Abstract
Background:
Premature canities is a common yet unfathomed disorder. The evidence for the role of micronutrient deficiency in premature canities is not well established.
Aim:
The present study was undertaken to evaluate the micronutrient levels in Indian patients with premature canities as compared to controls.
Materials and Methods:
We conducted a case–control study in 52 self-reporting patients with premature canities (<20 years age). Micronutrient levels including serum Vitamin B12, biotin, and folic acid were assessed and compared among the patients and controls.
Results:
We observed that mean serum Vitamin B12 (198.07 ± 88.98 pg/ml in cases vs. 343.07 ± 143.06 pg/ml in controls, P = 0.000), folic acid (6.22 ± 2.46 ng/ml in cases vs. 8.49 ± 4.18 ng/ml in controls, P = 0.01), and biotin (252.71 ± 18.79 pg/ml in cases vs. 266.47 ± 30.44 pg/ml in controls, P = 0.013) levels were significantly lower in cases as compared to the controls.
Conclusion:
In view of the dark hair and many prevailing myths, premature canities is a significant problem in Asians with profound psychosocial impact. This study unveils the association with Vitamin B12, folic acid, and biotin deficiencies. Larger studies are recommended to arrive on a logical conclusion.
Key words: Biotin, graying of hair, India, micronutrient deficiency, premature canities, Vitamin B12
INTRODUCTION
Graying of hair is often considered as a sign of aging and loss of vitality. In Asian races, it assumes a special significance as even a few gray strands stand out prominently amid the dark-colored hair. Premature graying of hair or canities has been an elusive entity with little being known about its clinical manifestations or etiopathogenesis. The age incidence is also reported variably with respect to race and ethnicity.[1] It is not only mainly considered to be a genetic disorder with an autosomal dominant inheritance[2] but has also been reported as a part of many syndromes such as Waardenburg syndrome, Book's syndrome, and progeria. Other factors implicated include nutritional deficiencies and oxidative stress. The alteration seen in hair color with malnutrition is well known. Although the role of various micronutrients such as biotin, Vitamin B12, zinc, copper, selenium, and iron in the etiopathogenesis of premature canities has been speculated, adequate evidence is deficient due to lack of properly designed systematic studies. In wake of lack of evidence, multiple multivitamin and trace mineral supplements are marketed as a panacea to the problem of premature graying of hair. This adds to the economic burden of the disease without reducing the suffering.
The present study was designed to evaluate the status of various micronutrients in Indian patients with premature canities and matched controls.
MATERIALS AND METHODS
Fifty-two cases of premature canities and matched controls were recruited from the outpatient department of dermatology of a tertiary care setup. The Institutional Ethical Committee approval was taken before the initiation of the study. The cases included patients of Indian origin presenting with onset of graying before the age of 20 years. Cases with graying of hair as a part of other conditions such as vitiligo, syndromes such as Waardenburg and progeria, cutaneous disease involving the scalp or cases who had dyed their hair in the past 6 months were excluded as this could interfere with evaluation of graying. Furthermore, cases with febrile illness within 3 months of onset of graying were excluded from the study. The control group included healthy patients of Indian origin below 20 years of age attending the dermatology outpatient department for benign conditions. A written informed consent was obtained from each participant in both the groups. Subjects who were smokers, pregnant, lactating, taking systemic medications or multivitamin supplements, or who had systemic illness were excluded from both the groups.
The participants were asked about their dietary habits (vegetarian or nonvegetarian; egg intake being included in the latter). A detailed history about the onset and progression of graying of scalp hair was taken. A complete general physical examination was conducted especially to assess for any signs of malnourishment. Anthropometric measurements and body mass index (BMI) estimation were recorded for all patients. In addition, a complete scalp examination was done to record the area and site of involvement and severity of graying assessed by Graying Severity Score (GSS).[3]
All patients and controls underwent hematological and biochemical examination as well. An estimation of serum micronutrient levels of biotin (Vitamin H), Vitamin B12, and folic acid was done for all cases and controls. Serum biotin level was estimated by the commercially available kit, Cusabio (Genxbio) with the help of Ranskan Sprint enzyme-linked immunosorbent assay reader, Ranbaxy Diagnostics. Serum Vitamin B12 and folic acid level was estimated by commercially available kit (Cobas, Roche Diagnostics, US) with the help of Elecsys 2010 Immunoassay System, Roche Diagnostics. All the three tests were based on the competitive principle.
The collected data were statistically analyzed; unpaired t-test was used to compare the micronutrient levels between the two groups. Statistical software SPSS version 17.0 (SPSS Inc., Chicago, USA) was used to conduct the statistical analysis and P < 0.05 was considered statistically significant.
RESULTS
The mean age of cases was 14.5 ± 4.2 years (range 7–20 years) while that of controls was 14.6 ± 2.9 years (range 5–20 years; P = 0.7). Both the groups had 25 boys and 27 girls each. Assessment of dietary habits showed that [Table 1] both the groups to be comparable with an almost similar number of nonvegetarians. Similarly, the BMI estimation of the cases and controls was also comparable. The severity of graying as assessed by GSS revealed that 17 patients had mild (Grade I GSS), 32 had moderate (Grade II GSS), and 3 had severe (Grade III GSS) premature canities.
Table 1.
Comparison of diet, body mass index, and protein levels among cases and controls
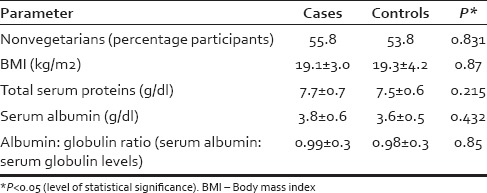
On general physical examination, three of the cases (5.8%) had significant pallor, with one of them also having atrophic glossitis. These three cases had a hemoglobin level of <10 mg/dl suggestive of anemia, whereas none of the controls had anemia. The mean value for erythrocyte sedimentation rate was raised in cases as compared to controls (14.5 ± 13.5 mm vs. 8.3 ± 4.9 mm in 1st h) with the difference being statistically significant (P = 0.004). The other evaluated hematological parameters including fasting and postprandial blood sugars, serum bilirubin, serum glutamic pyruvic transaminase, alkaline phosphatase (ALP), calcium, phosphate, total proteins, and albumin were found to be comparable in both the groups [Table 1].
With respect to the micronutrients evaluated, the mean Vitamin B12 levels were found to be significantly lower in cases as compared to controls (198.1 ± 89 pg/ml vs. 343.1 ± 143.1 pg/ml; P = 0.000) [Table 2]. Among the cases, 29 (55.8%) participants were documented to have Vitamin B12 deficiency against 9 (17.3%) participants in the control group. It was also noticed that the percentage of participants with Vitamin B12 deficiency in the cases increased as severity of graying increased [Table 3]. Approximately one-third (35.29%) of the patients with Grade 1 canities had Vitamin B12 deficiency whereas around two-third (62.5%) patients with Grade 2 canities and all the patients with Grade 3 canities had Vitamin B12 deficiency [Figure 1]. This finding was found to be statistically significant with P = 0.042.
Table 2.
The mean serum levels of micronutrients in cases and controls

Table 3.
Vitamin B12 and folic acid deficiency in the 3 grades of premature canities
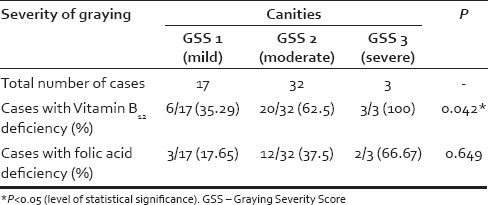
Figure 1.
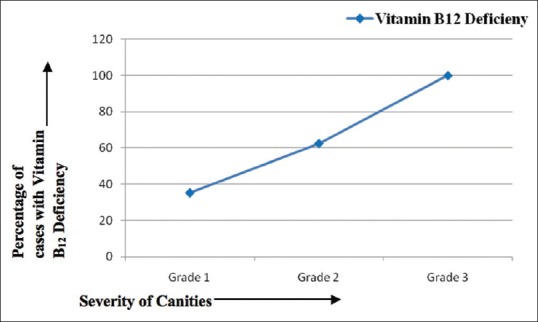
Severity of graying on X-axis and percentage of patients with Vitamin B12 deficiency on Y-axis. With increase in severity, the percentage of patients with Vitamin B12 deficiency is seen to rise
The mean serum folic acid levels were also lower in cases as compared to controls (6.22 ± 2.46 vs. 8.49 ± 4.18 ng/ml), the difference being statistically significant [P = 0.01; Table 2]. A total of twenty cases (38.5%) had folic acid deficiency as compared to 19 controls (36.5%). Among the cases, 13 of the 20 cases with folic acid deficiency also had a concomitant Vitamin B12 deficiency, whereas among the controls, only four (21%) had a concomitant Vitamin B12 deficiency. Serum folic acid levels correlated positively (r = 0.173) with serum Vitamin B12 levels though not significantly (P = 0.221). As in case of Vitamin B12, with an increase in the severity of graying the number of participants with folic acid deficiency also increased [Table 3 and Figure 2].
Figure 2.
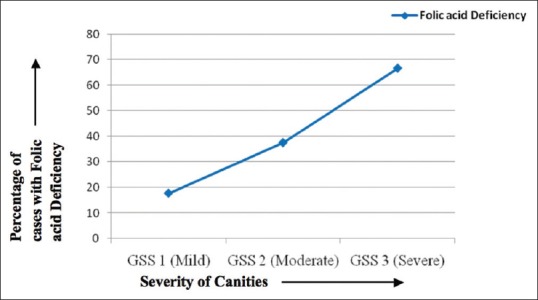
Severity of graying on X-axis and percentage of patients with folic acid deficiency on Y-axis. With increase in severity, the percentage of patients with folic acid deficiency is also seen to rise
None of the cases or controls was deficient in biotin; however, the mean serum biotin levels were on the lower normal side in cases (mean level being 252.7 ± 18.8 pg/ml) as compared to controls (mean being 266.5 ± 30.4 pg/ml). This difference was also found to be statistically significant [P = 0.013; Table 2].
DISCUSSION
Premature graying is a common condition but still largely unexplored. It is known that the age incidence of graying varies with race as well as ethnicity. The precise definition of premature onset of graying still eludes us, as it has been variably attributed to an onset of graying of hair before the age of 20 years in Caucasians and 30 years in Africans.[2,4] There are no studies published so far, which define premature graying in Indian population or in Asian population at large; though a cutoff at the age of 25 years has been once considered.[5] For the purpose of our study, we defined premature graying if it occurred before the age of 20 years.
Premature graying has a significant impact on the patient's psyche as graying of hair is often equated with senility and adds a sense of guilt, hampering a person's self-confidence.[6] Usually, the process is progressive and permanent though there are occasional reports of re-pigmentation of previously nonpigmented hair.[7,8,9]
Multiple hypotheses have been proposed to explain the etiopathogenesis of premature canities though none have been conclusively proven. These include an exhaustion of enzymes involved in melanogenesis, impaired DNA repair, loss of telomerase activity, antioxidant mechanisms, and antiapoptotic signals including the loss of Bcl-2 and decreased stem cell factor.[10,11,12,13,14,15,16] Malnourishment, both in the form of micronutrient deficiency as well as gross protein malnutrition, has been reported to induce a change in hair color from a darker to a lighter shade. This is amply demonstrated in cases with intermittent protein malnutrition, which leads to the “flag” sign of kwashiorkor (signe de la bandera) characterized by alternating white (abnormal) and dark bands along the individual hair shafts. This has been attributed to deficiency of the amino acid tyrosine which is converted to DOPA (3,4 dihydroxyphenylalanine) as the initial step of melanogenesis. None of our patients were found to have significant protein deficiency which was evaluated by BMI measurements and serum protein estimation. BMI was used as a parameter for detecting gross malnutrition. It was found to be comparable between both the groups. Serum proteins were used as surrogate markers for malnutrition, and these were also found to be comparable between both the groups. Hence, any gross protein malnutrition was ruled out in both the groups.
The role of micronutrient deficiencies has long been suspected in the etiopathogenesis of premature canities and is to some extent responsible for the widespread practice of substitution therapy with micronutrients in these cases. However, definitive evidence has been lacking in English literature with no published reference studies. Our study focused on the evaluation of micronutrient deficiency in these patients. As these micronutrients, especially Vitamin B12, could be influenced by the dietary habits, an assessment of the dietary habits (vegetarian or nonvegetarian) was undertaken for both cases and control group. The only external source of Vitamin B12 is animal food; thus vegetarians are more predisposed to develop such a deficiency. In our patient population, no significant difference in dietary habits was found between the two groups; thus, diet as a confounding factor for the mean serum Vitamin B12 differences between cases and controls was ruled out.
Our study documented a higher prevalence of Vitamin B12 deficiency in cases (n = 29, 55.8%) as compared to controls (n = 9, 17.3%). In addition, there were lower mean Vitamin B12 levels found in the patient group. These findings strongly implicate the role of Vitamin B12 deficiency in the etiopathogenesis of premature graying and thus its possible role in treatment. In the current clinical practice, Vitamin B12 is not routinely given to patients of premature canities. Although premature gray hair has been reported with Vitamin B12 deficiency,[4,17] this is the first study to document the same. Pernicious anemia is also known to be associated with premature graying.[18] In a controlled study of 125 patients with pernicious anemia, 11% had premature graying, defined as onset before the age of 20, compared with 2% in the control group.[19] It is not known whether the association between pernicious anemia and canities is due to autoimmune etiology or through the Vitamin B12 deficiency, though anecdotal case reports of reversible pigmentation of hair with Vitamin B12 replacement therapy do exist in literature.[20,21,22]
In the present study, an almost similar percentage of cases and controls were found to have folic acid deficiency (n = 20, 38.5% vs. n = 19, 36.5%). Nevertheless, the mean folic acid levels were found to be significantly lower in cases. It was also noted that 65% of cases with folic acid deficiency had concomitant Vitamin B12 deficiency as compared to 21% of controls. In addition, the serum folic acid levels correlated positively (Spearman ρ = 0.173) with serum Vitamin B12 levels though this was not significant (P = 0.221). It is a well-known fact that Vitamin B12 deficiency impairs the metabolism of folic acid, leading to a functional folate deficiency (the folate trap). In the present study, we got a deficiency of both the micronutrients suggesting possible role of both Vitamin B12 and folic acid in premature canities.
Biotin deficiency has also been implicated in the etiopathogenesis of premature canities. It is reportedly used as a treatment modality though there is a lack of documentary evidence, both for its deficiency and efficacy. In the present study, serum biotin levels were found to be toward the lower side of normal in cases (mean: 252.71 ± 18.79 pg/ml) as compared to controls (mean: 266.47 ± 30.44 pg/ml), and this difference was found to be statistically significant (P = 0.013). Our findings are in concordance with the previous reports documenting that an absolute deficiency of biotin is rare.[23,24]
There are reports implicating various other nutrients (including pantothenic acid, zinc, selenium, and copper) in the possible pathogenesis of premature canities; however, all these parameters were not evaluated in the present study due to resource constraints. In a study from Iran, serum copper levels were found to be lower in cases of premature canities, serum iron levels were found to be lower in controls, and serum zinc levels did not show a significant difference between cases and controls.[25] In a recent Indian study,[26] serum ferritin, calcium, and Vitamin D3 were found to be lower in cases compared to controls. However, the mean hemoglobin, TIBC, serum iron, and Vitamin B12 were comparable.
As the etiopathogenesis of premature graying is poorly understood, there are no evidence-based treatment guidelines. The most commonly followed approach is the use of multivitamin supplements constituted by 5–10 mg of biotin, 100 mg of calcium pantothenate, and many trace elements such as zinc, selenium, and copper in varying concentrations. In addition, some preparations contain varying doses of iron, N-acetyl cysteine, and grape seed extracts. There have been few anecdotal reports of re-pigmentation of hair after ingestion of large doses of p-aminobenzoic acid (PABA).[2,27,28] In a study, intake of 100 mg thrice daily of PABA caused a temporary darkening of hair.[2,29] Zarafonetis reported re-pigmentation of hair with daily intake of 12–20 g of PABA.[28] Pasricha reported the successful use of 200 mg of calcium pantothenate daily in two adolescent girls with premature graying.[30] In yet another study, combination of calcium pantothenate with gray hair avulsion was reported to be more effective than calcium pantothenate alone.[31]
As there are no case–control or randomized controlled trials documenting efficacy of any of these agents, the patients are often treated empirically. Such prescriptions can have a huge impact on the patients' budget, which is particularly precarious in a developing country like India.
CONCLUSION
Premature canities is a common disorder that assumes importance in the Indian population, with dark brown to black hair, hence even a few strands of gray hair stand out prominently. This can have a tremendous psychological impact on the patients, especially affecting the adolescents. This case–control study documents a deficiency of Vitamin B12 and folic acid in the patients evaluated and lower levels of biotin without any obvious biotin deficiency in the cases. Small sample size is the major limitation of this study. Therefore, future studies with larger sample sizes are recommended to arrive at a definite conclusion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Keogh EV, Walsh RJ. Rate of greying of human hair. Nature. 1965;207:877–8. doi: 10.1038/207877a0. [DOI] [PubMed] [Google Scholar]
- 2.Trüeb RM. Pharmacologic interventions in aging hair. Clin Interv Aging. 2006;1:121–9. doi: 10.2147/ciia.2006.1.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singal A, Daulatabad D, Grover C. Graying severity score: A useful tool for evaluation of premature canities. Indian Dermatol Online J. 2016;7:164–7. doi: 10.4103/2229-5178.182372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odom RB, James WD, Berger TG. Diseases of the skin appendages. In: James WD, Berger TG, Odom RB, editors. Andrew's Diseases of the Skin Clinical Dermatology. 9th ed. Philadelphia: WS Saunders; 2000. p. 955. [Google Scholar]
- 5.Pasricha JS, Verma K. Treatment of Skin Diseases. 5th ed. New Delhi: Mehta Publishers; 2008. Diseases of the appendages; p. 289. [Google Scholar]
- 6.Daulatabad D, Grover C, Singal A. Quality of life and psychological impact of premature canities: A study from North India. Pigment Int. 2016;3:24–8. [Google Scholar]
- 7.Verbov J. Erosive candidiasis of the scalp, followed by the reappearance of black hair after 40 years. Br J Dermatol. 1981;105:595–8. doi: 10.1111/j.1365-2133.1981.tb00806.x. [DOI] [PubMed] [Google Scholar]
- 8.Shaffrali FC, McDonagh AJ, Messenger AG. Hair darkening in porphyria cutanea tarda. Br J Dermatol. 2002;146:325–9. doi: 10.1046/j.1365-2133.2002.04591.x. [DOI] [PubMed] [Google Scholar]
- 9.Comaish S. White scalp hairs turning black – An unusual reversal of the ageing process. Br J Dermatol. 1972;86:513–4. doi: 10.1111/j.1365-2133.1972.tb16105.x. [DOI] [PubMed] [Google Scholar]
- 10.Arck PC, Overall R, Spatz K, Liezman C, Handjiski B, Klapp BF, et al. Towards a “free radical theory of graying”: Melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006;20:1567–9. doi: 10.1096/fj.05-4039fje. [DOI] [PubMed] [Google Scholar]
- 11.Ancans J, Tobin DJ, Hoogduijn MJ, Smit NP, Wakamatsu K, Thody AJ. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp Cell Res. 2001;268:26–35. doi: 10.1006/excr.2001.5251. [DOI] [PubMed] [Google Scholar]
- 12.Commo S, Gaillard O, Bernard BA. Human hair greying is linked to a specific depletion of hair follicle melanocytes affecting both the bulb and the outer root sheath. Br J Dermatol. 2004;150:435–43. doi: 10.1046/j.1365-2133.2004.05787.x. [DOI] [PubMed] [Google Scholar]
- 13.Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: Incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–4. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- 15.Müller-Röver S, Rossiter H, Lindner G, Peters EM, Kupper TS, Paus R. Hair follicle apoptosis and Bcl-2. J Investig Dermatol Symp Proc. 1999;4:272–7. doi: 10.1038/sj.jidsp.5640228. [DOI] [PubMed] [Google Scholar]
- 16.Eskandani M, Golchai J, Pirooznia N, Hasannia S. Oxidative stress level and tyrosinase activity in vitiligo patients. Indian J Dermatol. 2010;55:15–9. doi: 10.4103/0019-5154.60344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortonne JP. Vitiligo and other disorders of hypopigmentation. In: Jorizzo JL, Rapini R, Bolognia JL, editors. Dermatology. 2nd ed. Missouri: Mosby Elsevier; 2008. p. 936. [Google Scholar]
- 18.Gawkrodges DJ, Hunter JA. Premature ageing syndromes. In: Graham Brown RA, Srakany I, Monk BE, editors. Skin Disorders in the Elderly. London: Blackwell Scientific Publications; 1988. p. 47. [Google Scholar]
- 19.Dawber RP. Integumentary associations of pernicious anaemia. Br J Dermatol. 1970;82:221–3. doi: 10.1111/j.1365-2133.1970.tb12428.x. [DOI] [PubMed] [Google Scholar]
- 20.Carmel R. Hair and fingernail changes in acquired and congenital pernicious anemia. Arch Intern Med. 1985;145:484–5. [PubMed] [Google Scholar]
- 21.Noppakun N, Swasdikul D. Reversible hyperpigmentation of skin and nails with white hair due to Vitamin B12 deficiency. Arch Dermatol. 1986;122:896–9. [PubMed] [Google Scholar]
- 22.Niiyama S, Mukai H. Reversible cutaneous hyperpigmentation and nails with white hair due to Vitamin B12 deficiency. Eur J Dermatol. 2007;17:551–2. doi: 10.1684/ejd.2007.0285. [DOI] [PubMed] [Google Scholar]
- 23.Nyhan WL. Inborn errors of biotin metabolism. Arch Dermatol. 1987;123:1696–8. [PubMed] [Google Scholar]
- 24.Mukhopadhyay D, Das MK, Dhar S, Mukhopadhyay M. Multiple carboxylase deficiency (late onset) due to deficiency of biotinidase. Indian J Dermatol. 2014;59:502–4. doi: 10.4103/0019-5154.139910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fatemi Naieni F, Ebrahimi B, Vakilian HR, Shahmoradi Z. Serum iron, zinc, and copper concentration in premature graying of hair. Biol Trace Elem Res. 2012;146:30–4. doi: 10.1007/s12011-011-9223-6. [DOI] [PubMed] [Google Scholar]
- 26.Bhat RM, Sharma R, Pinto AC, Dandekeri S, Martis J. Epidemiological and investigative study of premature graying of hair in higher secondary and pre-university school children. Int J Trichology. 2013;5:17–21. doi: 10.4103/0974-7753.114706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 28.Zarafonetis CJ. Darkening of gray hair during para-amino-benzoic acid therapy. J Invest Dermatol. 1950;15:399–401. doi: 10.1038/jid.1950.121. [DOI] [PubMed] [Google Scholar]
- 29.Wadhwa SL, Khopkar U, Nischal KC. Hair and scalp disorders. In: Valia RG, Valia AR, editors. IADVL Textbook of Dermatology. 3rd ed. Mumbai: Bhalani Publishing House; 2008. pp. 864–948. [Google Scholar]
- 30.Pasricha SJ. Successful treatment of gray hairs with high dose calcium pantothenate. Indian J Dermatol Venereol Leprol. 1981;47:311–3. [Google Scholar]
- 31.Pasricha JS. Effect of grey hair evulsion on the response to calcium pantothenate in premature grey hairs. Indian J Dermatol Venereol Leprol. 1986;52:77–80. [PubMed] [Google Scholar]


