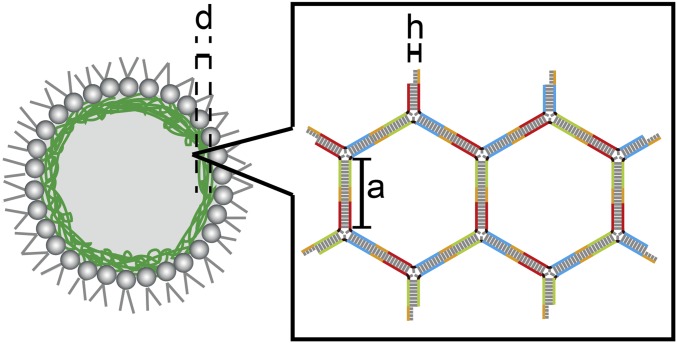
Significance
Although liposomes and lipid droplets have been used for numerous applications, the fragility of the lipid membrane causes an unintentional collapse, which is problematic for advanced applications. To solve this problem, we constructed an artificial cytoskeleton with DNA nanotechnology (a DNA cytoskeleton). The DNA cytoskeleton is a DNA network formed underneath the membrane of positively charged lipids through electrostatic interactions without the need for special handling. The DNA cytoskeleton significantly improves mechanical stability and, therefore, confers tolerance against osmotic shock to liposomes like the cytoskeleton in live cells. Because of its biocompatibility and the easiness of implementing design changes, the DNA cytoskeleton could become a tool for great stabilizer of liposomes and lipid droplets.
Keywords: cytoskeleton, self-assembly, DNA gel, lipid droplet, liposome
Abstract
Cell-sized liposomes and droplets coated with lipid layers have been used as platforms for understanding live cells, constructing artificial cells, and implementing functional biomedical tools such as biosensing platforms and drug delivery systems. However, these systems are very fragile, which results from the absence of cytoskeletons in these systems. Here, we construct an artificial cytoskeleton using DNA nanostructures. The designed DNA oligomers form a Y-shaped nanostructure and connect to each other with their complementary sticky ends to form networks. To undercoat lipid membranes with this DNA network, we used cationic lipids that attract negatively charged DNA. By encapsulating the DNA into the droplets, we successfully created a DNA shell underneath the membrane. The DNA shells increased interfacial tension, elastic modulus, and shear modulus of the droplet surface, consequently stabilizing the lipid droplets. Such drastic changes in stability were detected only when the DNA shell was in the gel phase. Furthermore, we demonstrate that liposomes with the DNA gel shell are substantially tolerant against outer osmotic shock. These results clearly show the DNA gel shell is a stabilizer of the lipid membrane akin to the cytoskeleton in live cells.
Liposomes have been used as artificial cell models to understand cell shape, membrane protein function, and lipid−protein interaction, among other biological functions (1–3). In addition, liposomes have been used as a platform for biosensing and as drug delivery systems (DDS) because of their excellent biocompatibility and biodegradability (4). However, liposomes collapse easily against environmental shifts and mechanical forces because of their low bending modulus. The fragility of liposomes causes uncontrolled leakage of the entrapped compounds and thus inhibits their use in biomedical applications and artificial cells experiments.
In contrast, cell membranes are tolerant against environmental shifts and mechanical forces. The stability of cell membrane arises from the cytoskeleton underneath the membrane. The major component of cytoskeletons is actin (5). Actin gels show high elasticity (6), which ensures the stability of cell membranes against various forces. For liposomes, the use of actin filaments as a cytoskeleton is not an optimal strategy for the following three reasons: First, although actin bundles and actomyosin rings have been reconstituted in artificial cells (7, 8), formation of an actin cortex underneath artificial membranes has been still challenging. Second, actin is hard to modify by chemical and genetic means because of its essentiality for cell growth. Third, the physicochemical properties of actin gels are still unclear (9, 10). Hence, the cytoskeleton of liposomes should be constructed with defined and designable materials. To accomplish this aim, DNA nanotechnology, which uses limited components with high designability in a nanometer scale (11), is a feasible candidate to construct cytoskeleton structures in artificial cells.
DNA nanostructures form three- or four-way junctions, connect with each other through their complementary sticky ends, and are reported to become gels by forming network structures (11–14). DNA gels have several advantages as the cytoskeleton of liposomes compared with other polymers: (i) the gelation temperature of DNA gels is easily controlled by changing the DNA sequences and nanostructures, (ii) the interaction between DNA gels and lipid membranes can be controlled because the negatively charged DNA has an attractive force with the positively charged membrane (15), and (iii) DNA sequence is easily designed with computer software [such as Nucleic Acid Package (NUPACK) (16) and Cadnano (17)] and easily chemically modified (11).
Here, we implemented a DNA cytoskeleton by enforced localization of Y-shaped DNA nanostructures (hereafter referred to as Y-motif DNA) on lipid membranes using the attractive interaction between DNA and positively charged lipids. We succeeded in preparing droplets and liposomes that have a shell-like structure of self-assembled DNA underneath the membrane. The DNA shell in the gel phase increased the mechanical strength of the membrane, such as the interfacial tension of the droplet surface and the critical pressure where the droplets collapse. Therefore, the artificial cytoskeleton made from the DNA gel shell works to enhance the membrane stability akin to the cytoskeleton in cells.
Materials and Methods
Materials.
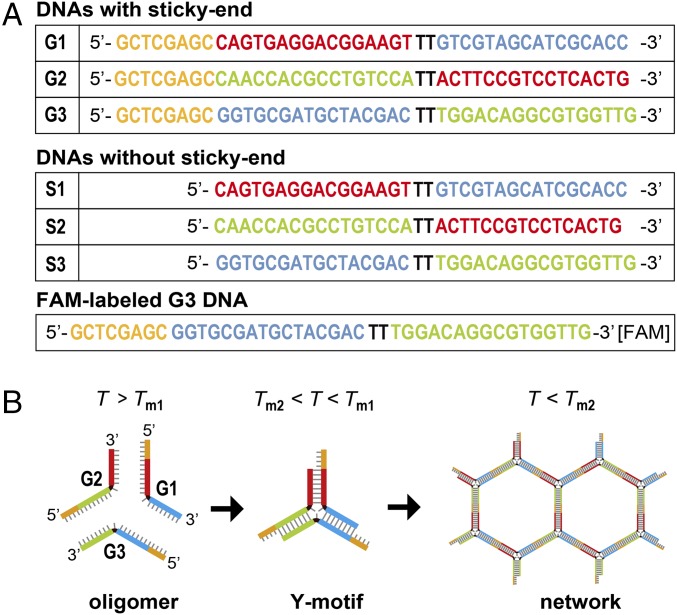
To prepare the artificial cells, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC; Avanti Polar Lipids), positively charged lipid 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) (chloride salt; Avanti Polar Lipids) and mineral oil (Nacalai Tesque) were used to prepare artificial cells. We used a fluorescently labeled lipid, 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (Rh-PE) (ammonium salt; Avanti Polar Lipids) to visualize the lipid membrane. We designed DNA oligomers that assemble to form nanostructures, both with sticky ends (G1 to G3) and without sticky ends (S1 to S3) (Fig. 1A), and obtained them as lyophilized powders (salt-free grade purification; Eurofins Genomics). To visualize DNA with sticky ends (G1 to G3), we replaced 1/120 of G3 with 6-carboxy-fluorescein (FAM)-labeled DNA G3, where the FAM was attached to the 3′ end of G3. In the case of DNA without sticky ends (S1 to S3), we mixed the oligomers with double-stranded DNA (dsDNA)-binding dye SYBR-Green I (Takara Bio), diluted with distilled water to 1:30,000. The designed DNA nanostructures were dissolved in distilled water to a concentration of 2 mM and stored at −20 °C until use. All materials were used without further purification.
Fig. 1.
DNA sequences used to form Y-motif DNA. (A) DNA sequences with or without sticky ends, which are marked as orange. Each color (red, green, and blue) shows its complementary sequence. (B) Schematic illustration of hybridization of DNA with sticky ends (orange) by decreasing the temperature (T). The three different DNA oligomers form a Y motif of dsDNA with their complementary regions below Tm1, and the Y-motif DNAs form a network structure by annealing with their sticky ends below Tm2.
Hybridization of DNA in Bulk.
DNA oligomers were dissolved in a buffer [20 mM Tris⋅HCl (pH 8.0) and 350 mM NaCl] at room temperature (∼20 °C). Equimolar solutions of DNA were mixed with dyes (1/120 of total oligodeoxynucleotide amount) to an approximate concentration of 18 μM. After heating at 80 °C for 10 min, the DNA solution was gradually cooled to 10 °C at a rate of 0.01 °C/s using a thermal cycler (T-Gradient; Biometra). The hybridized DNA with sticky ends (G1−G3) has two melting points; G1 to G3 forms a Y motif below Tm1, and the Y-motif units form duplexes at Tm2 (Fig. 1B). On the other hand, DNA without sticky ends (S1 to S3) has only one melting point at Tm1. In other words, DNA S1−S3 cannot form a network structure like DNA G1 to G3. The melting temperatures were estimated to be Tm1 = 69 °C and Tm2 = 49 °C, according to previous reports (18).
Preparation of Droplets and Liposomes.
Microdroplets covered with a lipid layer were prepared as reported previously (1, 19). Briefly, dry lipid films with Rh-PE (0.1 mol% of total lipid amount) were prepared on the bottom of a glass tube. These lipids were dissolved in mineral oil to a concentration of ∼2 mM by ultrasonication for 90 min using a sonicator with an output power of 90 W (Branson). The solution of hybridized DNA (i.e., DNA microspheres of G1 to G3 or DNA solution of S1 to S3) was dispersed in the lipids-in-oil (∼1/30 of total volume) by pipetting to prepare droplets covered with a lipid layer. An aliquot containing the droplet-encapsulated DNA was placed on a silicone-coated cover glass (Matsunami Glass) to prevent the droplets from sticking to the glass plate.
We used a droplet transfer method, as reported previously, to prepare the liposomes (20–22). POPC in mineral oil at a concentration of ∼2 mM was used to form the outer membrane. The outer solution of the liposomes was the same as the inner buffer solution. These procedures allowed us to obtain relatively abundant and large liposomes. The liposome solution was poured between two glass plates with a 0.5-mm silicone spacer for observation.
Fluorescence Observation of Artificial Cells with DNA.
Liposomes and droplets with DNA were observed by confocal laser scanning fluorescence microscopy (CLSM) (Olympus IX83 with FV1200; Olympus). Fluorescent images of Rh-PE and FAM, which were excited by a mercury lamp or a laser (488 nm and 473 nm for DNA and lipid membrane, respectively), were obtained using fluorescence filter sets (U-FGW and U-FBNA for mercury lamp; 530 nm to 550 nm and 470 nm to 495 nm for laser; Olympus). The pinhole size was fixed to be ∼1 μm. The obtained images were analyzed by National Institutes of Health Image J software. We evaluated the fluidity of DNA assemblies with fluorescence recovery after photobleaching (FRAP) experiments using CLSM. After photobleaching (bleach radius 1 μm to 3 μm, bleach time 1 s to 2 s for DNA and 10 min for lipid, laser power 100%), fluorescence recovery was recorded according to the manufacturer’s instructions (frame rate, 120 frame/s) and analyzed to determine the diffusion constant D by using software (Diffusion measurement package; Olympus) based on a 2D diffusion equation, described by Axelrod et al. (23).
Micropipette Aspiration of Artificial Cells.
To investigate how the DNA gel, underneath the artificial cell membrane, affects the mechanical properties of the membrane, we aspirated the droplets with the DNA by using a micropipette manipulator system (MMO-202ND and MN-4; Narishige) and microinjector (IM-11-2; Narishige) with a differential pressure transducer (DP15; Validyne), which was equipped with a microscope (Axiovert 40CFL; Carl Zeiss). We prepared glass micropipettes with the inner diameter of the tip being ∼5 μm by pulling glass capillaries (inner diameter 0.5 mm, GC-1; Narishige) with a puller (PC-10; Narishige) and a microforge (MF-900; Narishige).
Results and Discussion
Spontaneous Formation of DNA Spheres in Bulk.
As a material for the artificial cytoskeleton, we designed three sequences of DNA oligomers that assemble to form a Y motif. To ensure the flexibility for efficient Y-motif formation, TT sequences without hybridization pairs were inserted at the center of the Y motif (Fig. 1). We designed Y-motif DNA to have sticky ends to connect with each other to form a gel-like network structure.
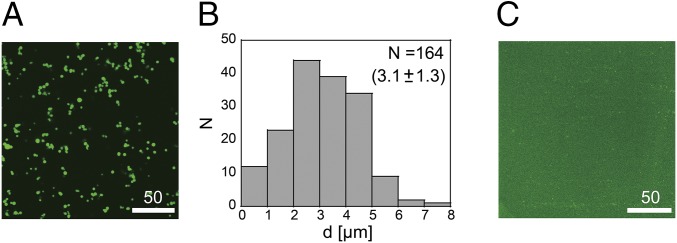
Before preparing DNA gel network inside artificial cells, we observed the hybridization of Y-motif DNAs in a bulk solution by using CLSM. Fig. 2A shows a typical CLSM image of hybridized Y-motif DNA with sticky ends (sequences G1 to G3). Similar to previous reports (14), micrometer-sized fluorescent spheres were observed. Because the fluorescence dye (SYBR-Green I) binds dsDNA, the green fluorescence indicates that the microspheres incorporated dsDNA structure. The distribution of the average diameter (d) from the binarized image (Fig. 2B) shows the average size is 3.1 μm. In testing hybridized DNA without sticky ends (sequences S1 to S3), no microspheres were observed (Fig. 2C).
Fig. 2.
(A) CLSM image of the microspheres incorporating hybridized DNA oligomers with sticky ends. (Scale bar, 50 μm.) (B) Histogram of the average diameter of the DNA microspheres. (C) CLSM image of the microspheres incorporating hybridized DNA oligomers without sticky ends. (Scale bar, 50 μm.)
DNA Shell in DOTAP Droplets.
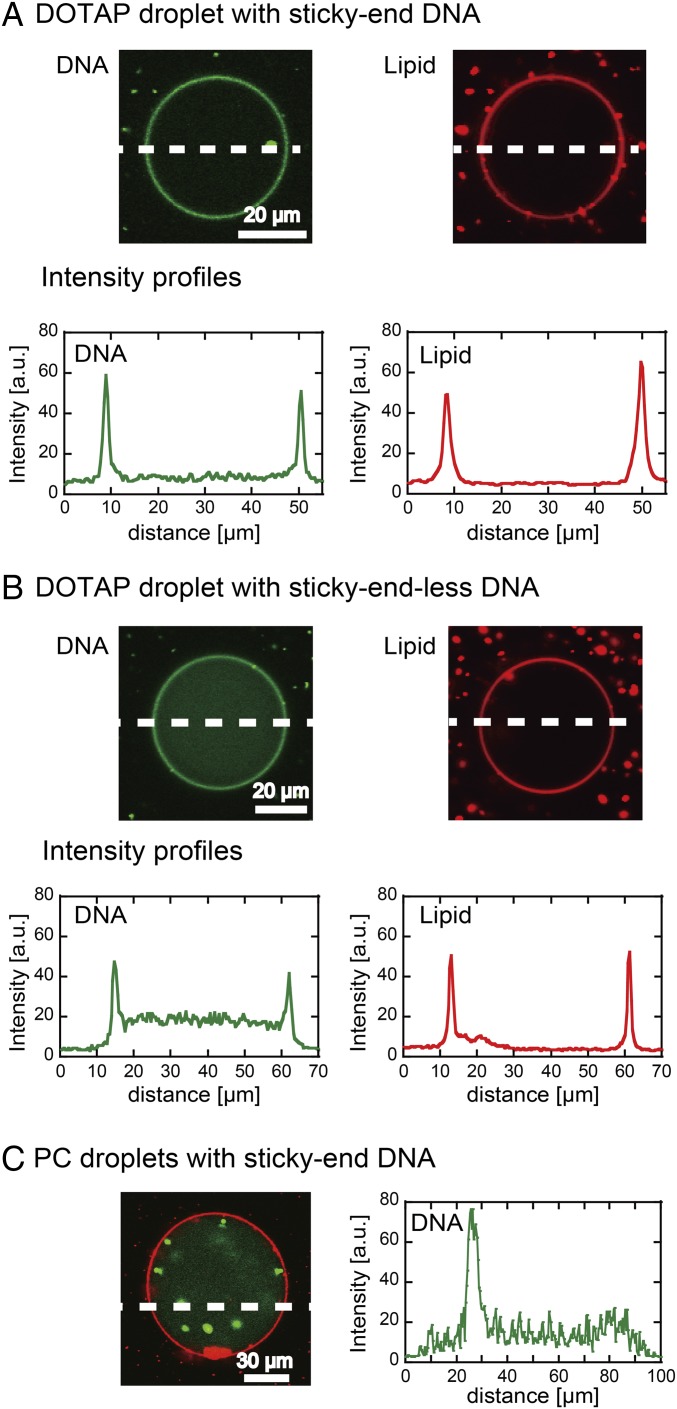
To localize DNA structures near lipid membranes, we used electrostatic interactions between negatively charged DNA and the positively charged lipid DOTAP. First, we encapsulated the solution of the hybridized sticky-end DNA into droplets coated with a DOTAP monolayer. Fig. 3A shows a typical cross-section image of the DOTAP droplet, where the DOTAP membrane and DNA are shown in red and green, respectively. Intensity profiles demonstrate that the DNA microspheres spontaneously accumulated near the DOTAP membrane to cover the interior surface of the droplet like a shell (DNA shell). The DNA shell had a homogeneous thickness of ∼1 μm, which is smaller than the diameter of the DNA microspheres in bulk (∼3 μm, Fig. 2B). We found the thickness of DNA microspheres deposited on the DOTAP membrane to be 1 μm to 2 μm, analyzed by atomic force microscopy (AFM) (Fig. S1 and Topology and Elasticity of DNA Microgels on Lipid Membrane). This value is similar to the thickness of DNA shell (∼1 μm). If the accumulation of the DNA microspheres is due to electrostatic interaction with the DOTAP membrane rather than the DNA gelation, Y-motif DNAs without sticky ends (S1 to S3) should also accumulate on the membrane. In fact, the sticky-end–less DNA accumulation was observed, although the level was lower than that observed when using DNA microspheres with sticky ends (Fig. 3A). This difference may result from the fact that high concentrations of DNA molecules have a large entropy and that DNA molecules diffusing in the center of the lipid droplet are not influenced by the membrane. This DNA shell was not observed for droplets covered with a zwitterionic PC membrane (Fig. 3C), which is moderately negatively charged in this buffer condition (24). The DNA microspheres formed inside the droplet, not on its surface (Fig. 3C). These results strongly suggest that the electrostatic interaction between the DNA microspheres and the lipid membrane is useful to construct the DNA shell structure inside the droplet covered with the lipid layer.
Fig. 3.
(A and B) Cross-section images of DOTAP droplets encapsulating DNA (A) with and (B) without sticky ends. The DNA and lipid membrane are shown in green and red, respectively. Fluorescence intensity profiles along the broken lines are shown. (Scale bars, 20 μm.) (C) Cross-section image of PC droplets after the encapsulation of DNA microspheres with sticky ends. Fluorescence intensity profile along the broken line is shown. (Scale bar, 30 μm.) a.u., arbitrary unit.
Fig. S1.
AFM images of DOTAP membranes on (A) glass and (B) the deposited DNA microgels on the membrane after removing buffer solution by drying. (C) Force indentation curve of the DNA microgel for wet sample.
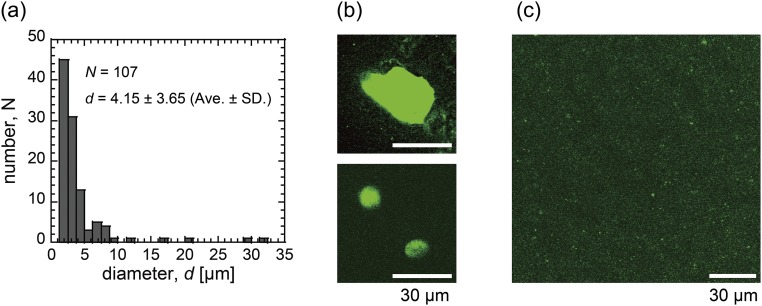
To confirm adhesion among microgels on the DOTAP membrane, we analyzed the size of the collected DNA shells by centrifugation after droplet rapture (Fig. S2 and Collection of DNA Shells from Droplets). The collected shells had a larger size than the initial DNA microspheres in bulk, ∼3 μm. Therefore, we concluded that the encapsulated DNA microspheres fused with one another and spread out on the DOTAP membrane (Figs. S1 and S2).
Fig. S2.
(A) Histogram of diameters of the collected DNA gel shells from the DOTAP droplets. (B and C) Images of the collected DNA gel shells (B) before and (C) 30 min after the addition of DNase solution. (Scale bars, 30 μm.) Ave, average.
Fluidity of DNA Shell in DOTAP Droplets.
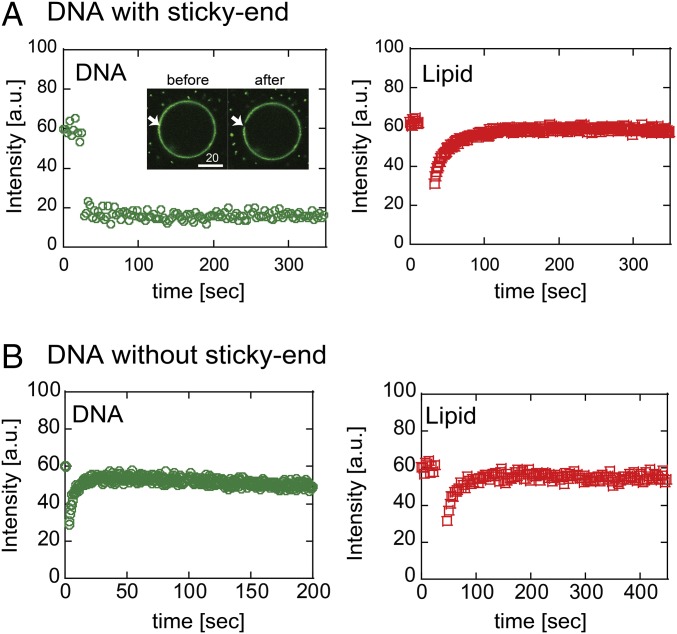
The thin structure of the DNA shell in DOTAP droplets raises concerns that the DNA shell is not in gel phase. To test this possibility, we performed FRAP experiments to analyze the fluidity of the assembled DNA and membrane lipids. In the case of sticky-end DNA shell, the fluorescence intensity did not recover 10 min after photobleaching (Fig. 4A, Left), indicating that fluidity of the DNA shell was extremely low. Therefore, we concluded that the DNA shell was in gel phase. However, the fluorescence intensity of membrane lipids was recovered soon after the photobleaching, and the estimated diffusion constant (D) was on the order of 10 μm2/s (Fig. 4A, Right). This diffusion coefficient is comparable to that of lipids without DNA, as reported previously (25, 26).
Fig. 4.
FRAP curves of DNA shell (green circle) and lipids (red square). (A) DOTAP droplet with shell structure of DNA with sticky ends. The two images show before and after photobleaching. (Scale bar, 20 μm.) (B) DOTAP droplet encapsulating DNA without sticky ends. a.u., arbitrary unit.
FRAP experiments for the DNA without sticky ends support the gel structure of the DNA shell originated from the sticky-end arrangement (Fig. 4B). The fluorescent intensity of the DNA without sticky ends recovered soon after photobleaching, indicating that the sticky-end–less DNA was not in gel phase. The estimated D of the DNA without sticky ends was on the order of 1 μm2/s (Fig. 4B, Left). The D of lipids of the droplets did not depend on whether the DNA is in gel phase or not (Fig. 4B, Right), suggesting that the DNA molecules without sticky ends are just localized at the membrane. Taken together, the observed slow fluidity of the DNA gel shell likely originates from the DNA gel shell structure via adhesion at the sticky ends, and the encapsulated DNA microspheres may connect to each other on the membrane. In addition, similar to the cytoskeleton, the DNA gel shell does not impede lipid diffusion.
Micropipette Aspiration of the Droplets with DNA.
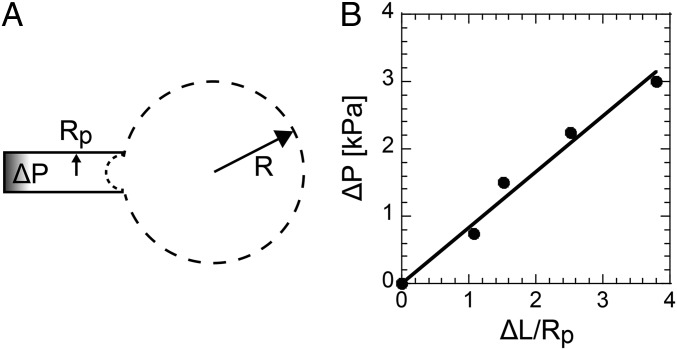
We showed that the DNA microspheres spontaneously form shell structure in gel phase at the interior surface of DOTAP droplets. To investigate the degree to which the DNA shell mechanically stabilizes the lipid membrane, interfacial tension at the droplet surface was evaluated by micropipette aspiration (27–29). As illustrated in Fig. 5A, a spherical droplet with a radius R0 (∼30 μm to 50 μm) was aspirated into the micropipette with a radius Rp (∼2.5 μm, Rp << R0) under an aspiration pressure (∆P). When the aspiration length (∆L) is small enough (∆L/Rp < 5), the ∆L/Rp increases linearly with ∆P (Fig. 5B). Above a critical pressure (∆P*), the droplet collapses and flows into the micropipette. The value of ∆P* for droplets with sticky-end DNA shell was greater than 10 kPa, which was much higher than that of droplets with sticky-end–less DNA and droplets without DNA, which were less than 0.2 kPa. Under the condition ∆L/Rp = 1, the surface tension (γ) of the droplet is derived from the Young−Laplace law,
where R is radius of the droplet. The surface tension of droplets with the DNA gel shell (γG) was 4 ± 2 mN/m, which was about 4 times greater than that of droplets with sticky-end–less DNA in the sol phase (S1 to S3) (γS = 1 ± 0.5 mN/m), and of droplets without DNA (γ0 = 1 ± 0.7 mN/m). A control experiment showed that the effect of DOTAP adsorption on the glass capillary was negligible for the measurement. The values of γS and γ0 were similar to the values of PC droplets without DNA measured by the Wilhelmy plate method (19) and the micropipette aspiration method (27). These values are summarized in Table 1. Accordingly, we derived the shear modulus (μ) and the area expansion modulus (dilation elasticity, κ) based on the following equations (30, 31) under the condition of 1 < ∆L/Rp < 5 (linear response region in Fig. 5B), respectively:
where A0 is the initial surface area of spherical droplets with R0, and ∆A is the increase in surface area from the aspiration. The obtained κ values and μ values of droplets with the DNA gel shell were higher than those of droplets with sticky-end–less DNA in sol phase and without DNA, as summarized in Table 1. These results mean that the DNA shell mechanically supports the lipid membrane. In addition, the ratio κ/μ is much greater than 1, which means that the DNA gel shell droplets easily deform with shearing force, but are not likely to change surface area.
Fig. 5.
Micropipette aspiration of a droplet. (A) Sketch of an aspirated droplet into a micropipette with an inner radius Rp under an aspiration pressure ΔP. (B) Relationship between the ΔP and the normalized aspiration length ΔL/Rp.
Table 1.
Comparison of observed mechanical parameters of droplets with/without DNA gel
| Droplet types | Interfacial tension, mN/m | Area expansion modulus, mN/m | Shear modulus, mN/m |
| Sticky-end DNA in DOTAP droplets | γG = 4 ± 2 | κG = 640 ± 390 | μG = 0.8 ± 0.1 |
| Sticky-end–less DNA in DOTAP droplets | γS = 1 ± 0.5 | κS < 220 | μS < 0.5 |
| DOTAP droplets without DNA | γ0 = 1 ± 0.7 | κ0 < 15 | μ0 < 0.4 |
Stability of Liposomes with the DNA Gel Shells.
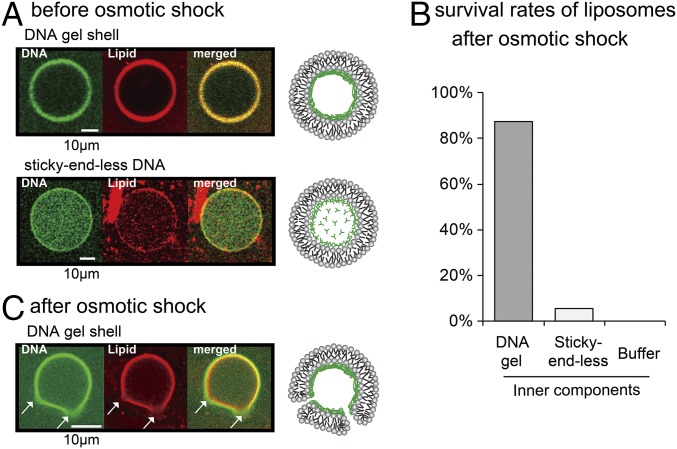
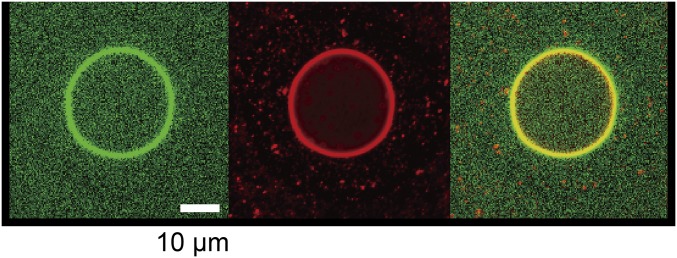
By using the droplet transfer method, we created liposomes with a DNA gel shell. Zwitterionic PC lipid was used for the outer leaflet of the liposome because positively charged DOTAP strongly adsorbs on glass (15). Fig. 6A clearly shows successfully prepared asymmetric liposome with the DNA gel shells (green), because the fluorescent-labeled lipids were only contained in the PC layer, that is, the outer membrane was maintained. To analyze stability of the liposomes with the DNA gel shell, an osmotic stress was applied by addition of hypertonic sorbitol as an osmolyte. The hypertonic treatment resulted in collapse of almost all liposomes containing sticky-end–less DNA or without DNA (Fig. 6B, center and right). However, most liposomes with DNA gel shell survived and retained their spherical shapes after the hypertonic treatment (Fig. 6B, left). In addition, the DNase did not penetrate the membrane (Fig. S3), suggesting that the liposome membrane is sufficiently stable to isolate its inner solution from the surrounding solution. When a much higher concentration of sorbitol was added (2 times higher pressure), micrometer-sized pores appeared on the liposome membrane and on the DNA shell at the same position (Fig. 6C). The pores maintained their size, and the membrane did not rupture. This result suggests that the liposome membrane and the DNA shell are strongly bound to each other, as illustrated in Fig. 6C, Far Right. In general, osmotically stressed liposomes cause strong fluctuations in the membrane and result in membrane rupture (32). Encapsulation of polymer gels inside the liposomes prevents membrane rapture, but results in liposome shrinkage and inhibits release of the encapsulated materials (33). These results strongly suggest that the DNA shell stabilizes liposomes. Therefore, the presence of the DNA gel shell underneath the liposomal membrane mechanically strengthens the membrane structure and prevents the membrane rupture, similar to the cytoskeleton in live cells.
Fig. 6.
(A and C) Liposomes with DNA (A) before and (C) after the osmotic shock treatment. Confocal images show (Left) DNA (green), (Center) lipids of outer leaflet (red), and (Right) the merged images. Schematic illustrations of the confocal images are shown together (Far Right). Arrows in C indicate location of the pores. (Scale bars, 10 μm.) (B) Survival rates after osmotic treatment.
Fig. S3.
Confocal image of a liposome with DNA gel shell captured 1 h after the addition of DNase in the outer media. The images show (Left) DNA (green), (Center) lipids of outer leaflet (red), and (Right) the merged images. (Scale bar, 10 μm.)
To compare the mechanical strength of the DNA shell with that of polymer gels and with that of cells with elastic modulus ranging from 100 Pa to 10 kPa (34), we measured the elasticity of DNA microgels deposited on the DOTAP membrane by AFM (Fig. S1C). The average elasticity of the gels was 155 ± 28 kPa (average ± SE; n = 7). This degree of elasticity is comparable to that of 10 wt% polyacrylamide (35, 36) and 2 mol/L agarose (37) gels.
Estimation of Strength of DNA Cytoskeleton from the Aspect of Enthalpy of DNA Gel Shell.
Enthalpy is a major factor in determining the strength of the DNA cytoskeleton. When the aspiration length increased up to the critical value ∆Lc (>Rp), DOTAP droplets with the DNA cytoskeleton collapsed and flowed into the pipette. Experimentally, the value of ∆Lc was ∼7.5 μm for droplets with R = 20 μm. We assume that a part of DNA gel shell collapsed into Y-motif DNA by detaching the sticky ends. In other words, an increase in the interfacial energy of the DNA cytoskeleton with increasing surface area (∆EDNA) is hypothesized to be equal to enthalpy of the sticky ends of the collapsed DNA shell (∆HDNA), that is, ∆EDNA = ∆HDNA. When the droplets aspirate into the micropipette with the aspirated length ∆Lc (as illustrated in Fig. 5A), the interfacial energy of DNA shell increases according to the equation
where γG and γ0 are interfacial tensions of droplets with and without DNA, respectively, and γG = 4 mN/m and γ0 = 1 mN/m. The increase in the interfacial energy (∆EDNA) was estimated to be 3.5 10─13 J from the experimentally obtained values Rp = 2.5 μm and ∆Lc = 7.5 μm. The basic structure of the DNA gel network is a six-member ring (Fig. 7). Therefore, one DNA ring would collapse with energy equal to triple the enthalpy of the sticky end, that is, 3∆Hsticky end. When a number (Nring) of DNA rings collapse into the Y motif, the total enthalpy of the DNA rings is expressed as
From a previous study (18), the ∆Hsticky end is reported to be 12 10─19 J. When the ∆EDNA was equal to the ∆HDNA, the number of DNA rings (Nring) would be 2.5 105.
Fig. 7.
Schematic images of DOTAP droplet and the DNA gel network.
To test the validity of these calculations based on the estimated number of DNA rings, we derived the number of DNA rings (Nring) geometrically from a slide of the DNA ring, a = 14.28 nm (G1 to G3 are 42 base pairs) and diameter of the dsDNA, h = 2 nm. The minimum volume of the DNA rings occupied (Vring) for Nring = 105 is estimated as
Because the thickness of DNA shell (d) was ∼1 μm (Fig. 3A), the increased ∆EDNA can collapse a part of the DNA gel network with an area of 0.4 μm2. If energy is used not only to collapse the double helical structure of DNA but also to resolve the entanglement and the electrostatic interaction among DNA rings, the size of the collapsed area will be smaller. In other words, the appearance of such a small pore on the DNA gel network might lead the droplet flow into the pipette above the critical length (ΔLc). This phenomenon corresponds to the fact that the DNA shell has a small shear modulus (μ) and a large area expansion modulus (κ), i.e., κ/μ >> 1 (Table 1), which is similar to cells with cytoskeletons, such as red blood cells and white cells (30, 31, 38).
Topology and Elasticity of DNA Microgels on Lipid Membrane
To investigate the topology and elastic modulus of DNA microgels on DOTAP (chloride salt; Avanti Polar Lipids) lipid membrane, we used an atomic force microscope (AFM) (MFP-3D; Asylum Research) equipped with a tetrahedral cantilever (OMCL-AC240TS-C3; Olympus). The elastic modulus of the gels was determined by deforming the surface and fitting the force–indentation curve to the Hertz (Sneddon’s variation) model (48), given by the following equation:
Here, F is the applied force, which is calculated using κ (spring constant of the cantilever), and δ is the indentation, given by the difference between piezo height and cantilever deflection. The elastic modulus, E, and Poisson’s ratio, ν, define the material properties. The Poisson’s ratio ν was assumed to be 0.5, like the value of the polymer gel (49, 50). The cantilever properties were defined by the opening angle (α = 34°) and the cantilever spring constant (κ = 2 N/m). Analysis of the approach curve yielded the elastic modulus E.
To prepare lipid layers, we deposited DOTAP in chloroform solution on the glass slide. AFM topology images revealed that the lipid lamellar structure had a height of ∼10 nm (Fig. S1A). Then, we deposited a drop of DNA microgel solution on the lipid membrane to obtain the topology image for the dry samples (Fig. S1B). This method was not applied for wet samples. The height of the deposited microgel was ∼1 μm to 2 μm, which is similar to the estimated thickness of DNA shell (1 μm). The topology image also indicated a DNA structure volume of 30 μm3 to 50 μm3. It means that two or three microgels with an initial diameter of ∼3 μm fuse and spread out together on the membrane. We analyzed the elasticity of the microgels for wet samples from the force−indentation curves (Fig. S1C). The average elasticity was 155 ± 28 kPa (average ± SE; number of measurements n = 7).
Collection of DNA Shells from Droplets
We collected DNA shells from DOTAP droplets with a diameter of 30 μm to 50 μm by centrifugation (2,000 × g for 5 min) after droplet rapture using an extraction buffer [40 mM Tris⋅HCl (pH 8.0), 10 mM NaCl, 6 mM MgCl2, and 10 mM CaCl2]. A histogram of the sizes of collected DNA gels is shown in Fig. S2A (4.15 ± 3.65 μm). The collected microgels showed a larger distribution in size and slightly higher average, compared with those of the initial DNA microgels (3.1 ± 1.3 μm; average ± SD). Representative images of the collected DNA shells are shown in Fig. S2B. A representative image 30 min after addition of DNase at 40 °C is shown in Fig. S2C.
Conclusion
We successfully prepared cell-sized liposomes and lipid droplets with an artificial cytoskeleton by using DNA nanostructures. The DNA oligomers formed Y motifs and connected to each other with their sticky ends to form network structure in gel phase. The hybridized DNA formed microsphere gels in bulk. By encapsulating the DNA microgels into droplets coated by a cationic lipid membrane, DNA spontaneously formed a shell structure underneath the membrane via electrostatic interactions. The DNA gel network increased interfacial tension and area expansion modulus of the droplet surface to a degree similar to the enthalpy of the sticky ends of the DNA. Such drastic changes were detected only with DNA skeletons in the gel phase. Furthermore, we demonstrate that liposomes with the DNA skeleton are substantially stabilized and tolerant against osmotic stress. These results clearly demonstrate that the DNA gel skeleton is a stabilizer of the artificial cells. Yang et al. (39) reported DNA nanorings as templates to prepare nanometer-sized liposomes inside. Meanwhile, our DNA cytoskeleton mechanically supported the micrometer-sized liposomes from the inside. Thus, our method will contribute to the extension of DNA−liposome hybrid systems.
The DNA gel network structures on inner membranes show highly similar traits to cytoskeletons of live cells. Thus, the DNA gel shell can be characterized as a cytoskeleton for artificial cells, including liposomes and lipid droplets. The use of a DNA cytoskeleton has many remarkable merits. (i) DNA has excellent biocompatibility and biodegradability, which is important for DDS. We confirmed that the DNA gels have little effect on viability of living cells (Fig. S4 and Biocompatibility), and that they are degradable by the addition of DNase solution (Fig. S2C). In addition, previous studies reported that DOTAP also do not show harmful effects on live cells (40–42). (ii) The DNA nanostructure is easy to assemble and dissemble by temperature changes and illumination via DNA sequence design (43). (iii) The mechanical properties of DNA gels are also controllable via DNA sequence design by changing the number and length of arms (sticky ends) in DNA gels (11), which is difficult to achieve with biopolymer gels. (iv) DNA has versatile potential by combining logic gates (44), actuators (45), and switches (46), which may also be applicable for our artificial cell systems. (v) Because DNA gels are not crystallized, as reported by Rovigatti et al. (47), the DNA gels can rearrange their structures in response to their surrounding circumstances. Furthermore, our proposed liposomes with DNA cytoskeletons are much closer to the living cells compared with the conventional liposomes without skeletal structures. Therefore, liposomes with a DNA cytoskeleton have the potential to become an ideal platform to understand how cells regulate their shapes with cytoskeleton from physicochemical point of view.
Fig. S4.
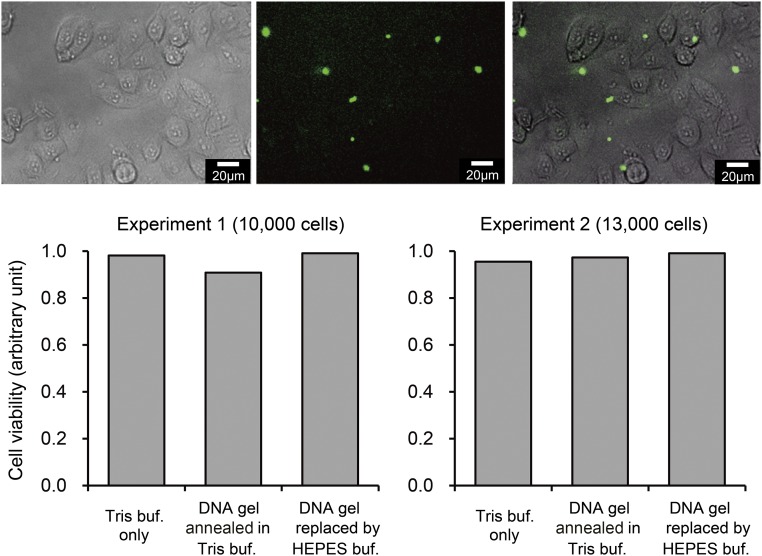
(Upper) Fluorescent and phase contrast microscopic images of cells after 24 h of incubation with DNA microgels (shown in green: annealing in Tris-buffer, then replaced by Hepes-buffer). (Scale bar, 20 μm.) (Lower) Cell viability after 24 h of incubation with DNA microgels, determined by the MTT assay (two independent experiments). Presence of DNA microgels did not significantly affect the viability of HeLa cells. buf, buffer.
Biocompatibility
To investigate the biocompatibility of DNA microgels, we tested viability of cultured human cells on the DNA gel. Viability of HeLa cells (obtained from ATCC, CCL-2) was examined using a standard 3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit (Cell Proliferation Kit I; Roche). The DNA microgel was prepared, as described in Hybridization of DNA in Bulk, with slight modification of buffer conditions as follows: Annealing was done in Tris buffer, and Tris buffer was then replaced with Hepes buffer. The DNA gel solutions (17.8 μL), diluted in DMEM (Nacalai Tesque) supplemented with 10% FCS, were immediately mixed with the cells. The final concentration was 17.8 × 102 μL per well. One hundred microliters of solution with 10,000 cells per well or 13,000 cells per well was plated for 3 h before the DNA gel were added. When 96-well plates were used, 5 μL of the culture medium was removed from each well. Five microliters of the solution containing DNA gel was immediately added to each well. The plate was then gently swirled to mix the existing media and incubated for 24 h at 37 °C. Ten microliters of MTT reagent was added after obtaining the images of the DNA gel and the cells (Fig. S4, Upper). After 4 h incubation at 37 °C, solubilization solution was added. After 20 h of incubation, the resultant formazan crystals were dissolved, and the absorbance intensity was measured using spectrometer (Nano-drop One; Thermo Scientific) at 600 nm with reference wavelength of 750 nm. The cell viability after 24 h incubation with DNA microgels was determined from the MTT assay (Fig. S4, Lower). The results demonstrated that DNA microgels did not significantly affect the viability of cells.
Acknowledgments
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grants 16812285 and 14J10002 (to M.M.); 24104005, 22220001, and 15H01715 (to S.M.); 24104002 and 26280097 (to M.T.); and 15H05463, 15KT0081, 16H00796, and N16H01443 (to M.Y.); and by Japan Agency for Medical Research and Development-Core Research for Evolutional Science and Technology (AMED-CREST), Japan Science and Technology Agency (JST) Grant 16gm0810001h0102 (to S.-i.M.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702208114/-/DCSupplemental.
References
- 1.Yanagisawa M, Sakaue T, Yoshikawa K. Characteristic behavior of crowding macromolecules confined in cell-sized droplets. Int Rev Cell Mol Biol. 2014;307:175–204. doi: 10.1016/B978-0-12-800046-5.00007-2. [DOI] [PubMed] [Google Scholar]
- 2.Noireaux V, Maeda YT, Libchaber A. Development of an artificial cell, from self-organization to computation and self-reproduction. Proc Natl Acad Sci USA. 2011;108:3473–3480. doi: 10.1073/pnas.1017075108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stano P, Luisi PL. Semi-synthetic minimal cells: Origin and recent developments. Curr Opin Biotechnol. 2013;24:633–638. doi: 10.1016/j.copbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Lian T, Ho RJ. Trends and developments in liposome drug delivery systems. J Pharm Sci. 2001;90:667–680. doi: 10.1002/jps.1023. [DOI] [PubMed] [Google Scholar]
- 5.Noireaux V, et al. Growing an actin gel on spherical surfaces. Biophys J. 2000;78:1643–1654. doi: 10.1016/S0006-3495(00)76716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janmey PA, et al. The mechanical properties of actin gels. Elastic modulus and filament motions. J Biol Chem. 1994;269:32503–32513. [PubMed] [Google Scholar]
- 7.Takiguchi K, Yamada A, Negishi M, Tanaka-Takiguchi Y, Yoshikawa K. Entrapping desired amounts of actin filaments and molecular motor proteins in giant liposomes. Langmuir. 2008;24:11323–11326. doi: 10.1021/la802031n. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki M, Chiba M, Eguchi H, Ohki T, Ishiwata S. Cell-sized spherical confinement induces the spontaneous formation of contractile actomyosin rings in vitro. Nat Cell Biol. 2015;17:480–489. doi: 10.1038/ncb3142. [DOI] [PubMed] [Google Scholar]
- 9.Takiguchi K, et al. Chapter 3 - Construction of cell-sized liposomes encapsulating actin and actin-cross-linking proteins. Methods Enzymol. 2009;464:31–53. doi: 10.1016/S0076-6879(09)64003-9. [DOI] [PubMed] [Google Scholar]
- 10.Pontani LL, et al. Reconstitution of an actin cortex inside a liposome. Biophys J. 2009;96:192–198. doi: 10.1016/j.bpj.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roh YH, Ruiz RC, Peng S, Lee JB, Luo D. Engineering DNA-based functional materials. Chem Soc Rev. 2011;40:5730–5744. doi: 10.1039/c1cs15162b. [DOI] [PubMed] [Google Scholar]
- 12.Ma S, et al. Fabrication of microgel particles with complex shape via selective polymerization of aqueous two-phase systems. Small. 2012;8:2356–2360. doi: 10.1002/smll.201102715. [DOI] [PubMed] [Google Scholar]
- 13.Liu J. Oligonucleotide-functionalized hydrogels as stimuli responsive materials and biosensors. Soft Matter. 2011;7:6757–6767. [Google Scholar]
- 14.Matsuura K, Masumoto K, Igami Y, Fujioka T, Kimizuka N. In situ observation of spherical DNA assembly in water and the controlled release of bound dyes. Biomacromolecules. 2007;8:2726–2732. doi: 10.1021/bm070357w. [DOI] [PubMed] [Google Scholar]
- 15.Helwa Y, Dave N, Liu J. Electrostatically directed liposome adsorption, internalization and fusion on hydrogel microparticles. Soft Matter. 2013;9:6151–6158. [Google Scholar]
- 16.Zadeh JN, et al. NUPACK: Analysis and design of nucleic acid systems. J Comput Chem. 2011;32:170–173. doi: 10.1002/jcc.21596. [DOI] [PubMed] [Google Scholar]
- 17.Douglas SM, et al. Rapid prototyping of 3D DNA-origami shapes with caDNAno. Nucleic Acids Res. 2009;37:5001–5006. doi: 10.1093/nar/gkp436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SantaLucia J., Jr A unified view of polymer, dumbbell, and oligonucleotide DNA nearest-neighbor thermodynamics. Proc Natl Acad Sci USA. 1998;95:1460–1465. doi: 10.1073/pnas.95.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanagisawa M, Yoshida T, Furuta M, Nakata S, Tokita M. Adhesive force between paired microdroplets coated with lipid monolayers. Soft Matter. 2013;9:5891–5897. [Google Scholar]
- 20.Yanagisawa M, Iwamoto M, Kato A, Yoshikawa K, Oiki S. Oriented reconstitution of a membrane protein in a giant unilamellar vesicle: Experimental verification with the potassium channel KcsA. J Am Chem Soc. 2011;133:11774–11779. doi: 10.1021/ja2040859. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara K, Yanagisawa M. Generation of giant unilamellar liposomes containing biomacromolecules at physiological intracellular concentrations using hypertonic conditions. ACS Synth Biol. 2014;3:870–874. doi: 10.1021/sb4001917. [DOI] [PubMed] [Google Scholar]
- 22.Morita T, Narita T, Mukai S, Yanagisawa M, Tokita M. Phase behaviors of agarose gel. AIP Adv. 2013;3:042128. [Google Scholar]
- 23.Axelrod D, Koppel DE, Schlessinger J, Elson E, Webb WW. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976;16:1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egawa H, Furusawa K. Liposome adhesion on mica surface studied by atomic force microscopy. Langmuir. 1999;15:1660–1666. [Google Scholar]
- 25.Negishi M, Sakaue T, Yoshikawa K. Mismatch of bulk viscosity reduces interfacial diffusivity at an aqueous-oil system. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;81:020901. doi: 10.1103/PhysRevE.81.020901. [DOI] [PubMed] [Google Scholar]
- 26.Walder RB, Honciuc A, Schwartz DK. Phospholipid diffusion at the oil-water interface. J Phys Chem B. 2010;114:11484–11488. doi: 10.1021/jp1053869. [DOI] [PubMed] [Google Scholar]
- 27.Delacotte J, Gourier C, Pincet F. Interfacial pressure and phospholipid density at emulsion droplet interface using fluorescence microscopy. Colloids Surf B Biointerfaces. 2014;117:545–548. doi: 10.1016/j.colsurfb.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 28.Tsamantakis C, Masliyah J, Yeung A, Gentzis T. Investigation of the interfacial properties of water-in-diluted-bitumen emulsions using micropipette techniques. J Colloid Interface Sci. 2005;284:176–183. doi: 10.1016/j.jcis.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Yeung A, Dabros T, Masliyah J, Czarnecki J. Micropipette: A new technique in emulsion research. Colloids Surf A Physicochem Eng Asp. 2000;174:169–181. [Google Scholar]
- 30.Merkel R, et al. A micromechanic study of cell polarity and plasma membrane cell body coupling in Dictyostelium. Biophys J. 2000;79:707–719. doi: 10.1016/S0006-3495(00)76329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lelievre J, Bucherer C, Geiger S, Lacombe C, Vereycken V. Blood cell biomechanics evaluated by the single-cell micromanipulation. J Phys III. 1995;5:1689–1706. [Google Scholar]
- 32.Ohno M, Hamada T, Takiguchi K, Homma M. Dynamic behavior of giant liposomes at desired osmotic pressures. Langmuir. 2009;25:11680–11685. doi: 10.1021/la900777g. [DOI] [PubMed] [Google Scholar]
- 33.Campillo C, Pepin-Donat B, Viallat A. Responsive viscoelastic giant lipid vesicles filled with a poly(N-isopropylacrylamide) artificial cytoskeleton. Soft Matter. 2007;3:1421–1427. doi: 10.1039/b710474j. [DOI] [PubMed] [Google Scholar]
- 34.Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter. 2007;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 35.Wyss HM, Franke T, Mele E, Weitz DA. Capillary micromechanics: Measuring the elasticity of microscopic soft objects. Soft Matter. 2010;6:4550–4555. [Google Scholar]
- 36.Denisin AK, Pruitt BL. Tuning the range of polyacrylamide gel stiffness for mechanobiology applications. ACS Appl Mater Interfaces. 2016;8:21893–21902. doi: 10.1021/acsami.5b09344. [DOI] [PubMed] [Google Scholar]
- 37.Normand V, Lootens DL, Amici E, Plucknett KP, Aymard P. New insight into agarose gel mechanical properties. Biomacromolecules. 2000;1:730–738. doi: 10.1021/bm005583j. [DOI] [PubMed] [Google Scholar]
- 38.Lenormand G, Hénon S, Richert A, Siméon J, Gallet F. Direct measurement of the area expansion and shear moduli of the human red blood cell membrane skeleton. Biophys J. 2001;81:43–56. doi: 10.1016/S0006-3495(01)75678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, et al. Self-assembly of size-controlled liposomes on DNA nanotemplates. Nat Chem. 2016;8:476–483. doi: 10.1038/nchem.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y, Szoka FC., Jr Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996;35:5616–5623. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 41.Porteous DJ, et al. Evidence for safety and efficacy of DOTAP cationic liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Ther. 1997;4:210–218. doi: 10.1038/sj.gt.3300390. [DOI] [PubMed] [Google Scholar]
- 42.Wrobel I, Collins D. Fusion of cationic liposomes with mammalian cells occurs after endocytosis. Biochim Biophys Acta Biomembranes. 1995;1235:296–304. doi: 10.1016/0005-2736(95)80017-a. [DOI] [PubMed] [Google Scholar]
- 43.Bomboi F, et al. Re-entrant DNA gels. Nat Commun. 2016;7:13191. doi: 10.1038/ncomms13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takinoue M, Kiga D, Shohda K, Suyama A. Experiments and simulation models of a basic computation element of an autonomous molecular computing system. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;78:041921. doi: 10.1103/PhysRevE.78.041921. [DOI] [PubMed] [Google Scholar]
- 45.Simmel FC, Yurke B. Using DNA to construct and power a nanoactuator. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;63:041913. doi: 10.1103/PhysRevE.63.041913. [DOI] [PubMed] [Google Scholar]
- 46.Wang F, Liu X, Willner I. DNA switches: from principles to applications. Angew Chem Int Ed Engl. 2015;54:1098–1129. doi: 10.1002/anie.201404652. [DOI] [PubMed] [Google Scholar]
- 47.Rovigatti L, Smallenburg F, Romano F, Sciortino F. Gels of DNA nanostars never crystallize. ACS Nano. 2014;8:3567–3574. doi: 10.1021/nn501138w. [DOI] [PubMed] [Google Scholar]
- 48.Radmacher M, Fritz M, Hansma PK. Imaging soft samples with the atomic force microscope: Gelatin in water and propanol. Biophys J. 1995;69:264–270. doi: 10.1016/S0006-3495(95)79897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheppard S, Sweet S, Benedict AJ. Elasticity of purified gelatin jellies as a function of hydrogen-ion concentration. J Am Chem Soc. 1922;44:1857–1866. [Google Scholar]
- 50.Boudou T, Ohayon J, Picart C, Tracqui P. An extended relationship for the characterization of Young’s modulus and Poisson’s ratio of tunable polyacrylamide gels. Biorheology. 2006;43:721–728. [PubMed] [Google Scholar]