Abstract
While the hippocampus has long been identified as a structure integral to memory, the relationship between morphology and function has yet to be fully explained. We present an analysis of hippocampal dentation, a morphological feature previously unexplored in regard to its relationship with episodic memory. “Hippocampal dentation” in this case refers to surface convolutions, primarily present in the CA1/subiculum on the inferior aspect of the hippocampus. Hippocampal dentation was visualized using ultra-high resolution structural MRI and evaluated using a novel visual rating scale. The degree of hippocampal dentation was found to vary considerably across individuals, and was positively associated with verbal memory recall and visual memory recognition in a sample of 22 healthy adults. This study is the first to characterize the variation in hippocampal dentation in a healthy cohort and to demonstrate its association with aspects of episodic memory.
Keywords: episodic memory, healthy adults, hippocampus, ultra high resolution MRI
Introduction
The role of the hippocampus in declarative memory has been implicated and extensively investigated since the surgical removal of the medial temporal lobe of Patient H. M. (Scoville and Milner, 1957). Although he suffered from severe declarative memory deficits, H.M.'s motor memory and working memory remained functionally intact, leading to the theory that these types of memory are processed separately (Corkin, 1984). Declarative memory is defined by the ability to recall everyday facts (semantic memory) and personal experience (episodic memory) (Eichenbaum, 2004; Squire, 1992), with consolidation occurring principally in the hippocampus (Duvernoy et al, 2013; Eichenbaum, 2000; Eichenbaum, 2004; Squire et al., 2004).
Regarding hippocampal structure, the dentate gyrus of the human hippocampus derives its name from its ridged, “tooth-like” appearance on its intraventricular aspect (also known as the margo denticulatus) that is unique to humans and higher primates (Duvernoy et al, 2013). Similarly, the CA1/subicular region of the hippocampus proper also may show an undulating contour that takes on a “dentated” appearance on MRI, though to a highly variable degree. In some individuals the undulations become quite prominent and form folds in the inferior aspect of the hippocampus (Duvernoy et al, 2013) as can be seen clearly in high resolution images in the sagittal plane through the body and tail of the hippocampus (Fig. 1). We have coined the term “hippocampal dentation” to refer to this morphologic feature of the inferior aspect of the hippocampus proper, most notably CA1, which has been observed previously (Simic et al., 1997; Van Groen et al, 2008) but not quantified or described empirically in regard to its dramatic variation in prominence between individuals. Given that there is considerable variability in how the border between CA1 and the subiculum is defined (Ding and Van Hoesen, 2015; Insausti and Amaral, 2012), we are using the more general term of CA1/subiculum. Of note, the contour of CA1 and CA4 seem to be somewhat correlated, though not perfectly so; however, dentation seems to be independent of the complex fine-scale folding of the granule cell layer (see cell stain sagittal sections at http://neurosciencelibrary.org/Specimens/primates/human/sections/sagittal-cell/husa0782.jpg or Fig. 7.18B in Duvernoy's atlas of the human hippocampus (Duvernoy et al., 2013)).
Figure 1.
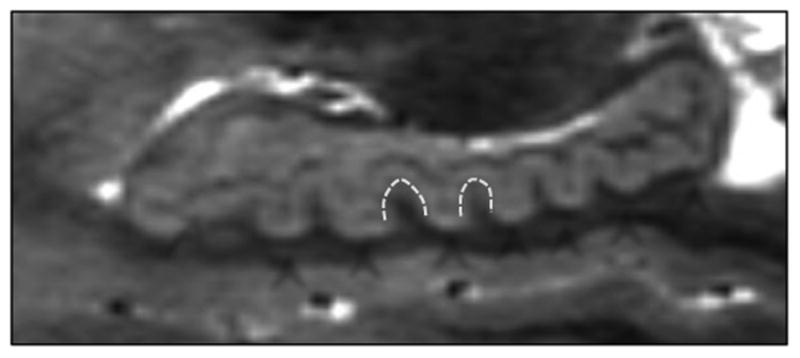
This image depicts an ultra-high resolution sagittal view of the hippocampus, which is necessary to clearly differentiate the gray matter of CA1/subiculum and CA4/hilar region from the dark band of white matter constituted by the strata radiatum, lacunosum, and moleculare (SRLM). Arrows indicate the dark SRLM layer and the CA1/subiculum and CA4 areas; arrowheads indicate the dentes on the hippocampal body and tail, of interest in this study.
Figure 7.
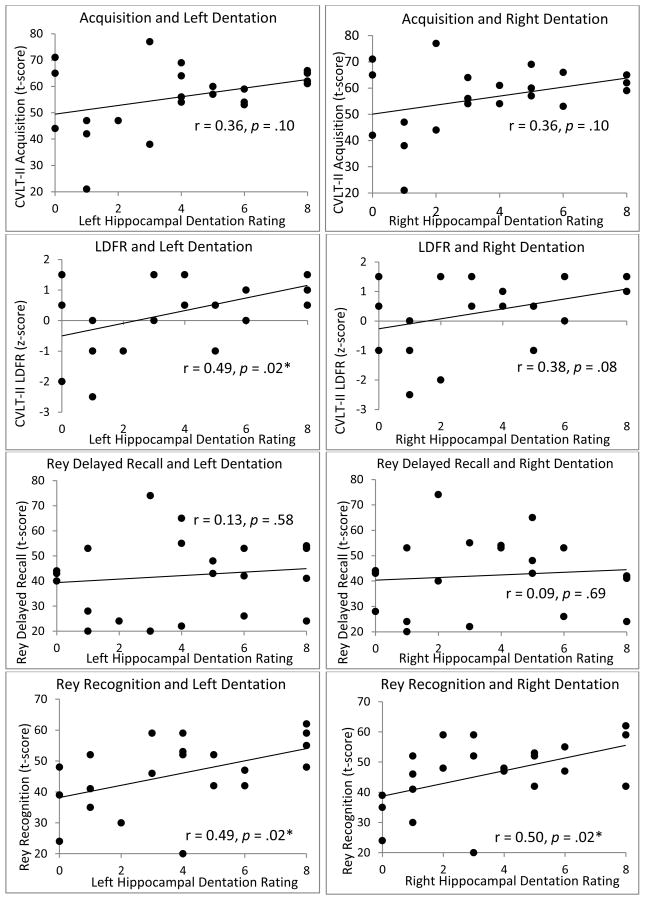
Comprehensive graphs of dentation and episodic memory.
Given that the CA1 cell layer is known to play a role in episodic memory (Bartsch et al, 2011; Farovik et al, 2010; Zola-Morgan et al, 1986), our observation of significant variability in hippocampal dentation led us to theorize a functional role of hippocampal dentation. Based on previous research indicating a positive association between cortical gyrification and cognitive functioning (Luders et al., 2008), we hypothesized that a greater degree of total hippocampal dentation would be associated with better episodic memory performance in healthy adults. Specifically, hippocampal dentation was hypothesized to reflect larger CA1/subiculum surface area and increased capacity for episodic memory functioning. We also predicted hemispheric specialization of this relationship - that is, that greater left hippocampal dentation would be associated with better verbal memory, while greater right hippocampal dentation would be associated with better visual memory. Furthermore, some research suggests that the anterior (body) and posterior (tail) regions of the hippocampus may have different functional roles (Bast et al, 2009; Maguire et al, 2000), and may be differentially impacted in pathologic states (Maller et al, 2007). However, debate remains over the specific functional roles along the long axis of the hippocampus (Strange et al, 2014), indicating a need for further investigation of this topic (Poppenk et al, 2013). Therefore, as an exploratory hypothesis, we predicted that the dentation of the body and tail of the hippocampus may be differentially involved in episodic memory, reflected by variation in the degree of hippocampal dentation along its long axis.
Materials and Methods
Participants
Twenty-two right-handed, English-speaking healthy adults (10 males, 12 females), with a mean age of 29.3 years (SD = 9.82, range: 20 to 57 years), were included in the analysis. Sixty-eight percent of participants identified their race as Caucasian, 27% as African-American, and 5% as another race. On average, participants were highly educated, with a mean of 16.7 years of education (SD = 2.55, range: 12-22 years). Participants with MRI contraindications, known neurological conditions, or recent neuropsychological testing (i.e., past 5 years) were excluded from the study. The Institutional Review Board at our university approved the use of human subjects for this study. Written informed consent was obtained from each study participant prior to participating.
Neuropsychological Assessment
Each participant completed a neuropsychological test battery to assess memory and general cognitive function lasting approximately 90 minutes. Participants completed one verbal episodic memory measure, the California Verbal Learning Test, Second Edition (CVLT-II) (Delis et al, 2000). This standardized measure involves five initial learning trials of a semantically-related 16-word list to measure learning (Acquisition score, based on total number of words recalled in five learning trials combined). An interference list is then presented, followed by a Short-Delay Free Recall and a Short-Delay Cued Recall. A 20-minute delay precedes a Long-Delay Free Recall (LDFR), a Long-Delay ed Recall, and a Recognition Trial. Acquisition (t-scores) and LDFR (z-scores) were considered outcome variables of interest. Scores were standardized based on participants' age and gender in accordance with standard scoring procedures (Delis et al, 2000).
Participants completed one non-verbal memory measure, the Rey Complex Figure Test (Meyers and Meyers, 1996). This standardized measure involves copying a complex geometric figure with 18 components onto a blank piece of paper. Participants are later asked to draw the figure from memory in an Immediate Recall trial (3 minutes following initial administration) and in a Delayed Recall trial (30 minutes after Immediate Recall trial). Following the Delayed Recall, participants complete a Recognition trial of individual components of the complex figure. This measure was scored based on standard clinical scoring procedures; specific scoring criteria for this measure are outlined in the technical manual (Meyers and Meyers, 1996). Outcome variables of interest included Delayed Recall (t-scores) and Rey Recognition (t-scores), corrected for participants' age in accordance with standard scoring procedures.
In order to obtain an intelligence quotient estimate, participants completed the Wechsler Abbreviated Scale of Intelligence (WASI), two-subtest version (Matrix Reasoning and Vocabulary subtests). This IQ score served as a brief estimate of overall intellectual functioning.
MRI acquisition
Participants underwent a structural MRI scanning protocol, conducted at our university on a 3T Philips Achieva scanner. The scanning protocol consisted of a standard clinical T1-weighted MPRAGE scan followed by a series of 12 to 16 ultra high-resolution (0.5×0.5×0.75mm) whole brain T2-weighted scans acquired using a variable flip-angle turbo spin-echo sequence (BrainView - Philips Healthcare, Einthoven, Netherlands). Each ultra high-resolution volume was acquired in only 6 minutes but had very low signal-to-noise ratio (SNR), therefore multiple scans were acquired, co-registered to the first scan, and averaged to produce final images with excellent SNR. The number of high-resolution scans acquired was determined by the participant's tolerance for a prolonged scanning session. Scans marred by head motion artifacts were excluded from post-processing. We refer to this method as high-resolution multiple image co-registration and averaging (HR-MICRA). Co-registration and averaging was performed using FMRIB's Linear Image Registration Tool (FSL-FLIRT; http://fsl.fmrib.ox.ac.uk/fsl/fsl-4.1.9/flirt) (Jenkinson et al, 2002; Jenkinson and Smith, 2001). The high resolution of HR-MICRA images in all 3 planes is necessary for precise and accurate visualization of hippocampal internal architecture and surface structure along the entire hippocampal length.
Dentation assessment
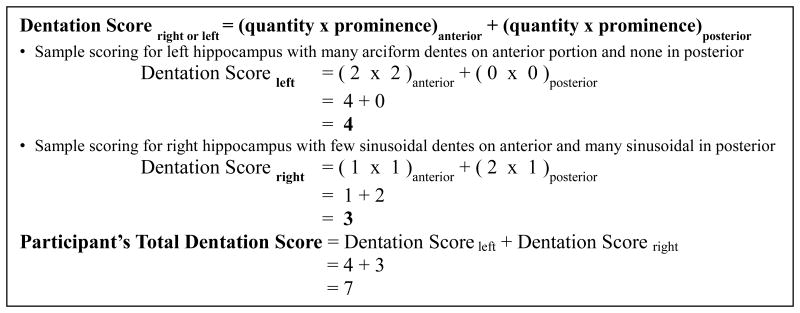
Through our experience reviewing high-resolution hippocampal images it became apparent that hippocampal dentation varies across individuals both in terms of the number of dentes and in the height of dentes (i.e., depth of folds). From this observation we developed a multi-point visual rating system that incorporates both the quantity and the prominence of dentes in the body and tail of the hippocampus separately. A visual rating scale was used in this case because the morphologic features being described were observed to be highly visually salient, and thus a visual rating was selected for simplicity and reliability. Regarding quantity, two points were assigned for four or more dentes, one point was assigned for one to three dentes, and zero points were assigned when no dentes were visible in a given area. Prominence was characterized as arciform (two points), sinusoidal (one point), or absent (zero points) (Table 1). Figure 2 B and C demonstrate the qualitative differences between arciform and sinusoidal dentes. It should be noted that prominence was observed to be independent from quantity. That is, an individual may have many arciform or many sinusoidal dentes, or few arciform or few sinusoidal dentes, but each case is qualitatively distinct. Thus, measuring hippocampal dentation with these two parameters allowed for a more comprehensive description of this variation, beyond simply counting the number of dentes. The body (anterior) and tail (posterior) regions were scored separately, the demarcation between these being defined by a coronal plane through the quadrigeminal plate in the dorsal midbrain, identified for each participant. Any dentation that may be seen in the inferior aspect of the head is typically only a single dente that is on the margin of the head and body and is always similar in size and shape to that of the dentes in the body, and would thus be included in the anterior division in our framework. Prominence and quantity scores were multiplied for each segment and summed for each hippocampus. Based on this system, dentation ranged from 0-16 for total dentation, 0-8 for either right or left hippocampal dentation, and 0-4 for anterior or posterior dentation within each hippocampus.
Table 1.
Zero, one, or two points are assigned separately for quantity (number of dentes) and prominence of dentation in each hippocampal sub-region (i.e., left anterior, left posterior, right anterior, and right posterior). Of note, while quantity and prominence are scored independently, a score of zero on one parameter (i.e., quantity or prominence) necessarily implies a score of zero on the other parameter for that region since a hippocampal region with zero dentes will also have absent prominence.
| Quantity | Points | Prominence | Points |
|---|---|---|---|
| None | 0 | Absent | 0 |
| 1-3 dentes | 1 | Sinusoidal | 1 |
| 4 or more dentes | 2 | Arciform | 2 |
Figure 2.
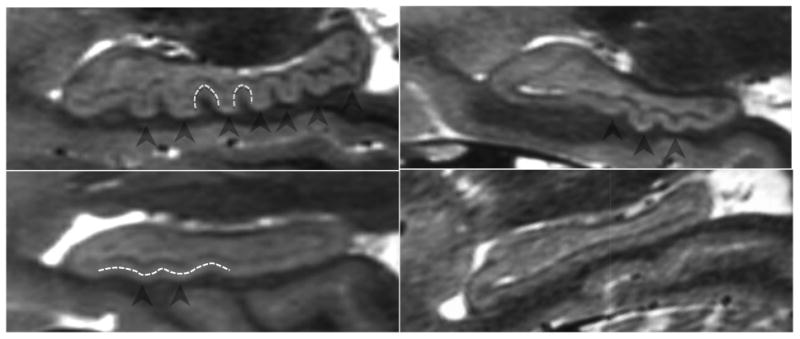
Examples of variation in degree of dentation among healthy adults. A depicts a high degree of dentation with many prominent, or arciform dentes. B depicts few arciform dentes. C shows few, less prominent, or sinusoidal dentes. D shows a hippocampus with no apparent dentation. Arrowheads indicate individual dentes. Dashed white lines illustrate the contour of arciform (A) and sinusoidal (C) dentation.
While multiple dentes can often be visualized in a single sagittal plane (Fig. 2), dentation wraps around the surface of the hippocampus such that dentation is present in the inferior-lateral surface of the anterior hippocampus and the medial surface of the posterior hippocampus (Fig. 3). All dentation is not visible in any single plane. For this reason, raters examined all sagittal slices to view dentation visible through the full width of the hippocampus (Fig. 4). Similarly, due to the fact that the tail of the hippocampus curves inward (toward the midline) as it extends posteriorly, all coronal slices through the tail were also visualized in the coronal plane to confirm the presence of and fully characterize additional dentation that may not be clearly seen in only sagittal views (Fig. 5). The volumetric nature of the image datasets allows simultaneous visualization of slice intersections in multiple planes of view. 3D Slicer was used in this study, though many image review packages would allow similar functionality (https://www.slicer.org/) (Fedorov et al, 2012). See Figure 6 for a complete example of dentation scoring.
Figure 3.
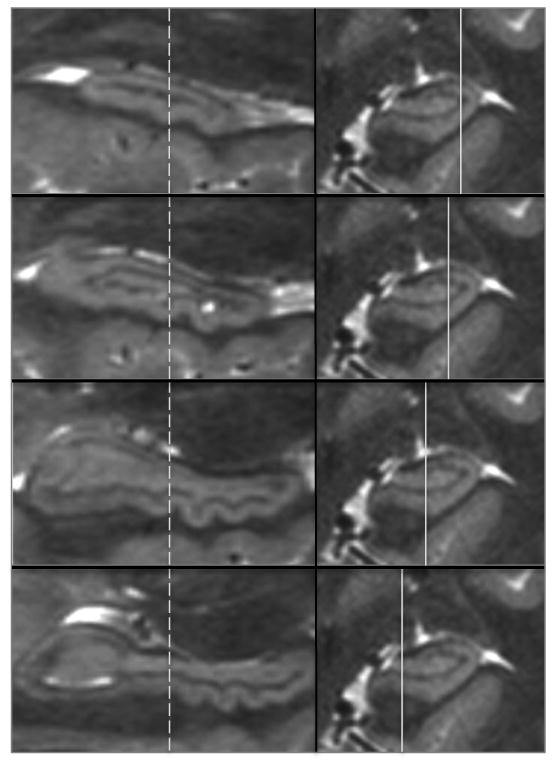
Panels on the left show sagittal views of the hippocampus at locations from lateral to medial as indicated by the solid lines in the panels on the right. The dashed lines indicate the coronal plane of section of the images on the right, which is identical in A-D. A single dente indicated by the arrows shows that it is visible in the lateral-most pane (A) and through the inferior aspect of the hippocampus (B & C), but is not apparent in pane D at this point along the anterior-posterior axis of the hippocampus. However, in D more posterior dentes can be seen because dentation shifts from being most prominent in the infero-lateral aspect anteriorly to the infero-medial aspect posteriorly.
Figure 4.
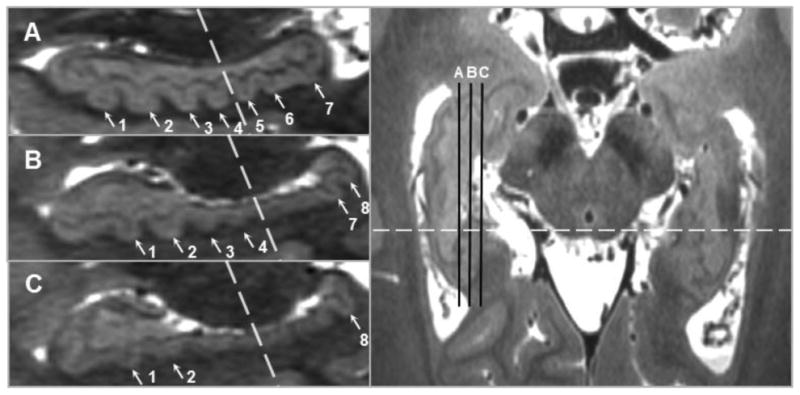
Dentation is assessed by scrolling through sequential sagittal slices because specific dentes may be only visible in more lateral or more medial slices, particularly in the hippocampal tail. The position of sagittal slices A, B, and C are indicated by the black lines in the axial slice in the right panel; the dashed lines indicate the boundary between anterior and posterior. Slice A shows 4 anterior and 3 posterior dentes (1-7), while in Slice B the first two posterior dentes cannot be seen (5-6) but an eighth emerges, and in Slice C only the eighth posterior dente is visible.
Figure 5.
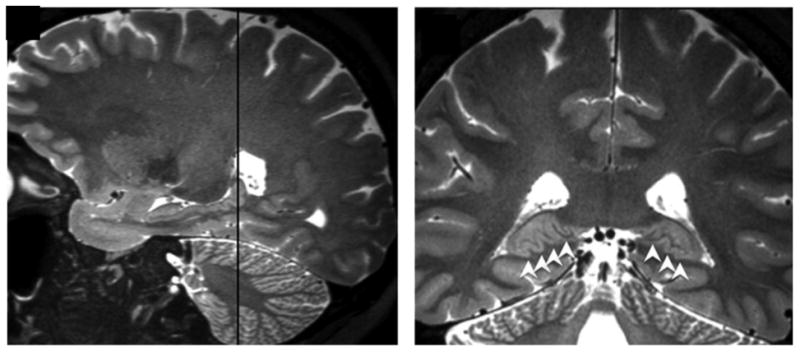
Image A depicts a sagittal view of prominent posterior hippocampal dentation. B shows the corresponding coronal view (location indicated by the dashed line in A). This illustrates how posterior hippocampal dentation is often viewed more clearly in the coronal plane due to the inward (medial) curvature of the hippocampal tail. Dentation is shown with white arrowheads in B.
Figure 6.
Guide for calculating right and left dentation scores. Quantity and prominence are multiplied to form anterior and posterior scores, and then summed to obtain the right or left hippocampal dentation score.
In development of this novel visual rating scale, we considered several alternative options, including simply counting the number of dentes, measuring the depth of folds, measuring the curvilinear length of all dentes in a given sagittal slice, and measuring the curvilinear length along a curved section reconstruction. Each of these methods was implemented on a small number of test cases and it became readily apparent that there were methodological limitations with all of them, including a failure to capture the surface contour represented by both depth and quantity of dentes, difficulties producing consistent results, and a failure to effectively capture the visual complexity of dentation. Of those considered, the most obvious alternative would be simply counting the number of dentes. This may be a reasonable approach when the resolution of the images is insufficient to clearly and consistently depict all of the hippocampal sublayers and the detailed contour and depth of each dente, for example, in the case of a common T1w MPRAGE image, in which only the “bumps” of the tips of the dentes may be visible. However, since in this study we have access to much higher resolution images with detailed contrast between layers, we felt that simply counting dentes would ignore additional contour data that was present. Additionally, our multiscale visual rating system was preferred based on the observation that individual dentes are not identical in their size, shape, or surface area, and thus each dente is not considered to necessarily be an identical unit of measure, as would be assumed if dentes were simply counted. We therefore did not assume that the precise number of dentes was the most direct reflection of the complex visible contour we wished to highlight in this study (i.e., we do not believe that there is a simple linear relationship between the exact number of dentes and their significance regarding memory). Nonetheless, simply counting dentes may be a reasonable alternative, particularly when ultra high resolution imaging is not available, and may be a valuable area of exploration in the future to make the study of dentation more generally available. Finally, it is important to note that our visual rating scale is not presumed to be the only way to characterize dentation, but rather was developed in order to provide an initial exploration into this construct and to capture and quantify the visually salient features of dentation available with our high-resolution images.
Hippocampal Volumetry
Although hippocampal volume is not the focus of this paper, an estimate of volumetry was included for comparison. Hippocampal volumes were estimated using the FreeSurfer Analysis Pipeline. The anatomical scans (MPRAGE) were segmented in order to obtain volumes of brain tissues including right and left hippocampi. See Dale, Fischl & Sereno for more information on this technique (Dale et al, 1999). Automated hippocampal volume segmentation was visually examined for accuracy, with 2 participants requiring manual adjustment to exclude erroneous labeling of ventricular voxels.
Statistical Analyses
Independent and dependent variables were examined for normality and found to have skewness and kurtosis values less than the absolute value of 2.0. Correlations are reported as Pearson's r coefficients. No univariate outliers were found based on a cutoff of standardized scores greater than 3.0. No multivariate outliers were found for the four main memory outcome variables with left and right dentation (Mahalonobis' Distance of less than 15.0 and Cook's Distance less than 1.0). Visual inspection of bivariate scatter plots revealed a linear relationship between the variables of interest. Visual inspection of bivariate scatter plots of residual and predicted data points revealed potential mild heteroscedasticity in the data for the relationship between long-delay free recall with left dentation. Examination of male and female data separately indicates that heteroscedasticity is driven by female participants in the sample, with homoscedasticity in the data for male participants only. Transformations, including the square root and logarithmic transformations were explored; however, homoscedasticity was not achieved with these transformations, therefore, the data were analyzed in their original form to preserve interpretation of constructs being studied. Implications are discussed.
Pearson correlations were conducted for total dentation, as well as separately for right and left hippocampal dentation with the four episodic memory measures. Although hypotheses were made based on hemispheric specialization, correlations were conducted with both right and left hippocampal dentation in order to explore specificity of these hypotheses (i.e., to determine whether correlations existed only in the hypothesized hemisphere). Additionally, although hypotheses we one-tailed in nature, two-tailed significance testing was used for all analyses in order to maintain more strict criteria for significance and conservative conclusions due to the novel nature of the study and due to the theoretical possibility that instead a negative relationship may exist.
In order to establish inter-rater reliability of this novel visual assessment tool, dentation was scored by two board-certified neuroimaging experts trained in this scoring system: a neurologist (LV) and a neuroradiologist (JC). Dentation was scored a second time by the first rater after randomizing the order of the scans in order to assess intra-rater reliability. Main analyses were conducted using a single rater's dentation ratings completed in a single review session.
Results
Development of the novel rating scale utilized in this study was examined for statistical reliability. Inter-rater agreement, as measured by the intraclass correlation coefficient (ICC), was 0.95 for left and right dentation considered together. The ICC for intra-rater reliability was 0.95. ICC(3,1) analyses were conducted according to methods outlined in Shrout and Fleiss (Shrout and Fleiss, 1979). These results indicate excellent consistency within and between raters, supporting the utility of this rating scale as a way to characterize and describe the visible features of dentation and to demonstrate the phenomenon of its variability.
A full range of hippocampal dentation was observed in a healthy adult population with no known neurological conditions (rangeright = 0-8, rangeleft = 0-8, rangetotal = 0-16; Mtotal = 7.4, SD = 5.2). Demographic variables including age (p = .20), years of education (p = .52), and IQ (p = .28) showed no significant relationship with total dentation.
The group showed no difference between left (M = 3.95, SD = 2.75) and right (M = 3.45, SD = 2.65) hippocampal dentation, t(21) = -1.76, p = .09. An independent samples t-test revealed a significant difference between both right and left dentation in males (MRight = 4.70, SDRight = 2.63; MLeft = 5.40, SDLeft = 2.95) and females (MRight = 2.42, SDRight = 2.27; MLeft = 2.75, SDLeft = 1.96), with males having a greater degree of both right, t(20) = 2.19, p = .04, and left hippocampal dentation compared to females, t(20) = 2.52, p = .02. No significant difference was found between males and females in LDFR, Acquisition, Rey Delay, or Rey Recognition scores (p > .05).
Main analyses revealed a significant positive association between total dentation and the number of words recalled after a 20 minute delay (CVLT-II long delay free recall score (LDFR)) (r = 0.45, p = .04) as well as the ability to recognize components of the complex figure previously drawn (Rey Recognition score) (r = 0.51, p = .02). The relationship between total dentation and list learning (CVLT-II Acquisition score) was not statistically significant at the p < .05 level (r = 0.37, p = .09). Finally, no relationship was observed between total hippocampal dentation and the ability to draw a complex figure from memory following a 30-minute delay (Rey Delay score) (r = 0.11, p = .62).
In order to test hypotheses of hemispheric specialization, analyses were conducted between dentation scores of the right and left hippocampi independently with each of the four main memory outcome variables (Acquisition, LDFR, Rey Delay, Rey Recognition) (Fig. 7). These analyses revealed a significant positive correlation between LDFR and left dentation but no significant relationship between LDFR and right dentation, supporting our a priori hypothesis of hemispheric specialization, though a non-significant trend was observed between right dentation and LDFR. Associations between Acquisition and both left and right dentation were non-significant at the p < .05 level. Analysis of visual memory measures revealed a positive association between Rey Recognition and both left and right hippocampal dentation. No association was observed between Rey Delay and left or right hippocampal dentation (Table 2, Fig. 7).
Table 2.
Comprehensive correlational analyses. All statistics are reported as two-tailed Pearson correlations except correlations with age, reported as two-tailed Spearman correlations.
| Pearson Correlation Matrix between Hippocampal Dentation and Memory | ||||
|---|---|---|---|---|
|
| ||||
| Hippocampal Dentation | Hippocampal VolumeA | |||
|
|
|
|||
| Right | Left | Right | Left | |
| Demographic Variables | ||||
| AgeB | 0.33 (p = .13) | 0.27 (p = .22) | 0.37 (p = .09) | 0.28 (p = .20) |
| Years of Education | 0.09 (p = .68) | 0.18 (p = .42) | 0.20 (p = .36) | 0.11 (p = .63) |
| IQ Estimate | 0.22 (p = .33) | 0.25 (p = .26) | 0.33 (p = .13) | 0.39 (p = .08) |
| Episodic Memory | ||||
| CVLT-II Acquisition | 0.36 (p = .10) | 0.36 (p = .10) | 0.51 (p = . 02)* | 0.47 (p = .03)* |
| CVLT-II LDFR | 0.38 (p = .08) | 0.49 (p = .02)* | 0.41 (p = .06) | 0.32 (p = .15) |
| Rey Delay | 0.09 (p = .69) | 0.13 (p = .58) | 0.19 (p = .40) | 0.11 (p = .63) |
| Rey Recognition | 0.50 (p = .02)* | 0.49 (p = .02)* | 0.001 (p = .99) | 0.12 (p = .57) |
Raw hippocampal volume (uncorrected for whole brain volume).
Values represent Spearman ρ values and associated significance levels. A priori hypotheses are noted in bold type.
Denotes significance at the p < .05 level, uncorrected.
To determine whether differences in hemispheric specialization were statistically significant, a comparison of r-values using the Fisher r-to-z transformation was conducted. No significant differences were found between any of the r-values for the left and right hippocampi when compared for each of the four episodic memory variables.
An additional hypothesis of the study involved examining the relationship between memory and dentation in the body versus tail of the hippocampus. Uncorrected Pearson correlations revealed interesting relationships between dentation of the hippocampal body and CVLT-II Acquisition (p = .05), LDFR (p = .02), and Rey Recognition (p = .007), but not Rey Delay (p = .38) (Table 3). However, due to the exploratory nature of this hypothesis, a Holm-Bonferroni correction for multiple comparisons was applied, resulting in no statistically significant effects at the p < .05 level.
Table 3.
Pearson correlation values are displayed for associations between episodic memory variables and anterior (body) and posterior (tail) dentation. Uncorrected r-values and p-values are displayed; no analyses were significant following correction for multiple comparisons.
| Regional Specialization of Hippocampal Dentation and Episodic Memory | ||
|---|---|---|
|
| ||
| Hippocampal Dentation | ||
|
| ||
| Anterior | Posterior | |
| Episodic Memory | ||
| CVLT-II Acquisition | 0.43 (p = .05) | 0.25 (p = .26) |
| CVLT-II LDFR | 0.51 (p = .02) | 0.31 (p = .16) |
| Rey Delay | 0.20 (p = .38) | 0.01 (p = .97) |
| Rey Recognition | 0.56 (p = .007) | 0.36 (p = .10) |
Parallel analyses were conducted to examine the relationship between hippocampal volume and memory. Both right and left raw hippocampal volume were correlated with Acquisition (a measure of initial verbal learning) (Table 2), but not with other memory variables (a trend was observed between right hippocampal volume and Long-Delay Free Recall). We conducted additional analyses to directly compare the strength of the memory-dentation correlations to the memory-volume correlations in order to determine whether statistically significant differences existed between the correlation values. The correlation between Rey Recognition and left hippocampal dentation (r = 0.50) was significantly larger than that of Rey Recognition and left hippocampal volume (r = 0.001) (p = .04). No difference was found when comparing other relationships between volume and dentation (i.e., Acquisition, LDFR, or Rey Delay (p > .05)). No significant relationships were found between hippocampal dentation and raw hippocampal volume (Table 4).
Table 4.
Pearson correlations between raw hippocampal volume and hippocampal dentation.
| Pearson Correlation Matrix between Dentation and Volume | ||
|---|---|---|
| Hippocampal Dentation | ||
|
| ||
| Hippocampal Volume | Right | Left |
| Right | 0.28 (p = .20) | 0.33 (p = .13) |
| Left | 0.38 (p = .08) | 0.29 (p = .19) |
Discussion
The results of this study revealed three main novel findings: first, there is considerable heterogeneity in the degree of hippocampal dentation across a healthy adult study sample; second, our data suggest that hippocampal dentation is significantly associated with aspects of episodic memory in healthy individuals; and third, hippocampal dentation correlates were found with memory, but not with hippocampal volume.
Our finding of significant variability in dentation, a morphological feature of the human hippocampus, in healthy adults attests to the complexity of this brain structure. We described that dentation ranges from completely absent to pronounced and numerous among healthy adults. This is consistent with anatomical sources describing variation in similar ridges in the margo denticulatus of the dentate gyrus (i.e., variable up to 15 dentes) (Duvernoy et al, 2013). While the presence of dentation is readily observed in high-resolution sagittal images by radiologists and neuroimaging researchers, it has received only brief mention in the anatomic literature. For example, Duvernoy's atlas, considered by many to be the standard reference for hippocampal anatomy, only describes this feature in the captions of a few figures as the “folded inferior aspect of CA1” (Duvernoy et al, 2013). By contrast, digitation of the hippocampal head, which has been described and studied (Ding and Van Hoesen, 2015; Gertz et al, 1972; Oppenheim et al, 1998), was not included in this analysis. Digitation is similar to dentation in that it also represents folds of CA1 but on the superior aspect of the hippocampal head, visible in the coronal plane (Fig. 8), versus the inferior aspect of the hippocampal body and tail respectively. Digitation is ubiquitously seen in the normal hippocampal head with virtually all healthy individuals having two, three, or rarely four folds that are readily apparent in coronal images (Fig. 8) (Ding and Van Hoesen, 2015; Gertz et al., 1972). Additionally, the hippocampal head is often examined separately from the body and tail in relation to functional significance (Poppenk et al, 2013; Poppenk and Moscovitch, 2011). For these reasons, digitation of the hippocampal head was not included in the current study, but its relationship to dentation and episodic memory may be an area for future investigation. Of note, in the pathologic state, digitation can show significant variation and may be completely obliterated in cases of marked hippocampal atrophy as seen in temporal lobe epilepsy (Oppenheim et al, 1998). Similarly, in our clinical experience we have commonly observed an asymmetric loss of dentation in epilepsy patients with hippocampal atrophy, though a comprehensive description of this is beyond the scope of the present work in healthy adults.
Figure 8.
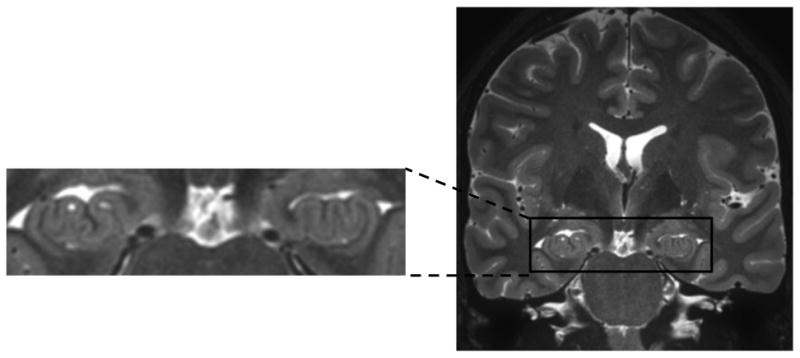
This image depicts an ultra-high resolution coronal view of the hippocampal head, highlighting an example of prominent hippocampal digitation, not examined as part of the current study.
Our data demonstrates gender differences in hippocampal dentation, with males having a greater degree of total hippocampal dentation, despite observing no gender differences in episodic memory performance. Due to the small number of participants of each gender in this sample (12 females, 10 males) it is difficult to assess whether observed differences are reflective of underlying gender differences in male and female hippocampal structure or are merely due to differences in gender groups that are unique to our sample. Previous studies using voxel-based morphometry (Protopopescu et al., 2008) and functional magnetic resonance imaging (Goldstein et al, 2010) suggest that sex hormones can contribute to hippocampal gray matter volume and activation, respectively, in females. More research in larger samples is needed to confidently establish any gender differences in dentation that may exist.
The second finding of this study is the positive association between degree of hippocampal dentation and episodic memory, indicating functional significance of this novel morphological feature. Total hippocampal dentation was positively associated with aspects of verbal and visual episodic memory performance. Specifically, individuals with a greater degree of hippocampal dentation recalled more words from a word list after a delay (LDFR) and were able to better recognize the components of a complex figure after a delay (Rey Recognition). In addition, a non-significant trend was observed with Acquisition scores, warranting further investigation with a larger sample. The relationship between hippocampal dentation and episodic memory is a novel finding in memory research and structural neuroimaging and presents the advent of a promising new direction for future research.
In regard to hemispheric specialization, left dentation is positively associated with better verbal memory performance, evidenced by better delayed recall of a word list (LDFR). While correlations between right hippocampal dentation and verbal memory did not did not meet statistical significance, the absolute differences between the left-sided and right-sided correlations were small, such that there were no significant differences in direct comparison of effect sizes. This suggests that although some of the left-sided correlations met criteria for statistical significance and right-sided correlations did not, the differences were sufficiently small that we cannot make definitive conclusions regarding hemispheric specialization of the role of dentation in regard to verbal memory function.
Additionally, dentation (both left and right) was significantly related to visual recognition memory on the Rey Complex Figure Test, although dentation showed no association with delayed visual memory performance on a trial involving construction of a complex figure. Possible confounds in this measure of visual memory include differences in participants' drawing abilities, which is likely to be independent of visual memory but may have strongly impacted scores on the Rey Complex Figure Test. In support of this hypothesis, a significant number of participants in this study had below average copying scores on the initial trial of the test (i.e., deficits in constructional or drawing skills), indicating a participant's theoretical “baseline” maximum performance that is not accounted for in subsequent recall performances in normative scoring procedures. Participants' approaches to drawing the figure (e.g., gestalt vs. piecemeal) may have influenced their subsequent immediate and delayed recall scores, but may have been less influential on visual recognition. Finally, some previous research has shown a lack of specificity between right temporal lobe dysfunction and poor visual memory performance on figural reproduction tasks (Barr et al., 1997). These hypotheses provide possible explanations for why no relationship was seen between hippocampal dentation and recall of a complex figure, when recognition of the same figure was significantly related to dentation.
To explore the possibility of regional specialization of function within the hippocampus, analyses were conducted to determine whether episodic memory showed a different relationship with dentation of the anterior (body) versus the posterior (tail) portions of the hippocampus. This approach was based on existing hypotheses of a functional difference between these regions (Kim, 2015; Maguire et al, 2000; Maguire et al, 2006). Left and right hippocampi were considered together when analyzing body and tail dentation due to the fact that correlation values were not significantly different between these two, and to specifically address the question of regional specialization independent of hemispheric specialization. Moderate correlation values were observed for verbal learning, verbal delayed recall, and visual recognition; however, analyses were non-significant following a correction for multiple comparisons. Although definitive conclusions regarding regional specialization of the hippocampus cannot be made based on this small sample size, it is certainly a topic of consideration for future research.
The third finding of the current study is that hippocampal dentation does not simply represent a surrogate measure of hippocampal volume but appears to reflect a unique and largely independent morphological feature of the human hippocampus. Data from the present study indicated no significant association between hippocampal dentation and hippocampal volume, suggesting that these two features may represent independent constructs with little redundancy. In addition, uncorrected hippocampal volume showed a positive association with verbal learning (i.e., encoding), but not with verbal memory (i.e., recall) or with visual memory. This finding is consistent with previous literature indicating mixed results between hippocampal volume and episodic memory performance in healthy adults. Analysis of effect sizes revealed that the relationship between dentation and memory was significantly different from the null correlations observed between hippocampal volume and visual memory recognition, but not for verbal recall. This finding implicates hippocampal dentation as an important direction for future research.
To put our findings in context, the structure-function relationship that has been most commonly explored in the existing literature is that of total hippocampal volume and episodic memory functioning. A number of studies have found a positive relationship between hippocampal volume and memory in individuals with disorders associated with memory impairment, including Alzheimer's Disease (Chan et al., 2001; Thompson et al., 2004), epilepsy (Hermann et al, 2009; Sass et al, 1992), and depression (Sheline et al, 2003; Sheline et al, 1999). However, when describing this relationship in a healthy adult population, it is difficult to find consensus across the literature.
Previous studies have published both significant positive (Pohlack et al, 2014) and negative correlations (Chantôme et al, 1999; Foster et al, 1999) between hippocampal volume and memory in healthy adults, and null results have also been noted (de Toledo-Morrell et al, 2000; Raz et al., 1998) (see Van Petten, 2004 for review). One study found a significant negative correlation between episodic memory and the ratio of both left and right hippocampal volume to total brain volume in a sample of young adults. Authors of this study concluded that neuropsychological relationships between hippocampal volume and memory may differ between healthy and disease populations, such that healthy adults have a negative relationship between hippocampal volume and memory while disease populations show a positive relationship (Chantôme et al, 1999). Some subsequent research has proposed that the volume-memory relationship may be moderated by age (Van Petten, 2004). Mixed results in the hippocampal volume literature, considered together with the bias against publishing null results, raise the possibility that no relationship exists between hippocampal volume and memory in the healthy adult population. However, we would like to emphasize that total hippocampal volume was used in this study simply as a comparison based on common and established methods, rather than as the core finding of this paper.
While total hippocampal volume is not consistently associated with memory, specific subfield analyses may be more promising. Smaller CA1 and CA3/dentate gyrus volumes have been implicated in memory loss in patients with amnestic mild cognitive impairment (Yassa et al, 2010). Several studies suggest that the CA1 subfield may be particularly important for episodic/autobiographical memory, evidenced by focal lesions in CA1 (Bartsch et al, 2011; Zola-Morgan et al, 1986). Analysis of hippocampal morphology has been shown to detect surface deformations associated with dementia (Csernansky et al., 2005), schizophrenia (Csernansky et al, 1998), depression in older adults (Ballmaier et al, 2008), and temporal lobe epilepsy (Maccotta et al, 2015).
The recent research implicating a relationship between hippocampal subfield volumes and episodic memory, particularly in the CA1 subfield layer (Bartsch et al, 2011; Yassa et al, 2010), is consistent with our findings in that prominent dentation reflects folds in CA1 and greater surface area of this layer. This morphology is similar to gyrification of the cerebral cortex in humans and higher order mammals. Cortical gyrification has been found to correlate with IQ in healthy adults (Luders et al, 2012; Luders et al, 2008). We hypothesized a similar construct in the human hippocampus, with more dentation producing an increase in CA1/subiculum surface area and allowing the capacity for better episodic memory functioning.
The ultra-high resolution 3T imaging technique used in this study allows for detailed imaging of the hippocampus, clearly depicting dentation. While the tooth-like appearance of the dentate gyrus has been observed previously, this is the first study to our knowledge to offer a system to quantify and formally describe variation in hippocampal dentation in either healthy or disease populations. This study employed a novel visual rating scale to assess hippocampal dentation. Though visual rating scales are inherently subjective, this scale showed excellent intra- and inter-rater reliability (ICC > .90). It should be clearly understood that the purpose of this study was not to demonstrate the definitive method to comprehensively and precisely quantify dentation, but simply to demonstrate the existence of this phenomenon in a sample of healthy adults, for which our visual rating scale is an adequate tool. Based on these initial positive findings, we believe it is worth exploring additional analytics to more objectively characterize hippocampal dentation. As stated earlier, we hypothesize that human hippocampal dentation reflects increased surface area of the CA1/subiculum layer, allowing for better functional capacity, analogous to the gyrification of the cerebral neocortex. Conducting an initial study focusing on the visual qualities of patterns of dentation allowed authors to refrain from operating on this assumption until the presence of this relationship has been demonstrated - that is, describing the relationship prior to assuming its hypothesized cause. This hypothesis (i.e., an investigation of surface area) should be considered an important future direction in this area, but will involve a non-trivial methodological and technical undertaking to develop the computational tools to consistently and accurately measure CA1/subiculum surface area. By contrast, our rating scale is simple but well suited to capture the salient features of dentation for the purpose of an initial exploration, and our findings justify the development of more sophisticated tools. Comparing hippocampal dentation with CA1/subiculum subfield volume is an important next step to further evaluate independence of these constructs. Hippocampal CA1/subiculum volume per se is not examined as a variable in this initial report due to the lack of consensus in the literature regarding precise methods and definitions for CA1 subfield segmentation. Yushkevich and colleagues, representing the Hippocampal Subfields Group, recently compared 21 different protocols for subfield segmentation, finding that significant differences exist between published protocols, specifically for the CA1 subfield at the subiculum border (Yushkevich et al, 2015). Subfield volumetry is an active and important area of investigation in the hippocampal research community, and understanding the relationship between CA1 volume, surface area, and dentation will be an important future direction. Lastly, it should be noted that our visual rating scale emphasizes shape of this contoured feature, which cannot be directly captured by either surface area or volume analyses, and may provide additional information.
Statistical limitations of the study include a small sample size, leading to limited power to detect subtle effects. Future research should include larger samples of healthy adults to further study this phenomenon. A second statistical limitation of the current study is potential heteroscedasticity observed in the sample. In females with higher dentation scores, verbal memory was more consistently elevated. This supports the need for future research with larger samples in this area. Although a wide range of IQ and education level was present in the sample, the average IQ and education was higher than the average adult population (IQ: M = 116.09, SD = 15.48, range = 82-133; years of education: M = 16.68, SD = 2.55, range = 12-22 years). The high educational background of this sample is likely attributable in part to study recruitment on and around a medical center and university campus. Associations included medium to large effect sizes (medium effect size: 0.30 < r < 0.50, large effect size: r > 0.50) (Cohen, 1992). No corrections were made for multiple comparisons in a priori directional hypotheses (associations with total hippocampal dentation, and left and right hippocampal dentation), decreasing the likelihood of Type II Error. However, corrections for multiple comparisons were used for anterior and posterior dentation analyses, due to the fact that these analyses were considered exploratory in nature, though uncorrected p-values were reported as well. As correlational research, this project does not allow authors to speculate regarding directionality of this effect (i.e., whether dentation allows the capacity for superior memory or whether memory practice may alter the shape of the hippocampus). Future research should explore this concept, potentially through the implementation of memory interventions and longitudinal analyses. Finally, as previously mentioned, visual rating scales are inherently subjective in nature, which is a limitation of this study; however, this scale was created in order to simply quantify the two parameters in which dentation was observed to vary and demonstrated good consistency within and between raters. More sophisticated approaches to characterize dentation may follow after the groundwork laid by this report. Additionally, digitation of the hippocampal head was not examined in conjunction with dentation in this analysis due to functional specialization along the long-axis of the hippocampus and due to a hypothesized independence of these constructs. Unlike dentation, digitation has been studied and well-described in past research. However, it may be of interested to investigate the relationship between dentation and digitation in future research.
Conclusions
Previous studies have demonstrated positive associations between increased cortical convolution (i.e., gyrification) and more efficient cognitive functioning in multiple brain regions (Luders et al., 2008). In this study, a wide range of hippocampal dentation was observed in a healthy adult cohort and associated with better episodic memory functioning. To our knowledge, this is the first report to describe the dramatic variation in hippocampal dentation and demonstrate its association with memory. Future research is needed to determine whether hippocampal dentation may predict memory performance in disease populations where memory impairment is prevalent and central to the disorder (e.g., Alzheimer's disease, temporal lobe epilepsy, Major Depressive Disorder). Future directions of this research include exploring potential diagnostic and prognostic utility of patterns of dentation in disease states as well as serving as an outcome measure for interventions. Furthermore, demonstrating the dramatic variability in dentation across individuals has important implications for automated hippocampal segmentation methods.
Supplementary Material
Highlights.
A range of hippocampal dentation was described in a sample of healthy adults.
Hippocampal dentation was related to episodic memory performance in healthy adults.
Hippocampal dentation did not correlate with traditional hippocampal volume.
Acknowledgments
Funding for this study was provided by NIH Grant K23EB008452. Support in study design and preparation of this manuscript was provided by Dr. Jerzy Szaflarski and Dr. Jane Allendorfer. Biostatistics consultation provided by Dr. Richard Kennedy. The authors of this study have no conflicts of interest to disclose.
Grant Sponsor: National Institutes of Health; Grant Number: K23EB008452
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ballmaier M, Narr KL, Toga AW, Elderkin-Thompson V, Thompson PM, Hamilton L, Haroon E, Pham D, Heinz A, Kumar A. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am J Psychiatry. 2008;165(2):229–37. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr WB, Chelune GJ, Hermann BP, Loring DW, Perrine K, Strauss E, Trenerry MR, Westerveld M. The use of figural reproduction tests as measures of nonverbal memory in epilepsy surgery candidates. J Int Neuropsychol Soc. 1997;3(5):435–43. [PubMed] [Google Scholar]
- Bartsch T, Dohring J, Rohr A, Jansen O, Deuschl G. CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc Natl Acad Sci U S A. 2011;108(42):17562–7. doi: 10.1073/pnas.1110266108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T, Wilson IA, Witter MP, Morris RG. From rapid place learning to behavioral performance: a key role for the intermediate hippocampus. PLoS Biol. 2009;7(4):e1000089. doi: 10.1371/journal.pbio.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, Rossor AM, Stevens JM, Cipolotti L, Rossor MN. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer's disease. Ann Neurol. 2001;49(4):433–42. [PubMed] [Google Scholar]
- Chantôme M, Perruchet P, Hasboun D, Dormont D, Sahel M, Sourour N, Zouaoui A, Marsault C, Duyme M. Is there a negative correlation between explicit memory and hippocampal volume? Neuroimage. 1999;10(5):589–95. doi: 10.1006/nimg.1999.0486. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112(1):155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Corkin S. Lasting consequences of bilateral medial temporal lobectomy: clinical course and experimental findings in H.M. Seminars in Neurology. 1984;4(2):249–259. [Google Scholar]
- Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, Grenander U, Miller MI. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci U S A. 1998;95(19):11406–11. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Swank J, Miller JP, Gado M, McKeel D, Miller MI, Morris JC. Preclinical detection of Alzheimer's disease: hippocampal shape and volume predict dementia onset in the elderly. Neuroimage. 2005;25(3):783–92. doi: 10.1016/j.neuroimage.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- de Toledo-Morrell L, Dickerson B, Sullivan MP, Spanovic C, Wilson R, Bennett DA. Hemispheric differences in hippocampal volume predict verbal and spatial memory performance in patients with Alzheimer's disease. Hippocampus. 2000;10(2):136–42. doi: 10.1002/(SICI)1098-1063(2000)10:2<136::AID-HIPO2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test - Second Edition. New York, NY: The Psychological Corporation; 2000. [Google Scholar]
- Ding SL, Van Hoesen GW. Organization and Detailed Parcellation of Human Hippocampal Head and Body Regions Based on a Combined Analysis of Cyto- and Chemoarchitecture. J Comp Neurol. 2015;523(15):2233–53. doi: 10.1002/cne.23786. [DOI] [PubMed] [Google Scholar]
- Duvernoy H, Cattin F, Risold PY. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. 4 ed. New York, NY: Springer Publishing Company. p. 2013;27:40, 187. [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1(1):41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44(1):109–20. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, Eichenbaum H. Distinct roles for dorsal CA3 and CA1 in memory for sequential nonspatial events. Learn Mem. 2010;17(1):12–17. doi: 10.1101/lm.1616209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323–41. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JK, Meikle A, Goodson G, Mayes AR, Howard M, Sünram SI, Cezayirli E, Roberts N. The hippocampus and delayed recall: bigger is not necessarily better? Memory. 1999;7(5-6):715–32. doi: 10.1080/096582199387823. [DOI] [PubMed] [Google Scholar]
- Gertz SD, Lindenberg R, Piavis GW. Structural variations in the rostral human hippocampus. Johns Hopkins Med J. 1972;130(6):367–76. [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci. 2010;30(2):431–8. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Lin JJ, Jones JE, Seidenberg M. The emerging architecture of neuropsychological impairment in epilepsy. Neurol Clin. 2009;27(4):881–907. doi: 10.1016/j.ncl.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Amaral DG. Chapter 24 - Hippocampal Formation A2 - Mai, Jürgen K. In: Paxinos G, editor. The Human Nervous System (Third Edition) San Diego: Academic Press; 2012. pp. 896–942. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kim H. Encoding and retrieval along the long axis of the hippocampus and their relationships with dorsal attention and default mode networks: The HERNET model. Hippocampus. 2015;25(4):500–10. doi: 10.1002/hipo.22387. [DOI] [PubMed] [Google Scholar]
- Luders E, Kurth F, Mayer EA, Toga AW, Narr KL, Gaser C. The unique brain anatomy of meditation practitioners: alterations in cortical gyrification. Front Hum Neurosci. 2012;6:34. doi: 10.3389/fnhum.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Bilder RM, Szeszko PR, Gurbani MN, Hamilton L, Toga AW, Gaser C. Mapping the relationship between cortical convolution and intelligence: effects of gender. Cereb Cortex. 2008;18(9):2019–26. doi: 10.1093/cercor/bhm227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccotta L, Moseley ED, Benzinger TL, Hogan RE. Beyond the CA1 subfield: Local hippocampal shape changes in MRI-negative temporal lobe epilepsy. Epilepsia. 2015;56(5):780–8. doi: 10.1111/epi.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97(8):4398–403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Woollett K, Spiers HJ. London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus. 2006;16(12):1091–101. doi: 10.1002/hipo.20233. [DOI] [PubMed] [Google Scholar]
- Maller JJ, Daskalakis ZJ, Fitzgerald PB. Hippocampal volumetrics in depression: the importance of the posterior tail. Hippocampus. 2007;17(11):1023–7. doi: 10.1002/hipo.20339. [DOI] [PubMed] [Google Scholar]
- Meyers J, Meyers K. Rey Complex Figure Test and Recognition Trial. Lutz, FL: Psychological Assessment Resources; 1996. [Google Scholar]
- Oppenheim C, Dormont D, Biondi A, Lehericy S, Hasboun D, Clemenceau S, Baulac M, Marsault C. Loss of digitations of the hippocampal head on high-resolution fast spin-echo MR: a sign of mesial temporal sclerosis. AJNR Am J Neuroradiol. 1998;19(3):457–63. [PMC free article] [PubMed] [Google Scholar]
- Pohlack ST, Meyer P, Cacciaglia R, Liebscher C, Ridder S, Flor H. Bigger is better! Hippocampal volume and declarative memory performance in healthy young men. Brain Struct Funct. 2014;219(1):255–67. doi: 10.1007/s00429-012-0497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17(5):230–40. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Moscovitch M. A hippocampal marker of recollection memory ability among healthy young adults: contributions of posterior and anterior segments. Neuron. 2011;72(6):931–7. doi: 10.1016/j.neuron.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, McEwen B, Silbersweig D, Stern E. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18(10):985–8. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Dupuis J, Acker J. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12(1):95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Sass KJ, Sass A, Westerveld M, Lencz T, Novelly RA, Kim JH, Spencer DD. Specificity in the correlation of verbal memory and hippocampal neuron loss: dissociation of memory, language, and verbal intellectual ability. J Clin Exp Neuropsychol. 1992;14(5):662–72. doi: 10.1080/01688639208402854. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516–8. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19(12):5034–43. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Simic G, Kostovic I, Winblad B, Bogdanovic N. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer's disease. J Comp Neurol. 1997;379(4):482–94. doi: 10.1002/(sici)1096-9861(19970324)379:4<482::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15(10):655–69. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, De Zubicaray GI, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM, et al. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22(4):1754–66. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Kadish I, Ver Hoef L, Wyss J. The Limbic System. In: Conn PM, editor. Neuroscience in Medicine. 3. Totowa, NJ: Humana Press; 2008. p. 384. [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42(10):1394–413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010;51(3):1242–52. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Amaral RS, Augustinack JC, Bender AR, Bernstein JD, Boccardi M, Bocchetta M, Burggren AC, Carr VA, Chakravarty MM, et al. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: towards a harmonized segmentation protocol. Neuroimage. 2015;111:526–41. doi: 10.1016/j.neuroimage.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6(10):2950–67. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Web References
- http://neurosciencelibrary.org/Specimens/primates/human/sections/sagittal-cell/husa0782.j Date Accessed: 2/22/2017, Source: www.brainmuseum.org
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.