of eliminating the central spindle by ablation of PRC1, a microtubule bundling protein that is critical to the formation of the central spindle. Without a central spindle, the asters guide the equatorial cortical accumulation of anillin and actin, and of the passenger proteins, which organize into a subcortical ring in anaphase. Furrowing goes to completion, but abscission to create two daughter cells fails. We conclude the central spindle bundle is required for abscission but not for furrowing in mammalian cells.
INTRODUCTION
Cytokinesis is the mechanism by which the genetic complement and the cellular cytoplasm are accurately and permanently segregated at the end of mitosis. Its proper completion is fundamental to the maintenance of the euploid genome and thus involves a coordinated series of changes whose temporal and spatial accuracy cannot be left to chance.
Cytokinesis occurs through the interaction of actin and myosin II on the cell cortex at the position of the spindle equator during late anaphase, creating the contractile event that ultimately separates the two daughter cells (Mabuchi, 1986). Evidence obtained from various systems, including invertebrate embryos and mammalian culture cells, demonstrates that a microtubule-dependent process transmits the signal for cytokinesis (Rappaport, 1986; Cao and Wang, 1996). Signaling communicates the spindle position to the cell cortex, to ensure that effective furrowing occurs at the precise midpoint of the spindle after the chromatid sets are well separated.
The anaphase spindle has two distinct components, astral arrays of microtubules that emanate from the two spindle poles and that do not interact, and a central spindle that is comprised of an array of tightly bundled antiparallel microtubules that arise from the two spindle poles and interdigitate in the equatorial region of the cell. The relative importance of the two microtubule arrays to furrowing seems to be divergent in different systems. Sea urchin embryos and Caenorhabditis elegans cells seem to require astral arrays for furrowing, whereas Drosophila melanogaster cells require the central spindle (for review, see Glotzer, 2004). This divergence may be due to differences in embryonic and nonembryonic cell cleavage mechanisms.
Mechanisms of cell cleavage are highly variable. Therefore, results in C. elegans or D. melanogaster do not necessarily predict cleavage requirements in mammalian cells. In mammalian cells the bulk of evidence, based on micromanipulation studies, supports an important role for the central spindle in furrow formation (Cao and Wang, 1996). On the other hand, it has been demonstrated that astral microtubules also can play a role in the positioning of cleavage apparatus (Rieder et al., 1997) and of the passenger proteins in mammalian cells (Murata-Hori and Wang, 2002). Interestingly, the absence of overlapping microtubules does not inhibit passenger protein localization or furrowing in cells with monopolar spindles (Canman et al., 2003), suggesting the central spindle is not required for furrowing.
The central spindle, created by PRC1 (Jiang et al., 1998; Mollinari et al., 2002; Kurasawa et al., 2004) and the central-spindlin complex, consisting of a kinesin-like protein ZEN-4/MKLP1 and a Rho-family GAP, CYK4/MgcRacGAP (Mishima et al., 2002), serves as a platform that enables the proper positioning at the anaphase spindle equator of proteins required for furrow progression. Such proteins include the passenger proteins, a group of proteins (INCENP, Survivin, Aurora B, Borealin, and TD-60) that seem to play a key role in the proper completion of cytokinesis in mammalian cells (Martineau et al., 1995; Mackay et al., 1998; Skoufias et al., 2001; Terada, 2001; Gassmann et al., 2004; Sampath et al., 2004).
Using a combination of small interfering RNA (siRNA) ablation of PRC1 and both time-lapse and immunofluorescence microscopy, we have here addressed the role of the central spindle bundle in furrow completion and in final cell abscission. We have found that furrowing proceeds to completion in the absence of the central spindle bundle, followed by failure of abscission, and reversion of the furrow an hour later to create a binucleate cell. In the absence of the anaphase spindle bundle, the passenger proteins organize into a subcortical ring instead of forming a telophase disc. This ring coincides with the deposition of actin and anillin at the cell cortex and thus coincides with the position of the cleavage event. The passenger proteins seem to be most prominent near the tips of astral microtubules. Our observations lead us to conclude that the central spindle bundle is not obligatory for completion of furrowing in mammalian cells but is required for the final abscission event.
MATERIALS AND METHODS
Cell Culture
HeLa cells were grown as a monolayer in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT), and maintained in a humid incubator at 37°C in a 5% CO2 environment. When appropriate, 1 mg/ml geneticin was added to the medium for selection purposes.
siRNA Oligonucleotides and Transfection
We targeted the human PRC1 cDNA sequence with RNA oligomers (Dharmacon Research, LaFayette, CO) as described previously (Mollinari et al., 2002). HeLa cells, plated on poly-d-lysine–coated coverslips or glass-bottom dishes, were transfected with RNA oligomers by using Oligofectamine 2000 (Invitrogen) in Optimem (Invitrogen) medium. The transfection conditions were modified depending on the dish size, according to manufacturer's instructions. Twenty-four hours after transfection, HeLa cells on coverslips were fixed and analyzed by immunofluorescence microscopy. For double transfection of RNA oligomers and the hemagglutinin (HA)-Survivin construct (Skoufias et al., 2000), Lipofectamine (Invitrogen) in Optimem was used.
Immunofluorescence Microscopy
Cells, grown on poly-d-lysine–coated glass coverslips for immunofluorescence microscopy, were fixed with 2% paraformaldehyde-phosphate-buffered saline for 20 min at 37°C, washed 5 min with phosphate-buffered saline (PBS), permeabilized with 0.1% Triton X-100 in PBS for 3 min, and washed three times for 5 min with PBS. Immunofluorescence was also performed on HeLa cells permeabilized 30 s at room temperature before fixation. Cells were then processed with primary and secondary antibodies and counterstained with TOTO3, a dimeric cyanine nucleic acid stain (Molecular Probes, Eugene, OR) or 4,6-diamidino-2-phenylindole (DAPI). The PRC1 affinity-purified rabbit antibody (Jiang et al., 1998) and JH human autoimmune serum (Andreassen et al., 1991) were diluted 500 times. The rabbit antibodies against MKLP1 (Santa Cruz Biotechnology, Santa Cruz, CA), human anillin (a kind gift from Dr. Chris Field, Harvard University, Cambridge, MA), RB6K and MgcRacGAP (kind gifts from Dr. Bruno Goud, Institute Curie, Paris, France, and Dr. Toshio Kitamura, University of Tokyo, Tokyo, Japan, respectively), and Survivin (Novus Biochemical, Littleton, CO) were diluted 250 times. The monoclonal anti AIM-1 (Aurora B) antibody (BD Transduction Laboratories, Lexington, KY) was diluted 300 times. The mouse monoclonal antibody (mAb) against CENP-E (Chan et al., 1999) was diluted 200 times. The anti-tyrosinated α-tubulin mAb (Lafanechere et al., 1998) was diluted 300 times. For actin staining, rhodamine phalloidin (Molecular Probes) was added to fixed HeLa cells for 10 min at room temperature. Secondary antibodies, including fluorescein isothiocyanate-conjugated affinity-purified donkey anti-mouse, rat, and rabbit IgG; Cy5-conjugated affinity-purified donkey anti-mouse and rabbit IgG; Cy3-conjugated affinity-purified donkey anti-mouse and rabbit IgG; and rhodamine-conjugated antihuman IgG, were all used at 2.5 μg/ml (Jackson ImmunoResearch Laboratories, West Grove, PA).
Images were collected either with a 2-photon Zeiss or a Nikon EZ-C1 confocal microscope. Images shown in this work represent projections of multiple scans across the entire cell (0.8-μm step, unless specified in the figure legend), or are mid-cell optical sections.
Western Blots
For Western blots, HeLa cells were trypsinized 24 h after transfection, extracted in SDS-PAGE sample buffer, subjected to PAGE, and transferred to nitrocellulose sheets as described previously (Mollinari et al., 2002). Blots were exposed to antibodies described above for immunofluorescence, including rabbit polyclonal anti-PRC1 (1:1000), anti-MgcRacGAP (1:1000), anti-RB6K (1:500), and anti-MKLP1 (1:1000); and mouse monoclonal anti-Aurora B (1: 500). Blots were then exposed to horseradish peroxidase-conjugated donkey secondary antibodies (1:2500; Tago Biosource International, Camarillo, CA, or Jackson ImmunoResearch Laboratories, 1:1000), for 1 h and then developed with enhanced chemiluminescence (Pierce Chemical, Rockford, IL).
Time-Lapse Microscopy
HeLa cells were transfected with phosphorylated enhanced green fluorescent protein (pEGFP)-α-tubulin (a kind gift from Dr. Patricia Wadsworth, University of Massachusetts, Amherst, MA) by using FuGENE6 (Roche Diagnostics, Indianapolis, IN). Two days after transfection, cells were subjected to selection for stably integrated pEGFP-α-tubulin with 300 μg/ml G418 (Invitrogen). Two clonal rounds of selection were performed to select for green fluorescent protein (GFP)-α-tubulin–positive cells. For time-lapse microscopy, stable HeLa cells expressing GFP-α-tubulin were plated at 50% density in glass-bottom dishes (Nalge Nunc, Naperville, IL) in phenol red-free DMEM (Invitrogen) supplemented with 10 mM HEPES solution. One day after plating, cells were transfected as described above. At 24 h after transfection, fresh medium containing 10 mM HEPES and 10 ng/ml Hoechst 33342 was added to the cells for 10 min and then replaced by Hoechst-free medium before observation. A Zeiss Axiovert microscope equipped with a piezoelectric focus and a CoolSnap HQ charge-coupled device camera (Roper Scientific, Trenton, NJ) was used for image collection. The system was controlled by MetaMorph software (Universal Imaging, Downingtown, PA). Wide-field images in two fluorescence channels, plus phase contrast, were collected as a series of 5–10 Z-steps every 30 s during rapid anaphase or 10 min for longer observations during telophase. Image analysis was performed using MetaMorph and Volocity LE software (Improvision, Lexington, MA).
RESULTS
Time-Lapse Imaging of HeLa Cells in the Absence of PRC1 and the Central Spindle
Suppression of PRC1 function by antibody microinjection and by siRNA ablation causes cell cleavage failure. However, furrowing is frequently evident in anaphase cells in the absence of a central spindle bundle (Mollinari et al., 2002), suggesting that cell furrowing can at least initiate in some cells after loss of PRC1 and the central spindle.
Using real-time imaging, we now demonstrate that furrowing in the absence of PRC1 and of the central spindle goes to completion in all cells that we have recorded (n = 30) and that the furrow subsequently reverts, forming a binucleate cell. For a proper analysis of the phenotype associated with the lack of PRC1 activity, we have used a HeLa cell line stably expressing GFP-α-tubulin. Cells were transfected with siRNA targeting PRC1 and were used for in vivo imaging 24 h after transfection. We have previously shown that siRNA strongly suppresses PRC1 expression in HeLa, leading to strong suppression of PRC1 on Western blots at 24 h posttransfection (Mollinari et al., 2002; Figure 2B). DNA was visualized using Hoechst 33342, which is cell permeable in the growth medium. Figure 1A shows time frame images from a typical recording (Video 1) of a cell, labeled with Hoechst (blue) and with GFP-α-tubulin (green), lacking a central spindle (see corresponding high-resolution images in Figure 2).
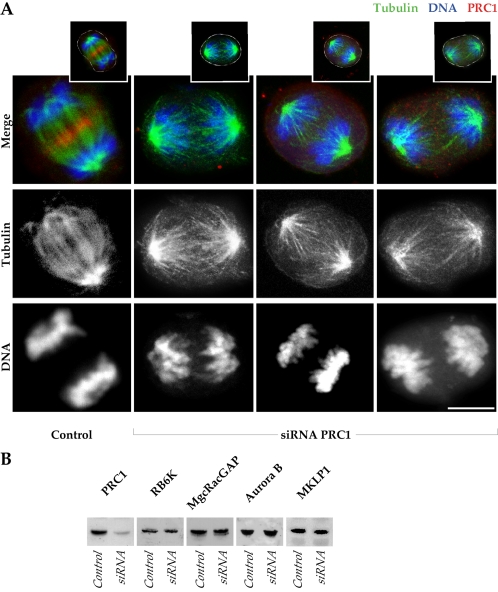
Figure 2.
Loss of the central spindle bundle but retention of anaphase spindle proteins after ablation of PRC1. (A) One control HeLa cell, and three examples of cells negative for PRC1 after siRNA ablation, are shown in anaphase (projections of 0.5-μm optical sections). In the merge, the control shows an accumulation of PRC1 (red) in the midzone of the mitotic spindle (green) and the segregated chromosomes (blue). In the absence of PRC1 (absence of red), chromosomes are segregated, although bridges are not established between the microtubules of the two half-spindles. Channel separations are shown to indicate in detail microtubule arrays in anaphase in the absence of PRC1. Insets above indicate the cell outlines in white. The siRNA cells are in late anaphase, whereas the control is in early telophase. Bar, 10 μm. (B) Western blots of HeLa cell extracts collected 24 h after transfection with PRC1 siRNA, compared with control HeLa at equivalent protein loadings. The PRC1 level is substantially suppressed by siRNA treatment at 24 h, whereas other proteins of the central spindle (MKLP1 and MgcRacGAP), and proteins required for cell cleavage (RB6K and Aurora B), are not diminished by ablation of PRC1.
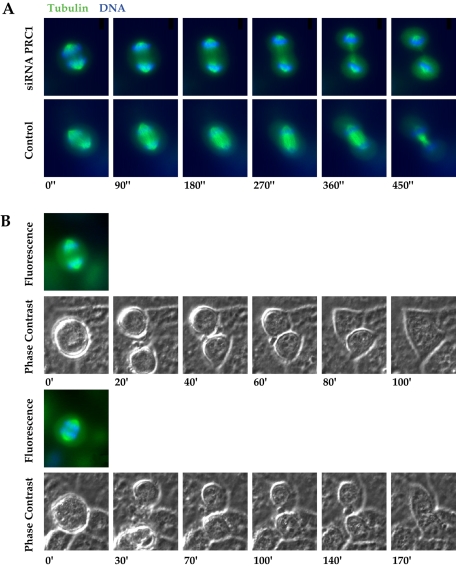
Figure 1.
Cells lacking a central spindle bundle undergo complete furrowing and then rejoin after failure of abscission. (A) Time frames from time-lapse videomicroscopy show progression of the cleavage furrow in a PRC1-ablated cell (top images) compared with a control (bottom images). Furrowing is complete in the absence of a central spindle bundle, and furrow progression occurs in approximately the same time course as in the control. Times indicated are in seconds. Images were collected of cells expressing EGFP-α-tubulin (green) and counterstained with Hoechst DNA dye (blue). The absence of a central spindle bundle is evident in the PRC1-ablated cell. See Video 1 for the full movie. (B) Cells that lack the central spindle bundle rejoin after furrowing is complete. Time frames from time-lapse videomicroscopy show phase contrast images of two cells that have undergone complete furrowing in the absence of a central spindle bundle and then rejoin more than an hour after furrowing has completed (time course to rejoining was variable but never <1 h after furrowing). Times indicated are in minutes. Color images above each initial frame show the same cells imaged for EGFP-α-tubulin (green) and counterstained with Hoechst DNA dye (blue) to indicate the absence of a central spindle bundle. The two cells were recorded in the same microscopic field. See Video 2 for the full movie.
By comparison, a control cell contains a strong tubulin signal between the separated chromosomes. The absence of the central spindle bundle correlates completely with the absence of PRC1, as we have shown previously, whereas a central spindle bundle is never absent in control anaphase cells. In the absence of a central spindle, we normally observed a rotation of one of the two spindle poles, a greater distance between the sets of separated chromosomes than in controls, and a somewhat greater velocity of furrowing. In cells lacking the central spindle, we observed that division nearly reached completion only to regress ∼1 h later, after a failure of abscission (Figure 1B and Video 2). Cells that completed furrowing in the absence of an organized midzone frequently showed torsion of the two cleaving cells relative to each other and a more proximal association than was evident in controls.
Thus, time-lapse images demonstrate that furrowing was robust and continued to near completion in the absence of an anaphase spindle bundle. In the examples shown, cells remained separated for more than an hour, tethered by a cytoplasmic bridge, and then fused again after the failure of complete cleavage (Figure 1B). The block in the last step of cleavage indicates that the process of abscission is evidently compromised by the absence of PRC1.
A panel of anaphase cells compares the presence and distribution of microtubules and of PRC1 in control and siRNA-transfected HeLa cells observed during anaphase (Figure 2A). The control contained microtubule bundles (green) between the separating chromosomes (blue), and PRC1 (red) had accumulated at the equatorial position of the spindle. By comparison, cells in which PRC1 was ablated (absence of PRC1 signal) had little evident association between the two spindle halves. One function of the interpolar spindle bundle seemed to be to maintain chromosome and spindle orientation, because chromosomes frequently rotated away from the axis of separation, sometimes substantially (for examples, see images in Figures 3 and 4).
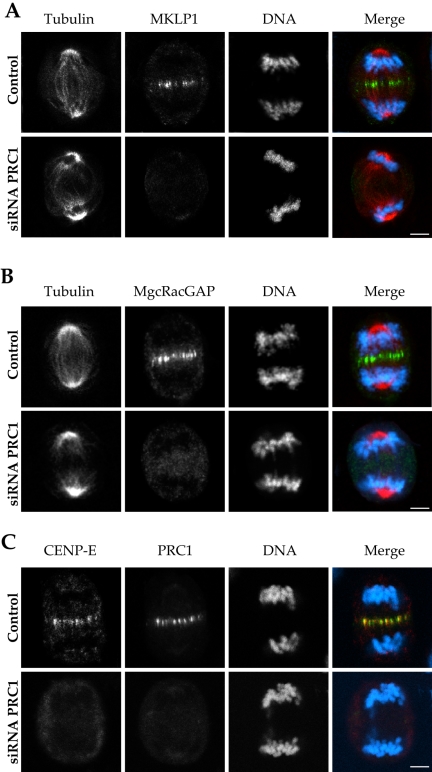
Figure 3.
MKLP1, MgcRacGAP, and CENP-E disperse during anaphase after central spindle ablation. Control and siRNA-treated HeLa cells showing the localization of MKLP1, MgcRacGAP, and CENP-E. In the absence of a central spindle after PRC1 ablation, MKLP1 (A) and MgcRacGAP (B) are dispersed in the cytoplasm. (A and B) Microtubules are shown in red, chromosomes in blue, and MKLP1 (A) and MgcRacGAP (B) in green. In C, a control cell shows colocalization of CENP-E (green) and PRC1 (red) in the midzone. In contrast, an siRNA-treated HeLa cell shows undetectable PRC1 levels, and CENP-E is dispersed in the cytoplasm. Bar, 5 μm.
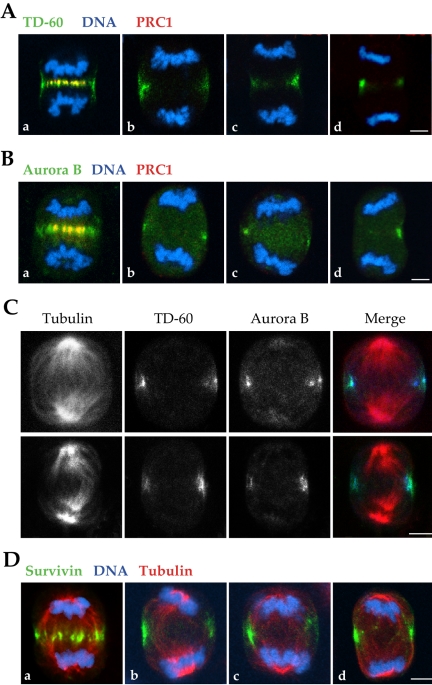
Figure 4.
In the absence of central spindle, passenger proteins associate with the cortex at the position of furrowing. (A) In the absence of PRC1, TD-60 is absent from the central region of the cell but is associated with the cell cortex at the midpoint between chromosome sets (b–d). In a control cell (a), PRC1 and TD-60 colocalize at the central spindle. HeLa cells were stained for TD-60 (green), PRC1 (red), and DNA (blue). (B) As observed for TD-60, Aurora B is also absent from the central region of the cell and is only found at the cell cortex at the position of furrowing. HeLa cells were stained for Aurora B (green), PRC1 (red), and DNA (blue). (C) TD-60 and Aurora B distribution on the cortex correlates with astral microtubule tips in anaphase. Two examples of siRNA-treated cells are shown, stained for TD-60 (green), Aurora B (blue), and α-tubulin (red). The spindles show a lack of a stable interdigitation between the microtubules of the two half-spindles. Astral microtubules seem to be prominent, and the passenger proteins TD-60 and Aurora B are limited to the cell cortex and localize at the tips of the astral microtubule arrays. (D) Survivin localizes to the cell cortex in the absence of PRC1. HeLa cells were stained for Survivin (green), α-tubulin (red), and DNA (blue). As for the other passenger proteins, the absence of a central spindle (b–d) limits Survivin to the cell cortex during anaphase. In contrast, in a control cell (a), Survivin is distributed throughout the entire equatorial diameter of the cell. Bar, 5 μm.
Transfected cells in Figure 2A were imaged 24 h after introduction of PRC1 siRNA. At this time, >80% of mitotic cells showed typical spindle derangements and had no evidence of PRC1 staining (our unpublished data). A Western blot confirmed that there was substantial suppression of PRC1 in the transfected population (Figure 2B). By contrast, an assay of a battery of proteins involved in cell cleavage showed that siRNA suppression of PRC1 did not cause secondary suppression of RB6K, MgcRacGAP, Aurora B, or MKLP1 (Figure 2B).
In the Absence of PRC1, the Centralspindlin Components Are Absent from the Anaphase Spindle
The central spindle is a platform on which proteins accumulate during late anaphase, and its components such as MKLP1 have been proposed to be required for localization of the passenger proteins to the spindle equator in anaphase (Straight and Field, 2000). Our results with PRC1 ablation raise the question as to the distribution, interaction, and function of the passenger proteins and of the heterotetrameric centralspindlin complex of MKLP1 and MgcRacGAP (Mishima et al., 2002) during anaphase in the absence of the anaphase spindle bundle. MKLP1 is a kinesin family microtubule motor that is present on the anaphase mitotic spindle and that associates with MgcRacGAP, an activating protein for the small G protein Rho (Hirose et al., 2001). Like PRC1, MKLP1 and MgcRacGAP normally localize to the equatorial position of the spindle in late anaphase.
In contrast to our results with PRC1 ablation in mammalian cells, ablation of MgcRacGAP homologue Cyk-4 (Jantsch-Plunger et al., 2000; Dechant and Glotzer, 2003) or of the MKLP1 homologue ZEN-4 in C. elegans (Raich et al., 1998) resulted in only partial furrowing, and suppression of pavarotti, the MKLP1 homologue in D. melanogaster, (Adams et al., 1998) completely suppressed furrow formation. It was therefore important to follow the fate of MKLP1 and of MgcRacGAP in the absence of PRC1.
During anaphase in control cells, both MKLP1 and MgcRacGAP accumulated at the spindle equator (Figure 3, A and B). In contrast, neither MKLP1 nor MgcRacGAP was evident on the central spindle in cells that lacked PRC1 (Figure 3, A and B) despite their continued presence in PRC1-ablated cells (Figure 2B). Lack of signal seems to reflect loss of the proteins from the anaphase spindle. Although the signal intensity on microtubule plus ends must be diminished by the lack of a spindle bundle, other microtubule plus end-associated proteins, such as the passenger proteins, are still evident in PRC1-depleted anaphase (e.g., Aurora B, Figure 6D, b). We conclude that MKLP1 and MgcRacGAP are both dependent on PRC1 for proper localization during anaphase. The two components of centralspindlin may be complexed with PRC1, because MgcRacGAP binds directly to PRC1 through a domain that is required for its spindle association (Ban et al., 2004).
Figure 6.
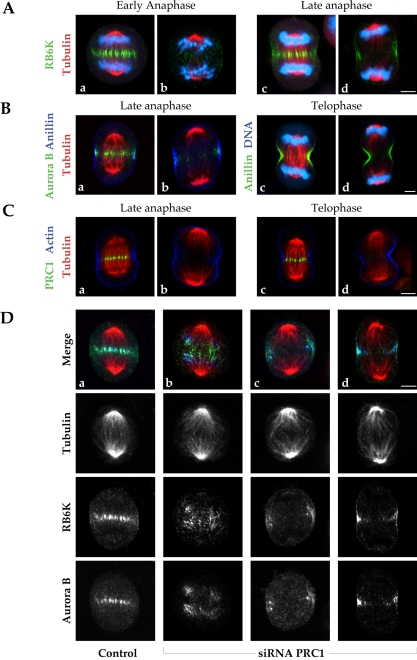
Anillin, actin, and RBK6 localize to the equatorial cell cortex in the absence of a central spindle. (A) RB6K is normally found throughout the equatorial midzone during late anaphase in control cells (a and c). In cells lacking PRC1 (b and d), RB6K is fully dispersed at the beginning of anaphase, but associates with the equatorial cortex when furrowing initiates. Cells were labeled for RB6K (green), α-tubulin (red), and DNA (blue). (B) Normal anillin association with the cortex in late anaphase and telophase, as shown in control cell (a and c), is not affected by the lack of PRC1 (b and d), here identified by the absence of a central spindle bundle. Anaphase cells (a and b) were stained for anillin (blue), α-tubulin (red), and Aurora B (green). Telophase cells (c and d) were stained for anillin (green), α-tubulin (red), and DNA (blue). Bar, 5 μm. (C) Actin cortical distribution found in untreated cells in late anaphase (a) and telophase (c) is not altered by the absence of a central spindle bundle in PRC1 ablated cells (b, anaphase; d, telophase). HeLa cells were stained for actin (blue), α-tubulin (red), and PRC1 (green). (D) RB6K associates with the equatorial cortex in the absence of PRC1. In a control cell (a) RB6K colocalizes with Aurora B and with astral microtubule plus ends at the cortex, and at the central spindle bundle. In siRNA-treated cells (b–d) RB6K is present at the equatorial cortex with Aurora B, although it apparently associates with astral microtubule plus ends more internally, both in early anaphase (b) and in late anaphase/telophase (c and d). Bar, 5 μm.
Another microtubule motor protein, CENP-E, migrates to the spindle equator along the central spindle bundle during anaphase (Yen et al., 1992; Liao et al., 1994). Although it is not known to have a role in cell division, we assayed for its localization in the absence of PRC1, to determine whether motor proteins other than MKLP1 might be dispersed. In control cells, CENP-E colocalized with PRC1 at the spindle midzone and did not extend beyond the microtubule array. Its presence at the anaphase spindle equator was also dependent on PRC1, because CENP-E did not localize to the anaphase mitotic spindle midzone in the absence of PRC1 (Figure 3C).
Passenger Proteins Localize to the Cell Cortex as a Ring during Anaphase in the Absence of a Central Spindle
In the absence of a central spindle, and of the proteins apparently dependent on it for anaphase spindle localization, MKLP1, MgcRacGAP, and CENP-E, furrowing nonetheless progresses to completion. The passenger proteins normally localize to the position of the anaphase central spindle equator and at this position create a transitory telophase disc that contacts the cell cortex (Martineau et al., 1995; Adams et al., 2001; Terada, 2001). The position of the passenger proteins at the cell cortex coincides with the position of furrow initiation, even in cells where furrowing is nonuniform (Martineau et al., 1995; Eckley et al., 1997). It was therefore important to determine whether ablation of PRC1 altered the distribution of passenger proteins in anaphase.
When PRC1 was present in late anaphase, both TD-60 and Aurora B associated with discs that corresponded in position to PRC1 on the spindle equator, and extended outward toward the cell cortex (Figure 4A, a and B, a). In the absence of PRC1, there was no evidence for a complete telophase disc, but TD-60 and Aurora B localized nonetheless to the cell cortex at the equator of the anaphase spindle (Figure 4A, b–d, and B, b–d).
Double labeling of two passenger proteins, Aurora B and TD-60, after siRNA suppression of PRC1, showed that they colocalized to the cell cortex at a position approximately halfway between the spindle poles (Figure 4C) and seemed to correlate in position with the ends of microtubules of the two disorganized half-spindles where the microtubules intersected with the cell cortex. Despite their unusual distribution, their position seemed to coincide with the cell cortex (Figure 4C).
Compared with controls that contained PRC1, neither protein participated in forming a complete telophase disc (Figure 4, A and B). It thus seems that although the full telophase disc organizes on the anaphase spindle bundle, what is essential for its localization to the cell cortex at the anaphase cell equator may be an association of the passenger proteins with the plus ends of the microtubules deriving from the two half-spindles, as was reported for INCENP localization in cells with monastral spindles (Canman et al., 2003). Such localization would permit stable association with the cell cortex at the termini of the facing parabolic arrays of the half-spindle microtubules. We also noted that where microtubule ends were unevenly distributed at the equator, the signal for the passenger proteins seemed to be stronger on the side where microtubules were more abundant (Figure 4, C and D, b). This result is in accord with the recent demonstration that astral microtubules play a role in the cortical organization of passenger proteins (Murata-Hori and Wang, 2002).
A similarly altered distribution also was observed for the passenger protein Survivin (Figure 4D). In the absence of a central spindle, as was observed for Aurora B and TD-60, Survivin also is found at the cortex at the equatorial position. The cortical localization observed here with Survivin antibody was confirmed by observations after PRC1 ablation in HeLa cells overexpressing HA-Survivin fusion protein (Skoufias et al., 2001) (our unpublished data).
Thus, the localization of the passenger proteins to the equatorial cortex occurs in the apparent absence of the centralspindlin components and of CENP-E. Positioning of the passenger proteins to the spindle equator during anaphase therefore does not seem to be dependent on the plus-ward microtubule motor proteins MKLP1 or CENP-E. Although it has been suggested that MKLP1 localizes to the central spindle in late mitosis by association with passenger proteins (Severson et al., 2000), we found no evidence for recruitment of MKLP1 to the position of the passenger proteins after PRC1 ablation.
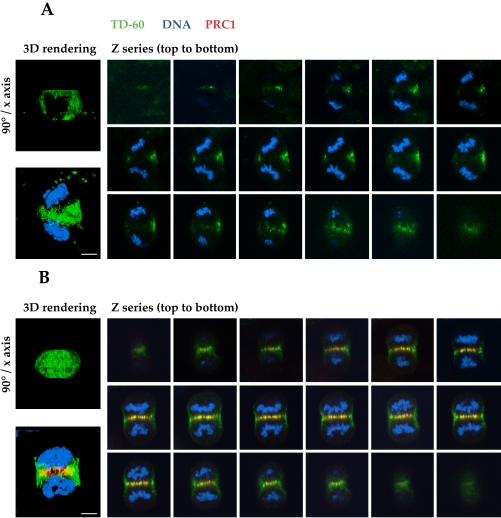
Figure 4 suggests that the passenger proteins accumulate at the equatorial position between the spindle poles as a ring on the cortex, rather than as a disc, during late anaphase. It is important to note that the passenger proteins were evident at the cell cortex equator before any furrow formation in anaphase and that their local abundance was therefore not due to passive accumulation at this site during furrowing. Furthermore, we have confirmed this impression by collecting optical sections through control and PRC1-suppressed cells in late mitosis. The result shows clearly that TD-60 is distributed as a ring on the cortex of the cell in the absence of PRC1, so that the immunofluorescence signal only crosses through the cell in the top or bottom optical sections (Figure 5A). The ring, clearly visible in the three-dimensional rendering of the Z-series showing TD-60 alone rotated 90° around the horizontal axis (Figure 5A, left image, above; Video 3) is typically asymmetric in distribution, due to spindle rotation, as noted above. In contrast, control cells display TD-60 throughout the equatorial region in the cell interior, colocalizing with PRC1 at the spindle equator (Figure 5B). For comparison with the TD-60 ring formed in the absence of PRC1, a full telophase disc is clearly visible in the three-dimensional rendering of the Z-series showing TD-60 alone, rotated 90° around the horizontal axis (Figure 5B, left image, above; Video 4).
Figure 5.
Optical sections show that TD-60 is distributed as a ring on the cortex of anaphase cells lacking a central spindle. (A) Gallery of images taken by optical sectioning through an entire cell in anaphase. In the absence of PRC1 (no red stain) TD-60 distribution (green) is limited to the cell cortex, where it forms a subcortical ring. This distribution implies that the central spindle is essential for the formation of a telophase disc. (B) Gallery of images of a control cell, showing that in the presence of PRC1 (red), TD-60 (green) is distributed throughout the equatorial diameter, forming a complete disc structure. Note the torsion of the chromosome sets in the cell. HeLa cells were stained for TD-60 (green), PRC1 (red), and DNA (blue). (A and B) Images to the left are three-dimensional renderings of the Z-series, showing TD-60 alone (above) rotated 90° around the horizontal axis, to show the ring or disc structure of TD-60 in the absence or presence of PRC1, respectively, and (below) the composite of the Z-series for all colors. See Videos 3 and 4 for rotations of the three-dimensional renderings of the ring or disc structure, respectively. Bars, 5 μm.
Anillin, Actin, and RB6K Localize to the Cell Cortex at the Furrow Position in the Absence of a Central Spindle
HeLa cells transfected with siRNA for PRC1 are able to complete furrowing, and passenger proteins correctly make contact with the cortex at the intersection of the two half-spindles, although the central spindle is absent. On the basis of these observations, we have further asked whether the other key components of the cleavage process were properly localized in the absence of PRC1 and the central spindle. RB6K is a motor protein of the kinesin family associated with the central spindle during mitosis (Hill et al., 2000; Fontijn et al., 2001). Overexpression experiments and RB6K antibody microinjection have interfered with cytokinesis, leading to binucleated cells (Hill et al., 2000). Furthermore, it has recently been demonstrated that RB6K binds Aurora B and is required for its translocation to the equator of the anaphase spindle (Gruneberg et al., 2004). In accord with this observation, Aurora B was closely associated with RB6K both during early anaphase (Figure 6D, b), and during late anaphase (Figure 6D, c and d) in the absence of PRC1.
In the absence of PRC1, RB6K was not present in the interior of the anaphase cell but rather exhibited a cortical association comparable with that shown by the passenger proteins during late anaphase (Figure 6A, d). This distribution was preceded by localization of RB6K to small foci distributed among the tips of the microtubules of the disorganized spindle during early anaphase (Figure 6A, b). Actin and anillin, an actin-associated protein that is required for cell cleavage (Oegema et al., 2000), were properly localized at the cortex in siRNA-transfected HeLa cells, as in controls (Figure 6B, c and d, and C). This result contrasts with the absence of actin and anillin from the anaphase cortex in D. melanogaster cells that lack pavarotti. The absence of PRC1 caused no evident alteration of actin distribution during furrow progression (Figure 6C). The position of astral microtubule ends seemed to correlate with the presence of passenger proteins, RB6K, actin, anillin at the cortex, and, finally, with furrow position.
DISCUSSION
It is evident that the mitotic spindle dictates the furrow position in higher eukaryotes (Rappaport, 1986). There are two discrete elements of the anaphase spindle, the central spindle bundle and the spindle asters. In different cell systems which of these may be the critical determinant for furrow formation may vary (Bonaccorsi et al., 1998; Powers et al., 1998; Jantsch-Plunger et al., 2000). In mammalian cells, evidence has favored a role for the central spindle bundle in the induction of the furrow (Cao and Wang, 1996; Wheatley and Wang, 1996), but recent evidence has supported a role for the asters in delivery of the passenger proteins (Murata-Hori and Wang, 2002; Canman et al., 2003).
PRC1, a microtubule bundling protein, is absolutely required for the formation of the central spindle bundle in anaphase cells. Both siRNA ablation and antibody microinjection have established that the central spindle is absent when PRC1 function is compromised (Mollinari et al., 2002). The capacity to ablate the central spindle has presented us with a unique system to address both the requirement for the central spindle bundle in recruitment of proteins required for cell cleavage and to address whether furrowing proceeds in its absence. We have here demonstrated that the central spindle bundle is not required for cell furrowing but that its absence instead prevents the final abscission event that creates two daughter cells.
The Central Spindle Bundle and Spindle Orientation
The most evident role for the central spindle bundle is to maintain the correct orientation of the two separated half-spindles and their associated chromatids. In the absence of PRC1, we have observed that the two half-spindles and the chromatid sets frequently rotate relative to each other. The central spindle bundle is not, however, required to maintain a separation between the two separated chromatid sets. The extensive elongation that we observe in cells without a central spindle is in broad accord with conclusions drawn from studies involving laser ablation in mitotic cells (Aist et al., 1993).
Comparison with the Role of PRC1 Homologues in Other Organisms
Recent work has demonstrated the role of PRC1 homologues in cell cleavage in other organisms. Mutation or ablation of the PRC1 homologue fascetto in D. melanogaster has demonstrated that its absence results in an abnormally thin microtubule bridge during telophase and causes failure of late cleavage events, accompanied by dispersal of actin (Verni et al., 2004). Its absence thus does not abolish central spindle formation but makes the central spindle abnormal. In contrast, mutation or RNA interference (RNAi) ablation of the PRC1 homologue in C. elegans, SPD-1, shows that it is required to bundle microtubules in vivo (Verbrugghe and White, 2004). Despite this requirement, cytokinesis goes to completion in most cells in its absence.
The distinct roles of the homologues are broadly in accord with a role for astral arrays in cleavage in C. elegans and for a central spindle bundle in D. melanogaster (Glotzer, 2004). Our evidence agrees well with the results in C. elegans, in that we also find the central spindle absent but furrow completion. However, the possibility of failure of abscission was not addressed in the work on C. elegans. Unlike our results, ZEN-4/MKLP1 localized to the equatorial furrow in the absence of SPD-1 in C. elegans. Interestingly, cleavage seemed to require ZEN-4/MKLP1 or CYK-4/MgcRacGAP even in the absence of a central spindle, because RNAi depletion of ZEN-4 or CYK-4 in mutant SPD-1 cells caused cleavage failure. It is not yet clear whether localization of the centralspindlin components to the cortical furrow was required for their function. It will be of great interest to determine whether ZEN-4/MKLP1 or CYK-4/MgcRacGAP are similarly important to furrow completion in PRC1-depleted mammalian cells, even when dispersed.
A Role for the Central Spindle in the Final Abscission Event
The central spindle bundle is apparently required for the formation of the highly condensed microtubule bundle, the midbody, and for the final abscission of the cleaving cell. Furrowed cells lack a normal midbody in the absence of PRC1 and may therefore fail to recruit or organize elements required for the abscission event. PRC1 itself may be among those elements required for abscission, because we have shown that a domain of PRC1 associates with the spindle midzone of cleaving cells independent of the capacity of native PRC1 to associate with microtubules (Mollinari et al., 2002). Such localization could entail indirect association of PRC1 with microtubules through association with the microtubule motor protein KIF4 (Kurasawa et al., 2004). We have found that localization of MKLP1 to the midzone depends on PRC1. An isoform of MKLP1, CHO1, is required for abscission (Matuliene and Kuriyama, 2002, 2004). The absence of CHO1 does not prevent the formation of a normal central spindle bundle in anaphase, because the other MKLP1 isoform is still present, but its absence suppresses formation of a midbody matrix in late telophase (Matuliene and Kuriyama, 2004). This requirement may at least partially explain the abscission failure after PRC1 ablation.
It has recently been demonstrated that cell abscission is an event independent of furrowing. All of mitosis, including furrowing, can proceed in the absence of centrosomes (Hinchcliffe et al., 2001), but the final abscission event requires centrosomes and is accompanied by the migration of the mother centriole to the midbody at the time of abscission (Piel et al., 2001). It is possible that migration of the mother centriole requires intact midzone microtubules to track toward the point of abscission.
CONCLUSIONS
We have found that the central spindle is not required to induce or promote furrowing in telophase. Astral microtubules convey all the essential information required to place the passenger proteins and RB6K at the correct location on the cortex where they colocalize with actin and anillin, which in turn permit furrowing to proceed. Furthermore, we have found that either PRC1 itself or the central spindle bundle is required for abscission at the end of cleavage in mammalian cells. Which element is critical to the abscission event remains to be determined.
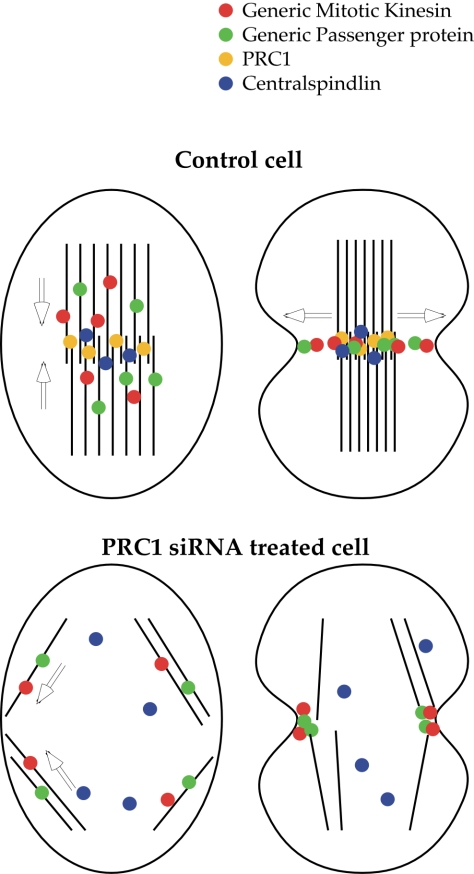
As detailed in Figure 7, our results support the likelihood that although the central spindle bundle may be normally central to furrow induction in mammalian cells, astral microtubules offer a redundant mechanism that ensures furrowing in the absence of the central spindle bundle, by permitting migration of the passenger proteins Aurora B (Murata-Hori and Wang, 2002) and INCENP (Canman et al., 2003) to the cortex, so that either the central spindle bundle or astral microtubules in isolation may be sufficient to induce complete furrowing.
Figure 7.
Model of the distribution and function of the anaphase spindle elements in control and PRC1-ablated spindles. In a control anaphase, the passenger proteins and motor proteins first migrate toward the spindle midzone on the anaphase spindle bundle, containing PRC1 and centralspindlin (left). The passenger proteins and RB6K then disperse laterally to contact the cortex where furrowing will initiate (right). In siRNA-treated cells, the absence of PRC1 causes the dispersal of centralspindlin and loss of parallel bundles in the spindle midzone. The kinesins and passenger proteins in consequence follow a secondary path on astral microtubules (left) to converge as a subcortical ring at the position of the spindle equator (right). The position of RB6K and of the passenger proteins then coincides with the position of the ingressing furrow (right).
Supplementary Material
Acknowledgments
We thank Drs. P. Wadsworth, L. Lafanechere, B. Goud, T. Kitamura, C. Field, and W. Jiang for kindly supplying pEGFP-α-tubulin, tyrosinated-α-tubulin mAb, RB6K and MgcRacGAP antibodies, anillin antibody, and affinity-purified PRC1 antibody, respectively. We also thank Dr. T. Gautier for helping with the acquisition of images using the two-photon Zeiss confocal at the Institut Albert Bonniot of Grenoble. This work was supported by funding from la Ligue Contre le Cancer (Equipe Labelisée) (to R.L.M.). C. M. was a postdoctoral fellow of la Ligue Contre le Cancer. S.A.J. and T.J.Y. were supported by grants from the National Institutes of Health (GM-44762 and CA-099423), Core grants CA-75138 and CA-06927, and an Appropriation from the Commonwealth of Pennsylvania.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-04-0346) on December 22, 2004.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Adams, R. R., Carmena, M., and Earnshaw, W. C. (2001). Chromosomal passengers and the (Aurora) ABCs of mitosis. Trends Cell Biol. 11, 49-54. [DOI] [PubMed] [Google Scholar]
- Adams, R. R., Tavares, A. A., Salzberg, A., Bellen, H. J., and Glover, D. M. (1998). pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 12, 1483-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aist, J. R., Liang, H., and Berns, M. W. (1993). Astral and spindle forces in PtK2 cells during anaphase B: a laser microbeam study. J. Cell Sci. 104, 1207-1216. [DOI] [PubMed] [Google Scholar]
- Andreassen, P. R., Palmer, D. K., Wener, M. H., and Margolis, R. L. (1991). Telophase disc: a new mammalian mitotic organelle that bisects telophase cells with a possible function in cytokinesis. J. Cell Sci. 99, 523-534. [DOI] [PubMed] [Google Scholar]
- Ban, R., Irino, Y., Fukami, K., and Tanaka, H. (2004). Human mitotic spindle-associated protein PRC1 inhibits MgcRacGAP activity towards Cdc42 during the metaphase. J. Biol. Chem. 279, 16394-16402. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi, S., Giansanti, M. G., and Gatti, M. (1998). Spindle self-organization and cytokinesis during male meiosis in asterless mutants of Drosophila melanogaster. J. Cell Biol. 142, 751-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canman, J. C., Cameron, L. A., Maddox, P. S., Straight, A., Tirnauer, J. S., Mitchison, T. J., Fang, G., Kapoor, T. M., and Salmon, E. D. (2003). Determining the position of the cell division plane. Nature 424, 1074-1078. [DOI] [PubMed] [Google Scholar]
- Cao, L. G., and Wang, Y. L. (1996). Signals from the spindle midzone are required for the stimulation of cytokinesis in cultured epithelial cells. Mol. Biol. Cell 7, 225-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, G. K., Jablonski, S. A., Sudakin, V., Hittle, J. C., and Yen, T. J. (1999). Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J. Cell Biol. 146, 941-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechant, R., and Glotzer, M. (2003). Centrosome separation and central spindle assembly act in redundant pathways that regulate microtubule density and trigger cleavage furrow formation. Dev Cell 4, 333-344. [DOI] [PubMed] [Google Scholar]
- Eckley, D. M., Ainsztein, A. M., Mackay, A. M., Goldberg, I. G., and Earnshaw, W. C. (1997). Chromosomal proteins and cytokinesis: patterns of cleavage furrow formation and inner centromere protein positioning in mitotic heterokaryons and mid-anaphase cells. J. Cell Biol. 136, 1169-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontijn, R. D., Goud, B., Echard, A., Jollivet, F., van Marle, J., Pannekoek, H., and Horrevoets, A. J. (2001). The human kinesin-like protein RB6K is under tight cell cycle control and is essential for cytokinesis. Mol. Cell. Biol. 21, 2944-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann, R., Carvalho, A., Henzing, A. J., Ruchaud, S., Hudson, D. F., Honda, R., Nigg, E. A., Gerloff, D. L., and Earnshaw, W. C. (2004). Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol. 166, 179-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer, M. (2004). Cleavage furrow positioning. J. Cell Biol. 164, 347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneberg, U., Neef, R., Honda, R., Nigg, E. A., and Barr, F. A. (2004). Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J. Cell Biol. 166, 167-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, E., Clarke, M., and Barr, F. A. (2000). The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis. EMBO J. 19, 5711-5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchcliffe, E. H., Miller, F. J., Cham, M., Khodjakov, A., and Sluder, G. (2001). Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science 291, 1547-1550. [DOI] [PubMed] [Google Scholar]
- Hirose, K., Kawashima, T., Iwamoto, I., Nosaka, T., and Kitamura, T. (2001). MgcRacGAP is involved in cytokinesis through associating with mitotic spindle and midbody. J. Biol. Chem. 276, 5821-5828. [DOI] [PubMed] [Google Scholar]
- Jantsch-Plunger, V., Gonczy, P., Romano, A., Schnabel, H., Hamill, D., Schnabel, R., Hyman, A. A., and Glotzer, M. (2000). CYK-4, a Rho family GTPase activating protein (GAP) required for central spindle formation and cytokinesis. J. Cell Biol. 149, 1391-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, W., Jimenez, G., Wells, N. J., Hope, T. J., Wahl, G. M., Hunter, T., and Fukunaga, R. (1998). PRC1: a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol. Cell 2, 877-885. [DOI] [PubMed] [Google Scholar]
- Kurasawa, Y., Earnshaw, W. C., Mochizuki, Y., Dohmae, N., and Todokoro, K. (2004). Essential roles of KIF4 and its binding partner PRC1 in organized central spindle midzone formation. EMBO J. 23, 3237-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafanechere, L., Courtay-Cahen, C., Kawakami, T., Jacrot, M., Rudiger, M., Wehland, J., Job, D., and Margolis, R. L. (1998). Suppression of tubulin tyrosine ligase during tumor growth. J. Cell Sci. 111, 171-181. [DOI] [PubMed] [Google Scholar]
- Liao, H., Li, G., and Yen, T. J. (1994). Mitotic regulation of microtubule cross-linking activity of CENP-E kinetochore protein. Science 265, 394-398. [DOI] [PubMed] [Google Scholar]
- Mabuchi, I. (1986). Biochemical aspects of cytokinesis. Int. Rev. Cytol. 101, 175-213. [DOI] [PubMed] [Google Scholar]
- Mackay, A. M., Ainsztein, A. M., Eckley, D. M., and Earnshaw, W. C. (1998). A dominant mutant of inner centromere protein (INCENP), a chromosomal protein, disrupts prometaphase congression and cytokinesis. J. Cell Biol. 140, 991-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau, S. N., Andreassen, P. R., and Margolis, R. L. (1995). Delay of HeLa cell cleavage into interphase using dihydrocytochalasin B: retention of a postmitotic spindle and telophase disc correlates with synchronous cleavage recovery. J. Cell Biol. 131, 191-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuliene, J., and Kuriyama, R. (2002). Kinesin-like protein CHO1 is required for the formation of midbody matrix and the completion of cytokinesis in mammalian cells. Mol. Biol. Cell 13, 1832-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuliene, J., and Kuriyama, R. (2004). Role of the midbody matrix in cytokinesis: RNAi and genetic rescue analysis of the mammalian motor protein CHO1. Mol. Biol. Cell 15, 3083-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima, M., Kaitna, S., and Glotzer, M. (2002). Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev. Cell 2, 41-54. [DOI] [PubMed] [Google Scholar]
- Mollinari, C., Kleman, J. P., Jiang, W., Schoehn, G., Hunter, T., and Margolis, R. L. (2002). PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J. Cell Biol. 157, 1175-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata-Hori, M., and Wang, Y. L. (2002). Both midzone and astral microtubules are involved in the delivery of cytokinesis signals: insights from the mobility of aurora B. J. Cell Biol. 159, 45-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema, K., Savoian, M. S., Mitchison, T. J., and Field, C. M. (2000). Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis. J. Cell Biol. 150, 539-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel, M., Nordberg, J., Euteneuer, U., and Bornens, M. (2001). Centrosome-dependent exit of cytokinesis in animal cells. Science 291, 1550-1553. [DOI] [PubMed] [Google Scholar]
- Powers, J., Bossinger, O., Rose, D., Strome, S., and Saxton, W. (1998). A nematode kinesin required for cleavage furrow advancement. Curr. Biol. 8, 1133-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich, W. B., Moran, A. N., Rothman, J. H., and Hardin, J. (1998). Cytokinesis and midzone microtubule organization in Caenorhabditis elegans require the kinesin-like protein ZEN-4. Mol. Biol. Cell 9, 2037-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport, R. (1986). Establishment of the mechanism of cytokinesis in animal cells. Int. Rev. Cytol. 105, 245-281. [DOI] [PubMed] [Google Scholar]
- Rieder, C. L., Khodjakov, A., Paliulis, L. V., Fortier, T. M., Cole, R. W., and Sluder, G. (1997). Mitosis in vertebrate somatic cells with two spindles: implications for the metaphase/anaphase transition checkpoint and cleavage. Proc. Natl. Acad. Sci. USA 94, 5107-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath, S. C., Ohi, R., Leismann, O., Salic, A., Pozniakovski, A., and Funabiki, H. (2004). The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell 118, 187-202. [DOI] [PubMed] [Google Scholar]
- Severson, A. F., Hamill, D. R., Carter, J. C., Schumacher, J., and Bowerman, B. (2000). The aurora-related kinase AIR-2 recruits ZEN-4/CeMKLP1 to the mitotic spindle at metaphase and is required for cytokinesis. Curr. Biol. 10, 1162-1171. [DOI] [PubMed] [Google Scholar]
- Skoufias, D. A., Andreassen, P. R., Lacroix, F. B., Wilson, L., and Margolis, R. L. (2001). Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc. Natl. Acad. Sci. USA 98, 4492-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoufias, D. A., Mollinari, C., Lacroix, F. B., and Margolis, R. L. (2000). Human Survivin is a kinetochore-associated passenger protein. J. Cell Biol. 151, 1575-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight, A. F., and Field, C. M. (2000). Microtubules, membranes and cytokinesis. Curr. Biol. 10, R760-R770. [DOI] [PubMed] [Google Scholar]
- Terada, Y. (2001). Role of chromosomal passenger complex in chromosome segregation and cytokinesis. Cell Struct. Funct. 26, 653-657. [DOI] [PubMed] [Google Scholar]
- Verbrugghe, K. J., and White, J. G. (2004). SPD-1 is required for the formation of the spindle midzone but is not essential for the completion of cytokinesis in C. elegans embryos. Curr. Biol. 14, 1755-1760. [DOI] [PubMed] [Google Scholar]
- Verni, F., Somma, M. P., Gunsalus, K. C., Bonaccorsi, S., Belloni, G., Goldberg, M. L., and Gatti, M. (2004). Feo, the Drosophila homolog of PRC1, is required for central-spindle formation and cytokinesis. Curr. Biol. 14, 1569-1575. [DOI] [PubMed] [Google Scholar]
- Wheatley, S. P., and Wang, Y. (1996). Midzone microtubule bundles are continuously required for cytokinesis in cultured epithelial cells. J. Cell Biol. 135, 981-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen, T. J., Li, G., Schaar, B. T., Szilak, I., and Cleveland, D. W. (1992). CENP-E is a putative kinetochore motor that accumulates just before mitosis. Nature 359, 536-539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.