Abstract
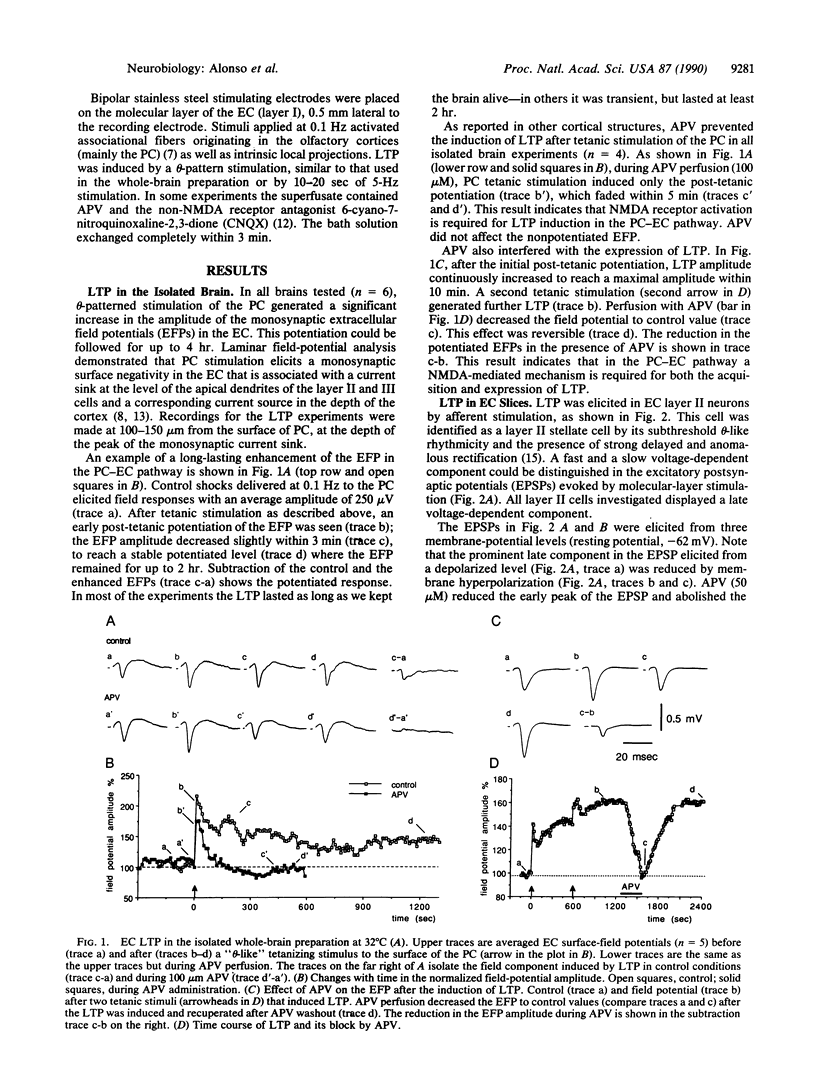
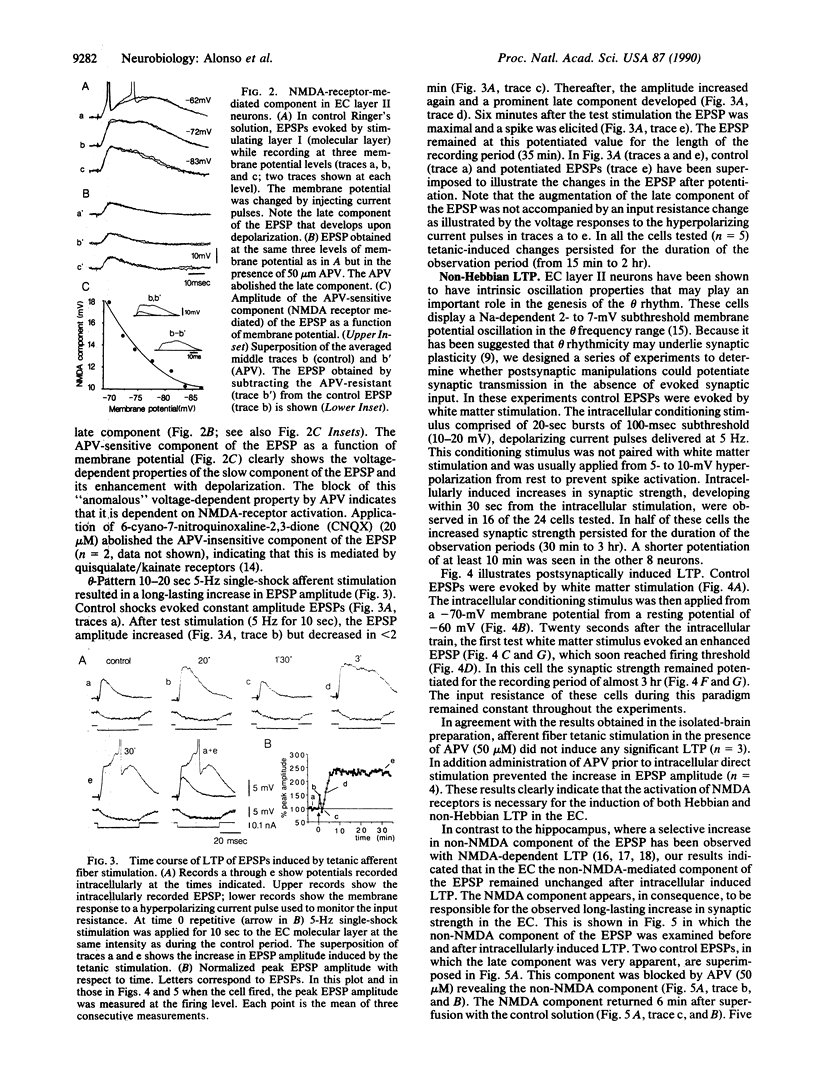
Long-term potentiation (LTP) was investigated in the mammalian entorhinal cortex by using two in vitro preparations, the isolated brain and the entorhinal cortex slice. Hebbian and non-Hebbian types of LTP appear to be present in layer II entorhinal cortex cells as demonstrated using two protocols: (i) tetanic stimulation of the piriform-entorhinal cortex afferent pathway to generate homosynaptic potentiation and (ii) postsynaptic subthreshold rhythmic membrane potential manipulation not paired to presynaptic activation, which gives rise to non-Hebbian LTP. The induction and the expression of both types of LTP were found to be dependent on activation of N-methyl-D-aspartate receptors as shown by their sensitivity to the receptor agonist D-2-amino-5-phosphonovalerate. This is in contrast to LTP in the hippocampus [Zalutsky, R. A. & Nicoll, R. A. (1990) Science 248, 1619-1624], where LTP is expressed by quisqualate receptors. Since, in the entorhinal cortex, LTP is linked to a selective increase of the N-methyl-D-aspartate-receptor-mediated synaptic responses, this enhancement is most likely due to postsynaptic factors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso A., Llinás R. R. Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature. 1989 Nov 9;342(6246):175–177. doi: 10.1038/342175a0. [DOI] [PubMed] [Google Scholar]
- Andersen P., Holmqvist B., Voorhoeve P. E. Entorhinal activation of dentate granule cells. Acta Physiol Scand. 1966 Apr;66(4):448–460. doi: 10.1111/j.1748-1716.1966.tb03223.x. [DOI] [PubMed] [Google Scholar]
- Artola A., Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987 Dec 17;330(6149):649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Blake J. F., Brown M. W., Collingridge G. L. CNQX blocks acidic amino acid induced depolarizations and synaptic components mediated by non-NMDA receptors in rat hippocampal slices. Neurosci Lett. 1988 Jun 29;89(2):182–186. doi: 10.1016/0304-3940(88)90378-3. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeijinga P. H., Van Groen T. Inputs from the olfactory bulb and olfactory cortex to the entorhinal cortex in the cat. II. Physiological studies. Exp Brain Res. 1984;57(1):40–48. doi: 10.1007/BF00231130. [DOI] [PubMed] [Google Scholar]
- Cain D. P. Long-term potentiation and kindling: how similar are the mechanisms? Trends Neurosci. 1989 Jan;12(1):6–10. doi: 10.1016/0166-2236(89)90146-x. [DOI] [PubMed] [Google Scholar]
- Davies J., Francis A. A., Jones A. W., Watkins J. C. 2-Amino-5-phosphonovalerate (2APV), a potent and selective antagonist of amino acid-induced and synaptic excitation. Neurosci Lett. 1981 Jan 1;21(1):77–81. doi: 10.1016/0304-3940(81)90061-6. [DOI] [PubMed] [Google Scholar]
- Fletcher E. J., Lodge D. Glycine reverses antagonism of N-methyl-D-aspartate (NMDA) by 1-hydroxy-3-aminopyrrolidone-2 (HA-966) but not by D-2-amino-5-phosphonovalerate (D-AP5) on rat cortical slices. Eur J Pharmacol. 1988 Jun 22;151(1):161–162. doi: 10.1016/0014-2999(88)90711-x. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Wigström H. Physiological mechanisms underlying long-term potentiation. Trends Neurosci. 1988 Apr;11(4):156–162. doi: 10.1016/0166-2236(88)90142-7. [DOI] [PubMed] [Google Scholar]
- Kauer J. A., Malenka R. C., Nicoll R. A. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron. 1988 Dec;1(10):911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- Kimura F., Nishigori A., Shirokawa T., Tsumoto T. Long-term potentiation and N-methyl-D-aspartate receptors in the visual cortex of young rats. J Physiol. 1989 Jul;414:125–144. doi: 10.1113/jphysiol.1989.sp017680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek J. E., Price J. L. Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in the rat and cat. J Comp Neurol. 1977 Apr 15;172(4):723–752. doi: 10.1002/cne.901720409. [DOI] [PubMed] [Google Scholar]
- Larson J., Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986 May 23;232(4753):985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- Llinás R., Mühlethaler M. An electrophysiological study of the in vitro, perfused brain stem-cerebellum of adult guinea-pig. J Physiol. 1988 Oct;404:215–240. doi: 10.1113/jphysiol.1988.sp017287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Porietis A. V., Wojtowicz J. M. L-Aspartic acid induces a region of negative slope conductance in the current-voltage relationship of cultured spinal cord neurons. Brain Res. 1982 Apr 8;237(1):248–253. doi: 10.1016/0006-8993(82)90575-3. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Zucker R. S., Nicoll R. A. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988 Oct 7;242(4875):81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- Muller D., Joly M., Lynch G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science. 1988 Dec 23;242(4886):1694–1697. doi: 10.1126/science.2904701. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Racine R. J., Milgram N. W., Hafner S. Long-term potentiation phenomena in the rat limbic forebrain. Brain Res. 1983 Feb 7;260(2):217–231. doi: 10.1016/0006-8993(83)90676-5. [DOI] [PubMed] [Google Scholar]
- Sah P., Hestrin S., Nicoll R. A. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989 Nov 10;246(4931):815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- Teyler T. J., DiScenna P. Long-term potentiation. Annu Rev Neurosci. 1987;10:131–161. doi: 10.1146/annurev.ne.10.030187.001023. [DOI] [PubMed] [Google Scholar]
- Van Groen T., Lopes da Silva F. H., Wadman W. J. Synaptic organization of olfactory inputs and local circuits in the entorhinal cortex: a current source density analysis in the cat. Exp Brain Res. 1987;67(3):615–622. doi: 10.1007/BF00247292. [DOI] [PubMed] [Google Scholar]
- Zalutsky R. A., Nicoll R. A. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990 Jun 29;248(4963):1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]