Significance
Many animals, especially humans, carry out activities collectively because the benefits of doing so exceed those that can be achieved individually. But how can collective action evolve when individuals receive the benefits of cooperation regardless of whether they pay the costs of participation? Collective action may be especially difficult to achieve when groups are large, because each individual’s contribution has a small effect on the outcome. We show that group augmentation theory helps explain one of the most striking examples of collective action in nonhuman primates, territorial boundary patrolling by male chimpanzees: Males bear the short-term costs of patrolling even when they have little to gain immediately because patrolling enhances group size, increasing the male’s chances of future reproduction.
Keywords: chimpanzees, Pan troglodytes, collective action, cooperation, territoriality
Abstract
How can collective action evolve when individuals benefit from cooperation regardless of whether they pay its participation costs? According to one influential perspective, collective action problems are common, especially when groups are large, but may be solved when individuals who have more to gain from the collective good or can produce it at low costs provide it to others as a byproduct. Several results from a 20-y study of one of the most striking examples of collective action in nonhuman animals, territorial boundary patrolling by male chimpanzees, are consistent with these ideas. Individuals were more likely to patrol when (i) they had more to gain because they had many offspring in the group; (ii) they incurred relatively low costs because of their high dominance rank and superior physical condition; and (iii) the group size was relatively small. However, several other findings were better explained by group augmentation theory, which proposes that individuals should bear the short-term costs of collective action even when they have little to gain immediately if such action leads to increases in group size and long-term increases in reproductive success. In support of this theory, (i) individual patrolling effort was higher and less variable than participation in intergroup aggression in other primate species; (ii) males often patrolled when they had no offspring or maternal relatives in the group; and (iii) the aggregate patrolling effort of the group did not decrease with group size. We propose that group augmentation theory deserves more consideration in research on collective action.
Many animals, especially humans, carry out activities collectively despite costs associated with those activities because the net benefits derived from doing so exceed those that can be achieved individually (1). How, however, can collective action evolve when all group members receive the benefits of cooperation even if they fail to pay the costs of participation? Understanding the factors that facilitate and impede collective action is a central problem in evolutionary biology and other disciplines, including economics, political science, psychology, and sociology.
According to Olson’s The Logic of Collective Action (2), collective action problems are common but can be solved by the “exploitation of the great by the small”: most collective action will be undertaken by a small number of individuals who receive the highest benefits and/or pay the lowest participation costs and who thus produce collective goods as byproducts available to free-riders (2). Although theoretical work has provided support for Olson’s perspective (3), findings from studies of participation in between-group aggression, a frequent type of collective action in primates and other group-living animals, have been particularly difficult to interpret (4, 5). As predicted, in many species that form dominance hierarchies, high-ranking individuals participate in intergroup encounters more often than do low-ranking individuals (6–13). Nevertheless, their reasons for doing so are unresolved. They may derive relatively high direct fitness benefits because they have many offspring who will gain from the collective good they provide, i.e., the home-range or territory and the fitness-limiting resources it contains (14, 15). Alternatively, the costs of aggression may vary inversely with rank because high-ranking individuals are typically in the prime of their lives and are in better physical condition than low-ranking individuals (4). The effect of indirect fitness benefits (16) on individual variation in participation also remains unclear. Some studies indicate that individuals with many relatives in the group cooperate frequently in between-group aggression, but others reveal that the number of kin has no effect (17–19). Disentangling the influence of dominance rank from kinship is difficult because high-ranking individuals often live with more close kin than do lower-ranking animals (20).
Another challenge to explaining participation in between-group aggression and other forms of costly collective action is posed by Olson’s “group size paradox” (2), which holds that collective action is particularly difficult to achieve in large groups. One reason for this difficulty is that when groups are large, defections by single individuals have relatively small impacts on the probability of success. Also, in many scenarios, each individual’s share of the collective good is smaller in large groups than in small groups because in large groups it must be divided among more individuals. Collective action in large groups is also puzzling from the perspective of kin selection theory (21), because the extent to which members of the philopatric sex [who typically are the main participants in between-group aggression (5)] are more closely related to each other than to members of competing groups decreases quickly with group size (22). Comparative analyses indicating that cooperative territoriality commonly occurs in primate species who live in small groups (5) and field studies demonstrating that individual participation in intergroup encounters decreases with group size (11, 12, 23) have been interpreted as evidence of the difficulty of achieving collective action in large groups. Nevertheless, how some species, including humans, regularly engage in collective action despite living in large groups remains unexplained.
Olson’s thesis is usually framed in terms of the immediate or short-term costs and benefits of participation in collective action. In contrast, the group augmentation hypothesis, originally formulated to explain why nonbreeding individuals help raise unrelated immature group members in cooperatively breeding species, adds a long-term view to explain group-level cooperation. It proposes that helpers gain fitness benefits by enlarging groups if the recruits produced as a result of helping increase the fitness of helpers (24). The increased fitness of helpers resulting from recruit production can be immediate or short-term (e.g., when increased group size dilutes predation risk and thus increases helper survivorship) but also can occur in the future when helpers become breeders and are assisted by recruits. When the long-term reproductive skew is low, so that helpers have high probabilities of attaining breeding positions, and life-long natal philopatry occurs, so that helpers live with recruits long enough to benefit from their presence, individuals will participate in collective action when they can afford to pay the cost, even if they do not immediately benefit (25). Individual participation in collective action by members of the philopatric sex is expected to be particularly high and nonvariable when between-group competition has a strong effect on fitness, because individuals that are “stuck” in the same group have fitness interests in common (26). Free-riders may increase their short-term reproductive success by avoiding the costs of collective action, but they do so at the cost of decreasing the long-term survival of the group if it fails to grow or maintain its size; nonparticipants suffer this cost alongside the individuals they had cheated (26, 27). Here group augmentation theory predicts that even if individual effort in collective action decreases with group size for the reasons outlined by Olson (2), this decrease will not necessarily result in decreased aggregate group effort and thus in a failure of collective action (28). Rather, large groups can produce the same or even greater levels of aggregate effort as smaller groups at lower individual costs because these costs are spread over more participants through “load-lightening” (29, 30).
In this paper, we contrast predictions from collective action and group augmentation theory to examine one of the most dramatic forms of collective action in mammals: territorial boundary patrolling by chimpanzees. Patrols are conspicuous events that occur when multiple individuals, typically male, travel to the peripheries of their territories and sometimes deep into those of their neighbors (31, 32). Patrollers become hypervigilant and behave in other ways that suggest they are actively searching for neighbors (33). Collective action theory predicts that average participation will be low and highly variable among individuals according to their short-term benefits, because of features distinguishing chimpanzee patrols from between-group aggression in other animals.
Unlike most other primate species in which group members are in constant association, chimpanzees live in societies with high fission–fusion dynamics: Group members associate in temporary parties that vary in size, duration, and composition (34). Individuals can thus free-ride not only by refraining from joining patrols when they are present at their start but also by choosing to limit their presence in large parties that contain many males, given that the probability of a patrol happening on a given day is related to maximum number of males who associate with each other per day (32). In contrast, fewer opportunities for free-riding in between-group aggression occur in species that typically form more cohesive groups. Individuals of such species can opt out of joining an intergroup encounter that takes place when two groups happen to meet at contested resources (e.g., fruiting trees in areas of territory overlap). However, unlike the decision to join patrols by chimpanzees, participation in intergroup aggression is not always entirely voluntary, because attacks by members of other groups demand immediate responses. Finally, patrols have considerable short-term opportunity and energy costs (35), given that they have a mean duration of 134 min, cover long distances (mean = 2.5 km), and are associated with elevations of testosterone (36) and cortisol (37) levels about 25% higher than mean values.
Chimpanzee societies also differ from those of most other species who display between-group aggression in ways that group augmentation theory predicts should lead to relatively high participation rates, with individual participation only weakly affected by immediate and short-term benefits. First, the influence of success in between-group competition on fitness and the effect of group size on success in between-group competition are probably unusually high in chimpanzees. Chimpanzees are one of the few mammals in which between-group aggression is a major source of mortality (38–40). Chimpanzees in large groups have been reported to kill most or all of the males in smaller groups over periods lasting several months or years, acquiring portions of their territory in the process (33, 41, 42). Territorial expansion can lead to the transfer of parous females from the losing to the winning group (41, 42); it also increases the amount of food available to females in the winning group and thereby can increase their fertility (43). Second, male chimpanzees are philopatric and remain in their natal groups for their entire lives (44). Because males can live for more than 50 y, this philopatry creates the potential for those who patrol when young to gain future benefits from the additional group members their patrolling efforts can produce (28).
We use 20 y of behavioral, demographic, and genetic data to examine individual variation in participation in patrols by male chimpanzees at Ngogo in Kibale National Park, Uganda. Like chimpanzees elsewhere, those at Kibale live in groups or “communities” (41), terms that we hereafter use interchangeably. The Ngogo community is unusually large and over the course of the study varied between ∼140 and 206 members, including 24–44 males aged 13 y or older. Thirteen is the youngest age at conception by an Ngogo male (45) and when males begin to patrol regularly (31). In keeping with the large size of their group, Ngogo males are only slightly more closely related to males in their own group than to males in competing groups (46); thus kin selection is likely an insufficient explanation for patrolling. We tested predictions derived from collective action theory by investigating how male participation in patrols varied with the potential short-term benefits and costs of patrolling. We used the number of living offspring and close maternal relatives that each male had at the time a given patrol occurred as proxies for individual variation in the potential direct and indirect benefits of patrolling, respectively, and used male dominance rank and age as proxies for costs. We also measured long-term reproductive skew among males to determine whether, and how often, males patrolled when they had no offspring or maternal relatives in the group and whether such males subsequently reproduced. These males would not have obtained immediate direct or indirect benefits by patrolling but could gain deferred fitness benefits over the long-term via subsequent reproduction and group augmentation. Finally, we examined the relationships between group size and individual and aggregate patrolling effort to determine whether per capita effort declined as the number of males in the group increased and, if it did, whether overall effort was independent of or positively related to group size.
Results
Average Participation Rates and Patrol Size.
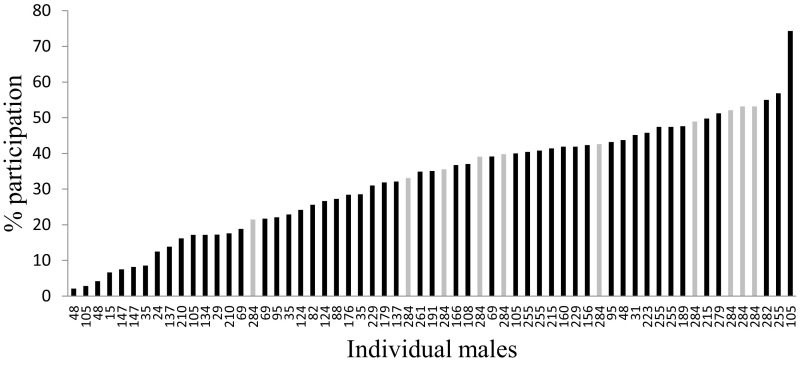
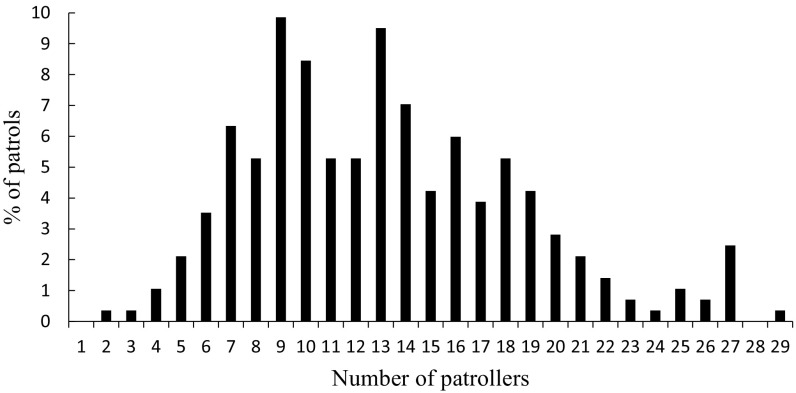
Despite ample opportunities and short-term incentives for free-riding, male chimpanzees patrolled frequently. On average, a male participated in 33% of the patrols that occurred while he was present in the group and of patrolling age (≥13 y) (Fig. 1). Some males died early or matured late during the study period and consequently were present for relatively few patrols. Their samples therefore are not likely to be representative. In fact, the average participation rate for the 10 males who were present in the group for all 284 patrols was considerably higher (42%) (Fig. 1). Because of the high rate of individual participation, patrols were large (mean ± SD = 13.2 ± 5.4 individuals, range = 2–29 individuals) (Fig. S1) and consisted of a large percentage of the total number of males (mean ± SD = 37.5 ± 15.5%, range = 6.7–85.2%).
Fig. 1.
Individual variation in patrol participation, calculated as the percentage of patrols a male participated in out of the patrols that occurred while he was alive and of patrolling age (≥13 y). Each bar represents the percentage of patrols in which an individual male participated. Values are ordered from left to right along the x axis from low to high; the number of patrols that occurred while the male was alive and of patrolling age is indicated below each bar. Light gray bars indicate the 10 males who were alive and of patrolling age for all 284 patrols.
Fig. S1.
Variation in patrol size (the number of males aged ≥13 y participating in a patrol). n = 284 patrols.
Individual Variation in Patrol Participation.
Despite the high overall participation rate, individual patrolling frequency varied considerably, with males joining 2–74% of patrols. However, participation varied substantially less among the 10 best-sampled males (21.5–53.2%) (Fig. 1).
We constructed a general linear mixed model (GLMM) (47) to determine the relationship between patrol participation and five factors hypothesized to influence the immediate short-term benefits (number of offspring, number of close maternal relatives, group size) and costs (dominance rank, age) of patrolling (Table 1). The comparison between the null and full model was statistically significant (likelihood ratio test: χ2 = 130.73, df = 5, P < 0.001).
Table 1.
Results of GLMM investigating the effects of male dominance rank, paternity success, maternal relatedness, and group size on patrol participation by individual male chimpanzees
| Estimate | SE | 95% CI | |
| Intercept | 0.27 | 0.14 | 0.05, 0.63 |
| Paternity success | 0.31 | 0.12 | 0.08, 0.68 |
| Rank | 0.28 | 0.08 | 0.08, 0.52 |
| Age | 0.09 | 0.15 | −0.18, 0.48 |
| Maternal relatedness | 0.08 | 0.09 | −0.11, 0.25 |
| Male group size | −2.21 | 0.16 | −3.97, −1.90 |
Estimates, their SEs, and 95% CIs are shown. The 95% CIs that do not overlap 0 are indicated by boldface type. n = 179 patrols.
The short-term direct fitness benefits that males can obtain by defending their community’s territory should increase as a function of their current number of offspring. We thus used a male’s paternity success at the time of patrols to assess the relationship between short-term direct fitness benefits and patrol participation. Although promiscuous mating results in low paternity certainty, male chimpanzees may have some information about their overall paternity success based on their mating histories (48, 49). Paternity success was positively and significantly related to patrol participation (Table 1). Despite this positive association, males often patrolled when they had no offspring in the group [29.8% (1,098/3,747) of all individual male patrolling events].
Male chimpanzees form strong social bonds with their close maternal kin (50, 51), whom they presumably recognize via familiarity, as in other primates (52). In contrast, chimpanzees do not appear to bias their behavior toward more distant maternal kin and collateral paternal kin (e.g., paternal siblings) (50, 53, 54). We thus predicted that males with several close maternal relatives (i.e., mothers, maternal brothers, maternal sisters, and maternal sisters’ offspring) living in the group would be particularly motivated to patrol. However, the number of close maternal relatives was unrelated to patrol participation (Table 1). As with paternity success, males frequently patrolled when they had no close maternal relatives in the group (1,225/3,747 = 32.7%). Patrolling by males who had neither living offspring nor close maternal relatives was also quite common (989/3,747 = 26.4%).
We used an individual’s dominance rank at the time of patrols to assess the relationship between direct fitness costs and patrol participation. High-ranking males often sire many offspring, but a positive relationship between rank and participation in patrols that occurs independently of rank-related variation in reproductive success would indicate that relative paternity success is an insufficient explanation for variation in patrolling (48, 55–58). One likely possibility is that high-ranking individuals experience relatively low costs of patrolling because of their superior physical condition (59–61). Consistent with this prediction, dominance rank was positively related to patrol participation (Table 1).
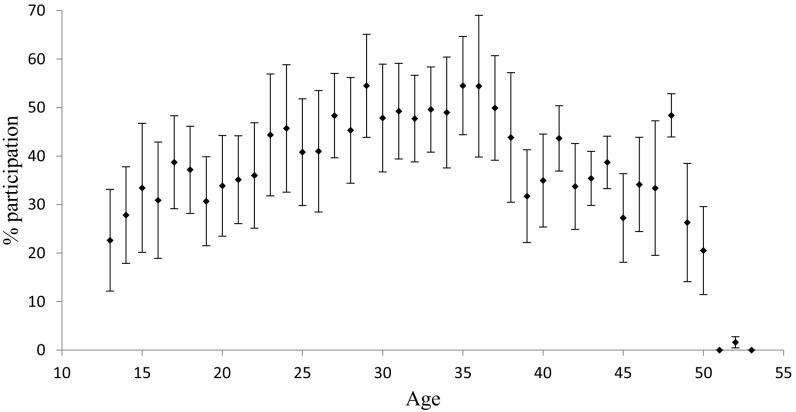
We also assessed how direct fitness costs might influence patrol participation by examining the relationship between participation and male age, which varied from 13 to 53 y (mean ± SD = 25.8 ± 9.6 y). We entered age as a squared term to account for the possibility that patrolling costs were highest for adolescents (who have not finished growing) and old males (who have experienced considerable senescence). Age was unrelated to patrol participation (Table 1), perhaps because age was strongly correlated with dominance rank (Pearson’s R = 0.58), which in turn was more strongly correlated with patrol participation than was age. A univariate analysis revealed the expected inverted U-shaped relationship between age and patrolling participation (Fig. S2).
Fig. S2.
Age and patrolling participation, calculated as the number of patrols in which a male participated relative to the number that occurred while he was alive and of patrolling age (≥13 y). We calculated patrol participation and age (on January 1) for each male on a yearly basis. Data points represent the means of these yearly values; error bars represent SDs.
Group Size and Individual and Aggregate Patrolling Effort.
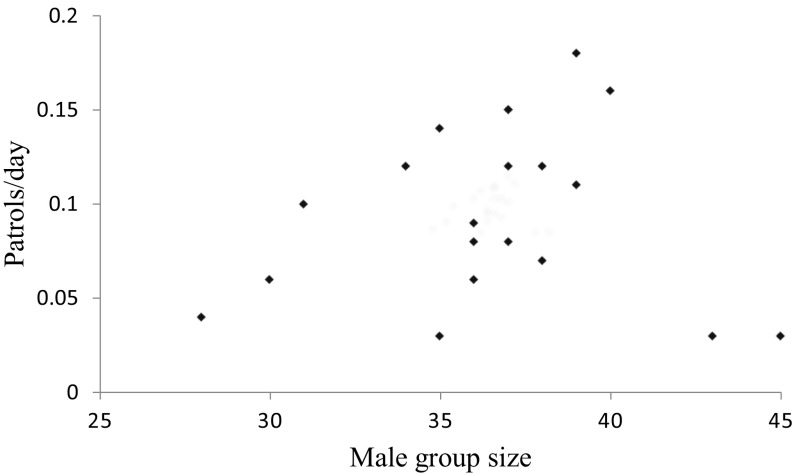
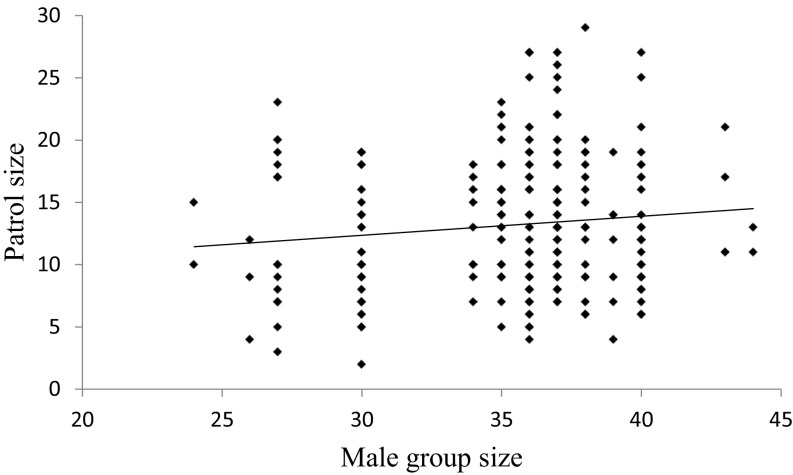
We investigated the relationship between group size and individual patrol participation by counting the number of males ≥13 y old living in the group on the day each patrol occurred (mean ± SD = 35.5 ± 3.9 males, range = 24–44). Individual males were less likely to participate when the number of potential patrollers in the group was relatively large (Table 1). Although similar results from previous research have been interpreted as evidence for collective action breaking down in large groups (11, 12, 23), two additional analyses that evaluated aggregate patrolling effort (i.e., the total amount of patrolling effort produced by the group as a whole) indicated that this breakdown did not occur. First, the number of patrols per day did not decrease with increasing group size (Pearson r = 0.06, P = 0.70, n = 20 y) (Fig. 2). Second, patrol size, assayed by the number of participating males, increased with group size, albeit weakly and nonsignificantly, rather than decreased (Pearson’s r = 0.11, P = 0.06, n = 284 patrols) (Fig. 3).
Fig. 2.
The relationship between the aggregate patrolling effort (the number of patrols per day in each year of the study) and group size (the number of males aged ≥13 y in the group on January 1 of each year). n = 20 y.
Fig. 3.
The relationship between the aggregate patrolling effort (patrol size: the number of males in the patrol) and group size (number of males aged ≥13 y in the group on the day the patrol occurred). n = 284 patrols.
The Ngogo chimpanzees expanded their territory by 22% (6.4 km2) in 2009 (33), and they might subsequently have required more patrolling effort to protect the larger territory. This difference in territorial size introduces a potential confound in the preceding analyses of group size and aggregate patrolling effort: Total aggregate patrolling effort may not have decreased with group size, but effort per unit of territory could still have fallen. Two additional analyses revealed that such was not the case. The number of patrols per day (Pearson’s r = −0.17, P = 0.94, n = 20 y) and patrol size (Pearson’s r = −0.01, P = 0.84, n = 284 patrols) were uncorrelated with male group size after we divided these two measures of aggregate patrolling effort by the contemporaneous territory size.
Long-Term Male Reproductive Success.
Group augmentation theory predicts that individuals will bear the short-term costs of collective action, even if they have little to gain immediately, if it increases group size, which in turn improves an individual’s future reproduction. We assessed this possibility by analyzing paternities for a sample of 113 offspring born during the study period. Among the 11 males present for all 113 births, the mean number of offspring sired was 3.3. This number is well above the replacement value of two offspring produced over the lifetime of an individual and indicates a growing population, especially considering that male chimpanzees can have reproductive careers that span up to 41 y (45).
This high average male reproductive success was coupled with reproductive skew sufficiently low as to be statistically undetectable, as indicated by a Nonacs’ binomial skew index (B) (62) of 0.0031 that had 95% CIs overlapping 0 (−0.0086 to 0.0024). The CI included the minimum possible B value (−0.0086), indicating that males might actually have shared reproduction equally. Two of the 11 males present for all births during the study period each fathered the highest number of offspring (seven). Only one male failed to father any offspring; however, his mating success was high over most of the study period, so it is likely that he was sterile.
Eleven males patrolled when they had no living offspring in the group. Only four of these males failed to sire any offspring subsequently. All four were alive and of reproductive age for relatively short times during the study period (mean = 8.6 y), either because they died relatively young (two males, aged 22 and 26 y) or because they were included in the sample relatively late (at the start of 2007 and 2012, respectively).
Discussion
Considerable research on collective action and between-group aggression in animals has focused on two key predictions: (i) collective action problems are common but can be solved through exploitation of the great by the small, whereby a minority of individuals who derive the highest benefits and/or pay the lowest costs by performing the collective action produce the common good, and (ii) collective action is especially difficult to achieve in large groups (the group size paradox). In support of these predictions, male chimpanzees were more likely to participate in territorial boundary patrols when they had more offspring living in the group (and thus stood to gain higher direct fitness benefits) and when they were high ranking (and thus presumably faced relatively low direct fitness costs). The effect of age, another measure of direct fitness costs, was equivocal: Although patrol participation was unrelated to age in the multivariate analysis, univariate analysis revealed a clear inverted-U shaped relationship, with males patrolling less frequently during adolescence and old age when it is presumably most costly. Additionally, individual participation in patrols decreased with group size, as assayed by the number of males of patrolling age living in the group on the day the patrol occurred.
However, four other results did not fully accord with the predictions of collective action theory. First, the average participation rate for the best-sampled males was quite high (42%). For comparison, in vervet monkeys, which also display a male bias in participation in between-group aggression, individual males participated in an average of only 25.3% of aggressive intergroup encounters (7). In blue monkeys, in which participation is strongly female-biased, the female who participated most often in intergroup encounters did so less often (17.9% of cases) (10) than the male who joined the fewest patrols among the best-sampled chimpanzees (21.5%). Second, individual variation in patrol participation by male chimpanzees was lower than the variation in participation in intergroup encounters documented in other primate species, in which several individuals of the more frequently participating sex consistently failed to take part (4, 63). For example, in a study of blue monkeys, the female who participated most often in intergroup encounters did so nine times more often than the female who participated least frequently but did participate a nonzero number of times (10). In contrast, participation in patrols by the best-sampled male chimpanzees varied only by a factor of 2.5 (Fig. 1). A strong test of the hypothesis that male chimpanzees patrol more often and with less variation than other primates who participate in intergroup encounters will require more data from additional species. Third, the number of close maternal relatives males possessed did not affect their participation in patrols, and males frequently patrolled even when they had no close maternal relatives or offspring in the community to protect. Finally, although individual participation in patrolling decreased with group size, this decrease in individual participation did not reduce the overall frequency of patrols or the mean number of males per patrol, either of which would have decreased the effectiveness of collective action or might even have led to its failure. Instead, as group size increased, the total amount of collective action remained more or less constant, even as the mean level of individual participation, and hence the level of costs per individual, decreased (i.e., load-lightening occurred).
Although close inspection of Figs. 2 and 3 suggests that aggregate patrolling effort may have decreased when group size was at its maximum (43–45 males), this decrease does not necessarily indicate a reduction or failure of collective action. Male group size achieved its maximum after 2009 when the Ngogo chimpanzees expanded their territory to the northeast after frequently patrolling and killing many individuals in this area over the previous 10 y (33). The reduction in aggregate patrolling effort is likely to have occurred because success in between-group competition decreased the need or benefits of patrolling.
Although these latter results are at odds with a strict interpretation of Olson’s collective action theory, they are congruent with expectations from group augmentation theory, which suggests how cooperation can evolve when the short-term costs of helping behavior are outweighed by the long-term benefits of living in a large group. Males presumably patrolled at high rates and with little regard to variation in short-term fitness benefits, even when group size was large, because they were likely to reproduce in the future. This likelihood, in turn, is linked to two factors that are themselves probably a function of large group size. First, the high average reproductive success of male chimpanzees at Ngogo is related to the facts that survivorship at most ages is considerably higher and life expectancy at birth is longer than in other, smaller chimpanzee groups (64). Longevity is typically the single most important factor influencing reproductive success in nonhuman primates (14). Although an unusually abundant food supply is a probable proximate cause for high survivorship at Ngogo (65, 66), this same food supply is acquired (33) and maintained through the collective patrolling efforts of male chimpanzees (cf. ref. 43). Male chimpanzees at Ngogo patrol more frequently than do males in smaller chimpanzee groups (67), some of which have been observed to curtail patrolling drastically following large reductions in group size (68). Success in between-group competition has potential consequences for male reproduction beyond increased survival, including increased retention of natal females, recruitment of parous females from other groups, and increased female fertility. Whether these factors apply at Ngogo is the subject of ongoing research.
A second factor producing a high probability of future reproduction by individual males at Ngogo is the low degree of reproductive skew. As in other primate species living in multimale, multifemale groups (14), the strength of the relationship between male dominance rank and reproductive success decreases with group size in chimpanzees (48).This leveling of reproductive success should provide an incentive for all males to work hard to increase group size so as to maximize their reproductive opportunities (cf. ref. 69).
Why does group augmentation lead to the evolution of more extensive collective action in chimpanzees than in most or all other group-living primate species for which group size is positively associated with success in between-group competition? The imbalance-of-power hypothesis emphasizes that fission–fusion dynamics play a key role by creating low-cost opportunities for large parties to kill members of smaller parties in neighboring groups (38). Fission–fusion dynamics may also facilitate the evolution of patrolling and intergroup killing in another way: by placing lower constraints on the evolution of large group size in chimpanzees compared with other primates. In many female-philopatric primate species, the reproductive success of individual females is highest in medium-sized groups, reflecting the balance between the benefits and costs of group living. Although success in between-group competition for food increases with group size, so too does within-group competition for food (70, 71). However the flexible fission–fusion social system of chimpanzees allows them to adjust the size of their temporary foraging parties facultatively according to food availability (72). Moreover, access to mates rather than access to food is the main factor limiting male reproductive success (73), and success in between-group competition has a major impact on male chimpanzee access to mates (74). The importance of large group size for success in between-group competition in chimpanzees may help explain why both genetic data (75) and long-term behavioral observations (41) indicate that permanent group fissions are much rarer in chimpanzees than in female philopatric primate species. In the only documented chimpanzee group fission, male members of the larger postfission group systematically killed all the male members of the smaller postfission group and recruited most of their females (41).
Taken together, the results presented here contribute to our knowledge of between-group aggression displayed by animals in general and chimpanzees in particular. Our findings furnish insights into how male chimpanzees living in a large group with low average relatedness among its members solve the potential collective action problem created by territorial boundary patrols. Although some of our results were consistent with the predictions of collective action theory, other results suggest that it will be important to move beyond this short-term perspective to understand how animals, including humans, solve the cooperation problem posed by between-group aggression and other forms of collective action. Specifically, we propose that greater attention be paid to the long-term direct benefits individuals obtain by living in large groups via group augmentation. A deeper understanding of the multiple direct benefits individuals accrue by cooperating with others is likely to emerge as a consequence (27).
Materials and Methods
The Ngogo chimpanzees have been studied continuously since 1995. Ages of individuals already present in 1995 were estimated based on visual criteria and genetically derived pedigrees; ages of individuals born after 1995 are known to within a few days or months. We assayed dominance ranks from submissive vocalizations and decided agonistic encounters. We performed extensive genetic analyses on fecal samples to determine paternity success, maternal relatedness, and reproductive skew. Further methodological details are described in SI Materials and Methods.
Fieldwork at Ngogo was approved by the Uganda Wildlife Authority and the Ugandan National Council for Science and Technology and was judged to be exempt from formal review by Institutional Animal Care and Use and Committees at Arizona State University, Yale University, and the University of Michigan because of its purely noninvasive, observational nature.
SI Materials and Methods
Study Site, Subjects, and the Sample.
The Ngogo study site straddles the equator (0°29′53′′ N, 30°5′0′′ E) and lies in the center of Kibale National Park in southwest Uganda. Neighboring chimpanzees live around the entire periphery of the ∼35-km2 Ngogo chimpanzee territory, making it necessary for male chimpanzees to patrol and defend the entire boundary of their range. Chimpanzees at Ngogo have been observed continuously since 1995. Because male chimpanzees carry out territorial boundary patrols silently and surreptitiously, they are difficult to observe and document at the start of study when subjects are not very well habituated to human presence. Therefore we started to record this behavior systematically only after the first year of study. The observations we report here are based on 284 patrols recorded over 2,621 d between September 1996 and August 2015. All analyses of patrolling males include only those aged ≥13 y at the time the patrol occurred.
Age.
Most males in our sample were already alive at the start of continuous long-term observations at Ngogo in 1995. We estimated their ages based on visual assessment of growth, development, and signs of senescence. We also assessed their ages at the start of the study using comparative data from other well-studied populations of eastern chimpanzees and detailed information about the reproductive histories of individuals established through observation and genetics. Other males were born during the study period, and we knew their ages to within a few days or months (64).
Dominance Rank.
We assigned dominance ranks based on the direction of pant-grunts, a call given by low-ranking males to high-ranking males, and decided agonistic encounters between dyads. Ordinal ranks (R) were assigned to each male on a yearly basis. Here we first entered data on decided dominance interactions between each male dyad into an actor–receiver matrix in which “wins” (pant-grunts received plus aggressive acts that induced submission) were above the diagonal. We then used MatMan software (76) to order the males in a hierarchy that minimized the number of males and to calculate Landau’s index of linearity (H′) modified to account for empty cells. We also used MatMan to determine whether the hierarchy was significantly linear by comparing the observed H′ to the H′ calculated on10,000 randomly permuted matrices; the results were highly significant for all years. In the few cases in which males had tied R, they were given the same R value. Because the number of males varied across years, and thus the same rank had different meanings in different years (e.g., a male with R = 24 is extremely low ranking when the group consists of 24 males but is midranking when the group consists of 44 males), we standardized R to the number of males aged ≥13 y in the hierarchy (nm) using the following formula: (nm − R)/(nm − 1) (77). We could not calculate R for some very young males because we rarely saw them receive pant-grunts or win an agonistic interaction (78). We assigned all these males a standardized dominance rank of 0 to indicate that we lacked data suggesting that they were higher ranking than any other male in the hierarchy, even though there may have been actual rank differences among them. The possible consequences of this potential source of error in the dominance rank variable are likely to be small, because only 1,702 (16.9%) of the 10,079 male-subject/patrol combinations in our total dataset involved males assigned a rank of 0.
Maternal Relatedness.
We calculated maternal relatedness as the sum of the male’s expected average relatedness value (r) with close, readily recognizable maternal relatives alive on the date of the patrol (i.e., mother: r = 0.5, maternal brother: r = 0.25, maternal sister: r = 0.25, maternal sister’s daughter: r = 0.125, maternal sister’s son: r = 0.125). For example, a male with one maternal brother (r = 0.25) alive at the time of the patrol would be assigned a maternal relatedness value of 0.25, whereas a male with a mother (r = 0.5), maternal sister (r = 0.25), and maternal sister’s son (r = 0.125) would be assigned a maternal relatedness value of 0.875. Maternal relatedness values ranged from 0 to 1.875, with a mean (± SD) of 0.502 (± 0.492).
Paternity Success.
We calculated paternity success as the sum of the male’s relatedness values to his living offspring (r = 0.5) on the date of the patrol. For example, a male with one living offspring on the date of the patrol (r = 0.5) would be assigned a paternity success value of 0.5, and a male with three living offspring would be assigned a paternity success value of 1.5. Paternity success values ranged from 0–3.5, with a mean (± SD) of 0.8 (± 0.9). We used a total of 122 paternities to generate paternity success values.
Genetic Analyses.
We used genetic analyses based on noninvasively collected fecal samples to determine the kinship relationships used in the paternity success and maternal relatedness variables. Paternity success was determined by assigning paternity through likelihood-based parentage analyses of 19–44 autosomal microsatellite loci (79), the sharing of at least one allele at each of 13 X-linked microsatellite loci (for father–daughter dyads only), and the sharing of the same 13 microsatellite loci Y-chromosome haplotypes (for father–son dyads only). For the great majority of dyads, we determined maternal relatedness by constructing pedigrees based on genetic paternity and maternity assignments. Like genetic paternity assignments, genetic maternity assignments were based on likelihood-based parentage analyses of 19–44 autosomal microsatellite loci and markers with sex-specific patterns of inheritance (mothers had to share at least one allele at 13 X-linked microsatellite loci and the same mitochondrial DNA haplotype with both daughters and sons). A few individuals, especially those who were older at the start of the study, could not be placed directly into the pedigree because their mother and/or father had died before sampling and genotyping were possible and therefore was not directly identified through genetic parentage analyses. We determined the maternal sibship status of these dyads through a combination of relatedness values at the autosomal and X-linked loci and mtDNA and Y-chromosome haplotype-sharing information. We used a set of decision rules that have been shown previously to generate acceptably low false-positive and false-negative rates of maternal sibship status assignment in a large, independent sample of dyads whose maternal sibship status was known directly from genetic maternity analyses as described above. (See refs. 50 and 53 for further details of analyses used to determine kinship relationships and the laboratory methods used to produce reliable genetic data from noninvasively collected DNA source material.)
Statistical Analyses.
We conducted GLMM analyses (47) fit with Laplace approximation to determine the factors that influenced patrolling frequency by individual male chimpanzees. This model was based on a subset of 179 patrols that occurred after June 4, 2003, when our knowledge of maternal genetic relationships and paternity at the time patrols occurred was nearly complete (45, 48, 50, 53). We considered patrols as the units of analysis and used whether a male did (1) or did not (2) participate in a patrol as the binary response variable. We investigated the influence of five fixed effects on patrol participation by individual males: (i) paternity success; (ii) dominance rank; (iii) age; (iv) maternal relatedness; and (v) male group size. For each patrol, we entered for each male his specific individual value for each of the first four variables above; the fifth variable was the same for all males. We Z-transformed the fixed effect predictor variables so that each had a mean of 0 and an SD of 1. In addition to these five fixed effects, we also included male identity and date of the patrol as random effects. Random differences between males may impact how the fixed effects influence patrol participation. To account for this impact, we incorporated the random slopes of fixed effects within individuals in the model (80). We conducted GLMMs in R (version 3.2.1; R Core Team 2015) using the glmer function of the R package lme4 (81). We examined collinearity among our fixed effects with the vif (variance inflation factor) function in R and found that all were substantially lower (range: 1–2) than what is commonly considered “high,” (i.e., 5–10). We visually inspected the distribution of the fitted random effects to ensure that they were not highly skewed or bimodal. To examine model stability, we compared the estimates from the full models with those from reduced models in which we excluded one level at a time in each of the two random effects. Results of these analyses were quantitatively similar.
To evaluate the statistical significance of the fixed effects, we used a multistage bootstrapping procedure that accounted for the structure of our data to generate 95% CIs for each estimate. We refit models after each of 1,000 resamples with replacement first from the date of patrol level and then from the male identity level. We then converted the combined bootstrap estimates into a matrix from which we calculated the 2.5th and 97.5th percentiles for each estimate. We considered estimates whose 95% CIs did not include 0 to be statistically significant predictors of patrol participation.
We carried out two additional analyses to determine whether large group size led to a failure of collective action and a reduction in the production of the public good. First, we analyzed the relationship between male group size and patrolling effort (n = 20 y). We used the number of patrols⋅d−1⋅y−1 to assay patrolling effort, including only days on which we observed the chimpanzees for ≥6 h. We correlated this number with the number of male chimpanzees in the community, assayed as the number of males aged ≥13 y on January 1 of the year. Second, we correlated the number of males participating in each patrol with male group size at the time of the patrol (n = 284). If large group size negatively affects aggregate collective action, then patrols should occur less often and be smaller as groups increase in size. To control for the possible effect of variation in territory size on these analyses, we repeated them while dividing our two measures of aggregate patrolling effort by the contemporaneous territory size of the Ngogo chimpanzee community. The Ngogo chimpanzees maintained a territory that covered 28.76 km2 until 2009. Thereafter they expanded their territory at the expense of neighbors to the northeast, increasing it to encompass 35.16 km2 (33).
In a final analysis, we calculated Nonacs’ B index (62) to quantify levels of reproductive skew among males who fathered 113 offspring during the 20 y we sampled patrols. Our knowledge of paternity for offspring conceived during this period was nearly complete (45, 48, 50, 53).
The B index is the difference between the observed and expected variance in paternity success. The expected variance reflects a situation in which every male has an equal probability of fathering any given offspring and is calculated as the variance of the binomial distribution. The B index is the only reproductive skew index that adjusts for variation in the length of time that individuals spend as group members. We captured this variation by entering, for each male, the number of conceptions in the group while he was present in the group and was ≥13 y old (mean = 65.7, range = 3–113).
Data Sharing.
Data used in this paper are available at datadryad.org (doi:10.5061/dryad.kk33f).
Acknowledgments
We thank The Uganda Wildlife Authority and the Uganda National Council for Science and Technology for research permission; Makerere University Biological Field Station for logistical support; G. Mbabazi, L. Ngandizi, A. Tumusiime, and A. Twineomujuni for field assistance; S. Amsler, B. Pav, A. Sandel, and M. Sobolewski for contributing patrol data; C. Rowney, A. Heilman, and A. Nicklisch for laboratory assistance; R. Mundry for assistance with the GLMM; S. Angedakin and the late J. Lwanga for project management; and J. B. Silk for discussions. Our research has been supported by National Science Foundation Awards SBR-9253590, BCS-0215622, and IOB-0516644, NIH Grant R01AG049395, the Detroit Zoological Society, the Leakey Foundation, the National Geographic Society, Primate Conservation Inc., the Wenner-Gren Foundation, the President’s Strategic Initiative Fund of Arizona State University, Boston University, the University of Michigan, Yale University, the Max Planck Society, and the Institute of Human Origins.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data used in this paper are available at datadryad.org (doi:10.5061/dryad.kk33f).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701582114/-/DCSupplemental.
References
- 1.Esteban J, Ray D. Collective action and the group size paradox. Am Polit Sci Rev. 2001;95:663–672. [Google Scholar]
- 2.Olson M. The Logic of Collective Action. Harvard Univ Press; Cambridge, MA: 1965. [Google Scholar]
- 3.Gavrilets S. Collective action problem in heterogeneous groups. Philos Trans R Soc Lond B Biol Sci. 2015;370:20150016. doi: 10.1098/rstb.2015.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitchen DM, Beehner JC. Factors affecting individual participation in group-level aggression among non-human primates. Behaviour. 2007;144:1551–1581. [Google Scholar]
- 5.Willems EP, Hellriegel B, van Schaik CP. The collective action problem in primate territory economics. Proc R Soc B Biol Sci. 2013;280:20130081. doi: 10.1098/rspb.2013.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Belle S, Garber PA, Estrada A, Di Fiore A. Social and genetic factors mediating male participation in collective group defence in black howler monkeys. Anim Behav. 2014;98:7–17. [Google Scholar]
- 7.Arseneau TJM, Taucher AL, van Schaik CP, Willems EP. Male monkeys fight in between-group conflicts as protective parents and reluctant recruits. Anim Behav. 2015;110:39–50. [Google Scholar]
- 8.Kitchen DM, Seyfarth RM, Cheney DL, Seyfarth RM. Factors mediating inter-group encounters in savannah baboons (Papio cynocephalus ursinus) Behaviour. 2004;141:197–218. [Google Scholar]
- 9.Majolo B, Ventura R, Koyama NF. Sex, rank and age differences in the Japanese macaque (Macaca fuscata yakui) participation in inter-group encounters. Ethology. 2005;111:455–468. [Google Scholar]
- 10.Cords M. Variable participation in the defense of communal feeding territories by blue monkeys in the Kakamega Forest, Kenya. Behaviour. 2007;144:1537–1550. [Google Scholar]
- 11.Koch F, Signer J, Kappeler PM, Fichtel C. Intergroup encounters in Verreaux’s sifakas (Propithecus verreauxi): Who fights and why? Behav Ecol Sociobiol. 2016;70:797–808. doi: 10.1007/s00265-016-2105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonanni R, Valsecchi P, Natoli E. Pattern of individual participation and cheating in conflicts between groups of free-ranging dogs. Anim Behav. 2010;79:957–968. [Google Scholar]
- 13.Cheney DL. Intergroup encounters among free-ranging vervet monkeys. Folia Primatol (Basel) 1981;35:124–146. doi: 10.1159/000155970. [DOI] [PubMed] [Google Scholar]
- 14.Alberts SC. Magnitude and sources of variation in male reproductive performance. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. The Evolution of Primate Societies. Univ of Chicago Press; Chicago: 2012. pp. 412–431. [Google Scholar]
- 15.Pusey AE. Magnitude and sources of variation in female reproductive performance. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. The Evolution of Primate Societies. Univ of Chicago Press; Chicago: 2012. pp. 343–366. [Google Scholar]
- 16.Hamilton WD. The evolution of altruistic behavior. Am Nat. 1963;97:354–356. [Google Scholar]
- 17.Kitchen DM, Horwich RH, James RA. Subordinate male black howler monkey (Alouatta pigra) responses to loud calls: Experimental evidence for the effects of intra-group male relationships and age. Behaviour. 2004;141:703–723. [Google Scholar]
- 18.Nunn CL, Deaner RO. Patterns of participation and free riding in territorial conflicts among ringtailed lemurs (Lemur catta) Behav Ecol Sociobiol. 2004;57:50–61. [Google Scholar]
- 19.Nunn CL. Collective benefits, free-riders, and male extra-group conflict. In: Kappeler PM, editor. Primate Males: Causes and Consequences of Variation in Group Composition. Cambridge Univ Press; Cambridge, UK: 2000. pp. 192–204. [Google Scholar]
- 20.Silk JB. Social components of fitness in primate groups. Science. 2007;317:1347–1351. doi: 10.1126/science.1140734. [DOI] [PubMed] [Google Scholar]
- 21.Zefferman MR, Mathew S. An evolutionary theory of large-scale human warfare: Group-structured cultural selection. Evol Anthropol. 2015;24:50–61. doi: 10.1002/evan.21439. [DOI] [PubMed] [Google Scholar]
- 22.Lukas D, Reynolds V, Boesch C, Vigilant L. To what extent does living in a group mean living with kin? Mol Ecol. 2005;14:2181–2196. doi: 10.1111/j.1365-294X.2005.02560.x. [DOI] [PubMed] [Google Scholar]
- 23.Crofoot MC, Gilby IC. Cheating monkeys undermine group strength in enemy territory. Proc Natl Acad Sci USA. 2012;109:501–505. doi: 10.1073/pnas.1115937109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kokko H, Johnstone RA, Clutton-Brock TH. The evolution of cooperative breeding through group augmentation. Proc R Soc Lond B Biol Sci. 2001;268(1463):187–196. doi: 10.1098/rspb.2000.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clutton-Brock TH, et al. Individual contributions to babysitting in a cooperative mongoose, Suricata suricatta. Proc R Soc Lond B Biol Sci. 2000;267(1440):301–305. doi: 10.1098/rspb.2000.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright J. Cooperation theory meets cooperative breeding: Exposing some ugly truths about social prestige, reciprocity and group augmentation. Behav Processes. 2007;76:142–148. doi: 10.1016/j.beproc.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Clutton-Brock T. Breeding together: Kin selection and mutualism in cooperative vertebrates. Science. 2002;296:69–72. doi: 10.1126/science.296.5565.69. [DOI] [PubMed] [Google Scholar]
- 28.Kingma SA, Santema P, Taborsky M, Komdeur J. Group augmentation and the evolution of cooperation. Trends Ecol Evol. 2014;29:476–484. doi: 10.1016/j.tree.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Cockburn A. Evolution of helping behavior in cooperatively breeding birds. Annu Rev Ecol Sytematics. 1998;29:141–177. [Google Scholar]
- 30.Crick HQP. Load-lightening in cooperatively breeding birds and the cost of reproduction. Ibis (Lond 1859) 1992;134(1):56–61. [Google Scholar]
- 31.Watts DP, Mitani JC. Boundary patrols and intergroup encounters in wild chimpanzees. Behaviour. 2001;138:299–327. [Google Scholar]
- 32.Mitani JC, Watts DP. Correlates of territorial boundary patrol behaviour in wild chimpanzees. Anim Behav. 2005;70:1079–1086. [Google Scholar]
- 33.Mitani JC, Watts DP, Amsler SJ. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr Biol. 2010;20(12):R507–R508. doi: 10.1016/j.cub.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Nishida T. The social group of wild chimpanzees in the Mahale Mountains. Primates. 1968;9:167–224. [Google Scholar]
- 35.Amsler SJ. Energetic costs of territorial boundary patrols by wild chimpanzees. Am J Primatol. 2010;72:93–103. doi: 10.1002/ajp.20757. [DOI] [PubMed] [Google Scholar]
- 36.Sobolewski ME, Brown JL, Mitani JC. Territoriality, tolerance and testosterone in wild chimpanzees. Anim Behav. 2012;84:1469–1474. [Google Scholar]
- 37.Wittig RM, et al. Social support reduces stress hormone levels in wild chimpanzees across stressful events and everyday affiliations. Nat Commun. 2016;7:13361. doi: 10.1038/ncomms13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wrangham RW. Evolution of coalitionary killing. Am J Phys Anthropol. 1999;42:1–30. doi: 10.1002/(sici)1096-8644(1999)110:29+<1::aid-ajpa2>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Thompson FJ, Marshall HH, Vitikainen EIK, Cant MA. Causes and consequences of intergroup conflict in cooperative banded mongooses. Anim Behav. 2017;126:31–40. [Google Scholar]
- 40.Mech LD, Adams LG, Meier TJ, Burch JW, Dale TW. The Wolves of Denali. Univ of Minnesota Press; Minneapolis: 1998. [Google Scholar]
- 41.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. The Belknap Press of Harvard Univ Press; Cambridge, MA: 1986. [Google Scholar]
- 42.Nishida T, Hiraiwa-Hasegawa M, Hasegawa KYT. Group extinction and female transfer in wild chimpanzees in the Mahale Mountains National Park, Tanzania. Z Tierpsychol. 1985;67:281–301. [Google Scholar]
- 43.Williams JM, Oehlert G, Pusey AE. Why do male chimpanzees defend a group range? Anim Behav. 2004;68:523–532. [Google Scholar]
- 44.Nishida T, Kawanaka K. Inter-unit-group relationships among wild chimpanzees of the Mahali mountains. Kyoto Univ African Studies. 1972;7:131–167. [Google Scholar]
- 45.Langergraber KE, et al. Generation times in wild chimpanzees and gorillas suggest earlier divergence times in great ape and human evolution. Proc Natl Acad Sci USA. 2012;109:15716–15721. doi: 10.1073/pnas.1211740109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langergraber K, et al. Genetic differentiation and the evolution of cooperation in chimpanzees and humans. Proc Biol Sci. 2011;278:2546–2552. doi: 10.1098/rspb.2010.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baayen RH. Analyzing Linguistic Data: A Practical Introduction to Statistics Using R. Cambridge Univ Press; Cambridge, UK: 2008. [Google Scholar]
- 48.Langergraber KE, Mitani JC, Watts DP, Vigilant L. Male-female socio-spatial relationships and reproduction in wild chimpanzees. Behav Ecol Sociobiol. 2013;67:861–873. [Google Scholar]
- 49.Murray CM, Stanton MA, Lonsdorf EV, Wroblewski EE, Pusey AE. Chimpanzee fathers bias their behaviour towards their offspring. R Soc Open Sci. 2016;3:160441. doi: 10.1098/rsos.160441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langergraber KE, Mitani JC, Vigilant L. The limited impact of kinship on cooperation in wild chimpanzees. Proc Natl Acad Sci USA. 2007;104:7786–7790. doi: 10.1073/pnas.0611449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitani JC. Male chimpanzees form enduring and equitable social bonds. Anim Behav. 2009;77:633–640. [Google Scholar]
- 52.Langergraber KE. Cooperation among kin. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. The Evolution of Primate Societies. Univ of Chicago Press; Chicago: 2012. pp. 491–513. [Google Scholar]
- 53.Langergraber K, Mitani J, Vigilant L. Kinship and social bonds in female chimpanzees (Pan troglodytes) Am J Primatol. 2009;71:840–851. doi: 10.1002/ajp.20711. [DOI] [PubMed] [Google Scholar]
- 54.Lehmann J, Fickenscher G, Boesch C. Kin biased investment in wild chimpanzees. Behaviour. 2006;143:931–955. [Google Scholar]
- 55.Wroblewski EE, et al. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim Behav. 2009;77:873–885. doi: 10.1016/j.anbehav.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inoue E, Inoue-Murayama M, Vigilant L, Takenaka O, Nishida T. Relatedness in wild chimpanzees: Influence of paternity, male philopatry, and demographic factors. Am J Phys Anthropol. 2008;137:256–262. doi: 10.1002/ajpa.20865. [DOI] [PubMed] [Google Scholar]
- 57.Newton-Fisher NE, Thompson ME, Reynolds V, Boesch C, Vigilant L. Paternity and social rank in wild chimpanzees (Pan troglodytes) from the Budongo Forest, Uganda. Am J Phys Anthropol. 2010;142:417–428. doi: 10.1002/ajpa.21241. [DOI] [PubMed] [Google Scholar]
- 58.Vigilant L, Hofreiter M, Siedel H, Boesch C. Paternity and relatedness in wild chimpanzee communities. Proc Natl Acad Sci USA. 2001;98:12890–12895. doi: 10.1073/pnas.231320498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pusey AE, Oehlert GW, Williams JM, Goodall J. Influence of ecological and social factors on body mass of wild chimpanzees. Int J Primatol. 2005;26:3–31. [Google Scholar]
- 60.Archie EA, Altmann J, Alberts SC. Social status predicts wound healing in wild baboons. Proc Natl Acad Sci USA. 2012;109:9017–9022. doi: 10.1073/pnas.1206391109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 62.Nonacs P. Measuring and using skew in the study of social behavior and evolution. Am Nat. 2000;156:577–589. doi: 10.1086/316995. [DOI] [PubMed] [Google Scholar]
- 63.Willems EP, Arseneau TJM, Schleuning X, van Schaik CP. Communal range defence in primates as a public goods dilemma. Philos Trans R Soc B Biol Sci. 2015;370:20150003. doi: 10.1098/rstb.2015.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wood BM, Watts DP, Mitani JC, Langergraber KE. Favorable ecological circumstances promote life expectancy in chimpanzees similar to that of human hunter-gatherers. J Hum Evol. 2017;105:41–56. doi: 10.1016/j.jhevol.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Potts KB, Chapman CA, Lwanga JS. Floristic heterogeneity between forested sites in Kibale National Park, Uganda: Insights into the fine-scale determinants of density in a large-bodied frugivorous primate. J Anim Ecol. 2009;78:1269–1277. doi: 10.1111/j.1365-2656.2009.01578.x. [DOI] [PubMed] [Google Scholar]
- 66.Potts KB, Watts DP, Wrangham RW. Comparative feeding ecology of two communities of chimpanzees (Pan troglodytes) in Kibale National Park, Uganda. Int J Primatol. 2011;32:669–690. [Google Scholar]
- 67.Mitani JC. Demographic influences on the behavior of chimpanzees. Primates. 2006;47:6–13. doi: 10.1007/s10329-005-0139-7. [DOI] [PubMed] [Google Scholar]
- 68.Boesch C, Boesch-Achermann H. The Chimpanzees of the Taï Forest. Oxford Univ Press; Oxford, UK: 2000. [Google Scholar]
- 69.Bowles S. Group competition, reproductive leveling, and the evolution of human altruism. Science. 2006;314:1569–1572. doi: 10.1126/science.1134829. [DOI] [PubMed] [Google Scholar]
- 70.Wrangham RW. An ecological model of female-bonded primate groups. Behaviour. 1980;75:262–300. [Google Scholar]
- 71.Majolo B, de Bortoli Vizioli A, Schino G. Costs and benefits of group living in primates: Group size effects on behaviour and demography. Anim Behav. 2008;76:1235–1247. [Google Scholar]
- 72.Mitani JC, Watts DP, Lwanga JS. Ecological and social correlates of chimpanzee party size and gregariousness. In: Hohmann G, Boesch C, editors. Behavioural Diversity in Chimpanzees and Bonobos. Cambridge Univ Press; Cambridge, UK: 2002. pp. 102–111. [Google Scholar]
- 73.Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man 1871-1971. Aldine; Chicago: 1972. pp. 136–179. [Google Scholar]
- 74.Boesch C, Kohou G, Néné H, Vigilant L. Male competition and paternity in wild chimpanzees of the Taï forest. Am J Phys Anthropol. 2006;130:103–115. doi: 10.1002/ajpa.20341. [DOI] [PubMed] [Google Scholar]
- 75.Langergraber KE, et al. How old are chimpanzee communities? Time to the most recent common ancestor of the Y-chromosome in highly patrilocal societies. J Hum Evol. 2014;69:1–7. doi: 10.1016/j.jhevol.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 76.de Vries H, Netto WJ, Hanegraaf PLH. Matman: A program for the analysis of sociometric matrices and behavioural transition matrices. Behaviour. 1993;125:157–175. [Google Scholar]
- 77.Muller MN, Thompson ME, Wrangham RW. Male chimpanzees prefer mating with old females. Curr Biol. 2006;16:2234–2238. doi: 10.1016/j.cub.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 78.Sandel AA, Reddy RB, Mitani JC. Adolescent male chimpanzees do not form a dominance hierarchy with their peers. Primates. 2017;58:39–49. doi: 10.1007/s10329-016-0553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marshall TC, Slate J, Kruuk LEB, Pemberton JM. Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. [DOI] [PubMed] [Google Scholar]
- 80.Schielzeth H, Forstmeier W. Conclusions beyond support: Overconfident estimates in mixed models. Behav Ecol. 2009;20:416–420. doi: 10.1093/beheco/arn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]