Abstract
Background:
Telogen effluvium (TE) is a type of acquired, diffuse alopecia that occurs due to an abnormal shift of scalp hair follicles from anagen to telogen, leading to premature shedding of hair. Previous studies have suggested the existence of a neuroimmunologic “brain-hair follicle” axis, in which mast cells have been implicated as an important link between the nervous system and immunologic system.
Objective:
The current study sought to investigate the role of mast cell presence and mast cell degranulation in the pathogenesis of TE.
Materials and Methods:
Mast cells were counted using Giemsa and tryptase immunohistochemical stains in scalp biopsy specimens with the pathologic diagnosis of TE (TE, n = 10), alopecia areata (AA, n = 7), and androgenic alopecia (ANDRO, n = 9).
Results:
We found significant (P < 0.001) group-level differences between the mean mast cell counts per high-power fields for each type of alopecia studied. Tukey post hoc analysis showed the mean mast cell count for TE to be significantly larger than AA for both Giemsa (P = 0.002) and tryptase (P = 0.006); significantly larger than ANDRO for both Giemsa (P < 0.001) and tryptase (P < 0.001); and significantly larger when compared to normal scalp skin for both Giemsa (P < 0.001) and tryptase (P < 0.001). No significant difference of mean mast cell counts was observed for AA compared to ANDRO for Giemsa (P = 0.373) or tryptase (P = 0.598) stains.
Conclusion:
Our findings suggest that mast cells could play a role in mediating stress-induced hair loss seen in TE.
Key words: Alopecia areata, mast cells, telogen effluvium
INTRODUCTION
Telogen effluvium (TE) is a type of acquired, diffuse, nonscarring alopecia that occurs due to an abnormal shift of scalp hair follicles from anagen to telogen, leading to premature shedding of hair. While data on the epidemiology of TE is limited, it remains one of the most common forms of nonscarring hair loss for which patients present for clinical evaluation.[1] TE can occur at any age, and for reasons that are unclear women are more likely than men to present for the evaluation of acute TE.[2] The major clinical finding in TE is an acute or chronic decrease in scalp hair density.
Acute TE involves hair loss 2–4 months following a stressful inciting event. A wide variety of inciting factors have been associated with the induction of TE based on clinical observations. These factors include but are not limited to thyroid dysfunction,[3] nutritional deficiencies,[4] rapid weight loss,[5] significant blood loss, childbirth,[6] a myriad of prescription medications,[7] and any physiologic or psychological stressor.[8] However, the specific etiology of hair loss in TE still remains unclear. Several studies using mouse models have implicated a neuroimmunologic mechanism to account for hair loss in TE.[9,10,11] Implicated specifically at the distal end of the “brain-hair follicle” axis are mast cells which have been shown to modulate follicular cycling during times of stress.[12,13]
The current study sought to investigate the role of mast cell presence and mast cell degranulation in the pathogenesis of TE compared to two other nonscarring alopecias: Alopecia areata (AA) and androgenic alopecia (ANDRO). Identifying differences in mast cell presence and activity between these three alopecias may further elucidate their pathogeneses and provide insight into possible therapeutic interventions for these conditions.
MATERIALS AND METHODS
Case selection
The approval for this study was obtained from the Saint Louis University Office of Research Administration. Our dermatopathology laboratory information system was searched for scalp biopsy specimens with a final pathologic diagnosis of TE, AA, or ANDRO from 2006 to 2011. Formalin-fixed and paraffin-embedded tissue was obtained from the dermatopathology laboratory at Saint Louis University in St. Louis, MO. Ten cases of TE, seven cases of AA, and nine cases of ANDRO were obtained and reviewed by a board-certified dermatopathologist (CIV) for diagnostic accuracy and inclusion in the study. Eight elliptical scalp excision specimens for benign nevi were selected, and the tips of these excision specimens were used as normal skin controls.
Calculating the number of mast cells in each type of alopecia
Mast cells were counted using 4-μm transverse sections with specific attention made to highlight the areas of greatest inflammation. Follicular and nonfollicular mast cells were included in the study. All specimens were stained with the histochemical marker Giemsa (Artisan Giemsa Stain Kit; Dako Denmark A/S, Denmark), which provides metachromatic staining of mast cell granules, and the immunohistochemical marker tryptase (Monoclonal mouse antihuman mast cell tryptase; Dako, Denmark A/S, Denmark), a main mast cell constituent, for identification of mast cells within each specimen.[14] Alopecia cases were reviewed by the dermatopathology fellow (NA) and a board-certified dermatopathologist (CIV) who used three high-power fields (HPF) (×40) to count the number of mast cells within each specimen. The three counts were averaged, and the mean number of mast cells per HPF for each specimen was used to calculate an overall total mean/HPF for TE, AA, and ANDRO. The same protocol was used for controls of normal scalp skin.
Statistical analysis
One-way analysis of variance (ANOVA) was used to determine statistically significant difference between the three group mean mast cell counts. Tukey post hoc analysis was then employed to calculate the statistical significance of mean mast cell counts between each group and each stain. Student's t-test was used to compare the mean mast cell counts using Giemsa versus tryptase for each type of alopecia studied.
RESULTS
Telogen effluvium mast cell counts
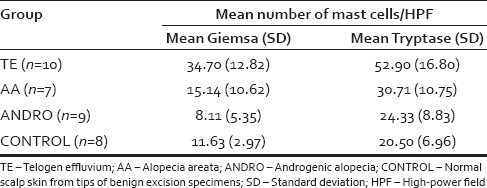
A total of ten specimens of previously diagnosed TE [Figure 1] were examined. When using Giemsa for the identification of mast cells, the calculated mean number of mast cells per HPF for individual TE specimens ranged from 18 to 53 with a total mean for all specimens of 34.70 and a standard deviation (SD) of 12.82. When using tryptase for the identification of mast cells, the calculated mean number of mast cells per HPF for individual TE specimens ranged from 21 to 74 with a total mean for all specimens of 52.90 and a SD of 16.80 [Table 1].
Figure 1.
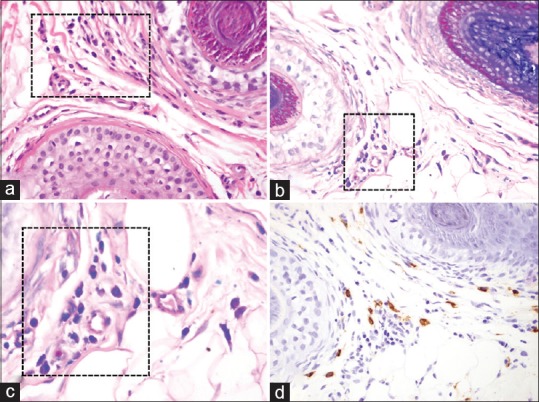
Telogen effluvium scalp biopsy specimen sections demonstrating the presence of perifollicular mast cells (black boxes). (a - H and E, ×40; b - Giemsa, ×40; c - Giemsa, ×100; d - Tryptase, ×40)
Table 1.
Mean number of mast cells per high-power field for each type of alopecia and normal scalp biopsies
Alopecia areata mast cell counts
A total of seven specimens of previously diagnosed AA [Figure 2] were examined. When using Giemsa for the identification of mast cells, the calculated mean number of mast cells per HPF for individual AA specimens ranged from 2 to 31 with a total mean for all specimens of 15.14 and a SD of 10.62. When using tryptase for the identification of mast cells, the calculated mean number of mast cells per HPF for individual AA specimens ranged from 11 to 40 with a total mean for all specimens of 30.71 and a SD of 10.75 [Table 1].
Figure 2.
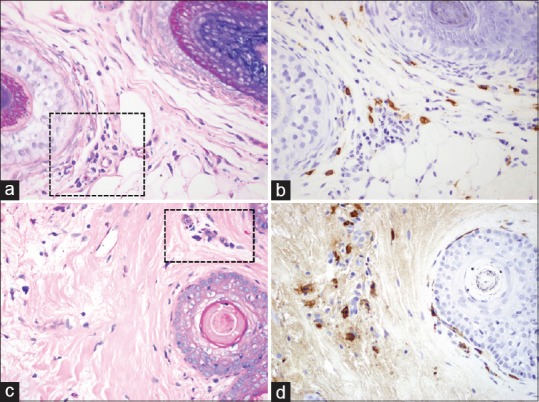
Mast cell presence in telogen effluvium compared to alopecia areata (black boxes indicate mast cells). (a) Telogen effluvium (Giemsa, ×40); (b) Telogen effluvium (Tryptase, ×40); (c) Alopecia areata (Giemsa, ×40); (d) Alopecia areata (Tryptase, ×40)
Androgenic alopecia mast cell counts
A total of nine specimens of previously diagnosed ANDRO were examined. When using Giemsa for identification of mast cells, the calculated mean number of mast cells per HPF for individual ANDRO specimens ranged from 1 to 18 with a total mean for all specimens of 8.11 and a SD of 5.35. When using tryptase for the identification of mast cells, the calculated mean number of mast cells per HPF for individual ANDRO specimens ranged from 7 to 34 with a total mean for all specimens of 24.33 and a SD of 8.83 [Table 1].
Normal scalp skin controls (CONTROL) mast cell counts
A total of eight normal scalp skin specimens were examined. When using Giemsa for the identification of mast cells, the calculated total mean number of mast cells per HPF for all specimens (n = 8) was 11.63 with a SD of 2.97. When using tryptase for the identification of mast cells, the calculated total mean number of mast cells per HPF for all specimens (n = 8) was 20.50 with a SD of 6.97 [Table 1].
Statistical analysis
One-way ANOVA demonstrated statistically significant group-level differences [Figure 3] in the mean mast cells counts across the three types of alopecia in both Giemsa and tryptase as well as for TE compared to normal scalp skin controls [Table 1]. In addition, Student's t-test analysis showed that within each type of alopecia, the tryptase staining mast cell mean was higher than the Giemsa staining mast cell mean.
Figure 3.
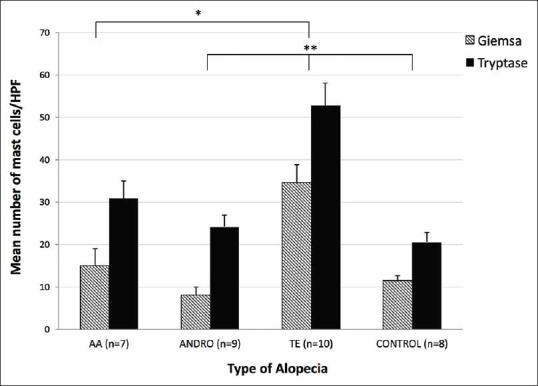
Mean number of mast cells (+standard error mean) per high-power field in telogen effluvium, alopecia areata, androgenic alopecia and normal (CONTROL) scalp biopsies. Tukey post hoc analysis showed the mean mast cell count for the telogen effluvium group to be significantly larger than androgenic alopecia (Giemsa, *P = 0.002; Tryptase, *P = 0.006) and CONTROL (Giemsa and Tryptase, *P < 0.001) and also showed the mean mast cell count for telogen effluvium to be significantly larger than alopecia areata (Giemsa and Tryptase, **P < 0.001)
DISCUSSION
TE is a type of acquired, diffuse, nonscarring alopecia which involves hair loss 2–4 months following a stressful inciting event. The relationship between psychoemotional stress and hair loss was first elucidated by Selye in 1950.[15] Selye's observation led to multiple investigations of the link between stress and sudden hair loss, many of which date back to the 1950s.[16,17] Although a medically benign condition, alopecia can have a significant negative impact on one's self-image, leading to a decreased quality of life.[18,19] Despite the fact that an association between hair loss and stress is readily acknowledged by patients and physicians, a clear-cut pathophysiological mechanism explaining the connection between psychoemotional stress and hair loss in humans remains to be demonstrated. Our analysis of scalp biopsy specimens demonstrated that TE specimens have a significantly higher number of mast cell counts per HPF compared to normal control skin, AA, and ANDRO specimens, indicating that mast cells could play a role in the pathophysiology of hair loss in TE.
In an attempt to further elucidate the pathophysiology of stress-induced hair loss, Arck et al. proposed a substanceP(SP)-dependent neuroimmunological “brain-hair follicle” axis.[11] Following the induction of a stressful event, the hypothalamic-pituitary-adrenal (HPA) stress response axis is activated. Many well-studied hormones that are released along the HPA axis, such as corticotropin-releasing hormone, adrenocorticotropic hormone, and glucocorticoids, have been associated with hair loss in mice.[20,21,22] In addition, increased HPA axis tone is thought to stimulate SP release from sensory nerve fibers in the skin, which densely innervate scalp hair follicles.[23] Locally released SP has been shown to induce hair follicle keratinocyte apoptosis by activating macrophages and mast cells[24,25] to release several hair growth inhibiting bioactive molecules such as tumor necrosis factor-α,[26] interleukin-1,[27,28] and proteases.[29] Furthermore, neurokinin-1 receptor (NK-1R) and SP knockout mice have been shown to be resistant to stress-triggered premature induction of catagen and hair follicle apoptosis, which suggests that mast cells express NK-1R and that the cross-talk between SP and NK-1R on mast cells is essential in mediating stress-related hair loss.[13] Nerve growth factor (NGF) has also been shown to promote outgrowth of SP+ nerve fibers as well as degranulate mast cells downstream of SP.[30,31] The administration of NGF-neutralizing antibodies following a stressful stimulus has been shown to abrogate premature onset of catagen, the number of perifollicular mast cells, and hair-follicle keratinocyte apoptosis in murine skin.[32]
Several murine studies indicate that mast cells play a distal, intermediary role in stress-induced hair loss, serving as a link between the nervous system and immunologic system, as evidenced by the proximity of mast cells and sensory nerve endings in both normal[33] and inflamed[34] skin. The relationship between dermal SP+ sensory nerve endings and mast cells may be bidirectional. SP degranulates mast cells[35] inducing cytokine[36,37] and NGF expression and selective release of proteases. The combination of proteases and NGF subsequently promotes survival and outgrowth of sensory nerve fibers[30,31] through protease-activating receptors on the surface of sensory nerve endings.[38] Thus, mast cells act as modulators of hair follicle growth and regression[25,26,27,28,29,30,31,32,33,34,35,36,37,38,39] by means of SP and NGF. Mast cells have also been reported to play a major role in AA. Bertolini et al.[40] found the number, degranulation, and proliferation of perifollicular mast cells as well as mast cell-CD8+ T-cell physical contacts to be significantly increased in human AA lesions compared to healthy control skin. They proposed the interaction of mast cells with CD8+ T-cells causes the mast cells to switch from an immune-inhibitory to a pro-inflammatory phenotype that causes the anagen hair follicle to lose its immune privilege by upregulating major histocompatibility complex (MHC) class I molecules and downregulating the expression of immune privilege guardian molecules such as transforming growth factor beta-1. In addition, Peters et al.[41] showed that cultured human anagen hair follicles exposed to SP promoted catagen transformation and increased MHC class I expression, suggesting that the loss of immune privilege also plays a role in stress-induced hair loss.
By analyzing human scalp biopsy specimens, the results of the current study corroborate the putative “brain-hair follicle” axis[11] and contribute to the growing body of murine model, in vivo evidence that stress-induced hair loss is a neuroimmunologic phenomenon with mast cells playing an essential role at the distal end of the cascade. Currently, systemic treatment with NK-1R antagonists[13] anti-NGF neutralizing antibodies[32] and topical minoxidil[42] has been shown to reduce stress-induced hair loss in murine models. Physicians should not underestimate the emotional impact of hair loss as it can often provoke distress that is out of proportion to its objective severity. As the investigation into the “brain-skin connection” continues, it is essential for researchers and clinicians to understand the hair follicle as a peripheral target of a myriad of bioactive molecules involved in the stress cascade. Theoretically, any step or molecule implicated in this cascade could serve as a therapeutic target, and clinical trials are needed. Our results suggest that mast cells could be implicated in the pathogenesis of hair loss in patients diagnosed with TE and that modulation of either systemic or perifollicular mast cell activity could be a promising therapeutic approach for these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hordinsky MK. Medical treatment of noncicatricial alopecia. Semin Cutan Med Surg. 2006;25:51–5. doi: 10.1016/j.sder.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Harrison S, Sinclair R. Telogen effluvium. Clin Exp Dermatol. 2002;27:389–5. doi: 10.1046/j.1365-2230.2002.01080.x. [DOI] [PubMed] [Google Scholar]
- 3.Lo Sicco K, McGuire S, English JC., 3rd A retrospective study of thyroid structural abnormalities in alopecia patients. Dermatoendocrinol. 2011;3:251–4. doi: 10.4161/derm.3.4.16838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez-Mora N, Goren A, Velasco C, Bermudez F. Acute telogen effluvium onset event is associated with the presence of female androgenetic alopecia: Potential therapeutic implications. Dermatol Ther. 2014;27:159–62. doi: 10.1111/dth.12101. [DOI] [PubMed] [Google Scholar]
- 5.Krusinski PA. Letter: Telogen effluvium secondary to weight loss and therapy with chorionic gonadotropin. Arch Dermatol. 1976;112:556. doi: 10.1001/archderm.1976.01630280074027. [DOI] [PubMed] [Google Scholar]
- 6.Gizlenti S, Ekmekci TR. The changes in the hair cycle during gestation and the post-partum period. J Eur Acad Dermatol Venereol. 2014;28:878–81. doi: 10.1111/jdv.12188. [DOI] [PubMed] [Google Scholar]
- 7.Patel M, Harrison S, Sinclair R. Drugs and hair loss. Dermatol Clin. 2013;31:67–73. doi: 10.1016/j.det.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Rebora A. Telogen effluvium. Dermatology. 1997;195:209–12. doi: 10.1159/000245944. [DOI] [PubMed] [Google Scholar]
- 9.Peters EM, Arck PC, Paus R. Hair growth inhibition by psychoemotional stress: A mouse model for neural mechanisms in hair growth control. Exp Dermatol. 2006;15:1–13. doi: 10.1111/j.0906-6705.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- 10.Arck PC, Handjiski B, Peters EM, Peter AS, Hagen E, Fischer A, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003;162:803–14. doi: 10.1016/S0002-9440(10)63877-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arck PC, Handjiski B, Hagen E, Joachim R, Klapp BF, Paus R. Indications for a 'brain-hair follicle axis (BHA)': Inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. FASEB J. 2001;15:2536–8. doi: 10.1096/fj.00-0699fje. [DOI] [PubMed] [Google Scholar]
- 12.Peters EM, Kuhlmei A, Tobin DJ, Müller-Röver S, Klapp BF, Arck PC. Stress exposure modulates peptidergic innervation and degranulates mast cells in murine skin. Brain Behav Immun. 2005;19:252–62. doi: 10.1016/j.bbi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Arck PC, Handjiski B, Kuhlmei A, Peters EM, Knackstedt M, Peter A, et al. Mast cell deficient and neurokinin-1 receptor knockout mice are protected from stress-induced hair growth inhibition. J Mol Med (Berl) 2005;83:386–96. doi: 10.1007/s00109-004-0627-z. [DOI] [PubMed] [Google Scholar]
- 14.Hogan AD, Schwartz LB. Markers of mast cell degranulation. Methods. 1997;13:43–52. doi: 10.1006/meth.1997.0494. [DOI] [PubMed] [Google Scholar]
- 15.Selye H. The Physiology and Pathology of Exposure to Stress. Oxford, England: Acta, Inc; 1950. [Google Scholar]
- 16.Greenberg SI. Alopecia areata, a psychiatric survey. AMA Arch Derm. 1955;72:454–7. doi: 10.1001/archderm.1955.03730350056010. [DOI] [PubMed] [Google Scholar]
- 17.Macalpine I. Is alopecia areata psychosomatic? A psychiatric study. Br J Dermatol. 1958;70:117–31. doi: 10.1111/j.1365-2133.1958.tb13304.x. [DOI] [PubMed] [Google Scholar]
- 18.Hunt N, McHale S. The psychological impact of alopecia. BMJ. 2005;331:951–3. doi: 10.1136/bmj.331.7522.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cartwright T, Endean N, Porter A. Illness perceptions, coping and quality of life in patients with alopecia. Br J Dermatol. 2009;160:1034–9. doi: 10.1111/j.1365-2133.2008.09014.x. [DOI] [PubMed] [Google Scholar]
- 20.Farooqi IS, Jones MK, Evans M, O'Rahilly S, Hodges JR. Triple H syndrome: A novel autoimmune endocrinopathy characterized by dysfunction of the hippocampus, hair follicle, and hypothalamic-pituitary adrenal axis. J Clin Endocrinol Metab. 2000;85:2644–8. doi: 10.1210/jcem.85.8.6753. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Kim CH, Choi YJ, Ayala AG, Amirikachi M, Ro JY. Juxtaglomerular cell tumor of kidney with CD34 and CD117 immunoreactivity: Report of 5 cases. Arch Pathol Lab Med. 2006;130:707–11. doi: 10.5858/2006-130-707-JCTOKW. [DOI] [PubMed] [Google Scholar]
- 22.Paus R, Botchkarev VA, Botchkareva NV, Mecklenburg L, Luger T, Slominski A. The skin POMC system (SPS).Leads and lessons from the hair follicle. Ann N Y Acad Sci. 1999;885:350–63. doi: 10.1111/j.1749-6632.1999.tb08690.x. [DOI] [PubMed] [Google Scholar]
- 23.Ericson M, Gabrielson A, Worel S, Lee WS, Hordinsky MK. SubstanceP(SP) in innervated and non-innervated blood vessels in the skin of patients with symptomatic scalp. Exp Dermatol. 1999;8:344–5. [PubMed] [Google Scholar]
- 24.Suzuki R, Furuno T, McKay DM, Wolvers D, Teshima R, Nakanishi M, et al. Direct neurite-mast cell communication in vitro occurs via the neuropeptide substance P. J Immunol. 1999;163:2410–5. [PubMed] [Google Scholar]
- 25.Maurer M, Fischer E, Handjiski B, von Stebut E, Algermissen B, Bavandi A, et al. Activated skin mast cells are involved in murine hair follicle regression (catagen) Lab Invest. 1997;77:319–32. [PubMed] [Google Scholar]
- 26.Rückert R, Lindner G, Bulfone-Paus S, Paus R. High-dose proinflammatory cytokines induce apoptosis of hair bulb keratinocytes in vivo. Br J Dermatol. 2000;143:1036–9. doi: 10.1046/j.1365-2133.2000.03784.x. [DOI] [PubMed] [Google Scholar]
- 27.Berman AS, Chancellor-Freeland C, Zhu G, Black PH. Substance P primes murine peritoneal macrophages for an augmented proinflammatory cytokine response to lipopolysaccharide. Neuroimmunomodulation. 1996;3:141–9. doi: 10.1159/000097239. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann R, Happle R. Alopecia areata 1: Clinical aspects, etiology, pathogenesis. Hautarzt. 1999;50:W222–31. doi: 10.1007/s001050050895. [DOI] [PubMed] [Google Scholar]
- 29.Hou L, Kapas S, Cruchley AT, Macey MG, Harriott P, Chinni C, et al. Immunolocalization of protease-activated receptor-2 in skin: Receptor activation stimulates interleukin-8 secretion by keratinocytes in vitro. Immunology. 1998;94:356–62. doi: 10.1046/j.1365-2567.1998.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, et al. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994;91:3739–43. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang Z, Nilsson G. IgE receptor-mediated release of nerve growth factor by mast cells. Clin Exp Allergy. 2000;30:1379–86. doi: 10.1046/j.1365-2222.2000.00906.x. [DOI] [PubMed] [Google Scholar]
- 32.Peters EM, Handjiski B, Kuhlmei A, Hagen E, Bielas H, Braun A, et al. Neurogenic inflammation in stress-induced termination of murine hair growth is promoted by nerve growth factor. Am J Pathol. 2004;165:259–71. doi: 10.1016/S0002-9440(10)63294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Botchkarev VA, Eichmüller S, Peters EM, Pietsch P, Johansson O, Maurer M, et al. A simple immunofluorescence technique for simultaneous visualization of mast cells and nerve fibers reveals selectivity and hair cycle – Dependent changes in mast cell – Nerve fiber contacts in murine skin. Arch Dermatol Res. 1997;289:292–302. doi: 10.1007/s004030050195. [DOI] [PubMed] [Google Scholar]
- 34.Naukkarinen A, Harvima I, Paukkonen K, Aalto ML, Horsmanheimo M. Immunohistochemical analysis of sensory nerves and neuropeptides, and their contacts with mast cells in developing and mature psoriatic lesions. Arch Dermatol Res. 1993;285:341–6. doi: 10.1007/BF00371834. [DOI] [PubMed] [Google Scholar]
- 35.Paus R, Heinzelmann T, Robicsek S, Czarnetzki BM, Maurer M. Substance P stimulates murine epidermal keratinocyte proliferation and dermal mast cell degranulation in situ. Arch Dermatol Res. 1995;287:500–2. doi: 10.1007/BF00373436. [DOI] [PubMed] [Google Scholar]
- 36.Matsuda H, Kawakita K, Kiso Y, Nakano T, Kitamura Y. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol. 1989;142:927–31. [PubMed] [Google Scholar]
- 37.Foreman JC. Substance P and calcitonin gene-related peptide: Effects on mast cells and in human skin. Int Arch Allergy Appl Immunol. 1987;82:366–71. doi: 10.1159/000234229. [DOI] [PubMed] [Google Scholar]
- 38.Steinhoff M, Ständer S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139:1479–88. doi: 10.1001/archderm.139.11.1479. [DOI] [PubMed] [Google Scholar]
- 39.Maurer M, Paus R, Czarnetzki BM. Mast cells as modulators of hair follicle cycling. Exp Dermatol. 1995;4(4 Pt 2):266–71. doi: 10.1111/j.1600-0625.1995.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 40.Bertolini M, Zilio F, Rossi A, Kleditzsch P, Emelianov VE, Gilhar A, et al. Abnormal interactions between perifollicular mast cells and CD8+T-cells may contribute to the pathogenesis of alopecia areata. PLoS One. 2014;9:e94260. doi: 10.1371/journal.pone.0094260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters EM, Liotiri S, Bodó E, Hagen E, Bíró T, Arck PC, et al. Probing the effects of stress mediators on the human hair follicle: Substance P holds central position. Am J Pathol. 2007;171:1872–86. doi: 10.2353/ajpath.2007.061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arck PC, Handjiski B, Peters EM, Hagen E, Klapp BF, Paus R. Topical minoxidil counteracts stress-induced hair growth inhibition in mice. Exp Dermatol. 2003;12:580–90. doi: 10.1034/j.1600-0625.2003.00028.x. [DOI] [PubMed] [Google Scholar]


