Abstract
The Na,K-ATPase, consisting of α- and β-subunits, regulates intracellular ion homeostasis. Recent studies have demonstrated that Na,K-ATPase also regulates epithelial cell tight junction structure and functions. Consistent with an important role in the regulation of epithelial cell structure, both Na,K-ATPase enzyme activity and subunit levels are altered in carcinoma. Previously, we have shown that repletion of Na,K-ATPase β1-subunit (Na,K-β) in highly motile Moloney sarcoma virus-transformed Madin-Darby canine kidney (MSV-MDCK) cells suppressed their motility. However, until now, the mechanism by which Na,K-β reduces cell motility remained elusive. Here, we demonstrate that Na,K-β localizes to lamellipodia and suppresses cell motility by a novel signaling mechanism involving a cross-talk between Na,K-ATPase α1-subunit (Na,K-α) and Na,K-β with proteins involved in phosphatidylinositol 3-kinase (PI3-kinase) signaling pathway. We show that Na,K-α associates with the regulatory subunit of PI3-kinase and Na,K-β binds to annexin II. These molecular interactions locally activate PI3-kinase at the lamellipodia and suppress cell motility in MSV-MDCK cells, independent of Na,K-ATPase ion transport activity. Thus, these results demonstrate a new role for Na,K-ATPase in regulating carcinoma cell motility.
INTRODUCTION
Na,K-ATPase is a ubiquitous plasma membrane-bound enzyme that is composed of two essential noncovalently linked subunits, viz., Na,K-α, which is the catalytic subunit, and Na,K-β, which is required for the translation (Rajasekaran et al., 2004), transport, stabilization, and function of Na,K-α at the plasma membrane (Chow and Forte, 1995; Geering, 2001). Localized to the basolateral plasma membrane in most epithelial cell types, Na,K-ATPase catalyzes an ATP-dependent transport of three sodium ions out and two potassium ions into the cell per pump cycle. This generates a transmembrane sodium gradient that regulates the vectorial transport function of epithelial cells. Polarized phenotype of epithelial cells with distinct apical and basolateral plasma membrane domains separated by tight junctions is crucial for the directional transport of ions and metabolites across the epithelial cell layer (Simons and Fuller, 1985; Rodriguez-Boulan and Nelson, 1989). We have shown recently that Na,K-ATPase function is necessary for the formation and maintenance of tight junctions in epithelial cells (Rajasekaran et al., 2001a; Rajasekaran et al., 2003), suggesting that Na,K-ATPase plays an essential role in regulating the transport and polarized phenotype of epithelial cells.
Loss of the polarized phenotype of epithelial cells is a characteristic of carcinoma and correlates with their invasiveness and metastatic potential (Birchmeier et al., 1996). Consistent with an important role for Na,K-ATPase in the regulation of polarized phenotype of epithelial cells, both Na,K-ATPase enzyme activity and subunit levels are reduced in carcinoma (Rajasekaran and Rajasekaran, 2003). Na,K-β levels were highly reduced in an invasive form of human renal clear cell carcinoma (Rajasekaran et al., 1999), androgen-dependent prostate cancer (Blok et al., 1999), in early stages of urothelial cancer (Espineda et al., 2003), as well as in poorly differentiated, highly motile carcinoma cell lines obtained from various tissues (Espineda et al., 2004), suggesting a functional link between reduced Na,K-β expression and cancer progression. In contrast, the levels of Na,K-α did not reveal a consistent pattern in different carcinomas.
Increased cell motility is a prerequisite for invasion and metastasis, and in general most of the invasive carcinoma cell lines are highly motile (Stetler-Stevenson et al., 1993). We have shown that Moloney sarcoma virus-transformed Madin-Darby canine kidney (MDCK) cells (MSV-MDCK) express highly reduced levels of Na,K-β, and repletion of Na,K-β expression in MSV-MDCK cells significantly reduced their motility (Rajasekaran et al., 2001b). This result indicated a motility suppressor function for Na,K-β in epithelial cells. Although the levels of Na,K-α were decreased in MSV-MDCK cells, repletion of Na,K-α was not sufficient to reduce their motility (Rajasekaran et al., 2001b).
The mechanisms controlling cell motility in carcinoma are poorly understood. Cell motility is orchestrated by the continuous remodeling of the actin cytoskeleton and members of the Rho family of small GTPases are known to control actin dynamics. For example, Rac1 induces the formation of lamellipodia in fibroblasts (Hall, 1998). Lamellipodia are involved in the formation of the leading edge of motile cells, development of adhesion to the substrate (Small et al., 2002), and defining the sites of developing cell-cell contact points (Ehrlich et al., 2002). Guanine nucleotide exchange factors (GEFs) induce the activation of Rac1 by accelerating the exchange of GTP for GDP. The binding of pleckstrin homology (PH) domain of GEFs to phosphatidylinositol (3,4,5)-trisphosphate (PIP3), a product of PI3-kinase activation in the membrane, is believed to stimulate the exchange activity of GEF and activate Rac1 (Welch et al., 2003). There is a dynamic local accumulation of PI3-kinase products at the leading edge of migrating cells (Servant et al., 2000) and at the nascent cell-cell contact points (Ehrlich et al., 2002). PI3-kinase is thus implicated in the activation of Rac1 and the formation of lamellipodia.
In this study, we have characterized the molecular mechanism by which Na,K-β1 suppresses motility of MSV-MDCK cells. We show that protein–protein interactions of Na,K-α1 and Na,K-β1 with proteins involved in PI3-kinase signaling mediate the activation of Rac1 and suppression of cell motility in MSV-MDCK cells.
MATERIALS AND METHODS
Cell Culture, Transfection Conditions, and Inhibitor Treatments
MSV-MDCK cells (DoCl1) obtained from American Type Culture Collection (Manassas, VA) were cultured in DMEM supplemented with 5% fetal bovine serum, 1 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. MSV-MDCK cells expressing full-length canine Na,K-β, hereafter referred to as MSV-β have been described previously as MSV-NaK-β-cl2 (Rajasekaran et al., 2001b). Canine Na,K-β cDNA, kindly provided by Dr. Robert Farley (University of Southern California, Los Angeles, CA), was mobilized into pEGFP-N3 (BD Biosciences Clontech, Palo Alto, CA), to obtain a green fluorescent protein (GFP) fusion at the C terminus of Na,K-β (Na,K-β-GFP). A cytoplasmic domain deletion mutant of Na,K-β generated by polymerase chain reaction (PCR) amplification by using appropriate primers was cloned into pEGFP-N3 (Na,K-βΔCD-GFP). Stable clones of MSV-MDCK cells expressing Na,K-β-GFP (MSV-β-GFP) and GFP-tagged Na,K-βΔCD (MSV-βΔCD-GFP), were obtained as described previously (Rajasekaran et al., 2001b). MSV-MDCK cells expressing GFP-tagged L61Rac1 (MSV-L61Rac1) were generated by transfecting L61Rac1-MIEG3 in MSV-MDCK cells, followed by sorting of the GFP-positive cells by using fluorescence activated cell sorting (FACS). MSV-MDCK cells coexpressing GFP-tagged Na,K-βΔCD and canine Na,K-α (a kind gift from Dr. Amir Askari, Medical College of Ohio, Toledo, OH) (MSV-βΔCD-GFP-α) were generated by cotransfection, followed by FACS sorting of GFP-positive cells. Wherever indicated, cells were treated with 60 nM wortmannin for 1 h, 50 μM LY294002 for 16 h, and 50 μM ouabain for 1 h.
Antibodies
Mouse monoclonal antibodies (mAbs) raised against Na,K-α (M7-PB-E9) and Na,K-β (M17-P5-F11) have been characterized and described previously (Abbott and Ball, 1993; Sun and Ball, 1994). Anti-phosphotyrosine mAb PY20, anti-PI3-kinase mAb, anti-Rac1 mAb, and anti-annexin II mAb were obtained from BD Transduction Laboratories (Lexington, KY). Texas Red-conjugated phalloidin was purchased from Molecular Probes (Eugene, OR). Cy3-labeled secondary mouse antibody was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA) and horseradish peroxidase (HRP)-anti-mouse antibody was from BD Transduction Laboratories.
Transwell Motility Assay
Transwell motility assay was performed as described previously (Keely et al., 1995; Rajasekaran et al., 2001b). Briefly, Transwell membrane cell culture inserts (8.0-μm pore size) were coated on the underside with 50 μg/ml rat tail collagen type I for 1 h at room temperature. Cells (105) resuspended in 1 ml of DMEM containing 5 mg/ml bovine serum albumin (BSA) were plated on the upper chamber. Cells were allowed to migrate for 18 h at 37°C, 5% CO2. The cells that migrated across the Transwell membrane, detached from it, fell to the bottom of the well, and attached to the tissue culture plastic of the bottom chamber were fixed and stained with crystal violet. The bottom chamber was washed with water and the cell-associated crystal violet was eluted with 10% acetic acid. Cell motility was quantified by measuring the absorbance of eluted crystal violet at a wavelength of 600 nm (Xu et al., 2001). Unless otherwise indicated, the absorbance corresponding to the number of MSV-MDCK cells that migrated across the membrane was considered as 100% and was used as control. Normal MDCK cells are highly nonmotile and fail to cross the Transwell membrane (Rajasekaran et al., 2001b).
Phalloidin Staining, Immunofluorescence, and Laser Scanning Confocal Microscopy
Cells plated on glass coverslips were fixed with 2% paraformaldehyde, permeabilized using 0.075% saponin and stained with Texas Red-conjugated phalloidin to visualize F-actin. For immunofluorescence using anti-Na,K-α or anti-Na,K-β antibodies, cells were fixed with chilled methanol. Epifluorescence was detected using an Olympus AX 70 microscope. Confocal microscope optical sections (12–15) generated at 1-μm intervals were used to create a projection of the cells, by using a Fluoview laser scanning confocal microscope (Olympus America, Melville, NY) and the Fluoview image analysis software (version 2.1.39).
Rac1 Activity Assay
The Rac1 interactive domain (residues 51–135) of human p21-activated kinase (PAK1) cloned in pGEX-2T vector was expressed in Escherichia coli and purified as a glutathione S-transferase (GST)-fusion protein immobilized to glutathione-coupled agarose as described previously (Zhu et al., 2000). For the Rac1 assay, GFP and Na,K-β expressing MSV-MDCK cells were washed with ice-cold phosphate-buffered saline (PBS) buffer once, before lysis in a buffer containing 50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 1 mM dithiothreitol, 10 μg/ml each of antipain, leupeptin, and pepstatin, and 1 mM phenylmethylsulfonyl fluoride (PMSF) at 4°C. Cell lysates were clarified by centrifugation at 13,000 × g at 4°C for 10 min and incubated with bacterially produced GST-PAK1 fusion protein, immobilized to glutathione-coupled agarose beads (10 μg/lysate sample) for 45 min at 4°C under constant agitation. The lysate-incubated beads were washed thrice with the lysis buffer, and the proteins bound to the beads were resolved on a 12% SDS-PAGE. The bound Rac1 was detected by an immunoblot analysis by using anti-Rac1 antibody and visualized by enhanced chemiluminescence (PerkinElmer Life and Analytical Sciences, Boston, MA). Quantification of the immunoblots was performed with the use of an ImageQuant software package (Amersham Biosciences, Piscataway, NJ). For comparison of the levels of active Rac1, the amount of GST-PAK-bound Rac1 was normalized to the total amount of Rac1 from cell lysates in each sample.
Immunoblotting and Immunoprecipitation
Cells grown to 70–80% confluence were chilled on ice, washed once with ice-cold PBS, lysed in a lysis buffer containing 20 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 1 mM sodium glycerolphosphate, 1 mM sodium orthovanadate, 1 mM PMSF, and 5 μg/ml each of antipain, leupeptin, and pepstatin. The lysates were sonicated thrice for 10 s and clarified by centrifugation at 13,000 rpm for 10 min at 4°C. The supernatants were collected and total protein was estimated using the Bio-Rad DC reagent (Bio-Rad, Hercules, CA) as per manufacturer's instructions. For immunoblot analysis, equal amounts of total protein were used. For immunoprecipitation, lysates corresponding to 1 mg of total cellular protein were incubated on a rotator overnight with antibody bound to protein A agarose beads at 4°C. The proteins bound to the beads were collected by centrifugation and washed thrice with lysis buffer, once with 25 mM Tris-HCl, pH 8.0, and separated by SDS-PAGE and transferred to nitrocellulose membrane (Schleicher & Schuell, Keene, NH). The blots were blocked with 5% nonfat dried milk in Tris-buffered saline (TBS) with 0.1% Tween 20 followed by incubation with primary antibody diluted in 5% BSA/TBS/0.1% Tween 20 at 4°C overnight. The primary antibody was washed thrice with TBS/0.1% Tween 20, and the blots were incubated with the secondary antibody conjugated with HRP diluted in 5% nonfat dried milk/TBS/0.1% Tween 20. The secondary antibody was washed with TBS/0.1% Tween 20 and the proteins were detected by using the enhanced chemiluminescence lighting system according to the manufacturer's recommendations (PerkinElmer Life and Analytical Sciences).
N-Glycosidase Treatment
N-Glycosidase F (New England Biolabs, Beverly, MA) digestion was performed as per the manufacturer's instructions. Untreated or treated lysate (10 μg) was resolved on a 10% SDS-PAGE and immunoblotted for Na,K-β as described above.
Cloning of Canine Annexin II
The regions of conserved amino acids were identified from the multiple sequence alignment of human, mouse, and chicken annexin II protein sequences. Degenerate primers were synthesized based on the amino acid sequences of the homologous regions and were used for RT-PCR. RT-PCR was performed on total RNA isolated from MDCK cells by using the Titan RT-PCR system (Roche Diagnostics, Indianapolis, IN). The DNA was cloned into pGEX-2T vector and was sequenced completely. The nucleotide sequence for dog annexin II cDNA was deposited into GenBank (accession no. AY422991).
Purification of GST-Fusion Proteins and GST-Pull-Down Assay
Dog annexin II (DAII), Na,K-α N-terminus containing amino acids 1–93 (αCD), and the cytoplasmic domain of Na,K-β containing amino acids 1–35 (βCD) were cloned in pGEX-5X vector (Invitrogen, Carlsbad, CA) and purified as described previously (Anilkumar et al., 2003). Briefly, E. coli BL-21 cells were transformed with GST-DAII or GST-βCD constructs. Expression of recombinant protein was induced by the addition of 0.25 mM isopropylthiogalactoside for 2 h. The bacterial cells were harvested and resuspended in a lysis buffer containing 50 mM Tris-HCl, pH 8.0, 100 mM NaCl, 2 mM MgCl2, 250 μg/ml lysozyme, 1 mM PMSF, and 10 μg/ml each of antipain, leupeptin, and pepstatin, and then sonicated. The lysates were centrifuged at 13,000 rpm at 4°C for 15 min. The supernatant was incubated with glutathione-coupled agarose beads (Amersham Biosciences) for 1 h at 4°C with constant agitation. Protein bound to the beads was washed thrice with lysis buffer and the amount of bound fusion protein was estimated using Coomassie-stained SDS gels. MSV-β cell lysates prepared as described in “Immunblotting and Immunoprecipitation,” were incubated with the indicated amounts of GST or GST-fusion proteins in separate tubes, at 4°C, overnight with constant agitation. The beads were washed thrice with lysis buffer and analyzed on SDS-PAGE.
MicroLiquid Chromatography Tandem Mass Spectrometry (μLC-MSMS)
GST-βCD and GST coupled to agarose beads were purified as described above and incubated with MSV-β cell lysates on a rotator overnight at 4°C. The beads were washed thrice with lysis buffer, and the proteins bound to the beads were resolved on a 10% SDS-polyacrylamide gel. The gel was stained with SYPRO Ruby (Molecular Probes). The protein bands pulled down by GST-βCD but not by GST were cut out from the SYPRO Ruby-stained SDS-polyacrylamide gel and subjected to in-gel digestion and extraction of peptides. The protein identification was performed by μLC-MSMS with data-dependent acquisition by using an ion-trap mass spectrometer (LCQ-DECA; Thermo Finnigan, San Jose, CA) (Gomez et al., 2003).
RESULTS
The Expression of Na,K-β Suppresses Cell Motility by Reorganization of the Actin Cytoskeleton
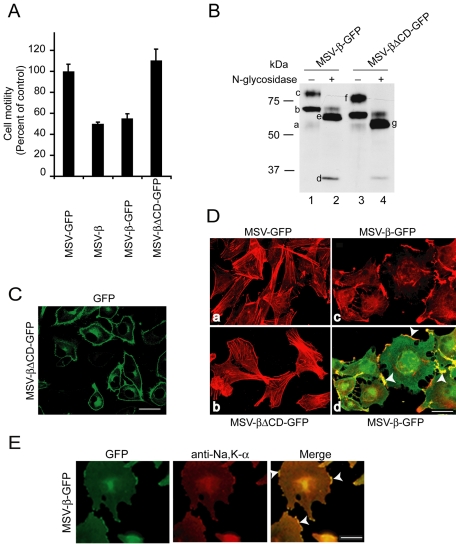
To study the mechanism by which Na,K-β controls cell motility, we used MSV-MDCK cells expressing additional exogenous canine Na,K-β. In a Transwell motility assay, MSV-MDCK cells expressing untagged (MSV-β) or GFP-tagged (MSV-β-GFP) Na,K-β showed only 50 ± 1.7 and 55 ± 4.5% motility, respectively, compared with MSV-MDCK cells expressing GFP (MSV-GFP) (Figure 1A). Although the cytoplasmic tail deletion mutant of Na,K-β (Na,K-βΔCD-GFP) was expressed at higher levels than the Na,K-β-GFP (Figure 1B), and was targeted to the plasma membrane (Figure 1C), cells expressing Na,K-βΔCD-GFP (MSV-βΔCD-GFP) did not show a reduction in the motility, suggesting that the cytoplasmic tail of Na,K-β is essential for the suppression of cell motility.
Figure 1.
Na,K-β expression suppresses cell motility by reorganization of the actin cytoskeleton. (A) Migration of MSV-MDCK cells expressing either GFP, Na,K-β, GFP-tagged Na,K-β, or GFP-tagged Na,K-βΔCD across Transwell filters. The error bars represent the SD of the mean of three independent measurements done in triplicates. (B) MSV-β-GFP and MSV-βΔCD-GFP cell lysates treated without or with N-glycosidase were separated on SDS-PAGE and blotted with anti-Na,K-β antibody. In lane 1, the faint 54-kDa band (a) indicates the endogenous fully glycosylated Na,K-β. The 80-kDa band (c) represents the fully glycosylated Na,K-β-GFP chimera, whereas the 65-kDa band (b) represents a high mannose form of the Na,K-β-GFP. In lane 2, the 33-kDa band (d) is the core Na,K-β protein, the band at 59 kDa (e) is the fully deglycosylated Na,K-β-GFP chimera, and the faint band above is incompletely digested form of the Na,K-β-GFP chimera. In lane 3, the 76-kDa band (f) represents the Na,K-βΔCD-GFP chimera. In lane 4, the 55-kDa band (g) represents the full deglycosylated Na,K-βΔCD-GFP chimera. (C) GFP fluorescence in MSV-βΔCD-GFP cells showing plasma membrane localization of Na,K-βΔCD-GFP. (D) Confocal microscope projections obtained from optical slices show actin organization (red) of MSV-GFP (a), MSV-βΔCD-GFP (b), and MSV-β-GFP (c), and merged image showing colocalization of Na,K-β-GFP with filamentous actin (yellow) at the lamellipodia (arrowhead) (d). The GFP fluorescence in a and b is not shown to reveal stress fibers clearly. (E) Immunofluorescence of MSV-β-GFP cells, stained with anti-Na,K-α antibody followed by anti-Cy3 antibody, Na,K-β-GFP fluorescence, and merged image showing colocalization of the two (yellow) at the lamellipodia (arrowhead). Bar, 25 μm.
Immunofluorescence analysis revealed that in MSV-β-GFP cells, lamellipodia were abundant and Na,K-β-GFP was concentrated at the actin-rich lamellipodia, whereas in MSV-βΔCD-GFP cells, lamellipodia were sparsely present like in MSV-GFP cells (Figure 1D). Both MSV-GFP and MSV-βΔCD-GFP cells showed abundant stress fibers unlike MSV-β-GFP cells (Figure 1D). Na,K-α colocalized with Na,K-β-GFP at the lamellipodia (Figure 1E). These results indicated that the exogenous expression of full-length Na,K-β induced lamellipodia in MSV-MDCK cells.
Na,K-β-mediated Reorganization of the Actin Cytoskeleton Is Rac1 Dependent
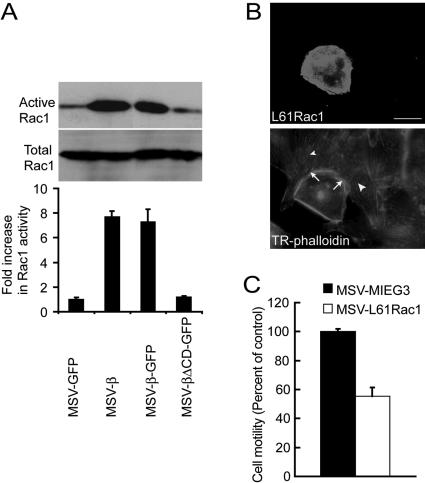
Formation of lamellipodia requires remodeling of the cortical actin cytoskeleton, which is governed by the activity of Rac1 in fibroblasts and epithelial cells (Ridley et al., 1992, 1995). To test whether Rac1 is involved in Na,K-β–mediated lamellipodia formation, we used an in vitro biochemical assay to determine the endogenous levels of active Rac1. In this assay, GST-PAK1, a GST-fusion protein containing the Rac1 binding domain of PAK1 that specifically binds to GTP bound Rac1 (active), is incubated with cell lysate and the bound, active Rac1 is detected by immunoblot analysis. MSV-MDCK cells expressing full-length Na,K-β showed seven- to eightfold increase in the levels of GST-PAK1–bound GTP-Rac1 compared with MSV-GFP and MSV-βΔCD-GFP cells (Figure 2A). The protein levels of Rac1 in the whole cell lysates as determined by immunoblot analysis were similar in all these cell lines. Although stress fibers were greatly reduced in MSV-β cells, RhoA activity determined by the amount of RhoA bound to GST-Rhotekin beads was not detectably altered in these same cells (our unpublished data), indicating that the Na,K-β–mediated reorganization of the actin cytoskeleton was independent of RhoA.
Figure 2.
Na,K-β–mediated reorganization of the actin cytoskeleton and suppression of cell motility is Rac1 dependent. (A) Immunoblot showing active and total endogenous Rac1 in MSV-GFP, MSV-β, MSV-β-GFP, and MSV-βΔCD-GFP cells. The graph represents mean ± SD of three independent experiments. (B) Fluorescence of MSV-MDCK cells transiently transfected with L61 Rac1 showing GFP-L61 Rac1 and Texas Red-labeled phalloidin. Arrows show the lamellipodia in a L61 Rac1-transfected cell. Arrowheads show the stress fibers in untransfected cells. Bar, 25 μm. (C) Migration of MSV-MDCK cells stably transfected with MIEG3 vector or L61 Rac1. The error bars show SD of the mean of three independent experiments done in triplicates.
To further substantiate the role of Rac1 in the suppression of motility, we generated MSV-MDCK cells expressing constitutively active L61 Rac1 (MSV-L61Rac1), a mutant that is defective in GTPase activity and is thought to exist constitutively in the GTP-bound form. MSV-L61Rac1 cells displayed large lamellipodia with Rac1 localized to the lamellipodia and revealed highly reduced stress fibers (Figure 2B). If Rac1 is involved in the suppression of cell motility in MSV-MDCK cells, then overexpression of constitutively active Rac1 should induce lamellipodia and reduce motility. As expected, a 45% reduction in the motility was observed in MSV-L61Rac1 cells compared with the vector-transfected MSV-MDCK cells (MSV-MIEG3) (Figure 2C). These results strongly indicated that increased Rac1 activity is associated with the suppression of motility in MSV-MDCK cells expressing Na,K-β.
PI3-Kinase Is Required for Na,K-β–mediated Suppression of Cell Motility and Enhancement of Rac1 Activity
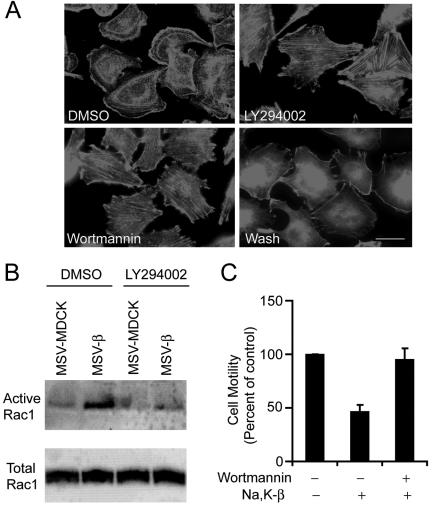
Although the precise mechanism is still not known, previous studies have demonstrated that PI3-kinase is involved in the activation of Rac1 (Hawkins et al., 1995; Welch et al., 2003). To test whether the activation of Rac1 is mediated by PI3-kinase, we treated MSV-β cells with specific pharmacological inhibitors of PI3-kinase such as wortmannin and LY294002. MSV-β cells treated with these PI3-kinase inhibitors showed drastically reduced lamellipodia and increased stress fibers compared with the untreated cells (Figure 3A). Withdrawal of wortmannin reinduced lamellipodia (Figure 3A), indicating that the effect of PI3-kinase on the induction of lamellipodia is reversible. Reduced levels of lamellipodia correlated with diminished levels of active Rac1, as determined by the amount of GST-PAK1–bound Rac1, whereas the levels of total Rac1 from the lysates remained unchanged in these cells (Figure 3B). Furthermore, inhibition of PI3-kinase activity resulted in increased motility of MSV-β cells (Figure 3C), indicating that PI3-kinase activity is essential for Na,K-β–mediated suppression of cell motility in MSV-MDCK cells.
Figure 3.
Requirement of PI3-kinase for Na,K-β–mediated suppression of cell motility and enhancement of Rac1 activity. (A) Phalloidin staining showing actin organization of MSV-β cells treated with DMSO, LY294002, wortmannin, or wortmannin followed by washout for 1 h (Wash). Bar, 25 μm. (B) Immunoblot showing active and total levels of endogenous Rac1 in DMSO- or LY294002-treated MSV-MDCK and MSV-β cells. (C) Migration of MSV-β cells treated with DMSO or wortmannin across Transwell filters. The error bars show SD of the mean of two independent experiments done in triplicates.
PI3-Kinase Is Activated in MSV-MDCK Cells Expressing Na,K-β
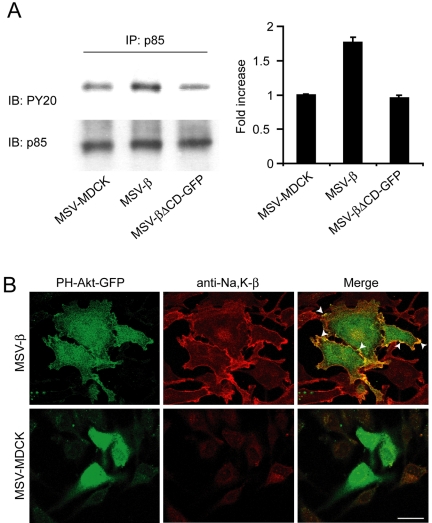
We then designed experiments to test whether PI3-kinase is activated in MSV-β cells. The class I PI3-kinase is composed of two subunits, a 110-kDa catalytic subunit and a 85-kDa regulatory subunit (p85). Tyrosine phosphorylation of p85 is coupled to activation of this subunit, which in turn activates the catalytic subunit (Gentili et al., 2002). Although total levels of p85 from the lysates, as determined by immunoblot analysis remained similar, MSV-β cells showed 1.8-fold higher levels of tyrosine-phosphorylated p85 compared with MSV-MDCK cells as well as MSV-βΔCD-GFP cells (Figure 4A). Furthermore, PH-Akt-GFP, a probe that specifically binds to PI3-kinase activation products and reflects local activation of PI3-kinase (Watton and Downward, 1999), colocalized with Na,K-β at the lamellipodia in MSV-β cells, whereas in MSV-MDCK cells it was present mostly in the cytoplasm (Figure 4B). Together, these results demonstrated that PI3-kinase is locally active, colocalizes with Na,K-β at the lamellipodia, and is involved in the activation of Rac1.
Figure 4.
Analysis of the activation of PI3-kinase in MSV-β cells. (A) MSV-MDCK, MSV-β, and MSV-βΔCD-GFP cells were lysed, and p85 was immunoprecipitated from 1 mg of total cell lysates. The amount of tyrosine phosphorylated p85 in the immunoprecipitates was normalized to total p85 detected by immunoblotting with anti-phosphotyrosine and anti-p85 antibodies, respectively. The graph represents mean ± SD from three independent experiments. (B) Colocalization (arrowheads) of PH-Akt-GFP and Na,K-β (stained with anti-Na,K-β antibody followed by anti-Cy3 antibody) in MSV-MDCK and MSV-β cells. Bar, 25 μm.
Na,K-β Cytoplasmic Tail Interacts with Annexin II in a PI3-Kinase-dependent Manner
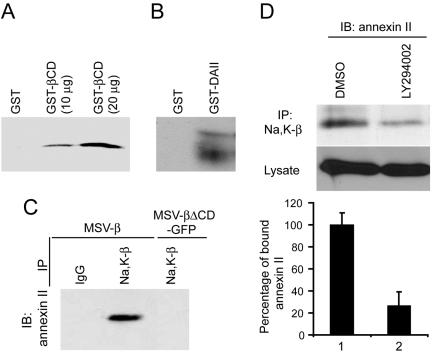
The requirement for the cytoplasmic domain of Na,K-β to suppress cell motility suggested that this domain might interact with protein/s involved in motility suppression. To test this possibility, we used the cytoplasmic domain of Na,K-β fused with GST (GST-βCD) to identify associated proteins by tandem mass spectrometry, as described in Materials and Methods. Annexin II was identified as a candidate protein that interacts with Na,K-β cytoplasmic tail. Annexin II binds anionic phospholipids such as PI3-kinase activation product (PIP3) (Waisman, 1995), associates with active Rac1 (Hansen et al., 2002), and is known to suppress cell motility (Waisman, 1995; Balch and Dedman, 1997; Oliferenko et al., 1999; Oliferenko et al., 2000; Liu et al., 2003). In a GST-pull down assay, as described in Materials and Methods, GST-βCD pulled down annexin II in a concentration-dependent manner from MSV-β cell lysates (Figure 5A). To further characterize the association of annexin II with Na,K-β, canine annexin II was cloned as described in Materials and Methods, and expressed as a GST-fusion protein (GST-DAII) in E. coli. The GST-DAII pulled down Na,K-β from MSV-β cell lysates (Figure 5B). To test whether Na,K-β association with annexin II occurs in vivo, we used coimmunoprecipitation analysis. A substantial amount of annexin II coimmunoprecipitated with anti-Na,K-β antibody from MSV-β cells but not from MSV-βΔCD-GFP cells (Figure 5C), which have low levels of active Rac1 (Figure 2A). This result suggested that annexin II binding to Na,K-β is essential for the activation of Rac1 as well as suppression of motility.
Figure 5.
Na,K-β cytoplasmic tail interacts with annexin II in a PI3-kinase dependent manner. (A) Ten or 20 μg of GST-βCD was incubated with 1 mg of MSV-β cell lysate and the annexin II pulled down by GST-βCD was determined by immunoblotting. (B) GST-annexin II (GST-DAII) was incubated with MSV-β cell lysate as described in Materials and Methods. The blots were then probed with anti-Na,K-β antibody to detect Na,K-β interaction with annexin II. (C) Na,K-β from MSV-β and MSV-βΔCD-GFP cell lysates was immunoprecipitated (IP) with anti-Na,K-β antibody and analyzed by immunoblotting (IB) for the presence of annexin II. (D) MSV-β cells were treated with DMSO or LY294002 and lysed. One milligram of total protein was used for immunoprecipitating Na,K-β. The amount of annexin II bound to Na,K-β was detected by immunoblotting. The graph represents the mean ± SD of three independent experiments.
We then tested whether Na,K-β association with annexin II is dependent on the activation of PI3-kinase. To examine this possibility, MSV-β cells were treated with LY294002, and the amount of annexin II coimmunoprecipitated with Na,K-β was quantified. Whereas LY294002 treatment did not affect the total levels of annexin II, there was a 74% reduction in the amount of annexin II coimmunoprecipitated with Na,K-β in LY294002-treated cells compared with dimethyl sulfoxide (DMSO)-treated cells (Figure 5D). These results demonstrated that annexin II binding to Na,K-β cytoplasmic tail is markedly dependent on the activation of PI3-kinase.
Na,K-α Associates with p85 Regulatory Subunit of PI3-Kinase
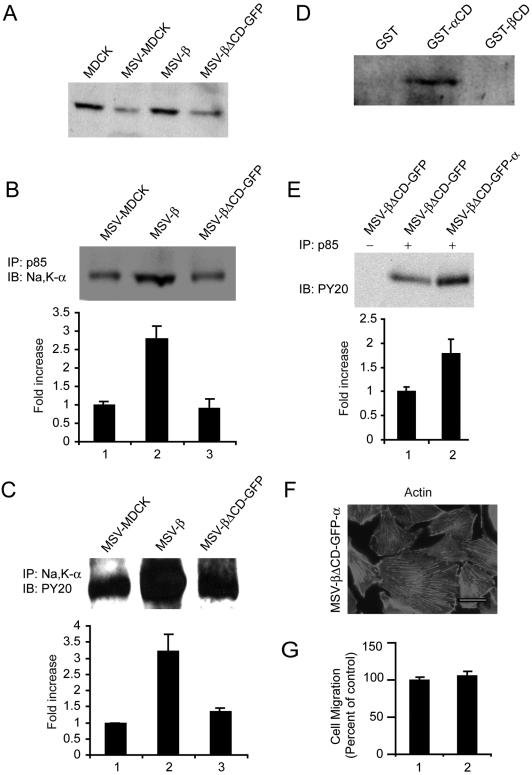
We then designed experiments to test whether the Na,K-ATPase subunits associate with and are involved in the activation of PI3-kinase. Immunoblot analysis revealed that MSV-β cells express sixfold more Na,K-α than MSV-MDCK vector (Rajasekaran et al., 2004) and MSV-βΔCD-GFP cells (Figure 6A). Immunoprecipitation analysis revealed that Na,K-α coimmunoprecipitated with p85 in MSV-MDCK, MSV-β, and MSV-βΔCD-GFP cells (Figure 6B). However, there was a 2.8-fold increase in the Na,K-α levels associated with p85 in MSV-β cells. Immunoprecipitation with anti-Na,K-α antibody followed by immunoblotting with anti-phosphotyrosine antibody revealed 3.2-fold more tyrosine phosphorylated p85 associated with Na,K-α in MSV-β cells compared with MSV-MDCK and MSV-βΔCD-GFP cells (Figure 6C). These results are consistent with increased levels of Na,K-α expressed in MSV-β cells. To further confirm association of Na,K-α with p85, we performed a GST-pulldown assay. It has been shown that the N terminus of Na,K-α containing a proline-rich motif is involved in the binding of p85 (Yudowski et al., 2000). Therefore, we generated GST-tagged N-terminal 93 amino acids of Na,K-α (GST-αCD) and performed a pull-down assay. The Na,K-β association with p85 was tested using GST-βCD. As shown in Figure 6D, the GST-αCD bound specifically to the p85, whereas neither GST alone nor GST-βCD pulled down p85. These results indicated that Na,K-α specifically associates with p85 and is involved in the activation of PI3-kinase signaling in MSV-β cells.
Figure 6.
Analysis of the role of Na,K-α in PI3-kinase signaling. (A) Immunoblot showing levels of Na,K-α from the lysates of indicated cells. (B) p85 was immunoprecipitated (IP) from 1 mg of MSV-MDCK, MSV-β and MSV-βΔCD-GFP cell lysates, and coprecipitating Na,K-α was detected by immunoblotting (IB). The graph represents mean ± SD of three independent experiments. (C) MSV-MDCK, MSV-β, and MSV-βΔCD-GFP cells were lysed and Na,K-α was immunoprecipitated. The precipitate was analyzed by immunoblotting for tyrosine phosphorylated p85 by using anti-phosphotyrosine antibody. The blots were stripped and reprobed for p85 to confirm the presence of p85 (our unpublished data). The graph represents mean ± SD of three independent experiments. (D) GST pull-down assay using GST, GST-αCD, or GST-βCD–coupled agarose beads with MSV-β cell lysate. The immunoblot shows the amount of p85 bound to GST-αCD. (E) The amount of tyrosine phosphorylated p85 in MSV-βΔCD-GFP cells and MSV-βΔCD-GFP cells transfected with Na,K-α (MSV-βΔCD-GFP-α) was determined by immunoprecipitating p85 from 1 mg of cell lysates, followed by blotting with anti-phosphotyrosine antibody. The graph represents mean ± SD of three independent experiments. (F) Phalloidin staining showing the actin organization of MSV-βΔCD-GFP-α cells. Bar, 25 μm. Note abundant stress fibers and lack of lamellipodia (G) Motility of MSV-βΔCD-GFP (1) and MSV-βΔCD-GFP-α (2) cells in a Transwell motility assay. The error bars show the SD of the mean of triplicate measurements done twice, expressed as a percentage of the control (MSV-βΔCD-GFP).
Increased tyrosine phosphorylated p85 seems to be due to the increased levels of Na,K-α expressed in MSV-β cells. To further confirm whether increased Na,K-α levels are associated with increased tyrosine phosphorylation of p85, we exogenously expressed Na,K-α and Na,K-βΔCD-GFP in MSV-MDCK cells (MSV-βΔCD-GFP-α). MSV-βΔCD-GFP cells express low levels of Na,K-α compared with MSV-β cells (Figure 6A). Consistent with our idea, MSV-βΔCD-GFP-α cells showed 1.8-fold more tyrosine phosphorylated p85 than MSV-βΔCD-GFP cells (Figure 6E). Strikingly, although tyrosine phosphorylated p85 levels were high like in MSV-β cells, MSV-βΔCD-GFP-α cells did not reveal lamellipodia and contained abundant stress fibers like MSV-βΔCD-GFP cells (Figure 6F). In addition, Transwell motility assay revealed that MSV-βΔCD-GFP-α cells were as motile as MSV-βΔCD-GFP cells (Figure 6G). Together, these results indicated that increased Na,K-α levels in MSV-β cells are associated with the activation of PI3-kinase. Nevertheless, this activation of PI3-kinase alone is not sufficient to suppress motility in MSV-MDCK cells, indicating that the downstream signaling involving Na,K-β is essential.
Suppression of Cell Motility by Na,K-ATPase Subunits Is Independent of Its Ion Transport Function
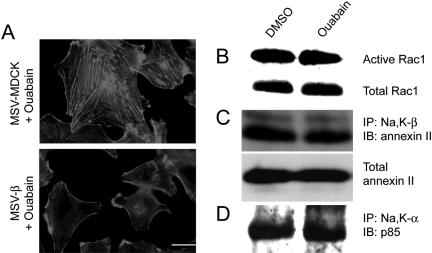
To determine whether the ion transport activity of Na,K-ATPase is required for Na,K-β–mediated suppression of cell motility, cells were treated with ouabain, a specific pharmacological inhibitor of Na,K-ATPase. We have shown earlier that treatment of MSV-β cells with 50 μM ouabain for 30 min completely inhibited Na,K-ATPase ion transport activity (Rajasekaran et al., 2001b). Whereas PI3-kinase inhibition increased the amount of stress fibers and reduced the Rac1 activity in MSV-β cells, inhibition of the ion transport activity of Na,K-ATPase by ouabain neither altered lamellipodial localization of Na,K-β nor increased the stress fiber content (Figure 7A) nor levels of active Rac1 (Figure 7B). Also, ouabain treatment did not affect annexin II binding to Na,K-β (Figure 7C) or Na,K-α binding to p85 (Figure 7D). These results strongly indicate that suppression of cell motility by Na,K-ATPase is independent of its pump function.
Figure 7.
Analysis of the role of Na,K-ATPase pump activity in PI3-kinase signaling and Rac1 activation. (A) Phalloidin staining showing actin organization in MSV-MDCK and MSV-β cells treated with ouabain. Bar, 25 μm. For B–D, MSV-β cells treated with DMSO or ouabain were used. Immunoblot showing the active and total levels of endogenous Rac1 (B), annexin II bound to Na,K-β (C), and p85 bound to Na,K-α (D). The blots are representative of three independent experiments.
DISCUSSION
Na,K-ATPase is a well studied molecule, best appreciated for its role in the regulation of ion homeostasis in mammalian cells. In this study, we demonstrate that Na,K-ATPase is localized to the actin-rich lamellipodia and is involved in controlling cell motility in carcinoma cells. Our results are consistent with a motility suppressor role for Na,K-β in epithelial cells. The cytoplasmic tail of Na,K-β is necessary for the lamellipodial localization of Na,K-ATPase, enhanced Rac1 activity, and suppression of cell motility in MSV-MDCK cells. We also demonstrate that suppression of cell motility is dependent on 1) the activation of PI3-kinase mediated by the association of N-terminus of Na,K-α with p85, and 2) the binding of the cytoplasmic tail of Na,K-β to annexin II. Finally, we show that inhibition of cell motility by Na,K-ATPase is independent of its ion transport function. Our results demonstrate that Na,K-β suppresses cell motility by a novel signaling mechanism involving cross-talk between the two subunits of Na,K-ATPase with proteins associated with PI3-kinase signaling.
Several lines of evidence strongly support that Na,K-β is specifically involved in the suppression of cell motility in MSV-MDCK cells. 1) MSV-MDCK cells have very low levels of Na,K-β and are highly motile. We have shown in three independent clones (MSV-β cl1 [Rajasekaran et al., 2001b], MSV-β cl2 [Rajasekaran et al., 2001b, this study], and MSV-β-GFP [this study]) that repletion of Na,K-β in MSV-MDCK cells suppresses motility. 2) Na,K-βΔCD-GFP, a cytoplasmic tail deletion mutant of Na,K-β, neither showed lamellipodial localization nor reduced the motility of MSV-MDCK cells. 3) The levels of active Rac1 in MSV-βΔCD-GFP cells were comparable with that of parental MSV-MDCK cells, indicating that the cytoplasmic tail of Na,K-β is essential for the signaling leading to the activation of Rac1. 4) Increased levels of Na,K-α in MSV-β cells are involved in the activation of PI3-kinase. Because Na,K-β facilitates the translation efficiency of Na,K-α in the endoplasmic reticulum (Rajasekaran et al., 2004), Na,K-β function also is involved indirectly in the activation of PI3-kinase by Na,K-α in MSV-β cells. 5) By increasing the levels of Na,K-α in MSV-βΔCD-GFP cells, which have very low levels of Na,K-α, we provided evidence that activation of PI3-kinase alone is not sufficient and that the association of Na,K-β with annexin II is also involved in the suppression of cell motility in MSV-MDCK cells. Together, these results demonstrate that reduced motility is primarily mediated by Na,K-β in MSV-MDCK cells.
We have recently shown that the transcription factor Snail known to suppress E-cadherin expression in carcinoma also reduces the transcription of Na,K-β (Espineda et al., 2004). Snail expression during normal development as well as during epithelial to mesenchymal transition induces motility of epithelial cells by reducing the expression of proteins such as E-cadherin (Nieto, 2002). Reduction of Na,K-β transcription by Snail (Espineda et al., 2004) and suppression of motility by repletion of Na,K-β in MSV-MDCK cells (this study), strongly suggests that high Na,K-β expression is associated with reduced motility in well differentiated epithelial cells. Based on these results, we suggest that Na,K-β is a motility suppressor in normal epithelial cells and that reduced levels of this protein are associated with increased motility of carcinoma cells.
Repletion of Na,K-β expression in MSV-MDCK cells induced dramatic reorganization of the actin cytoskeleton. Reduced motility of MSV-β cells correlated with abundant lamellipodia and substantially decreased amount of stress fibers. In general, induction of lamellipodia is associated with enhanced cell migration (Liliental et al., 2000; Sastry et al., 2002; Ridley et al., 2003). However, lamellipodia also can be coupled with decreased cell motility. For example, Chisel protein (normally expressed in heart and skeletal muscles), when exogenously expressed in myoblasts, induced lamellipodia, localized to lamellipodia and suppressed motility (Palmer et al., 2001). The mechanism by which Chisel protein suppresses motility is not known. Cell migration requires the polarization of the cells into a leading edge marked by lamellipodia and a trailing edge composed of focal adhesions (Ridley et al., 2003). In the absence of such a polarization, cell spreading is enhanced, leading to suppression of cell motility (Sander et al., 1999). We observed that MSV-β cells were more spread out compared with MSV-GFP and MSV-βΔCD-GFP cells (Figure 1D), which have reduced lamellipodia. This finding is consistent with the notion that exogenous expression of Na,K-β restricts cell movement by increased cell spreading and attachment to the substratum. Experiments are in progress in our laboratory to unravel the mechanism by which induction of lamellipodia leads to suppression of motility in MSV-β cells.
MSV-β cells were less motile and had highly reduced stress fibers. MSV-MDCK, MSV-βΔCD-GFP, and LY294002-treated MSV-β cells had abundant stress fibers, which correlated with increased motility of these cells. Increased amount of stress fibers provides enhanced contractility and promotes the migratory behavior of cells (Zhong et al., 1997). Therefore, it is possible that increased stress fibers might contribute to increased motility observed in MSV-MDCK, MSV-βΔCD-GFP, and LY294002-treated MSV-β cells. Formation of stress fibers is known to be regulated by the activity of RhoA (Ridley and Hall, 1992). However, we did not observe a difference in the levels of active RhoA in these cell lines. Because RhoA-independent mechanisms are known to modulate stress fiber formation and motility in mammalian cells (Ory et al., 2002), the possibility that such mechanisms are involved in the formation of stress fibers and enhanced motility in MSV-MDCK cells cannot be ruled out.
Na,K-β–mediated suppression of cell motility in MSV-MDCK cells involved PI3-kinase–dependent activation of Rac1. Strikingly, it has been shown that E-cadherin–mediated cell-cell adhesion also leads to PI3-kinase dependent Rac1 activation and suppression of cell motility (Braga et al., 1997; Hordijk et al., 1997; Takaishi et al., 1997; Sander et al., 1999; Yap and Kovacs, 2003). However, MSV-β cells, like MSV-MDCK cells, lack E-cadherin and cadherin-mediated cell-cell adhesion and adherens junction formation (Rajasekaran et al., 2001b). Therefore, Rac1 activation in MSV-β cells is independent of cadherin-mediated adhesion and is dependent on the expression of Na,K-β in these cells. Although MSV-β cells lack E-cadherin, interestingly, they do aggregate in an in vitro cell aggregation assay, indicating that Na,K-β might have cell-cell adhesion function (Rajasekaran et al., 2001b). Whether the activation of Rac1 by exogenous expression of Na,K-β is a result of Na,K-β–mediated cell-cell adhesion remains to be tested.
Our results suggest that increased p85 binding to Na,K-α due to increased expression of Na,K-α in MSV-β cells is involved in the activation of p85. Although MSV-βΔCD-GFP and MSV-MDCK cells showed comparable levels of tyrosine-phosphorylated p85, exogenous expression of Na,K-α in MSV-βΔCD-GFP cells increased the tyrosine phosphorylation of p85, supporting the idea that elevated levels of Na,K-α are involved in the increased phosphorylation of p85. The mechanism by which Na,K-α increases the tyrosine phosphorylation of p85 is currently not known. It is possible that specific kinases activated in MSV-β cells might be involved in increasing the tyrosine phosphorylation of p85 and facilitating the association of Na,K-α with p85. Future studies are necessary to understand this process. Interestingly, it has been reported that dopamine-mediated endocytosis and inhibition of Na,K-ATPase involves activation of PI3-kinase by Na,K-α binding to p85 (Yudowski et al., 2000), indicating that activation of PI3-kinase by binding to p85 is involved in the clearance of Na,K-ATPase from the plasma membrane. However, our results indicate that association of p85 with Na,K-α occurs under conditions where the pump is functional and is involved in the formation of lamellipodia. Although PI3-kinase activation is necessary, it is not sufficient for the suppression of motility. In MSV-βΔCD-GFP cells, exogenous expression of Na,K-α increased tyrosine-phosphorylated p85 levels, however, did not suppress motility, indicating that the downstream events involving the cytoplasmic tail of Na,K-β are essential for the suppression of motility in MSV-MDCK cells.
One of the striking findings reported in this study is the association of annexin II with the cytoplasmic tail of Na,K-β. Annexin II binds to PIP3 (Waisman, 1995), which is produced in cells due to the activation of PI3-kinase (Welch et al., 2003). Annexin II is found in the preparations of lamellae (Ghitescu et al., 2001) and is involved in the regulation of actin polymerization necessary for the delivery of macropinosomes to the plasma membrane (Merrifield et al., 2001). Annexin II also has been shown to inhibit cell migration (Balch and Dedman, 1997; Liu et al., 2003). The carboxy terminus of annexin II binds F-actin (Filipenko and Waisman, 2001), and this association might stabilize annexin II at the actin-rich lamellipodia. The binding of annexin II to CD44/H-CAM results in Rac1 dependent lamellipodia formation (Oliferenko et al., 1999; Oliferenko et al., 2000). Furthermore, it has been shown that annexin II coimmunoprecipitates with constitutively active Rac1 and is suggested to be involved in trapping the diffusive Rac1 complexes in the plasma membrane, causing local activation of Rac1 (Hansen et al., 2002). We have demonstrated that the association of the cytoplasmic tail of Na,K-β with annexin II is PI3-kinase dependent and that this binding is essential for the formation of lamellipodia and suppression of cell motility in MSV-MDCK cells. Because the cytoplasmic tail-deficient mutant of Na,K-β that fails to bind annexin II did not show increased Rac1 activity or suppression of motility, annexin II binding to Na,K-β is essential for the Na,K-β–mediated suppression of motility in MSV-MDCK cells.
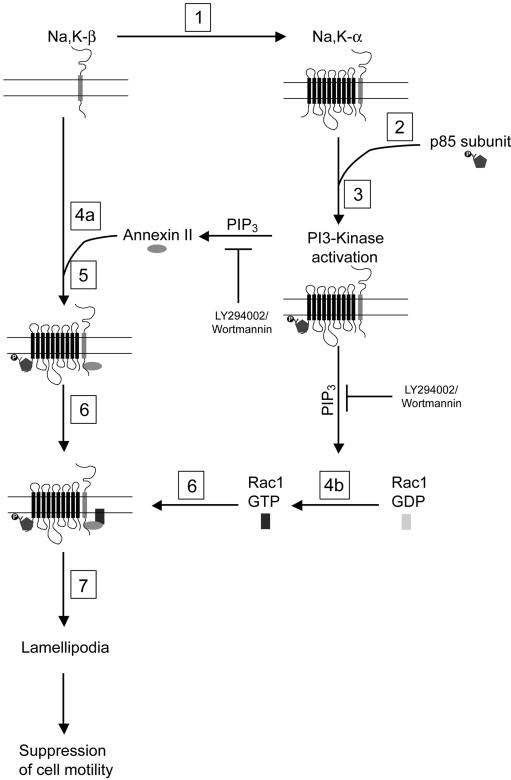
How might Na,K-ATPase be involved in the suppression of cell motility? Based on our results, we propose the following model (Figure 8) to explain the mechanism of Na,K-β–mediated suppression of cell motility. Repletion of Na,K-β in MSV-MDCK cells increases the levels of Na,K-α expression (Rajasekaran, 2004). Elevated Na,K-α levels are involved in its increased binding to p85 leading to the activation of PI3-kinase (Figure 6E). PI3-kinase activation increases the levels of PIP3 (Welch et al., 2003), which in turn activates Rac1 (Figure 3B; Welch et al., 2003) as well as facilitates binding of annexin II to Na,K-β (Figure 5D). Inhibition of PI3-kinase by LY294002 abrogates Rac1 activation (Figure 3B) and binding of annexin II to Na,K-β (Figure 5D). A complex containing Na,K-α, Na,K-β (Figure 1E), active PI3-kinase (as revealed by PH-Akt-GFP localization to the lamellipodia; Figure 4B), and annexin II (Ghitescu et al., 2001) localizes to the plasma membrane. Sequestration of active Rac1 into this complex by annexin II (Hansen et al., 2002) leads to the formation of lamellipodia and suppression of cell motility. Because pharmacological inhibition of Na,K-ATPase does not prevent lamellipodia formation or Rac1 activation (Figure 7, A and B), we suggest that protein–protein interactions via Na,K-α and Na,K-β, rather than ion transport function of Na,K-ATPase, are involved in the suppression of motility in MSV-MDCK cells. Moreover, because inhibition of PI3-kinase abolishes lamellipodia (Figure 3A), and suppression of cell motility (Figure 3C), Na,K-β–mediated suppression of cell motility in MSV-MDCK cells is dependent on the activation of PI3-kinase. Thus, these results demonstrate that Na,K-ATPase is a multifunctional protein, acting both as an enzyme as well as a signaling molecule involved in the regulation of cell motility in epithelial cells.
Figure 8.
Model showing the mechanism of Na,K-β–mediated suppression of cell motility. 1) Repletion of Na,K-β in MSV-MDCK cells increases Na,K-α levels. 2) High levels of Na,K-α lead to the increased tyrosine phosphorylation of p85 and its recruitment to the plasma membrane. 3) This causes activation of PI3-kinase and the generation of PIP3. Increased levels of PIP3 induce 4a) binding of annexin II to Na,K-β cytoplasmic tail and 4b) activation of Rac1. 5) A complex containing both Na,K-ATPase subunits, annexin II, and PI3 kinase is assembled at the plasma membrane. 6) Annexin II sequesters active Rac1 into this complex. 7) Formation of lamellipodia is accomplished, leading to suppression of cell motility.
Increased motility is a prerequisite for invasion and metastasis. Understanding molecular mechanisms involved in the regulation of cell motility in carcinoma cells should provide insights into novel therapeutic strategies to treat invasive and metastatic cancers. In this study, we have uncovered a novel role for Na,K-β in the suppression of cell motility in epithelial cells. We have shown that repletion of Na,K-β in MSV-MDCK cells significantly suppressed motility (Rajasekaran et al., 2001b). Reexpression of E-cadherin also suppressed motility in these cells (Rajasekaran et al., 2001b). However, coexpression of both these proteins suppressed the motility even further, indicating a synergistic role of these two proteins in the suppression of motility of carcinoma cells (Rajasekaran et al., 2001b). Therefore, loss of either E-cadherin or Na,K-β or both in carcinoma cells might be associated with their increased motility and invasive behavior. Ability to increase the expression of both these proteins in carcinoma cells should have significant therapeutic value to impede cancer cell invasion and metastasis.
Acknowledgments
We thank Dr. Greg Payne for critical reading of the manuscript, Dr. Tamas Balla for PH-Akt-GFP, and Dr. William James Ball, Jr., for Na,K-ATPase antibodies. This work was supported by National Institutes of Health grant DK-56216 and Department of Defense W81XWH-04-1-0132 grants.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-05-0427) on December 22, 2004.
References
- Abbott, A., and Ball, W. J., Jr. (1993). The epitope for the inhibitory antibody M7-PB-E9 contains Ser-646 and Asp-652 of the sheep Na+,K(+)-ATPase alpha-subunit. Biochemistry 32, 3511-3518. [DOI] [PubMed] [Google Scholar]
- Anilkumar, G., Rajasekaran, S. A., Wang, S., Hankinson, O., Bander, N. H., and Rajasekaran, A. K. (2003). Prostate-specific membrane antigen association with filamin A modulates its internalization and NAALADase activity. Cancer Res. 63, 2645-2648. [PubMed] [Google Scholar]
- Balch, C., and Dedman, J. R. (1997). Annexins II and V inhibit cell migration. Exp. Cell Res. 237, 259-263. [DOI] [PubMed] [Google Scholar]
- Birchmeier, C., Birchmeier, W., and Brand-Saberi, B. (1996). Epithelial-mesenchymal transitions in cancer progression. Acta Anat. 156, 217-226. [DOI] [PubMed] [Google Scholar]
- Blok, L. J., Chang, G. T., Steenbeek-Slotboom, M., van Weerden, W. M., Swarts, H. G., De Pont, J. J., van Steenbrugge, G. J., and Brinkmann, A. O. (1999). Regulation of expression of Na+,K+-ATPase in androgen-dependent and androgen-independent prostate cancer. Br. J. Cancer 81, 28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga, V. M., Machesky, L. M., Hall, A., and Hotchin, N. A. (1997). The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J. Cell Biol. 137, 1421-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, D. C., and Forte, J. G. (1995). Functional significance of the betasubunit for heterodimeric P-type ATPases. J. Exp. Biol. 198, 1-17. [DOI] [PubMed] [Google Scholar]
- Ehrlich, J. S., Hansen, M. D., and Nelson, W. J. (2002). Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev. Cell 3, 259-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espineda, C., Seligson, D. B., Ball, W. J., Jr., Rao, J., Palotie, A., Horvath, S., Huang, Y., Shi, T., and Rajasekaran, A. K. (2003). Analysis of the Na,K-ATPase alpha- and beta-subunit expression profiles of bladder cancer using tissue microarrays. Cancer 97, 1859-1868. [DOI] [PubMed] [Google Scholar]
- Espineda, C. E., Chang, J. H., Twiss, J., Rajasekaran, S. A., and Rajasekaran, A. K. (2004). Repression of Na,K-ATPase {beta}1-subunit by the transcription factor Snail in carcinoma. Mol. Biol. Cell 15, 1364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipenko, N. R., and Waisman, D. M. (2001). The C terminus of annexin II mediates binding to F-actin. J. Biol. Chem. 276, 5310-5315. [DOI] [PubMed] [Google Scholar]
- Geering, K. (2001). The functional role of beta subunits in oligomeric P-type ATPases. J. Bioenerg. Biomembr. 33, 425-438. [DOI] [PubMed] [Google Scholar]
- Gentili, C., Morelli, S., and Russo De Boland, A. (2002). Involvement of PI3-kinase and its association with c-Src in PTH-stimulated rat enterocytes. J. Cell. Biochem. 86, 773-783. [DOI] [PubMed] [Google Scholar]
- Ghitescu, L. D., Gugliucci, A., and Dumas, F. (2001). Actin and annexins I and II are among the main endothelial plasmalemma-associated proteins forming early glucose adducts in experimental diabetes. Diabetes 50, 1666-1674. [DOI] [PubMed] [Google Scholar]
- Gomez, S. M., Bil, K. Y., Aguilera, R., Nishio, J. N., Faull, K. F., and Whitelegge, J. P. (2003). Transit Peptide cleavage sites of integral thylakoid membrane proteins. Mol. Cell Proteomics 2, 1068-1085. [DOI] [PubMed] [Google Scholar]
- Hall, A. (1998). Rho GTPases and the actin cytoskeleton. Science 279, 509-514. [DOI] [PubMed] [Google Scholar]
- Hansen, M. D., Ehrlich, J. S., and Nelson, W. J. (2002). Molecular mechanism for orienting membrane and actin dynamics to nascent cell-cell contacts in epithelial cells. J. Biol. Chem. 277, 45371-45376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins, P. T., et al. (1995). platelet-derived growth factor stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr. Biol. 5, 393-403. [DOI] [PubMed] [Google Scholar]
- Hordijk, P. L., ten Klooster, J. P., van der Kammen, R. A., Michiels, F., Oomen, L. C., and Collard, J. G. (1997). Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science 278, 1464-1466. [DOI] [PubMed] [Google Scholar]
- Keely, P. J., Fong, A. M., Zutter, M. M., and Santoro, S. A. (1995). Alteration of collagen-dependent adhesion, motility, and morphogenesis by the expression of antisense alpha 2 integrin mRNA in mammary cells. J. Cell Sci. 108, 595-607. [DOI] [PubMed] [Google Scholar]
- Liliental, J., Moon, S. Y., Lesche, R., Mamillapalli, R., Li, D., Zheng, Y., Sun, H., and Wu, H. (2000). Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr. Biol. 10, 401-404. [DOI] [PubMed] [Google Scholar]
- Liu, J. W., Shen, J. J., Tanzillo-Swarts, A., Bhatia, B., Maldonado, C. M., Person, M. D., Lau, S. S., and Tang, D. G. (2003). Annexin II expression is reduced or lost in prostate cancer cells and its re-expression inhibits prostate cancer cell migration. Oncogene 22, 1475-1485. [DOI] [PubMed] [Google Scholar]
- Merrifield, C. J., Rescher, U., Almers, W., Proust, J., Gerke, V., Sechi, A. S., and Moss, S. E. (2001). Annexin 2 has an essential role in actin-based macropinocytic rocketing. Curr. Biol. 11, 1136-1141. [DOI] [PubMed] [Google Scholar]
- Nieto, M. A. (2002). The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell. Biol. 3, 155-166. [DOI] [PubMed] [Google Scholar]
- Oliferenko, S., Kaverina, I., Small, J. V., and Huber, L. A. (2000). Hyaluronic acid (HA) binding to CD44 activates Rac1 and induces lamellipodia outgrowth. J. Cell Biol. 148, 1159-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliferenko, S., Paiha, K., Harder, T., Gerke, V., Schwarzler, C., Schwarz, H., Beug, H., Gunthert, U., and Huber, L. A. (1999). Analysis of CD44-containing lipid rafts: recruitment of annexin II and stabilization by the actin cytoskeleton. J. Cell Biol. 146, 843-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory, S., Destaing, O., and Jurdic, P. (2002). Microtubule dynamics differentially regulates Rho and Rac activity and triggers Rho-independent stress fiber formation in macrophage polykaryons. Eur J. Cell Biol. 81, 351-362. [DOI] [PubMed] [Google Scholar]
- Palmer, S., et al. (2001). The small muscle-specific protein Csl modifies cell shape and promotes myocyte fusion in an insulin-like growth factor 1-dependent manner. J. Cell Biol. 153, 985-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran, A. K., and Rajasekaran, S. A. (2003). Role of Na-K-ATPase in the assembly of tight junctions. Am. J. Physiol. 285, F388-F396. [DOI] [PubMed] [Google Scholar]
- Rajasekaran, S. A., Ball, W. J., Jr., Bander, N. H., Liu, H., Pardee, J. D., and Rajasekaran, A. K. (1999). Reduced expression of beta-subunit of Na,K-ATPase in human clear-cell renal cell carcinoma. J. Urol. 162, 574-580. [PubMed] [Google Scholar]
- Rajasekaran, S. A., Gopal, J., Willis, D., Espineda, C., Twiss, J. L., and Rajasekaran, A. K. (2004). Na,K-ATPase beta1-subunit increases the translation efficiency of the alpha1-subunit in MSV-MDCK cells. Mol. Biol. Cell 15, 3224-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran, S. A., Hu, J., Gopal, J., Gallemore, R., Ryazantsev, S., Bok, D., and Rajasekaran, A. K. (2003). Na,K-ATPase inhibition alters tight junction structure and permeability in human retinal pigment epithelial cells. Am. J. Physiol. 284, C1497-C1507. [DOI] [PubMed] [Google Scholar]
- Rajasekaran, S. A., Palmer, L. G., Moon, S. Y., Peralta Soler, A., Apodaca, G. L., Harper, J. F., Zheng, Y., and Rajasekaran, A. K. (2001a). Na,K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells. Mol. Biol. Cell 12, 3717-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran, S. A., Palmer, L. G., Quan, K., Harper, J. F., Ball, W. J., Jr., Bander, N. H., Peralta Soler, A., and Rajasekaran, A. K. (2001b). Na,K-ATPase beta-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol. Biol. Cell 12, 279-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A. J., Comoglio, P. M., and Hall, A. (1995). Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol. Cell. Biol. 15, 1110-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A. J., and Hall, A. (1992). The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389-399. [DOI] [PubMed] [Google Scholar]
- Ridley, A. J., Paterson, H. F., Johnston, C. L., Diekmann, D., and Hall, A. (1992). The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell 70, 401-410. [DOI] [PubMed] [Google Scholar]
- Ridley, A. J., Schwartz, M. A., Burridge, K., Firtel, R. A., Ginsberg, M. H., Borisy, G., Parsons, J. T., and Horwitz, A. R. (2003). Cell migration: integrating signals from front to back. Science 302, 1704-1709. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan, E., and Nelson, W. J. (1989). Morphogenesis of the polarized epithelial cell phenotype. Science 245, 718-725. [DOI] [PubMed] [Google Scholar]
- Sander, E. E., ten Klooster, J. P., van Delft, S., van der Kammen, R. A., and Collard, J. G. (1999). Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 147, 1009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry, S. K., Lyons, P. D., Schaller, M. D., and Burridge, K. (2002). PTP-PEST controls motility through regulation of Rac1. J. Cell Sci. 115, 4305-4316. [DOI] [PubMed] [Google Scholar]
- Servant, G., Weiner, O. D., Herzmark, P., Balla, T., Sedat, J. W., and Bourne, H. R. (2000). Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287, 1037-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, K., and Fuller, S. D. (1985). Cell surface polarity in epithelia. Annu. Rev. Cell Biol. 1, 243-288. [DOI] [PubMed] [Google Scholar]
- Small, J. V., Stradal, T., Vignal, E., and Rottner, K. (2002). The lamellipodium: where motility begins. Trends Cell Biol. 12, 112-120. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson, W. G., Aznavoorian, S., and Liotta, L. A. (1993). Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu. Rev. Cell Biol. 9, 541-573. [DOI] [PubMed] [Google Scholar]
- Sun, Y., and Ball, W. J., Jr. (1994). Identification of antigenic sites on the Na+/K(+)-ATPase beta-subunit: their sequences and the effects of thiol reduction upon their structure. Biochim. Biophys. Acta 1207, 236-248. [DOI] [PubMed] [Google Scholar]
- Takaishi, K., Sasaki, T., Kotani, H., Nishioka, H., and Takai, Y. (1997). Regulation of cell-cell adhesion by Rac and Rho small G proteins in MDCK cells. J. Cell Biol. 139, 1047-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisman, D. M. (1995). Annexin II tetramer: structure and function. Mol. Cell. Biochem. 149–150, 301-322. [DOI] [PubMed] [Google Scholar]
- Watton, S. J., and Downward, J. (1999). Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr. Biol. 9, 433-436. [DOI] [PubMed] [Google Scholar]
- Welch, H. C., Coadwell, W. J., Stephens, L. R., and Hawkins, P. T. (2003). Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett. 546, 93-97. [DOI] [PubMed] [Google Scholar]
- Xu, J., Rodriguez, D., Petitclerc, E., Kim, J. J., Hangai, M., Moon, Y. S., Davis, G. E., Brooks, P. C., and Yuen, S. M. (2001). Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J. Cell Biol. 154, 1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap, A. S., and Kovacs, E. M. (2003). Direct cadherin-activated cell signaling: a view from the plasma membrane. J. Cell Biol. 160, 11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudowski, G. A., Efendiev, R., Pedemonte, C. H., Katz, A. I., Berggren, P. O., and Bertorello, A. M. (2000). Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+, K+-ATPase alpha subunit and regulates its trafficking. Proc. Natl. Acad. Sci. USA 97, 6556-6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, C., Kinch, M. S., and Burridge, K. (1997). Rho-stimulated contractility contributes to the fibroblastic phenotype of Ras-transformed epithelial cells. Mol. Biol. Cell 8, 2329-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, K., Debreceni, B., Li, R., and Zheng, Y. (2000). Identification of Rho GTPase-dependent sites in the Dbl homology domain of oncogenic Dbl that are required for transformation. J. Biol. Chem. 275, 25993-26001. [DOI] [PubMed] [Google Scholar]