Abstract
Glioblastoma (GBM) is the most common malignant brain tumor in adults with a median survival of 14.6 months. A contributing factor to GBM aggressiveness is the intratumoral expression of the potently immunosuppresive enzyme, indoleamine 2,3 dioxygenase 1 (IDO1). The enzymatic activity of IDO1 is associated with the conversion of tryptophan into downstream kynurenine (Kyn), which has previously been hypothesized to contribute toward the suppression of tumor immunity. Utilizing the syngeneic, immunocompetent, intracranial GL261 cell GBM model, we previously demonstrated that tumor cell-, but not non-tumor cell-IDO1, suppresses T cell-mediated brain tumor regression in mice. Paradoxically, we also showed that the survival advantage mediated by immune checkpoint blockade is abrogated by non-tumor cell IDO1-deficiency. Here, we have built on our past observations and confirm the maladaptive role of tumor cell IDO1 in a novel mouse GBM model. We also demonstrate that, non-tumor cells, rather than mouse GBM cells, are the dominant contributor to IDO1-mediated enzyme activity. Finally, we show the novel associations between maximally-effective immune-checkpoint blockade-mediated survival, non-tumor cell IDO1 and intra-GBM Kyn levels. These data suggest for the first time that, GBM cell-mediated immunosuppression is IDO1 enzyme independent, while the survival benefits of immune checkpoint blockade require non-tumor cell IDO1 enzyme activity. Given that current clinical inhibitors vary in their mechanism of action, in terms of targeting IDO1 enzyme activity versus enzyme-independent effects, this work suggests that choosing an appropriate IDO1 pharmacologic will maximize the effectiveness of future immune checkpoint blockade approaches.
Keywords: IDO1, glioma, tryptophan, kynurenine, immunosuppression, IDO2, TDO2
1. Introduction
Glioblastoma (GBM) is a primary malignant brain cancer in adults with a median overall survival of 14.6 months (1). Patients diagnosed with GBM typically undergo conventional therapy including surgical resection of the tumor, when possible, followed by irradiation and chemotherapy. Even with these aggressive treatments, the overall prognosis remains grim; highlighting the need for more effective and less toxic approaches. To address this clinical necessity, immunotherapy has been proposed as a potential beneficial strategy for GBM patients (2, 3), based on the recent demonstration of improved survival in terminal cancer patients that are otherwise refractory to treatment (4, 5). However, applying immunotherapy to GBM is challenging given the unique immunologic specialization of the central nervous system (CNS) that includes: anatomical barriers limiting lymphatic drainage, low MHC expression levels by parenchymal glial cells and immunosuppressive gene expression that increases with age (6).
Indoleamine 2,3-dioxygense 1 (IDO1) is an interferon-inducible mediator and rate-limiting enzyme associated with the conversion of tryptophan (Trp) into kynurenine (Kyn). This pathway is well-established to play an important role in driving cancer-induced immunosuppression and is a high-profile therapeutic target (7–9). In vitro, IDO1-mediated Trp depletion and/or Kyn accumulation, leads to effector T cell apoptosis (10) and/or naïve CD4+ T cell conversion into inducible regulatory T cells (Tregs; CD4+CD25+FoxP3+) (11). In support of its therapeutic targeting potential, multiple IDO1 enzyme or pathway inhibitors are being tested in clinical trials including epacadostat (Incyte), GDC-0919 (Genentech), PF-06840003 (Pfizer) and indoximod (D1-MT; New Link Genetics), with additional agents undergoing evaluation in the preclinical pipeline.
Our group previously demonstrated that, GBM cell- but not non-tumor cell-IDO1, facilitates increased Treg accumulation and negatively impacts overall survival in a syngeneic, immunocompetent, intracranial mouse GBM model (12). We also demonstrated the paradoxical observation showing that, maximal responsiveness to immune checkpoint blockade requires non-tumor cell IDO1 (13), providing evidence of non-overlapping immunomodulatory roles for IDO1 in GBM versus non-GBM cells. Based on the historical function of IDO1 as a canonical Trp catabolic enzyme, we next hypothesized that, IDO1 enzyme activity is primarily regulated by GBM cells - accounting for the tumor cell IDO1-induced immunosuppression that we previously discovered. Here, we unexpectedly found that, non-tumor cell-, rather than GBM cell-IDO1, predominantly contributes to the tryptophan catabolism in mouse GBM. We then confirmed the relevance of these findings by verifying that GBM-cell IDO1 negatively impacts overall survival in a novel orthotopic mouse glioma model. Finally, we confirm and expand upon the surprising requirement for non-GBM cell IDO1 to achieve maximal survival benefit after treatment with CTLA-4/PD-L1 blockade in mice with experimental brain tumors.
2. Materials and methods
2.1. Mice
Wild-type (WT) C57BL/6 (B6) mice (Cat# 000664) and systemic Ido1 knockout (Ido1−/−, B6 background; Cat# 005897) mice were purchased from Jackson Laboratories, maintained in the Northwestern University Center for Comparative Medicine Facility and intracranially-engrafted between the ages of 6 and 8 weeks. Tamoxifen-inducible transgenic mice that spontaneously develop glioma [tGBM; GFAP(ERT2)→Cre+/−; Ptenfl/fl; Rbfl/fl; p53fl/fl] were generously provided by Dr. Suzanne J. Baker, PhD (St. Jude Children’s Research Hospital, Memphis, TN) on a mixed FVB/B6 background and maintained as previously described (14). To obtain Ido1 deficient tGBM mice, Ido1−/− mice were mated with the tGBM founder line, followed by backcrossing to the founder line for 6 generations. Genotyping to confirm the status of all transgenes (Cre+/−, Ptenfl/fl, Rbfl/fl, p53fl/fl and Ido1−/−) were performed in accordance with established protocols. All mouse strains used in these studies were provided autoclaved food pellets and water ad libitum.
2.2. Primary GBM cell culture, cell proliferation, viability, mAb delivery, RNA isolation, reverse transcription and quantitative PCR (RT-PCR)
Procedures are described in the Supplementary Materials and Methods section.
2.3. Trp and Kyn analysis by high performance liquid chromatography (HPLC)
Procedures for HPLC sample processing and analysis have previously been described (15).
2.4. Statistical analysis
For all quantitative data, the normality of residuals and homogeneity of variances were analyzed with the Shapiro-Wilk test and Fligner-Killeen test, respectively. Two-way ANOVA with Tukey’s multiple comparison test were used for analysis when both assumptions were met, otherwise non-parametric analysis were used instead. Tryptophan (ng/mL) levels were log-transformed and then tested for the normality. Log-rank test was used to assess the statistical significance of Kaplan-Meier survival analysis. Differences were considered to be statistically significant when P < 0.05. Means ± SEM are presented throughout the manuscript.
3. Results
3.1. Non-tumor cell IDO1 predominantly contributes to kynurenine (Kyn) accumulation in mouse GBM
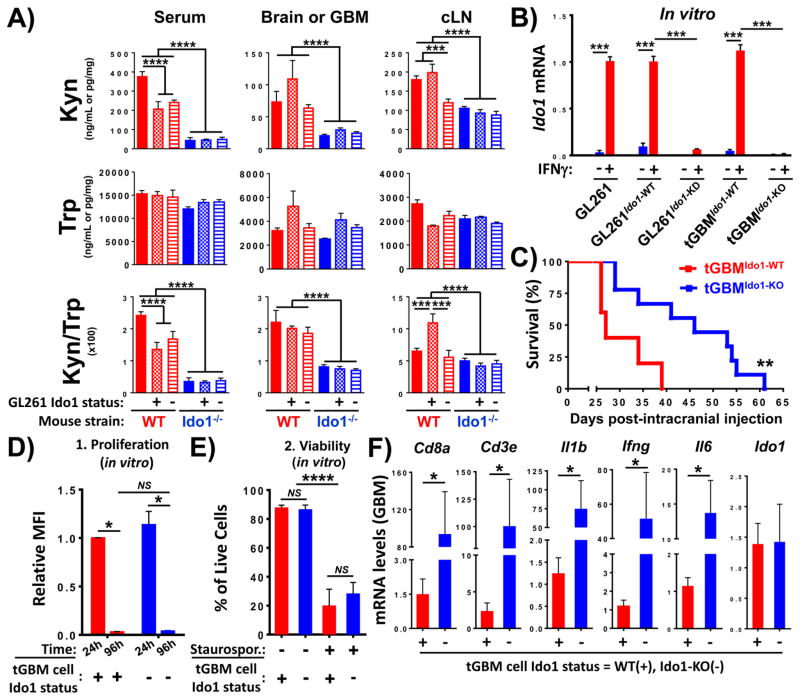
Our previous work demonstrated that IDO1 expression by mouse GL261 GBM cells, but not non-tumor cells, enhances the accumulation of tumor-infiltrating Treg and decreases the survival of brain tumor-bearing mice (12). This led to the hypothesis that, the majority of IDO1-mediated enzyme activity in brain tumors is attributable to GBM cells. To evaluate this hypothesis, Trp and Kyn levels were assessed in naïve mice that did not receive an intracranial injection, or in mice engrafted GL261 cells at 2 weeks post-intracranial injection (2wp-ic.). Universally, wild-type (WT) mice possess a higher Kyn level and Kyn/Trp ratio, when compared to Ido1−/− mice in the serum, naïve brain and brain tumor (Fig. 1A; P<0.001). In contrast, GBM cell IDO1 neither affects Kyn levels nor the Kyn/Trp ratio in mouse brain tumors. Taken together, these data suggest that non-tumor cell-, rather than GBM cell-IDO1, primarily contributes to IDO1 enzyme activity and Kyn accumulation in mouse brain tumors.
Figure 1. The effects of glioblastoma (GBM) cell and non-tumor cell IDO1 on enzyme activity, survival and inflammatory gene expression in a mouse brain tumor model.
(A) WT (red bars) or Ido1−/− (blue bars) mice were not injected (untreated and naïve; solid bars) or intracranially-engrafted 2×105 GL261 cells transduced with lentiviral particles encoding scrambled shRNA (GL261Ido1-WT; hatch-filled bars) or -shRNA specific to IDO1 (GL261 Ido1-KD; horizontal line-filled bars). Tryptophan (Trp) and kynurenine (Kyn) was quantified in the serum (left), uninjected normal brain or GBM (middle) and draining cervical lymph nodes (cLN; right) by HPLC in naïve (n=5/group) or tumor-bearing mice (n=4~7/group) at 2 weeks post-intracranial injection. (B) RT-PCR analysis of Ido1 mRNA expression in unmodified GL261 (GL261), scrambled-shRNA transduced GL261 (GL261Ido1-WT), IDO1-shRNA transduced GL261 (GL261Ido1-KD), as well as tGBMIdo1-WT and tGBMIdo1-KO cells isolated from symptomatic mice with tamoxifen-inducible wild-type [GFAP(ERT2)→Cre+/−; Ptenfl/fl; Rbfl/fl; p53fl/fl] or IDO1-deficient [GFAP(ERT2)→Cre+/−; Ptenfl/fl; Rbfl/fl; p53fl/fl; Ido1−/−] tumors and treated without (blue bars) or with 100 ng/mL mouse IFNγ (red bars) for 48 hours. All mRNA levels were normalized to unmodified GL261 cells at 48h post-stimulation with 100 ng/mL IFNγ. Data compiled from five independent experiments is shown. For each experiment, all groups were run in triplicate. (C) Kaplan-Meier survival analysis of WT FVB/B6 mice intracranially-injected 2×105 tGBMIdo1-WT (n=5) or tGBMIdo1-KO cells (n=9). One representative dataset from two experiments is shown. In vitro characterization of WT (red bars) and Ido1−/− (blue bars) tGBMIdo1-WT and tGBMIdo1-KO cells for proliferation (D) and viability (E). For viability studies, staurosporine (Staurospor.) was utilized to induce cell death. Cells negative for the Zombie and Annexin V dyes were gated as living cells. For both proliferation and viability assays, the quantitative data compiled from five independent experiments is shown. For each experiment, all groups were run in triplicate. (E) RT-PCR mRNA analysis of intratumoral gene expression for markers associated with T cells, proinflammatory cytokines and Ido1 in brain tumors isolated from WT FVB/B6 mice intracranially-injected 2×105 tGBMIdo1-WT (n=8) or tGBMIdo1-KO cells (n=8) at 3 weeks post-engraftment. Gene expression levels were compared to samples isolated from mice engrafted tGBMIdo1-WT cells. *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001.
To address the IDO1 expression potential in mouse GBM, unmodified- or GL261-cells transduced with scrambled shRNA (GL261Ido1-WT) or IDO1-targeted shRNA (GL261Ido1-KD) (16) were cultured with or without mouse IFNγ; a potent inducer of IDO1 expression (Fig. 1B) (17). Additionally, novel mouse GBM cell lines isolated from a tamoxifen-inducible mouse model of GBM [GFAP(ERT2)Cre+/−;p53fl/fl;pTENfl/fl;Rbfl/fl (14)] that was WT (tGBM Ido1-WT) or backcrossed with Ido−/− mice (tGBMIdo1-KO), were also analyzed for IDO1 expression potential. Mouse IDO1 mRNA was significantly increased in all WT mouse GBM cell lines, confirming the capability to express IDO1 (P<0.001). In contrast, all glioma lines targeted for IDO1 gene knockdown/deletion showed a ≥90% decrease in IDO1 mRNA expression (P<0.001).
To confirm the negative impact of IDO1 in GBM cells, which we previously evaluated in the GL261 brain tumor model (16), FVB/B6 mice were intracranially-engrafted novel tGBM Ido1-WT and tGBMIdo1-KO mouse glioma cells (Fig. 1C). The median survival of mice with IDO1-WT glioma is 27 days, which is significantly decreased when compared to mice with IDO1-deficient glioma that possessed a median survival of 46 days (P<0.01). Importantly, the rate of proliferation (MFI; mean fluorescence intensity) (Fig. 1D) and apoptosis (% cell viability) (Fig. 1E) was not different between the tGBM Ido1-WT and tGBMIdo1-KO mouse glioma cells, in vitro. These data confirm the maladaptive nature of IDO1 expression in an independent, immunocompetent mouse glioma model and suggest that the differences in survival are unrelated to tumor intrinsic cell growth or death properties and rather, likely represent an immune-mediated anti-tumor effect.
To characterize the immune regulation that might account for the differences in survival between mice engrafted tGBMIdo1-WT and tGBMIdo1-KO glioma cells, tumors were resected at 3 weeks post-intracranial engraftment and analyzed for mRNA expression of T cell-associated markers, Cd8a and Cd3e, as well as the proinflammatory cytokines, Il1b, Ifng and Il6 (Supplementary Table 1). All immune indicators were significantly decreased in the IDO1 WT-, when compared to the IDO1-deficient-brain tumor (P<0.05, respectively). Notably, the intratumoral IDO1 mRNA expression level was unchanged between mice engrafted tGBMIdo1-WT and tGBMIdo1-KO glioma cells, likely reflecting the compensatory IDO1 mRNA expression by non-tumor cells in the glioma microenvironment. Collectively, these data show a correlation between increased inflammatory gene expression and a benefit to survival in immunocompetent mice with intracranial GBM cell-IDO1 deficiency.
3.2. Immune checkpoint blockade-mediated survival and intracranial mouse GBM kynurenine (Kyn) levels
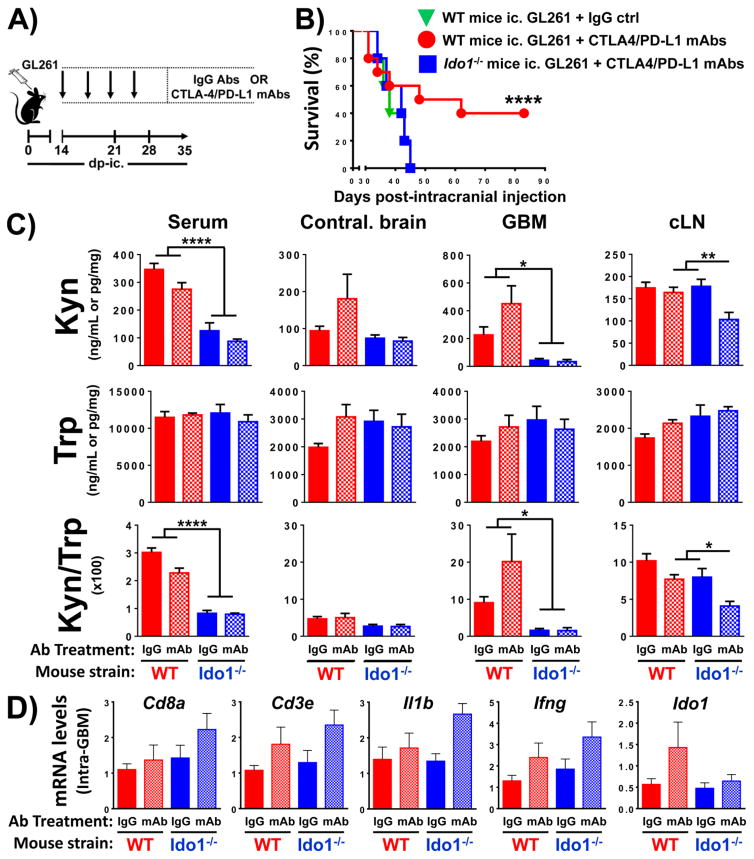
Previous work in a peripheral (outside of the CNS) B16 mouse melanoma model demonstrated that the therapeutic effects of CTLA-4- or PD-1-mediated immune checkpoint blockade, synergize with germline Ido1 deletion in mice (18). In contrast, we previously showed that the dual inhibition of CTLA-4/PD-L1 is less effective at achieving an overall survival benefit in Ido1−/−, when compared to WT mice with GL261 brain tumors (19). To further explore the requirement for germline IDO1 to achieve a maximal survival benefit from immune checkpoint therapy against brain tumors, WT and Ido1−/− mice engrafted unmodified GL261 were administered Syrian hamster IgG and rat IgG2B or CTLA-4 and PD-L1 mAbs at 2 weeks post-intracranial injection (Fig. 2A). While WT mice treated with CTLA-4/PD-L1 mAbs have a median survival of 55 days, Ido1−/− mice undergoing similar treatment possess a decreased median survival of 38 days (P<0.0001), similar to WT mice treated with IgG control antibodies (Fig. 2B). Notably, the CTLA-4/PD-L1-mediated survival benefit was durable in a subset of WT mice, while no Ido1−/− mice survived >45 days post-GBM cell engraftment (Fig. 2B). These data confirm the unexpected requirement for non-tumor cell IDO1 to achieve maximal survival in response to immune checkpoint blockade in mice with experimental GBM.
Figure 2. The effects of CTLA-4/PD-L1 blockade on survival, enzyme activity and inflammatory gene expression in wild-type (WT) and IDO1-deficient (Ido−/−) mice engrafted mouse glioblastoma (GBM).
(A) A timeline indicating a dosing schedule of control IgG antibodies (Abs) or CTLA-4 and PD-L1 mAbs. (B) Kaplan-Meier survival analysis of WT mice intracranially-engrafted 2×105 unmodified GL261 cells and treated with control IgG (green triangle, n=5) or CTLA-4 and PD-L1 mAbs (red circle, n=10). Additionally, Ido1−/− mice were engrafted 2×105 unmodified GL261 cells and treated with CTLA-4 and PD-L1 mAbs (blue square, n=10). One representative dataset from two independent experiments is shown. (C) WT (red bars) or Ido1−/− (blue bars) mice were intracranially-engrafted 2×105 GL261 cells followed by the treatment with control IgG (solid bars) or CTLA-4/PD-L1 mAbs (hatch-filled bars). Tryptophan (Trp) and kynurenine (Kyn) levels were measured at 3 weeks post-intracranial engraftment in the serum, contralateral brain without tumor from a tumor-bearing mouse (Contral. brain), GL261 GBM lysate and draining cervical lymph nodes (cLN) by HPLC. WT mice + IgG (n=10); WT mice + CTLA-4/PD-L1 mAbs (n=11); Ido1−/− mice + IgG (n=5); Ido1−/− mice + CTLA-4/PD-L1 mAbs (n=6). (D) RT-PCR analysis of intratumoral gene expression for markers associated with T lymphocytes, proinflammatory cytokines or Ido1 at 3 weeks post-intracranial engraftment of tumor cells isolated from WT and Ido1−/− mice intracranially-engrafted 2×105 unmodified GL261 and treated as shown in (A). Sample size is same as in (C). *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001.
Based on the paradoxical requirement for non-tumor cell IDO1 to achieve maximal therapeutic effectiveness with immune checkpoint blockade, we next explored the role of IDO1 enzyme activity and inflammatory gene expression in the experimental mouse GBM model. These findings, in combination with our other observations demonstrating the role of non-tumor cell IDO1 as a primary contributor to enzyme activity (Fig. 1A), led us to hypothesize that, non-tumor cell IDO1 contributes an essential pool of Kyn that facilitates responsiveness to immune checkpoint blockade. To pursue this question, Trp and Kyn levels were quantified in the sera, contralateral hemisphere without brain tumor (Contral. brain), GBM and the draining cervical lymph nodes in mice at 3 weeks post-GL261 cell engraftment (Fig. 2C). Notably, the mouse treatment schedule for administration of control IgG Abs and CTLA-4/PD-L1 mAbs were in accordance with the time line previously described (Fig. 2A). Trp levels are not affected by immune checkpoint inhibition regardless of the germline IDO1 status in mice. However, Kyn levels and the Kyn/Trp ratio are significantly increased in the sera and GBM of WT when compared to Ido1−/− mice (P<0.05, respectively). In contrast, there is no change in the Kyn levels and Kyn/Trp ratio of contralateral brain isolated from GBM-bearing WT and Ido1−/− mice, suggesting that the tryptophan catabolic effects of immune checkpoint blockade selectively affect different anatomical compartments in subjects with brain tumors. Unexpectedly, immune checkpoint blockade had no significant effect on intratumoral inflammatory gene expression associated with markers expressed by T cells, Cd8a and Cd3e, as well as the proinflammatory cytokines, Il1b and Ifng (Fig. 2D). Interestingly, while we observed a trend for increased intratumoral Ido1 expression in WT mice treated with immune checkpoint blockade, there was no trend in Ido1−/− mice, suggesting the possibility that CTLA-4/PD-L1 inhibition marginally increases IDO1 expression in non-tumor cells. Collectively, these data suggest the hypothesis whereby immune checkpoint inhibition requires increased Kyn levels, provided by non-tumor cell IDO1, to achieve maximal immune-mediated antitumor effects against GBM.
4. Discussion
The canonical function of IDO1 is associated with its ability to catalyze the conversion of Trp into Kyn, leading to the inhibition of T cell effector functions (20–22). However, not all immunosuppressive aspects of IDO1 can be explained through the Trp depletion/Kyn accumulation theory. Indoximod (D1-MT) is a non-enzymatic IDO1 pathway inhibitor (8, 23) that is currently in clinical trials, having previously shown antitumor activity in preclinical cancer models when combined with standard of care approaches (24). Additionally, mouse plasmacytoid DCs (pDCs) treated with transforming growth factor-β (TGF-β) cause IDO1 to mediate tolerogenic effects in T cells that are mechanistically independent from its enzymatic activity (25). The results from our current study provide further support toward the hypothesis that, IDO1 possesses a non-enzymatic, immunosuppressive function in mouse GBM cells. This is based on GBM cell IDO1 facilitating Treg accumulation and limiting mouse survival (16) through an IDO1 enzyme-independent mechanism (Fig. 1A), combined with non-GBM cell IDO1 possessing no effect on mouse survival (16) despite its primary contribution toward increasing Kyn levels in mouse GBM (Fig. 1A).
Given the current enthusiasm of combinatorial immunotherapy for the treatment of cancer, the results from this study demonstrate the importance of understanding the effects of different IDO1 targeting approaches that may vary in effectiveness, depending on the type of cancer being investigated. Our findings highlight the importance of developing future strategies that inhibit the noncanonical IDO1 activity in GBM cells, without disrupting the non-tumor cell IDO1-mediated enzyme activity associated with responsiveness to immune checkpoint blockade (Fig. 2B,C). We do recognize that our study is limited by the evaluation of single time points after tumor engraftment, rather than a rigorous time course analysis of Trp/Kyn and inflammatory gene expression levels. In the future, we also hope to better understand the noncanonical IDO1 effects in mouse GBM cells. To address this, while keeping in-mind the spontaneous tumor rejection that occurs in immunocompetent mice engrafted GL261Ido1-KD cells, our group has developed Ido1−/−Rag1−/− double knockout mice. This model will support the future evaluation of comparing IDO1 activity in the absence of GBM-infiltrating T cells, which is critical for comparing to the previous findings of Grohmann et al. reporting that, CTLA-4-induced immunotolerance is contingent upon effective Trp catabolism in dendritic cells (26). Given that DCs infiltrate intracranial GBM (27), it becomes more likely that non-tumor cells including microglia, macrophages and/or DCs, require IDO1 to mediate enzyme-dependent responsiveness to immune checkpoint inhibition.
It was surprising to find increased intratumoral mRNA expression for T cell markers and proinflammatory cytokines in mice that survive longer when engrafted IDO1-deficient GBM cells (Fig. 1C,F), but not in WT mice treated with CTLA-4/PD-L1 mAbs that also possess a substantial survival advantage (Fig. 2B,D). It is therefore important to keep in-mind our novel data showing that mouse GBM cells do not significantly contribute to IDO1-mediated Kyn accumulation in brain tumors (Fig. 1A), while the non-tumor cell IDO1 is required for maximal therapeutic response to CTLA-4/PD-L1 blockade (Fig. 2B) and associated with higher Kyn levels in WT-, when compared to Ido1−/−-mice engrafted GBM (Fig. 2C). Based on these collective observations, we hypothesize that the increased survival in mice intracranially-engrafted IDO1-deficient GBM cells reflects the inability of tumor cells to suppress T cell-mediated cytolytic effects. We further hypothesize that the diminished therapeutic response to CTLA-4/PD-L1 mAb treatment in Ido1−/− mice reflects the unexpected requirement for tryptophan catabolism to fuel T cell-mediated tumor rejection in brain tumors; independent of T cell infiltration and proinflammatory cytokine expression.
One surprising observation that was unexpected is the significantly increased Kyn/Trp ratio in draining cervical lymph nodes (cLN) of WT mice engrafted GL261Ido1-WT- when compared to GL261Ido1-KD glioma (Fig. 1A). Given that mice with GL261Ido1-WT brain tumors possess significantly increased Treg levels when compared to GL261Ido1-KD (16), it is possible that the increased Kyn/Trp ratio is somehow associated with enhanced Treg generation in the cLN. However, we find this possibility unlikely, given that there is no increase in the Kyn/Trp ratio in Ido1−/− mice engrafted IDO1-competent GL261 glioma, which also results in tumor-infiltrating Treg accumulation (16). Another interesting finding was the increased Kyn/Trp ratio in the GBM of WT when compared to Ido1−/− mice (Fig. 2C), which did not occur in the contralateral brain without tumor. This suggests that under normal conditions, IDO1 activity is neglible in the CNS, but is significantly increased by traumatic and/or proinflammatory events such as GBM development.
Taken together, our data demonstrate for the first time that GBM cell IDO1 suppresses tumor immunity through a mechanism independent of intratumoral Trp→Kyn catabolism in an immunocompetent, experimental mouse model of malignant glioma. In contrast, non-tumor cell IDO1 is required for the maximal therapeutic effect of immune checkpoint blockade and is associated with increased intratumoral Kyn levels in WT, when compared to Ido1−/− mice. Since the GL261 cell lines, techniques and time points utilized in the current study are identical to those that were previously reported(16), these findings are in alignment with the intriguing hypothesis that, IDO1 regulates brain tumor immunity through distinctly different mechanisms depending on the CNS cell type under consideration. Given that most IDO1 targeting strategies focus on inhibiting enzyme activity (28), our findings also provide novel considerations for designing second generation inhibitors that target the ill-defined immunosuppressive aspects of IDO1 in tumor cells. Ultimately, these data provide strong rationale for the continued pursuit of determining how IDO1 suppresses the immune response in malignant brain tumors and an extension and expansion of these analyses into human glioblastoma.
Supplementary Material
Supplementary Table S1. Quantitative PCR primer information
HIGHLIGHTS.
Non-tumor cell IDO1 predominantly contributes to Kyn accumulation in mouse GBM.
Immune checkpoint blockade requires IDO1 for maximal survival against mouse GBM.
Checkpoint-mediated survival is associated with higher Kyn levels in WT compared to IDO1KO mice.
Characterization of new glioma cell lines from wildtype and IDO1-deficient mice.
Acknowledgments
This work was supported NIH grants K99 NS082381 (D.A.W.), R00 NS082381 (D.A.W.), R01 MH101145 (RHM) and R01 NS097851-01 (D.A.W.), Robert H. Lurie Comprehensive Cancer Center – Zell Scholar Program of the Zell Family Foundation Gift (D.A.W.) and Cancer Research Institute – Clinic and Laboratory Integration Program. We thank Dr. Suzanne J. Baker, Ph.D. (St. Jude Children’s Research Hospital) for generously donating her tamoxifen-inducible mouse glioma model for these studies.
Footnotes
Conflict of Interest Disclosure: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Reardon DA, Gilbert MR, Wick W, Liau L. Immunotherapy for neuro-oncology: the critical rationale for combinatorial therapy. Neuro-oncology. 2015;17(Suppl 7):vii32–vii40. doi: 10.1093/neuonc/nov178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binder DC, Davis AA, Wainwright DA. Immunotherapy for cancer in the central nervous system: current and future directions. Oncoimmunology. 2015 doi: 10.1080/2162402X.2015.1082027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladomersky E, Genet M, Zhai L, Gritsina G, Lauing KL, Fangusaro J, Lulla RR, Lenzen A, Kumthekar P, Raizer JJ, Binder DC, James CD, Wainwright DA. Improving Vaccine Efficacy Against Malignant Glioma. Oncoimmunology. 2016 doi: 10.1080/2162402X.2016.1196311. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munn DH. Blocking IDO activity to enhance anti-tumor immunity. Frontiers in bioscience (Elite edition) 2012;4:734–45. doi: 10.2741/e414. [DOI] [PubMed] [Google Scholar]
- 8.Zhai L, Spranger S, Binder DC, Gritsina G, Lauing KL, Giles FJ, et al. Molecular Pathways: Targeting IDO1 and Other Tryptophan Dioxygenases for Cancer Immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015 doi: 10.1158/1078-0432.CCR-15-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature medicine. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 10.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, et al. T cell apoptosis by tryptophan catabolism. Cell death and differentiation. 2002;9:1069–77. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 11.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–8. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon K-S, Auffinger B, et al. IDO Expression in Brain Tumors Increases the Recruitment of Regulatory T Cells and Negatively Impacts Survival. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:6110–21. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim C, Tobias AL, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clinical Cancer Research. 2014 doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow LM, Endersby R, Zhu X, Rankin S, Qu C, Zhang J, et al. Cooperativity within and among Pten, p53, and Rb pathways induces high-grade astrocytoma in adult brain. Cancer Cell. 2011;19:305–16. doi: 10.1016/j.ccr.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhai L, Dey M, Lauing KL, Gritsina G, Kaur R, Lukas RV, et al. The kynurenine to tryptophan ratio as a prognostic tool for glioblastoma patients enrolling in immunotherapy. J Clin Neurosci. 2015 doi: 10.1016/j.jocn.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:6110–21. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlin JM, Borden EC, Sondel PM, Byrne GI. Biologic-response-modifier-induced indoleamine 2,3-dioxygenase activity in human peripheral blood mononuclear cell cultures. J Immunol. 1987;139:2414–8. [PubMed] [Google Scholar]
- 18.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. The Journal of experimental medicine. 2013;210:1389–402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim C, Tobias AL, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:5290–301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 21.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science (New York, NY) 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 22.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lob S, Konigsrainer A, Zieker D, Brucher BL, Rammensee HG, Opelz G, et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer immunology, immunotherapy : CII. 2009;58:153–7. doi: 10.1007/s00262-008-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyltryptophan correlates with antitumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 25.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–8. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 26.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 27.Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS medicine. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Röhrig UF, Majjigapu SR, Vogel P, Zoete V, Michielin O. Challenges in the Discovery of Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitors. Journal of Medicinal Chemistry. 2015 doi: 10.1021/acs.jmedchem.5b00326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Quantitative PCR primer information