Significance
New drugs are needed to combat multidrug-resistant tuberculosis. The electron transport chain (ETC) maintains the electrochemical potential across the cytoplasmic membrane and allows production of ATP, the energy currency of any living cell. The ETC of the tubercle bacilli contains two terminal oxidases, the cytochrome bc1:aa3 and the cytochrome bd oxidase. In this study, we used genetics and chemical biology approaches to demonstrate that simultaneous inhibition of both terminal oxidases stops respiration, kills nonreplicating drug-tolerant Mycobacterium tuberculosis, and eradicates infection in vivo at an extraordinarily fast rate. Exploiting this potent synthetic lethal interaction with new drugs promises to shorten tuberculosis chemotherapy.
Keywords: bioenergetics, oxidative phosphorylation, persisters, Q203, bedaquiline
Abstract
The recent discovery of small molecules targeting the cytochrome bc1:aa3 in Mycobacterium tuberculosis triggered interest in the terminal respiratory oxidases for antituberculosis drug development. The mycobacterial cytochrome bc1:aa3 consists of a menaquinone:cytochrome c reductase (bc1) and a cytochrome aa3-type oxidase. The clinical-stage drug candidate Q203 interferes with the function of the subunit b of the menaquinone:cytochrome c reductase. Despite the affinity of Q203 for the bc1:aa3 complex, the drug is only bacteriostatic and does not kill drug-tolerant persisters. This raises the possibility that the alternate terminal bd-type oxidase (cytochrome bd oxidase) is capable of maintaining a membrane potential and menaquinol oxidation in the presence of Q203. Here, we show that the electron flow through the cytochrome bd oxidase is sufficient to maintain respiration and ATP synthesis at a level high enough to protect M. tuberculosis from Q203-induced bacterial death. Upon genetic deletion of the cytochrome bd oxidase-encoding genes cydAB, Q203 inhibited mycobacterial respiration completely, became bactericidal, killed drug-tolerant mycobacterial persisters, and rapidly cleared M. tuberculosis infection in vivo. These results indicate a synthetic lethal interaction between the two terminal respiratory oxidases that can be exploited for anti-TB drug development. Our findings should be considered in the clinical development of drugs targeting the cytochrome bc1:aa3, as well as for the development of a drug combination targeting oxidative phosphorylation in M. tuberculosis.
The emergence and spread of drug resistance in pathogenic mycobacteria poses serious global health concerns. Tuberculosis (TB) continues to cause 1.4 million deaths in HIV-negative individuals and 10.4 million new cases in 2015 (1). It was recently evaluated that the number of TB cases in India is two to three times higher than previously estimated (2), suggesting that the global number of TB cases may be largely underestimated. Despite progress in public health management and the use of fixed-dose combinations, the number of multi- and extensively drug-resistant (M-XDR) TB cases continues to rise (1). According to the last WHO report, the proportion of multidrug-resistant tuberculosis among newly diagnosed cases is a staggering 5.6% (1). In 2015, 580,000 new patients were eligible for MDR-TB treatment. MDR-TB treatment is challenging because it requires the administration of second-line drugs for up to 2 y (3), with an estimated global success rate of 52% and an unacceptable mortality rate (3). There is a pressing clinical need for the development of new drugs able to shorten the treatment of MDR-TB to 6 mo or less. More than new drugs, a rational drug combination made of complementary agents is urgently needed. Despite increasing interest from the scientific community, the global drug pipeline remains thin: only a very few new chemical entities have entered clinical development in the last 40 y (4). The recent approval of bedaquiline (BDQ, Sirturo) represents a critical milestone in anti-TB drug discovery (5–7). Nevertheless, the successful advance of BDQ is overshadowed by the emergence of clinical resistance less than 3 y after its introduction to medical use (8). The rapid emergence of resistance is most likely linked to the absence of potent companion drugs. Indeed, BDQ is currently given in combination with weaker second- and third-line drugs, imposing a strong selection pressure for BDQ resistance. This reinforces the notion that a rational drug combination of complementary drugs is required to shorten the treatment time of MDR-TB.
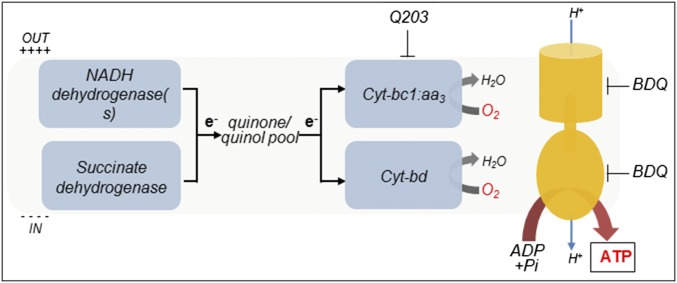
The discovery of BDQ, a potent inhibitor of the mycobacterial F1Fo-ATP synthase, validated oxidative phosphorylation (OxPhos) (Fig. 1) as an attractive drug target in M. tuberculosis. OxPhos is an ubiquitous metabolic pathway, in which the energy contained in nutrients is used to generate an electrochemical gradient, also called the proton motive force (pmf), that drives the synthesis of Adenosine Tri-Phosphate (ATP). The pmf is required for the survival of both replicating and nonreplicating (often referred to as dormant) mycobacteria (9, 10). Dissipation of the pmf leads to a rapid loss of cell viability and cell death. Therefore, drugs targeting enzymes involved in pmf generation are predicted to reduce time of therapy by killing phenotypic drug-resistant bacterial subpopulations (11).
Fig. 1.
Oxidative phosphorylation pathway in M. tuberculosis. The molecular targets of Q203 and bedaquiline (BDQ) are shown.
In M. tuberculosis, the generation of the pmf is mediated primarily by the proton-pumping components of the electron transport chain (ETC). Under aerobic conditions, the ETC of M. tuberculosis branches into two terminal oxidases; the proton-pumping cytochrome bc1-aa3 supercomplex (Cyt-bc1:aa3) and the less energy efficient, but higher-affinity cytochrome bd oxidase (Cyt-bd) (12–15). In recent years, the discovery of several small molecules targeting the Cyt-bc1:aa3 branch (16–21) has triggered interest in the heme–copper respiratory oxidase (18, 20). All small-molecule inhibitors discovered to date seem to target the cytochrome b subunit of the bc1 complex (16–21). The best characterized compounds targeting cytochrome bc1 are a series of imidazopyridine amides (IPA) (16, 18–20). The most advanced IPA derivative is Q203, a drug candidate currently in clinical trial phase I under a US FDA Investigational New Drug application (22). However, despite the reported susceptibility of the Cyt-bc1:aa3 to chemical inhibition, the influence of the alternative Cyt-bd terminal oxidase on the potency of Q203 and related drugs remains to be defined. Here, through a combination of chemical biology and genetic approaches, we reveal the existence of a synthetic lethal interaction between the Cyt-bc1:aa3 and the Cyt-bd terminal oxidases. A synthetic lethal interaction is a well-described phenomenon where the single inactivation of two genes has little effect on cell viability, whereas the simultaneous inactivation of both genes results in cell death (23). Upon chemical inhibition of the Cyt-bc1:aa3 complex, respiration through Cyt-bd is sufficient to maintain the viability of replicating and nonreplicating mycobacteria. However, simultaneous inhibition of both terminal oxidases was sufficient to inhibit respiration, kill phenotypic drug-resistant persisters, and rapidly eradicate M. tuberculosis infection in vivo.
Results
Q203 Is a Bacteriostatic Agent that Does Not Inhibit Oxygen Respiration.
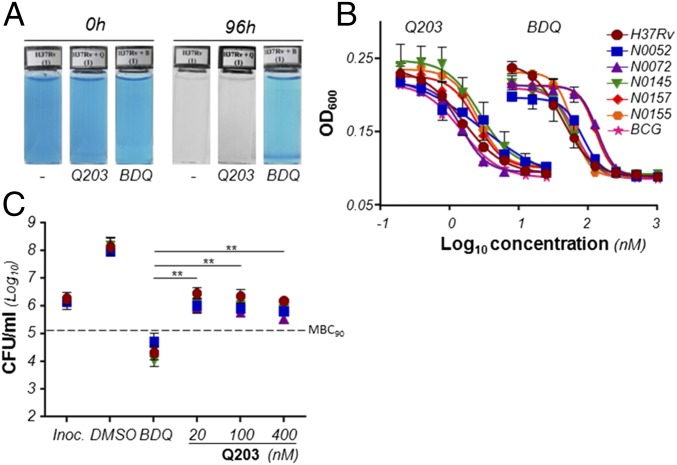
The metabolic consequences of the chemical inhibition of the mycobacterial Cyt-bc1:aa3 have not been studied in detail. A recent study revealed that Q203 and BDQ treatment triggered an increase in oxygen consumption rate (OCR) up to 16 h posttreatment, which is counterintuitive given the capacity of the drugs to interfere with respiration. Interestingly, increase in OCR was only observed at a very high dose of drugs (300× MIC), but not at an intermediate dose (30× MIC) (24). Consequently, we were interested in gaining more mechanistic insight into the ETC adaptations to Q203 and BDQ and their long-term effects on oxygen respiration. Using methylene blue as an oxygen probe, we made the observation that oxygen consumption was significantly inhibited by BDQ treatment over a 96-h period, but was unaffected by Q203 treatment (Fig. 2A). To ensure that these results were not an artifact due to the inability of inhibitors of the Cyt-bc1:aa3 to inhibit growth of laboratory strains of M. tuberculosis (17), we verified the potency of Q203 against five clinical isolates (N0052, N0072, N0145, N0157, N0155) from different M. tuberculosis lineages (25) and M. bovis bacillus Calmette–Guérin. We confirmed that Q203 has excellent growth inhibitory potency against all these strains (Fig. 2B). Q203 had a Minimum Inhibitory Concentration leading to 50% growth inhibition (MIC50) of 1.5–2.8 nM, whereas BDQ was active in a MIC50 range of 42–133 nM (Fig. 2B and Table S1). Altogether, these results suggested that chemical inhibition of the Cyt-bc1:aa3 terminal oxidase led to bacterial growth arrest without affecting oxygen consumption. Because BDQ inhibited oxygen respiration over a 96-h period, whereas Q203 did not (Fig. 2A), we were next interested in testing the correlation between inhibition of oxygen consumption and bacterial death. Interestingly, we observed that despite the superior potency of Q203 in the growth inhibition assay, the drug candidate was much less effective at killing M. tuberculosis compared with BDQ. BDQ was bactericidal against four strains of M. tuberculosis at a concentration 5- to 12-fold above its MIC50 (Fig. 2C), whereas Q203 was bacteriostatic even at doses exceeding 200-fold its MIC50 (Fig. 2C). Similar results were observed in Mycobacterium bovis bacillus Calmette–Guérin (Fig. S1). Because BDQ and Q203 target the same pathway (OxPhos), but have a striking difference on mycobacterial viability, we hypothesized that an alternate branch of the ETC may compensate for the chemical inhibition of the Cyt-bc1:aa3 terminal oxidase.
Fig. 2.
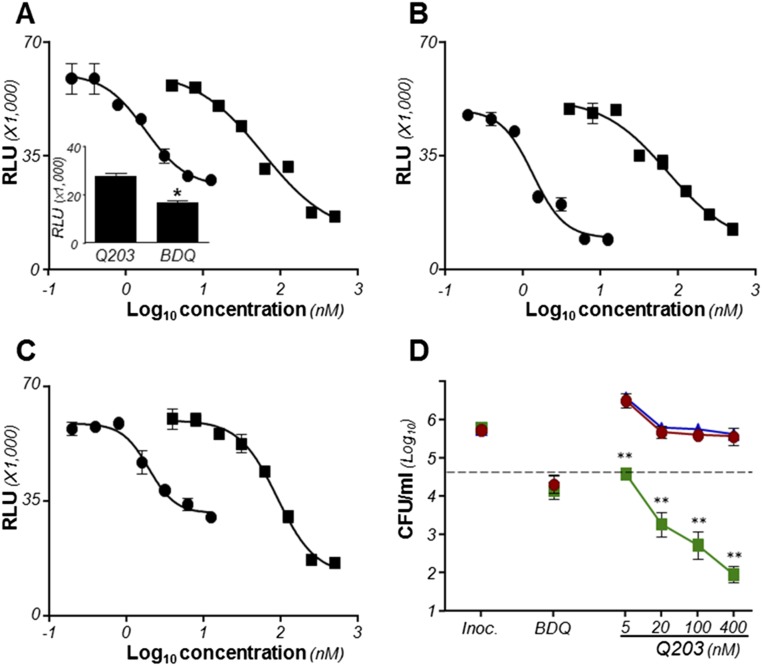
Q203 is a bacteriostatic agent that does not inhibit respiration in M. tuberculosis. (A) Oxygen consumption assay in M. tuberculosis H37Rv using the oxygen sensor Methylene Blue at 0.001%. (B) MIC50 of Q203 against M. tuberculosis H37Rv (red circles), bacillus Calmette–Guérin (pink stars), and the clinical isolates N0052 (blue squares), N0072 (purple triangles), N0145 (green inverted triangles), N0157 (red diamonds), and N0155 (orange hexagons) replicating in culture broth medium. Bacterial growth was measured by recording the Optical Density at 600 nm (OD600) after 5 d of incubation. (C) Bactericidal activity of Q203 and BDQ against M. tuberculosis H37Rv (red circles) and the clinical isolates N0052 (blue squares), N0072 (purple triangles), and N0145 (green triangles). The dotted line represents 90% bacterial killing compared with the initial inoculum (MBC90). **Statistical difference (P < 0.001, Student’s t test) between the potency of BDQ and Q203. All experiments were performed in triplicate and repeated at least once. BDQ was used as a control drug targeting oxidative phosphorylation in all experiments.
Table S1.
MIC50 and MBC90 of Q203 and BDQ against H37Rv, five M. tuberculosis clinical isolates, and M. bovis bacillus Calmette–Guérin
| Strains | Q203 | BDQ | ||||
| MIC50 (nM) | MBC90 (nM) | MBC90/MIC50 (ratio) | MIC50 (nM) | MBC90 (nM) | MBC90/MIC50 (ratio) | |
| H37Rv | 1.5 ± 0.1 | >400 | >267 | 42.1 ± 1.3 | ≤500 | ≤12 |
| N0072 | 1.5 ± 0.1 | >400 | >267 | 132.5 ± 4.4 | ≤500 | ≤4 |
| N0052 | 2.5 ± 0.4 | >400 | >160 | 75.3 ± 7.6 | ≤500 | ≤7 |
| N0145 | 2.8 ± 0.4 | >400 | >142 | 51.0 ± 5.3 | ≤500 | ≤10 |
| N0155 | 2.6 ± 0.1 | n.d. | n.d. | 57.6 ± 1.5 | n.d. | n.d. |
| N0157 | 2.4 ± 0.2 | n.d. | n.d. | 120.2 ± 5.9 | n.d. | n.d. |
| Bacillus Calmette–Guérin | 1.5 ± 0.2 | >400 | >267 | 61.6 ± 3.4 | ≤500 | ≤8.5 |
The ratio between the MIC50 and the MBC90 is shown. Experiments were performed in triplicate and repeated at least once. MIC50 results are expressed as the mean ± SDs of a representative experiment. n.d., not determined.
Fig. S1.
Effect of Q203 on the viability of M. bovis bacillus Calmette–Guérin. The dotted line represents 90% bacterial killing compared with the initial inoculum (MBC90). Inoc., inoculum size at the start of the experiment. Data are expressed as the mean ± SDs. The experiments were performed in triplicate and repeated once.
Cytochrome bd-Type Oxidase Compensates for Chemical Inhibition of the Cytochrome bc1:aa3 Branch.
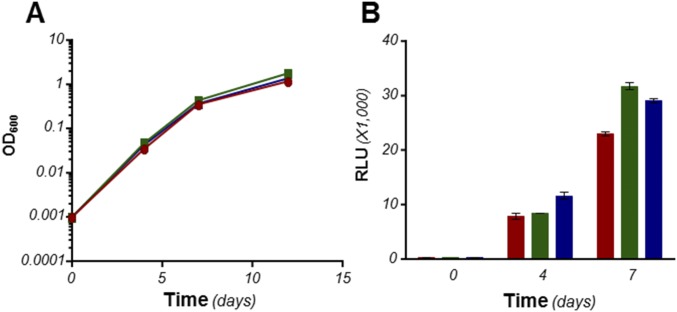
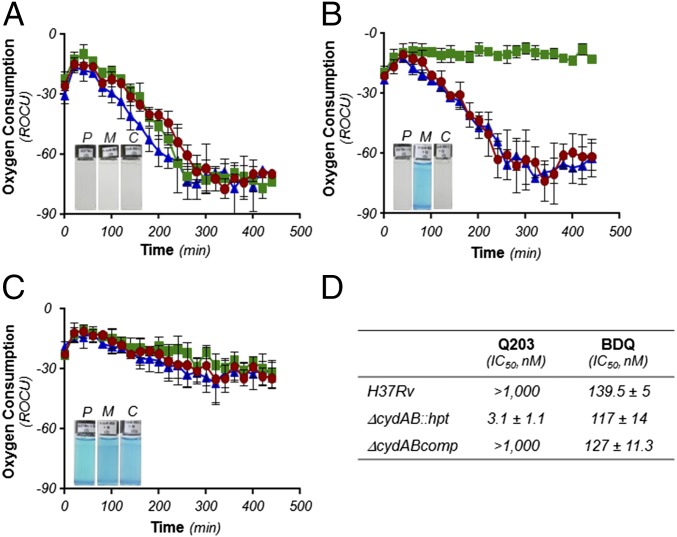
The involvement of Cyt-bd in a possible compensatory mechanism was investigated. The cydAB genes (coding for Cyt-bd) were deleted in M. tuberculosis H37Rv and Mycobacterium bovis bacillus Calmette–Guérin (bacillus Calmette–Guérin), leading to strains H37Rv ∆cydAB, and bacillus Calmette–Guérin ∆cydAB. Deletion of cydAB did not impact significantly on bacterial growth and ATP homeostasis (Fig. S2). The synthetic lethal interaction between the Cyt-bc1:aa3 and the Cyt-bd was evaluated by treating the mutant strains with Q203. Deletion of cydAB had a modest effect on the growth inhibitory potency of Q203 (Fig. S3), but a profound impact on the capacity of mycobacteria to respire with oxygen over a prolonged period (Fig. 3). Using methylene blue as an oxygen probe, we observed that treatment of H37Rv ∆cydAB or bacillus Calmette–Guérin ∆cydAB with Q203 led to an apparent complete inhibition of oxygen respiration (Fig. 3, Insets, and Fig. S4). This phenotype was reversed by expressing the cydAB operon in the mutant strains (∆cydABcomp strains) (Fig. 3, Insets, and Fig. S4). The inability of the Cyt-bd mutant to utilize oxygen was confirmed by measuring the Relative Oxygen Consumption rate (ROC) using the MitoXpress Oxygen probe in whole cells over a short period (Fig. 3). Under our experimental conditions, Q203 had no significant effect on oxygen respiration in the parental strain, but triggered a complete inhibition of oxygen consumption in H37Rv ∆cydAB at an IC50 of 3.1 nM (Fig. 3 B and D). These results were corroborated in inverted membrane vesicles with NADH as the electron donor (Fig. S5). Furthermore, Q203 treatment led to a decrease in ATP levels in the parental H37Rv strain, but to a lesser extent compared with BDQ treatment (Fig. 4A). Q203 treatment was more effective at disrupting ATP homeostasis in H37Rv ∆cydAB compared with the parental strain (Fig. 4B). Similar results were obtained in bacillus Calmette–Guérin (Fig. S6). Because the effect on oxygen consumption correlated with reduced ATP levels in Q203-treated ∆cydAB strains, we hypothesized that electron flow diverted to the Cyt-bd branch upon chemical inhibition of the Cyt-bc1:aa3 was sufficient to maintain cell viability. Consistent with this hypothesis, Q203 displayed a dose-dependent bactericidal effect against H37Rv ∆cydAB (Fig. 4D). Under the same conditions, the bactericidal potency of BDQ was unaffected by cydAB deletion (Fig. 4D). Similar results were obtained in bacillus Calmette–Guérin (Fig. S6D). Altogether, these findings established a strong synthetic lethal interaction between Cyt-bc1:aa3 and Cyt-bd and the requirement for at least one terminal oxidase to maintain cell viability in mycobacteria.
Fig. S2.
cydAB deletion had no significant impact on growth and ATP homeostasis in M. tuberculosis H37Rv. (A) Growth of M. tuberculosis strains H37Rv (red circles), H37Rv ΔcydAB (green squares), and H37Rv ΔcydABcomp (blue triangles) was monitored over a 12-d period. (B) ATP levels in replicating M. tuberculosis strains H37Rv (red bars), H37Rv ΔcydAB (green bars), and H37Rv ΔcydABcomp (blue bars). Data are expressed as mean ± SDs. The experiments were performed in triplicate.
Fig. S3.
cydAB deletion had a moderate effect on the MIC50 of Q203 MIC50 of Q203 against M. tuberculosis H37Rv (red circles), H37Rv ΔcydAB (green squares), and H37Rv ΔcydABcomp (blue triangles) strains replicating in culture broth medium (A and C). MIC50 of Q203 against bacillus Calmette–Guérin (red circles), bacillus Calmette–Guérin ΔcydAB (green squares), and bacillus Calmette–Guérin ΔcydABcomp (blue triangles) replicating in culture broth medium (B and D). Bacterial growth was quantified by recording the OD600 after 5 d of incubation.
Fig. 3.
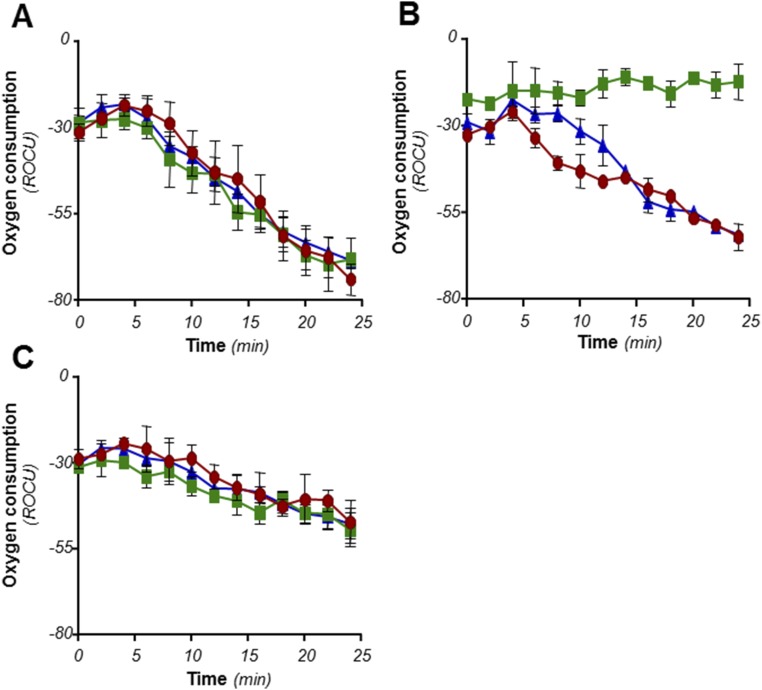
The alternate Cyt-bd terminal oxidase contributes to cellular respiration under aerobic conditions in M. tuberculosis. M. tuberculosis H37Rv (red circles), H37Rv ΔcydAB (green squares), and ΔcydABcomp (blue triangles) were incubated with the oxygen probe MitoXpress in the presence of 1% DMSO (A), Q203 at 400 nM (B), or BDQ at 500 nM (C). Kinetics of oxygen consumption was measured by recording the fluorescence (Ex380, Em650) over a 500-min period. Relative fluorescence units were converted into relative units of oxygen consumption (ROC). Insets: Oxygen consumption assay using methylene blue as oxygen sensor. P, H37Rv; M, H37Rv ΔcydAB; C, H37Rv ΔcydABcomp strains. (D) The inhibitory concentration (IC50) of Q203 and BDQ on oxygen consumption was measured using the MitoXpress oxygen probe. IC50 was calculated from measurement of the fluorescence read after 180 min of incubation at 37 °C. The experiments were performed in triplicate and repeated at least once. Data are expressed as the mean ± SDs of triplicates for each concentration of a representative experiment.
Fig. S4.
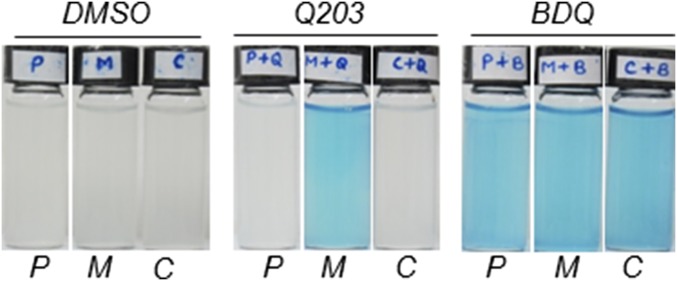
The alternate Cyt-bd terminal oxidase contributes to cellular respiration in bacillus Calmette–Guérin. Bacillus Calmette–Guérin (P), bacillus Calmette–Guérin ΔcydAB (M), and bacillus Calmette–Guérin ΔcydABcomp (C) were incubated with the oxygen probe Methylene blue in the presence of 1% DMSO, 400 nM Q203, or 500 nM BDQ in sealed tubes and incubated under an anaerobic atmosphere to prevent oxygen leak. Pictures were taken after 4 d of incubation at 37 °C.
Fig. S5.
The Cyt-bc1:aa3 and Cyt-bd contribute to oxygen respiration in mycobacterial inverted membrane vesicles. Inverted membrane vesicles from bacillus Calmette–Guérin parental (red circles), ΔcydAB (green squares), and ΔcydABcomp (blue triangles) strains were incubated with the oxygen probe MitoXpress in the presence of 1% DMSO (A), Q203 at 10 nM (B), or BDQ at 500 nM (C). Kinetic of oxygen consumption was measured by recording the fluorescence (Ex380, Em650) over a 30-min period. SDs of three replicates are shown. The experiment was repeated once.
Fig. 4.
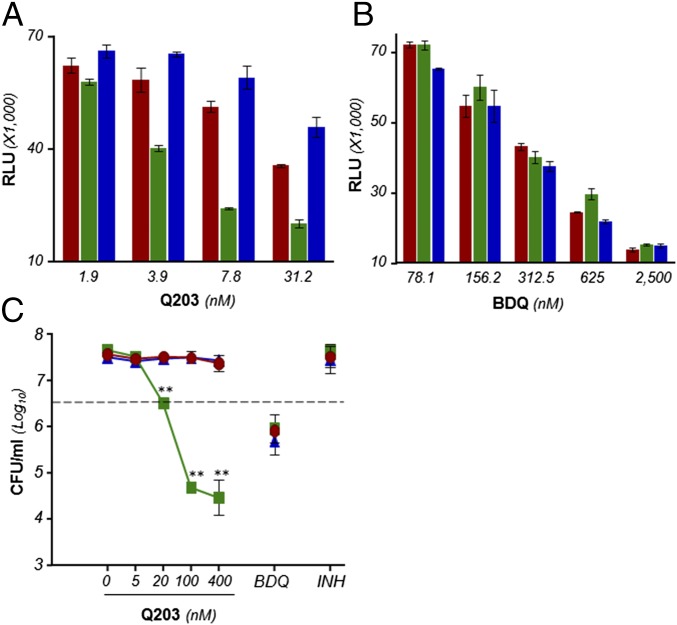
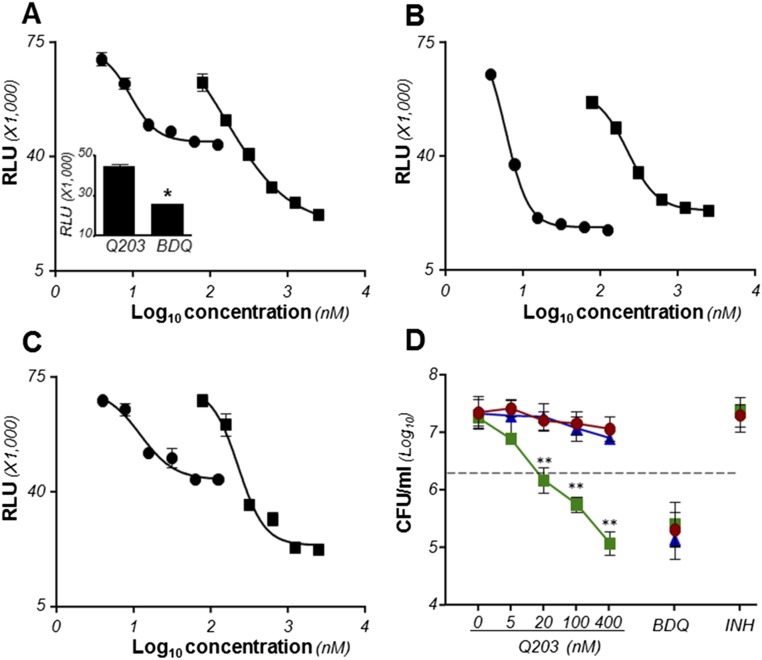
Q203 is bactericidal and triggers a rapid ATP depletion in M. tuberculosis H37Rv ΔcydAB strain. ATP levels were measured using a luciferase-based assay in H37Rv (A), H37Rv ΔcydAB (B), and H37Rv ΔcydABcomp (C) exposed to a dose-range of Q203 (circles) or BDQ (squares). Relative Light Units (RLU) were recorded after 24 h of incubation. Inset in A depicts the ATP levels in M. tuberculosis H37Rv treated with Q203 at 50 nM or BDQ at 500 nM (BDQ). *Statistical difference (P < 0.01, Student’s t test) in ATP level between Q203- and BDQ-treated bacteria. (D) Bactericidal potency of Q203 and BDQ against replicating M. tuberculosis H37Rv (red circles), H37Rv ΔcydAB (green squares), and H37Rv ΔcydABcomp (blue triangles) strains. The dotted line represents 90% bacterial killing compared with the initial inoculum (MBC90). **Statistical difference (P < 0.001, Student’s t test) in CFU number between H37Rv and H37Rv ΔcydAB treated with Q203. Inoc., inoculum size at the start of the experiment. Data are expressed as the mean ± SDs of triplicates for each concentration.
Fig. S6.
Q203 is bactericidal and triggers a rapid ATP depletion in bacillus Calmette–Guérin ΔcydAB. ATP levels were measured using a luciferase-based assay in bacillus Calmette–Guérin (A), bacillus Calmette–Guérin ΔcydAB (B), and bacillus Calmette–Guérin ΔcydABcomp (C) exposed to a dose-range of Q203 (circles) or BDQ (squares). Relative Light Units (RLU) were recorded after 24 h of incubation. Inset in A depicts the ATP levels in bacillus Calmette–Guérin treated with Q203 at 25 nM (Q203) or BDQ at 250 nM (BDQ). The dotted line represents 90% bacterial killing compared with the initial inoculum (MBC90). *Statistical difference (P < 0.01, Student’s t test) in ATP level between Q203- and BDQ-treated bacteria. (D) Bactericidal potency of Q203 and BDQ against bacillus Calmette–Guérin (red circles), bacillus Calmette–Guérin ΔcydAB (green squares), and bacillus Calmette–Guérin ΔcydABcomp (blue triangles) strains. **Statistical difference (P < 0.001, Student's t test) in CFU number between H37Rv and H37Rv ΔcydAB treated with Q203. Inoc., inoculum size at the start of the experiment. BDQ was used at 500 nM. Data are expressed as the mean ± SD of triplicates for each concentration.
Cytochrome bd-Type Oxidase Protects Nonreplicating Mycobacteria from Q203-Induced Bacterial Death.
Next, the impact of Q203 treatment on ATP homeostasis and viability of nutrient-starved, phenotypic drug-resistant mycobacteria was evaluated. Q203 treatment in the parental strain resulted in a dose-dependent reduction in ATP levels, but without affecting cell viability (Fig. 5 A and C). Under similar experimental conditions, BDQ was bactericidal (Fig. 5C). It was noted that ATP depletion induced by Q203 treatment in the parental strain was significantly less compared with BDQ treatment (Fig. 5 A and B). As observed under replicating conditions, Q203 treatment triggered a more profound ATP depletion in the nutrient-starved H37Rv ∆cydAB strain compared with the parental strain (Fig. 5A) and was bactericidal (Fig. 5C). The effect on cell viability was profound because Q203 at 100 nM killed more than 99.99% of the nonreplicating H37Rv ∆cydAB strain (Fig. 5C). The phenotype was reversed in the H37Rv ∆cydABcomp strain (Fig. 5C). Similar results were obtained in bacillus Calmette–Guérin (Fig. S7). These data further demonstrated that the respiratory terminal oxidases are jointly required for oxidative phosphorylation and that simultaneous inactivation of both has a striking effect on the viability of nonreplicating, phenotypically drug-resistant mycobacteria.
Fig. 5.
The Cyt-bc1:aa3 and the Cyt-bd terminal oxidases are jointly required for ATP homeostasis and survival in nutrient-starved, phenotypic drug-resistant persisters. ATP levels were quantified in nutrient-starved M. tuberculosis H37Rv (red bars), H37Rv ΔcydAB (green bars), and H37Rv ΔcydABcomp (blue bars) treated with a dose-range of Q203 (A) or BDQ (B). (C) Bactericidal potency of Q203, BDQ and isoniazid (INH) was evaluated against the M. tuberculosis strains H37Rv (red circles), H37Rv ΔcydAB (green squares), and H37Rv ΔcydABcomp (blue triangles). The dotted line represents 90% bacterial killing compared with the untreated control. **Statistical difference (P < 0.001, Student’s t test) in CFU number between H37Rv and H37Rv ΔcydAB treated with Q203. Results are expressed as mean ± SDs. Experiments were performed in triplicate and repeated once.
Fig. S7.
The Cyt-bc1:aa3 and the Cyt-bd are jointly required for ATP homeostasis and survival of nutrient-starved bacillus Calmette–Guérin. ATP levels were quantified in nutrient-starved bacillus Calmette–Guérin (A), bacillus Calmette–Guérin ΔcydAB (B), and bacillus Calmette–Guérin ΔcydABcomp (C) strains treated with a dose-range of Q203 (black circles) or BDQ (black squares). Inset in A depicts the ATP levels in bacillus Calmette–Guérin treated with Q203 at 62.5 nM (Q203) or BDQ at 1,250 nM (BDQ). *Statistical difference (P < 0.01, Student's t test) in ATP level between Q203- and BDQ-treated bacteria. (D) Bactericidal potency of Q203, BDQ, and isoniazid (INH) was evaluated against the strains bacillus Calmette–Guérin (red circles), bacillus Calmette–Guérin ΔcydAB (green squares), and bacillus Calmette–Guérin ΔcydABcomp (blue triangles) after 15 d of incubation. Bacterial counts were determined by CFU determination on nutrient–agar plates. The dotted line represents 90% bacterial killing compared with the untreated control. **Statistical difference (P < 0.001, Student's t test) in CFU number between H37Rv and H37Rv ΔcydAB treated with Q203. Data are expressed as mean ± SDs. The experiments were performed in triplicate and repeated two times.
Synthetic Lethal Interaction Between the Respiratory Terminal Oxidases During Infection.
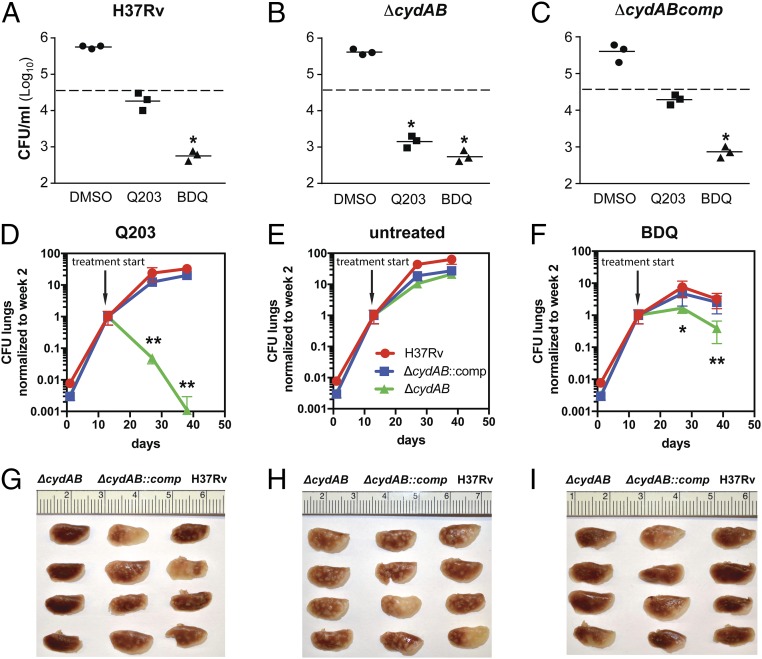
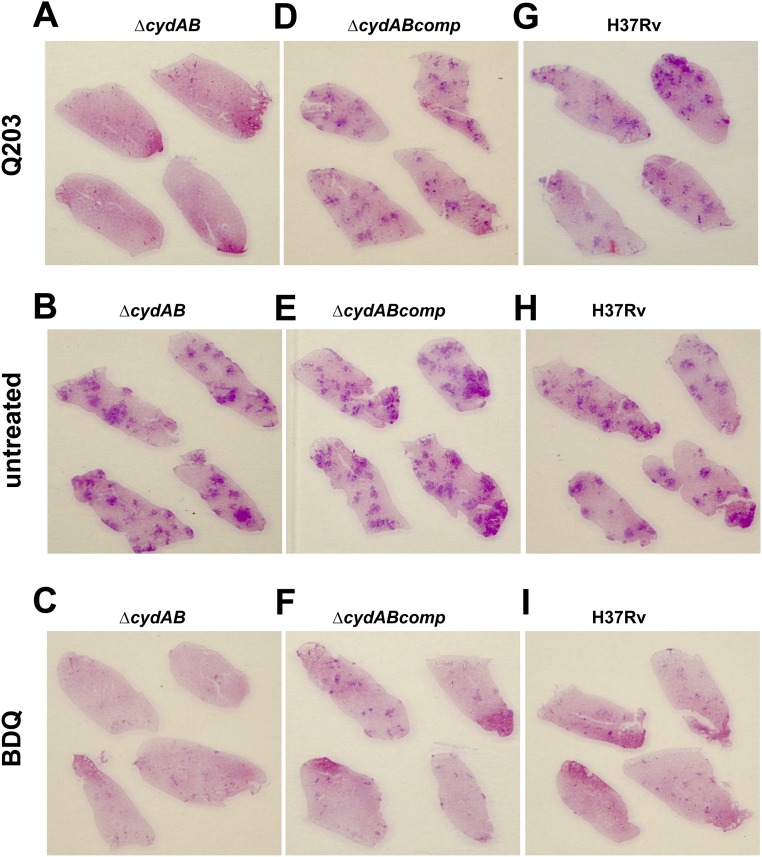
To test whether the synthetic lethal interaction between the Cyt-bc1:aa3 and the Cyt-bd was relevant during infection, the potency of Q203 was evaluated against the H37Rv, H37Rv ∆cydAB, and complemented strains replicating in THP-1 cells. Bacterial viability was evaluated after 5 d of treatment with Q203 or BDQ. Results revealed that the multiplication profile of the H37Rv ∆cydAB strain was comparable to the parental H37Rv strain (Fig. 6 A and B), suggesting that the Cyt-bd alone does not contribute to growth in macrophages. As reported before, Q203 was active against the parental H37Rv strain replicating in macrophages (18, 20). However, the effect of the drug candidate was bacteriostatic (Fig. 6A). In line with the in vitro phenotypes, Q203 was bactericidal against the H37Rv ∆cydAB strain replicating in THP-1 cells (Fig. 6B). BDQ was active against intracellular mycobacteria, regardless of the presence of the Cyt-bd (Fig. 6 A–C). Phenotypes were reverted in the H37Rv ∆cydABcomp strain (Fig. 6C). This result showed that oxygen respiration contributes to the virulence of M. tuberculosis in a macrophage model and that at least one of the terminal oxidases was required for respiration and energy production in an ex vivo infection model. This finding prompted us to investigate the joint essentiality of the terminal oxidases in a mouse model of tuberculosis. BALB/c mice infected by the aerosol route with the H37Rv, ∆cydAB, and ∆cydABcomp strains were treated with Q203 at 2 mg/kg, BDQ at 10 mg/kg, or with the vehicle control three times per week. The H37Rv ∆cydAB strain had no obvious attenuation phenotype during the course of the infection, but was dramatically more sensitive to Q203 compared with the parental H37Rv or ∆cydABcomp strains (Fig. 6 D–F). During the first 2 wk of treatment, Q203 reduced the bacterial load in the lungs of animals infected by the mutant strain by more than 99% (Fig. 6D). During the same time frame, Q203 had no significant efficacy against the parental strain (Fig. 6D). Strikingly, after 4 wk of treatment, the bacterial count in the mice infected by the H37Rv ∆cydAB strain and treated with Q203 had dropped below the limit of detection in 3 out of 5 mice (Fig. 6D). Using this suboptimal dosing regimen, Q203 had no significant effect against the parental H37Rv and the ∆cydABcomp strains (Fig. 6D). Although BDQ was effective against the parental strain, there was still an eightfold increase in sensitivity of the H37Rv ∆cydAB strain compared with the parental strain after 4 wk of BDQ treatment (Fig. 6F). It was interesting to note that the potency of Q203 against the H37Rv ∆cydAB strain was radically superior compared with BDQ (Fig. 6 D and F). Gross lung pathology (Fig. 6 G–I) and H&E staining (Fig. S8) corroborated these results. Lungs infected with H37Rv ∆cydAB and treated with Q203 showed no signs of typical lesions, nor any inflammation foci after 4 wk of treatment (Fig. 6G and Fig. S8A). In contrast, multiple lesions and inflamed foci were found in the lungs of the mice infected with the parental, or the complemented strain, that were treated by Q203 (Fig. 6G and Fig. S8 D and G). These results demonstrate the efficacy of a therapeutic approach that exploits the synthetic lethal interaction between Cyt-bc1:aa3 and Cyt-bd oxidases.
Fig. 6.
The Cyt-bc1:aa3 and Cyt-bd are jointly required for growth in macrophages and for virulence in a mouse model. THP-1 cells were infected with the strains H37Rv (A), H37Rv ΔcydAB (B), and H37Rv ΔcydABcomp (C) and treated with 1% DMSO (vehicle control), Q203, or BDQ. Viability of intracellular mycobacteria was determined after 5 d of treatment. Dotted line, initial bacterial load at 1 h postinfection. *Greater than or equal to 90% reduction in bacterial load compared with the initial bacterial load. The means and SDs of three replicates for each experiment are shown. The experiment was repeated once. BALB/c mice were aerosol-infected with either M. tuberculosis H37Rv (red circles), ∆cydAB (green squares), or ∆cydABcomp (blue triangles). Two weeks after infection, treatment was started by oral administration of Q203 at 2 mg/kg, BDQ at 10 mg/kg, or vehicle control three times a week. Bacillary burden (CFU) in lungs of treated animals was assessed after 2 and 4 wk treatment with either (D) Q203, (E) vehicle, or (F) BDQ. To compare drug efficacy between different strains, CFU counts were normalized to the time of treatment start (day 13 after infection). CFU counts are shown in Table S2. Gross pathology (G, H, I), and H&E staining (Fig. S8) was performed on all lung samples to determine severity of disease and level of inflammation. Error bars represent SDs of at least four replicates. An unpaired Student t test was performed between parental and ∆cydAB CFU counts. *P < 0.05; **P < 0.01.
Fig. S8.
Q203 treatment reduced disease severity and level of inflammation in the lungs of mice infected with the M. tuberculosis ∆cydAB strain. H&E staining was performed in lung sections of animals treated for 4 wk with either Q203 (A, D, and G), vehicle (B, E, and H), or BDQ (C, F, and I).
Table S2.
Mouse infection and treatment with Q203 or BDQ
| H37Rv | ΔcydAB | ΔcydABcomp | |||||
| Treatment | Time (day) | Average (CFU/mL) | SD (CFU/mL) | Average (CFU/mL) | SD (CFU/mL) | Average (CFU/mL) | SD (CFU/mL) |
| Vehicle | 1 | 4.0E+02 | 5.4E+01 | 7.6E+02 | 2.8E+02 | 4.7E+02 | 9.0E+01 |
| 13 | 6.5E+04 | 2.9E+04 | 2.4E+05 | 4.6E+04 | 1.5E+05 | 8.0E+04 | |
| 27 | 2.4E+06 | 7.5E+05 | 2.5E+06 | 7.0E+05 | 2.8E+06 | 1.0E+06 | |
| 38 | 3.3E+06 | 1.2E+06 | 5.1E+06 | 7.1E+05 | 4.0E+06 | 8.8E+05 | |
| BDQ | 1 | 4.0E+02 | 5.4E+01 | 7.6E+02 | 2.8E+02 | 4.7E+02 | 9.0E+01 |
| 13 | 6.5E+04 | 2.9E+04 | 2.4E+05 | 4.6E+04 | 1.5E+05 | 8.0E+04 | |
| 27 | 4.7E+05 | 2.5E+05 | 4.0E+05 | 1.6E+05 | 7.0E+05 | 5.0E+05 | |
| 38 | 2.1E+05 | 9.8E+04 | 9.8E+04 | 7.9E+04 | 3.9E+05 | 2.5E+05 | |
| Q203 | 1 | 4.0E+02 | 5.4E+01 | 7.6E+02 | 2.8E+02 | 4.7E+02 | 9.0E+01 |
| 13 | 6.5E+04 | 2.9E+04 | 2.4E+05 | 4.6E+04 | 1.5E+05 | 8.0E+04 | |
| 27 | 1.3E+06 | 7.0E+05 | 1.2E+04 | 5.0E+03 | 1.7E+06 | 4.5E+05 | |
| 38 | 1.8E+06 | 1.7E+05 | 3.3E+02 | 5.5E+02 | 2.9E+06 | 9.5E+05 | |
BALB/c mice were aerosol-infected with either the M. tuberculosis H37Rv, ΔcydAB, or ΔcydABcomp strains. Treatment with Q203, BDQ, or with the vehicle control was initiated 14 d postinfection. Bacillary burden (CFU) in lungs of infected animals was assessed after 2 wk (day 27) and 4 wk (day 38) of drug treatment. Bacterial burden was also assessed at day 1 and day 13 postinfection to confirm bacterial colonization before drug treatment. The average of four mice per time point and per condition is shown.
Discussion
M. tuberculosis is an obligate aerobe that can survive, but not replicate, under hypoxic conditions. The reasons for the strict dependence on oxygen for growth are poorly understood but illustrate the prominence of aerobic respiration and the terminal respiratory oxidases for the biology of this bacterium (11). In the past 10 y the discovery of drugs active against enzymes of the mycobacterial oxidative phosphorylation pathway, namely, inhibitors of ATP-synthase (BDQ) and Cyt-bc1:aa3 (imidazopyridine amides), have confirmed this vulnerability. Here, we show that rapid killing and bactericidal activity against M. tuberculosis can be achieved by exploiting the synthetic lethal interaction between the two terminal oxidases of the electron transport chain.
Synthetic lethal relationships likely arise in biological systems to create functional redundancies that mitigate the impact of loss-of-function mutations or inhibition of a single enzyme. The presence of two terminal respiratory oxidases is a perfect example of such a functional redundancy. In this study, we confirmed that chemical inhibition of the Cyt-bc1:aa3 branch by Q203 inhibited mycobacterial growth at a very low dose, but revealed that the drug candidate was not bactericidal even at a concentration 200-fold in excess of its MIC50. We demonstrated that respiration through the alternate Cyt-bd terminal oxidase alone is sufficient to maintain mycobacterial viability but insufficient to sustain growth. This discrepancy is likely due to a difference in energetic efficiency of the two terminal oxidases as the Cyt-bc1:aa3 complex pumps 6 protons per 2 electrons (H+/e− ratio of 3), whereas the ratio is only 1 H+/e− for Cyt-bd (11, 26, 27), and this might also explain the failure to isolate deletion mutants of genes that encode Cyt-bc1:aa3 in M. tuberculosis (28, 29). As a logical consequence of the functional redundancy, deletion of cydAB led to hypersusceptibility to Q203 with complete inhibition of oxygen consumption, an enhanced effect on ATP homeostasis, and bactericidal action at low dose against replicating and nonreplicating mycobacteria. Importantly, our results show that oxygen respiration is essential for the survival of nutrient-starved, phenotypic drug-resistant mycobacteria, validating avenues for drug development. Under the in vitro conditions used in this study, the presence of the Cyt-bd did not influence the potency of BDQ. A recent report demonstrated that the early killing rate of BDQ is enhanced in an M. tuberculosis ∆cydA strain (12). Our results are not necessarily in contradiction because in the present study, the bactericidal potency of BDQ was determined at only one late time point. The observation that the H37Rv ∆cydAB strain has a slight, yet significant increase in sensitivity to BDQ compared with the parental strain in the mouse model supports the previous observation (12). The most critical finding of this study is the rapid clearance of the H37Rv ∆cydAB strain by Q203 in a mouse model of tuberculosis. After 4 wk of treatment with Q203 at only 2 mg/kg, near-eradication of H37Rv ∆cydAB was achieved, whereas the same drug treatment had no significant effect against the parental strain. This result illustrates the powerful synthetic lethal interaction between both terminal oxidases and demonstrates that, at least in the microenvironment of the mouse lung, M. tuberculosis relies primarily on oxygen respiration to multiply and persist.
The synthetic lethal interaction between the Cyt-bc1:aa3 and the Cyt-bd could have consequences for the clinical development of Q203. Because the electron flow through the Cyt-bd is sufficient to maintain respiration and viability of Q203-treated mycobacteria, it is uncertain if drug candidates targeting the Cyt-bc1:aa3 will show efficacy in humans. An important characteristic of human tuberculosis disease is the manifestation of a range of lesions with different microenvironmental conditions, including varying oxygen tensions (30). The expression ratio of Cyt-bc1:aa3 and Cyt-bd is likely to play an integral role in the adaptation to this heterogeneity, further underlining the importance of a combination therapy targeting both terminal oxidases. It is possible that Q203, or other advanced derivatives, will be active against human tuberculosis when used in a combination drug therapy. However, based on the data presented here, it is unlikely that inhibition of the Cyt-bc1:aa3 alone would lead to bacterial sterilization under all physiological conditions. To unleash the full potential of drugs targeting the Cyt-bc1:aa3 branch, we advocate for the development of Cyt-bd inhibitors. It was previously suggested that interference with oxidative phosphorylation at multiple levels is a promising anti-TB strategy (24). Our data indicate that a drug combination targeting simultaneously the Cyt-bc1:aa3, the Cyt-bd, and the F1Fo-ATP synthase may represent the cornerstone of a complementary sterilizing drug combination for the treatment of MDR and XDR tuberculosis.
Materials and Methods
Strains and Growth Conditions.
M. tuberculosis H37Rv, derivative strains, and clinical isolates (25) were maintained in Middlebrook 7H9 broth medium supplemented with 0.2% glycerol, 0.05% Tween 80, and 10% ADS supplement. Hygromycin (75 μg/mL) or kanamycin (20 μg/mL) were used when required. Glycerol was omitted to determine drug potency. THP-1 cells were maintained in RPMI medium 1640 supplemented with 10% FBS, 2 mM l-glutamine, 10 mM sodium pyruvate, and kanamycin (50 µg/mL).
MIC50 and MBC90 Determination.
In this study, MIC50 was defined as the lowest concentration of compound that inhibited bacterial growth by 50%. MIC50 was determined by the broth microdilution method using a 96-well flat-bottom plate as described before (31). For MBC90 determination, mycobacterial inoculum adjusted at an OD600 of 0.005 was incubated in the presence of drugs for 10 d (replicating bacteria) or 15 d (nonreplicating mycobacteria) at 37 °C. Bacterial viability was determined by Colony Forming Units (CFUs) determination on agar plate. The Minimum Bactericidal Concentration leading to 90% reduction in CFU was defined as the MBC90.
Intracellular ATP quantification.
The intracellular ATP level was determined with the BacTiter-Glo Microbial Cell Viability Assay (Promega) (10).
Nutrient-Starved Culture.
Exponentially growing cultures of M. tuberculosis were harvested by centrifugation and washed twice with prewarmed DPBS (Thermo Fisher Scientific) supplemented with Ca2+, Mg 2+, and 0.025% Tween 80. Cell density was adjusted to OD600 of 0.15 and incubated for 2 wk at 37 °C before testing sensitivity to drugs.
Gene Knockout and Complementation.
Two sets of cydAB (Rv1623c-1622c) deletion strains were constructed independently in the K.P. laboratory and in the M.B. laboratory using similar strategies based on the use of the plasmid pYUB1471 (32). In the K.P. laboratory, the pYUB1471 containing the 5′ and 3′ flank of the cydAB locus was UV-irradiated (33) before electroporation into M. tuberculosis, whereas in the M.B. laboratory, specialized transduction was used as described previously (32). Complementation plasmids were created by either incorporating the cydABDC operon and its native promoter (330 bp upstream of the coding region) into the pMV306 vector (34) via Gibson cloning (35) (New England Biolabs), resulting in plasmid pMV306-cydABDC, or by cloning the cydAB genes in the pMV306 plasmid under the control of the hsp60 promoter, resulting in the plasmid pMV306-cydAB.
THP-1 Infection Model.
THP-1 cells were treated with 200 nM phorbol myristate acetate and were distributed at a density of 3 × 106 cells per well in 24-well plates. After 24 h of differentiation, the cell monolayers were infected with M. tuberculosis at a multiplicity of infection of 10 for 60 min. Prewarmed complete RPMI medium with or without the test drugs was added. Q203 was used at 250 nm, whereas BDQ was used at 1,000 nM. Mycobacterial viability was determined after 5 d of infection by CFU determination on agar plates.
Mouse Experiments and Pathology.
Mouse studies were performed in accordance with the National Institutes of Health guidelines following the recommendations in the Guide for the Care and Use of Laboratory Animals (36). The protocols used in this study were approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine (Protocol #20150208). Female BALB/c mice (The Jackson Laboratory) were infected via aerosol infection at a dose intended to yield an infection of 103 CFU per mouse. Drug dosing was initiated 13 d postinfection. Drugs were formulated in 20% d-α-Tocopherol polyethylene glycol 1000 succinate (TPGS) per 1% DMSO and administered via gavage three times per week. Infection in the lung was determined by CFU determination on agar plates at 13, 27, and 38 d. For pathological analysis and histological staining, lung samples were fixed in 10% (vol/vol) neutral formalin, paraffin embedment, and the tissues were sectioned at 5 µm. Sections were either stained with Hematoxylin & Eosin, or using the Kinyoun method for acid-fast bacilli.
Oxygen Consumption Assays.
Oxygen consumption in whole bacteria was measured using methylene blue or the MitoXpress Xtra–Oxygen Consumption Assay (Luxcel Biosciences).
Methylene blue-based assay. Mycobacteria culture adjusted to an OD600 of 0.3 were preincubated for 4 h in 2-mL screw-cap tubes in the presence of Q203 at 400 nM, BDQ at 500 nM, or 1% DMSO (vehicle control). Methylene blue at 0.001% was added to each tube. The tubes were then tightly sealed, an incubated in an anaerobic jar to avoid oxygen leak.
MitoXpress-based assay. The assay was performed in black 96-well plates (flat, clear bottom). One hundred fifty microliters of mycobacteria culture adjusted to an OD600 of 0.3 were preincubated for 6 h in the presence of Q203, BDQ, or 1% DMSO. Ten microliters of the MitoXpress oxygen probe was added to each well that was covered with a layer of high-sensitivity mineral oil to restrict oxygen back diffusion. Fluorescence (Ex: 380 nm, Em: 650 nm) was recorded on a BioTeK CYTATION 3 multimode reader.
SI Methods
Preparation of Inverted Membrane Vesicles.
Bacillus Calmette–Guérin was grown at 37 °C in 7H9-ADS medium. Bacteria were harvested by centrifugation when the cultures reached an OD600 of 0.8. Five grams of cells (wet weight) were suspended in 20 mL of 50 mM Mops-NaOH (pH 7.5), 2 mM MgCl2 supplemented with protease inhibitors (protease inhibitor mixture tablets, Roche). Lysozyme (1.2 mg/mL), 1,500 units of DNase I (Sigma), and 6.7 mM MgCl2 were added, and the cells were incubated under stirring conditions for 45 min at room temperature. The bacteria were then lysed by five passages using a precooled French pressure cell at 25,000 psi (M-110L, Microfluidiser). The lysate was centrifuged at 4,200 g at 4 °C for 20 min to remove unbroken bacteria. The supernatant was ultracentrifuged at 450,000 g for 1 h at 4 °C. The pellet of Inverted Membrane Vesicles (IMVs) was resuspended in an appropriate volume of 50 mM Mops-NaOH (pH 7.5), 2 mM MgCl2, and 15% glycerol. Protein estimation was performed using the BCA Protein assay (Thermo Fisher Scientific).
Oxygen Consumption Assay.
The MitoXpress oxygen probe was used to quantify oxygen consumption in IMVs. The IMVs (150 µL of 300 µg/mL) were preincubated for 5 min with a dose range of Q203 or bedaquiline in a prewarmed 50 mM Mes buffer (pH 6.5) supplemented with 2 mM MgCl2. NADH was added at a final concentration of 1 mM as electron donor. Ten microliters of the MitoXpress oxygen probe were added to each well and covered with a layer of high-sensitivity mineral oil. Fluorescence (Excitation: 380 nm, Emission: 650 nm) was recorded after 30 min of incubation using a BioTeK CYTATION 3 multimode reader.
Acknowledgments
We thank Sebastien Gagneux for the gift of the M. tuberculosis clinical isolates, Mei Chen and John Kim for technical support, and William R. Jacobs for phasmids and access to infrastructure. This research is supported by the Singapore Ministry of Health's National Medical Research Council under its Cooperative Basic Research Grant (Project Award NMRC/CBRG/0083/2015), and the Lee Kong Chian School of Medicine, Nanyang Technological University Start-Up Grant (to K.P.). M.B. and E.J.H. were financially supported by NIH Grants AI119573 and T32-GM007288, respectively. K.H. and G.M.C. were financially supported by the Marsden Fund, Royal Society, New Zealand.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706139114/-/DCSupplemental.
References
- 1.WHO . Global Tuberculosis Report 2016. WHO; Geneva: 2016. [Google Scholar]
- 2.Arinaminpathy N, et al. The number of privately treated tuberculosis cases in India: An estimation from drug sales data. Lancet Infect Dis. 2016;16:1255–1260. doi: 10.1016/S1473-3099(16)30259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anonymous (2016) WHO Treatment Guidelines for Drug-Resistant Tuberculosis, 2016 Update (WHO, Geneva) [PubMed]
- 4.Cole ST. Tuberculosis drug discovery needs public-private consortia. Drug Discov Today. 2017;22:477–478. doi: 10.1016/j.drudis.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Andries K, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 6.Diacon AH, et al. TMC207-C208 Study Group Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med. 2014;371:723–732. doi: 10.1056/NEJMoa1313865. [DOI] [PubMed] [Google Scholar]
- 7.Pym AS, et al. TMC207-C209 Study Group Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. Eur Respir J. 2016;47:564–574. doi: 10.1183/13993003.00724-2015. [DOI] [PubMed] [Google Scholar]
- 8.Bloemberg GV, et al. Acquired resistance to Bedaquiline and Delamanid in therapy for tuberculosis. N Engl J Med. 2015;373:1986–1988. doi: 10.1056/NEJMc1505196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koul A, et al. Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis. J Biol Chem. 2008;283:25273–25280. doi: 10.1074/jbc.M803899200. [DOI] [PubMed] [Google Scholar]
- 10.Rao SP, Alonso S, Rand L, Dick T, Pethe K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2008;105:11945–11950. doi: 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook GM, Hards K, Vilchèze C, Hartman T, Berney M. Energetics of respiration and oxidative phosphorylation in Mycobacteria. Microbiol Spectr. 2014;2:MGM2-0015-2013. doi: 10.1128/microbiolspec.MGM2-0015-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berney M, Hartman TE, Jacobs WR., Jr A Mycobacterium tuberculosis cytochrome bd oxidase mutant is hypersensitive to bedaquiline. MBio. 2014;5:e01275–e14. doi: 10.1128/mBio.01275-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook GM, et al. Physiology of mycobacteria. Adv Microbial Physiol. 2009;55:81-182, 318-189. doi: 10.1016/S0065-2911(09)05502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook GM, Greening C, Hards K, Berney M. Energetics of pathogenic bacteria and opportunities for drug development. Adv Microb Physiol. 2014;65:1–62. doi: 10.1016/bs.ampbs.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Poole RK, Cook GM. Redundancy of aerobic respiratory chains in bacteria? Routes, reasons and regulation. Adv Microb Physiol. 2000;43:165–224. doi: 10.1016/s0065-2911(00)43005-5. [DOI] [PubMed] [Google Scholar]
- 16.Abrahams KA, et al. Identification of novel imidazo[1,2-a]pyridine inhibitors targeting M. tuberculosis QcrB. PLoS One. 2012;7:e52951. doi: 10.1371/journal.pone.0052951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arora K, et al. Respiratory flexibility in response to inhibition of cytochrome C oxidase in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2014;58:6962–6965. doi: 10.1128/AAC.03486-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang S, et al. Lead optimization of a novel series of imidazo[1,2-a]pyridine amides leading to a clinical candidate (Q203) as a multi- and extensively-drug-resistant anti-tuberculosis agent. J Med Chem. 2014;57:5293–5305. doi: 10.1021/jm5003606. [DOI] [PubMed] [Google Scholar]
- 19.Moraski GC, et al. Advent of Imidazo[1,2-a]pyridine-3-carboxamides with potent multi- and extended drug resistant antituberculosis activity. ACS Med Chem Lett. 2011;2:466–470. doi: 10.1021/ml200036r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pethe K, et al. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat Med. 2013;19:1157–1160. doi: 10.1038/nm.3262. [DOI] [PubMed] [Google Scholar]
- 21.Rybniker J, et al. Lansoprazole is an antituberculous prodrug targeting cytochrome bc1. Nat Commun. 2015;6:7659. doi: 10.1038/ncomms8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. ClinicalTrials.gov (2015) A dose-escalation study to evaluate safety, tolerability and pharmacokinetics of single doses of Q203 in normal healthy, male and female volunteers. Available at https://clinicaltrials.gov/ct2/show/NCT02530710. Accessed April 10, 2017.
- 23.Zimmermann GR, Lehár J, Keith CT. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discov Today. 2007;12:34–42. doi: 10.1016/j.drudis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Lamprecht DA, et al. Turning the respiratory flexibility of Mycobacterium tuberculosis against itself. Nat Commun. 2016;7:12393. doi: 10.1038/ncomms12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose G, et al. Mapping of genotype-phenotype diversity among clinical isolates of mycobacterium tuberculosis by sequence-based transcriptional profiling. Genome Biol Evol. 2013;5:1849–1862. doi: 10.1093/gbe/evt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunte C, Palsdottir H, Trumpower BL. Protonmotive pathways and mechanisms in the cytochrome bc1 complex. FEBS Lett. 2003;545:39–46. doi: 10.1016/s0014-5793(03)00391-0. [DOI] [PubMed] [Google Scholar]
- 27.Zara V, Conte L, Trumpower BL. Biogenesis of the yeast cytochrome bc1 complex. Biochim Biophys Acta. 2009;1793:89–96. doi: 10.1016/j.bbamcr.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Matsoso LG, et al. Function of the cytochrome bc1-aa3 branch of the respiratory network in mycobacteria and network adaptation occurring in response to its disruption. J Bacteriol. 2005;187:6300–6308. doi: 10.1128/JB.187.18.6300-6308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 30.Barry CE, 3rd, et al. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pethe K, et al. A chemical genetic screen in Mycobacterium tuberculosis identifies carbon-source-dependent growth inhibitors devoid of in vivo efficacy. Nat Commun. 2010;1:57. doi: 10.1038/ncomms1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain P, et al. Specialized transduction designed for precise high-throughput unmarked deletions in Mycobacterium tuberculosis. MBio. 2014;5:e01245–e14. doi: 10.1128/mBio.01245-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinds J, et al. Enhanced gene replacement in mycobacteria. Microbiology. 1999;145:519–527. doi: 10.1099/13500872-145-3-519. [DOI] [PubMed] [Google Scholar]
- 34.Stover CK, et al. New use of BCG for recombinant vaccines. Nature. 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 35.Gibson DG. Synthesis of DNA fragments in yeast by one-step assembly of overlapping oligonucleotides. Nucleic Acids Res. 2009;37:6984–6990. doi: 10.1093/nar/gkp687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anonymous (Committee on Care and Use of Laboratory Animals) (1996) Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No. 85-23.