Dong and Zhao (1) attempt to provide perspective on our use of Raman spectroscopy in plant stress studies (2). Unfortunately, their experimental criticism is incorrect and their technical suggestions won’t work. The following points support these strong statements.
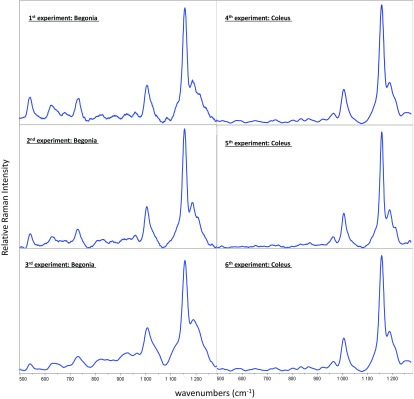
Dong and Zhao claim that Raman spectroscopy is limited by its “poor repeatability” (1). Our experimental data speak for themselves, as shown in Fig. 1. The spectra are remarkably consistent between experiments and across species, even though the red-leaved Begonias (Fig. 1) had higher levels of anthocyanins initially.
Fig. 1.
Texas A&M University data are shown reproducing Raman spectra from six separate experiments using Begonia and Coleus plant species.
After incorrectly criticizing the reproducibility of Raman spectra, Dong and Zhao (1) proceed to criticize our choice of spectroscopic lines, saying: “The Raman spectral features used in studies of Altangerel et al. … differ from those used in other studies. The most commonly used Raman peak for carotenoid measurement is 1,525 cm−1… whereas Altangerel et al. used much weaker peaks at 1,007 and 1,157 cm−1. For the determination of anthocyanins, previous studies have generally used the spectral features between 1,300 and 1,650 cm−1.”
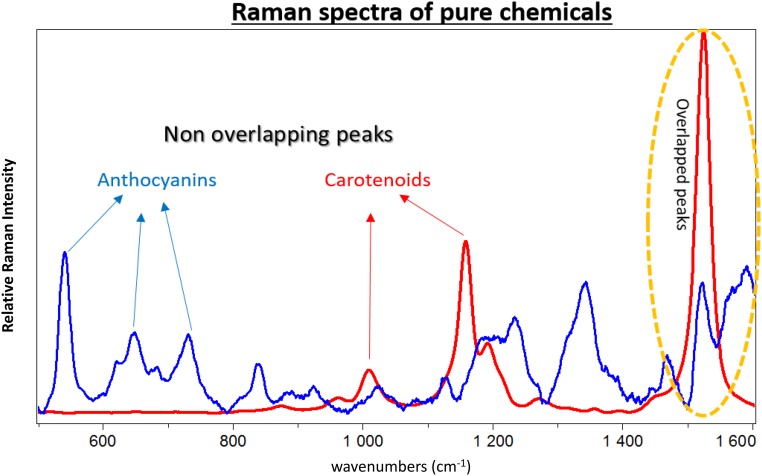
Dong and Zhao (1) do not understand our experiment. The Raman peak at 1,525 cm−1 is strong, but cannot be used for our purposes involving simultaneous detection of two molecules (2). We cannot use these peaks because anthocyanin’s 1,516–1,545 cm−1 peaks overlap with carotenoids’ 1,525 cm−1. We chose distinct nonoverlapping peaks for each molecule. Fig. 2 shows the Raman spectra of pure anthocyanin and carotenoid. (The anthocyanin peaks between 1,250 and 1,450 cm−1 overlapped with chlorophyll and fatty acid peaks, and therefore were not of interest for this study.)
Fig. 2.
Raman spectra of pure β-carotene (red) and pure callistephin chloride (blue).
Dong and Zhao (1) then proceed to make misleading suggestions concerning surface-enhanced Raman spectroscopy (SERS). The authors say: “Second, we strongly suggest the use of surface-enhanced Raman spectra to enhance the sensitivity of common Raman spectroscopy … The spraying of nanoparticles onto the plant surface will not affect on-site measurements in phenotyping” (1).
The Texas A&M University group has a substantial and successful experience in SERS (3) and the notion of “spraying nanoparticles” on leaves to enhance sensitivity is physically misguiding and biologically off base.
Physically, the advantage of SERS comes from, e.g., “hot spots” between nanoparticles. Thus, it takes a high density of nanoparticles to see SERS. But biologically, nanoparticles have been shown to have toxic effects in live plants and they can bind with molecules resulting in chemical changes in the plant (4).
The bottom line is that we disagree with the criticisms and suggestions of Dong and Zhao (1). Our paper (2) stands as written.
Footnotes
The authors declare no conflict of interest.
References
- 1.Dong D, Zhao C. Limitations and challenges of using Raman spectroscopy to detect the abiotic plant stress response. Proc Natl Acad Sci USA. 2017;114:E5486–E5487. doi: 10.1073/pnas.1707408114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altangerel N, et al. In vivo diagnostics of early abiotic plant stress response via Raman spectroscopy. Proc Natl Acad Sci USA. 2017;114:3393–3396. doi: 10.1073/pnas.1701328114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voronine DV, et al. Time-resolved surface-enhanced coherent sensing of nanoscale molecular complexes. Sci Rep. 2012;2:891. doi: 10.1038/srep00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ray PC, Yu H, Fu PP. Toxicity and environmental risks of nanomaterials: Challenges and future needs. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:1–35. doi: 10.1080/10590500802708267. [DOI] [PMC free article] [PubMed] [Google Scholar]