Significance
Many migraineurs report that their need to avoid light is driven mainly by how unpleasant it makes them feel. Seeking to understand why light is unpleasant, we show here that light can trigger the perception of chest tightness, shortness of breath, light-headedness, dry mouth, irritability, sadness, and fear (among other aversive symptoms identified), and that these perceptions are mediated by newly described neuronal pathways through which electrical signals generated by light travel from the eye through the hypothalamus to neurons that regulate autonomic functions and emotions. We conclude that the aversive nature of light during migraine is more complex than its association with headache intensification.
Keywords: photophobia, parasympathetic, sympathetic, emotions, colors
Abstract
Migraineurs avoid light because it intensifies their headache. However, this is not the only reason for their aversion to light. Studying migraineurs and control subjects, we found that lights triggered more changes in autonomic functions and negative emotions during, rather than in the absence of, migraine or in control subjects, and that the association between light and positive emotions was stronger in control subjects than migraineurs. Seeking to define a neuroanatomical substrate for these findings, we showed that, in rats, axons of retinal ganglion cells converge on hypothalamic neurons that project directly to nuclei in the brainstem and spinal cord that regulate parasympathetic and sympathetic functions and contain dopamine, histamine, orexin, melanin-concentrating hormone, oxytocin, and vasopressin. Although the rat studies define frameworks for conceptualizing how light triggers the symptoms described by patients, the human studies suggest that the aversive nature of light is more complex than its association with headache intensification.
Photophobia, defined as exacerbation of headache by light, is common among migraine patients undergoing acute attacks (1–3). Although most report that light increases their headache intensity, a significant portion reports that it is predominantly unpleasant. Either way, the need to avoid light renders migraineurs dysfunctional as they are forced to quit fundamental daily tasks to seek the comfort of darkness.
Following the notion that migraine-type photophobia is driven by disease-related hyperexcitable visual cortex (4–7), efforts to characterize photophobia have focused on the notion that light is avoided because it increases visual discomfort and headache intensity (2, 8–11) and because it gives rise to an uncomfortable sense of glare (8). Attempting to understand better how light intensifies the headache, we showed recently (i) that light exacerbates headache intensity in blind migraineurs who perceive light but have no sight as a result of loss of rods and cones, but not in blind migraineurs who lack light perception as a result of optic nerve degeneration; (ii) that retinal ganglion cells that contain melanopsin, a photoreceptor with peak sensitivity to blue light (12–14), converge on thalamic trigeminovascular neurons that relay nociceptive signals from the dura to the somatosensory and visual cortices (15); (iii) that certain colors of light exacerbate migraine headache more than others; and (iv) that the amplitude of the electrical signals that are generated in the retina and cortex of migraine patients with normal eyesight is larger in response to colors of light that hurt more compared with colors of light that hurt less (9). In the course of these studies, we observed cases in which the perception that light intensifies the headache was (i) driven by the spread of the headache from one side of the head to the other and/or from the front to the back (thus involving a large part of the cranium) and (ii) that headache begun to throb rather than actually increasing in intensity. We also documented cases in which migraineurs reported discomfort even when the light did not cause the headache to intensify, spread, or throb. In the open-ended interview that followed the documentation of headache intensity, spread, and throbbing, a pattern emerged in which patients reported that their aversion to light was the result of unwanted/unpleasant changes light caused in autonomic functions, affective responses, and physiological adjustments.
Accordingly, we hypothesized that certain colors of light worsen migraine symptoms and/or trigger new ones by interacting directly with hypothalamic neurons that regulate autonomic, affective, and/or physiological functions, and project to brainstem and spinal cord nuclei that contain parasympathetic and sympathetic preganglionic neurons, respectively. To test this hypothesis, we first determined the breadth of neurological responses which, when induced, can contribute to the aversion to light during migraine, and then mapped retinal projections to functionally/chemically identified hypothalamic neurons, including those regulating sympathetic and parasympathetic functions. Here we provide clinical and preclinical insights into the aversive nature of light during migraine.
Results
Clinical Study.
Subject screening, demographics, and categorization of symptoms.
Eighty-one patients diagnosed with migraine (16), photophobia, and no documented ocular diseases and 17 healthy subjects were recruited for this study. Light-induced symptoms were documented by using psychophysical assessments of responses to different colors of light during and between attacks or at any time for the healthy controls. Their demographic and headache characteristics are shown in Table S1. They were 41 ± 13 y of age (mean ± SD), mostly female (91%), with migraine history of 19 ± 14 y. Their attacks lasted 60 ± 57 h, and were associated with aura (36%), moderate to severe headache intensity (94%), unilateral location (69%), pulsating quality (72%), nausea or vomiting (81%), and phonophobia (81%). The 17 age-matched control subjects were 43 ± 16 y of age and were healthy with no history of migraine, photophobia, ocular diseases, or chronic pain.
Table S1.
Characteristics of migraineurs
| ID | Interictal/Ictal | Age (y) | Sex | Years with migraine | Attacks per month* | CM vs. EM | Visual aura | Usual duration with no treatment (h) | Unilateral location | Pulsating quality | Usual pain intensity moderate or severe | Aggravated by physical activity | Nausea and/or vomiting | Phonophobia† |
| 1 | INT | 29 | F | 16 | 8 | EM | MO | 48 | — | √ | — | — | √ | — |
| 2 | BTH | 38 | F | 22 | 5 | EM | MA | 72 | √ | √ | √ | √ | √ | — |
| 3 | BTH | 49 | F | 37 | 6 | EM | MA | 72 | √ | √ | √ | √ | √ | √ |
| 4 | INT | 34 | F | 19 | 9 | EM | MA | 48 | √ | √ | √ | √ | √ | √ |
| 5 | BTH | 57 | F | 7 | 1 | EM | MO | 168 | √ | √ | √ | — | √ | √ |
| 6 | BTH | 38 | F | 23 | 3 | EM | MO | 72 | √ | √ | √ | √ | √ | √ |
| 7 | BTH | 27 | F | 17 | 9 | EM | MA | 18 | √ | √ | √ | √ | — | √ |
| 8 | BTH | 49 | F | 28 | 4 | EM | MO | 72 | — | √ | √ | √ | √ | √ |
| 9 | INT | 31 | F | 5 | — | — | MO | 12 | √ | √ | √ | √ | √ | √ |
| 10 | BTH | 37 | F | 27 | 8 | EM | MA | 72 | — | √ | √ | √ | √ | √ |
| 11 | INT | 50 | F | 20 | 8 | EM | MO | 72 | √ | √ | √ | — | √ | √ |
| 12 | INT | 49 | F | 19 | 4.5 | EM | MO | 168 | √ | √ | √ | — | √ | √ |
| 13 | INT | 22 | F | 4 | 9 | EM | MA | 8 | √ | √ | √ | √ | √ | √ |
| 14 | BTH | 30 | F | 9 | 5.5 | EM | MA | ≥4 | — | — | √ | — | — | — |
| 15 | INT | 37 | F | 25 | 16 | CM | MO | ≥4 | √ | √ | √ | — | √ | √ |
| 16 | INT | 53 | F | 36 | 1 | EM | MO | 24 | √ | √ | √ | — | √ | √ |
| 17 | INT | 44 | F | 32 | 4.5 | EM | MO | 24 | √ | — | √ | √ | √ | √ |
| 18 | INT | 47 | M | 24 | 4.5 | EM | MO | 72 | √ | √ | √ | — | √ | — |
| 19 | INT | 53 | F | 23 | 4 | EM | MO | ≥4 | √ | √ | √ | √ | √ | √ |
| 20 | BTH | 46 | F | 9 | 1 | EM | MA | 72 | √ | — | √ | √ | √ | √ |
| 21 | BTH | 17 | M | 15 | 2.5 | EM | MO | 120 | √ | √ | √ | √ | √ | √ |
| 22 | INT | 58 | F | 45 | 15 | EM | MO | 84 | √ | √ | √ | √ | √ | √ |
| 23 | INT | 48 | F | 7 | 2.5 | CM | MO | 72 | — | — | √ | √ | √ | √ |
| 24 | INT | 40 | F | 19 | 7 | EM | MA | 36 | — | √ | √ | — | — | √ |
| 25 | BTH | 77 | F | 63 | 4 | EM | MO | 72 | √ | √ | √ | √ | √ | √ |
| 26 | INT | 39 | F | 23 | 2.5 | EM | MA | 48 | √ | √ | √ | √ | √ | √ |
| 27 | INT | 20 | F | 4 | 1 | EM | MA | 24 | √ | √ | √ | √ | √ | √ |
| 28 | ICT | 47 | F | 26 | >15 | CM | MO | ≥4 | — | — | √ | — | — | — |
| 29 | BTH | 29 | F | 20 | 9 | CM | MO | 72 | √ | √ | √ | √ | √ | √ |
| 30 | BTH | 41 | F | 27 | 1 | EM | MO | 72 | — | √ | √ | √ | √ | √ |
| 31 | INT | 25 | F | 9 | 4 | EM | MO | ≥4 | √ | √ | √ | √ | √ | √ |
| 32 | BTH | 42 | F | 2 | 2.5 | EM | MO | 96 | √ | √ | √ | √ | √ | √ |
| 33 | ICT | 33 | F | 12 | >15 | CM | MO | ≥4 | — | — | √ | — | — | — |
| 34 | INT | 27 | F | 15 | 9 | EM | MO | ≥4 | √ | √ | √ | √ | √ | √ |
| 35 | INT | 33 | F | 15 | 1.6 | EM | MA | 10 | √ | √ | √ | √ | √ | √ |
| 36 | INT | 20 | F | 3 | 3 | EM | MO | 72 | √ | √ | √ | √ | √ | — |
| 37 | INT | 34 | F | 9 | 2.5 | EM | MA | 48 | √ | √ | √ | — | √ | — |
| 38 | INT | 59 | M | 23 | >15 | EM | MO | ≥4 | √ | √ | √ | √ | √ | √ |
| 39 | INT | 26 | F | 16 | 2.5 | CM | MA | 24 | — | √ | √ | — | — | √ |
| 40 | INT | 55 | F | 16 | 5 | EM | MA | 72 | √ | — | √ | √ | √ | √ |
| 41 | INT | 33 | F | 25 | 2.5 | EM | MA | 72 | — | √ | √ | √ | √ | √ |
| 42 | BTH | 39 | F | 19 | 2.5 | EM | MO | 72 | √ | — | √ | — | √ | √ |
| 43 | INT | 28 | F | 17 | 8 | EM | MO | 4 | √ | √ | √ | √ | √ | √ |
| 44 | BTH | 46 | F | 28 | 12 | CM | MA | 48 | — | √ | √ | √ | √ | √ |
| 45 | INT | 23 | F | 2 | 1 | EM | MA | 10 | √ | — | √ | — | √ | √ |
| 46 | INT | 26 | F | 5 | 2.5 | EM | MA | 6 | — | √ | √ | √ | √ | √ |
| 47 | ICT | 49 | F | 41 | 4.5 | EM | MO | 24 | √ | √ | √ | √ | √ | √ |
| 48 | INT | 78 | F | 59 | 4 | EM | MO | 36 | — | — | — | — | — | — |
| 49 | BTH | 49 | F | 30 | 3 | EM | MO | 5 | √ | — | √ | √ | √ | √ |
| 50 | ICT | 47 | F | 26 | 12 | CM | MO | ≥4 | — | — | √ | — | √ | — |
| 51 | INT | 31 | F | 18.5 | 2.5 | EM | MO | 8 | — | √ | √ | — | √ | √ |
| 52 | ICT | 53 | F | 15 | 30 | CM | MO | 24 | √ | — | √ | √ | √ | √ |
| 53 | BTH | 42 | M | 13 | 12 | CM | MO | 15 | √ | √ | √ | √ | — | √ |
| 54 | BTH | 24 | F | 14 | 0.5 | EM | MO | 72 | — | √ | √ | √ | √ | √ |
| 55 | BTH | 39 | F | 23 | 10 | EM | MA | ≥4 | — | √ | √ | — | √ | √ |
| 56 | BTH | 38 | F | 2 | 4 | EM | MA | 72 | — | — | √ | √ | √ | √ |
| 57 | BTH | 49 | F | 34 | 2 | EM | MA | 180 | √ | √ | √ | √ | √ | √ |
| 58 | INT | 37 | F | 27 | 1.5 | EM | MO | 48 | √ | — | √ | √ | √ | √ |
| 59 | BTH | 36 | F | 27 | 7 | EM | MO | 168 | √ | — | √ | — | √ | √ |
| 60 | INT | 52 | F | 36 | 6 | EM | MO | 48 | √ | √ | √ | √ | √ | √ |
| 61 | BTH | 24 | F | 7 | 4 | EM | MO | 36 | √ | √ | √ | √ | — | √ |
| 62 | BTH | 33 | F | 21 | 17.5 | CM | MO | 168 | √ | √ | √ | √ | √ | √ |
| 63 | ICT | 44 | F | 19 | >15 | CM | MO | 24 | √ | √ | √ | — | — | √ |
| 64 | INT | 55 | F | 36 | — | — | MO | 12 | — | — | — | — | — | — |
| 65 | ICT | 41 | F | 15 | >15 | CM | MA | 240 | √ | √ | √ | √ | √ | √ |
| 66 | BTH | 56 | F | 4 | 16 | CM | MA | 72 | √ | √ | √ | √ | √ | √ |
| 67 | BTH | 63 | F | 15.5 | 16 | CM | MA | 72 | √ | √ | √ | √ | √ | √ |
| 68 | BTH | 49 | F | 14 | 16 | CM | MA | 36 | √ | √ | √ | √ | √ | √ |
| 69 | ICT | 30 | M | 3 | 25 | CM | MA | 72 | — | √ | √ | √ | √ | √ |
| 70 | ICT | 35 | F | 16 | 5.5 | EM | MO | ≥4 | √ | √ | — | — | — | √ |
| 71 | ICT | 29 | F | 2 | 8 | EM | MO | 24 | — | √ | √ | — | √ | √ |
| 72 | ICT | 52 | F | 27 | 9 | EM | MO | 24 | √ | — | √ | — | — | √ |
| 73 | INT | 27 | F | — | — | — | — | — | — | — | — | — | — | — |
| 74 | INT | 26 | F | 19 | 15 | CM | MO | 6 | √ | √ | √ | √ | √ | √ |
| 75 | ICT | 29 | F | 11 | 20 | CM | MA | 8 | — | √ | √ | — | √ | √ |
| 76 | BTH | 62 | M | 54 | 8 | EM | MO | 48 | — | — | √ | — | √ | √ |
| 77 | BTH | 46 | F | 26 | 15 | CM | MO | 72 | √ | √ | √ | √ | √ | √ |
| 78 | BTH | 28 | F | 10 | 6 | EM | MO | 72 | √ | √ | √ | √ | √ | — |
| 79 | INT | 45 | F | 20 | 4.5 | EM | MO | ≥4 | √ | — | √ | — | √ | — |
| 80 | INT | 64 | M | 45 | 1 | EM | MA | 36 | √ | — | √ | — | √ | — |
| 81 | BTH | 34 | F | 22 | 1.5 | EM | MO | 60 | √ | √ | √ | √ | — | √ |
BTH, completed both interictal and ictal visits; CM, chronic migraine; EM, episodic migraine; F, female; ICT, completed ictal visit only, INT, completed interictal visit only; M, male; MA, migraine with aura; MO, migraine without aura; —, no; √, yes.
All participants were screened and confirmed by a study physician to have migraine according to the International Classification of Headache Disorders (18).
All participants had photophobia as a criterion of inclusion in the study.
Light-induced symptoms were grouped as (i) hypothalamic-mediated autonomic, (ii) hypothalamic nonautonomic, (iii) affective negative, and (iv) affective positive responses. Description of hypothalamic-mediated autonomic responses included the perception of chest tightness, throat tightness, shortness of breath, fast breathing, faster than usual heart rate, light-headedness, dizziness, nausea, vomiting, dry mouth, salivation, rhinorrhea, stuffy sinuses, and lacrimation. Description of experiences we assigned to nonautonomic hypothalamic functions included thirst and hunger (regulation of feeding) and feeling drowsy, tired, sleepy, or fatigued and actual yawning (regulation of sleep). Description of affect was comprised of negative and positive emotions. Negative emotions were expressed most frequently with words such as intense, irritable, angry, nervous, hopeless, needy, agitated, sad, scared, cranky, upset, depressed, disappointed, jittery, worried, stressed, anxious, panic, and fear, and by actual crying. Positive emotions were expressed most frequently with words such as happy, relaxing, soothing, and calming.
Psychophysical studies assessing autonomic responses, hypothalamic functions, and affect in migraine patients and control subjects.
To determine if symptoms induced by nonselective (i.e., any of the five tested colors or intensity) and selective (white, blue, green, amber, red; regardless of intensity) photic stimuli depended on whether the participant was a control subject or a migraineur during ictal or interictal phase, we first compared the percentage of subjects in each of these groups reporting one or more symptoms we attributed to (i) hypothalamic-mediated autonomic responses, (ii) hypothalamic nonautonomic functions, (iii) negative emotions, and (iv) positive emotions.
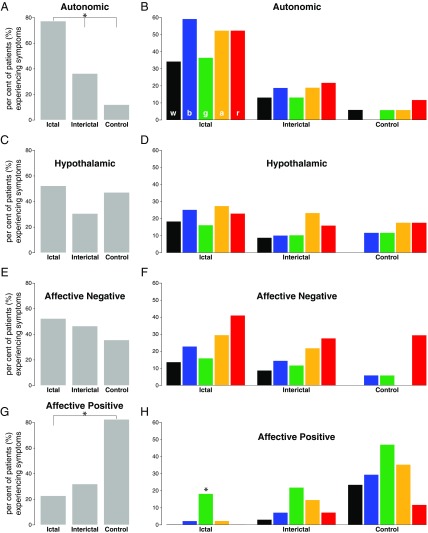
i) The proportion of migraine patients in which nonselective photic stimuli induced hypothalamic-mediated autonomic responses (Fig. 1A) was significantly higher during the ictal (nearly 80%) than during the interictal (<40%) phase or compared with the percentage of control subjects (P < 0.001; Tables 1 and 2). This was also the case for the selective photic stimuli (Fig. 1B), which ranged between 30% and 60% in the ictal group, 10% and 20% in the interictal group, and 0% and 10% in the control group. As shown in Fig. 1B, each of the five tested lights yielded a significantly higher number of autonomic responses in the ictal phase compared with the interictal phase or the control group (Tables 1 and 2). In contrast, the proportion of migraine patients in which nonselective as well as selective photic stimuli induced autonomic responses during the interictal phase (although higher) did not differ from the proportion of control subjects reporting these responses (Table 3).
ii) The proportion of migraine patients and control subjects reporting that nonselective photic stimuli induced alteration in (nonautonomic) hypothalamic functions (which ranged between 50% and 30%; Fig. 1C) was similar among the three groups (P > 0.05; Tables 1–3). This was also the case for the selective photic stimuli (Fig. 1D), which ranged between 10% and 20% among the three groups (P > 0.05 for each of the five tested lights; Tables 1–3).
iii) As with hypothalamic functions, the proportion of migraine patients and control subjects reporting that nonselective photic stimuli (usually in the low-intensity range of 5 and 10 cd/m2) provoked negative emotions (which ranged between 55% and 35%; Fig. 1E) was also similar among the three groups (P > 0.05; Tables 1–3). This was also the case for the selective photic stimuli (Fig. 1F), which ranged between 10% and 40%, 10% and 30%, and 0% and 30% in the ictal, interictal, and control groups, respectively (P > 0.05 for each of five tested lights; Tables 1–3).
iv) Unlike the previously described findings, the proportion of migraine patients and control subjects reporting that nonselective photic stimuli produced positive emotions (Fig. 1G) was significantly higher among the control subjects (80%) than among the ictal (approximately 20%), but not interictal (approximately 30%), migraine patients (P < 0.001; Tables 2 and 3). This was also the case for the selective photic stimuli (Fig. 1H), which ranged between 0% and 20% in the ictal group, 5% and 30% in the interictal group, and 10% and 50% in the control group. As depicted in Tables 1–3, each of the five tested lights yielded a significantly higher number of positive emotions in the control group compared with the ictal group (Table 2), but not the interictal group (Table 3). In contrast, the proportion of migraine patients in which nonselective as well as selective photic stimuli induced positive emotions during the interictal phase (although higher) did not differ from the proportion of migraine patients reporting positive emotions in the ictal phase (Table 1).
Fig. 1.
Effects of light and color on autonomic responses, hypothalamic functions, and affect. (A and B) Proportion of migraine patients and control subjects experiencing autonomic responses to nonselective (A; all colors combined) and selective [B; white (w), blue (b), green (g), amber (a), red (r)] photic stimuli. Autonomic responses included the perception of chest tightness, throat tightness, shortness of breath, fast breathing, faster-than-usual heart rate, light-headedness, dizziness, nausea, vomiting, dry mouth, salivation, rhinorrhea, stuffy sinus, and/or lacrimation. (C and D) Proportion of migraine patients and control subjects experiencing alteration in hypothalamic functions related to regulation of sleep and food intake in response to nonselective (C) and selective (D) photic stimuli. Hypothalamic responses included feeling sleepy, drowsy, tired, hungry, and/or thirsty. (E and F) Proportion of migraine patients and control subjects experiencing negative emotions in response to nonselective (E) and selective (F) photic stimuli. Emotions were classified negative when defined by participants as intense, irritable, angry, nervous, hopeless, needy, agitated, sad, scared, cranky, upset, depressed, disappointed, jittery, worried, stressed, anxious, “panic and fear,” and/or actual crying. (G and H) Proportion of migraine patients and control subjects experiencing positive emotions in response to nonselective (G) and selective (H) photic stimuli. Emotions were classified positive when defined by participants as happy, relaxing, soothing, and/or calming. Migraine patients were tested twice: once during the ictal and once during the interictal phase. Asterisk shows statistically significant P values (P < 0.03) considering Bonferroni-corrected α for multiple comparisons. Note that autonomic responses to light occurred most frequently during migraine and least frequently in control subjects, whereas most reports of positive emotions were provided by control subjects and the least by migraine patients undergoing acute attack. Also note lack of color effect in all aspects of the study except the percentage of patients experiencing positive emotions to green light during migraine (H) (P< 0.001).
Table 1.
Ictal vs. interictal comparisons of the percentage of patients who experienced symptoms from a specific group in response to different colors of light
| Color | Autonomic* | Hypothalamic | Affective negative | Affective positive |
| White | 0.0026† | 0.136 | 0.406 | 0.254 |
| Blue | <0.0001† | 0.180 | 0.263 | 0.250 |
| Green | 0.0036† | 0.363 | 0.509 | 0.645 |
| Amber | 0.0002† | 0.624 | 0.347 | 0.160 |
| Red | 0.0008† | 0.368 | 0.139 | 0.335 |
Comparing the response proportion to all colors combined yielded P < 0.0001.
Statistically significant P values considering Bonferroni-corrected α for multiple comparisons of ictal vs. interictal.
Table 2.
Ictal vs. healthy controls comparisons of the percentage of patients who experienced symptoms from a specific group in response to different colors of light
| Color | Autonomic* | Hypothalamic | Affective negative | Affective positive* |
| White | 0.0244 | 0.0588 | 0.110 | 0.00086† |
| Blue | <0.0001† | 0.258 | 0.126 | 0.00142† |
| Green | 0.0173 | 0.6818 | 0.298 | 0.02144 |
| Amber | 0.00086† | 0.4354 | 0.0114 | 0.00028† |
| Red | 0.0039† | 0.667 | 0.4066 | 0.02088 |
Comparing the response proportion to all colors combined yielded P < 0.0001.
Statistically significant P values considering Bonferroni-corrected α for multiple comparisons of ictal vs. healthy controls.
Table 3.
Interictal vs. healthy controls comparisons of the percentage of patients who experienced symptoms from a specific group in response to different colors of light
| Color | Autonomic | Hypothalamic | Affective negative | Affective positive |
| White | 0.407 | 0.210 | 0.208 | 0.003* |
| Blue | 0.530 | 0.842 | 0.342 | 0.055 |
| Green | 0.406 | 0.842 | 0.490 | 0.091 |
| Amber | 0.194 | 0.624 | 0.199 | 0.088 |
| Red | 0.358 | 0.865 | 0.881 | 0.542 |
Statistically significant P values considering Bonferroni-corrected α for multiple comparisons of interictal vs. healthy controls.
To determine if induction of hypothalamic-mediated autonomic responses, alteration of hypothalamic functions, and negative or positive affects depend on the color of light, we also performed a within-group analysis whereby we assessed the effects of white and the four different colors of light on the proportion of ictal and interictal migraine patients and control subjects who reported one or more symptoms of hypothalamic-mediated autonomic responses (Fig. 1B), alteration in hypothalamic functions (Fig. 1D), and negative (Fig. 1F) and positive emotions (Fig. 1H). This analysis revealed no color preference for induction of autonomic responses, alteration of hypothalamic functions, and provocation of negative emotions within any of the three groups (Fig. 1 B, D, and F). As indicted in Table 4, our post hoc nonparametric binomial comparison of all pairs (e.g., green vs. red) yielded P values that were lower than the Bonferroni-corrected α threshold for significance. In contrast, we found color preference in the ability to provoke positive emotions during migraine (i.e., in the ictal phase), but not in the interictal phase or in the control group (Fig. 1H). Although the percentage of migraine patients and control subjects reporting positive emotions when exposed to green light was higher than all other colors in all three groups, the post hoc nonparametric binomial proportion comparisons demonstrated significant differences between the response proportion to green vs. the remaining wavelengths (considering Bonferroni-corrected α threshold for significance) in the ictal group only (P < 0.007; Table 4).
Table 4.
Descriptive statistics and χ2 analyses examining proportion differences in response to the applied visual stimuli within various conditions
| Color | Autonomic | Hypothalamic | Affective negative | Affective positive | ||||||||
| Ictal | Interictal | HC | Ictal | Interictal | HC | Ictal | Interictal | HC | Ictal | Interictal | HC | |
| White | 15 | 9 | 1 | 8 | 6 | 0 | 6 | 6 | 0 | 0 | 2 | 4 |
| Blue | 26 | 13 | 0 | 11 | 7 | 2 | 10 | 10 | 1 | 1 | 5 | 5 |
| Green | 16 | 9 | 1 | 7 | 7 | 2 | 7 | 8 | 1 | 8 | 15 | 8 |
| Amber | 23 | 13 | 1 | 12 | 16 | 3 | 13 | 15 | 0 | 1 | 10 | 6 |
| Red | 23 | 15 | 2 | 10 | 11 | 3 | 18 | 19 | 5 | 0 | 5 | 2 |
| Total | 103 | 59 | 5 | 48 | 47 | 10 | 54 | 58 | 7 | 10 | 37 | 25 |
| χ2 | 4.524 | 2.441 | 0.600 | 1.792 | 7.362 | 0.400 | 8.778 | 9.759 | 4.571 | 9.800 | 14.216 | 4.000 |
| P value* | 0.340 | 0.655 | 0.896 | 0.774 | 0.118 | 0.940 | 0.067 | 0.045† | 0.102 | 0.007‡ | 0.007§ | 0.406 |
The ictal-phase experiment included 44 patients, the interictal experiment 69, and the HC group 17. Corresponding proportions are shown in Fig. 1. HC, healthy control.
χ2 test.
Post hoc nonparametric binomial proportion comparisons of all pairs yielded P values lower than the Bonferroni-corrected α threshold for significance.
Post hoc nonparametric binomial proportion comparisons suggest significant differences between the response proportion to green vs. the remaining wavelengths, considering Bonferroni-corrected α threshold for significance.
Post hoc nonparametric binomial proportion comparisons suggest significant differences between the response proportion to green vs. white only, considering Bonferroni-corrected α threshold for significance.
Preclinical Studies.
Retinal innervation of hypothalamic neurons that project to parasympathetic and sympathetic nuclei.
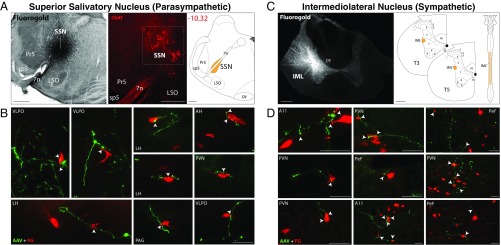
To delineate possible pathways for induction of autonomic responses to light, we searched for convergence of retinal axons on hypothalamic neurons that project to the parasympathetic superior salivatory nucleus (SSN) in the brainstem and the sympathetic intermediolateral nucleus (IML) in the spinal cord. To accomplish this, we first labeled retinal afferents in the hypothalamus by injecting a recombinant adenoassociated viral vector encoding for GFP (rAAV-GFP) into the eye of adult albino rats. Three weeks later, we retrogradely labeled hypothalamic neurons that project to the SSN and IML by filling each of these nuclei with the retrograde tracer Fluoro-Gold (FG; Methods). Neurons that project to the SSN and receive direct input from retinal ganglion cells were found mainly in the hypothalamic paraventricular nucleus (PVN) and lateral hypothalamus (LH) and perifornical area (PeF) nuclei, where GFP-positive retinal axons and axonal buttons were seen in close apposition to 4.6% and 2.4% of all FG-labeled neurons, respectively (Fig. 2 A and B). Outside these nuclei, occasional (<1%) apposition between GFP-positive retinal axons and FG-labeled neurons were observed in the anterior hypothalamus, ventrolateral preoptic nucleus, and periaqueductal gray (PAG; Fig. 2B). We classified these neurons as belonging to the retinohypothalamic-parasympathetic (RHP) pathway.
Fig. 2.
Retinal innervation of hypothalamic neurons that project to the SSN in the brainstem and IML of the spinal cord. (A) Iontophoretic injections of FG into the SSN (Left) were confirmed by staining sections containing the injection site with choline acetyltransferase (Middle), a marker of parasympathetic preganglionic neurons in the SSN. Location of SSN in the brainstem is represented on the right. (B) Anterogradely labeled retinal axons (green; GFP) shown in close apposition (arrowheads) with retrogradely labeled neurons in the hypothalamus, preoptic area, and PAG that project to the SSN. (C) Iontophoretic injections of FG into the IML (Left) and illustration of its location in the spinal cord (Right). (D) Anterogradely labeled retinal axons (green; GFP) shown in close apposition (arrowheads) with retrogradely labeled hypothalamic neurons that project to the IML. A11, dopaminergic hypothalamic nucleus; AAV, adenoassociated virus with reporter gene for GFP; AH, anterior hypothalamus; LSO, lateral superior olive; Pr5, principal sensory trigeminal nucleus; py, pyramidal tract; PVN, paraventricular hypothalamic nucleus; sp5, spinal trigeminal tract; T3/T5, thoracic spinal cord segments 3 and 5; VLPO, ventrolateral preoptic area; 7n, facial nerve; 4V, fourth ventricle. (Scale bars: A, 500 μm; C, 200 μm; B and D, 50 μm.)
Neurons that project to the IML and receive direct input from retinal ganglion cells were found in three hypothalamic nuclei: PVN, PeF, and the dopaminergic A11 nucleus. In these nuclei, GFP-positive axons were seen in close apposition to 3.4%, 18%, and 26% of all FG-labeled neurons, respectively (Fig. 2 C and D). We classified these neurons as belonging to the retinohypothalamic-sympathetic (RHS) pathway.
Retinal innervation of chemically identified hypothalamic neurons.
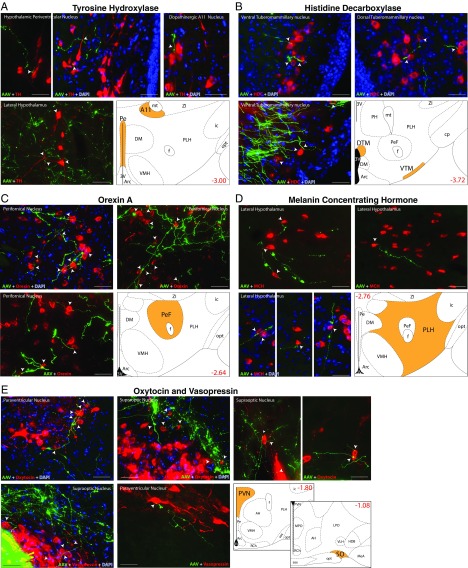
Given the large number of light-induced symptoms attributed to hypothalamic regulation of autonomic and endocrine functions, we further sought to identify the neuropeptides and/or neurotransmitter contained in the hypothalamic neurons that receive direct input from the retina. To accomplish this, we performed immunofluorescence with antibodies against biomarkers for dopamine/noradrenaline, orexin, melanin concentrating hormone (MCH), oxytocin, and vasopressin on neural tissue containing GFP-labeled retinal afferents in the hypothalamus (Methods). GFP-positive axons and varicosities were seen in close apposition to 9.7% and 6.7% of all tyrosine hydroxylase (TH)-labeled (dopaminergic/noradrenergic) neurons in the periventricular and A11 nuclei, respectively, and few (<1%) such neurons in the LH (Fig. 3A); 8.2% of all histidine decarboxylase (HDC)-labeled (histaminergic) neurons in the ventral tuberomammillary nucleus and few such neurons in the dorsal tuberomammillary (Fig. 3B); 30% of all orexinergic neurons in the perifornical area (Fig. 3C); 2.7% of all MCH-labeled neurons in the LH (Fig. 3D); and approximately 10% and 7% of all oxytocinergic and vasopressinergic neurons in the PVN and supraoptic nuclei, respectively (Fig. 3 E and F). Evidence of close apposition between retinal afferents and hypothalamic cell bodies or dendrites is provided in Fig. S1.
Fig. 3.
Retinal innervation of hypothalamic neurons containing the neurotransmitters dopamine and histamine and the neuropeptides orexin, MCH, oxytocin, and vasopressin. (A) Immunopositive TH neurons (red) in close apposition to retinal axons and varicosities (green). (B) Immunopositive histaminergic neurons in close apposition to retinal axons and varicosities. (C) Immunopositive orexinergic neurons in close apposition to retinal axons and varicosities. (D) Immunopositive MCH neurons in close apposition to retinal axons and varicosities. (E) Immunopositive oxytocinergic and vasopressinergic neurons in close apposition to retinal axons and varicosities. Reconstructions in lower right panels show locations of neurons in the different hypothalamic areas and nuclei. Numbers in red indicate distance from bregma. Arrowheads point to close appositions. (Scale bars: 50 μm.) Arc, arcuate nucleus; cp, cerebral peduncle; DM, dorsomedial hypothalamic nucleus; DTM, dorsal tuberomammillary nucleus; f, fornix; HDB, horizontal limb of the diagonal band; ic, internal capsule; LPO, lateral preoptic area; MeA, medial amygdaloid nucleus; MPO, medial preoptic nucleus; mt, mammillothalamic tract; opt, optic tract; Pe, periventricular nucleus; PH, posterior hypothalamic nucleus; PLH, peduncular part of the LH; RCh, retrochiasmatic area; SO, supraoptic nucleus; sox, supraoptic decussation; VLH, ventrolateral hypothalamic nucleus; VMH, ventromedial hypothalamus; VTM, ventral tuberomammillary nucleus; ZI, zona incerta; 3V, third ventricle. Other abbreviations are defined in Fig. 2.
Fig. S1.
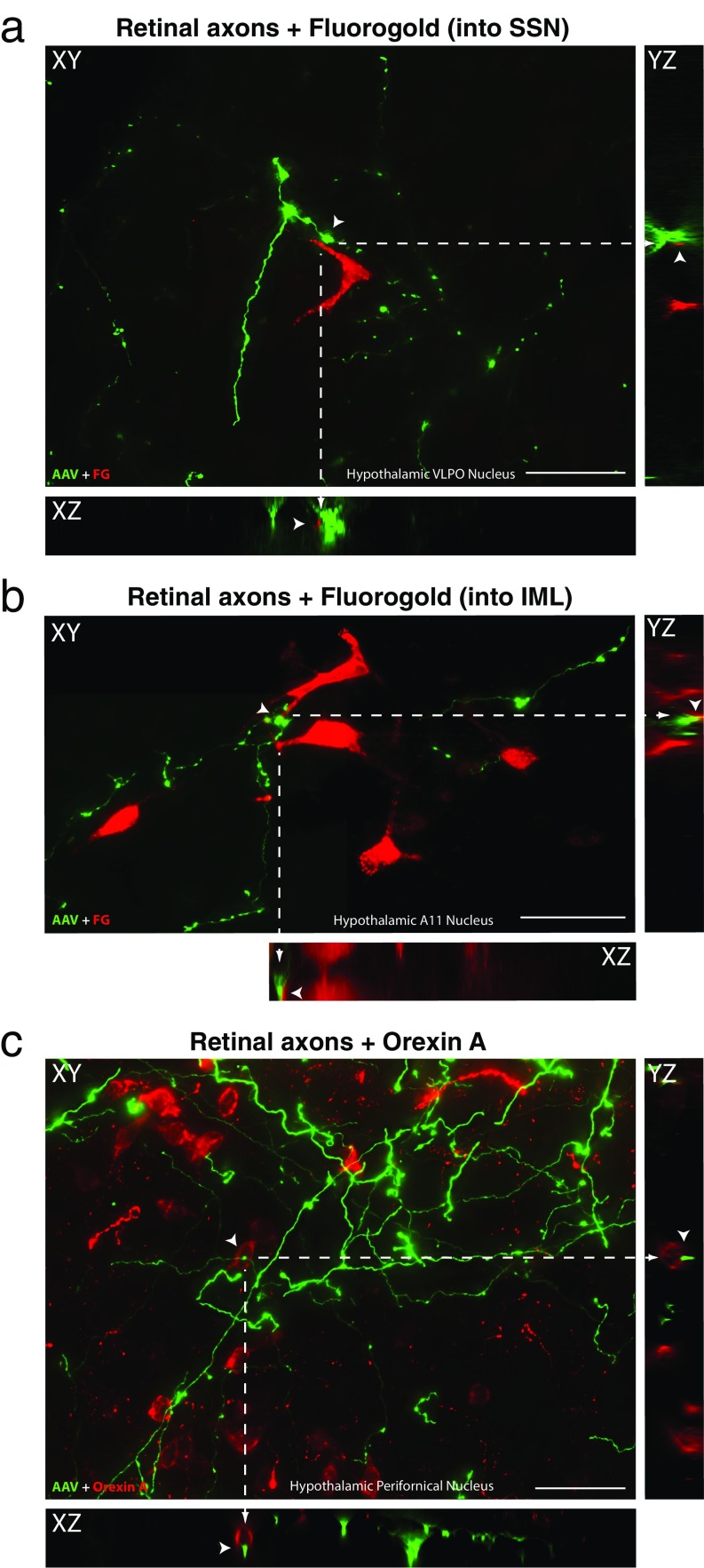
Close apposition between anterogradely labeled retinal axons and hypothalamic neurons. Examples of retinal axons (green; AAV-GFP) in close proximity (arrowheads) with hypothalamic neurons (red) that were (A) retrogradely labeled from the SSN in the brainstem, (B) retrogradely labeled from the IML in the spinal cord, and (C) chemically identified (Orexin A in this example) using immunofluorescence. A series of 2D (XY) images were obtained by scanning every 1 µm across the z plane. These images were used to create orthogonal views in the XZ and YZ planes to provide evidence that retinal fibers may contact neuronal somas and/or dendrites in the hypothalamus (as shown in Figs. 2 and 3). The XY image in (B) is a composite obtained by overlapping a small portion of two adjacent images. Definitive evidence for actual synapses, however, requires examination with EM. (Scale bars: 50 µm.)
Discussion
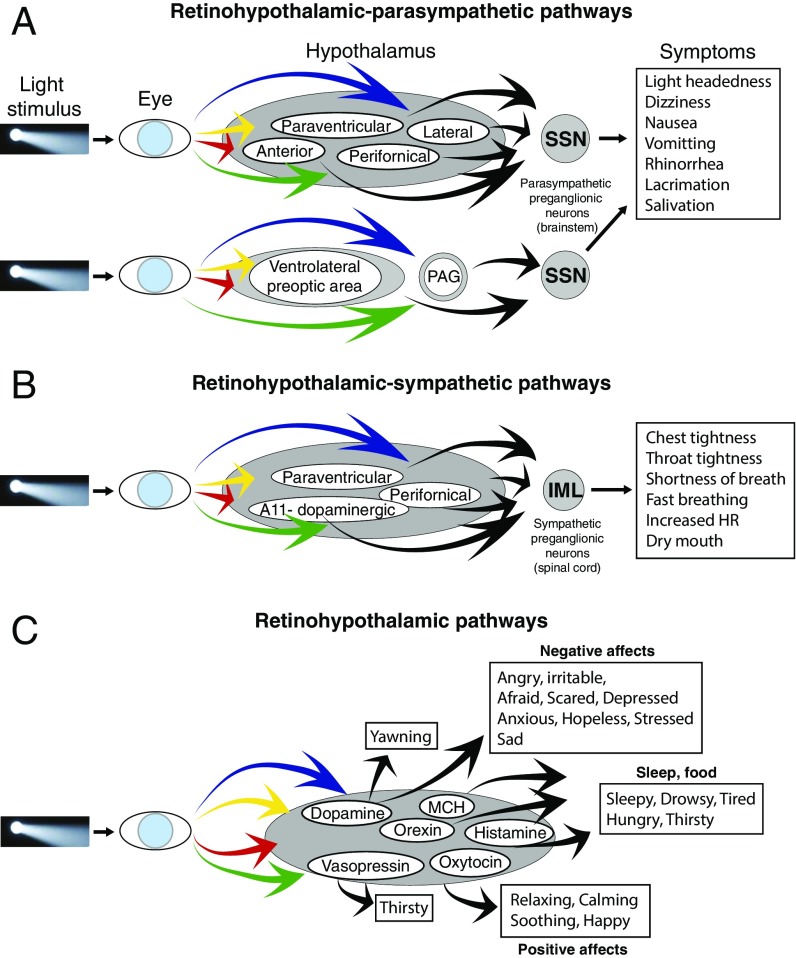
Clinically, the results of this multifaceted psychophysical study provide insight into migraine-type photophobia and induction of hypothalamic-mediated responses to light. By showing that light triggers a wide range of unpleasant sensations and emotions, the study stands to expand the definition of photophobia beyond the commonly used criteria of “headache (intensity) that is worsened by light.” Such expansion may explain why migraine patients avoid light even when it does not worsen their head pain. Preclinically, the study reports that axons of retinal ganglion cells converge on hypothalamic neurons that project to the SSN (i.e., RHP) and IML (i.e., RHS; Fig. 4 A and B). It also reports that retinal axons converge on dopaminergic/noradrenergic, histaminergic, orexinergic, MCHergic, oxytocinergic, and vasopressinergic hypothalamic neurons (Fig. 4C). These connections define an anatomical substrate for future studies on alterations of hypothalamic-mediated (autonomic and nonautonomic) functions during migraine. Because the induction of all hypothalamic-mediated unpleasant experiences was not influenced by the color of light, it is likely that the retinohypothalamic interactions observed in the study are independent of color processing by the visual cortex. This scenario differs greatly from the one showing that the perception of headache intensity is color-specific and most likely depends on sensory processing by the retinothalamocortical pathway (9).
Fig. 4.
Proposed pathways for modulation of autonomic responses, hypothalamic functions, and emotions by light. (A) Pathways for induction of symptoms associated with activation of the parasympathetic system. (B) Pathways for induction of symptoms associated with activation of the sympathetic system. (C) Pathways for inductions of symptoms mediated by dopamine, orexin, histamine, MCH, oxytocin, and vasopressin.
Hypothalamic-Mediated Autonomic Responses.
Hypothalamic-mediated autonomic responses are termed as such because, in the present study, autonomic responses are interpreted as originating in activation of hypothalamic neurons by light. The proportion of migraine patients in which photic stimuli induced autonomic responses was significantly higher during the ictal phase than during the interictal phase and compared with the percentage of control subjects. Building on the widely held view that autonomic regulation is altered during migraine (17–21), these findings offer insight into the possibility that it is hypothalamic regulation of parasympathetic and sympathetic functions that is abnormal during migraine, rather than the parasympathetic or sympathetic nervous systems themselves. However, because the induction of autonomic symptoms by light was altered during migraine only, when hypothalamic neurons are subjected to a barrage of nociceptive signals they receive from trigeminovascular/trigeminohypothalamic tract neurons (22, 23), one must keep in mind the possibility that convergence of nociceptive signals from the meninges and photic signals from the retina are required to produce the abnormal hypothalamic-mediated autonomic responses reported here. Such a scenario can also explain why light does not trigger abnormal autonomic responses in the interictal phase or in control subjects. Given that some of the symptoms induced by light include signs of sympathetic hyperresponsiveness (i.e., chest tightness, throat tightness, shortness of breath, fast breathing, faster-than-usual heart rate, dry mouth) whereas others point to parasympathetic hyper responsiveness (i.e., light-headedness, dizziness, nausea, vomiting, rhinorrhea, lacrimation), and based on the discovery of the RHP and RHS pathways, we propose that photic signals modulate the activity of hypothalamic neurons, which, in turn, activate preganglionic parasympathetic and sympathetic neurons in the SSN and IML, respectively (Fig. 4 A and B). Because many of the mentioned symptoms disappeared after a few minutes in the dark, it will be interesting to determine whether dark may have inhibitory effect on these RHP and RHS pathways in animals or on the incidence of autonomic symptoms in migraineurs.
Mechanistically, we propose that photic stimulation of RHP neurons in the paraventricular, lateral, perifornical, and anterior hypothalamic nuclei, as well as in the ventrolateral preoptic area and PAG, mediate the so-called parasympathetic symptoms. Supporting this proposal are previous documentations of neurons in these nuclei that project to the SSN (24–26), regulate parasympathetic functions (27, 28), and, independent of these, regulate a variety of circadian rhythms by firing differently under light and dark conditions (i.e., light-sensitive) (29–31). Similarly, we propose that photic stimulation of RHS neurons in the paraventricular, perifornical, and dopaminergic A11 nuclei mediate the sympathetic symptoms. Previous identification of neurons in these nuclei that project to the IML (32–34), regulate sympathetic functions (35–37), and respond to light (29–31) further supports this scenario. Therapeutically, the treatment of photophobia by blocking the sympathetic superior cervical ganglion (38, 39) may be mediated at least in part by the consequential reduction in some of the more unpleasant sympathetic responses to light described here.
Hypothalamic-Mediated Nonautonomic Responses.
Hypothalamic mediated nonautonomic responses are termed as such because the execution of sleep and food intake behaviors is believed to be regulated by the hypothalamus. The induction of hypothalamic-mediated nonautonomic responses to light was not specific to migraine patients or to the migraine attacks. It occurred in 10–20% of the participants, regardless of whether they were migraineurs or nonmigraineurs or whether they were at the ictal or interictal state. Because light modulation of physiological functions associated with sleep (i.e., feeling drowsy, tired, sleepy, fatigued) (29, 30, 40) and food intake (i.e., thirst, hunger) (41, 42) occur in all mammals, it was somewhat expected that light will trigger these symptoms to a certain extent in all participants. However, as sleep deprivation and extended fasting are among the most common migraine triggers (43–45), we were surprised by the findings that these hypothalamically regulated functions did not occur more often in the migraineurs than in the control subjects. Interpretation of this finding must take into consideration the relatively brief period (i.e., minutes) that patients were exposed to light, as it may differ greatly from real-life prolonged (i.e., hours) exposure to light. It should also take into consideration the possibility that migraine does not alter the fundamental physiological functions of hypothalamic neurons that mediate sleep and food intake.
Affective Responses.
The induction of negative emotions by light was not specific to migraine patients or to whether they were undergoing a migraine attack. Although it was reported by more migraine patients during the ictal than the interictal phase, and by more migraine patients during the interictal phase than the control subjects, the differences were insignificant. Because blue, red, and amber lights increase headache intensity more than green light (9), we initially thought that the incidence of negative emotions would be higher during exposure to all colors but green. The current findings, however, challenge our (oversimplistic) view that head pain alone may be the principal driver of negative emotions during migraine. In fact, the description of negative emotions in the interictal phase and in the control subjects suggests that the principal driver is light rather than pain. Although this explanation is reasonable, the understanding of how colors affect emotions, although heavily studied (46–48), is extremely limited for lack of hypothesis-driven experiments and scientific data (49).
In contrast, the incidence of light-induced positive emotions such as happiness, relaxing, soothing, and calming was significantly higher in the control subjects than in the ictal migraineurs. This observation unravels yet another perspective of the “dislike of light” during migraine. As for the effects of color on the induction of positive emotions among control subjects and interictal migraineurs, there was none, suggesting that the distribution of color preference in the absence of migraine is nearly even (i.e., the number of individuals who like red, blue, green and amber is similar). This was not the case, however, during migraine. In fact, the only color preference found in this study was the one showing that green is the only color capable of inducing positive emotions during acute attack. This preference may be secondary to the unique ability of green light to reduce headache intensity (9).
Retinal Innervation of Peptidergic Neurons.
The present study found that retinal axons contact dopaminergic/monoaminergic neurons in the periventricular and A11 nuclei, histaminergic neurons in the tuberomammillary nuclei, orexinergic neurons in the perifornical area (but not lateral or medial nuclei), MCHergic neurons in the LH, and oxytocinergic and vasopressinergic neurons in the paraventricular and supraoptic nuclei. They expand the scope of retinohypothalamic projections described previously (50–53). In the context of the present study, it is tempting to propose that modulation of these peptidergic hypothalamic neurons by light, which, at best, is incompletely documented (54–56), may trigger some of the affective, autonomic, and hypothalamic symptoms. Specifically, altered dopaminergic activity can facilitate anger and irritability (57–60), fear, panic, anxiety, and stress (61), altered oxytocinergic activity can reduce stress, anxiety, and fear and facilitate the relaxing, calming, soothing, and happy affects (62, 63); altered orexinergic, MCHergic, and histaminergic activity can facilitate the perception of sleepiness and hunger (64–66), altered vasopressinergic activity can facilitate thirst (67), and many of these peptidergic neurons can promote yawning (68, 69), salivation (70, 71), lacrimation, nasal congestion, and rhinorrhea (64). Given that many of these peptides and neurotransmitters regulate each other’s secretion, that they can be antagonistic to each other in one area of the brain and synergistic in another, and that their overall activity may depend on a variety of internal and external cues, we must acknowledge that the examples provided here are vastly oversimplified. Along this line, we must acknowledge that our classification of symptoms, although logical and phenotypically justified, is also oversimplified, as hypothalamic regulation of autonomic, endocrine, physiological, behavioral, and affective responses is achieved by reciprocal connections it makes with cortical, subcortical, and spinal cord neurons that play established role is the processing of sensory and visceral information as well as cognition and emotions.
Methods
Clinical Study.
All study visits took place at Beth Israel Deaconess Medical Center (BIDMC), Boston, MA (September 2010 to May 2015). The BIDMC Committee on Clinical Investigations approved the study, and all participants provided written informed consent. Patients were recruited from the BIDMC Comprehensive Headache Center, Neurology Clinic, and the primary care practice and from flyers placed in and around BIDMC and Harvard Medical School. Women and men who were 15–85 y old were potentially eligible for the study if they met the International Classification of Headache Disorders Committee (16) criteria for migraine with or without aura, were able to communicate in English, and were willing to attend a visit during an untreated migraine attack and when migraine-free for 3 d or more. Exclusion criteria included fewer than five headache-free days per month, chronic head or neck pain not attributed to migraine, chronic use of opioids (≥ 15 d/mo for previous consecutive 3 mo or longer), or the presence of an ocular disease. For this study, ocular diseases were defined as primary and persisting visual disorders such as glaucoma, macular degeneration, retinal degenerative diseases, cone dystrophy, rod dystrophy, achromatopsia, retinitis pigmentosa, Leber’s congenital amaurosis, albinism, night blindness, or cortical blindness as a result of posterior circulation stroke. Participants were permitted to stop the study or any phase of testing at any time. Age-matched control subjects were also recruited from BIDMC’s primary care practice and from flyers placed in and around BIDMC and Harvard Medical School. Their medical interview revealed no history of headache or migraine, no chronic pain or use of opioids, and no ocular diseases.
Psychophysical studies assessing patients’ responses to different colors of light during and in between migraine attacks.
The study included 81 migraine patients and 17 healthy subjects. Of the 81 migraineurs, 44 completed the psychophysical assessments during untreated migraine attack, whereas 69 completed it after being migraine-free for at least 3 d. Before testing, patients sat in a dimly lit room for 20 min. The light was then turned off for 3 min, and patients were asked to describe their symptoms (baseline). When a baseline had been established, participants were positioned in front of a full-field Ganzfeld ColorDome (Diagnosys), the light was turned on to the lowest intensity (1 cd⋅m−2) and then increased (1, 5, 20, 50, and 100 cd⋅m−2) every 30 seconds, and this was repeated for each color. The first light was white, the second was blue (447 ± 10 nm), the third was green (530 ± 10 nm), the fourth was amber (590 ± 10 nm), and the fifth was red (627 ± 10 nm). To minimize additive effects, patients sat in total darkness for 3 min between consecutive series of stimulation or until their headache intensity or their physiological and emotional responses returned to baseline level.
To assess the effects that different colors of light had, subjects were asked to describe what they experienced while looking at the light. To minimize bias, subjects were not provided with a list of words that describe different experiences. Rather, they were given the same verbal examples of physiological (light-headedness, shortness of breath) and emotional (depressed, anxious, happy, soothing) responses and were told to come up with their own words to describe what they felt. They were also told that they might experience some or none of these or experience other physiological or emotional responses, that they should not feel pressured to report anything unless they experienced it, that they could not go wrong, and that whatever they reported was right. Consequently, subjects used a wide variety of words to describe their experiences. In our previous study (9), we were able to quantitatively explore the effects of different colors of light and their intensities on headache rating during migraine. In the present study, however, we focused on documenting whether specific symptoms were elicited in response to the applied stimuli, rather than delineating those symptoms’ magnitudes. In keeping, reported symptoms were addressed as a binary variable (“yes” or “no”). Notably, most of the patients who reported a symptom as a response to a specific light color at a certain intensity (i.e., blue at 20 cd⋅m−2) persistently reported the same symptom at greater intensities of the same color (e.g., 50 and 100 cd⋅m−2).
To reduce the number of variables, we analyzed the most frequently used words and grouped them into four prespecified domains: (i) hypothalamic-mediated autonomic, (ii) hypothalamic (nonautonomic), (iii) affective negative, and (iv) affective positive responses (a full list of light-induced responses is provided in Results).
Preclinical Studies.
Animals.
All experimental procedures were approved by the institutional animal care and use committee at Harvard Medical School and Beth Israel Deaconess Medical Center and conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. We used 45 male Sprague–Dawley rats weighting 250–350 g. Rats were housed in a controlled environment (22 °C room temperature; 12-h light/dark cycle) with free access to food and water.
Anterograde labeling of retinal afferents.
To identify projection sites of retinal axons in the hypothalamus, we injected 5 μL rAAV-GFP into the vitreous body of one eye. By using a Nanofil syringe, rAAV-GFP (serotype 2; 7 × 1012 genome copies per milliliter) was injected under brief isoflurane anesthesia. Twenty-one days later, rats were deeply anesthetized with an overdose of pentobarbital sodium (100 mg/kg i.p.), and perfused with 200 mL heparinized saline solution, followed by 500 mL of 4% paraformaldehyde and 0.05% picric acid in 0.1 M PBS solution. Brains were removed from the skull, soaked in the fixative solution for 2 h, and cryoprotected in 30% sucrose phosphate buffer for 48 h. Brains were then frozen and cut into serial coronal sections (80-μm thick) by using a cryostat (Leica) and prepared for immunofluorescence.
Retrograde labeling of hypothalamic neurons that project to parasympathetic preganglionic SSN in the brainstem or preganglionic sympathetic IML in the spinal cord.
To determine whether retinal axons project to the vicinity of hypothalamic neurons that mediate parasympathetic and sympathetic responses, we injected the retrograde tracer FG (2% hydroxystilbamidine in dH2O; Fluorochrome) into the SSN of 16 rats and IML of 14 rats previously (17 d earlier) injected with the anterograde tracer rAAV-GFP into the eye. To inject these autonomic nuclei, rats were briefly anesthetized with a single dose of Brevital sodium (45 mg/kg i.p.) to allow endotracheal intubation. They were then mounted on a stereotaxic frame and connected to a gas anesthesia system for the rest of the procedure (O2/isoflurane 2.5% for craniotomy or laminectomy; 1–1.2% for maintenance, delivered at 100 mL/min). End-tidal CO2, respiratory and heart rate, blood oxygen saturation, and body temperature were continuously monitored and kept within a physiological range. For targeting the SSN, a small craniotomy was performed in the interparietal bone at ∼2 mm lateral/1.8 caudal to lambda. A glass micropipette (20–40-μm tip diameter) loaded with FG was lowered through the craniotomy into the SSN (8–9 mm depth) for microiontophoretic release of the tracer by applying direct positive current (5–10 μA, on/off cycles, 10 s per cycle) over 10–15 min as described elsewhere (15). A similar injection paradigm was used to fill the IML. To reach the IML, a partial laminectomy of T5 vertebra was performed. As the IML extends throughout the thoracic spinal cord, two or three microinjections were performed along the rostrocaudal axis by placing the glass micropipette 0.5–1.0 mm from the midline of the spinal cord and 0.7–1.0 mm deep. After the injection, micropipettes were pulled out of the brainstem/spinal cord, wounds were sutured and disinfected, pain medication was provided (Meloxicam SR, 4mg/kg; Zoopharm) and rats were put back in their cages. Three days after FG injections, rats were perfused, brain and spinal cord removed, and neural tissue prepared for staining as described earlier.
Immunofluorescence labeling of hypothalamic neuropeptides and neurotransmitters.
To determine which peptidergic neurons in the hypothalamus receive retinal input, free-floating sections containing GFP-positive retinal afferents were preincubated at room temperature in PBS solution containing 2% blocking serum and 1% Triton X-100 for 1 h. Sections were then incubated at 4 °C for 48 h in the latter solution with one of the following primary antibodies: (i) mouse anti-TH (1:5,000; Immunostar), (ii) goat anti-orexin A (1:2,500; Santa Cruz), (iii) rabbit anti-oxytocin (1:10,000; Immunostar), (iv) goat anti-vasopressin (1:1,000; Immunostar), (v) guinea pig anti-HDC (1:1,000; ARP), and (vi) rabbit anti-MCH (1:1,000; gift from Terry Maratos-Flier, Harvard Medical School, Boston, MA). The sections were washed multiple times and then incubated for 1–2 h at room temperature with the corresponding fluorescent secondary antibody (Alexa Fluor 594; Invitrogen) against the Igs of the animal in which the primary antibody was raised (dilution range, 1:200–1:1,000). Immunostained sections were serially mounted on glass slides and coverslipped with fluorescent mounting media (Vector).
Identification of FG injection sites.
To precisely identify and delimit FG injection sites in SSN or IML, free-floating sections were taken from rats injected 3 d earlier with FG into the SSN or IML and processed in the following sequence: (i) quenching with 3:1 methanol/PBS solution containing 1% H2O2 for 1 h; (ii) preincubation with PBS solution containing 2% normal goat serum and 1% Triton X-100 for 1 h; (iii) incubation with the rabbit anti-FG primary antibody (1:5,000; Millipore) for 48 h at 4 °C; (iv) rinsing with PBS solution and incubation with biotinylated goat anti-rabbit secondary antibody (1:500; Jackson ImmunoResearch) for 2 h; and (v) rinsing, amplification, and labeling using ABC complex and DAB-nickel kits (Vector).
Digital imaging of fluorescent labeling.
Digital imaging of GFP retinal afferents, FG-labeled neurons, and hypothalamic neurons immunopositive for the tested neuropeptides/neurotransmitters was performed by using epifluorescence scanning microscopy that compiled 1–1.5-μm-thick scans using z-stacking software (Leica). Immunofluorescence labeling of GFP and FG were detected by excitation/emission at 445/520 nm (green) and 360/408 nm (blue), respectively. For neuropeptides labeled with Alexa Fluor 594, the signal was detected by excitation/emission at 590/617 nm (red). In cases in which DAPI counterstaining was used, labeling was detected by excitation/emission at 358/461 nm (blue). Photomicrographs of colabeling were obtained by superimposition of green, red, and blue images. The anatomical delimitation and localization of fluorescent labeling was based on an atlas of the rat brain (72).
Calibration and Quantification of Photic Stimulation.
Repeated calibrations of the ColorDome with an International Light Technologies photometer (ILT1700) and an Ocean Optics Maya LSL spectrometer were used to verify that the different colors of light were delivered at equal luminance. For example, when the nominal luminance was set at 3 cd⋅m−2 in Espion software (Diagnosys), the luminance measurements for blue, green, amber, and red were 2.96, 3.0, 3.36, and 2.76 cd⋅m−2, respectively. To ensure that each of these colors appeared to be exactly the same luminance to our participants (i.e., taking into account differences in photopic sensitivity of the human retina), the ColorDome delivered different power with each photic stimulus. When the luminance measurements for blue, green, amber, and red were 2.96, 3.0, 3.36, and 2.76 cd⋅m−2, the respective power measurements were 32.2, 1.9, 2.1, and 4.9 μW⋅cm−2/nM.
Statistical Analysis.
Data obtained in the psychophysical studies in migraine patients and control subjects were analyzed by using SPSS Statistics (version 22; IBM). χ2 tests were used for comparing percentages of patients and controls (independent samples) who exhibited specific symptoms (autonomic, affective negative, affective positive, or hypothalamic) in response to the administered visual stimuli of different wave lengths (white, blue, green, amber, red) within and between the various conditions (ictal/interictal vs. healthy controls). To avoid bias of any kind, response to stimuli was processed as a binary variable based on whether subjects reported a reaction to a specific experimental condition, regardless of the used number of words. McNemar–Bowker tests were used to examine proportion differences between the migraine patients' conditions (ictal, interictal) so as to account for differences in repeated dichotomous measures. Post hoc nonparametric binomial proportion comparisons were used to detect effects of visual stimuli of different wavelengths (response proportion). As six analyses were conducted (one per symptom category), a Bonferroni correction was administered to yield a threshold α-value of 0.0083 (i.e., the standard 5% divided by six comparisons); this correction prevented a significant result by chance alone.
Acknowledgments
This research was supported by NIH Grants R37 NS079678 (to R.B.), R01 NS069847 (to R.B.), R21 NS090254 (to R.N.), and K24 NS77895 (to D.B.). This work was conducted with support from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102), and financial contributions from Harvard University and its affiliated academic health care centers.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708361114/-/DCSupplemental.
References
- 1.Liveing E. On Megrim, Sick Headache. Arts & Boeve Publishers; Nijmegen, The Netherlands: 1873. [Google Scholar]
- 2.Drummond PD. A quantitative assessment of photophobia in migraine and tension headache. Headache. 1986;26:465–469. doi: 10.1111/j.1526-4610.1986.hed2609465.x. [DOI] [PubMed] [Google Scholar]
- 3.Choi JY, et al. Usefulness of a photophobia questionnaire in patients with migraine. Cephalalgia. 2009;29:953–959. doi: 10.1111/j.1468-2982.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 4.Brennan KC. Turn down the lights!: An irritable occipital cortex in migraine without aura. Neurology. 2011;76:206–207. doi: 10.1212/WNL.0b013e3182074bfb. [DOI] [PubMed] [Google Scholar]
- 5.Boulloche N, et al. Photophobia in migraine: An interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry. 2010;81:978–984. doi: 10.1136/jnnp.2009.190223. [DOI] [PubMed] [Google Scholar]
- 6.Denuelle M, et al. A PET study of photophobia during spontaneous migraine attacks. Neurology. 2011;76:213–218. doi: 10.1212/WNL.0b013e3182074a57. [DOI] [PubMed] [Google Scholar]
- 7.Moulton EA, Becerra L, Borsook D. An fMRI case report of photophobia: Activation of the trigeminal nociceptive pathway. Pain. 2009;145:358–363. doi: 10.1016/j.pain.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanagaite J, et al. Light-induced discomfort and pain in migraine. Cephalalgia. 1997;17:733–741. doi: 10.1046/j.1468-2982.1997.1707733.x. [DOI] [PubMed] [Google Scholar]
- 9.Noseda R, et al. Migraine photophobia originating in cone-driven retinal pathways. Brain. 2016;139:1971–1986. doi: 10.1093/brain/aww119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Digre KB, Brennan KC. Shedding light on photophobia. J Neuroophthalmol. 2012;32:68–81. doi: 10.1097/WNO.0b013e3182474548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cucchiara B, Datta R, Aguirre GK, Idoko KE, Detre J. Measurement of visual sensitivity in migraine: Validation of two scales and correlation with visual cortex activation. Cephalalgia. 2015;35:585–592. doi: 10.1177/0333102414547782. [DOI] [PubMed] [Google Scholar]
- 12.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 13.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 14.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noseda R, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Headache Classification Committee of the International Headache Society (HIS) The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 17.Gelfand AA, Reider AC, Goadsby PJ. Cranial autonomic symptoms in pediatric migraine are the rule, not the exception. Neurology. 2013;81:431–436. doi: 10.1212/WNL.0b013e31829d872a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai TH, Fuh JL, Wang SJ. Cranial autonomic symptoms in migraine: Characteristics and comparison with cluster headache. J Neurol Neurosurg Psychiatry. 2009;80:1116–1119. doi: 10.1136/jnnp.2008.157743. [DOI] [PubMed] [Google Scholar]
- 19.Shechter A, Stewart WF, Silberstein SD, Lipton RB. Migraine and autonomic nervous system function: A population-based, case-control study. Neurology. 2002;58:422–427. doi: 10.1212/wnl.58.3.422. [DOI] [PubMed] [Google Scholar]
- 20.Goadsby PJ. Trigeminal autonomic cephalalgias. Pathophysiology and classification. Rev Neurol (Paris) 2005;161:692–695. doi: 10.1016/s0035-3787(05)85120-3. [DOI] [PubMed] [Google Scholar]
- 21.Goadsby PJ. Trigeminal autonomic cephalalgias: Fancy term or constructive change to the IHS classification? J Neurol Neurosurg Psychiatry. 2005;76:301–305. doi: 10.1136/jnnp.2004.036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malick A, Strassman RM, Burstein R. Trigeminohypothalamic and reticulohypothalamic tract neurons in the upper cervical spinal cord and caudal medulla of the rat. J Neurophysiol. 2000;84:2078–2112. doi: 10.1152/jn.2000.84.4.2078. [DOI] [PubMed] [Google Scholar]
- 23.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 24.Li C, et al. Projections from the hypothalamic paraventricular nucleus and the nucleus of the solitary tract to prechoroidal neurons in the superior salivatory nucleus: Pathways controlling rodent choroidal blood flow. Brain Res. 2010;1358:123–139. doi: 10.1016/j.brainres.2010.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: Axonal projections to the brainstem. J Comp Neurol. 2010;518:1460–1499. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spencer SE, Sawyer WB, Wada H, Platt KB, Loewy AD. CNS projections to the pterygopalatine parasympathetic preganglionic neurons in the rat: A retrograde transneuronal viral cell body labeling study. Brain Res. 1990;534:149–169. doi: 10.1016/0006-8993(90)90125-u. [DOI] [PubMed] [Google Scholar]
- 27.Dergacheva O, Yamanaka A, Schwartz AR, Polotsky VY, Mendelowitz D. Direct projections from hypothalamic orexin neurons to brainstem cardiac vagal neurons. Neuroscience. 2016;339:47–53. doi: 10.1016/j.neuroscience.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piñol RA, Jameson H, Popratiloff A, Lee NH, Mendelowitz D. Visualization of oxytocin release that mediates paired pulse facilitation in hypothalamic pathways to brainstem autonomic neurons. PLoS One. 2014;9:e112138. doi: 10.1371/journal.pone.0112138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 30.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SH, Dan Y. Neuromodulation of brain states. Neuron. 2012;76:209–222. doi: 10.1016/j.neuron.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cechetto DF, Saper CB. Neurochemical organization of the hypothalamic projection to the spinal cord in the rat. J Comp Neurol. 1988;272:579–604. doi: 10.1002/cne.902720410. [DOI] [PubMed] [Google Scholar]
- 33.Skagerberg G, Lindvall O. Organization of diencephalic dopamine neurones projecting to the spinal cord in the rat. Brain Res. 1985;342:340–351. doi: 10.1016/0006-8993(85)91134-5. [DOI] [PubMed] [Google Scholar]
- 34.van den Pol AN. Hypothalamic hypocretin (orexin): Robust innervation of the spinal cord. J Neurosci. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunn N, Womack M, Dart C, Barrett-Jolley R. Function and pharmacology of spinally-projecting sympathetic pre-autonomic neurones in the paraventricular nucleus of the hypothalamus. Curr Neuropharmacol. 2011;9:262–277. doi: 10.2174/157015911795596531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murakami M, et al. Involvement of the orexin system in sympathetic nerve regulation. Biochem Biophys Res Commun. 2015;460:1076–1081. doi: 10.1016/j.bbrc.2015.03.157. [DOI] [PubMed] [Google Scholar]
- 37.Shiuchi T, et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009;10:466–480. doi: 10.1016/j.cmet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 38.McCann JD, et al. A novel mechanism for benign essential blepharospasm. Ophthal Plast Reconstr Surg. 1999;15:384–389. doi: 10.1097/00002341-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Fine PG, Digre KB. A controlled trial of regional sympatholysis in the treatment of photo-oculodynia syndrome. J Neuroophthalmol. 1995;15:90–94. [PubMed] [Google Scholar]
- 40.Scammell TE, Saper CB. Orexins: Looking forward to sleep, back at addiction. Nat Med. 2007;13:126–128. doi: 10.1038/nm0207-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fonken LK, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107:18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patton DF, et al. Photic and pineal modulation of food anticipatory circadian activity rhythms in rodents. PLoS One. 2013;8:e81588. doi: 10.1371/journal.pone.0081588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosek A, Korczyn AD. Yom Kippur headache. Neurology. 1995;45:1953–1955. doi: 10.1212/wnl.45.11.1953. [DOI] [PubMed] [Google Scholar]
- 44.Mollaoğlu M. Trigger factors in migraine patients. J Health Psychol. 2013;18:984–994. doi: 10.1177/1359105312446773. [DOI] [PubMed] [Google Scholar]
- 45.Hauge AW, Kirchmann M, Olesen J. Characterization of consistent triggers of migraine with aura. Cephalalgia. 2011;31:416–438. doi: 10.1177/0333102410382795. [DOI] [PubMed] [Google Scholar]
- 46.Schaie KW. Scaling the association between colors and mood-tones. Am J Psychol. 1961;74:266–273. [Google Scholar]
- 47.Boyatzis CJ, Varghese R. Children’s emotional associations with colors. J Genet Psychol. 1994;155:77–85. doi: 10.1080/00221325.1994.9914760. [DOI] [PubMed] [Google Scholar]
- 48.Terwogt MM, Hoeksma JB. Colors and emotions: Preferences and combinations. J Gen Psychol. 1995;122:5–17. doi: 10.1080/00221309.1995.9921217. [DOI] [PubMed] [Google Scholar]
- 49.Elliot AJ, Maier MA, Moller AC, Friedman R, Meinhardt J. Color and psychological functioning: The effect of red on performance attainment. J Exp Psychol Gen. 2007;136:154–168. doi: 10.1037/0096-3445.136.1.154. [DOI] [PubMed] [Google Scholar]
- 50.Hattar S, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Canteras NS, Ribeiro-Barbosa ER, Goto M, Cipolla-Neto J, Swanson LW. The retinohypothalamic tract: Comparison of axonal projection patterns from four major targets. Brain Res Brain Res Rev. 2011;65:150–183. doi: 10.1016/j.brainresrev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 54.Castillo-Ruiz A, Gall AJ, Smale L, Nunez AA. Day-night differences in neural activation in histaminergic and serotonergic areas with putative projections to the cerebrospinal fluid in a diurnal brain. Neuroscience. 2013;250:352–363. doi: 10.1016/j.neuroscience.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dommett E, et al. How visual stimuli activate dopaminergic neurons at short latency. Science. 2005;307:1476–1479. doi: 10.1126/science.1107026. [DOI] [PubMed] [Google Scholar]
- 56.Adidharma W, Leach G, Yan L. Orexinergic signaling mediates light-induced neuronal activation in the dorsal raphe nucleus. Neuroscience. 2012;220:201–207. doi: 10.1016/j.neuroscience.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buckholtz JW, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. 2010;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mann JJ. The neurobiology of suicide. Nat Med. 1998;4:25–30. doi: 10.1038/nm0198-025. [DOI] [PubMed] [Google Scholar]
- 59.Schlüter T, et al. The impact of dopamine on aggression: An [18F]-FDOPA PET Study in healthy males. J Neurosci. 2013;33:16889–16896. doi: 10.1523/JNEUROSCI.1398-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosell DR, Siever LJ. The neurobiology of aggression and violence. CNS Spectr. 2015;20:254–279. doi: 10.1017/S109285291500019X. [DOI] [PubMed] [Google Scholar]
- 61.Liddell BJ, et al. A direct brainstem-amygdala-cortical ‘alarm’ system for subliminal signals of fear. Neuroimage. 2005;24:235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 62.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 63.Viviani D, et al. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- 64.Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 65.Hervieu G. Melanin-concentrating hormone functions in the nervous system: Food intake and stress. Expert Opin Ther Targets. 2003;7:495–511. doi: 10.1517/14728222.7.4.495. [DOI] [PubMed] [Google Scholar]
- 66.Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation: A unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17:1298–1303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trude lE, Bourque CW. Central clock excites vasopressin neurons by waking osmosensory afferents during late sleep. Nat Neurosci. 2010;13:467–474. doi: 10.1038/nn.2503. [DOI] [PubMed] [Google Scholar]
- 68.Collins GT, et al. Dopamine agonist-induced yawning in rats: A dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seki Y, Sato-Suzuki I, Kita I, Oguri M, Arita H. Yawning/cortical activation induced by microinjection of histamine into the paraventricular nucleus of the rat. Behav Brain Res. 2002;134:75–82. doi: 10.1016/s0166-4328(01)00454-5. [DOI] [PubMed] [Google Scholar]
- 70.Kanosue K, Nakayama T, Tanaka H, Yanase M, Yasuda H. Modes of action of local hypothalamic and skin thermal stimulation on salivary secretion in rats. J Physiol. 1990;424:459–471. doi: 10.1113/jphysiol.1990.sp018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 72.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th Ed Academic; San Diego: 2008. [Google Scholar]