Abstract
Annexin 2 is a calcium-dependent phospholipid-binding protein that has been implicated in a number of membranerelated events, including regulated exocytosis. In chromaffin cells, we previously reported that catecholamine secretion requires the translocation and formation of the annexin 2 tetramer near the exocytotic sites. Here, to obtain direct evidence for a role of annexin 2 in exocytosis, we modified its expression level in chromaffin cells by using the Semliki Forest virus expression system. Using a real-time assay for individual cells, we found that the reduction of cytosolic annexin 2, and the consequent decrease of annexin 2 tetramer at the cell periphery, strongly inhibited exocytosis, most likely at an early stage before membrane fusion. Secretion also was severely impaired in cells expressing a chimera that sequestered annexin 2 into cytosolic aggregates. Moreover, we demonstrate that secretagogue-evoked stimulation triggers the formation of lipid rafts in the plasma membrane, essential for exocytosis, and which can be attributed to the annexin 2 tetramer. We propose that annexin 2 acts as a calcium-dependent promoter of lipid microdomains required for structural and spatial organization of the exocytotic machinery.
INTRODUCTION
Annexins form an evolutionary conserved multigene family of proteins with members being expressed throughout the plant and animal kingdoms. The common characteristic of annexins is that they bind to negatively charged phospholipids in biological membranes in a Ca2+-dependent manner (for review, see Creutz, 1992; Gerke and Moss, 2002). As such, annexins have been implicated in various membrane trafficking events, including exocytosis, endocytosis, and cell-to-cell adhesion (Lecat and Lafont, 1999). The most compelling evidence for involvement in calcium-regulated exocytosis has been reported for annexin 2. Endogenous annexin 2 exists in part as a soluble monomer, p36, and in part as a heterotetrameric complex, p90, with its specific ligand the S100A10 protein also called p11 (Schafer and Heizmann, 1996). When complexed, the central S100A10 dimer links two annexin 2 chains in a highly symmetrical manner, creating a scaffold that can bridge opposing membrane surfaces (Lambert et al., 1997; Rety et al., 1999; Lewit-Bentley et al., 2000). Quick-freeze, deep-etch electron microscopic analysis has documented that annexin 2 forms cross-links between secretory granules and the plasma membrane in stimulated neuroendocrine cells (Nakata et al., 1990; Senda et al., 1994). In chromaffin cells, we (Sarafian et al., 1991) and others (Ali et al., 1989) have identified annexin 2 as one of the cytosolic proteins that can retard the rundown of secretory responsiveness to Ca2+ stimulation of permeabilized cells when added exogenously as a purified protein. In our assay, the tetrameric complex was more efficient than the monomeric annexin 2 protein, and phosphorylation by protein kinase C (PKC) was required (Sarafian et al., 1991). More recently, we demonstrated that a synthetic peptide corresponding to an NH2-terminal annexin 2 sequence containing the PKC phosphorylation site inhibits catecholamine secretion in response to nicotine when microinjected into chromaffin cells (Chasserot-Golaz et al., 1996). Together, these results strongly suggested, but did not prove, that annexin 2 plays an important role in calcium-regulated exocytosis. Hence, the functional implication of annexin 2 in exocytosis remains a controversial issue, because a peptide competing for the interaction of annexin 2 with p11 has no effect on secretion in permeabilized chromaffin cells (Ali and Burgoyne, 1990), although it significantly reduced Ca2+-triggered exocytotic membrane incorporation in endothelial cells (Konig et al., 1998). Moreover, expression of a chimeric protein that leads to the formation of cytosolic annexin 2 aggregates does not affect secretion in PC12 cells, another argument against the participation of annexin 2 in exocytosis (Graham et al., 1997).
In the present study, using a variety of direct means, we have revisited the role of annexin 2 in calcium-regulated exocytosis. Using chromaffin cells deficient in endogenous annexin 2, we demonstrate that the presence of annexin 2 at the cell periphery is a prerequisite for the docking and subsequent fusion of secretory granules with the plasma membrane. Our results suggest that the translocation of annexin 2 to the plasma membrane favors the formation of lipid microdomains that are required for granule exocytosis.
MATERIALS AND METHODS
Chromaffin Cells and [3H]Noradrenaline Release
Chromaffin cells were isolated from fresh bovine adrenal glands by retrograde perfusion with collagenase, purified on self-generating Percoll gradients, and maintained in culture as described previously (Bader et al., 1986). Catecholamine stores were labeled by incubation of cultured chromaffin cells with [3H]noradrenaline (13.3 Ci/mmol; Amersham Biosciences, Les Ulis, France) for 45 min. To trigger exocytosis, chromaffin cells were washed twice with Locke's solution (140 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 11 mM glucose, 0.56 mM ascorbic acid, and 15 mM HEPES, pH 7.2) and then stimulated 5 min with Locke's solution containing either 10 μM nicotine or 59 mM K+ (made by decreasing NaCl isosmotically). Permeabilization of chromaffin cells with streptolysin-O (SLO; Institut Pasteur, Paris, France) was performed as described previously (Sarafian et al., 1991). Briefly, cells were washed with calcium-free Locke's solution (containing 1 mM EGTA) and then permeabilized for 2 min at 37°C with streptolysin-O (18 U/ml) in 200 μl of calcium-free permeabilizing medium (150 mM glutamate, potassium salt, 10 mM PIPES, 5 mM nitrilotriacetic acid [NTA], 0.5 mM EGTA, 0.2% bovine serum albumin [BSA], 5 mM Mg-ATP, and 4.5 mM magnesium). Secretion was induced for 5 min with permeabilizing media containing 50 μM free calcium concentration. [3H]Noradrenaline release after stimulation was determined by measuring the radioactivity present in the incubation medium and in cells after precipitation with 10% (wt/vol) trichloroacetic acid (TCA). The amount of released [3H]noradrenaline is expressed as a percentage of total radioactivity present in the cells before stimulation.
Construction and Expression of Viral Vectors
Construction of pSFV1EGFPsubX was described previously (Knight, 1999). The first (natural) promoter is followed by enhanced green fluorescent protein (EGFP) and the second pSFV1 internal promoter by BamHI and SmaI cloning sites. For sense and antisense constructs, the fragment 9–970 of annexin 2 was ligated in-frame in sense and antisense orientation into BamHI- and SmaI-digested pSFV1EGFPsubX. The chimeric XM construct corresponding to the 54 first base of annexin 2 fused to the entire p11 sequence was generated as described previously (Harder and Gerke, 1993; Harder et al., 1993). The recombinant viral vectors and the SFV Helper 2 vector were linearized with SpeI, transcribed, and transfected into 107 baby hamster kidney (BHK) cells essentially as described previously (Liljestrom and Garoff, 1991). After 24 h, the virus was harvested, concentrated by centrifugation on sucrose gradient, and suspended in OptiMEM containing 0.2% fetal calf serum (FCS). The viral stocks were aliquoted and stored at –80°C. Helper 2-packadged recombinant viruses were activated by α-chymotrypsin digestion (1/20) for 30 min at room temperature, followed by aprotinin inactivation of α-chymotrypsin. The titer of viral stocks was determined by infecting BHK cells and counting the number of cells expressing GFP, under conditions of single virus infection (i.e., <10% of BHK cells infected). The titer of the viral stocks was typically in the order of 107 infectious units. Chromaffin cells on coated glass coverslips in 24-well plates were routinely infected in 0.4 ml of OptiMEM containing 0.2% FCS and cells on 3-cm plates with 1 ml of solution containing 10 infectious units per cell. Cells were used for functional studies between 24 and 48 h after infection to detect EGFP-expressing cells.
Electrochemical Measurement of Catecholamine Secretion from Single Chromaffin Cells
Cells cultured on 35-mm plates at a density of 7.5 × 105 cells/plate were washed with ascorbate-free Locke's solution and placed on the stage of an inverted microscope. A carbon fiber electrode was positioned in tangent contact with a single chromaffin cell by using a three-dimensional micromanipulator (Narishige, Tokyo, Japan). Catecholamine secretion was evoked by applying nicotine (100 μM) in ascorbate-free Locke's solution for 5 s to single cells by means of a glass micropipette (Femtotips; Eppendorf, Hamburg, Germany), and the amperometric response was measured as described previously (Vitale et al., 2001). The amplitude of secretion was quantified by measuring the area below the current curve by using the MacLab system.
Immunoblotting, Immunofluorescence, and Confocal Microscopy
One-dimensional SDS-gel electrophoresis was performed on 10% acrylamide gels in Tris-glycine buffer. The proteins were transferred to nitrocellulose sheets at a constant current of 120 mA for 1 h. Blots were developed using secondary antibodies coupled to horseradish peroxidase (HRP) (Amersham Biosciences), and the immunoreactive bands were detected using the enhanced chemiluminescence (ECL) system (Amersham Biosciences). For immunocytochemistry, chromaffin cells on coated glass coverslips were fixed as described previously (Chasserot-Golaz et al., 1996). GM1 labeling was performed on live chromaffin cells incubated 5 min with 8 μg/ml fluorescent cholera toxin B subunit (coupled with Alexa 488 or Alexa 598; Molecular Probes, Eugene, OR) in Locke's solution with or without 10 μM nicotine. Cells were then processed for immunofluorescence labeling (Chasserot-Golaz et al., 1996). The transient accessibility of dopamine β-hydroxylase (DBH) on the plasma membrane of stimulated chromaffin cells was tested by incubating cells for 5 min in Locke's solution containing 10 μM nicotine in the presence of anti-DBH antibodies diluted to 1:50. Staining for F-actin was performed with tetramethylrhodamine B isothiocyanate-conjugated phalloidin (0.5 μg/ml; Sigma-Aldrich, St. Louis, MO) for 15 min in the dark at room temperature.
Stained cells were visualized using a Zeiss confocal microscope LSM 510. Using the Zeiss CLSM instrument software 2.8, the amount of cholera toxin associated with the plasma membrane or the amount of phalloidin detected in the cell was measured and expressed as the average fluorescence intensity normalized to the corresponding surface area and divided by the total surface of each cell. This allows a quantitative cell-to-cell comparison of the fluorescence detected in cells.
Antibodies
Rabbit polyclonal antibodies raised against annexin 2 (p36) purified from bovine aorta were used at 1:200 dilution (generous gift from J. C. Cavadore, Institut National de la Santé et de la Recherche Médicale U-249, Montpellier, France). Mouse monoclonal antibodies against XM (H21) were used at a 1:5 dilution (Osborn et al., 1988; Harder and Gerke, 1993). Mouse monoclonal antibodies against p11 were used at a 1:50 dilution (BD Transduction Laboratories Lexington, KY). Rat polyclonal antibodies against DBH (EC.1.14.17.1) were used at a 1:50 dilution to specifically label secretory granules in chromaffin cells (Pollard et al., 1982; Perrin and Aunis, 1985). Rabbit polyclonal anti-chromogranin A antibodies were prepared in our laboratory (Ehrhart et al., 1986) and used at a 1:2000 dilution. Mouse monoclonal antibodies anti-synaptotagmine (mAb1D12) were used at a 1:200 dilution (generous gift from Dr. M Takahashi, Mitsubishi Kasei Institute of Life Sciences, Machida, Tokyo, Japan). Monoclonal anti-SNAP-25 antibodies were used at a 1:5000 dilution (Sternberger Monoclonals, Lutherville, MD). Mouse monoclonal antibodies anti-flotillin were used at a 1:500 dilution (BD Transduction Laboratories Lexington). Mouse monoclonal antibodies anti-transferrin receptor was used at a 1:1000 dilution (Zymed Laboratories, South San Francisco, CA). Cy2-antimouse, Cy3-anti-rabbit, and Cy5-anti-mouse were obtained from Amersham Biosciences.
Subcellular Fractionation
For subcellular fractionation, cultured chromaffin cells were collected and homogenized in 0.32 M sucrose, 10 mM Tris-HCl, pH 7.4, and then centrifuged at 800 × g for 15 min. The supernatant (cell lysate) was further centrifuged at 20 000 × g for 20 min. The 20,000-g pellet containing the crude membrane fraction was resuspended in sucrose 0.32 M (10 mM Tris-HCl, pH 7.4), layered on a continuous sucrose density gradient (1–2.2 M sucrose, 10 mM Tris-HCl, pH 7.4), and centrifuged for 90 min at 100,000 × g. Twelve 1-ml fractions were collected from the top to the bottom and analyzed for protein content by the Bradford procedure. The distribution of SNAP-25 (plasma membrane marker) and chromogranin A (chromaffin granule marker) was estimated in fractions 2–12 (40 μg of protein per fraction) by SDS-PAGE and immunoblotting. GM1 was detected in the cell lysate (Ly), the crude membrane fraction (CM), in fractions 2–3 containing plasma membranes (PMs) and in fractions 8–10 containing chromaffin granules (GMs). Two microliters of each of these fractions prepared from resting and nicotine-stimulated cells was dot blotted onto nitrocellulose filter strips, incubated with HRP-conjugated cholera toxin (10 ng/ml; Sigma-Aldrich), and visualized by ECL.
In some experiments, chromaffin cells were stimulated for 10 min with nicotine and then rapidly scrapped in 2 ml of 150 mM glutamate, potassium salt, 10 mM PIPES, pH 7.2, 5 mM NTA, 0.5 mM EGTA, 0.2% BSA, 5 mM Mg-ATP, 4.5 mM MgCl2, and 1 mM CaCl2, and centrifuged for 15 min at 100,000 × g. The pellet containing the crude membranes was homogenized in 500 μl of the same buffer with or without 30 μM filipin, further incubated for 30 min at 0°C, and then centrifuged for 15 min at 100,000 × g. Pellets and supernatants were solubilized in SDS-sample buffer, and the presence of p36 and p11 proteins was analyzed by electrophoresis and immunoblotting.
Flotation Gradient
Chromaffin cells on 10-cm dishes (5 × 106 cells) were washed in Locke's solution and stimulated with 10 μM nicotine for 10 min. Cells were lysed in 300 μl of TNE (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1 mM dithiothreitol, and CLAP protease inhibitor cocktail), 10% sucrose, and 1% Triton X-100 at 4°C. The cell pellet was resuspended and further incubated for 30 min on ice. Then, 600 μl of cold 60% Optiprep (Nycomed-Pharma, Oslo, Norway) was added to the extract, and the mix was transferred to a SW60 centrifuge tube (Beckman, Munich, Germany). The sample was overlaid with 900-μl step of each of 35, 30, 25, and 5% Optiprep in TNE, 1% Triton X-100. The gradients were spun for 17 h at 34,000 rpm at 4°C. Ten fractions from the top of the gradient were collected. The fractions were TCA precipitated and analyzed by Western blot with anti-p36, anti-p11, or anti-synaptotagmin antibodies followed by HRP-coupled secondary antibodies (Bio-Rad, Hercules, CA) and ECL (Amersham Buchler, Braunschweig, Germany). GM1 was detected as described above.
RESULTS
Reduction of Subplasmalemmal Annexin 2 Inhibits Exocytosis in Chromaffin Cells
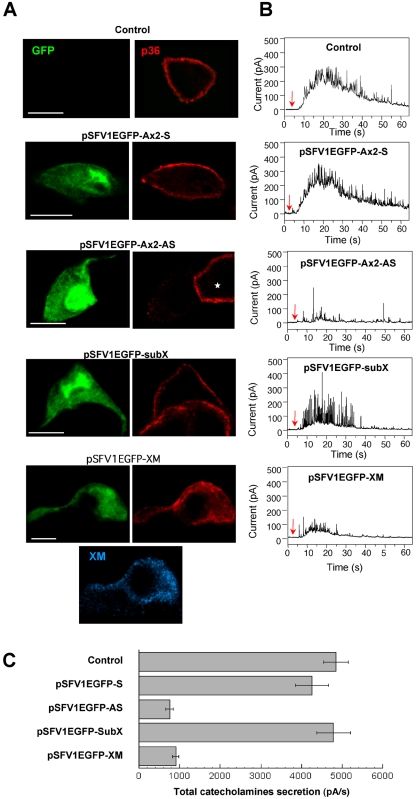
To probe the role of annexin 2 (p36) in exocytosis, we modified the expression level of endogenous annexin 2 in chromaffin cells by using the Semliki Forest virus expression system (Knight, 1999). Two types of recombinant virus were constructed: pSFV1EGFP-Ax2-AS, expressing the antisense sequence of p36 (annexin 2) to decrease levels of the endogenous protein, and pSFV1EGFP-XM, which expressed a p36/p11 chimera (XM) that aggregates cytosolic p36 and prevents the formation of the annexin 2 tetramers (Harder and Gerke, 1993; Harder et al., 1993). To identify infected cells, the EGFP marker was included in the viruses under the control of a second promoter. In control cells and cells infected with either an empty vector (subX) or a vector containing the p36 sense sequence (Ax2-S), secretagogue-evoked stimulation triggered the translocation of annexin 2 to the subplasmalemmal region (Figure 1A). Exocytotic capacity, as measured in parallel by using amperometry to resolve the frequency and kinetics of individual secretory granule release events, was unchanged by p36 sense expression (Figure 1B). Amperometric signals were characterized by a rapid increase in the oxidation current for generally 15–20 s and numerous sharp spikes reflecting the release of the contents of single secretory granules. In contrast, cells infected with the p36 antisense virus (Ax2-AS) exhibited an 80% reduction in the level of endogenous p36 as estimated by quantifying the fluorescence intensity (Figure 1A) and a 75% decrease in exocytotic activity (Figure 1, B and C). Similarly, sequestration of endogenous p36 into cytosolic aggregates by infection with pSFV1EGFP-XM strongly reduced the exocytotic response (Figure 1).
Figure 1.
Reduction of peripheral annexin 2 alters the exocytotic release of catecholamines in chromaffin cells. Chromaffin cells were infected with the following vectors: pSFV1EGFP-Ax2-S, a control vector containing the sense sequence of p36; pSFV1EGFP-Ax2-AS, a vector containing the antisense sequence of p36; pSFV1EGFP-XM, a vector expressing the dominant negative p36/p11 chimera XM; and pSFV1EGFP-SubX, the empty vector. To facilitate identification of the infected cells, the viruses coexpressed EGFP. Forty-eight hours after infection, cells were stimulated with 10 μM nicotine in Locke's solution, fixed, and immunostained with anti-p36 antibody. The XM chimeric protein was detected with the H21 monoclonal antibody (note that the anti-p36 antibody does not recognize the XM chimeric protein). (A) Confocal micrographs (bars, 10 μm). (B) Catecholamine release estimated by amperometry. The traces shown are typical responses to a local application of 100 μM nicotine for 5 s (arrow). (C) Amperometric responses were integrated to obtain the total catecholamine secretion expressed in pA per second. Data are the means of 25 cells/group from the same dish ± SEM. Similar results were obtained in three independent experiments performed on two culture preparations and infected with different batches of recombinant virus.
A more detailed amperometric analysis was undertaken on clearly defined spikes recorded at a higher resolution. Expression of p36 antisense or XM markedly decreased the number of nicotine-evoked individual exocytotic events (spikes) compared with control cells (Table 1). We examined the spike characteristics that provide information about the kinetics of fusion pore formation, expansion, and closure (Albillos et al., 1997; Burgoyne and Barclay, 2002). The amplitude (average height) and total charge carried by the residual spikes in infected chromaffin cells with reduced subplasmalemmal p36 were not decreased compared with noninfected control cells (Table 1), indicating that the reduction in secretion was not caused by depletion of granule catecholamine. Similarly, the overall shape of the spikes (rise time and fall time) and the mean values for the half-widths of the spikes were not affected (Table 1). Thus, inhibition of p36 translocation to the plasma membrane affects the number but not the kinetics of the single granule release events detected as amperometric spikes. This suggests that annexin 2 at the plasma membrane might be required for the recruitment and/or docking of secretory granules to sites of exocytosis rather than for the fusion event itself.
Table 1.
Analysis of amperometric spikes from infected chromaffin cells
| pSFV1EGFP-A×2S | pSFV1EGFP-A×2AS | pSFV1EGFP-SubX | pSFV1EGFP-XM | |
|---|---|---|---|---|
| Total cell recorded | 36 | 32 | 37 | 30 |
| Total number of spike | 1382 | 224 | 1299 | 182 |
| No. of spike/cell | 38±3 | 7±1 | 35±3 | 6±2 |
| Rise-time (ms) | 7.4±0.5 | 7.2±0.6 | 6.9±0.4 | 7.1±0.4 |
| Fall-time (ms) | 17±1.1 | 15.3±1.5 | 16.2±1.2 | 14.9±1.6 |
| Half width (ms) | 8.9±0.6 | 9.1±0.3 | 9.2±0.5 | 8.7±0.3 |
| Ave. height (pA) | 148±16 | 138±21 | 156±19 | 143±14 |
| Total charge/spike (pC) | 1.56±0.16 | 1.48±0.20 | 1.68±0.13 | 1.60±0.18 |
Amperometric responses were recorded on an expanded time base to avoid overlapping of the spikes. Spikes were only analyzed if they had a base width >10 ms and an amplitude >40 pA. In this way, analyzes were confined to spikes arising immediately beneath the carbon fiber to limit effects on the data of catecholamine diffusion from distant release sites. Spike characteristics were obtained through analysis of amperometric recordings by using Scope version 3.3 software.
Peripheral Annexin 2 Is Not Involved in the Actin Depolymerization Preceding Exocytosis
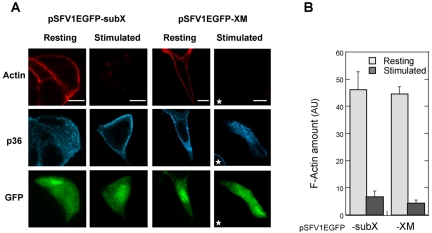
In chromaffin cells, actin filaments are concentrated under the plasma membrane. This actin network forms a barrier that prevents the docking of chromaffin granules to the plasma membrane. Stimulation triggers the reorganization of actin and partial removal of the barrier (Cheek and Burgoyne, 1986; Aunis and Bader, 1988; Sontag et al., 1988; Vitale et al., 1995), and these actin filament rearrangements are required for exocytosis. Because annexin 2 is an actinbinding protein, we investigated whether the reduction in peripheral p36 might affect this event. Therefore, actin filaments (F-actin) were visualized by rhodamine-phalloidin staining in cells transiently overexpressing the XM mutant. In resting chromaffin cells infected with control or XM-expressing virus, actin filaments localized to the cell periphery, in the form of a continuous cortical ring (Figure 2A). Stimulation with nicotine decreased the amount of F-actin detected in control and pSFV1EGFP-XM–infected cells by 84 and 89%, respectively (Figure 2, A and B), indicating that cortical actin similarly disassembled despite the blockade of p36 translocation in cells expressing XM. Thus, the presence of annexin 2 in the subplasmalemmal region is not a prerequisite for the actin depolymerization that necessarily precedes the recruitment and docking of secretory granules to the plasma membrane.
Figure 2.
Expression of the XM chimera blocks the translocation of annexin 2 but does not modify the actin depolymerization required for exocytosis. Chromaffin cells infected with pSFV1EGFP-subX or pSFV1EGFP-XM were maintained under resting conditions or stimulated for 5 min with 10 μM nicotine and then processed for immunocytochemistry. Cells were double labeled with anti-p36 antibodies and rhodamine-conjugated phalloidin. Images were recorded in the same optical section by a triple exposure procedure. Bars, 10 μm. The star indicates a noninfected cell. (B) The histogram represents a semiquantitative analysis of the amount of fluorescent phalloidin detected in resting and nicotine-stimulated cells (±SEM, n = 13). Actin was found to depolymerize similarly in infected cells in which p36 translocation was impaired in comparison with noninfected cells that had p36 in the periphery.
Secretagogue-Evoked Stimulation Triggers the Appearance of Lipid Rafts at the Sites of Exocytosis
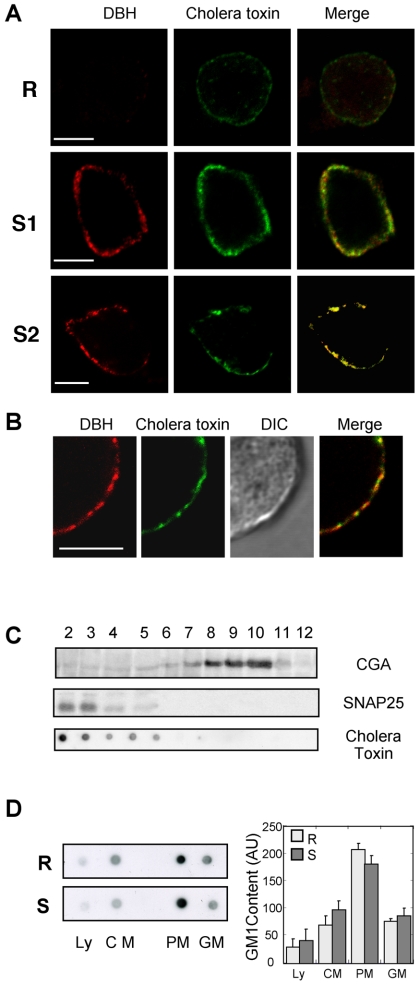
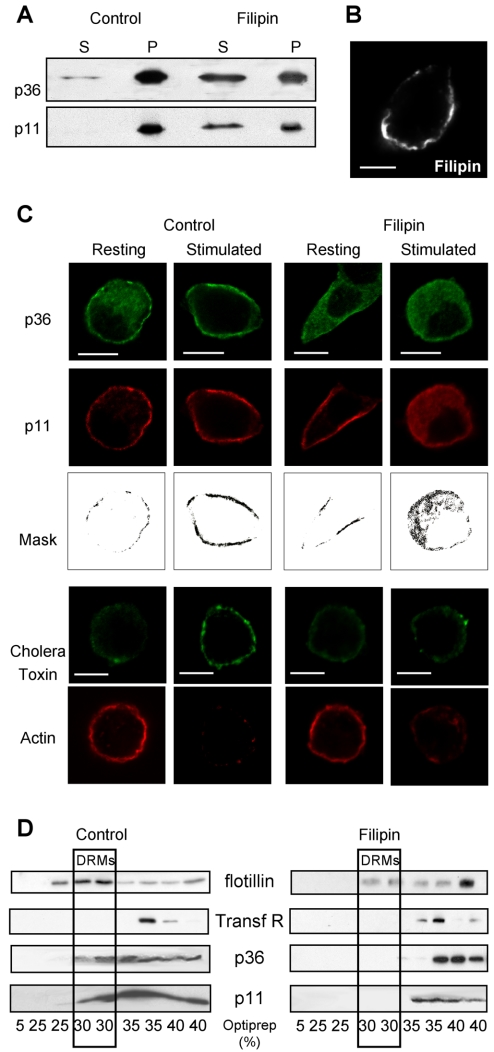
Annexin 2 has been recently described as a calcium-dependent promoter of lipid raft microdomains in membranes (Babiychuk and Draeger, 2000; Babiychuk et al., 2002; Mayran et al., 2003). Because lipid rafts may be important for exocytosis (Lang et al., 2001; Salaun et al., 2004), we have studied their formation in stimulated chromaffin cells. To monitor in parallel the exocytotic activity and the formation of rafts at the plasma membrane, we used an immunofluorescent approach. Exocytosis was visualized in living cells by adding anti-DBH antibodies into the incubation medium (Chasserot-Golaz et al., 1996). The granule-associated DBH becomes accessible to the antibody only at sites of exocytosis, leading to the appearance of fluorescent patches at the cell surface. In parallel, GM1-containing rafts were visualized using fluorescent cholera toxin (Harder et al., 1998; Janes et al., 1999). As illustrated in Figure 3A, resting chromaffin cells exhibited no DBH patches, confirming the low levels of baseline exocytotic activity in the absence of secretagogue, and displayed only a faint staining with cholera toxin at the cell surface. Stimulation with nicotine for 5 min triggered the appearance of a patchy pattern of DBH surface staining and concomitantly increased the binding of fluorescent cholera toxin (Figure 3A, S1). Note the colocalization between DBH and cholera toxin at the cell surface (Figure 3A, mask), indicating that the GM1-enriched microdomains correspond to the sites of exocytosis. Cholera toxin binding was similarly observed when stimulated cells were fixed before incubation with the toxin, excluding the possibility that GM1 clustering was caused by the toxin itself (Figure 3A, S2). As observed at higher magnification by differential interference contrast (DIC) imaging, the cell membrane remained uniform upon cell stimulation indicating that patches of cholera toxin were not due to morphological heterogeneity of the cell surface (Figure 3B).
Figure 3.
GM1-enriched microdomains colocalize to sites of exocytosis. (A) Chromaffin cells were maintained in the resting state (R) or stimulated 5 min with nicotine in the presence of anti-DBH antibodies (S1, S2). Cells were stimulated in the presence of Alexa-488 conjugated cholera toxin and then fixed (S1) or fixed before incubation with Alexa-488 conjugated cholera toxin (S2). DBH staining was revealed with Cy3-conjugated anti-rat antibodies. The weighted colocalization coefficient was ∼0.77 ± 0.02 (±SEM, n = 9). (B) Higher magnification images of a stimulated chromaffin cell double-labeled with DBH antibodies and cholera toxin and the corresponding DIC image. Bars, 5 μm. (C) Fractions 2–12 (40 μg of protein/fraction) collected from a continuous sucrose density gradient layered with the crude chromaffin membrane pellet were subjected to gel electrophoresis and immunodetection on nitrocellulose using anti-SNAP-25 (plasma membrane marker) and antichromogranin A (chromaffin granule marker) antibodies. To detect the ganglioside GM1, 2 μl of each fraction was dot blotted onto nitrocellulose and incubated with HRP-conjugated cholera toxin. (D) Resting or nicotine-stimulated chromaffin cells were lysed and processed for subcellular fractionation on sucrose gradients. Fractions corresponding to the Ly, CM, PM, and GM were probed for the presence of GM1 by using HRP-conjugated cholera toxin. Similar results were obtained in two independent fractionation performed with different cell cultures. The histogram represents a semiquantitative analysis of the cholera toxin binding detected in the different fractions under resting (R) and stimulating conditions (S). Data are given as the mean values ± SEM (n = 3).
To assess whether the increase in cholera toxin labeling resulted from a de novo synthesis of GM1 during the period of stimulation, we compared the amount of GM1 present in fractions collected from a continuous sucrose density gradient layered with crude membranes prepared from resting or stimulated cells. The amount of GM1 was determined by dot blot by using peroxidase-conjugated cholera toxin. As illustrated in Figure 3C, GM1 was essentially detected in fractions 2–3 containing the plasma membranes as assessed by following the distribution of SNAP-25. GM1 was not detected in the fractions containing secretory granules marked by chromogranin A (CGA). In addition, we found neither significant increase in the total cellular amount of GM1 nor increased level in plasma membranes prepared from resting or stimulated cells (Figure 3D). Thus, cell surface increase of cholera toxin labeling observed in stimulated cells was not due to the insertion of granule membranes into the plasma membrane by exocytosis or to the synthesis of GM1 during the period of stimulation. More likely, cholera toxin labeling resulted from the coalescence of small lipid microdomains into larger units. These larger rafts were either more easily detectable at the light microscopic level or they bound cholera toxin with an increased affinity due to multivalency effects given by the aggregated GM1 molecules (Arosio et al., 2004).
Annexin 2 Is Associated with Secretagogue-induced Lipid Rafts in Chromaffin Cells
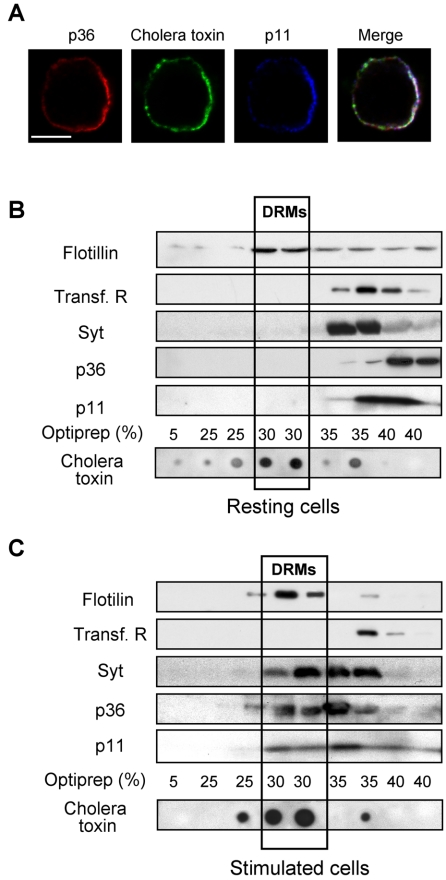
To investigate whether annexin 2 is present in the lipid rafts formed during exocytosis, we performed a triple labeling experiment by using anti-p36 and anti-p11 antibodies together with fluorescent cholera toxin. As illustrated in Figure 4A, areas of colocalization between p36, p11, and the cholera toxin-binding sites occurred in nicotine-stimulated cells after translocation of the cytosolic p36 to the subplasmalemmal region. This suggests that the annexin 2 tetramers formed at the plasma membrane upon cell stimulation (Chasserot-Golaz et al., 1996) preferentially associate to the GM1-containing microdomains. The presence of annexin 2 tetramers in the secretagogue-induced lipid rafts was further confirmed by subcellular fractionation. Rafts or detergent-resistant membranes (DRMs) can be isolated on the basis of their relative insolubility in Triton X-100. Resting and nicotine-stimulated chromaffin cells were solubilized in Triton X-100 and then centrifuged in a 5–35% Optiprep gradient to segregate the low-density rafts/DRMs (Harder et al., 1998). After centrifugation, the distribution of GM1 on the gradient was determined by dot blot by using peroxidase-conjugated cholera toxin, whereas cosegregating proteins were identified by SDS-electrophoresis and immunoblotting. As expected, GM1 gangliosides floated in the 30% Optiprep fraction (Figure 4, B and C). Flotillin was enriched in the same fractions, whereas transferrin receptors, which are not associated to DRMs, were present in the bottom of the tube. Caveolin was not detected (our unpublished data), in agreement with previous studies reporting low levels of caveolae in PC12 cells (Bilderback et al., 1999; Chamberlain et al., 2001). The total amount of GM1 and flotillin was not significantly modified in the Triton X-100 homogenates prepared from resting and stimulated cells (our unpublished data). However, GM1 and flotillin were concentrated in the 30% Optiprep fractions in gradients prepared from stimulated cells (Figure 4, B and C), confirming the formation of detergent-insoluble rafts during the exocytotic process.
Figure 4.
Annexin 2 is concentrated and recruited into rafts in stimulated chromaffin cells. (A) Chromaffin cells were stimulated 5 min with nicotine in the presence of cholera toxin, fixed, and double labeled with anti-p36 and anti-p11 antibodies. Images were recorded in the same optical section by a triple exposure procedure. The white pixels in the merge image correspond to the superimposition of red, green, and blue colors and reveal the areas of colocalization of p36 and p11 with the cholera toxin binding sites. The weighted colocalization coefficient is ∼0.85 ± 0.02 (±SEM, n = 9). Bars, 5 μm. (B and C) Resting or nicotine-stimulated chromaffin cells were extracted by 1% Triton X-100 at 4°C and fractionated on an Optiprep density gradient. Each fraction was resolved on SDS-PAGE and analyzed by Western blot/ECL to detect flotillin, transferrin receptors, p36, p11, and synaptotagmin. GM1 ganglioside was detected by dot blot with HRP-conjugated cholera toxin and visualized by ECL. Low-density fractions contained rafts/DRMs, as judged by the presence of GM1 and flotillin. Highdensity fractions contained the bulk of solubilized membrane proteins such as transferrin receptors.
Both p36 and p11 shifted into the GM1- and flotillin-containing DRMs in stimulated cells, together with synaptotagmin 1, a component of the exocytotic machinery known to be required for calcium-sensitive granule release in chromaffin cells (Voets et al., 2001). Note that none of these proteins were detected in DRMs prepared from resting cells (Figure 4B), indicating that their recruitment to rafts is intimately linked to exocytotic stimulation. Together, these experiments indicate that secretagogue-evoked stimulation in chromaffin cells triggers the formation of lipid rafts at the plasma membrane which recruit annexin 2 tetramers together with elements of the docking/fusion machinery.
Secretagogue-induced Rafts Depend on Cholesterol
In the following experiments, we investigated whether the cholera toxin-binding sites to which annexin 2 translocates in stimulated cells represent cholesterol-dependent microdomains. Using a cholesterol-sequestering agent, the polyen antibiotic filipin, we first examined whether the membrane association of annexin 2 was sensitive to the cholesterol clustering agent. Crude membranes prepared from nicotine-stimulated chromaffin cells were treated with filipin, centrifuged, and the amount of p36 and p11 detected in the pellet or released in the supernatant was analyzed by immunodetection. As illustrated in Figure 5A, filipin treatment led to a partial solubilization of both p36 and p11, suggesting that cholesterol was to some extent required to stabilize the calcium-dependent binding of annexin 2 tetramer to the plasma membrane. Consistent with this observation, addition of cholesterol has been reported to increase the binding of monomeric and tetrameric forms of annexin 2 on liposomes (Ayala-Sanmartin et al., 2001; Mayran et al., 2003).
Figure 5.
Filipin prevents the formation of annexin 2-containing rafts in stimulated cells. (A) Sequestration of cholesterol by filipin releases membrane-bound annexin 2. Crude membranes prepared from stimulated chromaffin cells were treated with filipin, centrifuged to separate the membrane-associated (P) from the soluble (S) proteins, and analyzed by SDS-PAGE and Western blot/ECL to detect p36 and p11. (B) Micrograph image of a chromaffin cell treated for 10 min with 10 μM filipin, which is fluorescent at 525 nm after excitation at 360 nm. Bar, 5 μm. (C) Confocal micrographs of chromaffin cells treated or not for 10 min with 10 μM filipin and maintained under resting conditions or stimulated with 10 μM nicotine. Cells were either double labeled with anti-p36 and anti-p11 antibodies or stimulated in the presence of cholera toxin and labeled with rhodamine-conjugated phalloidin. Masks representing the region of colocalization were generated by selecting the double-labeled pixels. Bar, 5 μm. (D) Nicotine-stimulated chromaffin cells were incubated in the presence or absence of 5 μM filipin, extracted by 1% Triton X-100 at 4°C with or without 5 μM filipin, and then fractionated on an Optiprep density gradient. Fractions were resolved on SDS-PAGE and analyzed by Western blot/ECL to detect the indicated proteins.
The effect of filipin on the distribution of p36 and p11 in resting and stimulated chromaffin cells is shown in Figure 5C. Chromaffin cells were exposed to 10 μM filipin before stimulation with nicotine. By fluorescent microscopy, we verified that filipin remained at the plasma membrane and therefore should not disrupt intracellular compartments such as secretory granules (Figure 5B). Under these experimental conditions, filipin also had no apparent effect on cell integrity as judged by the intact peripheral actin cytoskeleton observed in resting cells (Figure 5C). Treatment with filipin clearly reduced the amount of cholera toxin bound in stimulated cells, indicating that the secretagogue-evoked GM1-enriched microdomains represent genuine cholesterol-dependent rafts (Figure 5C). Moreover, p36 remained in the cytosol in filipin-treated cells (Figure 5C), supporting the idea that cholesterol is required to maintain annexin 2 at the plasma membrane after secretagogue-induced translocation. Although cholesterol depletion did not affect the peripheral distribution of p11 in resting chromaffin cells, it did cause p11 to fall into the cytosol upon cell stimulation (Figure 5C). This suggests that the interactions that recruit p11 to the plasma membrane in resting cells no longer suffice in stimulated cells when cholesterol is depleted. Subcellular fractionation performed on filipin-treated cells confirmed that p11 and p36 no longer floated on Optiprep density gradients (Figure 5D), consistent with the immunocytochemical results mentioned above indicating that the annexin 2 tetramers localize to cholesterol-dependent microdomains in stimulated cells. Together, these results lead us to conclude that secretagogue-evoked stimulation triggers the formation of cholesterol-dependent rafts in the plasma membrane, which are required to stabilize p11, and most likely the annexin 2 tetramer, near sites of exocytosis in chromaffin cells.
Raft/DRMs Dispersion by Cholesterol Sequestration Is Associated with an Inhibition of Exocytosis
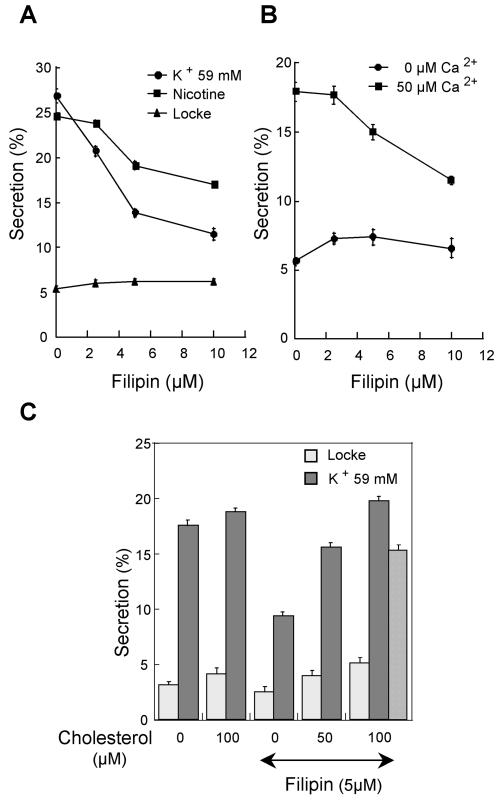
To evaluate the functional importance of the secretagogue-evoked lipid rafts in exocytosis, chromaffin cells were treated with various concentrations of filipin and catecholamine secretion was evoked. Figure 6A shows that filipin induced a dose-dependent inhibition of secretion in response to nicotine or a depolarizing concentration of potassium. Moreover, because cholesterol-dependent DRMs have been suggested to be important for clustering and regulation of neurotransmitter receptors and ion channels (Tsui-Pierchala et al., 2002), we also examined the effect of filipin on secretion from permeabilized chromaffin cells, to by-pass the nicotinic receptors and voltage-gated calcium channels. Filipin at 10 μM inhibited to a similar extent calcium-evoked catecholamine release from permeabilized cells (Figure 6B), indicating that the formation of cholesterol-dependent DRMs is required at a step distal to the activation of receptors and mobilization of cytosolic calcium. To confirm that the effect of filipin on exocytosis was due to cholesterol sequestration, we measured the influence of cholesterol addition on catecholamine secretion (Figure 6C). Cholesterol partially reversed the inhibitory effect of filipin, in line with the idea that cholesterol-dependent microdomains are important for exocytosis.
Figure 6.
Effect of filipin on [3H]noradrenaline secretion in response to various secretagogues. (A) Chromaffin cells were incubated for 10 min in Locke's solution containing the indicated concentrations of filipin and then stimulated for 10 min with 10 μM nicotine or 59 mM potassium. Basal release in the absence of stimulus (Locke) was recorded for 10 min. (B) Chromaffin cells incubated with the indicated concentrations of filipin were permeabilized and stimulated with 0 or 50 μM free calcium. (C) Chromaffin cells were incubated for 10 min in Locke's solution containing 5 μM filipin and the indicated concentrations of cholesterol. Hatched bar, cells were first exposed for 5 min to 5 μM filipin and then incubated for 10 min with cholesterol. Cells were subsequently stimulated for 10 min with 59 mM potassium. Extracellular fluids were collected and the radioactivity present in solutions and cells was assayed. Data are given as the mean of triplicate determinations on the same cell preparation. Similar results were obtained with three different cell preparations.
Annexin 2 Participates in the Organization of Lipid Rafts during Exocytosis
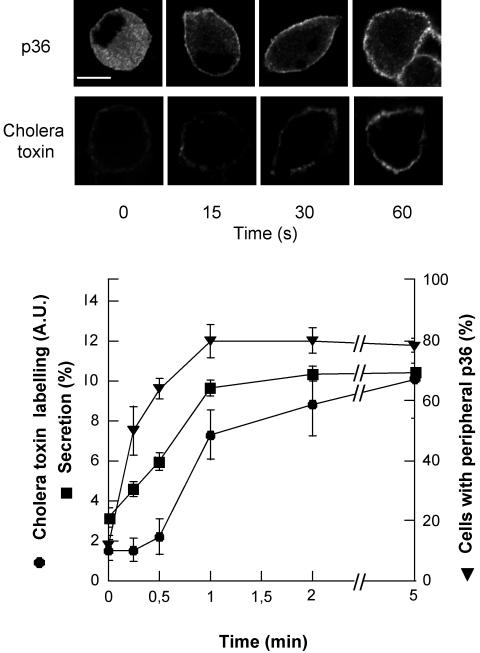
To probe the idea that annexin 2 contributes to the formation of lipid rafts in stimulated chromaffin cells, we first compared the time course of p36 translocation, raft formation, and catecholamine secretion in nicotine-stimulated chromaffin cells. As illustrated in Figure 7, the translocation of annexin 2 to the plasma membrane occurred as an early event in the exocytotic pathway that clearly preceded raft aggregation detected by cholera toxin labeling and [3H]noradrenaline secretion.
Figure 7.
Time course of annexin 2 translocation, raft formation and [3H]noradrenaline secretion in nicotine-stimulated chromaffin cells. Chromaffin cells labeled with [3H]noradrenaline were stimulated with 10 μM nicotine for the indicated periods of time. Extracellular fluids were then collected and the radioactivity present in solutions and in cells was measured. Data are given as the mean of triplicate determinations. In parallel, nicotine-stimulated chromaffin cells were fixed and labeled with cholera toxin or anti-p36 antibodies. The percentage of cells displaying a peripheral labeling of p36 was estimated by counting cells on randomly selected areas. Raft formation was assessed by a semiquantitative analysis of the cell surface cholera toxin binding and expressed as arbitrary units (±SEM; n = 10).
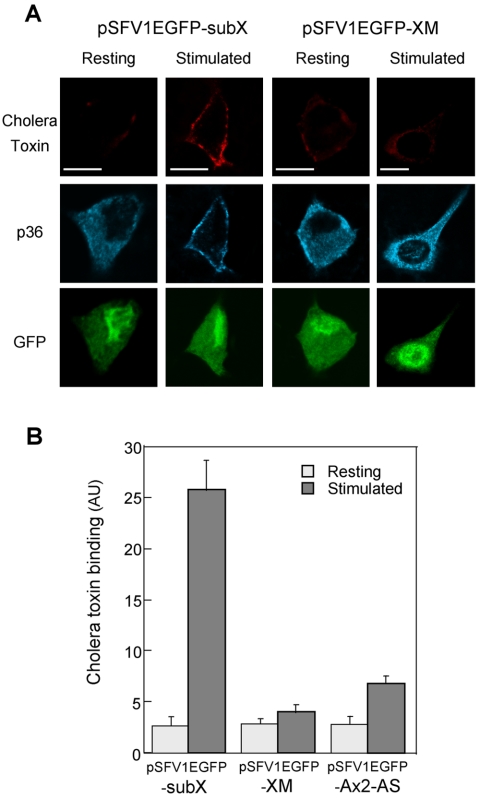
We then used cells expressing the XM chimera in which the translocation of p36 was impaired. Virus-infected cells identified with EGFP were stimulated with nicotine in the presence of cholera toxin to visualize the formation of rafts, fixed, and then stained with anti-p36 antibodies. As expected, stimulation of cells infected with a control vector triggered the translocation of p36 to the cell periphery and the concomitant appearance of rafts at the plasma membrane (Figure 8). In contrast, in chromaffin cells expressing the XM construct, p36 remained aggregated in the cytosol, and very little cell surface binding of cholera toxin was detected (Figure 8A). Semiquantitative analysis performed on cells expressing XM, and on cells with reduced endogenous p36 due to infection with the p36 antisense virus, confirmed that the amount of cholera toxin associated with the plasma membrane was closely related to the presence of p36 in the cell periphery (Figure 8B). Thus, the formation of rafts in secretagogue-stimulated cells depends on the translocation of p36 to the plasma membrane, suggesting that annexin 2 tetramers actively participate in the formation and/or the stabilization of the GM1-containing microdomains required for exocytosis.
Figure 8.
Reduction of peripheral annexin 2 inhibits raft formation in stimulated chromaffin cells. (A) Chromaffin cells infected with pSFV1EGFP-SubX or pSFV1EGFP-XM were maintained under resting conditions or stimulated with 10 μM nicotine in the presence of cholera toxin to visualize rafts. Cells were subsequently fixed and stained with anti-p36 antibodies. Confocal images were recorded in the same optical section by a triple exposure procedure Bars, 5 μm. (B) Semiquantitative analysis of the binding of cholera toxin to the plasma membrane in resting and stimulated chromaffin cells infected with pSFV1EGFP-SubX, pSFV1EGFP-XM, or pSFV1EGFP-AS expressed as arbitrary units (±SEM; n = 10).
DISCUSSION
We previously studied the distribution of annexin 2 (p36) and its cellular ligand p11 in chromaffin cells and found that p36 is located throughout the cytoplasm, whereas p11 is present exclusively in the subplasmalemmal region (Chasserot-Golaz et al., 1996). Secretagogue-evoked stimulation triggered the colocalization of p36 and p11 underneath the plasma membrane and the formation of the annexin 2 heterotetramer (p90) near exocytotic sites. Microinjection of a peptide that competes for the phosphorylation of annexin 2 by protein kinase C (PKC) prevented the translocation of annexin 2 and inhibited exocytosis (Chasserot-Golaz et al., 1996). This suggested that the presence of annexin 2 at the plasma membrane is required for exocytosis, although we could not exclude a direct inhibition of PKC activity in the microinjected cells. In this study, we attempted to obtain more direct evidence for the involvement of annexin 2 in calcium-regulated exocytosis. Using the Semliki Forest virus expression system, we show that expression of an antisense annexin 2 RNA decreases the synthesis of endogenous annexin 2, most probably by forming double-stranded RNA with the endogenous mRNA. This resulted in a marked inhibition of catecholamine secretion from chromaffin cells. Moreover, expression of the chimeric XM protein corresponding to p11 fused C-terminally to the first 18 residues of annexin 2 also produced a strong inhibition of chromaffin cell secretion. In Madin-Darby canine kidney cells, XM causes the aggregation of endogenous annexin 2 and p11 (Harder and Gerke, 1993; Harder et al., 1993). Similarly, in chromaffin cells, XM formed cytosolic aggregates that prevented the translocation of cytosolic p36 to the plasma membrane upon cell stimulation. Together, these results indicate that exocytosis is strongly inhibited when the formation of the annexin 2 tetramer at the plasma membrane is impaired by a reduction of functional p36. To our knowledge, this is the first direct demonstration of a functional role for annexin 2 in dense-core granule exocytosis, by using molecular tools in living cells. It should be mentioned that our results are in contrast to those of a previous study reporting that XM expression in stably transfected PC12 cell lines does not affect Ca2+-dependent secretion (Graham et al., 1997). However, stable XM expression resulted in an increase in the expression of endogenous annexin 2, and it cannot be ruled out that the endogenous nonaggregated pool of annexin 2 simply remained sufficient to maintain exocytotic activity in this cell line.
Despite 20 years of extensive study, the precise function of most of the annexins remains to be elucidated. Regulated exocytosis in neuroendocrine cells is a process that requires a specific reorganization of the cortical actin cytoskeleton to allow the recruitment and subsequent docking of secretory granules to the plasma membrane (Cheek and Burgoyne, 1986; Aunis and Bader, 1988; Lang et al., 2001). Because annexin 2 is an actinbinding protein (Gerke and Moss, 2002) that translocates from the cytosol to the plasma membrane in stimulated cells, it was conceivable that the protein, by interacting with cytoskeletal elements, might clear a path for secretory granules to move to the membrane. However, using rhodamine-conjugated phalloidin to visualize actin filaments, we could not correlate the strong inhibition of secretion induced by the XM fusion mutant to a stabilization of the cortical actin barrier. Thus, annexin 2 seems not to play an obvious role in promoting cortical actin depolymerization, although we cannot exclude other subtle modifications of the actin cytoskeleton that might be required in late stages of the exocytotic machinery.
Another possible function assigned to annexin 2 relates to the late fusion event. Indeed, it has been reported that phosphorylation by PKC triggers the fusion of purified secretory granules preaggregated by unphosphorylated p36 (Regnouf et al., 1995), suggesting that annexin 2 becomes fusogenic when phosphorylated by PKC. Because secretagogue-evoked stimulation activates PKC to phosphorylate endogenous annexin 2 in chromaffin cells (Delouche et al., 1997), annexin 2 has been proposed to mediate membrane fusion once the granule is brought in proximity to the plasma membrane by SNARE proteins (Regnouf et al., 1995). Our amperometric data do not support this hypothesis. We observed that reduction of annexin 2 expression level in chromaffin cells inhibited the number of exocytotic spikes, but the properties of the remaining spikes remained unchanged with respect to charge and kinetics. This suggests that annexin 2 is involved in the recruitment and/or docking of granules to the exocytotic sites. However, its implication in late events such as the formation of the fusion pore remains to be further investigated.
In many cell types, the association of annexin 2 with the plasma membrane seems to occur preferentially at sites of membrane microdomains, the so-called rafts rich in cholesterol, glycosphingolipids, and glycosyl phosphatidylinositol-anchored proteins (Gerke and Moss, 2002). Rafts have been implicated in numerous cellular processes, including signal transduction, molecular sorting, membrane trafficking events, and cell adhesion (Harder et al., 1998; Smart et al., 1999; Dermine et al., 2001). A role for rafts has been recently proposed in regulated exocytosis based on the findings that components of the exocytotic machinery such as syntaxin, SNAP-25, and VAMP2 are associated with rafts (Chamberlain et al., 2001; Lang et al., 2001; Salaun et al., 2004). Lipid rafts are highly dynamic structures that can be very small (a few tens to hundreds of nanometers in diameter) and dispersed but are able to coalesce into large micrometer-sized domains upon cellular stimulation, resulting in the clustering and recruitment of membrane components involved in specific signals or functions (Brown and London, 2000; Abrami et al., 2001; Brown, 2001; Pierini and Maxfield, 2001). Raft dynamics can be influenced by specific proteins, including annexin 2, which has been described as a promoter of lipid microdomain association (Babiychuk and Draeger, 2000). In chromaffin cells, subcellular fractionation experiments have revealed that the translocation of p36 from the cytosol to the cell periphery is accompanied by an increase of the protein in a Triton X-100–insoluble fraction (Chasserot-Golaz et al., 1996; Sagot et al., 1997). Because rafts have been defined by their low density and insolubility in Triton X-100, we examined whether annexin 2 might be involved in the formation of lipid rafts required for exocytosis. We show here that secretagogue-evoked stimulation triggers the Ca2+-dependent formation of GM1-containing domains at the plasma membrane in chromaffin cells. These domains are unlikely to reflect the incorporation of the granule membrane into the plasma membrane and as such be a consequence of the exocytotic process because GM1 cannot be detected in the chromaffin granule membrane. The de novo synthesis of GM1 seems also unlikely considering the small time window in which the cholera-binding sites occur in stimulated cells and the fact that total GM1 was similar in subcellular fractions prepared from resting and stimulated cells. Thus, it is possible that the increase in cholera toxin labeling observed in stimulated cells resulted from the coalescence of small GM1 microdomains into larger units that bound the pentavalent toxin with an increased affinity due to the multivalent display of the aggregated GM1 molecules (Arosio et al., 2004) or that became simply more easy to detect at the light microscopic level. Alternatively, we cannot exclude that GM1 motives are unmasked during stimulation. Centrifugation on density gradients to separate the low-density rafts and analysis of the cosegregating proteins revealed the specific association of the annexin 2-p11 tetramer to the lipid rafts formed in stimulated cells. Moreover, using chromaffin cells expressing the XM chimera or with reduced endogenous p36, we observed a close correlation between the recruitment of annexin 2 to the cell periphery, the enhancement of cholera toxin binding to the cell surface, and the exocytotic response, suggesting that the annexin 2 tetramer actively participates in the coalescence of GM1-containing microdomains and their stabilization into the larger rafts observed in stimulated cells. Finally, we found that cholesterol sequestration by filipin disrupts the annexin 2-containing rafts and in parallel inhibits catecholamine secretion evoked by various secretagogues, suggesting that the lipid microdomains formed in stimulated cells are required for exocytosis. Together, these results support the idea that lipid microdomains formed/stabilized in the plasma membrane by the annexin 2 tetramer play a key role in the organization of the exocytotic machinery in chromaffin cells.
How might annexin 2 influence the formation of lipid rafts in stimulated cells? Considering the binding domains of the annexin 2-p11 heterotetramer, different scenarios may be evoked. Oligomerization of annexin 2 can occur at the cytoplasmic leaflet of the plasma membrane. Because one molecule of annexin 2 can bind four molecules of phosphatidylserine (PS), annexin 2 may act directly to trap and cluster PS, thereby creating microdomains in the plasma membrane. As well, annexin 2 has recently been shown to be a phosphatidylinositol (4,5)-bisphosphate (PIP2)-binding protein (Hayes et al., 2004; Rescher et al., 2004), a major plasma membrane phosphoinositide required in exocytosis for the ATP-dependent priming reactions preceding fusion (Hay et al., 1995; Holz et al., 2000). Thus, another attractive hypothesis is that annexin 2 stabilizes PIP2 microdomains in the plasma membrane, which in turn recruit specific PIP2-binding proteins acting in the subsequent stages of exocytosis (Grishanin et al., 2004). In addition, the cortical actin cytoskeleton may provide constraints for the lateral mobility of rafts and increase their stability. Hence, the annexin 2 tetramer formed at the plasma membrane may participate in the formation of membrane-cytoskeleton complexes that could control raft assembly. Although additional experimental evidence is now required to explore these possibilities, it is of interest to mention that we found actin in the lipid rafts formed in stimulated cells (our unpublished data), suggesting that the cortical actin cytoskeleton may partner with annexin 2 to stabilize lipid raft domains and organize them into functional exocytotic sites.
The functional characteristics of the sites of exocytosis that ensure tethering of vesicles/granules to the appropriate active zones at the plasma membrane, as well as organization of the exocytotic machinery for rapid and efficient release, remain poorly understood, especially in neuroendocrine cells. Elements with an ability to compartmentalize the plasma membrane and thereby spatially and temporally organize the proteins required for docking and fusion may be crucial for speed and accuracy of the exocytotic process. As such, cholesterol-dependent lipid microdomains are ideally suited to bring together and efficiently assemble components of the exocytotic pathway, and the observations that SNAREs form cholesterol-dependent clusters in the plasma membrane are in line with this idea (Chamberlain et al., 2001; Lang et al., 2001). The present results provide for the first time a molecular support for the de novo formation of lipid rafts at the granule docking sites in stimulated neuroendocrine cells. Rise in intracellular calcium triggers the recruitment of cytosolic annexin 2 to the plasma membrane. We propose that p11 is the prime anchor for annexin 2 at plasma membrane. By engaging homophilic lateral interactions and binding to negatively charged phospholipids and phosphoinositides, annexin 2 tetramers could then induce raft clustering. Once formed, raft structures and the associated cholesterol may further stabilize the lipid–annexin 2 interactions, resulting in annexin 2-membrane scaffolds that may be required to assemble components of the exocytotic machinery. It is of interest to note that the calcium-dependent recruitment of annexin 2 to the plasma membrane offers the cell a mechanism to link spatial control of regulated exocytosis to cell surface receptor activation and calcium signaling.
Acknowledgments
We thank Dr. Michael A. Frohman for helpful comments and critically reading the manuscript and S. Grosch and T. Thahouly for technical assistance. We are grateful to F. Gonon (Centre National de la Recherche Scientifique Unité Mixte Recherche-5541, Bordeaux, France) for generously providing carbon fiber electrodes. We acknowledge the confocal microscopy facilities of Plateforme Imagerie In Vitro of IFR 37. This work was supported by the Association de la Recherche sur le Cancer (no. 3208).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-07-0627) on January 5, 2005.
References
- Abrami, L., Fivaz, M., Kobayashi, T., Kinoshita, T., Parton, R. G., and van der Goot, F. G. (2001). Cross-talk between caveolae and glycosylphosphatidylinositol-rich domains. J. Biol. Chem. 276, 30729-30736. [DOI] [PubMed] [Google Scholar]
- Albillos, A., Dernick, G., Horstmann, H., Almers, W., Alvarez de Toledo, G., and Lindau, M. (1997). The exocytotic event in chromaffin cells revealed by patch amperometry. Nature 389, 509-512. [DOI] [PubMed] [Google Scholar]
- Ali, S. M., and Burgoyne, R. D. (1990). The stimulatory effect of calpactin (annexin II) on calcium-dependent exocytosis in chromaffin cells: requirement for both the N-terminal and core domains of p36 and ATP. Cell. Signal. 2, 265-276. [DOI] [PubMed] [Google Scholar]
- Ali, S. M., Geisow, M. J., and Burgoyne, R. D. (1989). A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature 340, 313-315. [DOI] [PubMed] [Google Scholar]
- Arosio, D., Vrasidas, I., Valentini, P., Liskamp, R.M.J., Pieters, R. J., and Bernardi, A. (2004). Synthesis and cholera toxin binding properties of multivalent GM1 mimics. Org. Biomol. Chem. 2, 2113-2124. [DOI] [PubMed] [Google Scholar]
- Aunis, D., and Bader, M. F. (1988). The cytoskeleton as a barrier to exocytosis in secretory cells. J. Exp. Biol. 139, 253-266. [DOI] [PubMed] [Google Scholar]
- Ayala-Sanmartin, J., Henry, J. P., and Pradel, L. A. (2001). Cholesterol regulates membrane binding and aggregation by annexin 2 at submicromolar Ca(2+) concentration. Biochim. Biophys. Acta 1510, 18-28. [DOI] [PubMed] [Google Scholar]
- Babiychuk, E. B., and Draeger, A. (2000). Annexins in cell membrane dynamics. Ca(2+)-regulated association of lipid microdomains. J. Cell Biol. 150, 1113-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiychuk, E. B., Monastyrskaya, K., Burkhard, F. C., Wray, S., and Draeger, A. (2002). Modulating signaling events in smooth muscle: cleavage of annexin 2 abolishes its binding to lipid rafts. FASEB J. 16, 1177-1184. [DOI] [PubMed] [Google Scholar]
- Bader, M. F., Thierse, D., Aunis, D., Ahnert-Hilger, G., and Gratzl, M. (1986). Characterization of hormone and protein release from alpha-toxin-permeabilized chromaffin cells in primary culture. J. Biol. Chem. 261, 5777-5783. [PubMed] [Google Scholar]
- Bilderback, T. R., Gazula, V. R., Lisanti, M. P., and Dobrowsky, R. T. (1999). Caveolin interacts with Trk A and p75(NTR) and regulates neurotrophin signaling pathways. J. Biol. Chem. 274, 257-263. [DOI] [PubMed] [Google Scholar]
- Brown, D. A. (2001). Seeing is believing: visualization of rafts in model membranes. Proc. Natl. Acad. Sci. USA 98, 10517-10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D. A., and London, E. (2000). Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275, 17221-17224. [DOI] [PubMed] [Google Scholar]
- Burgoyne, R. D., and Barclay, J. W. (2002). Splitting the quantum: regulation of quantal release during vesicle fusion. Trends Neurosci. 25, 176-178. [DOI] [PubMed] [Google Scholar]
- Chamberlain, L. H., Burgoyne, R. D., and Gould, G. W. (2001). SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc. Natl. Acad. Sci. USA 98, 5619-5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasserot-Golaz, S., Vitale, N., Sagot, I., Delouche, B., Dirrig, S., Pradel, L. A., Henry, J. P., Aunis, D., and Bader, M. F. (1996). Annexin II in exocytosis: catecholamine secretion requires the translocation of p36 to the subplasmalemmal region in chromaffin cells. J. Cell Biol. 133, 1217-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek, T. R., and Burgoyne, R. D. (1986). Nicotine-evoked disassembly of cortical actin filaments in adrenal chromaffin cells. FEBS Lett. 207, 110-114. [DOI] [PubMed] [Google Scholar]
- Creutz, C. E. (1992). The annexins and exocytosis. Science 258, 924-931. [DOI] [PubMed] [Google Scholar]
- Delouche, B., Pradel, L. A., and Henry, J. P. (1997). Phosphorylation by PKC of annexin 2 in chromaffin cells stimulated by nicotine. J. Neurochem. 68, 1720-1727. [DOI] [PubMed] [Google Scholar]
- Dermine, J. F., Duclos, S., Garin, J., St-Louis, F., Rea, S., Parton, R. G., and Desjardins, M. (2001). Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J. Biol. Chem. 276, 18507-18512. [DOI] [PubMed] [Google Scholar]
- Ehrhart, M., Grube, D., Bader, M. F., Aunis, D., and Gratzl, M. (1986). Chromogranin A in the pancreatic islet: cellular and subcellular distribution. J. Histochem. Cytochem. 34, 1673-1682. [DOI] [PubMed] [Google Scholar]
- Gerke, V., and Moss, S. E. (2002). Annexins: from structure to function. Physiol. Rev. 82, 331-371. [DOI] [PubMed] [Google Scholar]
- Graham, M. E., Gerke, V., and Burgoyne, R. D. (1997). Modification of annexin II expression in PC12 cell lines does not affect Ca(2+)-dependent exocytosis. Mol. Biol. Cell 8, 431-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishanin, R. N., Kowalchyk, J. A., Klenchin, V. A., Ann, K., Earles, C. A., Chapman, E. R., Gerona, R.R.L., and Martin, T.F.J. (2004). CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron 43, 551-562. [DOI] [PubMed] [Google Scholar]
- Harder, T., and Gerke, V. (1993). The subcellular distribution of early endosomes is affected by the annexin II2p11(2) complex. J. Cell Biol. 123, 1119-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder, T., Scheiffele, P., Verkade, P., and Simons, K. (1998). Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141, 929-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder, T., Thiel, C., and Gerke, V. (1993). Formation of the annexin II2p112 complex upon differentiation of F9 teratocarcinoma cells. J. Cell Sci. 104, 1109-1117. [DOI] [PubMed] [Google Scholar]
- Hay, J. C., Fisette, P. L., Jenkins, G. H., Fukami, K., Takenawa, T., Anderson, R. A., and Martin, T.F.J. (1995). ATP-dependent inositide phosphorylation required for calcium-activated secretion. Nature 374, 173-177. [DOI] [PubMed] [Google Scholar]
- Hayes, M. J., Merrifield, C. J., Shao, D., Ayala-Sanmartin, J., Schorey, C. D., Levine, T. P., Proust, J., Curran, J., Bailly, M., and Moss, S. E. (2004). Annexin 2 binding to phosphatidylinositol 4,5-bisphosphate on endocytic vesicles is regulated by the stress response pathway. J. Biol. Chem. 279, 14157-14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz, R. W., Hlubek, M. D., Sorensen, S. D., Fisher, S. K., Balla, T., Ozaki, S., Prestwich, G. D., Stuenkel, E. L., and Bittner, M. A. (2000). A pleckstrin homology domain specific for phosphatidylinositol 4,5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important for exocytosis. J. Biol. Chem. 275, 17878-17885. [DOI] [PubMed] [Google Scholar]
- Janes, P. W., Ley, S. C., and Magee, A. I. (1999). Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 147, 447-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, D. E. (1999). Secretion from bovine chromaffin cells acutely expressing exogenous proteins using a recombinant Semliki Forest virus containing an EGFP reporter. Mol. Cell Neurosci. 14, 486-505. [DOI] [PubMed] [Google Scholar]
- Konig, J., Prenen, J., Nilius, B., and Gerke, V. (1998). The annexin II-p11 complex is involved in regulated exocytosis in bovine pulmonary artery endothelial cells. J. Biol. Chem. 273, 19679-19684. [DOI] [PubMed] [Google Scholar]
- Lambert, O., Gerke, V., Bader, M. F., Porte, F., and Brisson, A. (1997). Structural analysis of junctions formed between lipid membranes and several annexins by cryo-electron microscopy. J. Mol. Biol. 272, 42-55. [DOI] [PubMed] [Google Scholar]
- Lang, T., Bruns, D., Wenzel, D., Riedel, D., Holroyd, P., Thiele, C., and Jahn, R. (2001). SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 20, 2202-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecat, S., and Lafont, F. (1999). Annexins and their interacting proteins in membrane traffic. Protoplasma 207, 133-140. [Google Scholar]
- Lewit-Bentley, A., Rety, S., Sopkova-de Oliveira Santos, J., and Gerke, V. (2000). S100-annexin complexes: some insights from structural studies. Cell Biol. Int. 24, 799-802. [DOI] [PubMed] [Google Scholar]
- Liljestrom, P., and Garoff, H. (1991). A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology 9, 1356-1361. [DOI] [PubMed] [Google Scholar]
- Mayran, N., Parton, R. G., and Gruenberg, J. (2003). Annexin II regulates multivesicular endosome biogenesis in the degradation pathway of animal cells. EMBO J. 22, 3242-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata, T., Sobue, K., and Hirokawa, N. (1990). Conformational change and localization of calpactin I complex involved in exocytosis as revealed by quick-freeze, deep-etch electron microscopy and immunocytochemistry. J. Cell Biol. 110, 13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn, M., Johnsson, N., Wehland, J., and Weber, K. (1988). The submembranous location of p11 and its interaction with the p36 substrate of pp60 src kinase in situ. Exp. Cell Res. 175, 81-96. [DOI] [PubMed] [Google Scholar]
- Perrin, D., and Aunis, D. (1985). Reorganization of alpha-fodrin induced by stimulation in secretory cells. Nature 315, 589-592. [DOI] [PubMed] [Google Scholar]
- Pierini, L. M., and Maxfield, F. R. (2001). Flotillas of lipid rafts fore and aft. Proc. Natl. Acad. Sci. USA 98, 9471-9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard, H. B., Creutz, C. E., Fowler, V., Scott, J., and Pazoles, C. J. (1982). Calcium-dependent regulation of chromaffin granule movement, membrane contact, and fusion during exocytosis. Cold Spring Harb. Symp. Quant. Biol. 46, 2, 819-834. [DOI] [PubMed] [Google Scholar]
- Regnouf, F., Sagot, I., Delouche, B., Devilliers, G., Cartaud, J., Henry, J. P., and Pradel, L. A. (1995). “In vitro” phosphorylation of annexin 2 heterotetramer by PKC. Comparative properties of the unphosphorylated and phosphorylated annexin 2 on the aggregation and fusion of chromaffin granule membranes. J. Biol. Chem. 270, 27143-27150. [DOI] [PubMed] [Google Scholar]
- Rescher, U., Ruhe, D., Ludwig, C., Zobiack, N., and Gerke, V. (2004). Annexin 2 is a phosphatidylinositol (4,5)-bisphosphate binding protein recruited to actin assembly sites at cellular membranes. J. Cell Sci. 117, 3473-3480. [DOI] [PubMed] [Google Scholar]
- Rety, S., Sopkova, J., Renouard, M., Osterloh, D., Gerke, V., Tabaries, S., Russo-Marie, F., and Lewit-Bentley, A. (1999). The crystal structure of a complex of p11 with the annexin II N-terminal peptide. Nat. Struct. Biol. 6, 89-95. [DOI] [PubMed] [Google Scholar]
- Sagot, I., Regnouf, F., Henry, J. P., and Pradel, L. A. (1997). Translocation of cytosolic annexin 2 to a Triton-insoluble membrane subdomain upon nicotine stimulation of chromaffin cultured cells. FEBS Lett. 410, 229-234. [DOI] [PubMed] [Google Scholar]
- Salaun, C., James, D. J., and Chamberlain, L. H. (2004). Lipid rafts and the regulation of exocytosis. Traffic 5, 255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian, T., Pradel, L. A., Henry, J. P., Aunis, D., and Bader, M. F. (1991). The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires PKC. J. Cell Biol. 114, 1135-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, B. W., and Heizmann, C. W. (1996). The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem. Sci. 21, 134-140. [DOI] [PubMed] [Google Scholar]
- Senda, T., Okabe, T., Matsuda, M., and Fujita, H. (1994). Quick-freeze, deep-etch visualization of exocytosis in anterior pituitary secretory cells: localization and possible roles of actin and annexin II. Cell Tissue Res. 277, 51-60. [DOI] [PubMed] [Google Scholar]
- Smart, E. J., Graf, G. A., McNiven, M. A., Sessa, W. C., Engelman, J. A., Scherer, P. E., Okamoto, T., and Lisanti, M. P. (1999). Caveolins, liquidordered domains, and signal transduction. Mol. Cell Biol. 19, 7289-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag, J. M., Aunis, D., and Bader, M. F. (1988). Peripheral actin filaments control calcium-mediated catecholamine release from streptolysin-O-permeabilized chromaffin cells. Eur. J. Cell Biol. 46, 316-326. [PubMed] [Google Scholar]
- Tsui-Pierchala, B. A., Encinas, M., Milbrandt, J., and Johnson, E. M., Jr. (2002). Lipid rafts in neuronal signaling and function. Trends Neurosci. 25, 412-417. [DOI] [PubMed] [Google Scholar]
- Vitale, M. L., Seward, E. P., and Trifaro, J. M. (1995). Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron 14, 353-363. [DOI] [PubMed] [Google Scholar]
- Vitale, N., Caumont, A. S., Chasserot-Golaz, S., Du, G., Wu, S., Sciorra, V. A., Morris, A. J., Frohman, M. A., and Bader, M. F. (2001). Phospholipase D1: a key factor for the exocytotic machinery in neuroendocrine cells. EMBO J. 20, 2424-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets, T., Moser, T., Lund, P. E., Chow, R. H., Geppert, M., Sudhof, T. C., and Neher, E. (2001). Intracellular calcium dependence of large dense-core vesicle exocytosis in the absence of synaptotagmin I. Proc. Natl. Acad. Sci. USA 98, 11680-11685. [DOI] [PMC free article] [PubMed] [Google Scholar]