Abstract
Polyadenylation-induced translation is an important regulatory mechanism during metazoan development. During Xenopus oocyte meiotic progression, polyadenylation-induced translation is regulated by CPEB, which is activated by phosphorylation. XGef, a guanine exchange factor, is a CPEB-interacting protein involved in the early steps of progesterone-stimulated oocyte maturation. We find that XGef influences early oocyte maturation by directly influencing CPEB function. XGef and CPEB interact during oogenesis and oocyte maturation and are present in a c-mos messenger ribonucleoprotein (mRNP). Both proteins also interact directly in vitro. XGef overexpression increases the level of CPEB phosphorylated early during oocyte maturation, and this directly correlates with increased Mos protein accumulation and acceleration of meiotic resumption. To exert this effect, XGef must retain guanine exchange activity and the interaction with CPEB. Overexpression of a guanine exchange deficient version of XGef, which interacts with CPEB, does not enhance early CPEB phosphorylation. Overexpression of a version of XGef that has significantly reduced interaction with CPEB, but retains guanine exchange activity, decreases early CPEB phosphorylation and delays oocyte maturation. Injection of XGef antibodies into oocytes blocks progesterone-induced oocyte maturation and early CPEB phosphorylation. These findings indicate that XGef is involved in early CPEB activation and implicate GTPase signaling in this process.
INTRODUCTION
Polyadenylation-induced translation of stored maternal mRNAs is an important regulatory mechanism for ensuring appropriate spatial and temporal protein expression in the developing oocytes and embryos of many metazoans (Wickens et al., 2000; de Moor and Richter, 2001). Essentially, mRNAs with a short poly (A) tail are translationally repressed, whereas those with a long poly (A) tail are translationally activated. The active modulation of poly (A) tail length results in the repression or activation of translation of the mRNA (Richter, 2000). In Xenopus and mouse oocytes, mRNAs targeted for polyadenylation-induced translation contain a U-rich sequence, the cytoplasmic polyadenylation element (CPE), within the 3′ untranslated region (UTR). This sequence is bound by CPEB, which, depending on the mRNA and activation state of CPEB, assists in maintenance of translational repression of mRNA or mediates polyadenylation-induced translation (Mendez and Richter, 2001).
During meiotic progression in Xenopus oocytes (oocyte maturation), polyadenylation-induced translation plays an important role in activation of the Mos/mitogen-activated protein kinase (MAPK) pathway (Sheets et al., 1995). Briefly, meiosis can be reinitiated by the action of progesterone on the oocyte membrane, most likely through a seven-trans membrane G protein-coupled receptor (Zhu et al., 2003a,b), with perhaps some effects mediated by a classical progesterone receptor (Bayaa et al., 2000; Tian et al., 2000). Within minutes, there is a transient decrease in protein kinase A (PKA) activity (Nebreda and Ferby, 2000). Through not a completely defined pathway, the decrease in PKA activity leads to the activation of CPEB and the polyadenylation-induced translation of stored c-mos mRNA, thus activating the Mos/MAPK pathway. This pathway converges with the Cdc25 pathway, which is also activated by the decrease in PKA activity, to activate stored maturation promoting factor (MPF), a complex of cyclin B1 and cdc2 (Tunquist and Maller, 2003). Activated MPF then directs a number of key meiotic events, including chromatin condensation and nuclear envelope breakdown, or germinal vesicle breakdown (GVBD) (Sagata, 1997).
XGef was initially identified in a yeast two-hybrid screen for proteins interacting with CPEB. Functional analysis revealed that XGef is a guanine nucleotide exchange factor for Cdc42 in mammalian cells and that it influences Xenopus oocyte maturation before or coincident with the polyadenylation of c-mos mRNA (Reverte et al., 2003). The coincidence in timing of early CPEB activity and XGef influence on Mos protein accumulation suggests that XGef might influence c-mos mRNA polyadenylation by affecting CPEB activity. The current study was designed to examine the possibility that XGef might influence early CPEB activation during oocyte maturation.
CPEB activation in early oocyte maturation is regulated by phosphorylation. Serine 174 (S174), and perhaps serine 180 (S180), of CPEB are phosphorylated within 2 h of progesterone stimulation (Mendez et al., 2000a), and this is clearly necessary for proper CPEB function. Overexpression in oocytes of a version of CPEB containing alanine substitutions at S174 and S180 (CPEB AA) decreases the extent of polyadenylation of endogenous c-mos mRNA early during oocyte maturation (Charlesworth et al., 2004) and completely blocks polyadenylation of an injected c-mos mRNA 3′ UTR (Mendez et al., 2000a). In addition, overexpression of CPEB AA prevents endogenous Mos protein accumulation. This, perhaps in concert with the perturbation of expression of other proteins, leads to a complete block of oocyte maturation (Mendez et al., 2000a).
We have now determined that XGef has a direct influence on early CPEB activation. XGef is present in a complex containing CPEB and c-mos mRNA in prophase I oocytes and oocytes undergoing meiosis. Overexpression of XGef increases the level of early phosphorylated CPEB, and this directly correlates with an increase in Mos protein levels and acceleration of oocyte maturation. To determine how XGef might influence CPEB phosphorylation, we generated several mutated versions of XGef and tested their influence on early CPEB phosphorylation, Mos synthesis, and oocyte maturation. Using a version of XGef with very reduced binding to CPEB, we found that their interaction is necessary for the influence of XGef on CPEB phosphorylation. Using an exchange deficient version of XGef, we provide data that strongly suggests that XGef exchange factor activity is needed for the influence of XGef on early CPEB phosphorylation, thus implicating GTPase activity in this event. We also demonstrated that the amino-terminal 50 amino acids and the double homology (DH) domain of XGef are each involved in interacting with CPEB. Potential mechanisms for XGef mediation of early CPEB phosphorylation are discussed.
MATERIALS AND METHODS
Plasmid Constructs
The inserts from pACT2-XGef(1–461), pACT2-XGef(33–465), pACT2-XGef(65–465), pACT2-XGef(235–465), pACT2-XGef(268–465), pACT2-XGef(268–360), pACT2-XGef(1–360), pACT2-XGef(1–267), pACT2-XGef(65–360), pACT2-XGef(1–234), and pACT2-XGef(65–234) were removed by digestion with NcoI and XhoI and subcloned into the pHA-SP vector (Reverte et al., 2003), digested with the same enzymes, to generate pSP6HA-XGef deletion mutants. To generate pSP6-GST, pGST-CPEB-SP (Reverte et al., 2003) was digested with EcoRI and BglII to remove the CPEB insert, blunt ended with Mung Bean nuclease, and ligated. pSP6GST-XGef(1–465) was generated by polymerase chain reaction (PCR) amplification of pGEX2T-XGef(1–465) with primers SP6GST12For (5′-CCGCTCGAGTCAGAGCAGGGAGCAACTCC-3′) and SP6GST12Rev (5′-CCGCTCGAGTCAGAGCAGGGAGCAACTCC-3′). The product was then digested with EcoRI and XhoI and ligated into the pSP64-S vector cut with the same enzymes. To generate internal deletions, the QuikChange multisite-directed mutagenesis kit (Stratagene, La Jolla, CA) was used following the manufacturer's instructions. The oligonucleotides used for pSP6HA-XGef(Δ65–234) were dhfor1 (5′-CCATGGCTTGCTCTCGAGAATGAGCGGCACCTCCGAAGG-3′) and dhrev1 (5′-CTCGAGAGCAAGCCATGGTTTGGCCGTTGTCAGTCTCTTGCGC-3′); for pSP6HA-XGef(Δ50–234) were dhfor7 (5′-GGAACGTTGTCCATGGCTTGCTCTCGAGAATGAGCGGCACCTCC-3′) and dhrev7 (5′-GCAAGCCATGGACAACGTTCCTCTGGTTCCTCAACCAACTCTG-3′), and for pSP6HA-XGef(Δ50–77) were dhfor8 (5′-GGAACGTTGTCCATGGCATGACGTTTTCCGCGCTCGATGCGG-3′) and dhrev8 (5′-GAAAACGTCATGCCATGGACAACGTCCCTCTGGTTCCTCAACC-3′) using pSP6HA-XGef as the template. pAX142, pAX142-cdc42(12V), pAX142-dbl-HA1, and pAX142-XGEF have been described previously (Whitehead et al., 1999; Reverte et al., 2003). pAX142-XGef(65–360) was made by inserting the EcoR1 fragment of pSP6HA-XGef(65–360) into EcoRI-digested pAX142. The reporter construct used in the luciferase-coupled transcriptional assays (SREm)2 has been described previously (Westwick et al., 1998). pCMVnlac encodes the β-galactosidase gene under the control of the CMV promoter (Whitehead et al., 1999).
Production and Purification of Recombinant Proteins
The glutathione S-transferase-XGef(GST-XGef) fusion protein was expressed in Escherichia coli (BL21) by induction for 16 h with 2 mM isopropyl β-d-thiogalactoside (IPTG) at 20°C, purified on glutathione-Sepharose 4B beads (Amersham Biosciences, Piscataway, NJ), and concentrated in phosphate-buffered saline with 0.1% Triton X according to directions from the manufacturer. Expression of histidine (6x)-tagged CPEB (His-CPEB) was induced in BL21 cells for 3 h with 4 mM IPTG at 20°C and purified using nickel-agarose beads in binding buffer (20 mM Tris-HCl, pH 7.9, 0.5 M NaCl, 5 mM imidazole, and 0.25% Tween 20). After extensively washing in 20 mM Tris-HCl, pH 7.9, 0.5 M NaCl, and 60 mM imidazole, the protein tethered to the beads was saved in binding buffer at 4°C. If required, His-CPEB elution was performed with 20 mM Tris-HCl, pH 7.9, 0.5 M NaCl, and 1 M imidazole.
RNA Synthesis
Plasmids were linearized and used as templates for in vitro transcription with the SP6 or T7 mMessage mMachine kit (Ambion, Austin, TX) as described previously (Reverte et al., 2003).
Oocyte Culture and Microinjections
Oocytes from nonprimed Xenopus laevis adult females (Nasco, Fort Atkinson, WI) were obtained by digestion in 1× Barth's saline by using 2 mg/ml collagenase (type II; Sigma-Aldrich, St. Louis, MO) and 0.6 U/ml dispase (Roche Diagnostics, Indianapolis, IN) for 1.5 h. Prophase I oocytes were selected and cultured in 1× Barth's saline or OR2 medium with 10 μg/ml streptomycin (Invitrogen, Carlsbad, CA). Oocytes were injected as described previously (Reverte et al., 2001). The injected oocytes were then transferred to fresh 1× Barth's or OR2 medium and incubated 15–20 h at 18°C.
Protein Extracts and Immunoblot Analysis
To analyze the biochemical markers of oocyte maturation as well as for in vitro CPEB phosphorylation assays, extracts from prophase I and maturing oocytes were prepared by homogenization with 3 μl per oocyte extraction buffer (20 mM HEPES, pH 7.5, 100 mM NaCl, 10 mM β-glycerophosphate, 5 mM EGTA, 5 mM MgCl2, 100 mM sucrose, 2 mM NaVaO4, 20 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol [DTT], and 2 μM okadaic acid) with protease inhibitors (5 ng/μl leupeptin and 10 ng/μl each chymostatin, pepstatin, and aprotinin) and centrifugation (12.5 krpm, 4°C, 20 min). The clear cytoplasmic layer was removed and incubated with cytochalasin B and centrifuged again for 10 min. For protein interaction assays, extracts from prophase I oocytes were prepared as described previously (Reverte et al., 2003). For immunoprecipitation assays, extracts were prepared by pipetting oocytes up and down in 10 μl per oocyte 1× NET buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% NP-40, and 1 mM EDTA). In both cases, clarified extracts were obtained by centrifugation (14 krpm, 4°C, 10 min).
Immunoblotting was carried out after separation of protein extracts by 10–15% SDS-PAGE (Laemmli, 1970) or electrophoresis in 12.5–15% Anderson gels (Anderson et al., 1973) and transfer to polyvinylidene difluoride membranes (Millipore, Billerica, MA). Membranes were probed with the antibodies indicated, and bound antibodies were detected by enhanced chemiluminescence (Amersham Biosciences), as described previously (Reverte et al., 2003). Guinea pig polyclonal anti-CPEB antibody was produced by injection of gel purified His-CPEB into guinea pigs (Covance Research Products, Denver, CO) and used at a 1:3000 dilution. Anti-MAPK antibody (New England Biolabs, Beverly, MA) was used at a 1:1000 dilution. Horseradish peroxidase-conjugated anti-guinea pig IgG (Santa Cruz Biotechnology, Santa Cruz, CA) was used at a 1:7000 dilution.
H1 Kinase Assays
To assay MPF activity in oocytes, cytoplasmic extracts were prepared as described above. Ten microliters of clarified lysate was brought up to 20 μl with kinase buffer (20 mM HEPES pH 7.3, 20 mM EGTA, 10 mM MgCl2, 80 mM β-glycerophosphate, 1 mM DTT, 150 mM NaF, 100 mM ATP, and 3 μCi of [γ-32P]ATP) and 0.8 μg of Histone H1 (Ferby et al., 1999; Sigma-Aldrich). Reactions were performed at room temperature for 15 min, stopped by the addition of sample loading buffer, and analyzed by 12.5% SDS-PAGE followed by autoradiography.
Protein–Protein Interaction Assays
The binding of HA-XGef and GST-CPEB tethered to glutathione beads was carried out essentially as described previously (Reverte et al., 2003) with minor modifications. Oocytes were injected with hemagglutinin (HA)- or GST-tagged mRNAs and incubated overnight to allow translation and protein accumulation (Reverte et al., 2001). For the modified GST affinity precipitation assay, extracts from oocytes expressing individually either an HA- or GST-tagged protein were analyzed by immunoblotting with anti-HA or anti-GST antibodies, respectively, followed by densitometry. Extract containing equivalent amounts of GST or GST-CPEB was then preincubated with glutathione-Sepharose beads. Oocyte extract containing equivalent amounts of the various HA-tagged proteins was added to the GST or GST-CPEB beads. To maintain similar overall protein concentrations in each binding assay, extract from nonexpressing oocytes was added to samples containing lower volumes of HA-tagged protein-expressing extract. Binding conditions, washing and resuspension of the beads, and immunoblot analysis of the bound samples were performed as described previously (Reverte et al., 2003).
In Vitro Binding Assays
The in vitro binding assay for purified, bacterially expressed GST-XGef (0.5–1 μg immobilized on glutathione-Sepharose) and purified, bacterially expressed His-CPEB (1.5–2.5 μg) was carried out in binding buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.25% Triton-X, and protease inhibitors) and 0.5% bovine serum albumin for 20 min at 4°C. Beads were washed with the same buffer plus 200 mM NaCl, and samples were analyzed by 10% SDS-PAGE and immunoblotting by using guinea pig anti-CPEB antibody.
Coimmunoprecipitation of Endogenous CPEB and Endogenous XGef
Cleared oocyte extracts obtained as described above were first precleared using IgG-coated rProtein G Agarose beads (Invitrogen). XGef was immunoprecipitated from extracts using anti-XGef antibody and rProtein-G Agarose beads for 4 h at 4°C, and the immunoprecipitates were extensively washed with 1× NET buffer at room temperature and analyzed by SDS-PAGE and immunoblotting. As a control, IgG (normal rabbit IgG; Santa Cruz Biotechnology) coated rProtein G agarose beads were used.
Immunoprecipitation and Reverse Transcription (RT)-PCR
Protein extract from 300 oocytes was used for immunoprecipitation as describe above with minor modifications. After preclearing, the resulting lysates were divided into three fractions. Each fraction was mixed with beads plus either 1) nonspecific IgG from rabbit, 2) rabbit anti-XGef serum, or 3) rabbit anti-CPEB serum. After immunoprecipitation and extensive washing, immune complexes were eluted from the beads with 2× SDS loading buffer. RNA was phenol/chloroform deproteinized and then ethanol precipitated. First-strand cDNA was synthesized using SuperScript III first-strand synthesis system for RT-PCR (Invitrogen). In the second step, PCR was performed using Platinum TaqDNA polymerase (Invitrogen) and c-mos mRNA (Reverte et al., 2003) or β-actin mRNA-specific primers (sense, 5′-GCACGAGCCGCATAGAAAGGA-3′ and antisense, 5′-GCTTCTGTGAGCAGCACTGG-3′). Reamplification of PCR products was performed using the same primers as in the initial amplification. PCR products were resolved on a 1% agarose gel and visualized using ethidium bromide staining.
In Vitro CPEB Phosphorylation Assay
Phosphorylation assays were performed with 2.5 μg of bacterially expressed recombinant His-CPEB tethered to Ni-beads in 20 μl of mixture containing 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.1 mM EGTA, 0.5 mM DTT, 1 μM okadaic acid, 2 μM H-89 (PKA inhibitor), 30 μM ATP with 15 μl of oocyte extract, and 1.5 μl of [γ32P]ATP (3000 mCi/ml; PerkinElmer-Cetus, Wellesley, MA). The reaction mixture was incubated 20 min at room temperature. After extensive washing, phosphorylation of His-CPEB tethered to Ni-beads was analyzed by SDS-PAGE and autoradiography. Samples used for phosphopeptide analysis were prepared the same way, except that [γ32P]ATP was omitted.
Methods for Protein Sequence Analysis by Liquid Chromatography-Tandem Mass Spectrometry
Protein bands excised from gels were subjected to a modified in-gel trypsin digestion procedure (Shevchenko et al., 1996). Phosphopeptide analysis was performed by the Taplin Biological Mass Spectrometry Facility (Harvard Medical School, Boston, MA). Briefly, tryptic peptides were separated by nanoscale reverse-phase high-performance liquid chromatography (Peng and Gygi, 2001), subjected to electrospray ionization, and loaded into an LCQ DECAXP plus ion-trap mass spectrometer (Thermo Finnigan, San Jose, CA). Eluting peptides were detected, isolated, and fragmented to produce a tandem mass spectrum of specific fragment ions for each peptide. Peptide sequences (and hence protein identity) were determined by matching acquired peptide fragmentation patterns against fragmentation patterns from a protein database by using the software program Sequest (Thermo Finnigan; Eng et al., 1994). Modification of 80 mass units to serine, threonine, and tyrosine where included in the database searches to determine phosphopeptides. Each phosphopeptide that was determined by the Sequest program was also manually inspected in ensure confidence.
Transient Expression Reporter Gene Assays
COS-7 cells were maintained in DMEM (high glucose) supplemented with 10% fetal bovine serum (Sigma-Aldrich). For transient expression reporter gene assays, COS-7 cells were transfected by DEAE-dextran as described previously (Whitehead et al., 1995). Cells were allowed to recover for 30 h and were then starved in DMEM that was supplemented with 0.5% serum for 14 h before lysate preparation. Analysis of luciferase expression was as described previously with enhanced chemiluminescent reagents and a Monolight 3010 luminometer (Analytical Luminescence, San Diego, CA; Westwick et al., 1998). β-Galactosidase activity was determined using Lumi-Gal substrate (Lumigen, Southfield, MI) according to the manufacturer's instructions. All assays were performed in triplicate.
RESULTS
XGef and CPEB Interact In Vivo and Interact Directly In Vitro
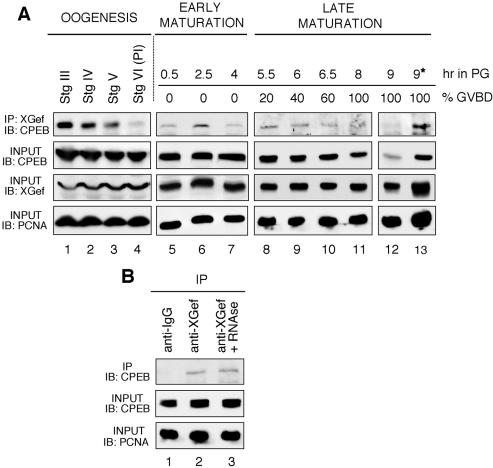
XGef overexpression in oocytes accelerates progesterone-stimulated polyadenylation and accumulation of c-mos mRNA (Reverte et al., 2003). XGef could achieve this by influencing the early activity of CPEB, perhaps through interaction with CPEB in oocytes. To address this possibility, we extended our previous studies showing that recombinant XGef interacts with endogenous CPEB and vice-versa (Reverte et al., 2003) and have demonstrated that endogenous XGef interacts with endogenous CPEB during oogenesis and oocyte maturation (Figure 1). XGef antibody coimmunoprecipitated CPEB from staged oocytes and oocytes undergoing progesterone-stimulated meiotic maturation (Figure 1A). XGef-CPEB interaction seemed to be stronger in stage III oocytes than in stage VI oocytes (Figure 1A, compare lanes 1 and 4). The level of interaction remained relatively consistent from stage VI oocytes through 100% GVBD oocytes (Figure 1, lanes 4–12). During oocyte maturation, 70–90% of CPEB is degraded (Reverte et al., 2001). This is evident as a decrease in CPEB signal as meiosis proceeds (Figure 1A, second panel, lanes 5–11) and is most apparent in extracts from oocytes 1 h after 100% GVBD (Figure 1A, second panel, lane 12). With a decreasing amount of CPEB, the amount that can be immunoprecipitated by XGef also obviously decreases and is barely detectable 1 h after GVBD (Figure 1A, top, lanes 5–12). We believe, however, that this decrease in signal 1 h after 100% GVBD is only due to the great decrease in CPEB amount, because when we use 5 times more of this extract for immunoprecipitation, we readily detect CPEB (Figure 1A, top, lane 13).
Figure 1.
XGef and CPEB interact throughout oogenesis and until 100% GVBD. (A) XGef and CPEB interact throughout oogenesis and oocyte maturation. Oocyte extracts were prepared from oocytes at different stages of oogenesis (lanes 1–4) and at intervals during progesterone-induced meiotic maturation (lanes 5–13). Protein complexes were immunoprecipitated with anti-XGef antibody from 50 (lanes 1–12) or 250 (*, lane 13) oocytes. Protein G-agarose–bound (IP: XGef) and input (middle) samples were analyzed by SDS-PAGE and immunoblotting with guinea pig anti-CPEB antibody (IB: CPEB). Input samples also were analyzed with anti-XGef antibodies and anti-PCNA antibodies to confirm equivalent sample loading (input, IB: XGef, IB: PCNA, lower two panels). PI, prophase I oocytes; IP, immunoprecipitation; IB, immunoblot; hr in PG, hours in progesterone. (B) Interaction between endogenous CPEB and endogenous XGef does not require RNA. Immunoprecipitations with the indicated antibodies were performed in the presence (+RNAse, lane 3) or absence (lanes 1 and 2) of 10 ng/μl RNAse A. Protein G-agarose–bound (IP) and input samples were analyzed as described above with either anti-CPEB or anti-PCNA antibodies (IB: CPEB or IB: PCNA).
The observation that XGef and CPEB can be coimmunoprecipitated in the absence (Figure 1A, lanes 1–4) or presence (lanes 5–13) of progesterone indicates that hormone induction is not required for their interaction. Moreover, the interaction between endogenous XGef and endogenous CPEB is not mediated by their mutual binding to RNA, because the interaction is maintained in immunoprecipitation reactions containing RNAse A (Figure 1B, compare lanes 2 and 3).
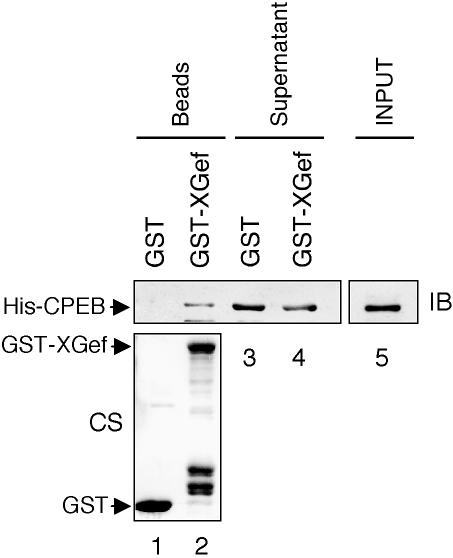
We examined whether XGef and CPEB interact directly using an in vitro pull-down assay. Using His-tagged CPEB (His-CPEB) and GST-tagged XGef (GST-XGef) purified after overexpression in bacteria, we demonstrated that XGef can interact directly with CPEB (Figure 2). His-CPEB interacted with glutathione beads coated with GST-XGef (Figure 2, lane 2), whereas His-CPEB did not interact with beads coated with GST alone (Figure 2, lane 1), although there is ample His-CPEB in the input sample (lane 5). Densitometry confirms that His-CPEB is depleted from the post-GST-XGef supernatant: 30% of the His-CPEB input is captured on GST-XGef beads (lane 2), leaving 60% of the input in the GST-XGef supernatant (lane 4). In contrast, GST beads captured 0% of the input (lane 1), leaving 94% in the GST supernatant (lane 3). Equivalent amounts of GST and GST-XGef are present on the beads (bottom). These results demonstrate that XGef interacts with CPEB during oogenesis and oocyte maturation and strongly suggest that the in vivo interaction is direct, thus increasing the likelihood of a functional connection between these proteins.
Figure 2.
XGef and CPEB interact directly in vitro. Bacterially expressed GST-XGef (or GST, as a control) was immobilized on glutathione-Sepharose beads that were then incubated with bacterially expressed, purified His-CPEB. The input CPEB protein (INPUT), bead bound fractions (Beads) and postbinding supernatant fractions (Supernatant) were analyzed by SDS-PAGE and immunoblotting with anti-CPEB antibodies (IB, top). Roughly equivalent amounts of GST and GST-XGef were present on the glutathione beads (bottom, Coomassie stained, CS, gel).
XGef and CPEB Are Components of a c-mos mRNP in Prophase I Oocytes and Early Maturing Oocytes
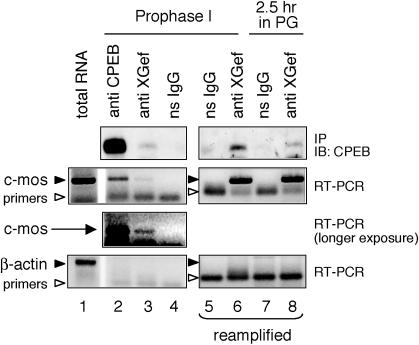
We next wondered whether XGef might interact with CPEB that also was bound to mRNA. To address this, we used RT-PCR to ask whether c-mos mRNA was present in XGef protein complexes immunoprecipitated from prophase I oocytes and oocytes in the early stages of meiotic resumption (Figure 3). RNA extracted from the immune complexes was analyzed by RT-PCR by using c-mos mRNA-specific primers (Reverte et al., 2003). XGef immune complexes from prophase I oocytes and from oocytes in early meiosis contained both CPEB protein and c-mos mRNA (Figure 3, top three panels, lanes 3, 6, and 8). Reamplification of the primary PCR products shown in lanes 3 and 4 confirms that c-mos mRNA is specifically immunoprecipitated with XGef antibodies and not ns IgG (second panel, compare lane 5 and 6). CPEB immune complexes from prophase I oocytes (lane 2, middle two panels) and from early maturing oocytes (our unpublished data) also contained c-mos mRNA, showing for the first time an endogenous CPEB protein/c-mos mRNA mRNP. The lower amount of c-mos RT-PCR product in the XGef immunoprecipitation versus the CPEB immunoprecipitation (Figure 3, second and third panels, compare lane 2 with lane 3) parallels the difference in amount of endogenous CPEB immunoprecipitated with anti-XGef versus anti-CPEB antibodies (Figure 3, top panel, CPEB immunoblot, lanes 2 and 3). Nonspecific IgG did not immunoprecipitate CPEB, c-mos mRNA, or actin mRNA (Figure 3, all panels, lanes 4, 5, and 7). In addition, no actin mRNA was detected in XGef or CPEB immunoprecipitations, even when the samples were reamplified, although the actin primers were clearly functional with total RNA (Figure 3, bottom). These results indicate that XGef interacts with CPEB bound to c-mos mRNA in both prophase I oocytes and in oocytes in early meiosis, reinforcing the possibility that XGef associates with the pool of CPEB that is involved in the regulation of c-mos mRNA polyadenylation-induced translation.
Figure 3.
XGef is present in an mRNP-containing CPEB protein and c-mos mRNA in both prophase I oocytes and early maturing oocytes. Protein complexes were immunoprecipitated from prophase I oocytes (lanes 2–6) and from oocytes that had been incubated in progesterone for 2.5 h (lanes 7 and 8). Antibodies used for immunoprecipitation included ns-IgG, anti-XGef and anti-CPEB. Immune complexes were analyzed by immunoblotting with anti-CPEB antibody (IP/IB: CPEB, top). RNA extracted from these complexes was analyzed by RT-PCR for the presence of c-mos mRNA (middle two panels) and β-actin mRNA (bottom). Lanes 5 and 6 represent reamplification of samples shown in lanes 4 and 3, respectively. Lanes 7 and 8 represent reamplification of ns IgG and anti-XGef immunoprecipitations from maturing oocytes where the primary amplification product was too low to visualize. Both c-mos mRNA and β-actin mRNA were successfully amplified from total ovary RNA (lane 1).
The Influence of XGef on Oocyte Maturation Requires an Interaction with CPEB
Our finding that XGef influences oocyte maturation before or at c-mos mRNA polyadenylation (Reverte et al., 2003), combined with our current demonstration of a direct interaction between CPEB and XGef, strongly suggests that XGef has an influence on the function of CPEB in oocyte maturation. To examine this more closely, we asked whether the interaction between XGef and CPEB was necessary for the influence of XGef on oocyte maturation. To do this, we first needed to map the domain of XGef required for interaction with GST-CPEB (Figure 4). From this information, we generated a version of XGef with very reduced binding to CPEB that was still capable of guanine nucleotide exchange (Figure 5). We then overexpressed this version of XGef in oocytes and assessed its influence on progesterone-induced oocyte maturation (Figure 6).
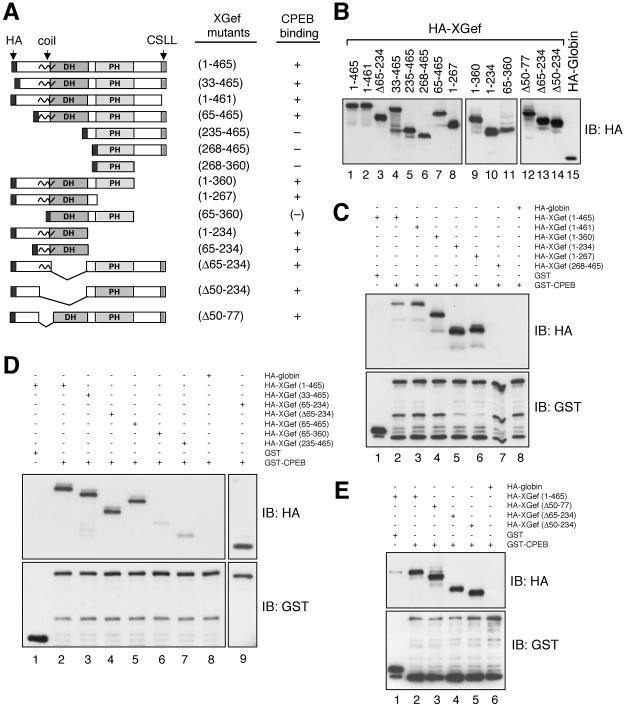
Figure 4.
Both the amino terminal and DH domains of XGef interact with CPEB. (A) Schematic diagram of wild-type and deletion mutant versions of HA-XGef. The HA-XGef wild-type or mutant name includes either the amino acids retained in the clone, or the Δ prefix to indicate which amino acids were removed. Interaction of the HA-XGef version with GST-CPEB is indicated by a plus sign, no interaction by a minus sign and very reduced interaction by (-). PH, pleckstrin homology domain; CSLL, amino terminal CAAX box; black squiggle, putative coiled-coil domain. (B) Oocyte extracts expressing various versions of HA-XGef were analyzed by SDS-PAGE and immunoblotting with HA antibody. HA-XGef(235–465) and HA-XGef(65–234) are not shown. (C–E) Representative interaction assays. Oocyte extract containing equivalent amounts of HA-XGef WT or a mutant HA-XGef version were combined with extract containing GST-CPEB or GST. Glutathioneagarose captured proteins were analyzed by SDS-PAGE and immunoblotting with anti-GST or anti-HA antibodies (IB: HA or IB: GST). The different combinations of fusion proteins combined into individual reactions are indicated in the grid above the picture. Immunoblots with only the most informative binding assays are shown.
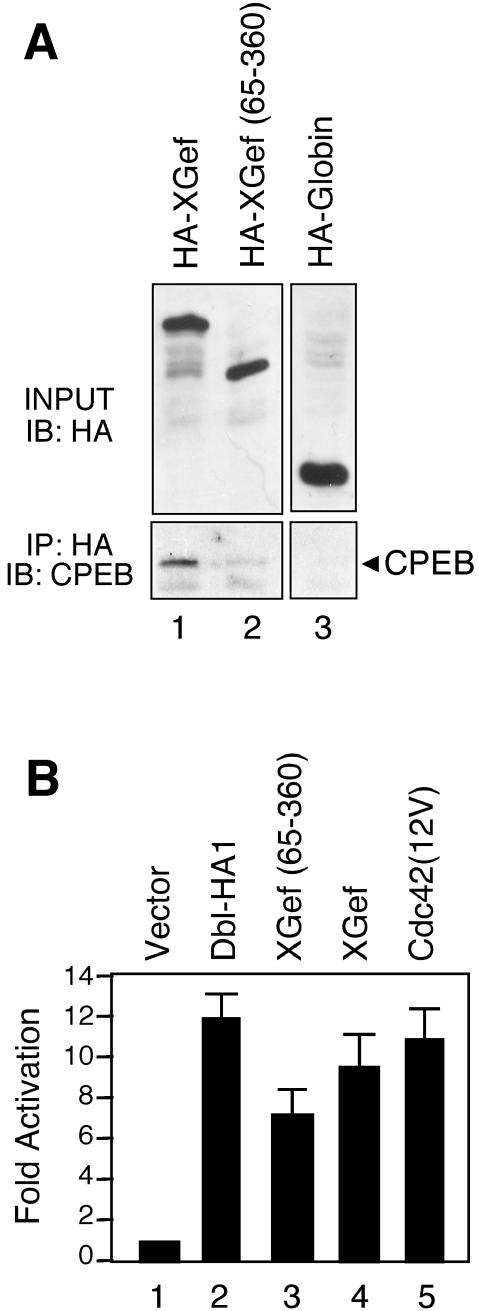
Figure 5.
XGef(65–360) has very reduced binding to endogenous CPEB and retains exchange factor activity. (A) Protein complexes from extracts prepared from oocytes overexpressing HA-XGef, HA-XGef(65–360), or HA-Globin were immunoprecipitated with anti-HA antibody. Input (INPUT) and protein G-agarose–bound (IP: HA) samples were analyzed by SDS-PAGE and immunoblotting with anti-HA (IB: HA) and anti-CPEB (IB: CPEB) antibodies, respectively. (B) Activation of (SREM)2-driven luciferase expression by XGef(65–360). NIH 3T3 cells were transiently transfected with 2.5 μg of the indicated constructs along with the reporter construct (SREm)2-luciferase (2.5 μg) and pCMVnlac (0.5 μg) as an internal control for transfection efficiency and growth inhibition. Luciferase and β-galactosidase activity were measured and expressed as fold activation relative to the level of activation seen with the vector control. Luciferase activity was then standardized relative to the β-galactosidase activity. Data shown are representative of three independent experiments performed on triplicate plates.
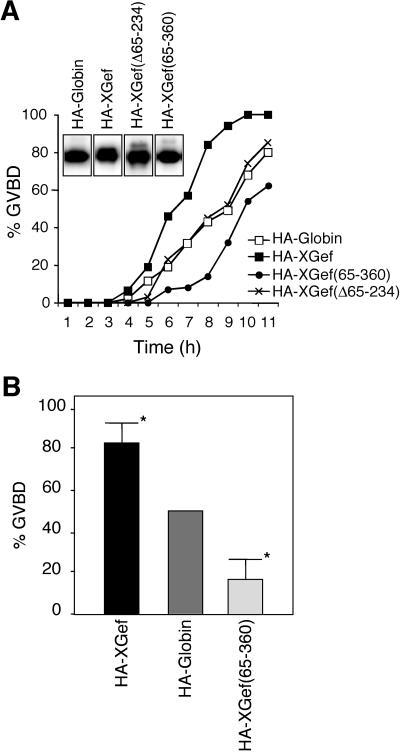
Figure 6.
Interaction between XGef and CPEB is necessary for the influence of XGef overexpression on oocyte maturation. (A) Effect of HA-XGef, HA-Globin, HA-XGef(65–360), and HA-XGef(Δ65–234) overexpression on progesterone-induced oocyte maturation, monitored by appearance of GVBD. Inset, anti-HA antibody immunoblot analysis of HA-XGef, HA-Globin, HA-XGef(65–360), and XGef(Δ65–234) levels in oocytes. (B) Histogram of the percentage of GVBD in oocytes overexpressing HA-XGef or HA-XGef(65–360) when Globin-overexpressing oocytes within the same experiment had reached 50% GVBD. Results from 10 independent experiments with at least 30 oocytes per injection were statistically analyzed using the Student's t test. *P < 0.01.
Both the Amino Terminus and DH Domain of XGef Interact with CPEB
Wild-type and deletion mutant versions of HA-tagged XGef (HA-XGef) were overexpressed in oocytes and tested for their ability to interact with oocyte-overexpressed GST-CPEB in glutathione agarose pull-down assays (Figure 4). A summary of our results in Figure 4A includes a schematic diagram of the versions of HA-XGef used. Versions of HA-XGef lacking both the amino terminal and DH domains do not interact with GST-CPEB [for example, HA-XGef(268–465), Figure 4C, lane 7], whereas versions of HA-XGef lacking the amino terminal domain but containing an intact DH domain retain an interaction with CPEB [for example, HA-XGef(65–465) and HA-XGef(65–234), Figure 4D, lanes 5 and 9]. Although the DH domain is clearly involved in the interaction with CPEB [compare HA-XGef(65–465) with HA-XGef(235–465), Figure 4D, lane 5 with lane 7], HA-XGef–containing internal deletions that remove the DH domain continue to interact with GST-CPEB, indicating that the amino terminal domain alone can interact with CPEB [for example, HA-XGef(Δ65–234) and HA-XGef(Δ50–234), Figure 4E, lanes 4 and 5]. In addition, the putative coiled coil domain does not contribute to the interaction between XGef and CPEB [HA-XGef(Δ50–77), Figure 4E, lane 3]. These results were confirmed by immunoprecipitation of HA-XGef with anti HA-antibodies, followed by immunoblotting for endogenous CPEB (our unpublished data). These data indicate that the amino terminal 50 amino acids and the DH domain are both capable of interacting with CPEB. Notably, a version of XGef containing only the DH through the PH domain [HA-XGef(65–360)] has a greatly reduced ability to interact with GST-CPEB (Figure 4D, lane 6). We suspect that, as found in other guanine nucleotide exchange factors (GEFs) (Schmidt and Hall, 2002), there may be an influence of the amino- and carboxy-terminal domains on the DH-PH domain, such that, when they are absent, the DH domain can only interact with CPEB very weakly.
XGef(65–360) Can Function as an Exchange Factor
An important requirement for our subsequent assays was that we generate a mutated version of XGef with diminished interaction with CPEB that was still able to function as a guanine nucleotide exchange factor. We confirmed that HA-XGef(65–360) has significantly reduced interaction with endogenous CPEB compared with wild-type HA-XGef by immunoprecipitation of HA-XGef(65–360) overexpressed in oocytes followed by a CPEB immunoblot (Figure 5A, compare lane 2 with lane 1, bottom). To determine whether HA-XGef(65–360) is catalytically active for nucleotide exchange in vivo, we compared full-length HA-XGef with HA-XGef(65–360) in a transcription-coupled luciferase assay (Figure 5B). (SREm)2-luciferase encodes the luciferase gene under the control of tandem serum response elements. We have shown previously that this reporter can be used as a reliable indicator of RhoGTPase activation in COS-7 cells (Westwick et al., 1998). Consistent with our previous results, a constitutively activated version of Cdc42 [Cdc42(12V)], and an activated version of the RhoGEF Dbl (Dbl-HA1), stimulated this reporter by 11- and 12-fold, respectively, compared with vector controls. In comparison, both HA-XGef and HA-XGef(65–360) showed a reduced but consistent ability to activate the reporter (9- and 7-fold, respectively). These results suggest that HA-XGef and HA-XGef(65–360) have roughly equivalent activity in vivo and are consistent with our previous observation that XGef is a weaker activator of mammalian Cdc42 than Dbl (Reverte et al., 2003).
Interaction between XGef and CPEB Is Necessary for the Influence of XGef Overexpression on Oocyte Maturation
To determine whether the interaction between XGef and CPEB is required for the influence of XGef on oocyte maturation, we overexpressed HA-XGef, HA-Globin, HA-XGef(65–360), and HA-XGef(Δ65–234) in oocytes (Figure 6) and induced meiotic maturation with progesterone. The time to GVBD was assessed to monitor the influence of these proteins on meiotic progression (Figure 6A; results showing analysis of some biochemical markers of meiosis in these oocytes are presented in Figure 8B). Overexpressed HA-XGef(Δ65–234), which lacks the DH domain and therefore exchange factor activity, has no influence on maturation compared with HA-Globin overexpression, as observed previously (Reverte et al., 2003). Overexpression of HA-XGef(65–360) significantly delays GVBD (P < 0.01) in comparison with HA-Globin (Figure 6B), whereas overexpression of wild-type HA-XGef significantly accelerates GVBD (P < 0.01), as observed previously (Reverte et al., 2003). These results strongly suggest that the interaction between XGef and CPEB is involved in the role of XGef in stimulating meiosis.
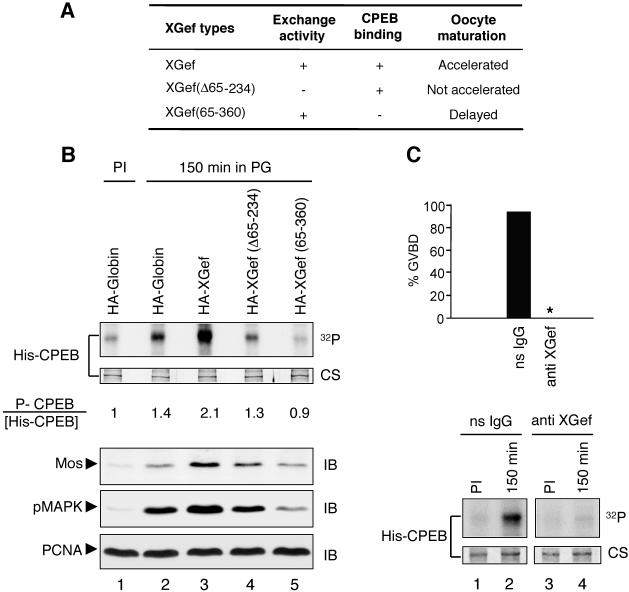
Figure 8.
XGef exchange factor activity and the interaction between XGef and CPEB are involved in early CPEB phosphorylation. (A) Summary of the influence of XGef-containing domain mutations on progesterone induced oocyte maturation. (B) His-CPEB WT bound to nickel beads was incubated with extracts prepared from oocytes overexpressing HA-XGef, HA-XGef(Δ65–234), and HA-XGef(65–360) isolated 150 min after progesterone addition, in the presence of [γ-32P]ATP. Extracts from HA-Globin–overexpressing oocytes selected at prophase I (PI) and 150 min after progesterone addition were analyzed in parallel as a control. The ratio of phosphorylated CPEB (32P) to His-CPEB (Coomassie stain, CS) is indicated. The levels of endogenous Mos protein and pMAPK in each sample were analyzed by SDS-PAGE and immunoblotting (IB) with anti-Mos and anti-pMAPK antibodies. Anti-PCNA antibodies were used to detect PCNA and confirm equivalent sample loading (bottom). Figure 6A shows the time course of GVBD for these oocytes. (C) Oocytes were injected with affinity-purified XGef antibody (anti-XGef) or nonspecific IgG (ns IgG), and progesterone was added. The percentage of GVBD observed 8 h after progesterone addition is indicated for each treatment in the top histogram (33 oocytes each). An asterisk (*) indicates that no oocytes underwent GVBD. His-CPEB WT bound to nickel beads was incubated with extracts prepared from ns IgG-(lanes 1 and 2) and anti-XGef antibody (lanes 3 and 4)-injected oocytes isolated at prophase I and 150 min after progesterone addition, in the presence of [γ-32P]ATP. The autoradiogram (32P) and CS image of the same gel are shown.
XGef Overexpression Enhances Early Phosphorylation of CPEB during Progesterone-stimulated Oocyte Maturation
Because early activation of CPEB by phosphorylation is involved in the polyadenylation-induced translation of c-mos mRNA (Mendez et al., 2000a; Charlesworth et al., 2004), we examined the possibility that XGef overexpression might influence early CPEB phosphorylation and therefore early CPEB activation. To do this, we used the in vitro phosphorylation assay developed by Mendez et al. (2000a) that recapitulates the early activating phosphorylation of endogenous CPEB. We monitored the timing, extent, and pattern of early CPEB phosphorylation in the presence and absence of overexpressed HA-XGef (Figure 7).
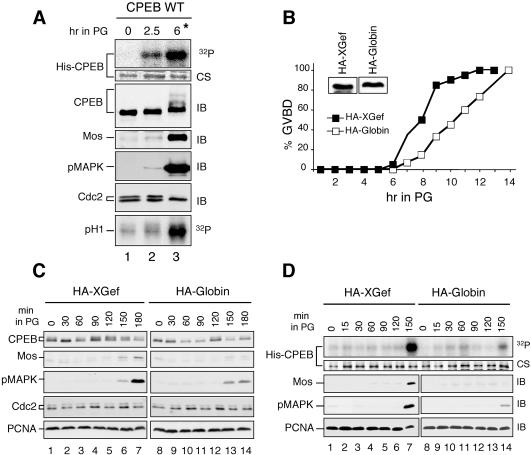
Figure 7.
XGef overexpression enhances early CPEB phosphorylation. (A) An in vitro phosphorylation assay using recombinant His-CPEB tethered to beads recapitulates early CPEB phosphorylation. His-CPEB WT was bound to nickel beads and incubated with extracts prepared from oocytes isolated at the indicated times after progesterone addition, in the presence of [γ-32P]ATP. Bead-bound, potentially phosphorylated His-CPEB was analyzed by one-dimensional SDS-PAGE and autoradiography (32P). Total CPEB bound to beads was visualized by Coomassie staining (CS). The same extracts used for phosphorylation assays were analyzed by SDS-PAGE and immunoblotting (IB) with anti-CPEB, anti-Mos, anti-pMAPK, and anti-Cdc2 antibodies. Samples were assayed for MPF activity by using an histone HI kinase assay (pH1, 32P, bottom). (B) Effect of HA-XGef and HA-Globin overexpression on oocyte maturation monitored by the appearance of GVBD. Inset, anti-HA antibody immunoblot analysis of HA-XGef and HA-Globin levels in oocytes. (C) Effect of HA-XGef (lanes 1–7) and HA-Globin (lanes 8–14) overexpression on Mos protein levels, MAPK phosphorylation, and cdc2 dephosphorylation, monitored by Anderson SDS-PAGE and immunoblot analysis (IB) with anti-CPEB, anti-Mos, anti-pMAPK, and anti-Cdc2 antibodies. Anti-PCNA antibodies were used to detect PCNA and confirm equivalent sample loading (bottom). (D) Effect of XGef overexpression on early CPEB phosphorylation. His-CPEB WT was immobilized on nickel beads and then incubated with extracts prepared from oocytes overexpressing either HA-XGef (lanes 1–7) or HA-Globin (lanes 8–14), isolated at the indicated times after progesterone addition, in the presence of [γ-32P]ATP. Top, autoradiogram of 32P His-CPEB (32P). Middle, CS of the same gel. Extracts used for the phosphorylation assay were analyzed by SDS-PAGE and immunoblotting with anti-Mos and anti-pMAPK antibodies (IB). Anti-PCNA antibodies were used to detect PCNA and confirm equivalent sample loading (bottom). These results were observed in three independent experiments.
We first confirmed that our early stage oocyte extracts recapitulate early phosphorylation of CPEB by comparing in vitro phosphorylation assays using extracts from prophase I oocytes and extracts from oocytes collected 2.5 h (early maturing oocytes) and 6 h (matured, GVBD oocytes) after progesterone stimulation (Figure 7A). Extracts were incubated with His-CPEB WT tethered to beads in the presence of [γ-32P]ATP. SDS-PAGE and autoradiography of the bead-bound material revealed that His-CPEB WT is phosphorylated in early maturing oocytes at 2.5 h and is phosphorylated to a greater extent in GVBD oocytes at 6 h (Figure 7A, top). This pattern parallels the early and late phosphorylation of endogenous CPEB (Figure 7A, third panel; Mendez et al., 2000a). CPEB immunoblots reveal the typical mobility shift of endogenous CPEB at GVBD, due to phosphorylation on multiple sites (Paris et al., 1991; Mendez et al., 2002). Late phosphorylation of CPEB and the mobility shift were coincident with the activation of MPF, revealed by complete dephosphorylation of Cdc2 and histone H1 kinase activity only at GVBD (Figure 7A, two bottom panels), as expected (de Moor and Richter, 1997; Mendez et al., 2002). Notably, phosphorylation of His-CPEB at 2.5 h coincided with the appearance of a low level of Mos protein and pMAPK. The further increase in Mos protein and pMAPK levels at GVBD is due to both a positive feedback loop (Howard et al., 1999) and stabilization of Mos protein (Nebreda et al., 1995).
To examine the influence of XGef on early CPEB phosphorylation, HA-XGef was overexpressed in oocytes, and then protein extracts from oocytes collected throughout the early stages of progesterone-stimulated oocyte maturation were used in in vitro phosphorylation assays (Figure 7, B–D). As expected (Reverte et al., 2003), HA-XGef overexpression accelerated the time to 100% GVBD (Figure 7B) as well as the appearance of Mos kinase and pMAPK, relative to Globin-overexpressing oocytes (Figure 7C, compare lanes 1–7 and lanes 8–14). Equal quantities of HA-XGef and HA-Globin were expressed (Figure 7B, inset), and these levels remained stable during maturation (our unpublished data). The persistent doublet of cdc2 kinase indicates that MPF is not active in these extracts (Figure 7A, bottom panels and C, bottom panel). In in vitro phosphorylation assays, early phosphorylation of CPEB was observed 150 min after progesterone stimulation (Figure 7D, lanes 7 and 14). Notably, in HA-XGef–overexpressing oocytes, the level of His-CPEB phosphorylation is greater than that observed in HA-Globin–overexpressing oocytes at 150 min postprogesterone. The appearance of Mos protein and pMAPK coincides with the initial increase in phosphorylated CPEB (Figure 7D, lane 7).
When we analyzed these samples much more closely using phosphopeptide analysis, we found that the phosphorylation pattern of His-CPEB incubated with extracts from HA-XGef–overexpressing oocytes was exactly the same as that obtained for His-CPEB incubated with extracts from HA-Globin–overexpressing oocytes, indicating that XGef overexpression does not lead to an aberrant pattern of His-CPEB phosphorylation. Importantly, using the more direct method of tandem mass spectrometry of peptide fragment ions, we confirmed previous results indicating that S174 is first phosphorylated 2.5 h after progesterone induction (Mendez et al., 2000a). In contrast, S180 is not phosphorylated to the same extent as S174: the relative amount of phosphorylated versus nonphosphorylated S174 was ∼1:2, whereas for S180, this ratio was ∼1:50. Previous work had suggested that S180 also might be a site for regulatory phosphorylation of CPEB, based on proximity to S174 and the similarity of the immediately surrounding sequence (Mendez et al., 2000a); however, our data suggest that this might not be the case. We conclude from these phosphorylation studies that His-XGef overexpression leads to an increase in the level of S174 phosphorylated CPEB, a modification that has been demonstrated to be vital for the early activation of endogenous CPEB (Mendez et al., 2000a).
XGef Exchange Factor Activity and an Interaction between XGef and CPEB Are Involved in the Early Phosphorylation of CPEB
To extend these studies, we assayed the effect of overexpressing HA-XGef(Δ65–234) and HA-XGef(65–360) on early CPEB phosphorylation (Figure 8). We found that exchange deficient HA-XGef(Δ65–234), which does not accelerate oocyte maturation, does not enhance the early phosphorylation of CPEB, in contrast with the influence of wild-type HA-XGef on CPEB phosphorylation (Figure 8B, compare lane 4 with lane 3). In fact, the levels of CPEB phosphorylation are similar to those obtained from oocytes overexpressing HA-Globin (compare lane 4 with lane 2). Overexpression of HA-XGef(65–360), which has very reduced binding to CPEB, produces early oocyte extracts that phosphorylate CPEB to levels well below those obtained with extracts from oocytes overexpressing HA-Globin (Figure 8B, lane 5 vs. lane 2). In addition, there is a direct correlation between the level of influence these XGef versions have on oocyte maturation and their influence on early CPEB phosphorylation (compare Figure 6A with 8B). Oocytes overexpressing HA-XGef reached GVBD ∼2 h earlier than HA-Globin–overexpressing oocytes, and extracts from these oocytes phosphorylated CPEB to a greater extent. In oocytes overexpressing HA-XGef(Δ65–234), time to 50% GVBD and the level of CPEB phosphorylation were about the same as those in controls. For HA-XGef(65–360), the time to 50% GVBD was delayed compared with controls, and the level of CPEB phosphorylation was lower than that found for control-injected oocytes. Notably, Mos protein levels and phosphorylation of MAPK also directly correlate with the level of CPEB phosphorylation and the time to GVBD (Figures 7, B–D, and 8B). In total, these results strongly support our finding of an involvement of XGef in early CPEB phosphorylation and go further to indicate that this involvement requires the interaction between XGef and CPEB and most likely XGef exchange factor activity. In addition, we have found a tight correlation between the timing of early CPEB phosphorylation, accumulation of Mos protein, and activation of MAPK.
XGef Antibody Blocks Early Phosphorylation of CPEB
Because XGef antibody can block c-mos mRNA polyadenylation, Mos protein accumulation, and progesterone-induced oocyte maturation (Reverte et al., 2003), we wondered whether XGef antibody also could block early CPEB phosphorylation (Figure 8C). As reported previously, anti-XGef antibody injection completely blocked oocyte maturation, whereas maturation was not affected when oocytes were injected with the same quantity of nonspecific rabbit antirat IgG (Figure 8C, top; Reverte et al., 2003). Extracts from these sets of oocytes, injected with either XGef or IgG antibodies, were used in in vitro phosphorylation assays. Notably, there was no phosphorylation of His-CPEB in early meiotic extracts when the oocytes were injected with XGef antibody before progesterone induction. Nonspecific IgG, in contrast, did not interfere with early phosphorylation of His-CPEB (Figure 8C, compare lane 4 with lane 2). An important aspect of this experiment is that the injected XGef antibody is presumably blocking the function of endogenous XGef(also see Reverte et al., 2003). We conclude that perturbation of endogenous XGef function, as well as overexpression of recombinant XGef, influences the early activating phosphorylation of CPEB.
DISCUSSION
XGef is a Rho family G protein that interacts with CPEB and is involved in progesterone-stimulated meiotic maturation in Xenopus oocytes before the polyadenylation of c-mos mRNA (Reverte et al., 2003). The timing of XGef function correlates with a requirement for CPEB activity in regulating c-mos mRNA polyadenylation. In this study, we therefore explored the role of XGef in early CPEB function in the Mos/MAPK pathway, which is activated by progesterone and necessary for timely oocyte maturation. We found that XGef interacts with CPEB throughout oogenesis and oocyte maturation and directly interacts with CPEB in vitro. In prophase I oocytes and oocytes in the early stages of meiosis, XGef and CPEB are present in a complex that also contains c-mos mRNA, suggesting that XGef is involved in a functional CPEB protein complex. To determine whether the XGef–CPEB interaction is required for the role of CPEB in progesterone-stimulated oocyte maturation, we mapped the XGef domains required for interaction with CPEB. We found that the amino-terminal 50 amino acids and the DH domain of XGef are each capable of facilitating the interaction with CPEB. We also identified a version of XGef [XGef(65–360)] with very reduced binding to CPEB that retains exchange factor activity. Interestingly, this mutant acts in a dominant negative manner when expressed in ovo, significantly delaying the onset of progesterone-stimulated GVBD. This result confirms that the interaction between XGef and CPEB is required for the influence of XGef on oocyte maturation. Because early CPEB phosphorylation is reported to be necessary for early CPEB activity in c-mos mRNA polyadenylation, we examined the potential influence of XGef overexpression on early CPEB phosphorylation. We found that wild-type XGef overexpression increases the level of S174 phosphorylated CPEB early during meiotic maturation. In addition, an exchange deficient version of XGef [XGef(Δ65–234)] does not influence early CPEB phosphorylation, whereas a version of XGef with greatly reduced binding to CPEB, significantly delays early CPEB phosphorylation. Notably, XGef antibody injection prevents early CPEB phosphorylation and blocks progesterone-induced oocyte maturation, presumably by blocking endogenous XGef function. We conclude from these studies that XGef mediates the early, activating phosphorylation of CPEB.
We observed a direct correlation between the level of early CPEB phosphorylation, Mos protein accumulation, and the time to 50% GVBD in control oocytes and oocytes overexpressing various versions of XGef. Although this is the first time this correlation has been extensively documented, it is not surprising given the established role of CPEB in regulating the early polyadenylation-induced translation of c-mos mRNA (Stebbins-Boaz et al., 1996) and the role of the Mos/MAPK pathway in timely meiotic progression (Fisher et al., 1999; Gross et al., 2000). Presumably, elevating early phosphorylated CPEB levels by overexpression of XGef results in increasing the amount of activated CPEB available to recruit more polyadenylation complexes to mRNA (Mendez et al., 2000b), leading to an increase in polyadenylation-induced translation of c-mos mRNA and stimulation of the Mos/MAPK pathway. In support of this, we previously demonstrated that XGef overexpression correlates with earlier c-mos mRNA poly (A) tail elongation and earlier achievement of maximal poly (A) tail length for the c-mos mRNA compared with controls (Reverte et al., 2003). Earlier appearance of Mos protein paralleled this earlier polyadenylation of c-mos mRNA (Reverte et al., 2003). It also follows that lower levels of phosphorylated CPEB would result in a lower degree of activation of polyadenylation-induced translation, and, in fact, we observe lower levels of Mos protein in samples with lower levels of early phosphorylated CPEB. Given the importance of the Mos/MAPK pathway in triggering events of meiotic maturation (Fisher et al., 1999; Gross et al., 2000; Palmer and Nebreda, 2000), the effects we see on meiosis are most likely due to the influence of XGef perturbation on Mos protein levels; however, it is certainly possible that the polyadenylation-induced translation of other mRNAs is influenced by the increase in CPEB activity and that these proteins also might impinge upon the timeliness of GVBD (Ferby et al., 1999; Charlesworth et al., 2004).
The finding that XGef is in a CPEB protein/c-mos mRNA mRNP in prophase I oocytes and oocytes undergoing meiosis, yet the influence of XGef on CPEB phosphorylation is only apparent ∼2 h after progesterone induction, indicates a requirement for a progesterone-stimulated event. Further support for this requirement comes from the observation that XGef overexpression increases the level of phosphorylated CPEB, but does not cause CPEB to be phosphorylated earlier than normal during oocyte maturation. Progesterone stimulation may activate CPEB-bound XGef for nucleotide exchange on a G protein by stimulating a regulatory domain of XGef, as seen with the activation of the exchange factor Vav (Aghazadeh et al., 2000). Another possibility is that progesterone stimulation activates an unidentified XGef binding protein required for stimulation of XGef nucleotide exchange activity. For example, activated Gα13, released after stimulation with lysophosphatidic acid or thrombin, binds to and activates the exchange factor activity of p115 RhoGEF in COS cells (Hart et al., 1998). Alternatively, progesterone stimulation may activate a pathway that works in parallel to or downstream of XGef-mediated GTPase activation to regulate the kinase or conditions necessary for CPEB phosphorylation. Certainly, given its proposed role in early CPEB phosphorylation (Mendez et al., 2000a), Aurora A kinase is a potential candidate for a kinase that might be acting in parallel to or downstream of XGef. However, the demonstration by several groups that Aurora A kinase is not activated until GVBD and that it in fact requires MPF for activation (Frank-Vaillant et al., 2000; Castro et al., 2003; Ma et al., 2003; Maton et al., 2003) raises the distinct possibility that Aurora A is not the endogenous kinase responsible for early phosphorylation of CPEB. Unfortunately, because of the paucity of information on the events occurring between the decrease in PKA activity and activation of CPEB, there are few clues as to prospective proteins that may be involved in the XGef-influenced pathway. We are therefore currently attempting to identify these proteins.
When we overexpress wild-type XGef, we are essentially doubling the concentration of XGef within the oocyte (our unpublished data). The resulting increase in CPEB phosphorylation and Mos protein levels suggests that there is an excess of suitable but stored CPEB in the cell that is easily recruited into an activated CPEB pool. The presence of a reserve CPEB pool also has been observed in experiments demonstrating that mRNAs containing a CPE are translationally repressed when injected into prophase oocytes (de Moor and Richter, 1999; Barkoff et al., 2000). Our observation also suggests that the endogenous concentration of XGef is limiting relative to the amount of CPEB in the oocyte. This proposal is supported by the fact that there is 3 ng of CPEB for every 1 ng of XGef per oocyte (Hake and Richter, 1994; Reverte et al., 2003). This set of observations, combined with the likelihood that XGef(Δ65–234) does not physically interact with a GTPase, provide a possible explanation for why XGef(Δ65–234) does not function in a dominant negative manner. Perhaps, overexpressed XGef(Δ65–234) interacts with the excess pool of CPEB, but the absence of a DH domain prevents it from interacting with a GTPase; the DH domain is critical for GTPase interaction in a number of GEFs (Aghazadeh et al., 1998; Liu et al., 1998; Worthylake et al., 2000). The XGef(Δ65–234):CPEB complex is not therefore recruited to the GTPase:kinase complex (see below), and no increase in phosphorylation of this particular CPEB molecule occurs. The remaining complexes of wild-type XGef and CPEB and their access to available GTPase are uninterrupted, and CPEB phosphorylation proceeds normally.
Our finding that the interaction between XGef and CPEB is necessary for the influence of XGef on CPEB phosphorylation can be interpreted two ways. One possibility is that the interaction is necessary for XGef to appropriately activate a GTPase. Regulatory interactions between GEFs and auxiliary proteins have been shown to influence both the temporal and spatial activation of GTPases (Krendel et al., 2002; Schmidt and Hall, 2002). Support for this possibility comes from our observations with the CPEB interaction impaired XGef mutant XGef(65–360), which still functions as an exchange factor for Cdc42 in mammalian cells, yet delays early CPEB phosphorylation. Although XGef(65–360) probably can function as an exchange factor in oocytes (Martínez and Hake, unpublished data), the interaction with CPEB may be required for the appropriate temporal or spatial activation of a GTPase in relation to its downstream targets. Proper activation of these targets would be required for the subsequent phosphorylation of CPEB. Alternatively, the interaction between XGef and CPEB may help target CPEB for phosphorylation by influencing recruitment of a kinase or accessibility of CPEB to an activated kinase. This is supported by the fact that XGef(65–360) can function as a dominant negative mutation, significantly decreasing the level of phosphorylated CPEB. The dominant negative effect might be achieved by preferential association of XGef(65–360) with components necessary for CPEB phosphorylation, thereby preventing access to these components by WT-XGef:CPEB complexes.
These two scenarios, that the XGef:CPEB interaction is necessary either for GTPase activation or for proper targeting of CPEB for phosphorylation, are not mutually exclusive, and there is precedent for signaling complexes that contain or recruit substrate, GEF, G protein, and kinase to the same location (Li et al., 2003). Based on our data, one possible model is that XGef, associated with a CPEB mRNP, recruits a Rho family G protein and a kinase. During progesterone-stimulated oocyte maturation, the G protein, activated by XGef, may then activate a kinase that phosphorylates CPEB, thus triggering polyadenylation-induced translation. A functional interaction between XGef and CPEB may not in fact be limited to Xenopus oocytes: Drosophila CG8606, which has 39% amino acid similarity with XGef (Reverte et al., 2003), interacts with a neuronal CPEB isoform from Drosophila (Si et al., 2003). We are currently working to identify the cognate G protein for XGef, as well as additional components of the XGef–CPEB protein complex.
Acknowledgments
We thank members of the Hake laboratory and Clare O'Connor for critical reading of the manuscript. This work was supported by R01-GM64702 from the National Institutes of General Medical Sciences (to L.E.H.) and Public Health Service Grant CA-77493 from the National Cancer Institute (to I.P.W.). L.E.H. is a Clare Boothe Luce Professor of Biology.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-07-0585) on January 5, 2005.
References
- Aghazadeh, B., Lowry, W. E., Huang, X. Y., and Rosen, M. K. (2000). Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell 102, 625-633. [DOI] [PubMed] [Google Scholar]
- Aghazadeh, B., Zhu, K., Kubiseski, T. J., Liu, G. A., Pawson, T., Zheng, Y., and Rosen, M. K. (1998). Structure and mutagenesis of the Dbl homology domain. Nat. Struct. Biol. 5, 1098-1107. [DOI] [PubMed] [Google Scholar]
- Anderson, C. W., Baum, P. R., and Gesteland, R. F. (1973). Processing of adenovirus 2-induced proteins. J. Virol. 12, 241-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoff, A. F., Dickson, K. S., Gray, N. K., and Wickens, M. (2000). Translational control of cyclin B1 mRNA during meiotic maturation: coordinated repression and cytoplasmic polyadenylation. Dev. Biol. 220, 97-109. [DOI] [PubMed] [Google Scholar]
- Bayaa, M., Booth, R. A., Sheng, Y., and Liu, X. J. (2000). The classical progesterone receptor mediates Xenopus oocyte maturation through a nongenomic mechanism. Proc. Natl. Acad. Sci. USA 97, 12607-12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, A., Mandart, E., Lorca, T., and Galas, S. (2003). Involvement of Aurora A kinase during meiosis I-II transition in Xenopus oocytes. J. Biol. Chem. 278, 2236-2241. [DOI] [PubMed] [Google Scholar]
- Charlesworth, A., Cox, L. L., and MacNicol, A. M. (2004). Cytoplasmic poly-adenylation element (CPE)- and CPE-binding protein (CPEB)-independent mechanisms regulate early class maternal mRNA translational activation in Xenopus oocytes. J. Biol. Chem. 279, 17650-17659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moor, C. H., and Richter, J. D. (1997). The Mos pathway regulates cytoplasmic polyadenylation in Xenopus oocytes. Mol. Cell. Biol. 17, 6419-6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moor, C. H., and Richter, J. D. (1999). Cytoplasmic polyadenylation elements mediate masking and unmasking of cyclin B1 mRNA. EMBO J. 18, 2294-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moor, C. H., and Richter, J. D. (2001). Translational control in vertebrate development. Int. Rev. Cytol. 203, 567-608. [DOI] [PubMed] [Google Scholar]
- Eng, J. K., McCormack, A. L., and Yates, J. R. (1994). An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass. Spectrom. 5, 976-989. [DOI] [PubMed] [Google Scholar]
- Ferby, I., Blazquez, M., Palmer, A., Eritja, R., and Nebreda, A. R. (1999). A novel p34(cdc2)-binding and activating protein that is necessary and sufficient to trigger G(2)/M progression in Xenopus oocytes. Genes Dev. 13, 2177-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, D. L., Brassac, T., Galas, S., and Doree, M. (1999). Dissociation of MAP kinase activation and MPF activation in hormone-stimulated maturation of Xenopus oocytes. Development 126, 4537-4546. [DOI] [PubMed] [Google Scholar]
- Frank-Vaillant, M., Haccard, O., Thibier, C., Ozon, R., Arlot-Bonnemains, Y., Prigent, C., and Jessus, C. (2000). Progesterone regulates the accumulation and the activation of Eg2 kinase in Xenopus oocytes. J. Cell Sci. 113, 1127-1138. [DOI] [PubMed] [Google Scholar]
- Gross, S. D., Schwab, M. S., Taieb, F. E., Lewellyn, A. L., Qian, Y. W., and Maller, J. L. (2000). The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90(Rsk). Curr. Biol. 10, 430-438. [DOI] [PubMed] [Google Scholar]
- Hake, L. E., and Richter, J. D. (1994). CPEB is a specificity factor that mediates cytoplasmic polyadenylation during Xenopus oocyte maturation. Cell 79, 617-627. [DOI] [PubMed] [Google Scholar]
- Hart, M. J., Jiang, X., Kozasa, T., Roscoe, W., Singer, W. D., Gilman, A. G., Sternweis, P. C., and Bollag, G. (1998). Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science 280, 2112-2114. [DOI] [PubMed] [Google Scholar]
- Howard, E. L., Charlesworth, A., Welk, J., and MacNicol, A. M. (1999). The mitogen-activated protein kinase signaling pathway stimulates mos mRNA cytoplasmic polyadenylation during Xenopus oocyte maturation. Mol. Cell. Biol. 19, 1990-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendel, M., Zenke, F. T., and Bokoch, G. M. (2002). Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat. Cell Biol. 4, 294-301. [DOI] [PubMed] [Google Scholar]
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- Li, Z., et al. (2003). Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell 114, 215-227. [DOI] [PubMed] [Google Scholar]
- Liu, X., et al. (1998). NMR structure and mutagenesis of the N-terminal Dbl homology domain of the nucleotide exchange factor Trio. Cell 95, 269-277. [DOI] [PubMed] [Google Scholar]
- Ma, C., Cummings, C., and Liu, X. J. (2003). Biphasic activation of Aurora-A kinase during the meiosis I-meiosis II transition in Xenopus oocytes. Mol. Cell. Biol. 23, 1703-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maton, G., Thibier, C., Castro, A., Lorca, T., Prigent, C., and Jessus, C. (2003). Cdc2-cyclin B triggers H3 kinase activation of Aurora-A in Xenopus oocytes. J. Biol. Chem. 278, 21439-21449. [DOI] [PubMed] [Google Scholar]
- Mendez, R., Barnard, D., and Richter, J. D. (2002). Differential mRNA translation and meiotic progression require Cdc2-mediated CPEB destruction. EMBO J. 21, 1833-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez, R., Hake, L. E., Andresson, T., Littlepage, L. E., Ruderman, J. V., and Richter, J. D. (2000a). Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature 404, 302-307. [DOI] [PubMed] [Google Scholar]
- Mendez, R., Murthy, K. G., Ryan, K., Manley, J. L., and Richter, J. D. (2000b). Phosphorylation of CPEB by Eg2 mediates the recruitment of CPSF into an active cytoplasmic polyadenylation complex. Mol. Cell 6, 1253-1259. [DOI] [PubMed] [Google Scholar]
- Mendez, R., and Richter, J. D. (2001). Translational control by CPEB: a means to the end. Nat. Rev. Mol. Cell Biol. 2, 521-529. [DOI] [PubMed] [Google Scholar]
- Nebreda, A. R., and Ferby, I. (2000). Regulation of the meiotic cell cycle in oocytes. Curr. Opin. Cell Biol. 12, 666-675. [DOI] [PubMed] [Google Scholar]
- Nebreda, A. R., Gannon, J. V., and Hunt, T. (1995). Newly synthesized protein(s) must associate with p34cdc2 to activate MAP kinase and MPF during progesterone-induced maturation of Xenopus oocytes. EMBO J. 14, 5597-5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, A., and Nebreda, A. R. (2000). The activation of MAP kinase and p34cdc2/cyclin B during the meiotic maturation of Xenopus oocytes. Prog. Cell Cycle Res. 4, 131-143. [DOI] [PubMed] [Google Scholar]
- Paris, J., Swenson, K., Piwnica-Worms, H., and Richter, J. D. (1991). Maturation-specific polyadenylation: in vitro activation by p34cdc2 and phosphorylation of a 58-kD CPE-binding protein. Genes Dev. 5, 1697-1708. [DOI] [PubMed] [Google Scholar]
- Peng, J., and Gygi, S. P. (2001). Proteomics: the move to mixtures. J. Mass. Spectrom. 36, 1083-1091. [DOI] [PubMed] [Google Scholar]
- Reverte, C. G., Ahearn, M. D., and Hake, L. E. (2001). CPEB degradation during Xenopus oocyte maturation requires a PEST domain and the 26S proteasome. Dev. Biol. 231, 447-458. [DOI] [PubMed] [Google Scholar]
- Reverte, C. G., Yuan, L., Keady, B. T., Lacza, C., Attfield, K. R., Mahon, G. M., Freeman, B., Whitehead, I. P., and Hake, L. E. (2003). XGef is a CPEB-interacting protein involved in Xenopus oocyte maturation. Dev. Biol. 255, 383-398. [DOI] [PubMed] [Google Scholar]
- Richter, J. D. (2000). Influence of Polyadenylation-induced translation on metazoan development and neuronal synaptic function. In: Translational Control of Gene Expression, ed. N. Sonenberg, J. Hershey, and M. Mathews, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 785-806.
- Sagata, N. (1997). What does Mos do in oocytes and somatic cells? Bioessays 19, 13-21. [DOI] [PubMed] [Google Scholar]
- Schmidt, A., and Hall, A. (2002). Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 16, 1587-1609. [DOI] [PubMed] [Google Scholar]
- Sheets, M. D., Wu, M., and Wickens, M. (1995). Polyadenylation of c-mos mRNA as a control point in Xenopus meiotic maturation. Nature 374, 511-516. [DOI] [PubMed] [Google Scholar]
- Shevchenko, A., Wilm, M., Vorm, O., and Mann, M. (1996). Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850-858. [DOI] [PubMed] [Google Scholar]
- Si, K., Giustetto, M., Etkin, A., Hsu, R., Janisiewicz, A. M., Miniaci, M. C., Kim, J. H., Zhu, H., and Kandel, E. R. (2003). A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in aplysia. Cell 115, 893-904. [DOI] [PubMed] [Google Scholar]
- Stebbins-Boaz, B., Hake, L. E., and Richter, J. D. (1996). CPEB controls the cytoplasmic polyadenylation of cyclin, Cdk2 and c-mos mRNAs and is necessary for oocyte maturation in Xenopus. EMBO J. 15, 2582-2592. [PMC free article] [PubMed] [Google Scholar]
- Tian, J., Kim, S., Heilig, E., and Ruderman, J. V. (2000). Identification of XPR-1, a progesterone receptor required for Xenopus oocyte activation. Proc. Natl. Acad. Sci. USA 97, 14358-14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunquist, B. J., and Maller, J. L. (2003). Under arrest: cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs. Genes Dev. 17, 683-710. [DOI] [PubMed] [Google Scholar]
- Westwick, J. K., Lee, R. J., Lambert, Q. T., Symons, M., Pestell, R. G., Der, C. J., and Whitehead, I. P. (1998). Transforming potential of Dbl family proteins correlates with transcription from the cyclin D1 promoter but not with activation of Jun NH2-terminal kinase, p38/Mpk2, serum response factor, or c-Jun. J. Biol. Chem. 273, 16739-16747. [DOI] [PubMed] [Google Scholar]
- Whitehead, I., Kirk, H., Tognon, C., Trigo-Gonzalez, G., and Kay, R. (1995). Expression cloning of lfc, a novel oncogene with structural similarities to guanine nucleotide exchange factors and to the regulatory region of protein kinase C. J. Biol. Chem. 270, 18388-18395. [DOI] [PubMed] [Google Scholar]
- Whitehead, I. P., et al. (1999). Dependence of Dbl and Dbs transformation on MEK and NF-kappaB activation. Mol. Cell. Biol. 19, 7759-7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens, M., Goodwin, E. B., Kimble, J., Strickland, S., and Hentze, M. (2000). Translational control of developmental decisions. In: Translational Control of Gene Expression, ed. N. Sonenberg, J.W.B. Hershey, and M. B. Mathews, Cold Spring Harbor New York: Cold Spring Harbor Press, 295-370.
- Worthylake, D. K., Rossman, K. L., and Sondek, J. (2000). Crystal structure of Rac1 in complex with the guanine nucleotide exchange region of Tiam1. Nature 408, 682-688. [DOI] [PubMed] [Google Scholar]
- Zhu, Y., Bond, J., and Thomas, P. (2003a). Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc. Natl. Acad. Sci. USA 100, 2237-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y., Rice, C. D., Pang, Y., Pace, M., and Thomas, P. (2003b). Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc. Natl. Acad. Sci. USA 100, 2231-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]