Abstract
Intravenous (i.v.) injection of a soluble myelin antigen can induce tolerance, which effectively ameliorates experimental autoimmune encephalomyelitis (EAE). We have previously shown that i.v. MOG induces tolerance in EAE and expands a subpopulation of tolerogenic CD11c+CD11b+ dendritic cells (DCs) with an immature phenotype having low expression of IA and co-stimulatory molecules CD40, CD86, and CD80. Here we further investigate the role of tolerogenic DCs in i.v. tolerance by injecting clodronate-loaded liposomes, which selectively deplete CD11c+CD11b+ and immature DCs, but not CD11c+CD8+ DCs and mature DCs. I.v. MOG-induced suppression of EAE was partially, yet significantly, blocked by CD11c+CD11b+ DC depletion. While i.v. MOG inhibited IA, CD40, CD80, CD86 expression and induced TGF-β, IL-27, IL-10 production in CD11c+CD11b+ DCs, these effects were abrogated after injection of clodronate-loaded liposomes. Depletion of CD11c+CD11b+ DCs also precluded i.v. autoantigen-induced T-cell tolerance, such as decreased production of IL-2, IFN-γ, IL-17 and numbers of IL-2+, IFN-γ+, and IL-17+ CD4+ T cells, as well as an increased proportion of CD4+CD25+Foxp3+ regulatory T cells and CD4+IL-10+Foxp3− Tr1 cells. CD11c+CD11b+ DCs, through low expression of IA and co-stimulatory molecules as well as high expression of TGF-β, IL-27 and IL-10, play an important role in i.v. tolerance-induced EAE suppression.
Keywords: Dendritic cell, Experimental autoimmune encephalomyelitis, Multiple sclerosis, immune tolerance
Introduction
Multiple sclerosis (MS) is a T-cell mediated autoimmune disease of the central nervous system (CNS) that is manifested clinically as weakness and progressive paralysis [1]. Experimental autoimmune encephalomyelitis (EAE), induced by immunization of susceptible mouse strains with myelin oligodendrocyte glycoprotein peptides (MOG) or other myelin components, provides a useful animal model for MS research [2, 3]. Myelin-reactive encephalitogenic Th1 and Th17 cells are critically involved in the initiation and development of EAE [4, 5]. On the other hand, Th2, regulatory T cells (Treg cells) and Tr1 cells are considered protective [6].
Intravenous (i.v.) injection of a soluble myelin antigen that has been used for EAE induction leads to the antigen-specific tolerance, which effectively ameliorates EAE [7]. Clonal deletion and anergy of antigen-specific Th1/Th17 cells and induction of regulatory T cells are the main mechanisms involved in the induction of i.v. tolerance [8].
Antigen presenting cells (APCs), including macrophages and dendritic cells (DCs), are important for Th cell differentiation [9, 10]. APCs provide Th cells not only with antigen stimulation (Signal 1) and co-stimulatory signals (Signal 2), but also with additional polarizing signals (Signal 3), such as inflammatory cytokines IL-12 (for Th1), IL-23 (for Th17), and immunomodulatory cytokines TGF-β, IL-27 and IL-10 (for T regulatory cells) [9, 11, 12]. DCs, as the most potent professional APCs, play an essential role in Th cells differentiation and thus are involved in the induction of tolerance [13, 14]. We have shown that i.v. MOG-induced tolerance in EAE mice is associated with an increased proportion of the CD11c+CD11b+ subpopulation of DCs, while a higher proportion of the CD11c+CD8+ subpopulation was observed in non-tolerized EAE mice [15]. These CD11c+CD11b+ DCs exhibited an immature phenotype, produced high levels of IL-10 and TGF-β, and effectively suppressed EAE upon adoptive transfer, demonstrating their tolerogenic nature [15].
In the present study, we have evaluated the role of CD11c+CD11b+ DCs in i.v. tolerance induction in EAE by depleting this DC population. Clodronate-loaded liposomes selectively deplete immature DCs (iDCs), but only minimally affect the mature DC population [16]. As CD11c+CD11b+ DCs exhibited an immature phenotype, we intraperitoneally (i.p.) injected clodronate-loaded liposomes to deplete CD11c+CD11b+ DCs in MOG-i.v. treated EAE mice and then defined their outcome clinically and immunologically. Indeed, the effects of i.v. MOG-injected tolerance were significantly abrogated after CD11c+CD11b+ DC depletion, demonstrating an important role of this DC population in i.v. tolerance induction.
RESULTS
Clodronate-loaded liposomes selectively deplete CD11c+CD11b+ DCs or iDCs
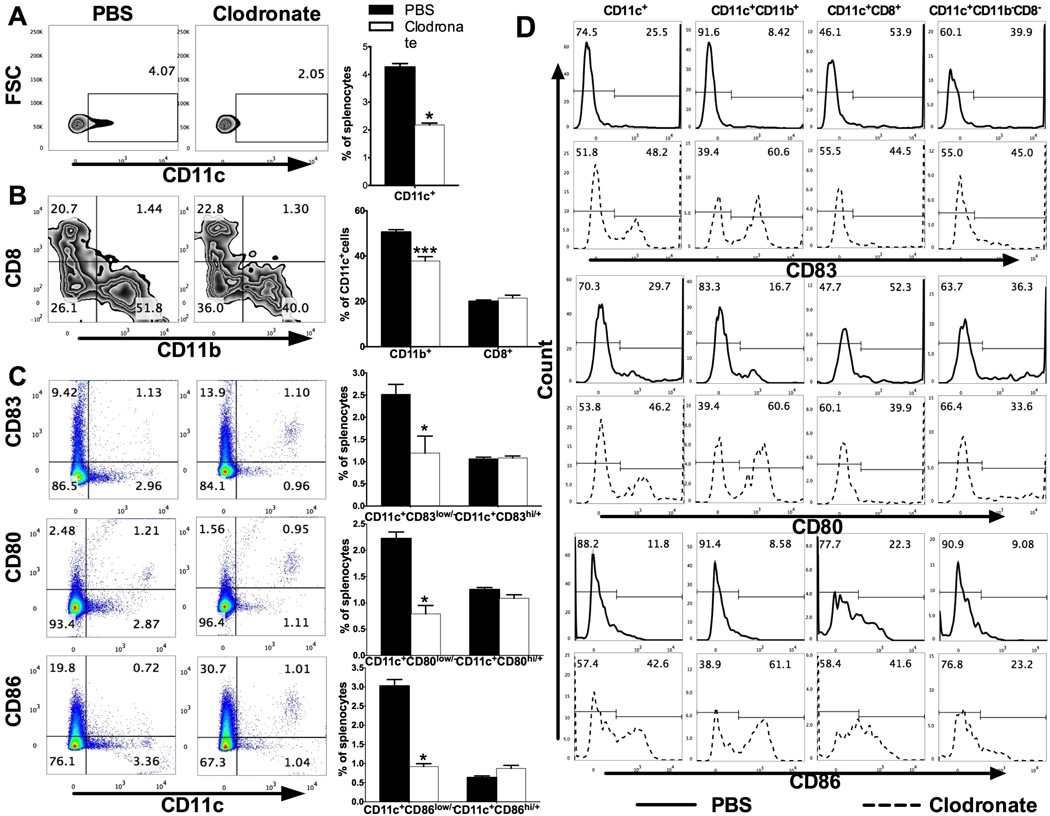
We first tested the efficacy of iDC depletion. Clodronate- or PBS-loaded liposomes were i.p. injected into naïve C57BL/6 mice; splenocytes were harvested 24 hours later and analyzed by flow cytometry as described [17]. Compared with PBS-loaded liposomes, clodronate-loaded liposomes largely depleted the DCs (CD11c+ cells, 4.07% vs. 2.05% among total splenocytes) and F4/80+ macrophages (16.0% vs. 4.67%; data not shown). When CD11b and CD8 markers were analyzed in gated CD11c+ DCs, a reduced percentage of CD11b+ (51.8% vs. 40.0%, P<0.001) was observed, with virtually no change in the CD8+ (20.7% vs. 22.8%) and an increased percentage of CD11b− CD8− DCs (Fig. 1A, B). Compared to those in PBS-treated mice, the absolute numbers of total CD11+ DCs and CD11b+CD11c+ DCs per spleen were significantly reduced in clodronate-treated mice (1.34±0.02 × 106 vs. 0.88±0.01 × 106, P<0.01; 0.70±0.01 × 106 vs. 0.36±0.01 ×106, P<0.001, respectively). The absolute number of CD8+CD11c+ DCs was also reduced (0.27±0.01 × 106 vs. 0.21±0.01 ×106, P<0.01), while there was no significant difference for the numbers of CD11b−CD8−CD11c+ DCs between the two groups (0.34±0.01 × 106 vs. 0.32±0.01 × 106). Thus, while clodronate-loaded liposomes reduced the numbers of total DCs and all DC subpopulations, the major reduction was in the CD11b+CD11c+ population.
Fig. 1. Clodronate-loaded liposomes selectively deplete CD11c+CD11b+ DCs or iDCs.
Naïve C57BL/6 mice were i.p. injected with PBS-loaded or clodronate-loaded liposomes, splenocytes were harvested at 24 hours later and analyzed with flow cytometry. (A) Percentages of DCs (CD11c+) among splenocytes (left). Results were statistically analyzed and shown as mean ± SEM (n=3 each group) (right). (B) CD11c+ cells were then gated and percentages of CD11b+ and CD8+ cells were analyzed (left). Results were statistically analyzed and shown as mean ± SEM (n=3 each group) (right). (C) Expression of CD11c vs. CD83, CD80, and CD86 in splenocytes (left). Results were statistically analyzed and shown as mean ± SEM (n=3 each group) (right). (D) Expression of CD83, CD80, and CD86 in total CD11c+ cells and in CD11b+, CD8+, CD11b−CD8− cells of gated CD11c+ cells. *p<0.05, ***p<0.001, unpaired Student’s t test. One representative of 3 experiments is shown.
Clodronate-loaded liposomes selectively reduced numbers (~3 fold) of CD83low/−, CD80low/− and CD86low/− iDCs, but minimally affected the CD83hi/+, CD80hi/+ and CD86hi/+ mature DCs (Fig. 1C). Mature markers of DCs, CD83, CD80, and CD86 in total CD11c+ cells and in CD11b+, CD8+, CD11b−CD8− cells of gated CD11c+ cells were analyzed. As shown in Fig. 1D, CD11c+CD11b+ cells contained many more immature DCs and were reduced by clodronate (CD83low/−: 91.6% vs. 39.4%, CD80low/−: 83.3% vs. 39.4%, CD86low/−: 91.4 vs. 38.9%), while these markers on CD8+, CD11b−CD8− DCs either remained unchanged or were slightly reduced. Thus, these data confirm an immature phenotype of CD11c+CD11b+ DCs, and the efficacy of clodronate-loaded liposomes in depleting these DCs.
Clodronate-loaded liposomes partially block i.v. MOG-induced EAE suppression
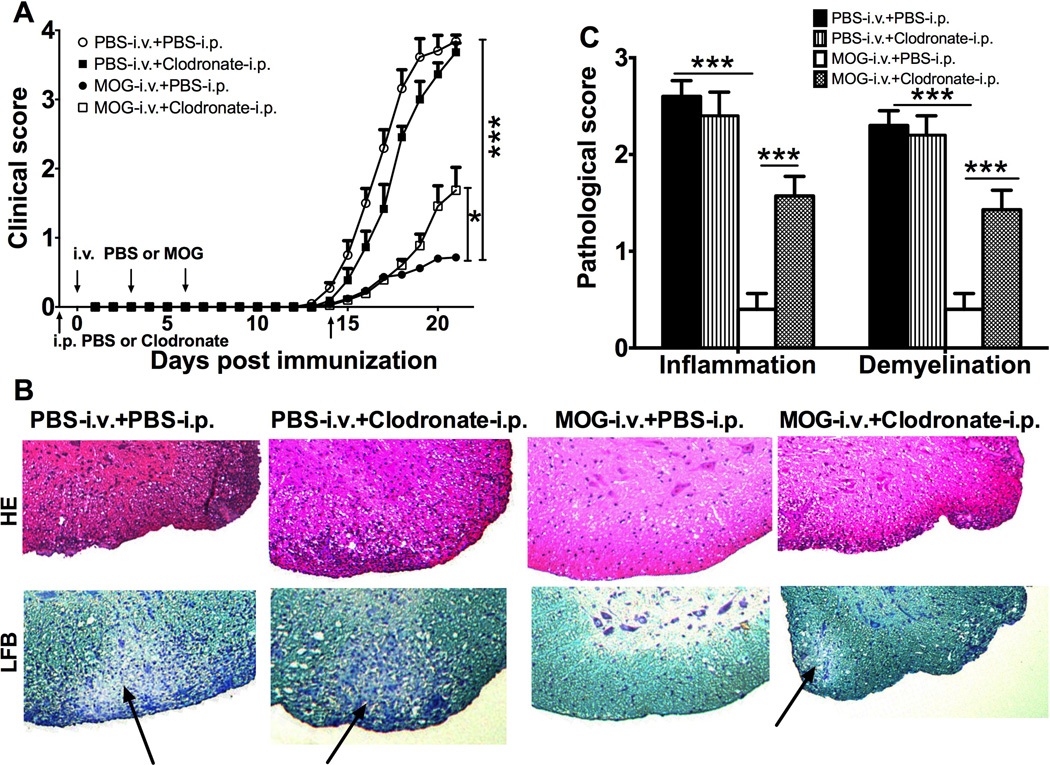
We investigated the effect of clodronate-loaded liposomes in i.v. tolerance induction in EAE. Mice were immunized with MOG35–55 in CFA to induce EAE. MOG35–55 peptide at 200 µg/mouse was i.v. injected to induce tolerance at days 0, 3, and 6 post immunization (p.i.), and PBS-i.v. served as control. Clodronate-loaded liposomes were i.p. injected 24 hours before and 14 days p.i., while mice that received PBS-loaded liposomes served as control. The onset of disease in control EAE mice (PBS-i.v. +PBS-i.p.) was observed at day 12–14 p.i., reaching the maximum clinical score by day 21 p.i. In contrast, a mild clinical signs of EAE were observed in MOG-i.v. tolerized mice (MOG-i.v. +PBS-i.p.). Injection of clodronate-loaded liposomes to tolerized mice (MOG-i.v.+clodronate-i.p.) partially, but significantly, blocked i.v. MOG-induced EAE suppression (Fig. 2A). While injection of clodronate-loaded liposome to untreated EAE mice (PBS-i.v. +clodronate-i.p.) did not influence clinical scores (Fig. 2A).
Fig. 2. Clodronate-loaded liposome partly block i.v. MOG-induced EAE suppression.
Mice were immunized with MOG35–55 in CFA to induce EAE. MOG35–55 or PBS was i.v. injected at days 0, 3, and 6 p.i. Clodronate- or PBS-loaded liposome was i.p. injected 24 hours before and 14 days p.i. (A) Clinical signs were scored daily following a 0–5 scale. Data were pooled from three independent experiments and presented as mean clinical score ± SEM (n=11–15 each group). (B) On day 21 p.i., spinal cords were harvested and 5-µm sections were stained with H&E and LFB. Demyelination areas are designated by arrows. (Magnifications, × 10). (C) Inflammation and demyelination were scored using a 0–3 scale and expressed as mean scores ± SEM (n=3 each group). *p<0.05, ***p<0.001, two-way ANOVA test. One representative of 3 experiments is shown.
Consistent with clinical signs, extensive inflammatory infiltrates and demyelination in the white matter of spinal cords were seen in untreated EAE mice, while they had been significantly reduced in tolerized mice (Fig. 2B, C). On the other hand, injection of clodronate-loaded liposomes to tolerized mice significantly increased their inflammation and demyelination scores, while the same injection did not affect scores in EAE mice (Fig. 2B, C). Thus, clodronate-loaded liposomes blocked i.v. MOG-induced inhibition of EAE both in clinical severity as well as CNS inflammation and demyelination.
DCs induced by i.v. tolerance have iDC phenotype, and are depleted by clodronate-loaded liposomes
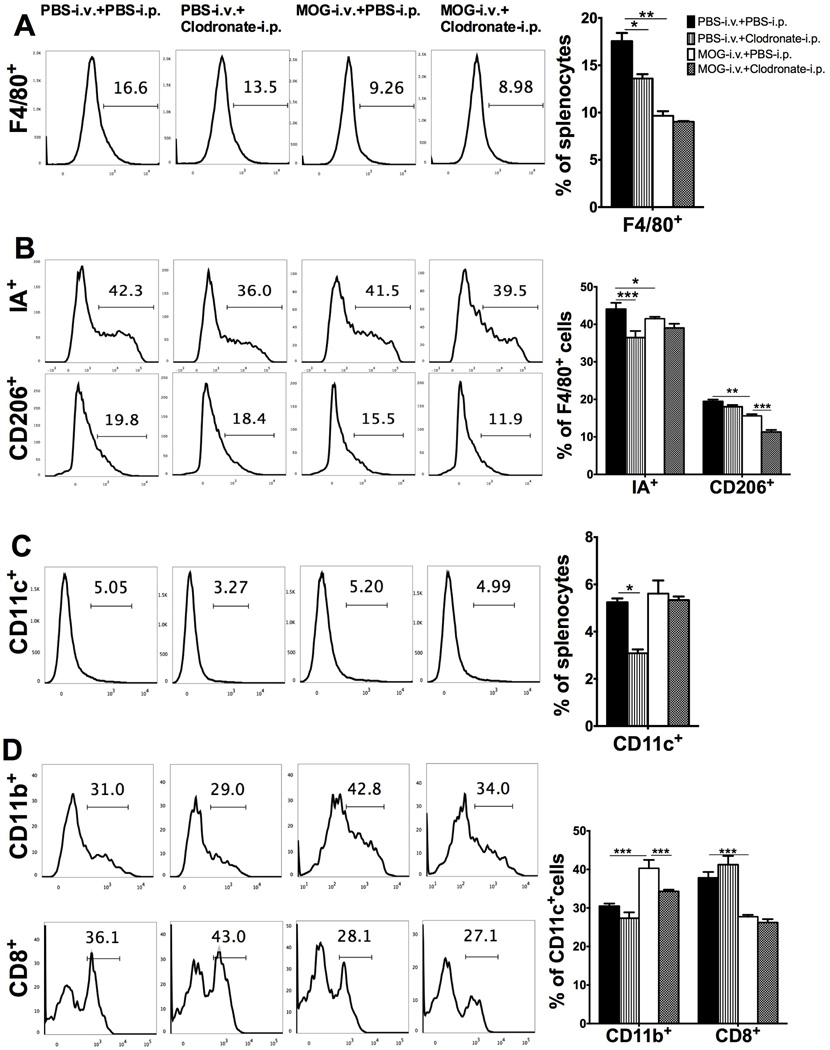
To test the depletion effect of clodronate-loaded liposomes on DC phenotype of EAE mice in vivo, splenocytes were isolated at day 21 p.i. and analyzed by flow cytometry. The percentages of macrophages (F4/80+ cells) and DCs (CD11c+ cells) in spleen cells of PBS-i.v.+clodronate-i.p. mice were lower than in untreated EAE (PBS-i.v.+PBS-i.p.) mice. Compared with untreated EAE mice, MOG-i.v. tolerized mice had decreased percentages of total macrophages (F4/80+; Fig. 3A) and both type 1 (M1; F4/80+IA+) and type 2 (M2; F4/80+CD206+) subtypes of these cells (Fig. 3B). Similarly, the proportion of CD11c+CD11b+ DCs was increased in tolerized mice, while this increase was significantly reduced after clodronate-loaded liposome injection (Fig. 3C, D). These results indicate that clodronate-loaded liposomes depleted both M2 macrophages and tolerogenic DCs in i.v. tolerized mice, and this effect may contribute to the observed blockade of MOG-induced EAE suppression. Interestingly, two reports showed that intracerebroventricular injection of clodronate-loaded liposomes effectively eliminated perivascular macrophages within the CNS [18, 19]. Whether i.v. injection of clodronate-loaded liposomes in our experiments had similar effect, and whether this contributed to the blockade of i.v. tolerance induction, remains to be determined in future studies.
Fig. 3. I.v. tolerance induced increase of CD11c+CD11b+ DCs is abrogated by injection of clodronate-loaded liposomes.
Mice immunized for EAE induction were i.v. injected with MOG or PBS, while PBS-loaded or clodronate-loaded liposomes were i.p. injected. Splenocytes were isolated 21 days p.i. and analyzed by flow cytometry. (A) FACS profile of macrophages (F4/80+ cells) in total splenocytes (left). Results were statistically analyzed and shown as mean ± SEM (n=3 each group) (right). (B) M1 (IA+) and M2 (CD206+) macrophages in gated F4/80+ cells (left). Results were statistically analyzed and shown as mean ± SEM (n=3 each group) (right). (C) DCs (CD11c+ cells) in total splenocytes (left). Results were statistically analyzed and shown as mean ± SEM (n=3 each group) (right). (D) CD11c+CD11b+ DCs (CD11b+ cells in gated CD11c+ cells) and CD11c+CD8+ DCs (CD8+ cells in gated CD11c+ cells) (left). Results were statistically analyzed and data shown as mean ± SEM (n=3 each group). *p<0.05, ** p<0.01, ***p<0.001, two-way ANOVA test. One representative of 3 experiments is shown.
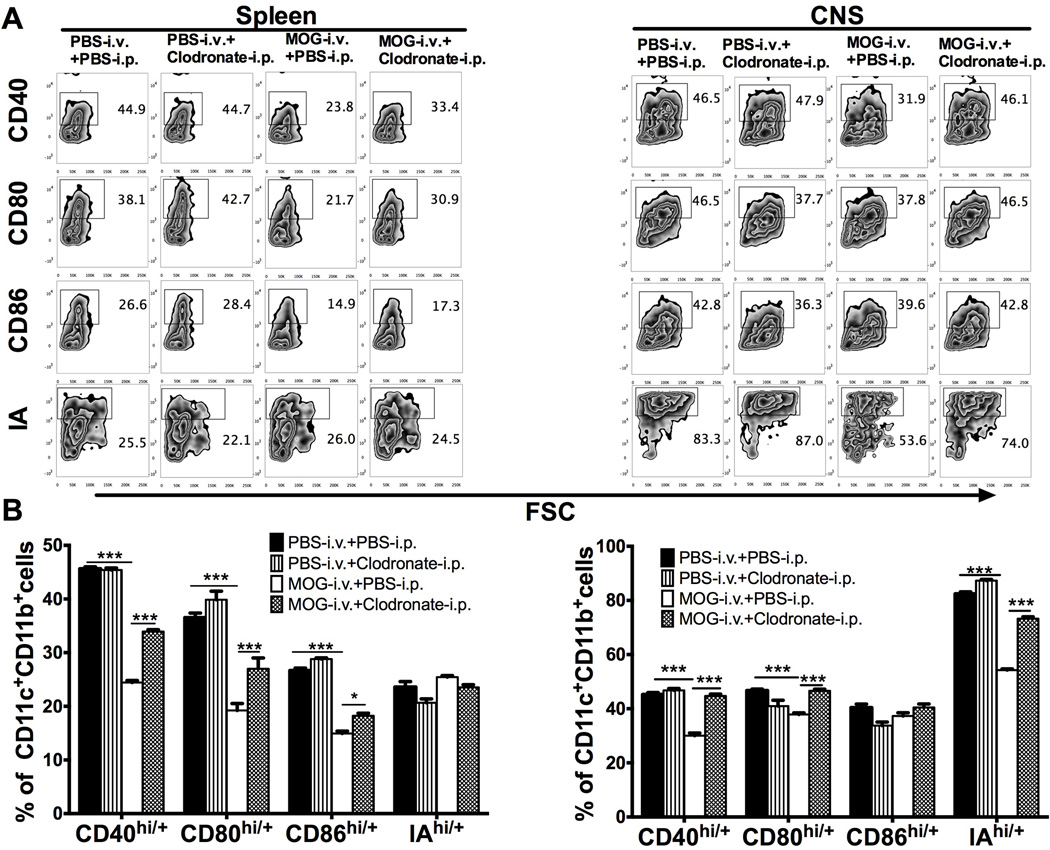
To further determine DC phenotype after i.v. tolerance and iDC depletion, splenocytes and CNS-infiltrating cells were analyzed at day 21 p.i. and the expression of IA and co-stimulatory molecules CD40, CD86, CD80 on CD11c+CD11b+ DCs was determined by flow cytometry. Compared to CD11c+CD11b+ DCs in untreated EAE mice, those in MOG-i.v. tolerized mice expressed lower levels of CD40, CD86, CD80 and IA, indicating an iDC phenotype, while the percentages of DCs expressing these molecules were upregulated after injection of clodronate-loaded liposomes to MOG-i.v. tolerized mice (Fig. 4). These results, together with those shown in Figure 3, indicate that clodronate-loaded liposomes significantly depleted i.v. autoantigen-induced iDC phenotype of CD11c+CD11b+ DCs.
Fig. 4. I.v. MOG-induced tolerogenic phenotype of CD11c+CD11b+ DCs is abrogated by injection of clodronate-loaded liposomes.
Mice immunized for EAE induction were i.v. injected with MOG or PBS, while PBS-loaded or clodronate-loaded liposomes were i.p. injected. Splenocytes and CNS infiltrating cells were isolated 21 days p.i. and analyzed by flow cytometry. (A) Expression of CD40, CD80, CD86 and IA in gated CD11c+CD11b+ DCs in splenocytes (left) and CNS infiltrating cells (right). (B) Results in (A) were analyzed and shown as mean ± SEM (n=3 each group). *p<0.05, ***p<0.001, two-way ANOVA test. One representative of 3 experiments is shown.
iDC depletion reduces i.v. MOG-induced IL-27 and TGF-β production
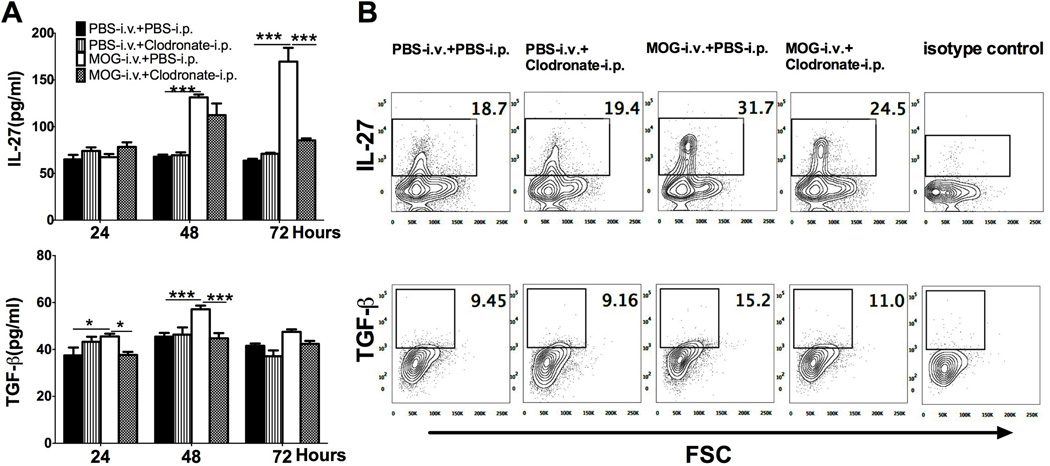
We then determined cytokine production of DCs after CD11c+CD11b+ or iDC depletion. Compared to splenocytes of untreated EAE mice, cells of MOG-i.v. mice produced significantly higher levels of IL-27 and TGF-β in culture supernatants, while these increases were reduced after injection of clodronate-loaded liposomes (Fig. 5A). When intracellular cytokine secretion in splenic CD11c+CD11b+ DCs was analyzed by flow cytometry, increased proportions of IL-27+and TGF-β+ DCs were observed in MOG-i.v. mice compared to untreated EAE mice, while injection of clodronate-loaded liposomes reduced their numbers (Fig. 5B). These results, together, showed that the production of i.v. MOG-induced immunomodulating cytokinesIL-27 and TGF-β can be reduced by depleting CD11c+CD11b+ DCs.
Fig. 5. iDC-depletion inhibits i.v. MOG-induced IL-27 and TGF-β production.
Mice immunized for EAE induction were i.v. injected with MOG or PBS, while PBS-loaded or clodronate-loaded liposomes were i.p. injected. Splenocytes were isolated 21 days p.i. and cultured in triplicate in 24-well plates with MOG35–55 for 24, 48 or 72 hours. (A) Supernatants were collected and analyzed for IL-27and TGF-β production by ELISA and shown as mean ± SEM (n=4 each group). (B) Intracellular secretion of TGF-β and IL-27 in gated CD11c+CD11b+ DCs was determined by flow cytometry. *p<0.05, ***p<0.001, two-way ANOVA test. One representative of 3 experiments is shown.
Effects of CD11c+CD11b+DC depletion on i.v. MOG-induced T-cell responses
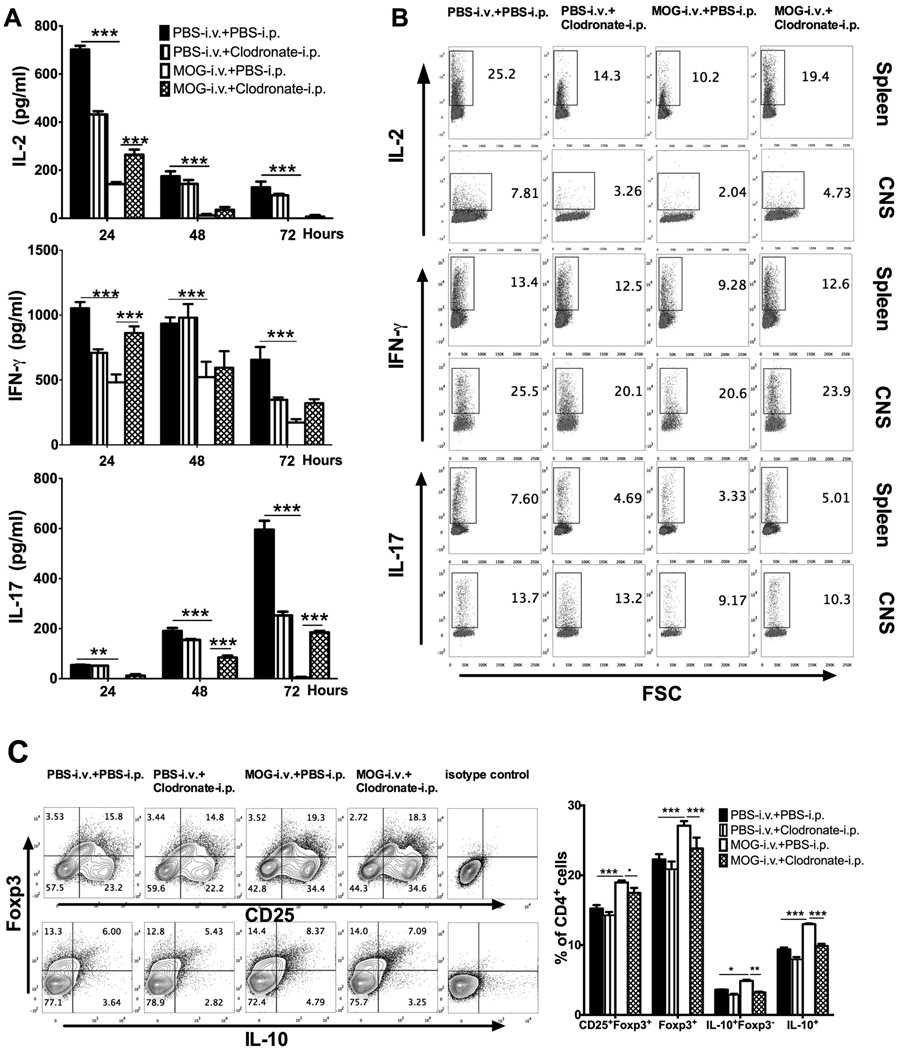
To study the effect of CD11c+CD11b+ DC depletion on T cell response after i.v. MOG-induced tolerance, cytokine production of splenocytes was analyzed in vitro. Production of these cytokines by MOG-i.v. tolerized mice compared to untreated EAE mice was significantly reduced, while injection of clodronate-loaded liposomes partially, but significantly, blocked this effect (Fig. 6A). Similar results were obtained for the percentages of Th1 cells (IL-2+CD4+, IFN-γ+CD4+) and Th17 cells (IL-17+CD4+) of splenocytes and CNS-infiltrating cells, as determined by flow cytometry (Fig. 6B). In contrast, numbers of both CD4+CD25+Foxp3+ Treg cells and CD4+IL-10+Foxp3− Tr1 cells [20, 21] in splenocytes were higher in MOG-i.v. tolerized mice than in untreated EAE mice, and the levels were significantly reduced after clodronate-loaded liposome injection (Fig. 6C). Thus, MOG-induced T cell tolerance is significantly diminished by CD11c+CD11b+ DC depletion.
Fig. 6. I.v. MOG-induced suppression of Th1/Th17 cells and increase of Treg cells and Tr1 cells is blocked by CD11c+CD11b+ DC depletion.
Mice immunized for EAE induction were i.v. injected with MOG or PBS, while PBS-loaded or clodronate-loaded liposomes were i.p. injected. Splenocytes and CNS infiltrating cells were isolated 21 days p.i. (A) Splenocytes triplicate cultured in 24-well plates restimulated with MOG35–55 for 24, 48 or 72 hours and supernatants were collected and analyzed for IL-2, IL-17, and IFN-γ production by ELISA and shown as mean ± SEM (n=4 each group). (B) Intracellular secretion of IL-2, IFN-γ and IL-17 in gated CD4+ T cells in splenocytes (top) and CNS infiltrating cells (bottom) were determined by flow cytometry. (C) Treg cells and Tr1 cells in splenocytes were determinate as CD25+ Foxp3+ and IL-10+ Foxp3− cells, respectively, in gated CD4+ T cells (left). Results were statistically analyzed and data shown as mean ± SEM (n=3 each group) (right). *p<0.05, ***p<0.001,***p<0.001, two-way ANOVA test. One representative of 3 experiments is shown.
Effect of iDC depletion on MOG-reactive T cells in co-cultures
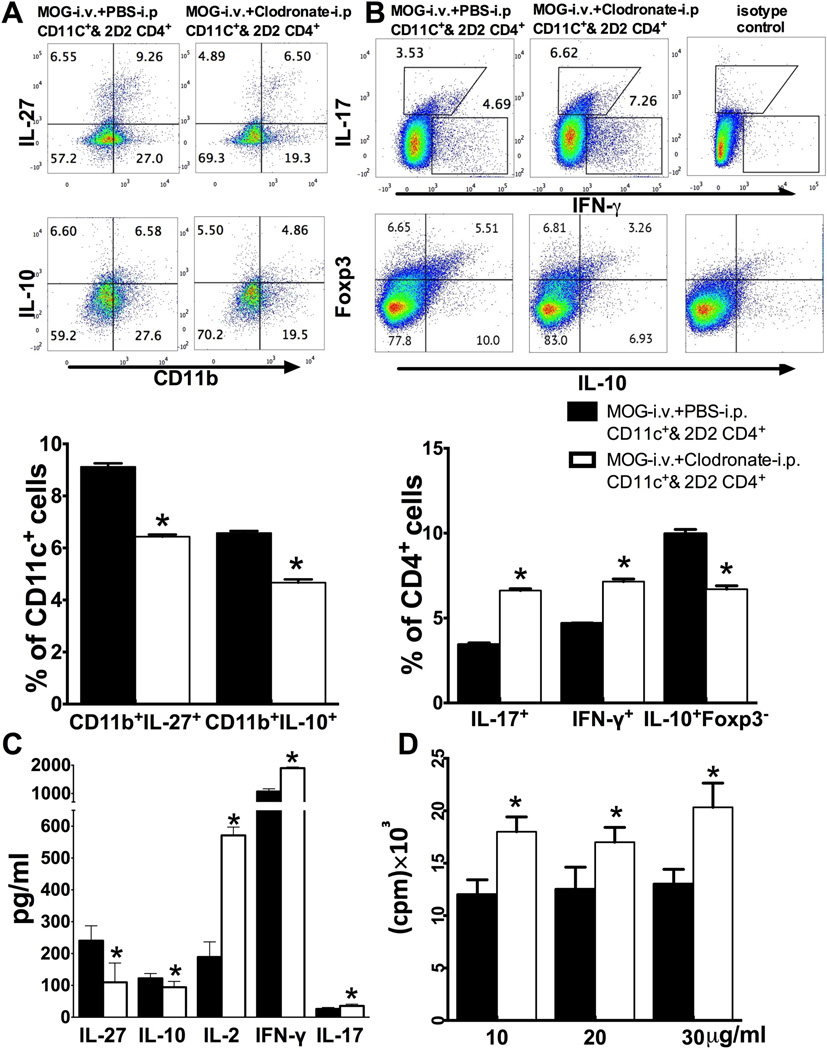
To test the direct effect of iDC depletion on T cell responses, CD11c+ cells were purified from the spleen of MOG-i.v. + PBS-i.p. mice and MOG-i.v. + clodronate-i.p. mice, then co-cultured with purified CD4+ T cells from 2D2 MOG TCR transgenic mice in the presence of MOG35–55 peptide for 3 days. As shown in Fig. 7A, reduced numbers of IL-27-and IL-10-producing CD11c+CD11b+DCs were observed in MOG-i.v. + clodronate-i.p. mice compared to MOG-i.v.+ PBS-i.p. mice. CD4+T cells co-cultured with DCs of MOG-i.v.+ clodronate-i.p. mice had higher proportions of IL-17+ and IFN-γ+ cells and lower proportions of IL-10+Foxp3− cells (Fig. 7B). Supernatants of CD4+ T cells co-cultured with DCs of MOG-i.v.+ clodronate-i.p. mice had significantly lower levels of immunomodulatory cytokines IL-27and IL-10, but higher concentrations of proinflammatory cytokines IL-2, IFN-γ and IL-17 compared to CD4+T cells co-cultured with DCs of MOG-i.v.+ PBS-i.p. mice (Fig. 7C). Further, CD4+ T cells co-cultured with DCs of MOG-i.v. + clodronate-i.p. mice exhibited significantly higher proliferative responses to MOG35–55 peptide stimulation (Fig. 7D). Together, these results indicate that iDC depletion significantly reduced MOG-i.v. induced tolerogenic functionality of CD11c+CD11b+ DCs.
Fig. 7. Effect of iDC depletion on MOG-reactive T cells in co-culture.
CD4+ T cells from naïve spleens of MOG TCR transgenic mice (2D2) were co-cultured with DCs from spleen of EAE mice treated with MOG-i.v.+PBS-i.p. (MOG-i.v.+PBS-i.p. DCs & 2D2 CD4) or MOG-i.v.+clodronate-i.p. (MOG-i.v.+clodronate-i.p. DCs & 2D2 CD4) in the presence of MOG35–55 for 3 days. (A) CD11c+ cells were gated and the intracellular secretion of IL-27 and IL-10 in CD11c+CD11b+ cells were analyzed (up) and expressed as mean ± SEM (n=3 each group; bottom). (B) CD4+ cells were gated and their intracellular IFN-γ, IL-17, Foxp3 and IL-10 were analyzed with flow cytometry (top) and data are shown as mean ± SEM (n=3 each group; bottom). (C) Culture supernatants were harvested and production of IL-27, IL-10, IL-2, IFN-γ, IL-17 was analyzed with ELISA. Data are expressed as mean ± SEM (n=4 each group). (D) MOG-induced T-cell proliferative responses were measured by 3H-methylthymidine incorporation and the values of cpm (counts per minute) are shown as mean ±SEM (n=3 each group). *p<0.05, unpaired Student’s t test. One representative of 3 experiments is shown.
Discussion
Immunological tolerance has been defined as a mechanism by which the immune system prevents pathologic autoreactivity against self and thus prevents autoimmune diseases [22]. Administration of specific encephalitogenic antigens by various routes, including mucosal, i.v., i.p., s.c. and oral, can induce immune tolerance in EAE [7, 23, 24]. DCs, as professional APCs, can be induced into a tolerogenic phenotype, with the ability to promote the function of regulatory T cells or inhibit activation of autoreactive T cells [25–27]. Our recent study also showed that tolerogenic CD11c+CD11b+ DCs, were significantly increased in tolerized mice after i.v. injection of myelin antigen MOG35–55 peptide [15]. In the present study we showed that this up-regulation of CD11c+CD11b+ DCs exhibited an immature phenotype and can be reduced by clodronate-loaded liposomes, which also abrogated i.v. MOG-induced suppression of EAE as well as decreased encephalitogenic IL-2-, IFN-γ-, and IL-17-producing CD4+ cells. Furthermore, recent studies have shown that M1 microglia/macrophages release pro-inflammatory cytokines and participate in the pathogenesis of EAE, while M2 cells produce anti-inflammatory cytokines and repair tissue [28, 29]. It has been shown that treatment with clodronate-loaded liposomes significantly reduced the numbers of CD45high CD11b+ macrophages within the CNS and slightly delayed the expansion of CD45dimCD11b+ microglia and infiltrating T cells [30]. In our study we found that clodronate-loaded liposomes selectively depleted M2 type, but not M1, macrophages in MOG-tolerized mice. This effect, together with reduced CD11c+CD11b+ tolerogenic DCs, might contribute to the observed blockade of MOG-induced EAE suppression by clodronate-loaded liposomes.
How DCs modulate the balance between autoimmunity and tolerance in vivo has not been fully elucidated. In general, tolerogenic DCs typically exhibit an immature phenotype characterized by low expression of IA and co-stimulatory molecules as well as low secretion of pro-inflammatory cytokines. In contrast, mature DCs facilitate activation of T cells and lead to inflammation [2, 9, 31]. In addition to the fact that co-stimulatory molecules expressed on DCs facilitate or inhibit the activity of effector cells, DCs secrete pro-or anti-inflammatory cytokines to modulate activation of CD4+ T cells. Further, iDCs exposed to necrotic cells or bacteria induce maturation and promote inflammation [32, 33], while binding and phagocytosis of apoptotic cells can inhibit the maturation of iDCs and render them tolerogenic [34–36].
Our group and others have shown that i.v. injection of myelin antigen into EAE mice induced by the same antigen results in apoptosis of a significant number of activated myelin-specific T cells [16, 37]. We have designed an immunotherapy using apoptotic cell-treated DCs to block EAE development in vivo [38, 39]. Our current results, together with previous studies, lead us to hypothesize that i.v. administration of the myelin antigen to EAE mice results in T cell apoptosis, and these apoptotic T cells can be ingested by iDCs, causing them to acquire a tolerogenic phenotype with lower expression of IA and co-stimulatory molecules [40, 41]. At the same time, DC deficiency in co-stimulatory molecules can induce regulatory T cells, and promote antigen-specific tolerance [42]. Indeed, Perruche et al. have shown that treatment of EAE mice with intact anti-CD3 antibody resulted in apoptosis of CD3+ T cells. These cells, when engulfed by phagocytes, promoted the latter to produce TGF-β, which induced CD4+Foxp3+ Treg cells in culture and contributed to immune tolerance in vivo [16]. When autoantigen-loaded DEC205+ DCs were injected, these cells induced TGF-beta production and Treg cells, and effectively suppressed EAE [43]. Similar results have also been observed in autoantigen-pulsed DCs [44]. In our present study, we found a higher proportion of TGF-β-producing CD11c+CD11b+ DCs in i.v. MOG tolerance mice compared to untreated EAE mice, accompanied by an increased proportion of CD4+CD25+Foxp3+ Treg cells, while these cells were largely reduced after depletion of CD11c+CD11b+ DCs. These results, together with the reports of other groups, indicate that induction of CD11c+CD11b+ DCs is an important step in the induction of Treg cells and, eventually, i.v. tolerance in EAE.
Another important immunomodulatory cytokine produced by APCs, including DCs and macrophages, is IL-27 [45–48]. DCs secreteIL-27 in response to Toll-like receptor (TLR) activation through a mechanism that involves the autocrine effects of interferon-β (IFN-β) [49]. The therapeutic effects of IFN-β administration on relapsing-remitting MS have been linked to the induction of IL-27 production by DCs [50]. IL-27 subunits (EBI3 and p28) and its receptor (WSX1) have been found to be upregulated in inflammatory cells in the CNS during EAE [51]. However, IL-27 administration inhibits EAE development [52]. IL-27R (WSX-1) deficient mice developed more severe EAE than WT mice, suggesting an anti-inflammatory effect of IL-27 [50, 53]. Mascanfroni et al. found that therapeutic vaccination with IL-27-conditioned DCs suppressed established relapsing-remitting EAE dependent on the expression of CD39. LPS pre-treatment with IL-27 on cDCs (F4/80−CD11c+CD11b+B220−MHC-II+LY6C− DCs) from naive mice up-regulated IL-27 expression, suggesting a positive feedback loop. It also led to a significantly decreased expression of IA, CD40, CD80, CD86, IL-12, IL-23 and an increased production of IL-10 and TGF-β1 by these cDCs [54]. In this study, suppression of EAE by i.v. MOG was associated with a higher proportion of CD11c+CD11b+DCs, with a low expression of IA and co-stimulatory molecules and a high production of IL-27, TGF-β and IL-10 as well.
IL-27 acts directly on T cells to suppress their differentiation into effector T cells. Pre-treatment of cDCs with IL-27 led to a significant decrease in the proliferative response of naïve 2D2 T cells to MOG35–55. Moreover, IL-27 treated cDCs had a decreased ability to induce IFN-γ and IL-17 production of CD4+T cells while their ability to promote IL-10+ and Foxp3+CD4+ T cells was increased [54]. Data from our previous studies show that IL-27 has a suppressive effect on EAE through an effect on encephalitogenic Th17 cells [50]. IL-27 also up-regulatedIL-10 in effector T cells that produce IFN-γ and T-bet, but not Foxp3, indicating a Tr1 phenotype. These IFN-γ+T-bet+Foxp3− cells resemble effector T cells, which have been identified as the main source of host-protective IL-10 during inflammation [52]. In the present study, we found a higher production of IL-27 in CD11b+CD11c+ DCs of i.v. MOG mice and an increased proportion of CD4+IL-10+Foxp3− Tr1 cells [20, 21]; we also found that these effects were significantly reduced after CD11b+CD11c+ DC-depletion. These results indicate that i.v. MOG-induced tolerance is achieved, at least in part, through the production of IL-27 in tolerogenic DCs, thus initializing the IL-27/Tr1 pathway [55].
In summary, our present study demonstrates that CD11b+CD11c+ DCs, upon i.v. MOG injection in EAE mice, facilitate development of both Treg cells and Tr1 cells through inhibiting co-stimulatory signals and inducing TGF-β and IL-27 signals, given that depleting these cells significantly blocked the effect of i.v. tolerance. Thus, induction of tolerogenic DCs is a key mechanism underlying i.v. autoantigen-induced tolerance.
Materials and Methods
Mice and antigen
Female C57BL/6 mice, 8–10 weeks of age, were purchased from the Jackson Laboratory. Mice were housed in the Thomas Jefferson University animal care facilities. All work was performed in accordance with the Thomas Jefferson University guidelines for animal use and care. Mouse MOG35–55 (MEVGWYRSPFSRVVHLYRNGK) peptide was purchased from Invitrogen (Carlsbad, CA).
Depletion of DCs in naïve mice
Female naïve C57BL/6 mice were i.p. injected with the same volume of PBS- or clodronate-loaded liposomes (Encapsula Nanosciences LLC) (2 mg clodronate/mouse). Spleen cells were then harvested 24 hours later and analyzed by flow cytometry to observe the depletion of DCs.
Induction of EAE, i.v. tolerance, and iDC depletion
Mice were injected subcutaneously (s.c.) with 200 µg of MOG35–55 in CFA containing 5 mg/ml Mycobacterium tuberculosis H37Ra (Difco Lab, Detroit, MI) over two sites on the back. All mice received 200 ng of pertussis toxin (List Biological Laboratories, Epsom, England) i.p. on days 0 and 2 post immunization (p.i.) [56]. To induce tolerance, MOG35–55 peptide (200 µg/mouse) was i.v. injected on days 0, 3, and 6 p.i., and mice that received the same volume (100 µl) of PBS in parallel served as controls. To deplete CD11c+CD11b+ DCs, mice were intraperitoneally (i.p.) injected with clodronate-loaded liposomes (2 mg clodronate/mouse) 1 day before and 14 days p.i. and mice that received the same volume of PBS-loaded liposomes served as control.
Clinical evaluation
Clinical EAE was assessed by a 0–5 scoring system described previously [57]. Briefly, 0: normal; 1: limp tail or waddling gait with tail tonicity; 2: waddling gait with limp tail (ataxia); 2.5: ataxia with partial limb paralysis; 3: full paralysis of one limb; 3.5: full paralysis of one limb with partial paralysis of second limb; 4: full paralysis of 2 limbs; 4.5: moribund and 5: death. Mice were examined daily in a blind fashion for signs of EAE.
Histopathology
For CNS histopathology assessment, mice were perfused with 30ml PBS via the heart to eliminate peripheral blood, and spinal cords were harvested at day 21 p.i. Tissues were treated with ethanol and xylene, and paraffin-embedded 5 µm sections were stained with H&E for assessment of inflammation and with Luxol fast blue (LFB) for demyelination. Slides were assessed in a blinded fashion for inflammation and demyelination using a 0–3 scale as described [58]. For inflammation 0: none; 1: a few inflammatory cells; 2: organization of perivascular infiltrates and 3: increasing severity of perivascular cuffing with extension into the adjacent tissue. For demyelination 0: none; 1: rare foci; 2: a few areas of demyelination and 3: large (confluent) areas of demyelination.
Isolation of splenocytes and CNS-infiltrating mononuclear cells
Mice were extensively perfused at day 21 p.i. and splenocytes were harvested by forcing the tissue through a sterile 70µm nylon cell strainer to generate a single-cell suspension. Erythrocytes in the cell pellets from spleen were lysed by adding NH4Cl-Tris buffer for 5 min at room temperature followed by washing and re-suspending, and viable cells were counted in 0.4% Trypan blue.
For preparation of infiltrating mononuclear cells (MNCs) from spinal cord and brain, we mechanically dissociated spinal cords and brain through a 70 µm cell nylon strainer and enzymatically digested by incubation with Liberase TM (Roche) at 37°C for 20–30 min. Infiltrating mononuclear cells were harvested using a Percoll (Sigma-Aldrich, St. Louis, MO) gradient (70/37%), washed, and viable cells were counted in 0.4% Trypan blue.
Cytokine profiles
Splenocytes at 1–2.5 ×106/ml were cultured in triplicate in RPMI1640 with 10% FBS in 24-well plates. Cells were restimulated with 20 µg/ml MOG35–55 for 24, 48 and 72 hours. Cell-free supernatants were collected and analyzed for the production of IL-27, TGF-β, IL-2, IFN-γ, and IL-17 by ELISA, using ELISA kits (R&D Systems, Minneapolis, MN).
Flow cytometry analysis
Splenocytes and CNS-infiltrating cells were suspended in complete RPMI 1640 with 10% FBS at a density of 2 × 106/ml. For surface-marker staining, cells were washed in FACS buffer (PBS containing 3% FBS and 0.02% NaN3), then incubated with fluorochrome-conjugated antibodies for 30 minutes on ice. For intracellular cytokine staining, cells were stimulated with PMA (50 ng/ml), ionomycin (500 ng/ml), and Golgi-Stop (1 mg/106 cells) for 4 hours in complete RPMI 1640 medium with 10% FBS. After being washed in FACS buffer cells were stained with fluorescent antibodies to surface markers, followed by intracellular staining in accordance with the manufacturer’s instructions. In brief, cells were fixed and permeabilized using Fix/Perm cell permeabilization reagents, followed by incubation with fluorescently labeled antibodies against intracellular cytokines. All antibodies and reagents used in this section were purchased from BD Biosciences (San Jose, CA), with the exception of PMA and ionomycin (both from Sigma-Aldrich). After the last wash, data were acquired by FACS Aria (BD Biosciences, San Jose, CA). Data were analyzed using FlowJo Software (Tree Star, Ashland, OR).
Purification of DCs and co-culture with MOG-reactive CD4+ T cells in vitro
DCs from splenocytes were prepared as previously described [59]. In brief, single cell suspensions derived from the spleen of EAE mice treated with MOG-i.v. + PBS-i.p. or MOG-i.v. + clodronate-i.p. were incubated with mouse CD11c MicroBeads (Miltenyi Biotec) and then subjected to positive selection through MACS separation columns. Cells selected on the basis of CD11c expression routinely consisted of 90% viable DCs. Single cell suspensions derived from the spleen of naïve MOG TCR transgenic mice (2D2, 6–10 weeks of age, Jackson Laboratory) were purified by positive selection with mouse CD4 MicroBeads (Miltenyi Biotec). CD4+ Cells (1 × 106/ml) were then incubated in 24-well culture plates with MOG (20 µg/ml) and co-cultured with DCs (1×105/ml) purified from spleen cells of MOG-i.v. + PBS-i.p. and MOG-i.v.+clodronate-i.p. mice respectively. After 3 days, cell-free supernatants were collected and analyzed for the production of IL-27, IL-10, IL-2, IFN-γ, and IL-17 by ELISA, using ELISA kits. Cells were then stained with CD4, CD11c, CD11b, CD25, IFN-γ and IL-17, IL-10, IL-27 antibodies (BD Biosciences) and analyzed with flow cytometry.
For cell proliferative response, triplicate aliquots (200 µl) of these co-culture cells were applied to 96-well plates at a density of 0.5×106/ml. MOG35–55 was added to appropriate wells at final concentrations of 10, 20, and 30 µg/ml. After 54 hours, cells were incubated with 1 μCi of 3H-methylthymidine (specific activity 42 Ci/mmol) for 18 hours. Cells were harvested on fiberglass filters and thymidine incorporation was measured at a scintillation counter.
Statistical analysis
Experimental data were analyzed using Prism software (GraphPad, La Jolla, CA, USA). Comparisons of data between experimental groups were performed by two-way ANOVA test or unpaired Student’s t tests. All tests were two-sided. Differences were considered significant at a value of p<0.05.
Acknowledgments
This study was supported by a grant from the National Institutes of Health. L.M.W. is partly supported by the Chinese Overseas Scholarship Program. We thank Katherine Regan for editorial assistance.
Footnotes
Disclosure of potential conflict of interest
The authors declare no financial or commercial conflict of interest.
Reference
- 1.Grigoriadis N, van Pesch V. A basic overview of multiple sclerosis immunopathology. Eur J Neurol. 2015;22(Suppl 2):3–13. doi: 10.1111/ene.12798. [DOI] [PubMed] [Google Scholar]
- 2.Hilliard BA, Kamoun M, Ventura E, Rostami A. Mechanisms of suppression of experimental autoimmune encephalomyelitis by intravenous administration of myelin basic protein: role of regulatory spleen cells. Exp Mol Pathol. 2000;68:29–37. doi: 10.1006/exmp.1999.2290. [DOI] [PubMed] [Google Scholar]
- 3.t Hart BA, van Kooyk Y, Geurts JJ, Gran B. The primate autoimmune encephalomyelitis model; a bridge between mouse and man. Ann Clin Transl Neurol. 2015;2:581–593. doi: 10.1002/acn3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rangachari M, Kuchroo VK. Using EAE to better understand principles of immune function and autoimmune pathology. J Autoimmun. 2013;45:31–39. doi: 10.1016/j.jaut.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luchtman DW, Ellwardt E, Larochelle C, Zipp F. IL-17 and related cytokines involved in the pathology and immunotherapy of multiple sclerosis: Current and future developments. Cytokine Growth Factor Rev. 2014;25:403–413. doi: 10.1016/j.cytogfr.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Jadidi-Niaragh F, Mirshafiey A. Regulatory T-cell as orchestra leader in immunosuppression process of multiple sclerosis. Immunopharmacol Immunotoxicol. 2011;33:545–567. doi: 10.3109/08923973.2010.513391. [DOI] [PubMed] [Google Scholar]
- 7.Pitkanen J, Peterson P. Autoimmune regulator: from loss of function to autoimmunity. Genes Immun. 2003;4:12–21. doi: 10.1038/sj.gene.6363929. [DOI] [PubMed] [Google Scholar]
- 8.Safavi F, Li H, Gonnella P, Mari ER, Rasouli J, Zhang GX, et al. c-kit plays a critical role in induction of intravenous tolerance in experimental autoimmune encephalomyelitis. Immunol Res. 2015;61:294–302. doi: 10.1007/s12026-015-8624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 10.Mohammad MG, Hassanpour M, Tsai VW, Li H, Ruitenberg MJ, Booth DW, et al. Dendritic cells and multiple sclerosis: disease, tolerance and therapy. Int J Mol Sci. 2012;14:547–562. doi: 10.3390/ijms14010547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegemund S, Schutze N, Freudenberg MA, Lutz MB, Straubinger RK, Alber G. Production of IL-12, IL-23 and IL-27p28 by bone marrow-derived conventional dendritic cells rather than macrophages after LPS/TLR4-dependent induction by Salmonella Enteritidis. Immunobiology. 2007;212:739–750. doi: 10.1016/j.imbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J Immunol. 2007;179:6421–6428. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 13.Zarnani AH, Moazzeni SM, Shokri F, Salehnia M, Jeddi-Tehrani M. Kinetics of murine decidual dendritic cells. Reproduction. 2007;133:275–283. doi: 10.1530/rep.1.01232. [DOI] [PubMed] [Google Scholar]
- 14.de Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer Semin Immunopathol. 2005;26:289–307. doi: 10.1007/s00281-004-0167-1. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Zhang GX, Chen Y, Xu H, Fitzgerald DC, Zhao Z, et al. CD11c+CD11b+ Dendritic Cells Play an Important Role in Intravenous Tolerance and the Suppression of Experimental Autoimmune Encephalomyelitis. The Journal of Immunology. 2008;181:2483–2493. doi: 10.4049/jimmunol.181.4.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perruche S, Zhang P, Liu Y, Saas P, Bluestone JA, Chen W. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat Med. 2008;14:528–535. doi: 10.1038/nm1749. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Zhang GX, Chen Y, Xu H, Fitzgerald DC, Zhao Z, et al. CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008;181:2483–2493. doi: 10.4049/jimmunol.181.4.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension. 2010;55:652–659. doi: 10.1161/HYPERTENSIONAHA.109.142836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galea I, Palin K, Newman TA, Van Rooijen N, Perry VH, Boche D. Mannose receptor expression specifically reveals perivascular macrophages in normal, injured, and diseased mouse brain. Glia. 2005;49:375–384. doi: 10.1002/glia.20124. [DOI] [PubMed] [Google Scholar]
- 20.Bohm L, Maxeiner J, Meyer-Martin H, Reuter S, Finotto S, Klein M, et al. IL-10 and regulatory T cells cooperate in allergen-specific immunotherapy to ameliorate allergic asthma. J Immunol. 2015;194:887–897. doi: 10.4049/jimmunol.1401612. [DOI] [PubMed] [Google Scholar]
- 21.Yao Y, Vent-Schmidt J, McGeough MD, Wong M, Hoffman HM, Steiner TS, et al. Tr1 Cells, but Not Foxp3+ Regulatory T Cells, Suppress NLRP3 Inflammasome Activation via an IL-10-Dependent Mechanism. J Immunol. 2015;195:488–497. doi: 10.4049/jimmunol.1403225. [DOI] [PubMed] [Google Scholar]
- 22.Faria AM, Weiner HL. Oral tolerance: therapeutic implications for autoimmune diseases. Clin Dev Immunol. 2006;13:143–157. doi: 10.1080/17402520600876804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasarello K, Gadamski R, Piotrowski P, Kurzepa K, Kwiatkowska-Patzer B, Lipkowski AW. Effect of oral administration of pig spinal cord hydrolysate on clinical and histopathological symptoms of experimental allergic encephalomyelitis in rats. Folia Neuropathol. 2015;53:128–138. doi: 10.5114/fn.2015.52409. [DOI] [PubMed] [Google Scholar]
- 24.Anderton SM. Peptide immunotherapy in experimental autoimmune encephalomyelitis. Biomed J. 2015;38:206–214. doi: 10.4103/2319-4170.158510. [DOI] [PubMed] [Google Scholar]
- 25.Comabella M, Montalban X, Munz C, Lunemann JD. Targeting dendritic cells to treat multiple sclerosis. Nat Rev Neurol. 2010;6:499–507. doi: 10.1038/nrneurol.2010.112. [DOI] [PubMed] [Google Scholar]
- 26.Steinbrink K, Graulich E, Kubsch S, Knop J, Enk AH. CD4(+) and CD8(+) anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–2476. doi: 10.1182/blood.v99.7.2468. [DOI] [PubMed] [Google Scholar]
- 27.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Z, Jiang JX, Zhang GX. Macrophages: a double-edged sword in experimental autoimmune encephalomyelitis. Immunol Lett. 2014;160:17–22. doi: 10.1016/j.imlet.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fux M, van Rooijen N, Owens T. Macrophage-independent T cell infiltration to the site of injury-induced brain inflammation. J Neuroimmunol. 2008;203:64–72. doi: 10.1016/j.jneuroim.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 31.Carreno LJ, Gonzalez PA, Bueno SM, Riedel CA, Kalergis AM. Modulation of the dendritic cell-T-cell synapse to promote pathogen immunity and prevent autoimmunity. Immunotherapy. 2011;3:6–11. doi: 10.2217/imt.11.38. [DOI] [PubMed] [Google Scholar]
- 32.Lange C, Durr M, Doster H, Melms A, Bischof F. Dendritic cell-regulatory T-cell interactions control self-directed immunity. Immunol Cell Biol. 2007;85:575–581. doi: 10.1038/sj.icb.7100088. [DOI] [PubMed] [Google Scholar]
- 33.Eisenblatter M, Buchal A, Gayum H, Jasny E, Renner Viveros P, Ulrichs T, et al. Nocardia farcinica activates human dendritic cells and induces secretion of interleukin-23 (IL-23) rather than IL-12p70. Infect Immun. 2012;80:4195–4202. doi: 10.1128/IAI.00741-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauter BAM, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191(3):423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, Lacy-Hulbert A. Inhibitory Effects of Apoptotic Cell Ingestion upon Endotoxin-Driven Myeloid Dendritic Cell Maturation. The Journal of Immunology. 2002;168:1627–1635. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson TA, Kazama H. Signals from dying cells: tolerance induction by the dendritic cell. Immunol Res. 2005;32:99–108. doi: 10.1385/IR:32:1-3:099. [DOI] [PubMed] [Google Scholar]
- 37.Zhang GX, Liu TT, Ventura ES, Chen Y, Rostami A. Reversal of spontaneous progressive autoimmune encephalomyelitis by myelin basic protein-induced clonal deletion. Autoimmunity. 1999;31:219–227. doi: 10.3109/08916939908994067. [DOI] [PubMed] [Google Scholar]
- 38.Zhou F, Lauretti E, di Meco A, Ciric B, Gonnella P, Zhang GX, et al. Intravenous transfer of apoptotic cell-treated dendritic cells leads to immune tolerance by blocking Th17 cell activity. Immunobiology. 2013;218:1069–1076. doi: 10.1016/j.imbio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou F, Ciric B, Zhang GX, Rostami A. Immunotherapy using lipopolysaccharide-stimulated bone marrow-derived dendritic cells to treat experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2014;178:447–458. doi: 10.1111/cei.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kleinclauss F, Perruche S, Masson E, de Carvalho Bittencourt M, Biichle S, Remy-Martin JP, et al. Intravenous apoptotic spleen cell infusion induces a TGF-beta-dependent regulatory T-cell expansion. Cell Death Differ. 2006;13:41–52. doi: 10.1038/sj.cdd.4401699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou F, Zhang GX, Rostami A. Apoptotic cell-treated dendritic cells induce immune tolerance by specifically inhibiting development of CD4 effector memory T cells. Immunol Res. 2015 doi: 10.1007/s12026-015-8676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klotz L, Dani I, Edenhofer F, Nolden L, Evert B, Paul B, et al. Peroxisome proliferator-activated receptor gamma control of dendritic cell function contributes to development of CD4+ T cell anergy. J Immunol. 2007;178:2122–2131. doi: 10.4049/jimmunol.178.4.2122. [DOI] [PubMed] [Google Scholar]
- 43.Ring S, Maas M, Nettelbeck DM, Enk AH, Mahnke K. Targeting of autoantigens to DEC205(+) dendritic cells in vivo suppresses experimental allergic encephalomyelitis in mice. J Immunol. 2013;191:2938–2947. doi: 10.4049/jimmunol.1202592. [DOI] [PubMed] [Google Scholar]
- 44.Mansilla MJ, Selles-Moreno C, Fabregas-Puig S, Amoedo J, Navarro-Barriuso J, Teniente-Serra A, et al. Beneficial effect of tolerogenic dendritic cells pulsed with MOG autoantigen in experimental autoimmune encephalomyelitis. CNS Neurosci Ther. 2015;21:222–230. doi: 10.1111/cns.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunter CA, Villarino A, Artis D, Scott P. The role of IL-27 in the development of T-cell responses during parasitic infections. Immunol Rev. 2004;202:106–114. doi: 10.1111/j.0105-2896.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- 46.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 47.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117:123–130. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 49.Molle C, Goldman M, Goriely S. Critical role of the IFN-stimulated gene factor 3 complex in TLR-mediated IL-27p28 gene expression revealing a two-step activation process. J Immunol. 2010;184:1784–1792. doi: 10.4049/jimmunol.0902005. [DOI] [PubMed] [Google Scholar]
- 50.Mitsdoerffer M, Kuchroo V. New pieces in the puzzle: how does interferon-beta really work in multiple sclerosis? Ann Neurol. 2009;65:487–488. doi: 10.1002/ana.21722. [DOI] [PubMed] [Google Scholar]
- 51.Li J, Gran B, Zhang GX, Rostami A, Kamoun M. IL-27 subunits and its receptor (WSX-1) mRNAs are markedly up-regulated in inflammatory cells in the CNS during experimental autoimmune encephalomyelitis. J Neurol Sci. 2005;232:3–9. doi: 10.1016/j.jns.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 52.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 53.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 54.Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol. 2013;14:1054–1063. doi: 10.1038/ni.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meka RR, Venkatesha SH, Dudics S, Acharya B, Moudgil KD. IL-27-induced modulation of autoimmunity and its therapeutic potential. Autoimmun Rev. 2015;14:1131–1141. doi: 10.1016/j.autrev.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 57.Stromnes IM, Goverman JM. Passive induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1952–1960. doi: 10.1038/nprot.2006.284. [DOI] [PubMed] [Google Scholar]
- 58.Yang J, Yan Y, Ciric B, Yu S, Guan Y, Xu H, et al. Evaluation of bone marrow- and brain-derived neural stem cells in therapy of central nervous system autoimmunity. Am J Pathol. 2010;177:1989–2001. doi: 10.2353/ajpath.2010.091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schlecht G, Mouries J, Poitrasson-Riviere M, Leclerc C, Dadaglio G. Purification of splenic dendritic cells induces maturation and capacity to stimulate Th1 response in vivo. Int Immunol. 2006;18:445–452. doi: 10.1093/intimm/dxh384. [DOI] [PubMed] [Google Scholar]