Abstract
Background:
Hirsutism is the presence of terminal hair in females in males pattern. It occurs in 5%–15% of women. Modified Ferriman–Gallwey (mFG) score of ≥ 8 is considered hirsutism, but there are populations with a low mFG score. In clinical practice, hirsutism is subjective. Although hirsutism is considered as a purely esthetic problem, it is associated with many underlying disorders, especially androgen excess disorders. Polycystic ovarian syndrome (PCOS) disease is the most common cause of androgen excess in females, and there are reports of its association with metabolic syndrome. Metabolic syndrome occurs alone in hirsutism.
Aims:
To assess mFG score in patients who consider themselves as hirsute. To study the association between metabolic syndrome and hirsutism.
Methods:
Hospital-based cross-sectional study design was adopted. A structured questionnaire was used to collect sociodemographic and clinical data. The severity of hirsutism was assessed using mFG score and metabolic syndrome was diagnosed by the American Heart Association criteria. mFG score was expressed as mean and Student's t-test and Chi-square statistic were used as the tests of significance. Logistic regression analysis was performed.
Results:
The mean mFG score was 5.5. Metabolic syndrome was present in 44%. About 65.2% of patients with score ≥8 had metabolic syndrome, whereas only 37.7% of patients with score <8 had metabolic syndrome (P = 0.019). Metabolic syndrome (P = 0.018) and PCOS (P = 0.003) were the significant variables in logistic regression analysis. Triglyceride levels ≥150 mg/dl and waist circumference ≥88 cm were the components of metabolic syndrome that were significantly associated with hirsutism (P = 0.006 in both).
Conclusions:
To find the ideal cutoff of mFG score to define hirsutism in our population, a population study among females in the reproductive age group has to be conducted. As there is a definite association of hirsutism and metabolic syndrome, and metabolic syndrome can result in cardiovascular complications, any women presenting with terminal hair in a male pattern should be evaluated for metabolic syndrome irrespective of the mFG score.
Key words: Hirsutism, metabolic syndrome, Modified Ferriman–Gallwey score, polycystic ovarian syndrome
INTRODUCTION
Hirsutism is defined as excessive growth of terminal hair in androgen-dependent areas of the body in women.[1] It is a common endocrine disorder responsible for a lot of anxiety and stress among young women and can be a threat to feminine identity. The severity of hirsutism and its acceptance depend on racial, cultural, and social factors. Hirsutism is broadly divided into androgen-and non-androgen induced. Polycystic ovarian syndrome (PCOS) is the most common cause of hyperandrogenic hirsutism, and idiopathic hirsutism is the most common cause of non-androgen-induced hirsutism. Both idiopathic hirsutism and PCOS account for 95% of hirsutism.[1] The spectrum of hirsutism varies from mild to severe. To quantify hirsutism, Ferriman and Gallwey introduced a scoring system in 1961 incorporating eleven androgen dependent sites.[2] This was later modified incorporating only nine sites and this modified Ferryman–Gallwey (mFG) scoring system is considered the standard scoring system that defines hirsutism quantitatively.[3,4,5] The prevalence of hirsutism depends on the method used to determine its presence, the population under study and the score that is used as the cutoff. Although conventionally, mFG score ≥8 is considered hirsutism, studies conducted in different races have shown that this cutoff value may not be applicable in many cases.[6,7,8,9,10] In clinical practice, it has often been suggested that real hirsutism is simply that, which the woman in question thinks is excessive.[1] We were interested in knowing the mFG score of our patients who consider themselves as hirsute. Metabolic syndrome is a constellation of interrelated risk factors of metabolic origin that promote the development of the atherosclerotic cardiovascular disease.[11,12,13] This is characterized by hyper-insulinemia, low glucose tolerance, dyslipidemia, hypertension, and obesity. Although the pathophysiology of metabolic syndrome is a subject of continuing controversy a causal relationship with insulin resistance has been suggested.[12] The association of PCOS, the most common cause of hirsutism and metabolic syndrome has been reported.[14,15] Hirsutism is often observed in patients with metabolic syndrome alone. Whether the metabolic syndrome diagnosed in hirsutism is due to the underlying PCOS or whether metabolic syndrome can occur as an independent association has not been reported from our part of the country. Hence, in addition to the mFG score, we also studied the association of metabolic syndrome and hirsutism.
METHODS
One hundred female patients with male pattern distribution of terminal hair who attended the Department of Dermatology and Venereology, of a tertiary care center in South India were subjected to the study after getting informed written consent. Nonconsenting patients and those patients without male pattern hair growth in the androgen sensitive areas were excluded from the study. Hospital-based, cross-sectional, descriptive type of the study design was used. The sample size was calculated using the formula “pq (Zα)2/d2”, where p represents the proportion of women with hirsutism and q the proportion without hirsutism. With p as 10.5%,[16] Zα of 1.96 with the significance level of P < 0.05 and precision as 6, the, sample size was calculated to be 100 (10.5 × 89.5× [1.96]2/ 6 × 6). Structured questionnaire was used to collect the data under the broad domains of sociodemographic characteristics (age, marital status, menstrual irregularities, family history, infertility, body weight, height, body mass index (BMI), waist circumference, and blood pressure), disease characteristics (mFG score, PCOS, metabolic syndrome, acne, acanthosis nigricans, cushingoid features, and virilization) and investigations (high-density lipoprotein [HDL] level, triglyceride (TG) level, fasting blood sugar (FBS), thyroid function tests, and abdominal ultrasound). Grading of hirsutism was done based on mFG scoring system.[3,4,5] The conventional definition of hirsutism based on mFG score ≥8 was used for studying the association with metabolic syndrome and other diseases. The presence of metabolic syndrome was assessed using the American Heart Association/National Heart, Lung, and Blood Institute criteria.[11] PCOS was diagnosed using the Gynaecology department. Data were entered into MS Exel. Mean was used for describing continuous variables and proportion for categorical variables. For univariate analysis, patients were categorized into two groups-those with hirsutism (mFG ≥8) and those without hirsutism (mFG <8). The association of variables such as acne, acanthosis nigricans, menstrual irregularities, infertility, family history of hirsutism, BMI, waist circumference, blood pressure, FBS, HDL, TG levels, and metabolic syndrome with hirsutism were studied. Student's t-test was used for testing the difference in mean in two groups, and Chi-square statistic was used for testing the difference in proportion in two groups. Logistic regression analysis was performed with those variables that were statistically significant in univariate analysis. The study was approved and conducted according to the institutional review board guidelines.
RESULTS
The age ranged from 17 to 77 years (mean 35.2 ± 13 years), with majority (29%) belonging to the age group of 31–40 years. Eighty percent of the patients were married. Majority (96%) had hirsutism for more than a year. The family history of hirsutism was present in 27%. Forty-three percent had menstrual irregularities. Nearly 26.3% of married women gave a history of infertility.
The mFG score ranged from 2 to 10 with a mean score of 5.5 ± 2.1. The most common score was 6 (20%) followed by 4 (17%), 8 (16%), 5 (12%), 3 (10%), 2 (9%), 3 (9%), and 10 (1%). Majority (77%) had score <8. Only 23% had score ≥8. All the 23 had mild hirsutism. The face was the most common site of involvement followed by the chest.
Table 1 shows the number- and percentage-wise distribution of each variable within the groups–mFG score ≥8 and <8 and the P value of the difference in proportion. Metabolic syndrome was present in 44%. 65.2% (15/23) patients with score ≥8 had metabolic syndrome whereas only 37.7% (29/77) of patients with score <8 had hirsutism (P = 0.019). The components of metabolic syndrome like waist circumference ≥88 cm was observed in 62%, HDL cholesterol <50 mg/dl in 55%, hypertension in 43%, diabetes in 34%, and elevated TG ≥150 in 33%. Among the patients with mFG score ≥8, 87% (20/23) had waist circumference ≥88 cm and in patients with score <8, there were only 54.5% (42/77) with waist circumference ≥88 cm (P 0.006). TGs ≥150 was seen in 56.5% (13/23) with mFG ≥ 8 and 26% (20/77) with mFG score <8 (P = 0.006). The proportion of other components in the mFG score ≥8 and <8, respectively, were: HDL cholesterol <50 mg/dl (69.6%, 50.6%; P = 0.11), hypertension (43.5%, 42.9%; P = 0.96), and diabetes (43.5%, 31.2%; P = 0.27).
Table 1.
Univariate analysis
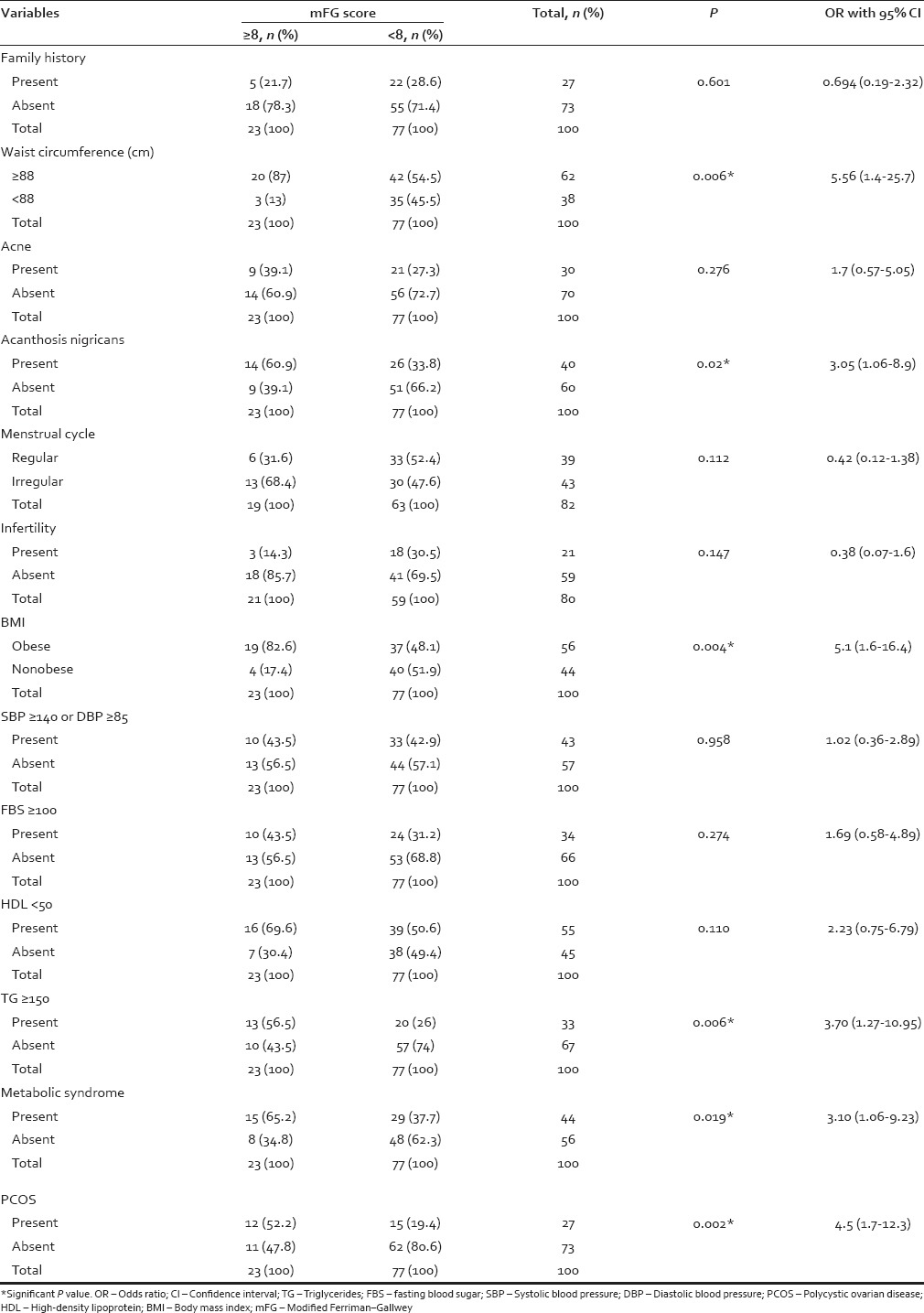
Fifty-six percent were preobese/obese. 82.6% (19/23) in mFG ≥8 group and 48.1% (37/77) in mFG <8 group were preobese/obese (P = 0.004). PCOS was present in 27%. 52.2% (12/23) in mFG ≥8 group and 19.4% (15/77) in mFG <8 group had PCOS (P = 0.002). Acanthosis nigricans was present in 40%. 60.9% (14/23) in mFG ≥8 group and 33.8% (26/77) in mFG <8 group (P = 0.02). Acne was seen in 30% and thyroid disorders in 19%. Table 1 shows the percentages of these disorders in the two groups.
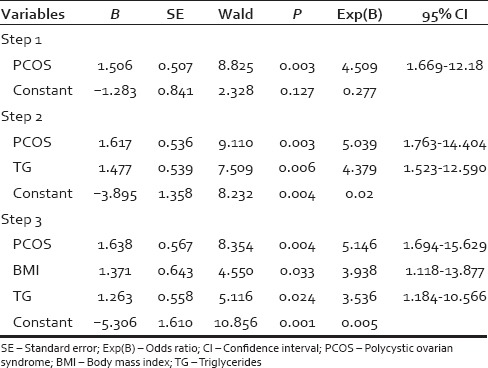
In the univariate analysis [Table 1] when the variables such as acne, acanthosis nigricans, menstrual history, infertility, family history of hirsutism, BMI, waist circumference, blood pressure, FBS, HDL, TG, and metabolic syndrome were cross-tabulated with mFG score, family history of hirsutism, acne, irregular cycles, infertility, hypertension, diabetes, HDL cholesterol <50 mg/dl, thyroid disorders did not achieve statistical significance. The statistically significant variables were PCOS (P = 0.002), BMI (P = 0.004), waist circumference ≥88 cm (P = 0.006.), hypertriglyceridemia ≥150 mg/dl (P = 0.006), metabolic syndrome (P = 0.019), and acanthosis nigricans (P = 0.02). From the statistically and clinically significant variables in the univariate analysis, logistic regression analysis was performed. Two logistic regression models were studied. In the first model acanthosis nigricans, PCOS and metabolic syndrome were chosen [Table 2]. The significant variables in the first model were PCOS (P = 0.003) and metabolic syndrome (P = 0.018). In the second model, all the variables significant in univariate analysis were incorporated [Table 3] and the significant variables were PCOS (P = 0.004), TGs ≥150 mg/dl (P = 0.024) and BMI (P = 0.033).
Table 2.
Logistic regression analysis: Model 1
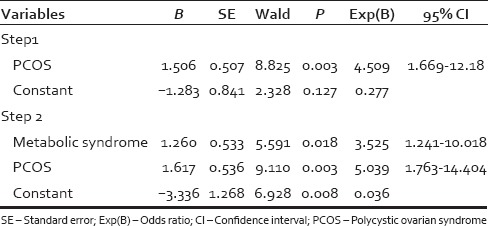
Table 3.
Logistic regression analysis: Model 2
DISCUSSION
Hirsutism although not a life-threatening disorder can negatively influence the psychological well-being. The acceptance of hirsutism depends on racial, cultural, and social factors. The modified Ferriman and Gallwey scoring system has become the standard scoring system, and the most important aspect is the selection of the cut-off value of the score to define hirsutism. Currently, many clinicians and researchers use mFG score of ≥8 as hirsutism. This score of ≥8 was put forward by Hatch et al., although Ferriman and Gallwey themselves have used different cutoffs in different studies.[2,3,4,5,6] As ethnicity and race can affect the terminal hair growth, the ideal cutoff value should be the 95th percentile value of the score of unselected women of reproductive age.[6] A specific statistical cutoff value may not reflect the subjective nature of hirsutism. We studied the mFG score of one hundred patients who considered themselves as having hirsutism, attending the Department of Dermatology and Venereology of a tertiary care center in South Kerala. The association of metabolic syndrome and other diseases were studied after categorizing patients into two groups; mFG score value ≥8 and <8.
The mean age (35.12) and the most common age range (31–40) was higher in our patients compared to other reports.[16,17,18] The duration of hirsutism (>1 year) was both in agreement and disagreement with other studies.[17,18,19,20] The family history of hirsutism was present in 23% of our patients, and the family history ranged from 16% to 45% in different studies.[18,19,20,21] Menstrual irregularities reported in 43% of our patients was also in agreement and disagreement with other studies.[17,18,19,20,21] Contradictory to previous reports 80% of our patients were married with 26.3% having a history of infertility.[18,20]
The mFG score ranged from 2 to 10 and was <8 in 77% and was consistent with the study by Noorbala and Kefaie and Sharma et al.;[16,19,21] however, it ranged from 10 to 34 in the study by Chhabra et al. and Ahmad et al.[18,20] The mean score was 12.4 and 13.5 in Delhi and Kashmir, respectively.[16,19] The low score in our study may be due to our inclusion criteria definition of hirsutism as terminal hair in at least one androgen dependent site rather than the definition of mFG score ≥8 used by others.
The majority of patients belonged to the preobese/obese category similar to that observed by Apridonidze et al.[22] Metabolic syndrome was present in 44% of our patients. Studies also report the prevalence of metabolic syndrome in 43% and 46% in patients with PCOS. Sixty-five percentage of patients with mFG score ≥8 had metabolic syndrome, whereas only 37.7% of patients with score <8 had metabolic syndrome. As in other studies the most common metabolic syndrome component and the second most common component was waist circumference ≥88 cm and HDL cholesterol <50 mg/dl, respectively, although these studies were in PCOS.[15,22] The lowest prevalent component in our study was serum TG ≥150 mg/dl, whereas in other studies, it was FBS.[15,22]
The prevalence of acanthosis nigricans (60.9%), acne (39.1%), and PCOS (52.2%) in those with MFG score ≥8 was almost similar to other studies with minimal variations.[17,18,19] None of our patients had ovarian tumors.
To study the association of hirsutism with metabolic syndrome and other clinical parameters, the conventional definition of mFG score ≥8 was used to define hirsutism. The study population were, thus, categorized into two, those with hirsutism (mFG score ≥8) and without hirsutism (mFG score <8). Mean age of those with mFG score <8 was 35.5 and those with mFG score of ≥8 was 34.3. There was no statistically significant difference (P 0.68) in the mean age of two groups ensuring comparability of the two groups. After univariate analysis, PCOS, BMI, waist circumference ≥88 cm, serum TGs level ≥150 mg/dl, metabolic syndrome, and acanthosis nigricans and were found to have a significant association with mFG ≥8 with P = 0.002, 0.004, 0.006, 0.006, 0.019, and 0.02, respectively. The findings of univariate analysis were comparable to previous reports.[17,18,19,20] In those patients with metabolic syndrome the chance that the patient will have mFG score ≥8 was 3.1 times when compared to those without metabolic syndrome. Among the components of metabolic syndrome, the significant components were waist circumference ≥88 cm and serum TGs more than 150 mg/dl. The patients with waist circumference ≥88 cm had 5.6 times chance for developing mFG score ≥8 when compared to those with waist circumference <88 cm. Elevated TG level was also found to associated with hirsutism with mFG score ≥8 and the odds was 3.7 times more than those without TG elevation. The odds was 5.1 times more among patients with increased BMI, 4.5 times with PCOS and 3 times for the patients with acanthosis nigricans to develop mFG score ≥8 than the patients without these associated diseases. Contrary to our expectation, infertility was more in patients with mFG score <8. The patients with infertility might have undergone treatment for infertility which could have led to an improvement of their hirsutism resulting in low mFG score in these patients.
When we looked at the variables that have achieved significance in the univariate analysis, it turned out that most of these variables were inter-related. PCOS is a known cause of hirsutism, and there are reports of association of metabolic syndrome and PCOS.[15,22] In our study, metabolic syndrome was seen in 44.4% (12/27) with PCOS, and 43.8% (32/43) without PCOS and this difference in proportion was not statistically significant. As metabolic syndrome was almost equally present in those with and without PCOS, metabolic syndrome can be considered an independent risk factor for hirsutism. The proportion of metabolic syndrome in PCOS was higher than reported by Ehrmann et al. and same as that reported by Apridonidze et al. and Glueck et al.[15,22,23] Acanthosis nigricans can occur in PCOS and metabolic syndrome. Increased BMI can occur in metabolic syndrome, and increased waist circumference can occur in those with increased BMI. Waist circumference ≥88 and elevated TG level ≥150 mg/dl are components of metabolic syndrome. These variables, since they can occur together can act as confounding variables. Logistic regression analysis was performed with the significant variables in univariate analysis. Two models were analyzed. In the first model, the variables incorporated were acanthosis nigricans, metabolic syndrome, and PCOS and in the second model BMI, acanthosis nigricans, PCOS, and instead of metabolic syndrome, the significant components of metabolic syndrome like waist circumference and elevated TG level were incorporated. In the first model, there was a statistically significant association of hirsutism with PCOS and metabolic syndrome. The patients with PCOS and those with metabolic syndrome have 5 and 3.5 times increased the chance of developing hirsutism. Acanthosis nigricans, though significant in univariate analysis, lost its significance in the logistic regression model. Acanthosis nigricans can occur in both metabolic syndrome and PCOS, and when analyzed individually it became significant, and when analyzed together it lost its significance. In the second model, instead of metabolic syndrome, the significant components of metabolic syndrome like waist circumference and elevated TG were incorporated along with PCOS, acanthosis nigricans and BMI. The patients with PCOS had 5 times, increased BMI had 3.9 times and elevated TGs had 3.1 times chance for developing hirsutism. Waist circumference though highly significant in univariate analysis lost its significance in logistic regression analysis as waist circumference ≥88 cm was seen in 91% with increased BMI. BMI can be a proxy measure for waist circumference ≥88 cm. Acanthosis nigricans lost its significance as it can occur in PCOS and increased BMI.
The sample size was adequate to study the mFG score, but when the patients were categorized into two groups within this sample, the sample size became inadequate. There is also the issue of generalizability of the study findings as the study setting was a tertiary care center. The androgen levels were not estimated in the study. Although in a study on hirsutism not estimating, the androgen levels is itself a limitation, it was not done, as the idea was to study the mFG score and association of metabolic syndrome.
The low mean mFG score in clinic attendees implies even lower score in unselected general population. This signifies the need for conducting population studies among females in the reproductive age group to determine a cutoff for mFG score to define hirsutism in our population.
CONCLUSION
Hirsutism still remains a major psychosocial problem among females. Hirsutism in any form remains disturbing to social sensibilities and conventions. Even though PCOS was the most significant association with hirsutism, metabolic syndrome and or its components also had a significant association with hirsutism. Logistic regression modeling has shown that in spite of the association of metabolic syndrome with PCOS, metabolic syndrome has become an independent risk factor. PCOS and metabolic syndrome have common clinical features and share the same pathogenesis. Insulin resistance and compensatory hyperinsulinemia is the key pathogenic event in both the conditions. The prevalence of 37.7% metabolic syndrome in those with mFG score <8 indicates that metabolic syndrome starts early even before hirsutism manifest and as metabolic syndrome progress the score increases. Hence terminal hair in an androgen dependent site can be considered as a surrogate marker for metabolic syndrome. So when patient complaints of terminal hair in androgen-dependent areas, the patient needs to be evaluated for metabolic syndrome in spite of the low mFG score. Metabolic syndrome is a condition that is preventable to a greater extent with lifestyle modification. As metabolic syndrome is associated with increased risk of cardiovascular disorders, early identification of metabolic syndrome is of prime importance. Prevention of the development of metabolic syndrome will be able to prevent hirsutism also. The successful treatment of hirsutism should incorporate lifestyle modification for sustained clinical response.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Messenger AG, de Berker DA, Sinclair RD. Disorders of hair. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. Vol. 4. West Sussex: Wiley-Blackwell; 2010. pp. 66.80–9. [Google Scholar]
- 2.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–7. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 3.Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: Implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815–30. doi: 10.1016/0002-9378(81)90746-8. [DOI] [PubMed] [Google Scholar]
- 4.Ferriman D, Purdie AW. Association of oligomenorrhoea, hirsuties, and infertility. Br Med J. 1965;2:69–72. doi: 10.1136/bmj.2.5453.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferriman D, Purdie AW. The aetiology of oligomenorrhoea and/or hirsuties: A study of 467 patients. Postgrad Med J. 1983;59:17–20. doi: 10.1136/pgmj.59.687.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escobar-Morreale HF, Carmina E, Dewailly D, Gambineri A, Kelestimur F, Moghetti P, et al. Epidemiology, diagnosis and management of hirsutism: A consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2012;18:146–70. doi: 10.1093/humupd/dmr042. [DOI] [PubMed] [Google Scholar]
- 7.Yildiz BO, Bolour S, Woods K, Moore A, Azziz R. Visually scoring hirsutism. Hum Reprod Update. 2010;16:51–64. doi: 10.1093/humupd/dmp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wijeyaratne CN, Balen AH, Barth JH, Belchetz PE. Clinical manifestations and insulin resistance (IR) in polycystic ovary syndrome (PCOS) among South Asians and Caucasians: Is there a difference? Clin Endocrinol (Oxf) 2002;57:343–50. doi: 10.1046/j.1365-2265.2002.01603.x. [DOI] [PubMed] [Google Scholar]
- 9.Cheewadhanaraks S, Peeyananjarassri K, Choksuchat C. Clinical diagnosis of hirsutism in Thai women. J Med Assoc Thai. 2004;87:459–63. [PubMed] [Google Scholar]
- 10.DeUgarte CM, Woods KS, Bartolucci AA, Azziz R. Degree of facial and body terminal hair growth in unselected black and white women: Toward a populational definition of hirsutism. J Clin Endocrinol Metab. 2006;91:1345–50. doi: 10.1210/jc.2004-2301. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 12.Reaven GM. Banting lecture 1988.Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med. 1989;149:1514–20. doi: 10.1001/archinte.149.7.1514. [DOI] [PubMed] [Google Scholar]
- 14.Orsino A, Van Eyk N, Hamilton J. Clinical features, investigations and management of adolescents with polycystic ovary syndrome. Paediatr Child Health. 2005;10:602–8. doi: 10.1093/pch/10.10.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN. PCOS/Troglitazone Study Group. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:48–53. doi: 10.1210/jc.2005-1329. [DOI] [PubMed] [Google Scholar]
- 16.Zargar AH, Wani AI, Masoodi SR, Laway BA, Bashir MI, Salahuddin M. Epidemiologic and etiologic aspects of hirsutism in Kashmiri women in the Indian subcontinent. Fertil Steril. 2002;77:674–8. doi: 10.1016/s0015-0282(01)03241-1. [DOI] [PubMed] [Google Scholar]
- 17.Sharma NL, Mahajan VK, Jindal R, Gupta M, Lath A. Hirsutism: Clinico-investigative profile of 50 Indian patients. Indian J Dermatol. 2008;53:111–4. doi: 10.4103/0019-5154.42387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chhabra S, Gautam RK, Kulshreshtha B, Prasad A, Sharma N. Hirsutism: A Clinico-investigative study. Int J Trichology. 2012;4:246–50. doi: 10.4103/0974-7753.111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma D, Shanker V, Tegta G, Gupta M, Verma GK. Clinico-investigative profile of patients of hirsutism in a tertiary level institution. Int J Trichology. 2012;4:69–74. doi: 10.4103/0974-7753.96904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad QM, Shah IH, Sameem F, Kamili QU, Sultan J. Hirsutism in Kashmir: An etiological study. Indian J Dermatol. 2009;54:80–2. doi: 10.4103/0019-5154.48997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noorbala MT, Kefaie P. The prevalence of hirsutism in adolescent girls in Yazd, Central Iran. Iran Red Crescent Med J. 2010;12:111–7. [Google Scholar]
- 22.Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929–35. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 23.Glueck CJ, Papanna R, Wang P, Goldenberg N, Sieve-Smith L. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism. 2003;52:908–15. doi: 10.1016/s0026-0495(03)00104-5. [DOI] [PubMed] [Google Scholar]


