Abstract
Objective:
The purpose of the study was to examine the substantivity of a new disinfectant against biofilm formation in the dental unit waterlines.
Materials and Methods:
Twenty dental units were selected for the study and divided into two groups: Group A (dental unit waterlines treated with the disinfectant) and Group B (untreated dental unit waterlines). Biofilm formation was monitored in both groups by removing the one dental unit waterline from each group for the period of 10 days. One inch of the dental unit waterline tube was cut at random site, and the inner lumen of the cut sections was analyzed using the scanning electron microscope (SEM) (TESCAN VEGA3 SBU).
Results:
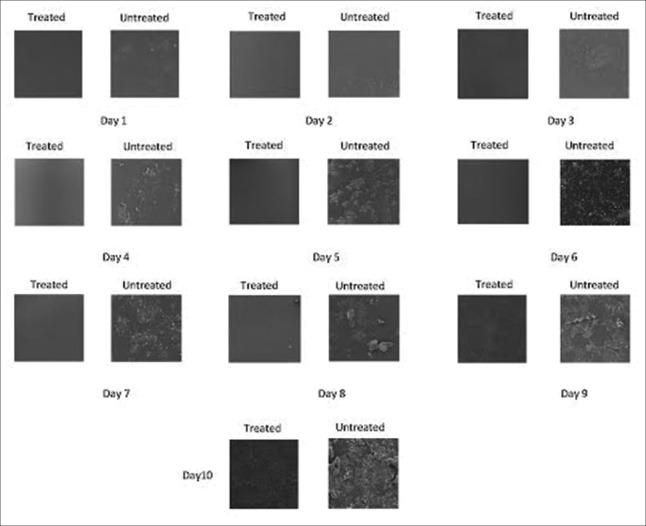
On examination, SEM images showed that there was no slime layer or bacterial cells seen in cut section for the period of 7 days in the treated dental waterlines, which means that there is no evident of biofilm formation. In the untreated dental unit waterline cut section, slime layer was observed from day 1.
Conclusion:
Disinfectant solution was proved to be effective for 7 days against biofilm formation. This technique could be used as a valid method for disinfection of dental unit waterlines.
Keywords: Biofilms, dental chair, disinfectant
INTRODUCTION
Dental unit waterlines are 3–4 narrow polyurethane tubes that carry water to the handpiece, coolant and they are contaminated by the formation of biofilm along the inner lumen of the tubing wall.[1] Numerous methods are suggested to maintain the microbial load in the dental unit water within 200 CFU/ml as per the American Dental Association recommendation.[2] Among them, flushing is the common method recommended in the clinical practice, but the biofilm acts as a reservoir releasing the planktonic organisms; thus, the higher level of microbial load can still persist and violate the basic infection control principle.[3,4,5] In this regard, disinfection method is the practical solution to remove biofilm from the dental unit waterlines as well as to provide safe water to the patients and dental staff.[6] Previous study conducted with the disinfectant solution (CleanCert, Unit, 16b Wyndham Place, Tisbury, Wiltshire SP3 6GS UK)proved that the disinfectant was efficient to eliminate the biofilm from the dental unit waterlines, but the substantivity of the disinfectant was not evaluated. Substantivity is the prolonged association between a material (Cleancert) and a substrate (polyurethane tube), an association that can be greater and more extended than would be expected from a simple deposition mechanism.[7] However, prolonged inhibition of biofilm formation is merely an indirect way to estimate the substantivity of disinfectant or disinfection procedure in the dental unit waterline.
Thus, the purpose of the study was to investigate the substantivity of the disinfectant in preventing biofilm formation in the dental unit waterlines.
MATERIALS AND METHODS
Study area
The study was conducted in a dental college, Chennai. Twenty dental chairs were selected and new plastic tubes were installed in all the dental chairs. Twenty chairs were divided into two groups of ten chairs each.
Group A: Treated dental unit waterlines with the disinfectant.
Group B: Untreated dental unit waterlines.
Dental unit waterline disinfection
In Group A, disinfection procedure was carried as follows: 200 ml of the disinfectant solution (Cleancert) was filled in the reservoir bottle and the solution was allowed to run through the dental unit waterlines till the disinfectant emerges out of the handpiece. The unit was turned off and the solution was left overnight in the dental chair. At the beginning of the next day, the remaining disinfectant was discarded and the bottle was washed with hot water. During the study period, conservative and endodontic procedure was carried out and all the dental chairs were supplied with ground water.
Untreated dental unit waterline
In Group B, dental chairs were not disinfected; this was done to have an insight into the progressive development of biofilm in the dental unit waterlines. Substantivity of the disinfectant and biofilm formation in the newly installed dental unit waterlines was analyzed for 10 days.
Method of sample collection
The study included removal of dental unit waterlines from the two groups, one on each day from each group. One inch of the dental unit waterline tube was cut at random site, and sterile blade was used to section the excised tubing part along the lengthwise and immediately processed for sample preparation.
Sample preparation
The samples were immersed in 2% glutaraldehyde fixative and the fixative was removed using phosphate buffer solution for 10–15 min. Then, 10 min dehydration process was carried out with a series of alcohol 30%, 50%, 70%, 90% and 100%. Finally, hexamethyldisilazane treatment is done for 10 min. The inner lumen wall of the cut section of the dental unit waterline obtained from both groups was analyzed from day 1 to day 10 using scanning electron microscopy (SEM).
RESULTS
The objectivity of the present study was to assess the substantivity of the disinfectant in the treated dental unit waterline and the progressive development of biofilm formation in the untreated new dental unit waterline for the period of 10 days. This was achieved using SEM.
The images obtained from SEM analysis allowed to estimate the difference and also the development of biofilm in the treated and untreated dental unit waterlines.
Effect of dental unit waterline disinfection
In Group A, from day 1 to day 7, no slime layer or microbial colonization seen in the inner lumen of the analyzed cut section sample collected from the treated dental unit waterlines; at day 8, multiple white deposits were seen which is probably calcium carbonate; it developed to a layer of organic matrix deposition at day 9; at day 10, microbial cell was seen scattered along the inner lumen of the tubing wall [Figure 1].
Figure 1.
Biofilm formation in the treated and untreated dental unit waterlines is shown for 10 days using scanning electron microscopy
Biofilm formation in the untreated dental unit waterline
In Group B, from day 1 to day 4, there was deposition of calcium carbonate along the inner lumen of the tubing wall and the rate of deposition was increasing progressively. On day 5–day 7, there was clustered of rods and cocci intermingled with organic matrix. The biofilm developed from a few individual cells and microcolonies scattered along the lumen walls to a mature biofilm with a dense population of coalescing microcolonies, which was seen on day 8–day 10 [Figure 1].
DISCUSSION
Contamination of the dental unit water was reported from 1963 which are due to the proliferating bacteria, fungi, protozoa along the inner lumen of the dental unit waterlines – biofilm.[8,9] Contaminated dental unit water used during treatment procedure is the serious threat to patients as well as the dental staff.[10] These are attributed to the complexity of the dental unit, so the solution for this problem is to disinfect the dental unit waterlines.[11] Various disinfectant was such as chlorhexidine gluconate, sodium hypochlorite, and povidone-iodine are used in the dental unit waterlines. Analyzing the substantivity of the disinfectant gives us a clue about the time interval to perform the disinfection procedure in the dental unit waterlines.[12,13,14,15]
The study was designed to analyze the substantivity of the new hypochlorous acid-based disinfecting solution against biofilm formation in the dental unit waterlines. The disinfectant was found to be effective for 7 days in the treated dental unit waterlines, and in the untreated dental unit waterlines, biofilm formation commenced within 24 h after installation in the dental chair. The appearance of white deposits on day 8 shows that biofilm formation can only be prevented for a certain period and it can never be completely eliminated. It is likely that under clinical conditions, the substantivity of disinfection solution can be enhanced by good quality of incoming water and proper usage of antiretraction valves.
The substantivity of the disinfectant may be attributed to the action of individual ingredient in the disinfection solution, namely, sodium chloride, hypochlorous acid, and sodium hypochlorite. The active ingredient in the disinfectant agent is stabilized hypochlorous acid which is a strong oxidizing agent which invades DNA and RNA of the invading pathogens, killing the pathogens by disrupting the oxidative phosphorylation, metabolic pathway involved in Adenosine triphosphate (ATP) generation.[6] Sodium chloride acts as a bleaching agent.
Biofilm formation analyzed in the untreated dental unit waterlines shows that microbial cells tend to get attached to the inner lumen of the tubing wall within 24 h as soon as water flows through the tube and biofilm continues to become dense as the day passes which emphasize the need for disinfecting the new dental unit waterlines immediately after installing in the dental chair. The result obtained from our study coincides with Tall et al., who proved in their study that once the dental unit waterline is connected to the water supply, biofilm formation begins within 8 h and reaches its climax within 6 days.[16]
Thus, the study suggests that one course of treatment with disinfecting solution may be effective for 1 week time against biofilm formation in the dental unit waterlines.
CONCLUSION
Disinfecting solution such as Cleancert was proved to be effective for 7 days against biofilm formation in the dental unit waterlines. This technique could be used as a valid method for disinfection of dental unit waterlines.
Financial support and sponsorship
Grant for the study was provided by the ICMR. The SEM was performed in Centre for Nanoscience and Technology, Anna University, Chennai.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The grant for the research was supported by Indian Council of Medical Research (ICMR). Authors are thankful to the management of Faculty of Dental Sciences, Sri Ramachandra University, Chennai, and Centre for Nanoscience and Technology, Anna University, Chennai, for providing the facilities and support for the successful completion of the work. We would also like to thank the dental team and the clinical engineering staff for their much appreciated contribution.
REFERENCES
- 1.Barbeau J, Nadeau C. Dental unit waterline microbiology: A cautionary tale. J Can Dent Assoc. 1997;63:775–9. [PubMed] [Google Scholar]
- 2.Dental unit waterlines: Approaching the year 2000. ADA Council on Scientific Affairs. J Am Dent Assoc. 1999;130:1653–64. [PubMed] [Google Scholar]
- 3.Williams HN, Kelley J, Folineo D, Williams GC, Hawley CL, Sibiski J. Assessing microbial contamination in clean water dental units and compliance with disinfection protocol. J Am Dent Assoc. 1994;125:1205–11. doi: 10.14219/jada.archive.1994.0164. [DOI] [PubMed] [Google Scholar]
- 4.Gross A, Devine MJ, Cutright DE. Microbial contamination of dental units and ultrasonic scalers. J Periodontol. 1976;47:670–3. doi: 10.1902/jop.1976.47.11.670. [DOI] [PubMed] [Google Scholar]
- 5.Meiller TF, Depaola LG, Kelley JI, Baqui AA, Turng BF, Falkler WA. Dental unit waterlines: Biofilms, disinfection and recurrence. J Am Dent Assoc. 1999;130:65–72. doi: 10.14219/jada.archive.1999.0030. [DOI] [PubMed] [Google Scholar]
- 6.Kim PJ, Cederberg RA, Puttaiah R. A pilot study of 2 methods for control of dental unit biofilms. Quintessence Int. 2000;31:41–8. [PubMed] [Google Scholar]
- 7.Greenstein G, Polson A. The role of local drug delivery in the management of periodontal diseases: A comprehensive review. J Periodontol. 1998;69:507–20. doi: 10.1902/jop.1998.69.5.507. [DOI] [PubMed] [Google Scholar]
- 8.Blake GC. The incidence and control of bacterial infection of dental units and ultrasonic scalers. Br Dent J. 1963;115:413–6. [Google Scholar]
- 9.Whitehouse RL, Peters E, Lizotte J, Lilge C. Influence of biofilms on microbial contamination in dental unit water. J Dent. 1991;19:290–5. doi: 10.1016/0300-5712(91)90075-a. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Atray D, Paiwal D, Balasubramanyam G, Duraiswamy P, Kulkarni S. Dental unit waterlines: Source of contamination and cross-infection. J Hosp Infect. 2010;74:99–111. doi: 10.1016/j.jhin.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Smith AJ, McHugh S, Aitken I, Hood J. Evaluation of the efficacy of Alpron disinfectant for dental unit water lines. Br Dent J. 2002;193:593–6. doi: 10.1038/sj.bdj.4801635. [DOI] [PubMed] [Google Scholar]
- 12.Douglas CW, Rothwell PS. Evaluation of a dental unit with a built-in decontamination system. Quintessence Int. 1991;22:721–6. [PubMed] [Google Scholar]
- 13.Abel LC, Miller RL, Micik RE, Ryge G. Studies on dental aerobiology. IV. Bacterial contamination of water delivered by dental units. J Dent Res. 1971;50:1567–9. doi: 10.1177/00220345710500063601. [DOI] [PubMed] [Google Scholar]
- 14.Smith AJ, Bagg J, Hood J. Use of chlorine dioxide to disinfect dental unit waterlines. J Hosp Infect. 2001;49:285–8. doi: 10.1053/jhin.2001.1085. [DOI] [PubMed] [Google Scholar]
- 15.Mills SE, Lauderdale PW, Mayhew RB. Reduction of microbial contamination in dental units with povidone-iodine 10% J Am Dent Assoc. 1986;113:280–4. doi: 10.14219/jada.archive.1986.0178. [DOI] [PubMed] [Google Scholar]
- 16.Tall BD, Williams HN, George KS, Gray RT, Walch M. Bacterial succession within a biofilm in water supply lines of dental air-water syringes. Canadian J Microbiology. 1995;41:647–4. doi: 10.1139/m95-088. [DOI] [PubMed] [Google Scholar]