Abstract
The polarized distribution of Na+,K+-ATPase plays a paramount physiological role, because either directly or through coupling with co- and countertransporters, it is responsible for the net movement of, for example, glucose, amino acids, Ca2+, K+, Cl-, and CO3H- across the whole epithelium. We report here that the β-subunit is a key factor in the polarized distribution of this enzyme. 1) Madin-Darby canine kidney (MDCK) cells (epithelial from dog kidney) express the Na+,K+-ATPase over the lateral side, but not on the basal and apical domains, as if the contact with a neighboring cell were crucial for the specific membrane location of this enzyme. 2) MDCK cells cocultured with other epithelial types (derived from human, cat, dog, pig, monkey, rabbit, mouse, hamster, and rat) express the enzyme in all (100%) homotypic MDCK/MDCK borders but rarely in heterotypic ones. 3) Although MDCK cells never express Na+,K+-ATPase at contacts with Chinese hamster ovary (CHO) cells, they do when CHO cells are transfected with β1-subunit from the dog kidney (CHO-β). 4) This may be attributed to the adhesive property of the β1-subunit, because an aggregation assay using CHO (mock-transfected) and CHO-β cells shows that the expression of dog β1-subunit in the plasma membrane does increase adhesiveness. 5) This adhesiveness does not involve adherens or tight junctions. 6) Transfection of β1-subunit forces CHO-β cells to coexpress endogenous α-subunit. Together, our results indicate that MDCK cells express Na+,K+-ATPase at a given border provided the contacting cell expresses the dog β1-subunit. The cell–cell interaction thus established would suffice to account for the polarized expression and positioning of Na+,K+-ATPase in epithelial cells.
INTRODUCTION
The membrane enzyme Na+,K+-ATPase of epithelial cells serves two different but integrated roles. The first is the translocation of ions across the plasma membrane as in other cell types (Skou, 1957; Skou, 1998). The second stems from its expression in a particular domain of the plasma membrane (polarization), in such a way that it propels the translocation of Na+ across the whole epithelium as proposed by Koefoed-Johnsen and Ussing (1958). In turn, a combination between the polarized distribution of Na+,K+-ATPase and the polarized expression of co-, countertransporters, and ion channels drives the net transport of, for example, glucose, amino acids, Ca2+, K+, Cl-, and CO3H- across epithelia (Schultz and Curran, 1969; Cereijido and Rotunno, 1971; Rabito and Karish, 1983). In keeping with these roles, Na+,K+-ATPase is found to reside on the basolateral surface in most epithelial cells (Cereijido et al., 1980; Ernst and Mills, 1980; Fambrough and Bayne, 1983; Kashgarian et al., 1985). In a few other epithelial cells, such as those of the choroid plexus (Wright, 1972), retinal pigment epithelium (Steinberg and Miller, 1979; Gundersen et al., 1991), and cockroach salivary gland (Just and Walz, 1994), this enzyme is expressed on the opposite side of the cells but always in a strictly polarized manner.
Na+,K+-ATPase is a heteromultimer comprised of three subunits. The 110-kDa α-subunit bears the Mg2+, ATP, Na+, K+, and ouabain binding sites and is therefore considered to be the catalytic subunit of the enzyme. The β-subunit is a glycoprotein of 40–60 kDa that seems to be involved in the structural and functional maturation of the holoenzyme (Geering et al., 1989; Ackermann and Geering, 1990) and subsequent transport of the α-subunit to the plasma membrane (Noguchi et al., 1987; Fambrough, 1988; Takeyasu and Kawakami, 1989). Ion transport requires the participation of both α- and β-subunits (Noguchi et al., 1987; Horowitz et al., 1990). These units assemble during or soon after biosynthesis (Fambrough and Bayne, 1983), a requirement to exit from the endoplasmic reticulum (Jaunin et al., 1992). Finally, there is a small γ-subunit (Forbush et al., 1978) that belongs to the FXYD family that modulates Na+,K+-ATPase activity (Sweadner et al., 2000); it is not referred to in the present work.
Although for macroscopic processes such as net transepithelial fluxes and electrical potentials it is irrelevant whether the pump is located at the basal or at the lateral side of the cells, the results of the present work suggest that the expression on the lateral plasma membrane facing the intercellular spaces may be decisive for the polarized distribution of the pump. We study this polarized expression in MDCK cells. We describe the lateral expression of Na+,K+-ATPase in pure MDCK monolayers and in coculture with other cell types, in particular when this other cell type has been transfected with the dog β1-subunit. We observe that the expression of this subunit in Chinese hamster ovary fibroblasts (CHO) cells up-regulates the coexpression of endogenous α-subunit at the cell surface, confers cell adhesiveness, and induces the expression of the pump in heterologous contacts when cocultured with MDCK. Together, these results indicate that the β-subunit plays a major role in the polarized expression of Na+,K+-ATPase.
The present results were partially presented in overall reviews (Cereijido et al., 2000, 2001, 2003, 2004; Shoshani and Contreras, 2001).
MATERIALS AND METHODS
Cell Culture
Starter cell cultures were obtained from the American Type Culture Collection (MDCK, CCL-34; LLC-PK1 CRL 1392; LLC-MK2, CCL-7; NRK-52E, CRL-1571; PtK2, CCL-56; VERO, CCL-81; COS-7, CRL-1651;CRFK, CCL-94; CF2TH, CRL-1430; 3T3, CL-173; and CHO, CCL-61; 293, CRL-1573). Ma104 cells (epithelial line derived from rhesus monkey kidney) were a generous gift from Dr. E. Rodríguez-Boulán (Cornell University, Ithaca, NY). MDCK and Ma104 cells were cloned, and all experiments reported here were performed in cells of clone 7 and 1, respectively. All cells were grown at 36.5°C in disposable plastic bottles (3250; Costar, Cambridge, MA) in a 5% CO2 atmosphere (Forma Scientific CO2 incubator, Steri-Cult 200). We used DMEM (430-1600; Invitrogen, Carlsbad, CA), with 100 U/ml penicillin, 100 μg/ml streptomycin (600-5145; Invitrogen), 0.8 U/ml insulin (Eli Lilly, Indianapolis, IN), and 10% fetal calf serum (FCS) (200-6170; Invitrogen), except for CHO cells that were cultured in a mixture of F12/DMEM. Cells were harvested with trypsin-EDTA and plated on dishes with or without glass coverslips.
Plasmid Construct and Transfection
The full-length cDNA of dog kidney Na+,K+-ATPase β1-subunit (cloned into pKS+βΔ11; a generous gift from Dr. R. Farley, University of Southern California School of Medicine, Los Angeles, CA) was subcloned into the expression vector pIRESneo (previously known as pCIN4 from BD Biosciences Clontech, Palo Alto, CA). Briefly, pKS+βΔ11 was restricted with SpeI and SacI, and the insert corresponding to the full-length β1 was then blunt end ligated to the pCIN4 vector that was previously restricted with EcoRV. Two positive clones of transformed bacteria were subjected to plasmid purification with QIAGEN plasmid midi kit (catalog no. 12143; QIAGEN, Valencia, CA). The transfection was as follows: CHO cells were plated at 90% density on glass coverslips placed in a culture dish of 3 cm in diameter, in a serum-free medium. Cells were then incubated for 5 h with a mixture of LipofectAMINE Plus (10964-013; Invitrogen) and 3 μg of the purified plasmid (pCIN4 [mock] or pCIN4-β), as indicated by the manufacturer. Thereafter, the transfection medium was removed, and the cells were incubated for additional 24 h with fresh serum-containing medium to allow recovery. Subsequently, the coverslips were removed and processed for immunofluorescence, to confirm the transient expression. Then, 0.8 mg/ml G418 was added to select stable clones expressing the dog Na+,K+-ATPase β1-subunit. Except for biotinylation and Takeichi's aggregation assays, in which various clones have been compared, all the experiments were done with clone 10c that was chosen because it expresses the dog Na+,K+-ATPase β1-subunit mainly in the plasma membrane. Stable clones are maintained with 0.2 mg/ml G418 in F12/DMEM mixture.
Mixed Monolayers
Cell mixture was performed as described previously (Contreras et al., 1995; Cereijido et al., 2002). Briefly, usually the cell type different from MDCK (CHO, CHO-β, Ma104, and normal rat kidney [NRK]) was prelabeled with 5- (and 6-){[(4-chloromethyl)benzoyl]amino}-tetramethylrhodamine (C-2927, CellTracker Orange CMTMR; Molecular Probes, Eugene, OR). This was achieved by incubating the cells for 1 h at 36.5°C, with CMTMR in dimethyl sulfoxide added to the medium to a final concentration of 6.3 μM. Cells were then washed three times with phosphate-buffered saline (PBS) solution, reincubated for 1 h in DMEM supplemented with 10% FCS. Then, the cells are trypsinized and the suspension is mixed with MDCK cells suspension, in equal parts, to be cocultured on glass coverslips and processed the day after for immunofluorescence assay.
Immunofluorescence Microscopy
For immunofluorescence microscopy, cells grown on coverslips were washed with PBS and then fixed and permeabilized with ice-cold methanol for 5 min. After washing with PBS, the cells were soaked in blocking solution (PBS containing 5% fetal bovine serum) for 1 h. The cells were then incubated with the first antibodies for 30 min at 37°C, washed 10 times quickly with PBS, and thereafter incubated with the secondary antibodies for 30 min at room temperature. All antibodies were diluted in blocking solution, and the following secondary antibodies were used: Alexa 488-conjugated goat anti-mouse IgG, (Molecular Probes) and CY5-goat anti-rabbit IgG (Zymed Laboratories, South San Francisco, CA). After washing, as indicated previously, specimens were mounted on glass slides with FluoroGuard antifade reagent (170–3140; Bio-Rad, Hercules, CA) and observed with a confocal microscope (Bio-Rad MRC-600 or Leica TCS SP2) (Leica, Hiena, Germany). The β1-subunit of the Na+,K+-ATPase was identified with a mouse monoclonal antibody donated by Dr. M. Caplan (Yale University, New Haven, CT), and the α-subunit with a polyclonal antibody (F592-594) donated by Dr. D. Fambrough (The Johns Hopkins University, Baltimore, MD). Acquisition and analysis were performed with the respective software (COMOS, Bio-Rad: LCS, Leica) and with Image J from National Institutes of Health (Bethesda, MD).
Immunoblot
All the extraction steps are performed at 4°C. Monolayers grown on 3-cm dish were washed three times with PBS, and then 200 μl of RIPA buffer containing 10 mM PIPES, pH 7.4, 150 mM NaCl, 2 mM EDTA plus 1% Triton X-100, 0.5% DocNa, 10% glycerol, and protease inhibitors (Complete, Mini; Roche Diagnostics, Indianapolis, IN) was added. The cells were scrapped with rubber policeman, and the cell lysate collected into a 1.5-ml microcentrifuge tube. This was repeated with another 200 μl of RIPA and then incubated for 30 min under continuous and vigorous shaking. The extract was sonicated for 30 s and centrifuged 20 min at 14,000 rpm in a microcentrifuge. The supernatant was recovered, and its protein content was measured with the BCA protein assay reagent (catalog no. 23225; Pierce Chemical, Rockfield, IL) and subsequently boiled in Laemmli sample buffer. The SDS-PAGE–resolved proteins were electro-transferred to polyvinylidene difluoride (PVDF) membrane (RPN 303F, Hybond-P; Amersham Biosciences, Piscataway, NJ), which were blocked with 5% dry defatted milk and 3% bovine serum albumin (BSA) in PBS. The proteins of interest were detected with the specific polyclonal or monoclonal antibodies indicated above, followed by species-appropriate peroxidase-conjugated antibodies (62-6120 and 62-6520, Zymed Laboratories; and A-9037, Sigma-Aldrich, St. Louis, MO) and a chemiluminescent detection system (RPN2132, ECL PLUS; Amersham Biosciences).
Biotinylation of Cell Surface Proteins
Confluent monolayers of stable clones of CHO cells expressing the dog β1-subunit or the empty vector were rinsed twice with 5 ml of ice-cold PBS containing 1 mM MgCl2 and 0.1 mM CaCl2 (CM-phosphate-buffered saline), followed by the addition of 3 ml of the same ice-cold solution containing 0.5 mg/ml freshly added SULFO-NHS-SS-biotin (Pierce Chemical) for 45 min. Fresh buffer and biotin were added and incubated another 45 min. For quenching, the plates were washed three times with CM-phosphate-buffered saline containing 100 mM glycine. The cells were scraped in RIPA buffer for 10 min at 4°C, and the homogenates were centrifuged at 14,000 rpm, for 10 min. Protein of each lysate was used for precipitation (16 h at 4°C) with 30 μl of Neutravidin beads (Pierce Chemical). The beads were washed four times with RIPA buffer, and the final pellets were resuspended in 100 μl of 4× Laemmli buffer and incubated 1 h at 37°C. The beads were pelleted, and the solubilized proteins were separated by SDS-PAGE, transferred to PVDF membranes and probed with antibodies directed against α- and β-subunits of the Na+,K+-ATPase as indicated in Immunoblot.
Aggregation Assays
CHO cells stably expressing dog β1-subunit or the empty vector and MDCK cells (as controls) were tested for their ability to aggregate with two different techniques: hanging drop suspension cultures and classical cell aggregation assay.
Hanging Drop Suspension Cultures (Thoreson and Reynolds, 2002). Cells were trypsinized in the presence of EDTA, washed twice in PBS, and resuspended in F12/DMEM without serum. Cells (1.5 × 104)in30 μl of media were suspended as hanging drops from the lid of a 24-well culture dish and allowed to aggregate overnight in a humid 5% CO2 incubator at 37°C. Corresponding wells were filled with PBS to prevent drying of the drops. Aggregation was evaluated 14–18 h after plating. To assay for tightness of cell-cell adhesion, cells were subjected to shear force by passing them 10 times through a standard 200-μl micropipette tip. Cells were observed through a light microscope with 5× phase contrast objective (DMIRE2; Leica). For quantification, after the pipetting stress, pictures (DC-300F; Leica) of individual fields of cells were scored for small (7–20 cells) or large (>20 cells) aggregates. The data presented here are from three experiments in which 12 pictures were analyzed for each cell type. Results are expressed as mean ± SE.
Classical Cell Aggregation Assay (Takeichi, 1977). Confluent monolayers were treated with 0.2% (wt/vol) trypsin and 1 mM EDTA at 37°C for 5 min and dispersed by moderate pipetting. Cells were resuspended in P buffer (145 mM NaCl, 10 mM HEPES, pH 7.4, 1.0 mM Na-pyruvate, 10 mM glucose, 3.0 mM CaCl2) complemented with Complete Mini (Roche Diagnostics) at 106 cells/ml, except for the Ca2+-dependent experiments in which DMEM with (1.8 mM) or without (5 μM) Ca2+ was used. Cell suspension was placed in 1.5-ml microfuge tubes precoated with BSA and rotated on a gyratory shaker at 37°C for 3 h. Aggregation was stopped by adding 2% (vol/vol) glutaraldehyde. The extent of aggregation was assessed by fluorescence-activated cell sorting (FACS) analysis of 50,000 events (FACS Vantage; BD Biosciences, San Jose, CA).
Transepithelial Electrical Resistance (TER)
The degree of sealing of the tight junctions was assessed by measuring the transepithelial electrical resistance (TER) (Cereijido et al., 1978, 2002). After incubation under a given condition, the filter with the monolayer was mounted as a flat sheet between two Lucite chambers with an exposed area of 0.69 cm2. Current was delivered via Ag/AgCl electrodes placed at 2.0 cm from the monolayer; the voltage deflection elicited was measured with a second set of electrodes placed at 1.0 mm from the membrane. Values of TER reported were obtained by subtracting the contribution of the filter and the bathing solution. A given monolayer was used only for a single determination and discarded to avoid leaks due to edge damage.
RESULTS
The Polarized Distribution of Na+,K+-ATPase
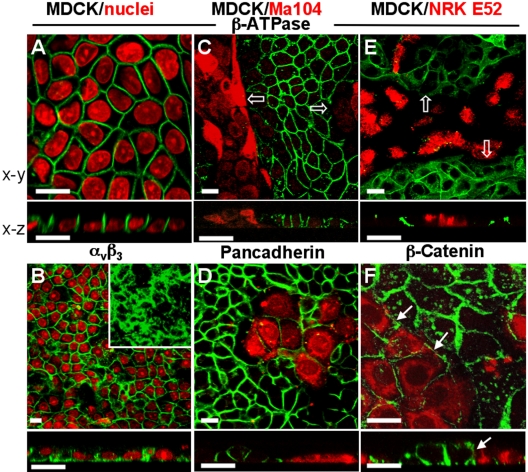
Studies with radioactive tracers, short-circuit currents, sidedness of the effect of inhibitors, and [3H]ouabain labeling had long anticipated that the Na+,K+-ATPase would be found on the basolateral side (Cereijido et al., 1980, 1981, 2000, 2001; Louvard, 1980; Rabito and Tchao, 1980; Contreras et al., 1989). Yet, the study of the distribution of this enzyme in at least 100 contacts (in each case) of confluent monolayers of epithelial cells by using immunofluorescence approach, provides some revealing details on this localization (Table 1 and Figure 1). Foremost, the enzyme is localized at the lateral border of the cell (Figure 1A) and not at the basolateral one. Figure 1B showing αvβ3-integrin staining at lateral as well as basal domains demonstrates that the confocal method used is able to detect a protein placed at this border and that the absence of β1-subunit at the basal membrane may not be attributed to a technical difficulty. The lateral expression of this subunit seems to depend on cell-cell contacts, as well as the nature of the neighboring cell. Thus, when this neighbor is another MDCK cell, expression of Na+,K+-ATPase is observed in 100% of the contacting borders. This percentage markedly decreases in contacts with other cell lines (Table 1). Actually, many borders that we have scored as positive in heterotypic contacts were in fact due to faint labeling presumably caused by immature contacts or rearrangement of cells in the monolayer. Not even the fact that the neighboring cell derives from the same animal species (e.g., dog thymus CF2TH cells) or from the same organ (e.g., kidney) of other animal species (e.g., PTK2, LLCPK1, and NRK-E52) ensures that MDCK cells would express Na+,K+-ATPase in the heterotypic border. A given MDCK cell that expresses its β-subunit in a homotypic contact does not express it on the other side, when this side contacts a rat kidney cell (Figure 1E, NRK-E52). The same observation is repeated with Ma104 (Contreras et al., 1995; Figure 1C), LLCMK2, human embryonic kidney 293, VERO, and CHO cells. This absence of expression of Na+,K+-ATPase in heterotypic contacts between epithelial cells may not be attributed to lack of intimate cell adhesion. Thus, Figure 1, D and F, shows in two different cocultures (MDCK/Ma104 and MDCK/NRK-E52) the expression of cadherin (anti-pancadherin antibody) and β-catenin in both homotypic and heterotypic borders. This is in agreement with our previous results demonstrating that monolayers of mixed epithelial cell types have the TER that could be expected from the TER of each cell type in pure monolayers, and their proportion in the mixture (Gonzalez-Mariscal et al., 1989; Contreras et al., 2002). Furthermore, mixed and pure monolayers had the same structure and arrangement of TJ strands in freeze fracture replicas (Gonzalez-Mariscal et al., 1989). Contreras et al. (2002) have shown that these heterotypic contacts also express molecules such as E-cadherin, ZO-1, and occludin. On the contrary, we were unable to detect Na+,K+-ATPase in contacts with cells lines of fibroblastic morphology such as 3T3 and COS-7.
Table 1.
Percentage of expression of Na+,K+-ATPase in MDCK cells contacts
| Cell line | Animal species | Organ | Cell morphology | ATPase |
|---|---|---|---|---|
| MDCK | Dog | Kidney | Epithelial | 100 |
| CRFK | Cat | Kidney | Epithelial | 45 |
| CF2TH | Dog | Thymus | NA | 37 |
| PTK2 | Marsupial rat | Kidney | Epithelial | 10 |
| LLCPK1 | Pig | Kidney | Epithelial | 6 |
| Ma104 | Monkey | Kidney | Epithelial | 0 |
| LLCMK2 | Monkey | Kidney | Epithelial | 0 |
| NRK E52 | Rat | Kidney | Epithelial | 0 |
| VERO | Monkey | Kidney | Epithelial | 0 |
| 293 | Human fetal | Kidney | Epithelial | 0 |
| CHO | Chinese hamster | Ovary | Epithelial | 0 |
| COS-7 | Monkey | Kidney | Fibroblast | 0 |
| 3T3 | Mouse | Embryo | Fibroblast | 0 |
Cells listed in the first column were labeled with CMTMR, mixed in 50:50 proportions with MDCK cells, plated at confluence and incubated overnight. Monolayers were then fixed, treated with a first antibody against the dog β1-subunit, and a second, fluoresceinated one. These were then observed by confocal microscopy as described in Figure 1. One or two hundred borders between MDCK/other cell type were analyzed and scored positive if they exhibited green fluorescence staining. Last column on the right shows the proportion of heterotypic borders exhibiting β1-subunit (except for the first line, where MDCK/MDCK have no heterotypic contacts). This subunit of Na+,K+-ATPase was present in 100% of the homotypic MDCK/MDCK contacts and in a lower proportion in heterotypic ones. NA, not available.
Figure 1.
MDCK cells express β1-subunits at homotypic but not at heterotypic contacts. Localization of the β1-subunit (A, C, and E, green) of Na+,K+-ATPase and cell adhesion proteins (B, D, and F, green) was studied by immunofluorescence assay in MDCK cells derived from dog kidney. (A) Monolayer of pure MDCK cells with nuclei stained with propidium iodide (red) shows that the β1-subunit is only expressed at the plasma membranes in the lateral domain where cells contact each other. (B) Integrin αVβ3 (green) shows the staining pattern of a membrane protein that is expressed at the lateral as well as the basal membrane domain (inset). (C) MDCK cells (unstained) cocultured with Ma104 ones (derived from monkey kidney) that were labeled beforehand with CMTMR (red). β1-Subunit in mixed monolayer is only expressed in homotypic borders (MDCK/MDCK) but not in heterotypic ones (MDCK/Ma104, empty arrows). (D) Pancadherin antibody (green) staining a conserved sequence of cadherins shows that these molecular species are present at all cell borders in mixed monolayers, regardless of whether this contact is homoor heterotypic. (E) Monolayer of mixed MDCK/NRK-E52 cells (epithelial line derived from normal rat kidney, red) showing that β-subunit (green) concentrates in homotypic MDCK/MDCK contacts, whereas β-catenin, an adherent junction marker, is clearly observed in all cell-cell contacts (F, filled arrows). Bars, 20 μm.
Overexpression of β-Subunit in CHO Cells
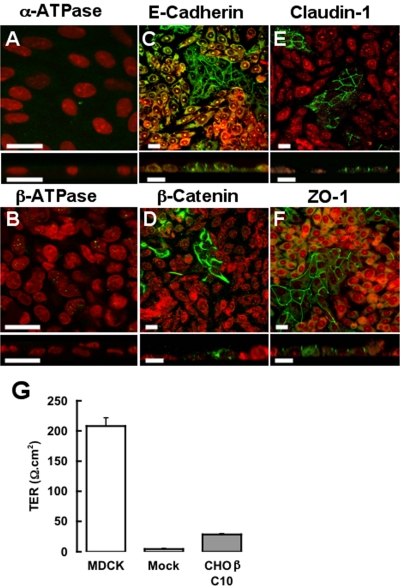
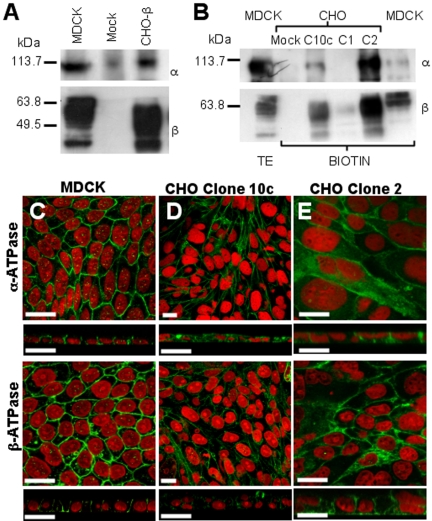
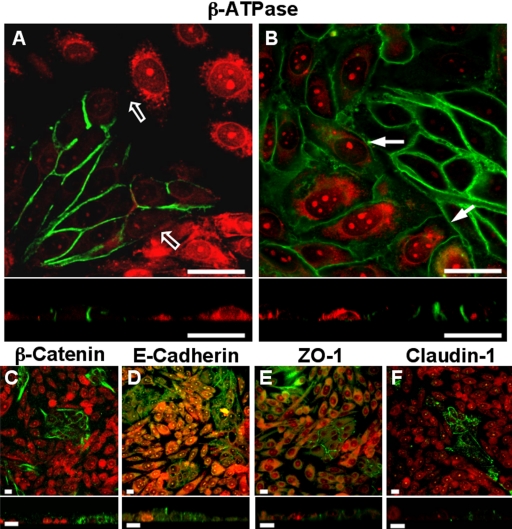
To study the role of the β-subunit in the polarized expression of Na+,K+-ATPase, we selected CHO cells, because MDCK cells do not express a detectable amount of Na+,K+-ATPase in its heterotypic borders with CHO (Table 1). As shown in Figure 2, endogenous Na+,K+-ATPase in CHO cells cannot be detected with anti-dog antibodies, an observation that is repeated with adhesion-associated proteins such as E-cadherin (Figure 2C), β-catenin (Figure 2D), claudin-1 (Figure 2E), or ZO-1 (Figure 2F), which are readily observed in MDCK cells. Furthermore, when cultured as layers on permeable supports, no considerable electrical resistance is observed (Figure 2G). Accordingly, we transfected CHO cells with the cDNA of dog β1-subunit and obtained stable clones (CHO-β; see Materials and Methods). Figure 3A shows that protein extracts of CHO-β cells assayed with an antibody against the dog β1-subunit exhibit a major glycosylated band (∼50 kDa) similar to the one detected in MDCK cells. This antibody does not recognize any β-subunit in the mock-transfected CHO fibroblasts. On the other hand, an antibody against the α-subunit of Na+,K+-ATPase that recognizes a C-terminal conserved motif, shows the typical ∼110-kDa band in the Western blot corresponding to α-subunit. Interestingly, the expression of endogenous α-subunit in CHO fibroblast is very low (Figure 3A, mock). Nevertheless, in CHO-β cells the expression of α-subunit is more pronounced, resembling that of MDCK cells. This could either reflect an increase in a gene expression or increased stability of synthesized α-subunit that is now able to reach the plasma membrane. To assess whether the expression of α-subunit induced by the overexpression of β1-subunit reaches the cell surface, we used a biotinylation assay (Figure 3B). Mock-transfected CHO cells do not express α- nor β1-subunit in their plasma membrane, whereas the different clones of CHO-β (C10, C1, and C2) express both of them at the surface. These findings are in accordance with the recent study from the Rajasekaran laboratory (Rajasekaran et al., 2004) indicating that in mammalian cells, the Na+,K+-ATPase β1-subunit is involved in facilitating the translation of the α1-subunit mRNA in the endoplasmic reticulum. Observations with immunofluorescence microscopy of MDCK, CHO, and CHO-β cells are in keeping with the biotinylation data. Thus, MDCK cells show a plasma membrane codistribution of both subunits (Figure 3C). Transverse optical sections confirm that the expression of Na+,K+-ATPase occurs mostly at the lateral borders. Mock-transfected CHO cells show no membrane staining (Figure 2A), whereas CHO-β cells (clone 10c and clone 2, Figure 3, D and E, respectively) show that both α- and β1-subunits are localized at the plasma membrane, even though a green staining spread throughout the cytoplasm is observed. This may be due to overexpressed protein that is trapped in intracellular compartments. Nonetheless, our observations suggest that the transfection of β1-subunit in CHO cells up-regulates the expression of the endogenous α ones.
Figure 2.
Absence of physiological and molecular evidence for tight and adherent junctions in wild CHO cells cultured in pure and mixed monolayers. Immunoassays of α- and β-subunits of Na+,K+-ATPase in pure monolayers of CHO cells (nuclei stained in red with propidium iodide) show no evidence of this enzyme (A and B). (C–F) Mixed monolayers of MDCK and CHO cells (red). E-cadherin (C) and β-catenin (D), which are specific markers of epithelial adherent junctions, as well as claudin-1 (E) and ZO-1 (F) corresponding to tight junctions, are only present at MDCK borders (green). (G) TER of monolayers of MDCK cells, mock-transfected CHO cells, and CHO-β. Bars, 20 μm.
Figure 3.
Expression of α- and β1-subunits in epithelial (MDCK) and fibroblastic CHO cells. (A) Western blots using an antibody against α-subunit (α) and dog β1-subunit (β). As expected, MDCK cells show a heavy mark due to glycosylated β1-subunits. Mock-transfected CHO cells do not show a β1-subunit band, whereas transfected ones (CHO-β) exhibit two bands corresponding to β1-subunits. Blotting with anti-α antibody shows heavy staining in MDCK cells. The antibody crossreacts with the α-subunit of CHO cells. As expected, CHO-β cells exhibit a much heavier staining. (B) Western blots of biotinylated α- and β1-subunits expressed on the cell surface. MDCK lane shows the usual migration pattern of both subunits of total extracts (TE) and cell surface extracts (BIOTIN). Mock-transfected CHO cells show no signal, whereas three different clones of CHO cells transfected with the dog β1-subunit (C10c, C1, and C2) express different levels of the exogenous protein at cell surface. Immunofluorescence confocal images of MDCK (C), 10c clone (D), and clone 2 of CHO-β (E) stained against α- and β-subunits of Na+,K+-ATPase. Each image is accompanied by the transverse section. Nuclei were stained with propidium iodide (red). MDCK cells show the typical chicken fence pattern of both subunits. Transverse sections show the exclusive lateral distribution of the Na+,K+-ATPase. CHO-β clones express the transfected dog β1-subunit as well as the endogenous α-subunit in the plasma membrane. Transverse optical sections below indicate that the Na+,K+-ATPase in CHO-β is not polarized. Bars, 20 μm.
Polarization of Na+,K+-ATPase in CHO Fibroblasts
Previous studies of various groups suggested that nonpolarized cells are capable of polarized plasma membrane delivery, but they lack the spatial segregation of distinct membrane targets (Musch et al., 1996; Yoshimori et al., 1996). Therefore, CHO-β fibroblasts were not expected to deliver the pump to the plasma membrane in a polarized manner unless appropriate extrinsic signals and asymmetric plasma membrane cues are established, leading to a differentiated apical/basolateral membrane domains formation (Shoshani and Contreras, 2001; Cereijido et al., 2003). Indeed, the transversal optical sections of transfected monolayers in Figure 3, D and E, indicate that the pump distribution in CHO-β cells is not polarized. Moreover, in transfected CHO-β cells (Figure 4, green) cocultured with mock-transfected CHO cells that were labeled beforehand with CMTMR (red), the plasma membrane expression of the β1-subunit is observed in both contacting and noncontacting cell borders, and, again, it is not restricted to a particular pole of the cell.
Figure 4.
CHO fibroblasts (red) cocultured with CHO-β stably expressing the β1-subunit (green) of dog Na+,K+-ATPase. Fibroblasts, either wild or transfected do not segregate from each other. CHO-β cells express the dog β1-subunit at the membrane in hetero-(empty arrow) and homotypic contacts, as well as free borders. Optical transverse sections (below) show the expression of β1-subunit all around the cell, confirming that fibroblasts do not express this protein polarizedly. Bars, 10 μm.
Transfection of the β1-Subunit Confers Cell Adhesiveness
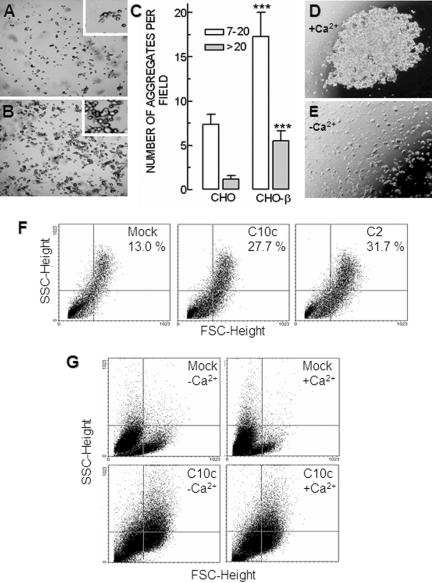
Fibroblastic CHO-β cells tend to form continuous layers, as if they establish an intimately registered vicinity (Figure 3D). Because these cells were not cotransfected with E-cadherin and do not exhibit a significant electrical resistance across themselves (28.3 ± 1.5 Ω·cm2; Figure 2G), it could imply that β-subunit is acting as an adhesion molecule, as observed by Gloor et al. (1990) in glial cells. Therefore, to test the adhesiveness conferred by the β-subunit to CHO cells, we used the two aggregation assays described in Materials and Methods. In the first method (Thoreson and Reynolds, 2002), CHO-β cells (Figure 5A) associate in small (7–20 cells) or large (>20 cells) aggregates in a higher proportion than mock-transfected cells (Figure 5B) (136 and 380% increase for small and large aggregates, respectively; p < 0.001) (Figure 5C). MDCK cells under the same experimental conditions clump spontaneously (Figure 5D), except when the medium was devoid of Ca2+ (Figure 5E). To ensure that the adhesiveness of CHO-β cells (clone 10c) does not depend on the chosen clone, we performed a comparative aggregation assay (Takeichi, 1977) followed by FACS analysis to four separate β1-subunit expressing CHO-β clones and MDCK cells. Our data reveal that the distribution of aggregates of β1-transfected clones resembles that of MDCK cells. All clones exhibit at least a 100% increase in adhesiveness with respect to mock-transfected CHO cells, as reflected by the relative density in the top right quadrant of the plot (Figure 5F). Furthermore, the adhesiveness in the absence (5 μM) or presence (1.8 mM) of Ca2+, of mock-transfected (mock) and β1-transfected CHO cells (C10c) also was analyzed by FACS (Figure 5G). The relative density in the top right quadrant of the plot, of mock-transfected cells (0.98 and 0.68%, respectively) and C10c cells (11.35 and 10.8%, respectively) suggests that adhesiveness does not depend on extracellular Ca2+. Together, these assays show that the β-subunit does confer adhesiveness to the cells.
Figure 5.
Cell aggregation assay. The adhesiveness of CHO cells transfected with the β-subunit was assayed following the two different aggregation assays described in Materials and Methods. (A) CHO cells transfected with the empty vector (mock) are mostly isolated or in small aggregates (inset). (B) CHO-β cells tend to aggregate in larger clumps (inset). (C) Clumps were scored into two groups: those with 7–20 cells (white bars) and those larger than 20 cells (gray bars). Groups of less than seven cells are not represented. The first two columns correspond to mock-transfected CHO cells that tend to group in small clumps of 7–20 cells. Transfection of β-subunit (last two columns) produces a 136% increase in the number of small clumps (p < 0.001) and a 380% in the number of large clumps (p < 0.001). Each column represents an average of 36 individual fields. MDCK cells at the tip of the hanging drop form a large clump in the presence of 1.8 mM of Ca2+ (D), whereas in the absence of Ca2+ the cells are dispersed (E). (F) Dot plots depicting cell complexity (SSC height) versus cell size (FSC height) of mock- and dog β1-transfected CHO cells (clones C10c and C2). All β1-transfected CHO cells exhibited at least a 100% increase in adhesiveness as estimated by the percentage of particles in the top right quadrant of the plot (higher size and complexity). (G) Dot plots of dog β1-(C10c) and mock-transfected CHO cells in the absence (-Ca2+) or presence (+Ca2+) of calcium ions in the bathing medium during aggregation assay.
Coculture of MDCK and CHO Cells
If it is true that MDCK cells express the Na+,K+-ATPase in homotypic borders because the neighboring cell simultaneously expresses the same type of β-subunit (Figure 6), it is expected that they will express the pump at heterotypic contacts with CHO-β cells. To test this possibility, we mixed the two cell types and observed that heterotypic borders between MDCK and CHO cells that do not express β-subunit from the dog do not show the presence of Na+,K+-ATPase (Figure 7A). Yet, MDCK cells cocultured with CHO-β cells do express the pump at both homo- and heterotypic contacts (Figure 7B). To discard the possibility that this expression at heterotypic contacts of MDCK cells were attributed to induction of E-cadherin or other bona fide adhesion-associated proteins in CHO-β cells, we stained the mixed monolayers with antibodies against several adhesion markers. Figure 7, C–F, shows that epithelial adherent proteins (E-cadherin and β-catenin) as well as tight junction proteins (claudin-1 and ZO-1) are not detected in CHO-β cells.
Figure 6.
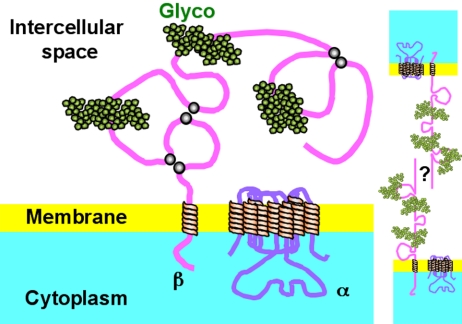
Schematic representation of Na+,K+-ATPases located at the lateral border of epithelial cells. The α-subunit with its 10 transmembrane domains is represented. The β-subunit is depicted with a single transmembrane segment and a long extracellular domain containing three S-S links (gray dots) and three glycosylation sites (green). The structure and function of the enzyme requires that both subunits interact closely and strongly; yet for clarity, these are represented as molecules placed far from each other. For the same reason, γ-subunit is omitted. On the right-hand side two β-subunits belonging to neighboring cells are represented as spanning the intercellular space. The present results suggest the possibility that a β-β linkage anchors the Na+,K+-ATPase at the lateral borders of epithelial cells. The question mark indicates that we ignore whether the β–β interaction would be a direct one or mediated by an as yet unknown molecule.
Figure 7.
Expression of dog β1-subunit in CHO cells induces MDCK cells to express their Na+,K+-ATPase at heterotypic borders. MDCK cells were cocultured with CHO fibroblasts (red) and assayed with antibody raised against the β1-subunit of the dog (green). (A) MDCK cells only express the protein in homotypic MDCK/MDCK borders but not in those contacting mock-transfected CHO cells (empty arrows). (B) MDCK cocultured with CHO-β, now express their β-subunit at both homo- and heterotypic contacts (filled arrows). This induction may not be attributed to adherens nor tight junctions as CHO-β/MDCK monolayers show that β-catenin (C), E-cadherin (D), ZO-1 (E), and claudin-1 (F) are only expressed by MDCK cells.
DISCUSSION
Although the asymmetry of epithelia was discovered by Émile Du Bois Raymond in the second half of the nineteenth century, it took the introduction of radioactive tracers, the devise of electrophysiological techniques, and another century to show that epithelia are in fact able to transport net amounts of a given substance all the way across themselves (for a historical sketch, see Cereijido et al., 2003, 2004). Eventually, the Na+,K+-ATPase, which had been shown to account for the transport of Na+ and K+ across the plasma membrane of individual cells, was found to be also the provider of electrochemical gradients that drive the net movement of sugars, amino acids, and other ion species. Yet to fulfill these transepithelial transports, it is not sufficient that the enzyme is placed at the plasma membrane of epithelial cells, but it also must be present in one of the poles only. This polarization is far from being understood, but several characteristics provide some clues: 1) Na+,K+-ATPase is not a basolateral protein as generally assumed, but just lateral (Figure 1). 2) The different isoforms of β-subunits have the typical structure of a cell attachment protein: short cytoplamic tail, a single transmembrane domain, and a long and highly glycosylated extracellular domain (Shull et al., 1986) (Figure 6); 3) in fact, β2-subunit was found to act as a cell attachment protein (Gloor et al., 1990) that has a high degree of homology with the β1-isoform expressed by MDCK cells. Accordingly, our first step was to demonstrate that transfected CHO cells do express this subunit (Figure 3A, CHO-β) and that this expression takes place at the plasma membrane (Figure 3B) and confers an adhesiveness that prompts the cells to adapt their borders and adopt an “epithelioid” shape (Figure 3, D and E). Yet, this is not a true epithelial arrangement, because CHO-β layers do not exhibit the typical electric resistance across (Figure 2G), and they fail to express molecular markers of TJs (Figures 2 and 7). We shall now elaborate on the observations mentioned above.
Expression of Transfected β-Subunits Confers Adhesiveness to CHO Cells
It may be stressed that the transfection of CHO cells with dog β-subunit is just an experimental tool to present this subunit to MDCK cells. The fact that upon transfection with β1-subunit CHO-β cells adjust their borders in closer contact, resembles the “epithelization” observed by Mcneill et al. (1990) upon transfection of E-cadherin in L-fibroblasts. Furthermore, these authors have shown that this epithelization brings about the polarization of Na+,K+-ATPase. Therefore, it is pertinent to point out that we have not transfected E-cadherin, nor found an endogenous E-cadherin either before nor after the expression of β1-subunit. Rajasekaran et al. (2001) demonstrated that MDCK cells whose polarity was impaired by transformation with Moloney Sarcoma Virus can partially recover some of their epithelial attributes upon transfection of Na+,K+-ATPase β1-subunit. This recovery is more evident when E-cadherin is cotransfected. A virus-transformed epithelial cell, however, may not be equated with a fibroblastic CHO cell, because they lack E-cadherin among other epithelial molecules. Therefore, it is not surprising that CHO cells would not express the pump in a polarized manner upon transfection of β1-subunit.
CHO-β cells would not show signs of forming TJs either. This can be tested through the value of the electrical resistance (ER) across the cell layer. ER has two main components in parallel: the transcellular and the paracellular route. Because in most epithelia with resistances below 1.0 KΩ·cm2 the resistance of the transcellular route is several orders of magnitude higher than the paracellular one, a measurement of ER across the whole cell layer only reflects the permeability of the paracellular route (Cereijido et al., 1983). Such is the case of MDCK monolayers studied in the present work (207 ± 14 Ω·cm2; Figure 2G). In turn, the ER of the paracellular route has two components arranged in series: the resistance of the tight junction and that of the intercellular space (ICS). In an epithelium with an ER above some 30 Ω·cm2, the contribution of the ICS is insignificant. The ER of CHO layers is instead negligible (Figure 2G). Even when upon transfection of β-subunit ER achieves 28.3 ± 1.5 Ω·cm2 (p < 0.001), this relatively low value may be attributed to a narrowing of the ICS. Together, transfection of β1-subunit, which confers adhesiveness and adoption of an “epithelial” shape of CHO-β cells, may not cause a true epithelization nor the synthesis and assembly of adherens and TJs.
β-Subunit Stabilizes the Lateral Distribution of the Sodium Pump in Epithelial Cells
Three models have been proposed for the polarized expression of Na+,K+-ATPase in epithelial cells. One involves intracellular sorting of newly synthesized proteins at the Golgi apparatus, followed by a vectorial delivery of the Na+,K+-ATPase molecules to a distinct surface domain (Caplan et al., 1986; Zurzolo and Rodriguez-Boulan, 1993). Unfortunately, so far multiple efforts to identify an addressing signal in the α-subunit of this enzyme were not successful (Gottardi and Caplan, 1993; Dunbar and Caplan, 2000; Dunbar et al., 2000). An alternative model emphasizes random delivery of newly synthesized Na+,K+-ATPase molecules to the entire plasma membrane, but selective retention of this protein at specific sites of the plasma membrane by attachment to the submembrane cytoskeleton (Nelson and Hammerton, 1989; Hammerton et al., 1991). Although the association of the cytoskeleton with already polarized Na+,K+-ATPase has not been disputed, this model might not explain why the enzyme binds to the cytoskeleton in the membrane facing the intercellular space, and not somewhere else. In the present work, we explored the possibility that, independently of the sorting and addressing mechanisms that handle Na+,K+-ATPase from the trans-Golgi to the plasma membrane, the specific position of this enzyme is primarily due to the retention provided by an anchorage between the β-subunits in neighboring cells.
The fact that MDCK cells do not express Na+,K+-ATPase in heterotypic borders with NRK-E52 cells that derive from the same organ (kidney) and express the same type of isoform (β1) suggests that the β-subunit association may be a species-specific one. Although this is in keeping with our central tenet regarding the role of the β-subunit, the specificity of the β-subunit association must await further studies using subunits derived from different animal species.
Studies from Geering's laboratory (Geering et al., 1989; Ackermann and Geering, 1990; Geering, 1990; Jaunin et al., 1992) indicate that α- and β-subunits are associated from their early posttranslation steps in the endoplasmic reticulum and were never observed to be delivered to the plasma membrane separately. In agreement with these observations, and with recent work from Rajasekaran laboratory (Rajasekaran et al., 2004), we show that the expression of transfected β1-subunit from the dog in CHO-β cells induces the coexpression of hamster α-subunit (Figure 3).
Actually, the relationship between the polarized expression of the pump (P) constituted by Na+,K+-ATPase and the attachment (A) provided by its β-subunit was expected on three different grounds: 1) The already mentioned observation from Gloor et al. (Gloor et al., 1990; Schmalzing et al., 1992; Muller-Husmann et al., 1993) that the β-subunit has the typical structure of an adhesion molecule and functions as such; 2) studies from the Takeyasu laboratory (Takeyasu et al., 2001; Okamura et al., 2003a,b) pointing out that early in evolution, an ancestor of this subunit was expressed independently of the α one, a circumstance that may still be present in organisms such as Caenorhabditis elegans and that shows that it may be involved in other functions besides of ion pumping; and 3) moreover, we have found in previous work that the occupancy of Na+,K+-ATPase by ouabain (Contreras et al., 1999) or other molecules able to inhibit the pump (Contreras et al., 2004) triggers a cascade of phosphorylations that results in retrieval of attaching molecules in the so called P→A mechanism. For further reviews of these aspects, see Cereijido et al. (2004).
In summary, although the α-subunit of the Na+,K+-ATPase accounts for most of the properties of the enzyme (ATP hydrolysis, Mg2+ binding, Na+ and K+ translocation, and ouabain binding), the β-subunit role seems to be reduced to a partner of α-subunit. Yet, in the present work, we observe that, due to the adhesiveness it confers, it may establish a linkage with a similar subunit located in a neighboring cell across the intercellular space and be responsible for the polarized expression of Na+,K+-ATPase in epithelial cells.
Acknowledgments
We thank Dr. Michael Caplan who have generously provided the antibody against the dog β1-subunit and Dr. Robert Farley for the cDNA coding for the dog kidney β1-subunit. This work was performed with economic support of the National Research Council of México (Consejo Nacional de Ciencia y Tecnologia) and a travel award for L. S. from The Royal Society (London) and the Mexican Academy of Science (Academia Mexicana de Ciencias).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-03-0267) on December 22, 2004.
References
- Ackermann, U., and Geering, K. (1990). Mutual dependence of Na,K-ATPase alpha- and beta-subunits for correct posttranslational processing and intracellular transport. FEBS Lett. 269, 105-108. [DOI] [PubMed] [Google Scholar]
- Caplan, M. J., Anderson, H. C., Palade, G. E., and Jamieson, J. D. (1986). Intracellular sorting and polarized cell surface delivery of (Na+,K+)ATPase, an endogenous component of MDCK cell basolateral plasma membranes. Cell 46, 623-631. [DOI] [PubMed] [Google Scholar]
- Cereijido, M., Contreras, R. G., and Shoshani, L. (2004). Cell adhesion, polarity, and epithelia in the dawn of metazoans. Physiol. Rev. 84, 1229-1262. [DOI] [PubMed] [Google Scholar]
- Cereijido, M., Contreras, R. G., Shoshani, L., and Garcia-Villegas, M. R. (2003). Membrane targeting. Prog. Biophys. Mol. Biol. 81, 81-115. [DOI] [PubMed] [Google Scholar]
- Cereijido, M., Ehrenfeld, J., Fernandez-Castelo, S., and Meza, I. (1981). Fluxes, junctions, and blisters in cultured monolayers of epithelioid cells (MDCK). Ann. N.Y. Acad. Sci. 372, 422-441. [DOI] [PubMed] [Google Scholar]
- Cereijido, M., Ehrenfeld, J., Meza, I., and Martinez-Palomo, A. (1980). Structural and functional membrane polarity in cultured monolayers of MDCK cells. J. Membr. Biol. 52, 147-159. [DOI] [PubMed] [Google Scholar]
- Cereijido, M., Gonzalez-Mariscal, L., and Borboa, L. (1983). Occluding junctions and paracellular pathways studied in monolayers of MDCK cells. J. Exp. Biol. 106, 205-215. [DOI] [PubMed] [Google Scholar]
- Cereijido, M., Robbins, E. S., Dolan, W. J., Rotunno, C. A., and Sabatini, D. D. (1978). Polarized monolayers formed by epithelial cells on a permeable and translucent support. J. Cell. Biol. 77, 853-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido, M., and Rotunno, C. A. (1971). Introduction to the Study of Biological Membranes, New York: Gordon & Breach, Science Publishers.
- Cereijido, M., Shoshani, L., and Contreras, R. G. (2000). Molecular physiology and pathophysiology of tight junctions. I. Biogenesis of tight junctions and epithelial polarity. Am. J. Physiol. 279, G477-G482. [DOI] [PubMed] [Google Scholar]
- Cereijido, M., Shoshani, L., and Contreras, R. G. (2001). The polarized distribution of Na+, K+-ATPase and active transport across epithelia. J. Membr. Biol. 184, 299-304. [DOI] [PubMed] [Google Scholar]
- Cereijido, M., Shoshani, L., and Contreras, R. G. (2002). Functional Analysis of the Tight Junctions. In: Cell–Cell Interactions, ed. T. P. Fleming, New York: Oxford University Press, 71-91.
- Contreras, R. G., Avila, G., Gutierrez, C., Bolivar, J. J., Gonzalez-Mariscal, L., Darzon, A., Beaty, G., Rodriguez-Boulan, E., and Cereijido, M. (1989). Repolarization of Na+-K+ pumps during establishment of epithelial monolayers. Am. J. Physiol. 257, C896-C905. [DOI] [PubMed] [Google Scholar]
- Contreras, R. G., Flores-Maldonado, C., Lazaro, A., Shoshani, L., Flores-Benitez, D., Larre, I., and Cereijido, M. (2004). Ouabain binding to Na+,K+-ATPase relaxes cell attachment and sends a specific signal (NACos) to the nucleus. J. Membr. Biol. 198, 147-158. [DOI] [PubMed] [Google Scholar]
- Contreras, R. G., Lazaro, A., Bolivar, J. J., Flores-Maldonado, C., Sanchez, S. H., Gonzalez-Mariscal, L., Garcia-Villegas, M. R., Valdes, J., and Cereijido, M. (1995). A novel type of cell-cell cooperation between epithelial cells. J. Membr. Biol. 145, 305-310. [DOI] [PubMed] [Google Scholar]
- Contreras, R. G., Shoshani, L., Flores-Maldonado, C., Lazaro, A., and Cereijido, M. (1999). Relationship between Na(+),K(+)-ATPase and cell attachment. J. Cell. Sci. 112, 4223-4232. [DOI] [PubMed] [Google Scholar]
- Contreras, R. G., Shoshani, L., Flores-Maldonado, C., Lazaro, A., Monroy, A. O., Roldan, L., Fiorentino, R., and Cereijido, M. (2002). E-Cadherin and tight junctions between epithelial cells of different animal species. Pflugers Arch. 444, 467-475. [DOI] [PubMed] [Google Scholar]
- Dunbar, L. A., Aronson, P., and Caplan, M. J. (2000). A transmembrane segment determines the steady-state localization of an ion-transporting adenosine triphosphatase. J. Cell. Biol. 148, 769-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar, L. A., and Caplan, M. J. (2000). The cell biology of ion pumps: sorting and regulation. Eur. J. Cell. Biol. 79, 557-563. [DOI] [PubMed] [Google Scholar]
- Ernst, S. A., and Mills, J. W. (1980). Autoradiographic localization of tritiated ouabain-sensitive sodium pump sites in ion transporting epithelia. J. Histochem. Cytochem. 28, 72-77. [DOI] [PubMed] [Google Scholar]
- Fambrough, D. M. (1988). The sodium pump becomes a family. Trends Neurosci. 11, 325-328. [DOI] [PubMed] [Google Scholar]
- Fambrough, D. M., and Bayne, E. K. (1983). Multiple forms of (Na+ + K+)-ATPase in the chicken. Selective detection of the major nerve, skeletal muscle, and kidney form by a mAb. J. Biol. Chem. 258, 3926-3935. [PubMed] [Google Scholar]
- Forbush, B., 3rd, Kaplan, J. H., and Hoffman, J. F. (1978). Characterization of a new photoaffinity derivative of ouabain: labeling of the large polypeptide and of a proteolipid component of the Na, K-ATPase. Biochemistry 17, 3667-3676. [DOI] [PubMed] [Google Scholar]
- Geering, K. (1990). Subunit assembly and functional maturation of Na,K-ATPase. J. Membr. Biol. 115, 109-121. [DOI] [PubMed] [Google Scholar]
- Geering, K., Theulaz, I., Verrey, F., Hauptle, M. T., and Rossier, B. C. (1989). A role for the beta-subunit in the expression of functional Na+-K+-ATPase in Xenopus oocytes. Am. J. Physiol. 257, C851-C858. [DOI] [PubMed] [Google Scholar]
- Gloor, S., Antonicek, H., Sweadner, K. J., Pagliusi, S., Frank, R., Moos, M., and Schachner, M. (1990). The adhesion molecule on glia (AMOG) is a homologue of the beta subunit of the Na,K-ATPase. J. Cell. Biol. 110, 165-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal, L., Chavez, D. R., Lazaro, A., and Cereijido, M. (1989). Establishment of tight junctions between cells from different animal species and different sealing capacities. J. Membr. Biol. 107, 43-56. [DOI] [PubMed] [Google Scholar]
- Gottardi, C. J., and Caplan, M. J. (1993). Molecular requirements for the cell-surface expression of multisubunit ion-transporting ATPases. Identification of protein domains that participate in Na,K-ATPase and H,K-ATPase subunit assembly. J. Biol. Chem. 268, 14342-14347. [PubMed] [Google Scholar]
- Gundersen, D., Orlowski, J., and Rodriguez-Boulan, E. (1991). Apical polarity of Na,K-ATPase in retinal pigment epithelium is linked to a reversal of the ankyrin-fodrin submembrane cytoskeleton. J. Cell. Biol. 112, 863-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerton, R. W., Krzeminski, K. A., Mays, R. W., Ryan, T. A., Wollner, D. A., and Nelson, W. J. (1991). Mechanism for regulating cell surface distribution of Na+,K(+)-ATPase in polarized epithelial cells. Science 254, 847-850. [DOI] [PubMed] [Google Scholar]
- Horowitz, B., Eakle, K. A., Scheiner-Bobis, G., Randolph, G. R., Chen, C. Y., Hitzeman, R. A., and Farley, R. A. (1990). Synthesis and assembly of functional mammalian Na,K-ATPase in yeast. J. Biol. Chem. 265, 4189-4192. [PubMed] [Google Scholar]
- Jaunin, P., Horisberger, J. D., Richter, K., Good, P. J., Rossier, B. C., and Geering, K. (1992). Processing, intracellular transport, and functional expression of endogenous and exogenous alpha-beta 3 Na,K-ATPase complexes in Xenopus oocytes. J. Biol. Chem. 267, 577-585. [PubMed] [Google Scholar]
- Just, F., and Walz, B. (1994). Immunocytochemical localization of Na+/K(+)-ATPase and V-H(+)-ATPase in the salivary glands of the cockroach, Periplaneta americana. Cell Tissue Res. 278, 161-170. [DOI] [PubMed] [Google Scholar]
- Kashgarian, M., Biemesderfer, D., Caplan, M., and Forbush, B., 3rd. (1985). mAb to Na,K-ATPase: immunocytochemical localization along nephron segments. Kidney Int. 28, 899-913. [DOI] [PubMed] [Google Scholar]
- Koefoed-Johnsen, V., and Ussing, H. H. (1958). The nature of the frog skin potential. Acta Physiol. Scand. 42, 298-308. [DOI] [PubMed] [Google Scholar]
- Louvard, D. (1980). Apical membrane aminopeptidase appears at site of cell-cell contact in cultured kidney epithelial cells. Proc. Natl. Acad. Sci. USA 77, 4132-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcneill, H., Ozawa, M., Kemler, R., and Nelson, W. J. (1990). Novel function of the cell-adhesion molecule uvomorulin as an inducer of cell-surface polarity. Cell 62, 309-316. [DOI] [PubMed] [Google Scholar]
- Muller-Husmann, G., Gloor, S., and Schachner, M. (1993). Functional characterization of beta isoforms of murine Na,K-ATPase. The adhesion molecule on glia (AMOG/beta 2), but not beta 1, promotes neurite outgrowth. J. Biol. Chem. 268, 26260-26267. [PubMed] [Google Scholar]
- Musch, A., Xu, H., Shields, D., and Rodriguez-Boulan, E. (1996). Transport of vesicular stomatitis virus G protein to the cell surface is signal mediated in polarized and nonpolarized cells. J. Cell. Biol. 133, 543-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, W. J., and Hammerton, R. W. (1989). A membrane-cytoskeletal complex containing Na+,K+-ATPase, ankyrin, and fodrin in Madin-Darby canine kidney (MDCK) cells: implications for the biogenesis of epithelial cell polarity. J. Cell. Biol. 108, 893-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi, S., Mishina, M., Kawamura, M., and Numa, S. (1987). Expression of functional (Na+ + K+)-ATPase from cloned cDNAs. FEBS Lett. 225, 27-32. [DOI] [PubMed] [Google Scholar]
- Okamura, H., Denawa, M., Ohniwa, R., and Takeyasu, K. (2003a). P-type ATPase superfamily: evidence for critical roles for kingdom evolution. Ann. N.Y. Acad. Sci. 986, 219-223. [DOI] [PubMed] [Google Scholar]
- Okamura, H., Yasuhara, J. C., Fambrough, D. M., and Takeyasu, K. (2003b). P-type ATPases in Caenorhabditis and Drosophila: implications for evolution of the P-type ATPase subunit families with special reference to the Na,K-ATPase and H,K-ATPase subgroup. J. Membr. Biol. 191, 13-24. [DOI] [PubMed] [Google Scholar]
- Rabito, C. A., and Karish, M. V. (1983). Polarized amino acid transport by an epithelial cell line of renal origin (LLC-PK1). The apical systems. J. Biol. Chem. 258, 2543-2547. [PubMed] [Google Scholar]
- Rabito, C. A., and Tchao, R. (1980). [3H]ouabain binding during the monolayer organization and cell cycle in MDCK cells. Am. J. Physiol. 238, C43-C48. [DOI] [PubMed] [Google Scholar]
- Rajasekaran, S. A., Gopal, J., Willis, D., Espineda, C., Twiss, J. L., and Rajasekaran, A. K. (2004). Na,K-ATPase {beta}1-Subunit Increases the Translation Efficiency of the {alpha}1-Subunit in MSV-MDCK Cells. Mol. Biol. Cell 15, 3224-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran, S. A., Palmer, L. G., Quan, K., Harper, J. F., Ball, W. J., Jr., Bander, N. H., Peralta, S. A., Rajasekaran, A. K. (2001). Na,K-ATPase beta-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol. Biol. Cell 12, 279-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalzing, G., Kroner, S., Schachner, M., and Gloor, S. (1992). The adhesion molecule on glia (AMOG/beta 2) and alpha 1 subunits assemble to functional sodium pumps in Xenopus oocytes. J. Biol. Chem. 267, 20212-20216. [PubMed] [Google Scholar]
- Schultz, S. G., and Curran, P. F. (1969). The role of sodium in non-electrolyte transport across animal cell membranes. Physiologist 12, 437-452. [PubMed] [Google Scholar]
- Shoshani, L., and Contreras, R. G. (2001). Biogenesis of Epithelial Polarity and Tight Junctions. In: Tight Junctions, ed. M. Cereijido and J. M. Anderson, Boca Raton FL: CRC Press, 165-197.
- Shull, G. E., Lane, L. K., and Lingrel, J. B. (1986). Amino-acid sequence of the beta-subunit of the (Na+ + K+)ATPase deduced from a cDNA. Nature 321, 429-431. [DOI] [PubMed] [Google Scholar]
- Skou, J. C. (1957). The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim. Biophys. Acta 23, 394-401. [DOI] [PubMed] [Google Scholar]
- Skou, J. C. (1998). Nobel Lecture. The identification of the sodium pump. Biosci. Rep. 18, 155-169. [DOI] [PubMed] [Google Scholar]
- Steinberg, R. H., and Miller, S. S. (1979). Transport and Membrane Properties of the Retinal Pigment Epithelium. In: The Retinal Pigment Epithelium, ed. K. M. Zinn and M. F. Marmor, Cambridge, MA: Harvard University Press, 205-225.
- Sweadner, K. J., Wetzel, R. K., and Arystarkhova, E. (2000). Genomic organization of the human FXYD2 gene encoding the gamma subunit of the Na,K-ATPase. Biochem. Biophys. Res. Commun. 279, 196-201. [DOI] [PubMed] [Google Scholar]
- Takeichi, M. (1977). Functional correlation between cell adhesive properties and some cell surface proteins. J. Cell. Biol. 75, 464-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeyasu, K., and Kawakami, K. (1989). [Na+,K(+)-ATPase: genes, expression and membrane insertion]. Seikagaku 61, 394-401. [PubMed] [Google Scholar]
- Takeyasu, K., Okamura, H., Yasuhara, J. C., Ogita, Y., and Yoshimura, S. H. (2001). P-type ATPase diversity and evolution: the origins of ouabain sensitivity and subunit assembly. Cell. Mol. Biol. 47, 325-333. [PubMed] [Google Scholar]
- Thoreson, M. A., and Reynolds, A. B. (2002). Altered expression of the catenin p120 in human cancer: implications for tumor progression. Differentiation 70, 583-589. [DOI] [PubMed] [Google Scholar]
- Wright, E. M. (1972). Mechanisms of ion transport across the choroid plexus. J. Physiol. 226, 545-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori, T., Keller, P., Roth, M. G., and Simons, K. (1996). Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J. Cell. Biol. 133, 247-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurzolo, C., and Rodriguez-Boulan, E. (1993). Delivery of Na+,K(+)-ATPase in polarized epithelial cells. Science 260, 550-552. [DOI] [PubMed] [Google Scholar]