Abstract
During mitosis, chromosome segregation is regulated by a spindle checkpoint mechanism. This checkpoint delays anaphase until all kinetochores are captured by microtubules from both spindle poles, chromosomes congress to the metaphase plate, and the tension between kinetochores and their attached microtubules is properly sensed. Although the spindle checkpoint can be activated in many different cell types, the role of this regulatory mechanism in rapidly dividing embryonic animal cells has remained controversial. Here, using time-lapse imaging of live embryonic cells, we show that chemical or mutational disruption of the mitotic spindle in early Caenorhabditis elegans embryos delays progression through mitosis. By reducing the function of conserved checkpoint genes in mutant embryos with defective mitotic spindles, we show that these delays require the spindle checkpoint. In the absence of a functional checkpoint, more severe defects in chromosome segregation are observed in mutants with abnormal mitotic spindles. We also show that the conserved kinesin CeMCAK, the CENP-F-related proteins HCP-1 and HCP-2, and the core kinetochore protein CeCENP-C all are required for this checkpoint. Our analysis indicates that spindle checkpoint mechanisms are functional in the rapidly dividing cells of an early animal embryo and that this checkpoint can prevent chromosome segregation defects during mitosis.
INTRODUCTION
During mitosis, some microtubules emanating from bipolar microtubule organizing centers grow toward chromosomes and attach to specialized chromosomal regions called kinetochores (Skibbens and Hieter, 1998; Cleveland et al., 2003). Once captured, sister chromatids are segregated, one to each of two daughter cells. Eukaryotic cells have evolved a mechanism called the spindle checkpoint, to monitor chromosomal segregation and increase the fidelity of mitosis (reviewed in Gardner and Burke, 2000; McIntosh et al., 2002). Until spindle microtubules from both poles capture and align all sister chromatid pairs at a metaphase plate, the spindle checkpoint produces a delay in the onset of anaphase. If this delay is bypassed by reducing checkpoint function, anaphase starts prematurely, and daughter cells may receive unequal complements of chromosomes. Such genomic instability can result in lethality and in tumorigenesis (Hartwell and Kastan, 1994; Basu et al., 1999).
Originally identified and characterized in Saccharomyces cerevisiae, seven spindle assembly checkpoint genes (MAD1, MAD2, MAD3, BUB1, BUB2, BUB3, and MPS1) are known to function at kinetochores to inhibit anaphase onset, or to mediate mitotic exit in the presence of spindle abnormalities (Hoyt et al., 1991; Li and Murray, 1991). Mutations in these genes reduce or eliminate the cell cycle delays that normally occur after treatment with microtubule depolymerizing drugs (Wang and Burke, 1995; Pangilinan and Spencer, 1996). Bub1 and Bub3 form a protein kinase complex that, in concert with Mps1, functions at kinetochores upstream of Mad1 and Mad2 (Roberts et al., 1994; Hardwick and Murray, 1995; Hardwick et al., 1996). One target of the spindle checkpoint signal is the anaphase-promoting complex/cyclosome, an E3 ubiquitin ligase that targets cell cycle proteins, including the securins Pds1 and Cut2, for proteosome-mediated degradation (Hwang et al., 1998).
Several checkpoint proteins are widely conserved, and new components continue to be identified in other organisms (Cleveland et al., 2003). A Bub1-related kinase, called hBubR1 in humans and Mad3 in other organisms, binds CENP-E, a kinesin motor protein that directly tethers kinetochores and kinetochore-bound checkpoint components to spindle microtubules and also is involved in checkpoint signaling (Chan et al., 1998; Abrieu et al., 2000; Weaver et al., 2003). Both CENP-E and hBUB-1 can bind another human protein called CENP-F, which is cell cycle regulated and localizes to kinetochores (Liao et al., 1995; Zhu et al., 1995). CENP-F levels are increased at unaligned kinetochores during meiosis in mouse spermatocytes, leading to suggestions that CENP-F might play a role in meiotic spindle checkpoint signaling (Eaker et al., 2001). It is not clear, however, what role, if any, CENP-F has in mitotic checkpoint signaling.
In Caenorhabditis elegans, homologues of MAD1 and MAD2, called mdf-1 and mdf-2, are required for viability and promote the arrest of mitotically proliferating premeiotic germ cells exposed to nocodazole (Kitagawa and Rose, 1999). The MDF-2 protein localizes to centrosomes and chromosomes in early embryonic cells, but its role during mitosis in the early embryo remains unknown. Other conserved kinetochore components have also been identified in C. elegans. Two inner kinetochore proteins CENP-A and CENP-C act upstream of KNL-1, a novel kinetochore protein, and are all required for kinetochore assembly and chromosome segregation (Buchwitz et al., 1999; Oegema et al., 2001; Desai et al., 2003). CeBUB-1 and two CENP-F-related proteins called HCP-1 and HCP-2 also localize to kinetochores in one-cell embryos. HCP-1 and HCP-2 play a role in chromosome segregation (Moore et al., 1999), but the role of CeBUB-1 is unknown. Finally, the kinesin MCAK (CeMCAK) localizes to the outer surface of kinetochores and to centrosomes and is required for mitotic spindle midzone formation (Grill et al., 2001). It is not known whether CeMCAK, CeBUB-1, HCP-1, HCP-2, or CeCENP-C are required for spindle checkpoint function.
Although several proteins that localize to kinetochores in the early C. elegans embryo are important for chromosome segregation, it is not known whether they have roles in spindle checkpoint activity. However, a recent study has found that destabilizing microtubules increased the number of metaphase-staged cells in early C. elegans embryos. Furthermore, reducing the function of mdf-2 and a Mad3-like gene called san-1 decreased the observed frequency of metaphase stage cells after microtubule destabilization, suggesting that a spindle checkpoint functions in the early embryo (Nystul et al., 2003). Studies in Drosophila have shown that microtubule inhibitors arrest early embryos with metaphase-like chromatin (Zalokar 1976; Foe and Alberts, 1983), and abnormally compacted chromosomes delay anaphase and result in nuclear “fallout” (Sullivan et al., 1993). However, the failure of microtubule depolymerizing drugs such as nocodazole to arrest mitosis in early embryonic cells has led to suggestions that the rapid mitotic divisions that occur in some early embryos do not use a spindle checkpoint (Murray and Hunt, 1997). Here, we show that both chemical and mutational disruption of the microtubule cytoskeleton in the early C. elegans embryo result in modest but reproducible mitotic delays at the transition to anaphase. We also show that conserved spindle checkpoint genes are required for these delays. Furthermore, we provide the first evidence that two CENP-F-like proteins, HCP-1 and HCP-2, the core kinetochore component CeCENP-C, and the mitotic kinesin CeMCAK are required, directly or indirectly, for spindle checkpoint function.
MATERIALS AND METHODS
Strains and Genetic Analyses
N2 Bristol was used as the wild-type strain and maintained according to standard methods (Brenner, 1974). The following alleles listed by chromosome number were used: apo-5(or358ts), dpy-5(e61), I; dnc-1(or404ts), dpy-20(e1282), him-8(e1489), unc-8(e49), IV; lin-2(e1309), X. The following strains carrying integrated green fluorescent protein (GFP) constructs were used to visualize centrosomes and DNA: AZ244: unc-119(ed3) III; ruIs57[unc-119(+) pie-1::GFP::tubulin]. GFP expressed in β-tubulin labels microtubules and centrosomes (Praitis et al., 2001); pJH4.52: his-11::GFP. GFP expressed in H2B histones throughout the cell cycle (Strome et al., 2001).
apo-5(or358ts) and dnc-1(or404ts) were isolated in a screen for temperature-sensitive (ts) maternal-effect embryonic-lethal mutations, using methods described previously (Encalada et al., 2000). Both or358 and or404 were backcrossed five times by using either him-8 or N2 males. Mutant worms were maintained at the permissive temperature (15°C). L4 larvae were shifted to the restrictive temperature (26.6°C) overnight before phenotypic analysis. Homozygous apo-5(or358ts) hermaphrodites produce 2% dead embryos at 15°C (5/244), whereas 100% dead embryos were produced by homozygous hermaphrodite L4 larvae shifted to 26.6°C (2469/2469). Genetic analysis of dnc-1(or404ts) indicates that homozygous mutant hermaphrodites produce 0.4% (3/750) dead embryos at the permissive temperature of 15°C, whereas 100% (600/600) of the embryos produced at 26.6°C failed to hatch. To test for zygotic requirements, homozygous unc-8 dnc-1(or404) hermaphrodites were crossed to him-8 males. Heterozygous progeny (with genotypes unc-8 or404/+; him-8/+) were singled and placed at 26.6°C. All embryos from heterozygous parents hatched, and one-quarter (100/400) of the progeny had the Unc phenotype and were fertile, suggesting no zygotic requirement for or404ts. Heterozygous apo-5 dpy-5/+; him-8/+ produced 16% dead embryos (155/996) at 26.6°C. Approximately 15% (123/841) of the embryos that hatched were Dpy, suggesting that the dead embryos produced by heterozygous parents reflect at least in part an essential but not fully penetrant zygotic requirement for apo-5.
The apo-5 mutant was mapped to linkage group (LG) III by crossing ts males from him strains and MT3751 (dpy-5 I; rol-6 II; unc-32 III) hermaphrodites. Progeny of the outcrossed hermaphrodites were examined for exclusion of the ts mutation by a homozygous marker chromosome, which indicated linkage. To map apo-5, Blister nonLin and Lin nonBlister recombinant progeny were picked from an apo-5/bli-3 lin-17 strain. Of 33 Lin nonBlister, six picked up apo-5, whereas eight of eight Blister nonLin picked up apo-5. These data position apo-5 (or358) at approximately –8.7 map units on LG III.
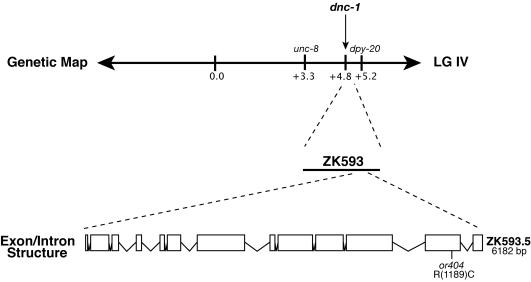
Cloning of dnc-1
or404ts was mapped to LG IV by using visible markers, following standard methods (Brenner, 1974). Unc nonDpy and Dpy nonUnc recombinant progeny were picked from an or404ts/unc-8 dpy-20 strain. In 22/28 Unc nonDpy recombinants, or404ts was linked to unc-8, and in 2/20 Dpy nonUnc recombinants, or404ts was linked to dpy-20. These data positioned or404ts to approximately +4.9 map units on chromosome IV. Previous analysis revealed that reducing the function by injection of single-stranded RNA (RNA interference, RNAi) of dynactin (dnc-1, ZK593.5), which maps at +4.8, produced embryos with P0 spindle defects similar to those of or404ts (Skop and White, 1998). Genomic DNA fragments of the gene ZK593.5 were polymerase chain reaction (PCR) amplified and sequenced from or404ts mutants and compared with those from lin-2 mutants, the strain used for mutagenesis. Three separate PCR amplifications were pooled and sequenced for each of the mutant strains. The sequenced DNA included 400 base pairs upstream and downstream of the start and stop codons, for a total of 6959 base pairs. Sequencing was performed at the University of Oregon DNA Sequencing Facility using a Beckman Coulter CEQ 800 genetic analysis sequencer. Sequence analysis revealed a missense mutation in or404ts at nucleotide position 5730 (cgt to tgt), corresponding to amino acid 1189 (arg to cys) that was not present in either lin-2 or the wild-type strain. To confirm the identity of or404ts, germline transformation of homozygous or404 mutants was performed using cosmid ZK593. The concentration of injected cosmid was 5 ng/μl, and a dominant rol-6 (pRF4) gene was coinjected and used as a marker for transformation, at a concentration of 50 ng/μl. Genomic yeast DNA was used as a carrier at a concentration of 50 ng/μl. Three stable transmitting lines were obtained; in all three lines the embryonic lethality of or404ts was complemented at the restrictive temperature.
Live Embryo Imaging
For live imaging of wild-type, apo-5(or358) and dnc-1(or404) embryos by using differential interference contrast (DIC) microscopy, gravid hermaphrodites were dissected and embryos were mounted in M9 buffer (Brenner, 1974) on 3% agarose pads, covered with a coverslip, and filmed as described previously (Encalada et al., 2000). To measure the duration of mitotic stages, embryos from hermaphrodites carrying GFP strains were mounted on 3% agarose pads, covered with a coverslip, and DIC and fluorescence time-lapse movies of some GFP strains were made using a spinning disk confocal microscope (PerkinElmer Life and Analytical Sciences, Boston, MA) as described in Hamill et al. (2002). Other fluorescence time-lapse movies were made using a Nikon Eclipse TE200-U spinning disk microscope mounted with a Hamamatsu digital camera ORCA-ER and a Wallac Ultraview confocal scanner. Z-series were taken for all movies, by using both DIC and epifluorescence (GFP) images, acquiring six focal planes at 1-μm intervals every 10 s, or seven focal planes at 1-μm intervals every 7 s. Time points measured were chromosome condensation (prophase, the first instance when chromosomes could be individually distinguished in fluorescent images), prometaphase (at the beginning of pronuclear envelope breakdown; PNEBD), metaphase, anaphase (first instance of separation of chromosomal masses), and nuclear envelope reformation (NER). PNEBD and NER were determined by analysis of DIC images, whereas the other stages were determined by analysis of fluorescent images. Images were assembled using Adobe Photoshop (version 7.0). Pairwise comparisons of mitotic timing were done using Student's t test statistics. We used the t test because this test is commonly used to compare the actual difference between two means in relation to the variation in the data (Brauchle et al., 2003). Student's t test was used for comparing the means of two treatments (with or without even number of replicates).
Nocodazole Treatment
To depolymerize microtubules in two-cell embryos, wild-type gravid hermaphrodites carrying the β-tubulin::GFP; histone2B::GFP construct were dissected in water, and embryos were placed directly on gasket poly-lysinecoated glass slides (Erie Scientific, Portsmouth, NH). One-cell embryos were observed in water by using a stereomicroscope until 4 min after the beginning of cytokinesis. The water was then removed almost entirely, and nocodazole was immediately added at concentrations of 20, 40, 50, or 100 μg/ml. At this time, centrosomes in newly formed AB and P1 blastomeres that have duplicated are small, and astral microtubules have disassembled from the previous (first) mitotic cell cycle. Application of nocodazole at this time prevented the reformation of astral microtubules during the second cell division. Although in earlier studies the eggshell was cracked to introduce nocodazole into the early embryo (Strome and Wood, 1983), we have found that nocodazole can effectively penetrate the early embryo eggshell without cracking, as determined by mispositioned mitotic spindles and the partial depletion of microtubules of one- and two-cell embryos (Kurz et al., 2002). Slides were overlaid with a 22 × 22-mm glass coverslip and sealed with melted petroleum jelly. Timing from PNEBD to NER was measured in embryos with small or undetectable mitotic spindles, by using a spinning disk microscope as outlined above. Timing of mitotic cell cycle of two-cell embryos did not significantly change with the different concentrations of nocodazole (our unpublished data).
RNA-mediated Interference
To analyze the phenotype of kinetochore or spindle assembly checkpoint genes, double-stranded RNA (dsRNA) was made in vitro and microinjected into the gonads of either wild-type or apo-5(or358ts) hermaphrodites, at a concentration of at least 1 mg/ml. Double-stranded RNA was made by PCR amplification from genomic N2 DNA with primers specific to each of the following genes: Cebub-1 (R06C7.8: ctcagtcacagagtcatcaaagcct, gctgtcgttactcatcagagcatct), hcp-1 (ZK1055.1: ccgagcatgaggagtcaatca, catgagcgatctgaactct), hcp-2 (T06E4.1: cagtcaacatctcagcatga, acgatgtgcatcgtttagctgac), mdf-1 (C50F4.11: tcttcgcagtttgtttggtg, cacgagcgatttctctttcc), mdf-2 (Y69A2AR.30a: cgggagaattcgaatcttaaact, ttccttcttccaccaattagtca), CeCENP-C (T03F1.9: ggaaatgtacggagcgaaaa, ccttgctcgtatgcatctct), CeMCAK (K11D9: gtgagtcgcatccttcgagaaga, ggagaattgtgatgtcacgagaag), and zyg-9 (F22B5.7: acttcgatgagctgcgagaatc, atctctcaacgagtgcgccatagt). Both the forward and reverse specific primers had extensions with T7 promoters that were used for the PCR amplifications with a T7 primer. PCR reactions were cleaned with phenol: chloroform and ethanol precipitated. In vitro transcription reactions containing 5× transcription buffer, 25 mM rNTPs (3.3 mM final concentration), 0.3% RNAsin, 10% dithiothreitol, water, T7 RNA polymerase, and the PCR product were incubated at 37°C for 2 h. Transcription reactions were subsequently cleaned by phenol:chloroform and ethanol precipitated. Pellet was eluted with 30–40 μl of deionized water and kept at –20°C. wrm-1 dsRNA was made from a cDNA clone (yk213d6; provided by Yuji Kohara, National Institute of Genetics, Nishima, Japan). Single-stranded RNA (ssRNA) from dhc-1 (T12E12.4) was made following the same protocols described above and using the following primers: aaggaaggagctcaacgaca, ctttccttcctgggtcttc. Adult hermaphrodites were injected with dhc-1 ssRNA at a concentration of 1 mg/ml. Worms injected with RNA were placed overnight at 26.6°C for next-day analysis. Hermaphrodites injected with dhc-1 ssRNA produced 36% (41/156) dead embryos 0–16.5 h postinjection and 100% (83/83) dead embryos 16.5–38.5 h postinjection. Adult hermaphrodites injected with hcp-1 and hcp-2 dsRNA produced 74/79 (94%) dead embryos ∼20–35 h postinjection at 26.6°C. Hermaphrodites injected with mdf-1 and mdf-2 dsRNA produced 6% (8/126) and 3% (7/238) dead embryos, respectively, 17–26 h postinjection, consistent with results from previous studies (Kitagawa and Rose, 1999). Hermaphrodites injected with Cebub-1 dsRNA, produced 99% (673/678) dead embryos 12.5–41 h postinjection, and those injected with CeMCAK dsRNA produced 92% (122/132) 17–27.5 h postinjection.
Immunocytochemistry
Homozygous mutant apo-5(or358) and dnc-1(or404) hermaphrodites and hermaphrodites injected with Cebub-1 and CeMCAK dsRNA were placed at 26.6°C overnight (17–20 h), and their embryos were fixed and stained the next day with an antibody against α-tubulin (DMIα; Sigma-Aldrich, St. Louis, MO). Hermaphrodites injected with hcp-1/2, CeMCAK, and Cebub-1 dsRNA were placed at 26.6°C overnight (17–20 h), and their embryos were fixed and stained with anti-CeBUB-1, CeMCAK (provided by Karen Oegema, Ludwig Institute for Cancer Research, University of California San Diego, La Jolla, CA), and HCP-1 antibodies (provided by Mark Roth, Fred Hutchinson Cancer Research Center, University of Washington, Seattle, WA), following previously described protocols (Moore et al., 1999; Oegema et al., 2001). Imaging of stained embryos was done on a Bio-Rad 2100 Radiance confocal microscope, by using a 60× oil immersion lens. Confocal images were assembled into figures using Adobe Photoshop (version 7.0).
RESULTS
A Functional Spindle Checkpoint in the Early C. elegans Embryo
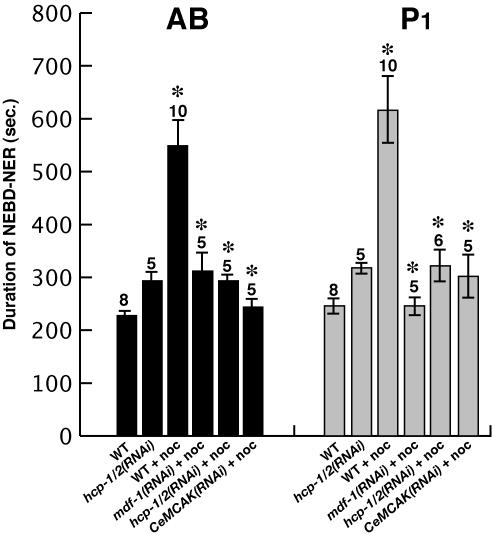
Microtubule instability can delay, and in some cases arrest, mitosis in many organisms. We examined this response in C. elegans, by using nocodazole to destabilize microtubules in two-cell stage embryos (see Materials and Methods). In earlier studies, nocodazole was shown to largely, although not entirely, deplete spindle microtubules in dividing one-cell stage C. elegans embryos, without arresting mitosis (Strome and Wood, 1983; Hyman and White, 1987). Exposing one-cell embryos to nocodazole produced variable effects (our unpublished data), possibly due to variability in the postfertilization age of one-cell embryos dissected from adults when exposed to this chemical. We therefore chose instead to expose early two-cell stage embryos to nocodazole, 4 min after the initiation of the cytokinetic furrow in dividing one-cell stage embryos (see Materials and Methods), normalizing embryos with respect to exposure time during the cell cycle. At this early time of exposure, the newly duplicated centrosomes in both the recently formed anterior (AB) and posterior (P1) daughters are small, with few astral microtubules. Exposure to nocodazole largely prevented reformation of astral microtubules later in the cell cycle (our unpublished data). We timed mitotic progression in untreated and nocodazole-treated early embryos from transgenic mothers that maternally express both β-tubulin::GFP and histone2B::GFP fusion proteins to label microtubules and chromosomes, respectively (Praitis et al., 2001; Strome et al., 2001). We made time-lapse movies of these embryos by using both DIC and epifluorescence optics, with a spinning disk confocal microscope (see Materials and Methods). Because chromosomes never formed metaphase plates and anaphase was abnormal in nocodazole-treated embryos, we timed mitosis from NEBD to NER by using DIC. In both the AB and P1 cells, the duration of this interval increased 2.5-fold compared with untreated wild-type embryos (n = 10; Figure 1 and Supplementary Table 1).
Figure 1.
Depletion of MDF-1, HCP-1/2, or CeMCAK restores normal mitotic timing in two-cell stage C. elegans embryos exposed to nocodazole. Timing of mitosis was measured from NEBD to NER. Mean durations in seconds are plotted with SEM error bars. Numbers above bars indicate sample number for each experiment. Asterisks denote significant differences as determined by Student's t test statistics for comparisons between nocodazole-treated and untreated wild-type embryos and between nocodazole-treated embryos depleted from MDF-1, HCP-1/2, and CeMCAK function and nocodazole-treated wild-type embryos. Nocodazole concentrations used are as follows: WT+noc, 20, 40, 50, and 100 μg/ml; mdf-1(RNAi)+noc, 100 μg/ml; and hcp-1/2(RNAi)+noc and CeMCAK(RNAi)+noc, 50 μg/ml.
If these mitotic delays are due to the activity of a spindle checkpoint, then reducing the function of checkpoint genes should restore more normal cell division timing in nocodazole-treated embryos. We therefore used RNAi to reduce the function of MDF-1, a C. elegans ortholog of the yeast spindle checkpoint protein Mad1p, in nocodazole-treated embryos. We found that the duration of mitosis was restored to the shorter time observed in untreated wild-type embryos (Figure 1 and Supplementary Table 1). We conclude that nocodazole-induced destabilization of microtubules in C. elegans embryos activates the spindle checkpoint to delay progression through mitosis.
Mitotic Delays in Mutant Embryos with Defective Spindles
As a further test of whether microtubule defects can prolong mitosis, we examined mitotic progression in mutant embryos with spindle defects in early embryonic cells, by using both Nomarski DIC and epifluorescence optics, and transgenic strains expressing β-tubulin::GFP and histone2B::GFP fusion proteins (see Materials and Methods). For these and all subsequent analyses of mutant embryos, we examined cell cycle progression during the first mitotic division of the zygote, when spindle defects first occur, in contrast to our use of two-cell stage embryos for nocodazole exposure.
We first timed cell cycle progression in dnc-1(or404) mutant embryos. The dnc-1 gene encodes a p150Glued dynactin subunit family member and is required for proper assembly and positioning of the mitotic spindle in early embryonic cells (Skop and White, 1998; Gonczy et al., 1999). We identified dnc-1(or404) in a genetic screen for temperature-sensitive embryonic-lethal mutants with cell division defects in early embryonic cells (Figure 2). We examined embryos produced by self-fertilization in homozygous dnc-1(or404) mutant hermaphrodites that were matured to adulthood at the restrictive temperature of 26.6°C (hereafter referred to as mutant embryos). Using time-lapse DIC microscopy to examine the first mitotic division in dnc-1(or404) mutant embryos, we found that pronuclei always met and a small posteriorly displaced spindle formed (Figure 4A; n = 12). This mutant phenotype resembles the relatively weak phenotype observed after partial depletion of dnc-1 by using RNAi, with more extensive RNAi depletion causing defects in pronuclear migration and more severe defects in spindle assembly (Skop and White, 1998; Gonczy et al., 1999). We conclude that dnc-1(or404) is a recessive, partial loss-of-function mutation. In addition to the spindle positioning and assembly defects, we also found that the average time required to progress from chromosome condensation to NER in dnc-1 mutant embryos was increased by 1.3-fold, compared with wild-type. Similarly, the time required to progress from the breakdown of the oocyte and sperm derived pronuclei (PNEBD, which corresponds to prometaphase) to anaphase onset was increased almost twofold, compared with wild type (Figure 3A and Table 1).
Figure 2.
Molecular cloning of dnc-1. dnc-1 maps approximately +4.8 map units, on LGIV (left of dpy-20). dnc-1 mutants were rescued by cosmid ZK593. A single allele of dnc-1 has a lesion in the predicted gene ZK593.5, as shown below exon 12. We identified dnc-1(or404ts) in a temperature-sensitive screen for embryonic lethal mutants described in Encalada et al. (2000).
Figure 4.
Microtubule cytoskeletal defects in apo-5 and dnc-1 mutant embryos. (A) Time-lapse Nomarski photomicrographs of a dividing wild-type one-cell stage embryo (a–d), and of two apo-5 (e–l), and one dnc-1 (m–p) mutant embryos undergoing their first mitotic division. Embryos are shown with anterior to the left and posterior to the right. Clearings in a, e, i, and m are the maternal and paternal pronuclei. Arrowheads point to centrosomes and arrows point to the middle of spindles. Notice short distance between nuclei in the newly formed two-cell apo-5 and dnc-1 embryos (h, l, and p), likely due to chromosome segregation defects. (B) Positioning and orientation of the first mitotic spindle in two wild-type (a and c) and two apo-5 (b and d) embryos. Lines inside embryos in a and b represent the position and length of the spindle during elongation. Numbers indicate the sequential order of movement of the mitotic spindle throughout mitosis, starting at pronuclear envelope breakdown (1) and ending at cytokinesis (5 or 6). The length of the spindle was drawn at 10-s intervals. Marks on quadrants on c and d represent the angle between centrosomes and the a-p axis (0°) at PNEBD and cytokinesis. Each mark represents a single scored spindle. (C) Laser scanning confocal images of α-tubulin (green) and DNA (TOTO, red) staining of one-cell stage wild-type and apo-5 embryos during metaphase and anaphase. Arrows point at short microtubules that do not reach the cortex of the embryo and arrowhead points to cytoplasmic tubulin fragments. Dots delineate the embryo cortex in apo-5 mutants.
Figure 3.
Depletion of spindle checkpoint proteins restores normal mitotic timing in one-cell mutant embryos with cytoskeletal abnormalities and delayed mitoses. Bars represent the duration of mitosis from prometaphase (PNEBD) to the onset of anaphase (A) or PNEBD to NER (B), in one-cell embryos. Numbers inside boxes indicate the duration of each mitotic stage in seconds. Mean durations are plotted with SEM error bars. Table 2 shows Student's t test statistical comparisons of mitotic timing between wild-type and mutant embryos. The p values for mitotic timing comparisons between apo-5 and the following double mutants at the p ≤ 0.05 level are shown in parentheses: apo-5; mdf-1(0.042); apo-5; mdf-2 (0.0082); apo-5; Cebub-1 (0.17); apo-5; wrm-1 (0.47); apo-5; hcp-1/2 (0.013); apo-5; CeMCAK (0.032); and apo-5; CeCENP-C (0.00067).
Table 1.
Duration of mitosis in one-cell embryos
| Embryo | Total mitosisa (s) ± SE (n) | PNEBD-anaphase (s) ± SE (n) | PNEBD-NERb (s) ± SE (n) |
|---|---|---|---|
| Wild type | 737.1 ± 32.7 (7) | 135.0 ± 6.0 (8) | 280.0 ± 8.5 (7) |
| dnc-1(or404) | 933.3 ± 288.7 (3) | 248.3 ± 51.0 (6) | |
| zyg-9(RNAi) | 1106.7 ± 79.6 (6) | 294.5 ± 18.3 (9) | |
| apo-5(or358) | 1010.0 ± 100.3 (7) | 251.3 ± 32.1 (8) | 497.5 ± 35.7 (8) |
| mdf-1(RNAi) | 865.0 ± 27.5 (5) | 160.0 ± 9.0 (7) | |
| mdf-2(RNAi) | 683.3 ± 84.1 (3) | 140.9 ± 8.8 (11) | |
| Cebub-1(RNAi) | 946.6 ± 35.3 (3) | 208.3 ± 8.7 (6) | |
| zyg-9(RNAi); mdf-1(RNAi) | na | 186.0 ± 19.9 (5) | |
| apo-5(or358); mdf-1(RNAi) | 866.0 ± 78.5 (5) | 168.3 ± 14.9 (6) | |
| apo-5(or358); mdf-2(RNAi) | 804.0 ± 55.6 (5) | 135.6 ± 11.4 (9) | |
| apo-5(or358); Cebub-1(RNAi) | 920.0 ± 101.2 (4) | 200.0 ± 11.3 (7) | |
| apo-5(or358); wrm-1(RNAi) | na | 210.0 ± 43.8 (4) | |
| hcp-1/2(RNAi) | 805.0 ± 33.3 (4) | 84.0 ± 14.4 (5) | |
| CeMCAK(RNAi) | 825.7 ± 73.4 (7) | 150.0 ± 8.9 (8) | |
| apo-5(or358); hcp1/2(RNAi) | 1210.0 ± 171.0 (3) | 118.9 ± 34.5 (8) | |
| apo-5(or358); CeMCAK(RNAi) | na | 161.7 ± 17.8 (12) | |
| dhc-1(RNAi) | 1350.0 ± 234.3 (3) | na | 520.0 ± 49.7 (7) |
| CeCENP-C(RNAi) | na | na | 347.5 ± 14.9 (4) |
| apo-5(or358); CeCENP-C(RNAi) | 842.0 ± 55.8 (5) | na | 302.9 ± 22.8 (7) |
na, not available.
Duration of timing from chromosome condensation (beginning of prophase) to NER
Duration of timing from PNEBD to NER was measured for wild-type, apo-5 embryos and for embryos in which clear segregation of chromosomes (anaphase) was not obvious
Although the mitotic delay in dnc-1 mutant embryos is consistent with activation of the spindle checkpoint, dynein has been implicated in nuclear envelope breakdown, and a reduction of dynein function can delay the onset of NEB in normal rat kidney cells (Salina et al., 2002). Because this delay occurs before the prometaphase-to-anaphase stage, when spindle checkpoint activity is functional, the delay in cell cycle progression we observe in dnc-1 mutant embryos likely reflects activation of a spindle checkpoint rather than a defect in dynein function during NEB.
We next used RNAi to examine embryos depleted of either ZYG-9, a XMAP215 homolog required for microtubule stability during mitosis (Matthews et al., 1998), or DHC-1, the heavy chain of cytoplasmic dynein, a microtubule minus-end directed motor that, like dynactin, is required for mitotic spindle assembly (Sweeney and Holzbaur, 1996; Skop and White, 1998; Gonczy et al., 1999). In one-cell stage zyg-9(RNAi) mutant embryos, as expected, astral microtubules were abnormally short and the first mitotic spindle was mispositioned (our unpublished data). The duration of mitosis in zyg-9(RNAi) embryos, from chromosome condensation to NER, was ∼1.5-fold longer than in wild type (Tables 1 and 2). This increase was largely due to a 2.2-fold lengthening of the interval from prometaphase to anaphase (Figure 3A and Table 1). In dhc-1(RNAi) embryos, severe spindle assembly defects preclude the use of metaphase and anaphase as landmarks (Skop and White, 1998; Gonczy et al., 1999). We therefore assessed the duration of mitosis by measuring the time from PNEBD to NER in one-cell dhc-1(RNAi) embryos by using DIC optics. Progression through this interval took almost twice as long compared with wild type (Figure 3B and Tables 1 and 2).
Table 2.
Student's t test statistics for pairwise comparisons of duration of mitosis
| mdf-1 (RNAi)a | mdf-2 (RNAi)a | Cebub-1 (RNAi)a | apo-5 (or358ts)a | dnc-1 (or404ts)a | zyg-9 (RNAi)a | dhc-1 (RNAi)b | hcp-1/2 (RNAi)a | CeMCAK (RNAi)a | CeCENP-C (RNAi)b | |
|---|---|---|---|---|---|---|---|---|---|---|
| Wild type | 0.042* | 0.59 | 5.7×10-5* | 0.0082* | 0.077 | 0.0049* | 0.0027* | 0.020* | 0.19 | 0.011* |
Numbers represent p values between each pairwise comparison. Asterisks denote significant differences at the p ≤ 0.05 level.
p values were calculated for comparisons of timing from PNEBD to anaphase onset
p values were calculated for comparisons of timing from PNEBD to NER
Finally, we examined cell cycle progression in apo-5(or358) mutant embryos. We identified apo-5(or358) in our screens for conditional embryonic-lethal mutants but have not yet determined its molecular identity (see Materials and Methods). Using time-lapse Nomarski DIC movies, we found that in most apo-5 mutant embryos (27/28), the position of the first mitotic spindle was unstable, often residing abnormally near the posterior pole during anaphase (Figure 4, A and B). We therefore chose the name apo-5 for anaphase spindle position-defective. We also used indirect immunofluorescence with an antibody that recognizes α-tubulin to examine microtubules in fixed one-cell stage apo-5(or358) and wild-type embryos. Compared with wild-type embryos (n = 12), mitotic astral microtubules were short in apo-5 mutants at metaphase (3/3) and anaphase (7/8), and short microtubule fragments were detected throughout the cytoplasm (Figure 4C). Analyses of one-cell apo-5 embryos containing β-tubulin::GFP and histone2B::GFP fusion constructs revealed that chromosomes do not align normally during metaphase and anaphase, presumably due to defects in kinetochore–microtubule attachment or stability (see Supplemental Movies). As in the other spindle defective mutants, we found that the time required to progress from chromosome condensation to NER was 1.4-fold longer in apo-5 mutant embryos, compared with wild type (Table 1). Nearly twofold delays occurred during the interval from PNEBD to anaphase (Figure 3 and Table 1). To summarize, we have found that mitotic spindle defects in dnc-1, zyg-9, dhc-1, and apo-5 mutant embryos are associated with modest but reproducible delays in progression through mitosis.
A Spindle Checkpoint Delays Mitosis and Reduces Chromosome Segregation Defects in Embryonic Cells with Abnormal Mitotic Spindles
To determine whether the mitotic delays in spindle-defective mutant embryonic cells require a spindle checkpoint, we used RNAi to reduce the function of MDF-1, MDF-2, and CeBUB-1, C. elegans orthologues of the yeast spindle checkpoint proteins Mad1p, Mad2p, and Bub1p. We reduced the function of MDF-1, MDF-2, and CeBUB-1 in apo-5 embryos and that of MDF-1 in zyg-9(RNAi) embryos. We found that reducing the function of MDF-1 decreased the mitotic delays observed in apo-5 and zyg-9 embryos, and reducing the function of MDF-2 restored normal timing to the first mitotic cell cycle of apo-5 embryos (Figure 3A). Cebub-1(RNAi) was less effective in rescuing the mitotic delays in apo-5 mutants, probably due to mitotic delays observed in wild-type embryos depleted of CeBUB-1 using RNAi (Figure 3A). As a negative control, we used RNAi to reduce the function of WRM-1, a β-catenin homolog not expected to influence checkpoint function, and we did not observe significant changes in the timing of mitosis in apo-5 embryos (Figure 3A). We conclude that a functional spindle checkpoint monitors proper spindle assembly during early embryonic cell divisions.
To address the potential biological significance of the spindle checkpoint in early C. elegans embryos, we compared the frequency of DNA segregation defects in apo-5 mutants with or without RNAi-mediated depletion of MDF-1 and MDF-2. Using time-lapse movies of one-cell embryos carrying a histone2B::GFP fusion, we observed lagging chromosomes in fewer than one-half of the dividing one-cell stage apo-5 embryos (Figure 5 and Table 3; see Supplemental Movies). Reducing MDF-1 and MDF-2 function in apo-5 mutants resulted in detectable chromosome segregation defects (lagging chromosomes and chromosome bridges) in nearly all embryos (Figure 5 and Table 3; see Supplemental Movies). We conclude that the spindle checkpoint can delay progression through mitosis and reduce the likelihood of chromosome segregation defects in embryonic cells with abnormal spindles.
Figure 5.
Increased presence of DNA bridges after disruption of MDF-1 function. Fluorescent spinning disk confocal images of one-cell stage apo-5 and apo-5; mdf-1 embryos showing histone2B::GFP signal. Insets show enlarged chromosomes. Arrow points to DNA bridges present in the double mutant.
Table 3.
Frequency of lagging chromosomes in one-cell C. elegans embryos
| Embryo | No. of embryos with lagging chromosomes (%) |
|---|---|
| WT | 0/18 (0) |
| apo-5(or358ts) | 10/18 (56) |
| mdf-1(RNAi) | 1/17 (6) |
| mdf-2(RNAi) | 1/11 (9) |
| apo-5(or358ts); mdf-1(RNAi) | 15/16 (94) |
| apo-5(or358ts); mdf-2(RNAi) | 8/10 (80) |
WT, wild type.
CeCENP-C, CeMCAK, and the Two CENP-F–like Proteins HCP-1 and HCP-2 Are Required for a Functional Spindle Checkpoint
Having established that a checkpoint delays mitosis in embryonic cells with defective spindles, we asked whether other known kinetochore proteins in C. elegans are required for a functional spindle checkpoint. Recently, two C. elegans proteins called HCP-1 and HCP-2, with limited homology to mammalian CENP-F, were shown to contribute redundantly to the proper segregation of chromosomes during embryogenesis (Moore et al., 1999). Intriguingly, mammalian CENP-F was recently implicated in meiotic spindle checkpoint function (Eaker et al., 2001). The conserved kinesin CeMCAK localizes to kinetochores in C. elegans embryos and is required for assembly of the mitotic spindle midzone (Grill et al., 2001). The localization of both HCP-1 and CeMCAK to kinetochores requires a core kinetochore protein called CeCENP-C (Moore and Roth, 2001; Oegema et al., 2001). To better define the requirements for a functional mitotic checkpoint, we asked whether HCP-1, HCP-2, CeMCAK, and CeCENP-C are required for the mitotic delays observed in embryonic cells with defective spindles.
To examine the role of HCP-1 and HCP-2 in early embryos, we used RNAi to reduce the function of both proteins simultaneously. Reducing the function of either HCP-1 or HCP-2 alone by RNAi does not result in significant embryonic lethality, but depletion of both proteins simultaneously [hereafter referred to as hcp-1/2(RNAi)], has been shown to result in 99% embryonic lethality, and in embryonic cells with abnormal amounts of chromosomal DNA, presumably due to chromosome segregation defects (Moore et al., 1999). We also found that depletion of both HCP-1 and HCP-2 resulted in a highly penetrant embryonic lethality (see Materials and Methods), and we examined the chromosome segregation defects during the first mitotic division of mutant embryos by using time-lapse microscopy (see next section). Because chromosomes in one-cell stage hcp-1/2(RNAi) embryos did not congress to form a metaphase plate, but did move toward opposite poles (see next section), we measured the time from pronuclear envelope breakdown to the anaphase-like movements of chromosomal masses to opposite poles. The duration of this interval was shorter in hcp-1/2(RNAi) embryos, compared with wild-type (Figure 3A and Table 1). We next used RNAi to deplete HCP-1/2 in one-cell stage apo-5(or358) mutant embryos (Figure 3A) and in two-cell stage wild-type embryos treated with nocodazole (Figure 1). We found that reducing HCP-1/2 restored normal mitotic timing to apo-5 and nocodazole-treated embryos. We also used RNAi to deplete CeMCAK function in one-cell apo-5(or358) mutant embryos (Figure 3A), and in nocodazole-treated two-cell stage wild-type embryos (Figure 1). In both cases, CeMCAK depletion restored a more normal timing of mitotic progression.
Finally, we examined mitotic progression in CeCENP-C–depleted embryos. As shown previously (Oegema et al., 2001), chromosomes do not separate in anaphase movements in one-cell CeCENP-C(RNAi) embryos. We thus measured mitosis in CeCENP-C(RNAi) embryos from PNEBD to NER and found that the timing of mitosis was similar to that of wild-type embryos. Reducing the function of CeCENP-C by RNAi in one-cell apo-5(or358) embryos restored nearly normal mitotic timing, compared with wild-type (Figure 3B and Table 1). We conclude that in addition to known checkpoint proteins, the kinetochore proteins HCP-1/2, CeMCAK, and CeCENP-C also are required for a functional mitotic spindle checkpoint.
Different Requirements for HCP-1/2 and CeMCAK in Spindle Midzone Assembly and Function
Depletion of the core kinetochore protein CENP-C seems to completely disrupt kinetochore assembly (Oegema et al., 2001), presumably accounting for the inactivity of the spindle checkpoint in CENP-C–depleted embryos. To address how the depletion of HCP-1/2 and CeMCAK might influence the spindle checkpoint function, and to assess whether these proteins act in shared pathways, we compared the mutant phenotypes of dividing one-cell stage embryos produced after RNAi depletion of HCP-1/2, CeMCAK, and CeBUB-1, a presumed checkpoint protein.
We first examined the structure of the mitotic spindle in fixed one-cell embryos, by using indirect immunofluorescence to detect microtubules (Figure 6A). As reported by others (Grill et al., 2001), we found that the spindle midzone was not detectable in CeMCAK-depleted embryos (7/8 embryos; Figure 6A). The spindle midzone also was absent in hcp-1/2(RNAi) embryos (9/10), but it was normal in Cebub-1(RNAi) embryos (8/8; Figure 6A). Surprisingly, cytokinesis seemed normal during the first mitotic division in embryos from all three genotypes, despite the absence of a central spindle (our unpublished data). This is in contrast to previous studies which showed that cytokinesis always fails in the absence of a central spindle after depletion of ZEN-4, an MKLP1-like kinesin-related protein (Raich et al., 1998).
Figure 6.
Spindle abnormalities in hcp-1/2(RNAi), CeMCAK(RNAi), and Cebub-1(RNAi) mutant embryos. (A) One-cell wild-type, hcp-1/2(RNAi), CeMCAK(RNAi), and Cebub-1(RNAi) embryos were fixed and stained to simultaneously visualize DNA (red) and α-tubulin (green). Arrows point to midzone spindle in wild-type and Cebub-1(RNAi) embryos, which is absent in hcp-1/2(RNAi) and CeMCAK(RNAi) embryos. (B) Still images from spinning disk confocal movies of wild-type, hcp-1/2(RNAi), CeMCAK(RNAi), and Cebub-1(RNAi) embryos expressing β-tubulin::GFP; histone::GfP constructs. Arrowheads in wild-type images point to spindle microtubule attachments between centrosomes and chromosomes. (C and D) Centrosome separation as a percentage of embryo length plotted for three wild-type (squares) and three hcp1/2(RNAi), (circles) (C) and CeMCAK(RNAi), (circles) (D) embryos. Arrows in both C and D indicate the time of anaphase onset in wild-type embryos.
We next analyzed the dynamics of spindle pole separation during the first mitotic division in wild-type, hcp-1/2(RNAi), CeMCAK(RNAi), and Cebub-1(RNAi) embryos (Figure 6B). The first mitotic spindles seemed normal at pronuclear envelope breakdown in hcp-1/2(RNAi), CeMCAK(RNAi), and Cebub-1(RNAi) embryos (Figure 6B, a, g, m, and s), but by the time chromosomes had formed a metaphase plate in wild-type embryos, chromosomes in hcp-1/2(RNAi) or Cebub-1(RNAi) embryos had not properly aligned to form a metaphase plate (Figure 6B, b, h, and t). Subsequently in hcp-1/2(RNAi) and CeMCAK(RNAi) embryos, the centrosomes and chromosomes rapidly moved toward opposite poles (Figure 6B, h–l, n–r). In contrast to the abrupt centrosome separation observed in hcp-1/2(RNAi) embryos, centrosomes separated more gradually and to more variable extents in CeMCAK(RNAi) embryos. The mitotic spindle in one-cell Cebub-1(RNAi) embryos elongated with nearly wild-type kinetics (Figure 6B, s–x; our unpublished data). Nevertheless, mitosis was clearly abnormal in Cebub-1(RNAi), progressing more slowly than in wild-type (Figures 3A) and with lagging chromosomes during anaphase (our unpublished data).
Finally, we analyzed the dynamics of spindle separation in hcp-1/2(RNAi), CeMCAK(RNAi), and Cebub-1(RNAi) embryos (Figure 6, C and D). Although centrosomes in dividing one-cell stage wild-type embryos moved apart gradually, with a slight increase in velocity during anaphase (Figure 6C, arrow), centrosomes separated abruptly in hcp-1/2(RNAi) embryos (Figure 6, B and C). These anaphase-like movements of chromosomes and centrosomes occurred ∼85 s after PNEBD in hcp-1/2(RNAi) embryos, compared with 135 s in wild type, suggesting that the mutant embryos entered anaphase precociously. In agreement with previous observations (Grill et al., 2001), we found that centrosomes often moved abnormally far apart from each other during the first division in CeMCAK(RNAi) embryos (4/10 embryos; Figure 6D). Despite the premature and abnormally rapid anaphase movements in hcp-1/2(RNAi) and CeMCAK(RNAi) embryos, cytokinesis occurred at approximately the same time as in wild type (Figure 6B; our unpublished data).
In summary, depletion of CeMCAK or HCP-1/2 resulted in similar mutant phenotypes, including the absence of a central spindle and the abnormal poleward movement of centrosomes during anaphase. In contrast, depletion of CeBUB-1 does not obviously influence central spindle structure or the movement of centrosomes during anaphase. Thus, HCP-1/2 and CeMCAK may act in similar spindle midzone assembly pathways and clearly differ in their requirements compared with a known checkpoint protein, CeBUB-1.
CeBUB-1 and CeMCAK Are Required for the Localization of HCP-1 to Kinetochores
To further compare the requirements for HCP-1/2, CeMCAK, and CeBUB-1, we examined their assembly onto kinetochores in wild-type and mutant embryos. Previous studies have shown that the localization to kinetochores of HCP-1, CeMCAK, and CeBUB-1 all require two core kinetochore components, CeCENP-A (HCP-3) and CeCENP-C (HCP-4) (Moore and Roth, 2001; Oegema et al., 2001; Desai et al., 2003). CeMCAK and CeBUB-1 localize independently of each other (Oegema et al., 2001), but it is not known whether HCP-1/2 localization to kinetochores depends on either CeMCAK or CeBUB-1. We first stained wild-type and hcp-1/2(RNAi) embryos with antibodies against CeMCAK and CeBUB-1. In agreement with an earlier report (Oegema et al., 2001), we found that CeBUB-1 localized to wild-type kinetochores during prophase (n = 6), prometaphase (n = 5), metaphase (n = 6; Figure 7A), and mid-anaphase (n = 8; Figure 7A). Also in agreement with previous work, CeMCAK localized in wild-type embryos to centrosomes during prophase (n = 8) and at centrosomes and kinetochores throughout prometaphase (n = 3), metaphase (n = 4; Figure 7A), anaphase (n = 6; Figure 7A), and telophase (n = 4). In hcp-1/2(RNAi) embryos, both CeBUB-1 (n = 14) and CeMCAK (n = 22) localized normally to kinetochores during mitosis (Figure 7A), suggesting that HCP-1 is not required for either the stability or the proper localization of these proteins. By contrast, in CeBUB-1–depleted embryos (12/12) and in CeMCAK-depleted embryos (36/37), HCP-1 was either absent or localized diffusely around DNA but apparently not at kinetochores (Figure 7B). Even though HCP-1 localization to kinetochores requires CeBUB-1, depletion of CeBUB-1 and HCP-1/2 results in distinct defects that do not clearly overlap beyond their shared requirement for checkpoint function. In particular, depletion of HCP-1/2 results in more severe spindle defects than does depletion of CeBUB-1. One possible explanation is that HCP-2 might localize to kinetochores independently of CeBUB-1 and HCP-1 and thus fulfill requirements for HCP-1/2 in CeBUB-1–depleted embryos. The lack of an HCP-2 antibody has prevented us from examining this issue further. In summary, HCP-1 localization to chromosomes depends on both CeBUB-1 and CeMCAK but not vice versa (Figure 7C).
Figure 7.
HCP-1 requires CeMCAK and CeBUB-1 for proper localization to kinetochores. (A) Confocal micrographs of metaphase and anaphase wild-type or hcp1/2(RNAi) embryos fixed and stain to visualize DNA (TOTO, red) and either CeBUB-1 or CeMCAK (green). (B) Confocal micrographs of wild-type, Cebub-1(RNAi) or CeMCAK(RNAi) embryos at prometaphase and metaphase fixed and stained with anti HCP-1 antibodies. HCP-1 is not detected at prometaphase and is not targeted to kinetochores at metaphase in Cebub-1(RNAi) embryos. Localization of HCP-1 is also disrupted at prometaphase and metaphase in CeMCAK(RNAi) embryos. (C) Dependency relationships between kinetochore and spindle checkpoint components position HCP-1 downstream of CeMCAK and CeBUB-1.
DISCUSSION
Mitotic checkpoints are known to delay progression through mitosis in yeast and vertebrate mutants with defective spindles (Gardner and Burke, 2000; Cleveland et al., 2003). We have shown here that a spindle checkpoint in C. elegans delays progression through mitosis when spindles are defective in rapidly dividing early embryonic cells. In addition to documenting requirements for known checkpoint proteins, we also have shown that the kinetochore proteins CeCENP-C, CeMCAK and two CENP-F-like proteins called HCP-1/2 are required for spindle checkpoint function.
A Functional Spindle Checkpoint Mechanism in an Early Animal Embryo
We have shown that a spindle assembly checkpoint in early C. elegans embryonic cells can delay mitosis both after treatment with nocodazole to destabilize microtubules and in several mutants with defective spindles. Although reproducible, the average duration of the delays is modest compared with those reported for other cell types after exposure to chemicals that stabilize or destabilize microtubules, including PtK1 cells (Waters et al., 1998) and sea urchin embryos (Sluder and Begg, 1983). In some cases, such as in HeLa cells, an apparently permanent arrest occurs at the metaphase/anaphase stage (Jordan et al., 1996). In C. elegans, a cell cycle delay or arrest in response to nocodazole, detected in fixed premeiotic germline cells as an increase in the number of histone H3-positive mitotic cells, was dependent on mdf-1 and mdf-2 function (Kitagawa and Rose, 1999). Furthermore, spindle checkpoint function is required for a suspended animation that occurs in response to hypoxia in early C. elegans embryos (Nystul et al., 2003). Our results suggest that a mitotic checkpoint mechanism functions at least during the first two divisions of the C. elegans embryo to produce modest delays in cell cycle progression. Perhaps the modest magnitude of these delays is sufficient to prevent some defects in chromosome segregation, with the duration limited by a need for coordinated cell divisions to properly position descendants during embryogenesis.
In contrast to our findings, studies of early embryonic cell cycles in Xenopus laevis have not identified a functional spindle checkpoint, although it is clear that mitotic checkpoint genes act later in embryogenesis and postembryonically (Cleveland et al., 2003). The first 12 embryonic cell divisions in Xenopus proceed without an effective mitotic checkpoint, because inhibition of spindle assembly does not prevent the regular oscillation of the maturation promoting factor (Gerhart et al., 1984). In vitro activation of the spindle checkpoint in early Xenopus extracts has been achieved only after adding sperm nuclei to generate a chromosome/cytoplasm ratio comparable with that present at the midblastula transition, when spindle checkpoints normally become active (Minshull et al., 1994).
The apparent absence of a spindle checkpoint in early Xenopus embryos has led to suggestions that the rapid early embryonic cell cycles observed in some organisms may be devoid of mitotic checkpoint regulation (Murray and Hunt, 1997; Hartwell and Weinert, 1989). In Drosophila melanogaster, mutational inactivation of the spindle checkpoint genes bub1, Rod, and Zw10 result in an accelerated exit from metaphase and chromosome segregation defects in larval brain cells and neuroblasts with defective mitotic spindles, but earlier embryonic defects have not been reported (Basu et al., 1999; Basto et al., 2000). However, maternal expression of these genes could mask earlier requirements during embryogenesis, and treatment of early Drosophila embryos with nocodazole does result in mitotic delays (Yu et al., 1998). Evidence for a functional spindle checkpoint during meiosis in mouse oocytes suggests that the checkpoint machinery is present in early mammalian embryos (Wassmann et al., 2003). Thus, it seems likely that other early embryos will be found to use, perhaps to varying degrees, a spindle checkpoint during early embryonic cell cycles.
One difference between C. elegans and most other eukaryotic organisms is that C. elegans chromosomes are holocentric, not centromeric (Pimpinelli and Goday, 1989; reviewed in Maddox et al., 2004). Microtubules attach along the entire length of holocentric chromosomes, and all C. elegans kinetochore proteins studied to date localize along the entire length of mitotic chromosomes, including the proteins that we report in this study to be involved in spindle checkpoint function (Figure 7, A and B). How the spindle checkpoint can monitor many attachment sites in holocentric chromosomes and how mechanistically distinct might be the monitoring mechanisms for monocentric chromosomes remain unknown.
Although we have documented mitotic delays in several different mutants, mitotic delays have not been observed in some C. elegans mutants with severe spindle assembly defects in early embryonic cells. For example, embryonic cells depleted of AIR-1, an aurora-A kinase required for centrosome maturation, produce a very small but still bipolar spindle with reduced centrosomes, yet the timing of progression through metaphase and anaphase was normal (Schumacher et al., 1998; Hannak et al., 2001). Similarly, we have found that embryos lacking SPD-5, a coiled-coil centrosomal protein required for centrosome maturation and assembly of a mitotic spindle (Hamill et al., 2002), exhibit normal mitotic timing (our unpublished data). Further investigation of spindle and kinetochore assembly in these exceptional mutants should provide further insights into the assembly of a functional spindle checkpoint.
Differential Control of Cell Division Timing in Early Embryos
In contrast to Xenopus and Drosophila, cell divisions in the early C. elegans embryo are asynchronous (Sulston et al., 1983). These early divisions seem to consist entirely of S phase and mitosis, with differences in division timing being due to different rates of DNA replication during S phase (Edgar and McGhee, 1988). The differences in timing were recently shown to depend in part on the widely conserved DNA replication checkpoint, which can delay progression through S phase in response to defects in DNA replication in early embryonic cells (Brauchle et al., 2003). Similarly in Drosophila, a DNA replication/damage checkpoint regulated by grapes and Mei-41/ATM has been found to operate during late syncytial embryonic divisions to ensure proper DNA replication before entry into mitosis (Fogarty et al., 1997; Sibon et al., 1999). In contrast to S phase, the duration of mitosis is roughly constant in different early C. elegans embryonic cells (Edgar and McGhee, 1988; Encalada et al., 2000), suggesting that the differential control of mitotic progression by the spindle checkpoint does not contribute to the asynchrony of early embryonic cell cycles.
Kinetochore Proteins and Spindle Checkpoint Function
The restoration of normal mitotic timing in embryos with defective spindles, after depletion of MDF-1, MDF-2, CeCENP-C, CeMCAK, HCP-1/2, and perhaps CeBUB-1, indicates that these proteins are involved in mitotic checkpoint activity in the early C. elegans embryo. This checkpoint function could be direct, despite other potential roles for some of these proteins in kinetochore and/or spindle midzone structure. For example, the poleward movement of centrosomes and chromosomes during anaphase in CeMCAK(RNAi) and hcp-1/2(RNAi) embryos suggests that kinetochore-microtubule interactions were not entirely absent and thus that these genes are not entirely required for kinetochore function (although this residual kinetochore function might be due to incomplete knockdown of CeMCAK and HCP-1/2 by RNAi). Furthermore, both CeMCAK and HCP-1 assemble onto kinetochores downstream of CENP-A and CENP-C, two core structural kinetochore proteins (Moore et al., 1999; Oegema et al., 2001). Thus, CeMCAK and HCP-1/2 do not seem to disrupt kinetochore structure generally and could be involved more directly in checkpoint assembly or function. Indeed, the precocious anaphase observed in hcp-1/2(RNAi) embryos is characteristic of other spindle checkpoint proteins (Gorbsky et al., 1998; Canman et al., 2002) and is consistent with the role of hcp-1/2 in checkpoint function.
However, we cannot rule out the possibility that these checkpoint requirements could be largely indirect, reflecting more general structural roles in kinetochore assembly. Indeed, it has been shown previously that CeCENP-C is necessary for kinetochore assembly in early embryonic cells (Oegema et al., 2001), and the lack of spindle checkpoint delays in CeCENP-C(RNAi) mutants could be attributed to the absence of kinetochores.
Kinetochore Proteins Function in a Complex Network of Spindle Midzone Formation and Spindle Checkpoint Pathways
Our analysis of the functional requirements for CeMCAK, HCP-1/2, and CeBUB-1 suggests that in addition to having spindle checkpoint roles, CeMCAK and HCP-1/2 also function in spindle midzone formation and spindle checkpoint pathways. CeBUB-1 might be required more specifically for proper mitotic checkpoint activity, although it also is required for chromosome segregation. Thus, our data together with data from previous studies define at least four overlapping classes of kinetochore proteins: core kinetochore components (CeCENP-A and CeCENP-C), spindle midzone formation factors (CeMCAK and HCP-1/2), chromosome segregation factors (CeMCAK, HCP-1/2, and CeBUB-1), and spindle checkpoint signaling proteins (MDF-1, MDF-2, CeCENP-C, CeMCAK, CeBUB-1, and HCP-1/2).
Although the requirements for these kinetochore proteins are complex and overlapping, we can detect some hierarchical relationships, or pathways. For example, embryos depleted of CeMCAK or CeBUB-1 fail to localize HCP-1 to kinetochores, whereas CeMCAK and CeBUB-1 localize to kinetochores in the absence of HCP-1/2, suggesting that both CeMCAK and CeBUB-1 act upstream of HCP-1/2 (Figure 7C). These results are in agreement with labeling experiments in U2OS osteosarcoma cells and HeLa cells, in which microinjection of anti-hBUB-1 and anti-hCENP-F antibodies suggest that hBUB-1 assembles onto kinetochores before hCENP-F, and with yeast two hybrid data suggesting that CENP-F binds Bub-1 (Jablonski et al., 1998). However, it is not clear why, in spite of both CeBUB-1 and CeMCAK being required for HCP-1 localization, hcp-1/2(RNAi) embryos seem to have a more severe phenotype than either CeMCAK(RNAi) or especially Cebub-1(RNAi) embryos. Moreover, Cebub-1(RNAi); CeMCAK(RNAi) double mutant embryos do not exhibit the severe phenotype of hcp-1/2(RNAi) embryos (our unpublished data). Thus, other proteins besides CeMCAK and CeBUB-1 might regulate HCP-1/2 function. The localization of HCP-2 is not known, and it would be interesting to learn whether HCP-2 is at kinetochores and functional in the absence of CeBUB-1 or CeMCAK. Although apparently redundant in function, HCP-1 and HCP-2 share only 54% similarity and could have different requirements for their localization to kinetochores.
CeMCAK, CeBUB-1, and HCP-1 have all been positioned downstream of two core kinetochore proteins, CeCENP-A and CeCENP-C (Moore et al., 1999; Oegema et al., 2001). Interestingly, the phenotypes of hcp-1/2(RNAi) and CeMCAK(RNAi) embryos differ substantially from the phenotypes of embryos depleted of CeCENP-A and CeCENP-C in that chromosomes do not exhibit poleward movement in CeCENP-A(RNAi) and CeCENP-C(RNAi) embryos (Oegema et al., 2001). The less general requirements for some kinetochore proteins further implies a hierarchy in functional assembly. However, the interplay and coordination between kinetochore and spindle checkpoint proteins seem complex and may not be easily explained by simple linear pathways. Moreover, until definitive null phenotypes have been defined, it may prove difficult to distinguish the requirements for different loci based only on RNAi-mediated depletion of proteins.
Is CeMCAK a Bridge between Kinetochores and Microtubules?
We think it is particularly interesting to consider a direct role for CeMCAK in checkpoint function. One possibility is that CeMCAK might link kinetochores to spindle microtubules, as proposed for the kinetochore kinesin CENP-E in vertebrate cells (Abrieu et al., 2000). The motor activity of CENP-E is thought to generate tension at the kinetochore/microtubule interface upon capture of microtubules, while also conveying checkpoint signaling activities to other checkpoint proteins (Abrieu et al., 2000). BLAST searches indicate that a CENP-E sequence homolog does not exist in C. elegans, and a functional homolog has not been identified. Three observations support the possibility that CeMCAK might act like CENP-E to influence checkpoint signaling. First, in contrast to the vertebrate MCAK family members that localize to inner centromeres between paired sister chromatid kinetochores (Wordeman and Mitchison, 1995; Walczak et al., 1996), CeMCAK localizes to outer kinetochores in C. elegans (Oegema et al., 2001). Moreover, whereas vertebrate MCAK colocalizes with Aurora B, an established inner centromere protein (Adams et al., 2001; Shannon and Salmon, 2002; Cleveland et al., 2003), CeMCAK localizes to kinetochores independent of the inner centromere proteins CeINCENP and AIR-2/CeAurora B (Oegema et al., 2001). Thus, CeMCAK is positioned to interact with kinetochore microtubules and influence checkpoint function, in contrast to some of its vertebrate relatives. Second, studies in Xenopus extracts have shown that XMCAK promotes microtubule depolymerization during mitotic spindle assembly (Walczak et al., 1996), and depolymerization at microtubule minusends can generate forces to pull chromosomes in the absence of ATP-dependent forces (Coue et al., 1991). Thus, CeMCAK may have activities appropriate for generating force at kinetochores. Finally, CeMCAK is required for HCP-1 localization to kinetochores, which we show, together with CeBUB-1, is required for checkpoint function. Thus, CeMCAK is closely linked to spindle checkpoint proteins and could transmit the contact/tension generated at the microtubule/kinetochore interface to mitotic checkpoint proteins. If, as these observations suggest, CeMCAK does function like vertebrate CENP-E, then animal cells apparently have evolved multiple solutions to the assembly of functional kinetochore and spindle checkpoint complexes during mitosis.
Supplementary Material
Acknowledgments
We thank Dan Burke, Julie Canman, Chris Doe, Judith Eisen, Tom Hays, and Marc Meneghini for helpful comments on the manuscript. We thank Yuji Kohara for providing cDNA clones, the Caenorhabditis elegans Genetics Center (funded by the National Institutes of Health) for strains, Joe Christison for preliminary studies of the hcp-1/2(RNAi) phenotype, and members of the Bowerman laboratory for helpful discussions. We are grateful to Karen Oegema for anti-CeBUB-1 and anti-CeMCAK antibodies and to Mark Roth for the anti-HCP-1 antibody. This work was supported by National Institutes of Health training grants (to S. E. and J. W.), by the American Cancer Society (to R. L.), and by National Institutes of Health research grant GM-49869 (to B. B.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-08-0712) on December 22, 2004.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Abrieu, A., Kahana, J. A., Wood, K. W., and Cleveland, D. W. (2000). CENP-E as an essential component of the mitotic checkpoint in vitro. Cell 102, 817-826. [DOI] [PubMed] [Google Scholar]
- Adams, R. R., Carmena, M., and Earnshaw, W. C. (2001). Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 11, 49-54. [DOI] [PubMed] [Google Scholar]
- Basto, R., Gomes, R., and Karess, R. E. (2000). Rough deal and Zw10 are required for the metaphase checkpoint in Drosophila. Nat. Cell Biol. 2, 939-943. [DOI] [PubMed] [Google Scholar]
- Basu, J., Bousbaa, H., Logarinho, E., Li, Z., Williams, B. C., Lopes, C., Sunkel, C. E., and Goldberg, M. L. (1999). Mutations in the essential spindle checkpoint gene bub1 cause chromosome missegregation and fail to block apoptosis in Drosophila. J. Cell Biol. 146, 13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauchle, M., Baumer, K., and Gonczy, P. (2003). Differential activation of the DNA replication checkpoint contributes to asynchrony of cell division in C. elegans embryos. Curr. Biol. 13, 819-827. [DOI] [PubMed] [Google Scholar]
- Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwitz, B. J., Ahmad, K., Moore, L. L., Roth, M. B., and Henikoff, S. (1999). A histone-H3-like protein in C. elegans. Nature 401, 547-548. [DOI] [PubMed] [Google Scholar]
- Canman, J. C., Salmon, E. D., and Fang, G. (2002). Inducing precocious anaphase in cultured mammalian cells. Cell Motil. Cytoskeleton 52, 61-65. [DOI] [PubMed] [Google Scholar]
- Chan, G. K., Schaar, B. T., and Yen, T. J. (1998). Characterization of the kinetochore binding domain of CENP-E reveals interactions with the kinetochore proteins CENP-F and hBUBR1. J. Cell Biol. 143, 49-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland, D. W., Mao, Y., and Sullivan, K. F. (2003). Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112, 407-421. [DOI] [PubMed] [Google Scholar]
- Coue, M., Lombillo, V. A., and McIntosh, J. R. (1991). Microtubule depolymerization promotes particle and chromosome movement in vitro. J. Cell Biol. 112, 1165-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, A., Rybina, S., Muller-Reichert, T., Shevchenko, A., Hyman, A., and Oegema, K. (2003). KNL-1 directs assembly of the microtubule-binding interface of the kinetochore in C. elegans. Genes Dev. 17, 2421-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaker, S., Pyle, A., Cobb, J., and Handel, M. A. (2001). Evidence for meiotic spindle checkpoint from analysis of spermatocytes from Robertsonian-chromosome heterozygous mice. J. Cell Sci. 114, 2953-2965. [DOI] [PubMed] [Google Scholar]
- Edgar, L. G., and McGhee, J. D. (1988). DNA synthesis and the control of embryonic gene expression in C. elegans. Cell 53, 589-599. [DOI] [PubMed] [Google Scholar]
- Encalada, S. E., Martin, P. R., Phillips, J. B., Lyczak, R., Hamill, D. R., Swan, K. A., and Bowerman, B. (2000). DNA replication defects delay cell division and disrupt cell polarity in early Caenorhabditis elegans embryos. Dev. Biol. 228, 225-238. [DOI] [PubMed] [Google Scholar]
- Foe, V. E., and Alberts, B. M. (1983). Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J. Cell Sci. 61, 31-70. [DOI] [PubMed] [Google Scholar]
- Fogarty, P., Campbell, S. D., Abu-Shumays, R., Phalle, B. S., Yu, K. R., Uy, G. L., Goldberg, M. L., and Sullivan, W. (1997). The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr. Biol. 7, 418-426. [DOI] [PubMed] [Google Scholar]
- Gardner, R. D., and Burke, D. J. (2000). The spindle checkpoint: two transitions, two pathways. Trends Cell Biol. 10, 154-158. [DOI] [PubMed] [Google Scholar]
- Gerhart, J., Wu, M., and Kirschner, M. (1984). Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J. Cell Biol. 98, 1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy, P., Pichler, S., Kirkham, M., and Hyman, A. A. (1999). Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. J. Cell Biol. 147, 135-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky, G. J., Chen, R. H., and Murray, A. W. (1998). Microinjection of antibody to Mad2 protein into mammalian cells in mitosis induces premature anaphase. J. Cell Biol. 141, 1193-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill, S. W., Gonczy, P., Stelzer, E. H., and Hyman, A. A. (2001). Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature 409, 630-633. [DOI] [PubMed] [Google Scholar]
- Hamill, D. R., Severson, A. F., Carter, J. C., and Bowerman, B. (2002). Centrosome maturation and mitotic spindle assembly in C. elegans require SPD-5, a protein with multiple coiled-coil domains. Dev. Cell 3, 673-684. [DOI] [PubMed] [Google Scholar]
- Hannak, E., Kirkham, M., Hyman, A. A., and Oegema, K. (2001). Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J. Cell Biol. 155, 1109-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick, K. G., and Murray, A. W. (1995). Mad1p, a phosphoprotein component of the spindle assembly checkpoint in budding yeast. J. Cell Biol. 131, 709-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick, K. G., Weiss, E., Luca, F. C., Winey, M., and Murray, A. W. (1996). Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science 273, 953-956. [DOI] [PubMed] [Google Scholar]
- Hartwell, L. H., and Weinert, T. A. (1989). Checkpoints: controls that ensure the order of cell cycle events. Science 246, 629-634. [DOI] [PubMed] [Google Scholar]
- Hartwell, L. H., and Kastan, M. B. (1994). Cell cycle control and cancer. Science 266, 1821-1828. [DOI] [PubMed] [Google Scholar]
- Hoyt, M. A., Totis, L., and Roberts, B. T. (1991). S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 66, 507-517. [DOI] [PubMed] [Google Scholar]
- Hwang, L. H., Lau, L. F., Smith, D. L., Mistrot, C. A., Hardwick, K. G., Hwang, E. S., Amon, A., and Murray, A. W. (1998). Budding yeast Cdc 20, a target of the spindle checkpoint. Science 279, 1041-1044. [DOI] [PubMed] [Google Scholar]
- Hyman, A. A., and White, J. G. (1987). Determination of cell division axes in the early embryogenesis of Caenorhabditis elegans. J. Cell Biol. 105, 2123-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski, S. A., Chan, G. K., Cooke, C. A., Earnshaw, W. C., and Yen, T. J. (1998). The hBUB1 and hBUBR1 kinases sequentially assemble onto kinetochores during prophase with hBUBR1 concentrating at the kinetochore plates in mitosis. Chromosoma 107, 386-396. [DOI] [PubMed] [Google Scholar]
- Jordan, M. A., Wendell, K., Gardiner, S., Derry, W. B., Copp, H., and Wilson, L. (1996). Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 56, 816-825. [PubMed] [Google Scholar]
- Kitagawa, R., and Rose, A. M. (1999). Components of the spindle-assembly checkpoint are essential in Caenorhabditis elegans. Nat. Cell Biol. 1, 514-521. [DOI] [PubMed] [Google Scholar]
- Kurz, T., Pintard, L., Willis, J. H., Hamill, D. R., Gonczy, P., Peter, M., and Bowerman, B. (2002). Cytoskeletal regulation by the Nedd8 ubiquitin-like protein modification pathway. Science 295, 1294-1298. [DOI] [PubMed] [Google Scholar]
- Li, R., and Murray, A. W. (1991). Feedback control of mitosis in budding yeast. Cell 66, 519-531. [DOI] [PubMed] [Google Scholar]
- Liao, H., Winkfein, R. J., Mack, G., Rattner, J. B., and Yen, T. J. (1995). CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J. Cell Biol. 130, 507-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox, P. S., Oegema, K., Desai, A., and Cheeseman, I. M. (2004). “Holo”er than thou: chromosome segregation and kinetochore function in C. elegans. Chromosome Res. 12, 641-653. [DOI] [PubMed] [Google Scholar]
- Matthews, L. R., Carter, P., Thierry-Mieg, D., and Kemphues, K. (1998). ZYG-9, a Caenorhabditis elegans protein required for microtubule organization and function, is a component of meiotic and mitotic spindle poles. J. Cell Biol. 141, 1159-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh, J. R., Grishchuk, E. L., and West, R. R. (2002). Chromosome–microtubule interactions during mitosis. Annu. Rev. Cell Dev. Biol. 18, 193-219. [DOI] [PubMed] [Google Scholar]
- Minshull, J., Sun, H., Tonks, N. K., and Murray, A. W. (1994). A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell 79, 475-486. [DOI] [PubMed] [Google Scholar]
- Moore, L. L., Morrison, M., and Roth, M. B. (1999). HCP-1, a protein involved in chromosome segregation, is localized to the centromere of mitotic chromosomes in Caenorhabditis elegans. J. Cell Biol. 147, 471-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, L. L., and Roth, M. B. (2001). HCP-4, a CENP-C-like protein in Caenorhabditis elegans, is required for resolution of sister centromeres. J. Cell Biol. 153, 1199-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, A. W., and Hunt, T. (1997). The Cell Cycle: An Introduction, Oxford, NY: Oxford University Press.
- Nystul, T. G., Goldmark, J. P., Padilla, P. A., and Roth, M. B. (2003). Suspended animation in C. elegans requires the spindle checkpoint. Science 302, 1038-1041. [DOI] [PubMed] [Google Scholar]
- Oegema, K., Desai, A., Rybina, S., Kirkham, M., and Hyman, A. A. (2001). Functional analysis of kinetochore assembly in Caenorhabditis elegans. J. Cell Biol. 153, 1209-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangilinan, F., and Spencer, F. (1996). Abnormal kinetochore structure activates the spindle assembly checkpoint in budding yeast. Mol. Biol. Cell 7, 1195-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpinelli, S., and Goday, C. (1989). Unusual kinetochores and chromatin diminution in Parascaris. Trends Genet. 5, 310-315. [DOI] [PubMed] [Google Scholar]
- Praitis, V., Casey, E., Collar, D., and Austin, J. (2001). Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157, 1217-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich, W. B., Moran, A. N., Rothman, J. H., and Hardin, J. (1998). Cytokinesis and midzone microtubule organization in Caenorhabditis elegans require the kinesin-like protein ZEN-4. Mol. Biol. Cell 9, 2037-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, J. M., Ashcroft, N., Donovan, P. J., and Golden, A. (1998). A highly conserved centrosomal kinase, AIR-1, is required for accurate cell cycle progression and segregation of developmental factors in Caenorhabditis elegans embryos. Development 125, 4391-4402. [DOI] [PubMed] [Google Scholar]
- Salina, D., Bodoor, K., Eckley, D. M., Schroer, T. A., Rattner, J. B., and Burke, B. (2002). Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell 108, 97-107. [DOI] [PubMed] [Google Scholar]
- Shannon, K. B., and Salmon, E. D. (2002). Chromosome dynamics: new light on Aurora B kinase function. Curr. Biol. 12, R458-R460. [DOI] [PubMed] [Google Scholar]
- Sibon, O. C., Laurencon, A., Hawley, R., and Theurkauf, W. E. (1999). The Drosophila ATM homologue Mei-41 has an essential checkpoint function at the midblastula transition. Curr. Biol. 9, 302-312. [DOI] [PubMed] [Google Scholar]
- Skibbens, R. V., and Hieter, P. (1998). Kinetochores and the checkpoint mechanism that monitors for defects in the chromosome segregation machinery. Annu. Rev. Genet. 32, 307-337. [DOI] [PubMed] [Google Scholar]
- Skop, A. R., and White, J. G. (1998). The dynactin complex is required for cleavage plane specification in early Caenorhabditis elegans embryos. Curr. Biol. 8, 1110-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder, G., and Begg, D. A. (1983). Control mechanisms of the cell cycle: role of the spatial arrangement of spindle components in the timing of mitotic events. J. Cell Biol. 97, 877-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome, S., Powers, J., Dunn, M., Reese, K., Malone, C. J., White, J., Seydoux, G., and Saxton, W. (2001). Spindle dynamics and the role of gamma-tubulin in early Caenorhabditis elegans embryos. Mol. Biol. Cell 12, 1751-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome, S., and Wood, W. B. (1983). Generation of asymmetry and segregation of germ-line granules in early C. elegans embryos. Cell 35, 15-25. [DOI] [PubMed] [Google Scholar]
- Sullivan, W., Daily, D. R., Fogarty, P., Yook, K. J., and Pimpinelli, S. (1993). Delays in anaphase initiation occur in individual nuclei of the syncytial Drosophila embryo. Mol. Biol. Cell 4, 885-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston, J. E., Schierenberg, E., White, J. G., and Thomson, J. N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64-119. [DOI] [PubMed] [Google Scholar]
- Sweeney, H. L., and Holzbaur, E. L. (1996). Mutational analysis of motor proteins. Annu. Rev. Physiol. 58, 751-792. [DOI] [PubMed] [Google Scholar]
- Walczak, C. E., Mitchison, T. J., and Desai, A. (1996). XKCM 1, a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell 84, 37-47. [DOI] [PubMed] [Google Scholar]
- Wang, Y., and Burke, D. J. (1995). Checkpoint genes required to delay cell division in response to nocodazole respond to impaired kinetochore function in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 15, 6838-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassmann, K., Niault, T., and Maro, B. (2003). Metaphase I arrest upon activation of the Mad2-dependent spindle checkpoint in mouse oocytes. Curr. Biol. 13, 1596-1608. [DOI] [PubMed] [Google Scholar]
- Waters, J. C., Chen, R. H., Murray, A. W., and Salmon, E. D. (1998). Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol. 141, 1181-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver, B. A., Bonday, Z. Q., Putkey, F. R., Kops, G. J., Silk, A. D., and Cleveland, D. W. (2003). Centromere-associated protein-E is essential for the mammalian mitotic checkpoint to prevent aneuploidy due to single chromosome loss. J. Cell Biol. 162, 551-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordeman, L., and Mitchison, T. J. (1995). Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J. Cell Biol. 128, 95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, K. R., Duronio, R. J., and Sullivan, W. (1998). Cell cycle checkpoints: safe passage through mitosis. In: Mechanisms of Cell Division: Frontiers in Biology. ed. S. Endow and D. Glover, New York: Oxford University Press, 1-41.
- Zalokar, M. (1976). Autoradiographic study of protein and RNA formation during early development of Drosophila eggs. Dev. Biol. 49, 425-437. [DOI] [PubMed] [Google Scholar]
- Zhu, X., Chang, K. H., He, D., Mancini, M. A., Brinkley, W. R., and Lee, W. H. (1995). The C terminus of mitosin is essential for its nuclear localization, centromere/kinetochore targeting, and dimerization. J. Biol. Chem. 270, 19545-19550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.