Abstract
Cytoplasmic dynein, a minus-end–directed microtubule motor, has been implicated in many cellular and developmental processes. Identification of specific cellular processes that rely directly on dynein would be facilitated by a means to induce specific and rapid inhibition of its function. We have identified conditional variants of a Caenorhabditis elegans dynein heavy chain (DHC-1) that lose function within a minute of a modest temperature upshift. Mutant embryos generated at elevated temperature show defects in centrosome separation, pronuclear migration, rotation of the centrosome/nucleus complex, bipolar spindle assembly, anaphase chromosome segregation, and cytokinesis. Our analyses of mutant embryos generated at permissive temperature and then upshifted quickly just before events of interest indicate that DHC-1 is required specifically for rotation of the centrosome/nucleus complex, for chromosome congression to a well ordered metaphase plate, and for timely initiation of anaphase. Our results do not support the view that DHC-1 is required for anaphase B separation of spindle poles and chromosomes. A P-loop mutation identified in two independent dominant temperature-sensitive alleles of dhc-1, when engineered into the DHC1 gene of Saccharomyces cerevisiae, conferred a dominant temperature-sensitive dynein loss-of-function phenotype. This suggests that temperature-sensitive mutations can be created for time-resolved function analyses of dyneins and perhaps other P-loop proteins in a variety of model systems.
INTRODUCTION
The active transport of proteins, RNAs, organelles, and chromosomes to specific destinations within cells is essential for normal development. In animal cells, two major classes of force-producing motor proteins, kinesins and dyneins, use the energy generated by ATP hydrolysis to drive the active transport of cargoes along microtubules, which act as directional tracks. Dyneins and kinesins with C-terminal motor domains move toward microtubule minus-ends, whereas kinesins with N-terminal motor domains move toward plus-ends (reviewed in Sawin and Scholey, 1991; Higuchi and Endow, 2002; Vale, 2003). The diverse forms of dyneins and kinesins and substantial experimental work have established that, despite the simplicity of bidirectional microtubule tracks, force generation and transport along them are complex. Specific processes require particular motors or sets of motors, and those motors are subject to precise regulatory controls. Although the full complement of microtubule motors has been identified in several model organisms, their functions and hence the mechanisms of many active transport processes remain poorly defined.
We focus here on questions about the functions of cytoplasmic dynein in mitosis and early patterning in C. elegans embryos. Cytoplasmic dynein is a multisubunit complex composed of two identical ∼500-kDa heavy chains (DHCs) and several intermediate, light intermediate, and light chains (reviewed in Milisav, 1998; King, 2000; Vale, 2003). DHC is the force-producing component of the complex (Gibbons et al., 1991; Ogawa, 1991; Asai and Koonce, 2001). The globular DHC head encompasses six AAA domains, four of which contain a conserved nucleotide-binding P-loop motif (Gibbons et al., 1991; Ogawa, 1991; King, 2000). Projecting from the head is an intrachain coiled-coil stalk extension that mediates microtubule binding (Gee et al., 1997; Koonce, 1997). The N-terminal tail of DHC interacts with the intermediate, light intermediate, and light chains and with the p150Glued component of dynactin, a multisubunit, dyneinassociated complex. These interacting proteins help regulate the activity, cargo specificity, and subcellular localization of dynein (reviewed in Milisav, 1998; Asai and Koonce, 2001; Vale, 2003).
Dynein is thought to participate in a variety of processes, including nuclear migration, centrosome separation, spindle formation, spindle alignment, chromosome segregation, nuclear envelope breakdown, mRNA localization, and organelle transport (Eshel et al., 1993; Li et al., 1993; Vaisberg et al., 1993; McGrail and Hays, 1997; Harada et al., 1998; Gönczy et al., 1999; Sharp et al., 2000a,b; Salina et al., 2002; Goshima and Vale, 2003). However, interpretation of the results of specific dynein inhibition experiments is complicated by the myriad of potential dynein functions. A failure of early dynein-dependent processes can have dramatic indirect effects on later processes of interest. Hence, a means to inactivate dynein function on a short timescale would be valuable.
We have determined that the let-354 complementation group in C. elegans (Howell and Rose, 1990; Mains et al., 1990) corresponds to the dhc-1 gene, which encodes a cytoplasmic dynein heavy chain, DHC-1 (Lye et al., 1987). Six alleles encode temperature-sensitive (ts) variants of DHC-1, and two of them have a missense mutation in a conserved P-loop residue. When we engineered it into the DHC1 gene in budding yeast, that mutation caused a severe dhc1 mutant phenotype that is ts. Using the C. elegans dhc-1 ts alleles and a microscope stage whose temperature could be adjusted rapidly, we have addressed questions about specific dynein functions in early embryogenesis. By allowing mutant embryos to undergo early development at permissive temperature and then inactivating DHC-1 with a fast temperature shift just before an event of interest, we found evidence that dynein is required directly for rotation of the centrosome/nucleus complex onto the proper embryonic axis, for correct chromosome congression, and for timely initiation of anaphase. We did not find evidence that dynein is directly needed for anaphase spindle pole separation or for cytokinesis.
MATERIALS AND METHODS
Worm Strains
C. elegans strains were maintained as described by Brenner (1974). N2 variety Bristol was used as the wild-type strain. Other strains used in this study were as follows: KR1584 let-354(h934) dpy-5(e61) unc-13(e450)/szT1[lon-2(e678) unc-29(e403)] I; +/szT1 X, and similar strains containing other let-354 recessive alleles: h72, h79, h90, h201, h267, h370, h390, h441, h482ts, h504, h508, h549, h693, h803, h809, h819, h841, h863, h866; SS578 let-354(h934) dpy-5(e61) unc-13(e450)/gaDp1 (I;f); BW503 let-354(ct42ts)/unc-11(e47) dpy-5(e61) I, and similar strains containing other let-354 dominant ts alleles: ct76ts, ct77ts, and sb42ts; EU654 dhc-1(or195ts) I; AZ212 unc-119(ed3); ruIs32[unc119(+) pie-1::gfp::histone H2B] III; WH204 unc-119(ed3); ojIs1[unc119(+) pie-1::gfp::tbb-2]; TH32 unc-119(ed3); ruIs32[unc119(+) pie-1::gfp::histone H2B] III; ddls6 [unc119(+) pie-1::gfp::tbg-1]; SS645 let-354(ct76ts)/unc-11(e47) dpy-5(e61) I; ruIs32[unc119(+) pie-1::gfp::histone H2B] III; SS651 let-354(ct76ts)/unc-11(e47) dpy-5(e61) I; ojIs1[unc119(+) pie-1::gfp::tbb-2]; SS824 dhc-1(or195ts) I; ruIs32[unc119(+) pie-1::gfp::histone H2B] III; ddls6 [unc119(+) pie-1::gfp::tbg-1]; and KR1737 hDf6 dpy-5(e61) unc-13(e450)I; hDp31(I;f).
Strains were kindly provided by Paul Mains (University of Calgary, Calgary, Alberta, Canada), Ann Rose (University of British Columbia, Vancouver, British Columbia, Canada), D. Hamill (Ohio Wesleyan University, Delaware, OH), B. Bowerman (University of Oregon, Eugene, OR), J. White (University of Wisconsin, Madison, WI), A. Hyman (Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany), A. Desai (University of California, San Diego, San Diego, CA), and the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN).
Transformation Rescue
let-354(h934) dpy-5 unc-13/gaDp1 adult hermaphrodites were injected with a mixture of 2 ng/μl cosmid T21E12 DNA (linearized with SacII), 2 ng/μl cosmid ZK973 DNA (linearized with NotI), and 100 ng/μl pRF4 rol-6(su1006) DNA (linearized with SmaI). Cosmid DNA was prepared using the QIAGEN (Valencia, CA) maxiprep protocol, and plasmid DNA was prepared using the QIAGEN miniprep protocol. Two independent F1 lines of rescued let-354(h934) dpy-5 unc-13 worms were obtained. Those lines were Dpy Unc, which obscured the Rol-6 phenotype caused by the presence of a rol-6(su1006)-bearing extrachromosomal array, so the presence of an array was confirmed in two ways. Mating of rescued Dpy Unc hermaphrodites to N2 males resulted in the production of some Rol-6 outcross progeny. Rescued Dpy Unc hermaphrodites fixed and stained with Hoechst 33342 contained in their oocytes six bivalents and an extrachromosomal DNA mass.
Sequencing
Genomic DNA was extracted from let-354/unc-11 dpy-5 heterozygous hermaphrodites bearing each of three dominant ts alleles of let-354 (ct42, ct76, and ct77) by using a standard phenol/chloroform extraction method (Maniatis et al., 1982) followed by column purification using QIAGEN Genomic 100/G columns. DNA segments spanning the predicted coding region (∼15 kb) of dhc-1 were polymerase chain reaction (PCR) amplified and sequenced by SeqWright DNA Sequencing (Houston, TX). We confirmed mutant changes by targeted sequencing of specific portions of PCR-amplified dhc-1 DNA from five to 10 heterozygous worms and from five to 10 N2 worms, by using an ABI 3700 prism automated fluorescent sequencer (Indiana University Molecular Biology Institute, Bloomington, IN).
Yeast Transformation
Yeast strains YEF473a and YEF473a dhc1Δ::HIS3 and plasmid CYDHC/GEM containing the full-length yeast dynein heavy chain gene DHC1 (a gift from K. Bloom, University of North Carolina, Chapel Hill, NC) were used to test whether the P-loop mutation found in let-354(ct77) and (ct42) would cause a dominant ts dhc1 mutant phenotype in yeast. The mutation was engineered into the yeast DHC1 gene by using PCR-based site-directed mutagenesis. A 1-kb fragment containing the third P-loop of DHC1 was cut from CYDHC/GEM, PCR amplified using primers 5′ GCG AAG AGT TCC GGA GTG CAT TAT TCA TAA TCA TTG TTT TAC CAG ATT CAG GTG GCC CAC AAA GG 3′ and 5′ ATA TGG TGG GTG CTT CTT CG 3′, and reintroduced back into the CYDHC/GEM plasmid to generate CYDHC*/GEM. To allow expression in yeast, the CYDHC* insert was transferred into pRS316 plasmid (a gift from A. Bender, Indiana University) and introduced into yeast by using either electroporation (protocol adapted from D. Gottschling, Fred Hutchinson Cancer Research Center, Seattle, WA; http://iprotocol.mit.edu/protocol/100.htm) or lithium acetate (Walhout and Vidal, 2001). Transformed yeast were selectively grown on URA- plates, and then grown for 1–4 h at 16 or 30°C in YPD medium (BD Biosciences Clontech, Palo Alto, CA). Cells were fixed by standard methanol/acetone fixation, stained with 4,6-diamidino-2-phenylindole (DAPI), and scored for one nucleus or two or more nuclei on a Zeiss (Carl Zeiss, Thornwood, NY) Axioskop by using differential interference contrast (DIC) and fluorescence microscopy. To confirm the sequence change, plasmid was isolated from transformed yeast and the relevant region was sequenced.
Analysis of Embryonic Development by Time-Lapse DIC and Confocal Fluorescence Microscopy
Embryos were mounted in M9 buffer (Brenner, 1974) on 2% agarose pads containing a fine-Teflon PTFE-insulated probe connected to a Cole-Parmer Instrument (Vernon Hills, IL) Digisense thermocouple thermometer to monitor temperature and covered with a coverslip. DIC images were obtained on a Zeiss Axioplan microscope with a Hamamatsu C2400 camera and video controller with an Argus-10 image processor (Hamamatsu City, Japan). Images were captured every 3 s by using NIH Image (version 1.62b7, developed by Wayne Rasband, National Institutes of Health, Bethesda, MD and available at http://rsb.info.nih.gov/nih-image). Fluorescence images were obtained on a Nikon Optiphot microscope with a Bio-Rad MRC600 scanning confocal system (Hercules, CA) and collected as a time series by using MRC600 software. Images were captured every 6 s for green fluorescent protein (GFP)::histone time-lapse movies or every 10 s for GFP::tubulin time-lapse movies. Stacks of images were manipulated to generate figures in NIH Image.
Rapid temperature-upshift experiments were performed using a temperature-control microscope stage (Supplemental Figure s1) in a microscope room held at 16°C. Embryos were prepared and mounted on slides with agar pads at 16°C, placed on the microscope stage, observed, and shifted to 25°C just before an event of interest. Based on the read-out from a thermocouple probe mounted in the agar pad immediately adjacent to embryos, a shift from 16 to 25°C took 24–40 s.
Antibodies and Immunofluorescence Microscopy
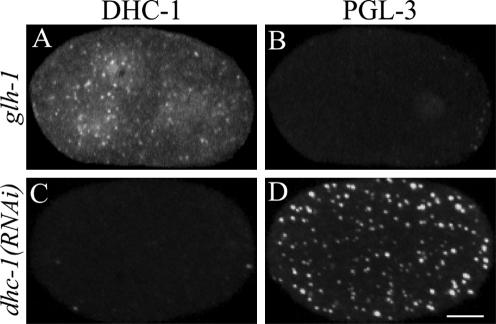
To generate antisera against C. elegans DHC-1, a 17-amino acid peptide, MDSGNESSIIQPPNLKC, corresponding to the N-terminal 16 amino acids plus an additional cysteine, was synthesized and conjugated to maleimide-activated keyhole limpet hemocyanin (Research Genetics, Huntsville, AL). The peptide was used to immunize rabbits (Cocalico Biologicals, Reamstown, PA). Affinity-purified antibodies were prepared by passage over a column of DHC-1 peptide coupled to Sulfolink coupling gel (Pierce Chemical, Rockford, IL). Bound antibodies were eluted with 100 mM glycine, pH 2.5, dialyzed against phosphate-buffered saline, and concentrated (5-fold). Figure 1 demonstrates that the antibody is specific for DHC-1.
Figure 1.
Test of anti-DHC-1 antibody specificity. Rabbit antibodies were raised against the amino terminal 16 amino acids of DHC-1, affinity purified, and used to stain dhc-1(+) glh-1(gk100) embryos and dhc-1(RNAi) embryos. Both types of embryos were dissected, fixed, and stained together on the same slide to ensure identical treatment. glh-1 mutant embryos lack detectable staining by rat anti-PGL-3 (a marker for P granules) (Meyer and Strome, unpublished data), allowing control dhc-1(+) glh-1 embryos to be distinguished from dhc-1(RNAi) embryos. (A and B) Control 2-cell embryo containing DHC-1 (A) and lacking PGL-3 (B). (C and D) Nearby 1-cell RNAi embryo lacking detectable DHC-1 (C) and containing PGL-3 (D). Images are projections of Z-series from a spinning disk confocal fluorescence microscope. Embryos in this and subsequent figures are oriented with posterior to the right. Bar, 10 μm.
For immunofluorescent staining of embryos, adult hermaphrodites were cut, fixed, and stained as described previously (Strome and Wood, 1983). The primary antibodies used were anti-DHC-1 at 1:200, anti-DNC-1 at 1:200 (a gift from H. Zhang and J. White, University of Wisconsin), and mouse 4A1 anti-α-tubulin at 1:50 (a gift from M. Fuller, Stanford University School of Medicine, Stanford, CA; Piperno and Fuller, 1985). The secondary antibodies used were Alexa488 goat anti-mouse and Alexa594 goat anti-rabbit (Molecular Probes, Eugene, OR). Embryos were counterstained with DAPI to visualize DNA. Anti-DHC-1 and anti-tubulin images were collected as Z-series of 1.0-μm slices on a Bio-Rad MRC600 microscope, or on a PerkinElmer Ultraview LCI30E spinning disk confocal microscope by using Ultraview software (PerkinElmer Life and Analytical Sciences, Boston, MA). DNA images were collected with a Hamamatsu ORCA-ER digital camera on a Nikon (Tokyo, Japan) Eclipse E800 microscope with MetaMorph (Universal Imaging, Downingtown, PA) software. Images were processed using Photoshop 7.0 (Adobe Systems, Palo Alto, CA).
RNA Interference (RNAi)
dhc-1 cDNA clones yk27b2 and yk35d8 were obtained from Y. Kohara (National Institute of Genetics, Mishima, Japan). Phagemid DNA and double-stranded RNA (dsRNA) for injection were prepared as described previously (Strome et al., 2001). dsRNA at a concentration of 1 mg/ml was injected into young adult hermaphrodites. Embryos produced by injected parents were analyzed 18–24 h postinjection. A dhc-1 dsRNA feeding construct was engineered by transferring a 3.1-kb dhc-1 cDNA fragment into plasmid L4440 and transforming HT115(DE3) bacterial cells (Invitrogen, Carlsbad, CA). The dsRNA-expressing bacteria were grown using the protocol of Kamath et al. (2000).
RESULTS
Temperature-sensitive Alleles of C. elegans dhc-1
To pursue studies of cytoplasmic dynein in early C. elegans embryos, we sought genetic mutations that would inhibit the function of its force-producing subunit, DHC-1. Two findings suggested that the let-354 complementation group represents alleles of the dhc-1 gene: 1) let-354 maps genetically to a region of chromosome I that contains dhc-1 (Howell and Rose, 1990; Mains et al., 1990; The C. elegans Sequencing Consortium, 1998), and 2) RNAi depletion of DHC-1 causes developmental defects similar to those seen in let-354 mutants (see below; Mains et al., 1990; Gönzcy et al., 1999). To determine whether the let-354 complementation group indeed corresponds to the dhc-1 gene, we first tested whether dhc-1 genomic DNA could rescue the larval lethal mutant phenotype of let-354 recessive alleles. Cotransformation of let-354(h934)/+ hermaphrodites with two overlapping cosmids (T21E12 and ZK973), which together encompass the dhc-1 coding sequence, as well as 13 other predicted genes, generated extrachromosomal transgenic arrays that rescued the lethality of let-354(h934)/let-354(h934) mutant progeny.
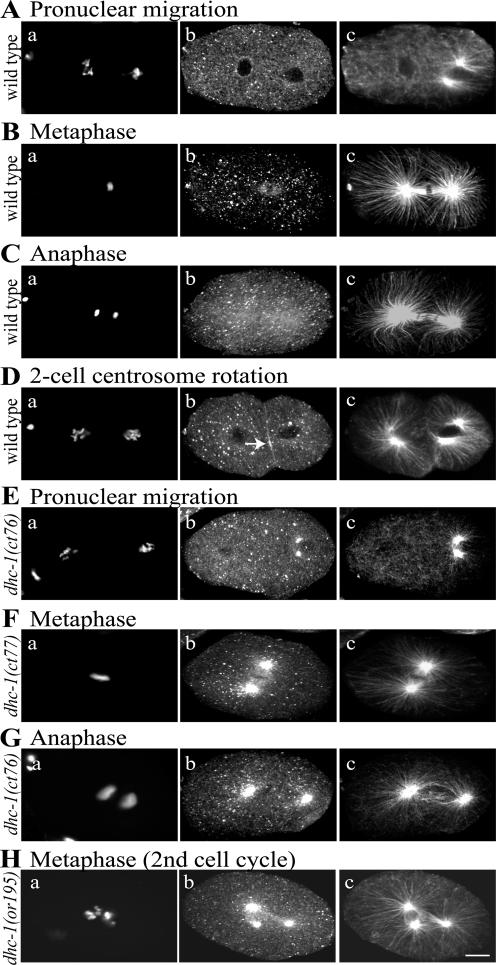
To determine whether let-354 mutations affect the expression or distribution of DHC-1, we compared DHC-1 staining in wild-type and mutant embryos (Figure 2), focusing on the dominant ts let-354 alleles isolated by Paul Mains (ct42, ct76, ct77, and sb42) (Mains et al., 1990). Animals homozygous for those alleles are not viable at any temperature. Heterozygotes are viable at 16°C and maternal-effect lethal at 25°C (see below). Affinity-purified antibodies revealed a distribution of DHC-1 in wild-type embryos similar to that described previously (Gönczy et al., 1999): punctate in cytoplasm, an elevated concentration on nuclear envelopes during pronuclear migration, enrichment in the central spindle during metaphase, and faint enrichment over the entire anaphase spindle (Figure 2, A–C). We also noted a transient accumulation of DHC-1 in a narrow cortical zone between the AB and P1 cells during rotation of the P1 centrosome-centrosome axis onto the anterior-posterior (AP) axis (Figure 2D). This supports the hypothesis that cortically anchored dynein in that cortical zone pulls on astral microtubules to generate force for the rotation (Skop and White, 1998).
Figure 2.
DHC-1 distribution in wild-type and dhc-1 embryos. Fixed embryos were stained with DAPI (a), affinity-purified rabbit anti-DHC-1 (b), and mouse anti-tubulin (c). Images in panels a are from a widefield fluorescence microscope. Images in b and c are projections of Z-series from a scanning confocal fluorescence microscope. All embryos displayed punctate DHC-1 staining in the cytoplasm; other localized concentrations are described below. (A–D) Wild-type embryos. (A) DHC-1 is lightly concentrated around the oocyte pronuclear envelope during pronuclear migration. (B) DHC-1 is lightly concentrated on the central spindle at metaphase; there is no concentration on spindle poles. (C) DHC-1 is faintly concentrated on the anaphase spindle and spindle poles. (D) During rotation of the P1 cell centrosome/nucleus complex (on the right), DHC-1 is concentrated at the cell periphery between AB and P1 in the region (arrow) toward which one centrosome (the lower one in c) is moving. (E–H) dhc-1 one-cell embryos from ct76/+, ct77/+, or or195 hermaphrodites shifted to 25°C for 24 h. At all stages, DHC-1 shows the normal distribution and in addition a dramatic accumulation on the microtubule organizing centers, initially the centrosomes associated with the sperm pronucleus (E) and later the poles of the mitotic spindle (F–H). Mutant embryos like that shown in H are multipolar due to continued centrosome replication after a failed first cleavage. The dhc-1 embryos shown in this figure contain uncharacteristically well-formed spindles, to highlight the concentration of DHC-1 on spindle poles. Similar pole concentrations also were observed in mutant embryos with small or monopolar spindles. Bar, 10 μm.
Embryos derived from mothers heterozygous for any of the dominant ts alleles of let-354 that had been cultured at 25°C for 24 h showed abnormally heavy concentrations of DHC-1 at prophase centrosomes and mitotic spindle poles (Figure 2, E–G). A recessive ts dhc-1 allele, or195 (Hamill et al., 2002), caused a similar accumulation of DHC-1 on centrosomes at 25°C (Figure 2H). Interestingly, DHC-1 concentration on centrosomes also was seen at permissive temperature (16°C) with both dominant and recessive ts alleles (Supplemental Figure s2, E and F). To determine whether the accumulation reflected aberrant behavior of the entire dynein–dynactin complex, we tested the effects of let-354 ts alleles on the distribution of DNC-1, the dynactin p150Glued ortholog in worms. It, too, concentrated heavily at centrosomes (Supplemental Figure s2, C and D). These observations, showing that both recessive and dominant ts let-354 alleles cause the dynein–dynactin complex to mislocalize, are consistent with the identity of let-354 as dhc-1.
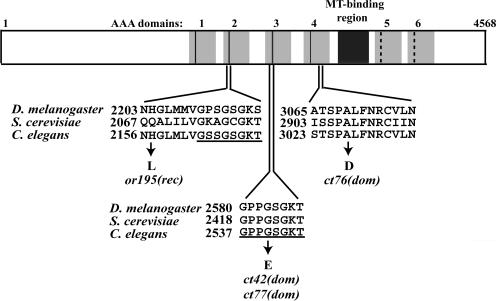
To search for specific let-354 ts mutant changes, we sequenced the dhc-1 coding regions of three dominant ts strains (Figure 3). In ct76, a conserved alanine in the Box VI motif of the fourth AAA domain is changed to an aspartic acid. In ct42 and ct77, the same G→A base change replaces glycine2540 in the third P-loop with glutamic acid. P-loops, with a consensus of GxxgxGKT/S (where x is anything and g is common), are key features of ATP-binding sites in dynein heavy chains and a variety of other NTPases, including myosins, kinesins, ATP synthase, and Ras (Walker et al., 1982; Gibbons et al., 1991; Shen et al., 1994; Kull et al., 1996; Deyrup et al., 1998). Glycine2540 corresponds to “g” at position 4 in the P-loop consensus. These results confirmed that the let-354 complementation group corresponds to the dhc-1 gene and raised the possibility that dynein heavy chains in other organisms might be rendered temperature sensitive by equivalent mutations.
Figure 3.
Amino acid changes caused by ts mutations in the dhc-1 gene. The six AAA domains are shown as gray boxes. The first four AAA domains have conserved P-loops (black lines), whereas the fifth and sixth AAA domains have diverged P-loops (dashed black lines). The coiled-coil, microtubule-binding stalk region is shown as a black box. The dominant ts dhc-1 allele ct76 contains a mutation (G→A) that causes an A3027D replacement in the Box VI element within AAA4. Two other dominant ts dhc-1 alleles, ct42 and ct77, both contain the same base pair substitution (G→A), which results in a G2540E change in the third P-loop (underlined). The recessive ts dhc-1 allele or195 has a mutation that causes an H2157L replacement near the second P-loop (underlined; Hamill et al., 2002). Accession numbers: Drosophila, P37276; S. cerevisiae, P36022; and C. elegans, NP491363.
An Engineered Dominant Temperature-sensitive DHC1 in Budding Yeast
To determine whether the ct42-ct77 P-loop mutation has a dominant ts effect on dynein in other species, the equivalent G-to-E codon change was tested in the DHC1 gene of Saccharomyces cerevisiae. A deletion-disruption mutation of DHC1 (dhc1Δ) impairs translocation of the daughter nucleus to the bud-cell at the end of mitosis. It is not lethal but causes a significant percentage of mutant cells to carry two or more nuclei (Eshel et al., 1993; Li et al., 1993). Yeast cells were transformed with either a mutant dhc1(G-E) gene or a wild-type DHC1 gene in a low copy number plasmid. The effects of each on dynein function were tested in wild-type and dhc1Δ backgrounds by counting multinucleate cells after growth at 16 or 30°C (Table 1; statistical analysis is in Supplemental Table s1). At both temperatures, the wild-type transgene had little effect on wild-type cells and substantially reduced the multinucleate phenotype of dhc1Δ cells. In contrast, the dhc1(G-E) transgene in the wild-type background at 30°C caused multinucleate cells at a frequency equivalent to that observed in dhc1Δ (∼17%). That effect was significantly reduced at 16°C but was not eliminated (3.2%). Interestingly, in the dhc1Δ background at 30°C, the dhc1(G-E) transgene caused a modest but significant increase in the percentage of multinucleate cells (23%) relative to that observed with dhc1Δ alone (17%), suggesting that the mutant motor has a slightly inhibitory effect on the dynein-independent mechanism for nuclear migration. These results demonstrate that the C. elegans dhc-1(ct42-ct77) G-E change, when placed in DHC of an evolutionarily distant organism, causes a dominant ts phenotype. This confirms in vivo that the third P-loop is essential for proper dynein function (Silvanovich et al., 2003; Reck-Peterson and Vale, 2004) and highlights the possibility that equivalent mutations can be used to create dominant ts versions of a variety of other P-loop proteins.
Table 1.
Dominant ts effect of a G→E change in the third P-loop of yeast DHC-1
| Yeast strain | Plasmid | Temp. (°C) | % Cells with >1 nucleusa | nb |
|---|---|---|---|---|
| Wild type | 30 | 0.2 | 403 | |
| 16 | 0 | 298 | ||
| Wild type | dhc1(G→E)c | 30 | 17.4 | 339 |
| 16 | 3.2 | 219 | ||
| Wild type | DHC1d | 30 | 0 | 328 |
| 16 | 0.8 | 380 | ||
| dhc1Δ | 30 | 17.3 | 347 | |
| 16 | 28.2 | 298 | ||
| dhc1Δ | dhc1(G→E)c | 30 | 23.2 | 387 |
| 16 | 31.2 | 269 | ||
| dhc1Δ | DHC1d | 30 | 2.1 | 334 |
| 16 | 4.0 | 373 |
Transformed or untransformed haploid cells were grown for 2—4 in YPD medium after selection on URA- plates. Statistical analysis of the data is in Supplemental Table s1.
Yeast cells were fixed and stained with DAPI. Dividing cells (i.e. cells with buds) were scored for the presence of more than one nucleus
Total number of dividing cells
Plasmid containing full-length dhc1 with a mutation that causes Gly2421 to be replaced by Glu
Plasmid containing full-length wild-type DHC-1
Processes Influenced by Inhibition of DHC-1
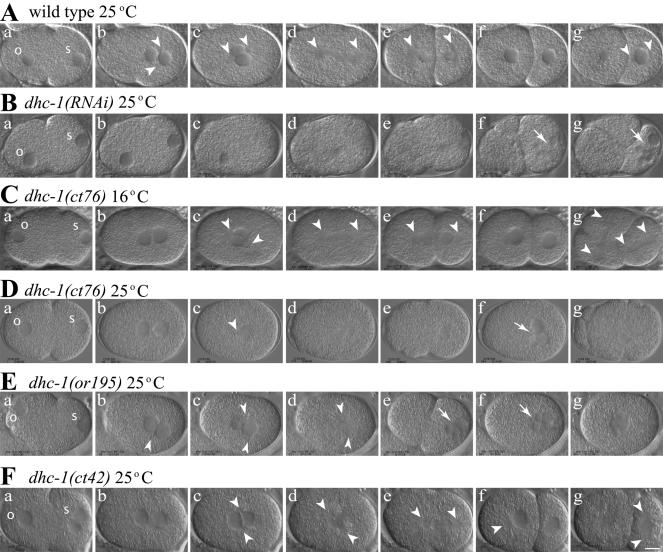
To assess which events in early embryogenesis are impaired by dhc-1 mutations, we compared wild-type, dhc-1(RNAi), and dhc-1 ts mutant embryos by time-lapse DIC microscopy (Figure 4, Videos 1–6, and Table 2). The progression of events in wild-type is illustrated in Figure 4A and in Video 1 (supplemental data). As described previously by Gönczy et al. (1999), RNAi depletion of DHC-1 from the maternal germline results in severe defects in 1-cell embryos (Figure 4B, Video 2, and Table 2). In embryos produced 24 h after dsRNA injection, the sperm pronucleus did not leave the posterior cortex, the oocyte pronucleus did not show a fast phase of migration, and hence pronuclei failed to meet before nuclear envelope breakdown (NEB) (Figure 4B, a–d). A bipolar spindle was not evident, and cytokinesis did not occur. Instead, RNAi embryos underwent dramatic cortical blebbing, and multiple nuclei formed in the posterior cytoplasm during telophase (Figure 4B, f and g). By 36 h after RNA injection, existing oocytes were aberrant and the gonad stopped producing new oocytes. This indicates that DHC-1 serves essential roles in the maternal germline and raises the possibility that defects in germline physiology contribute nonspecifically to the phenotypes observed in dhc-1(RNAi) embryos.
Figure 4.
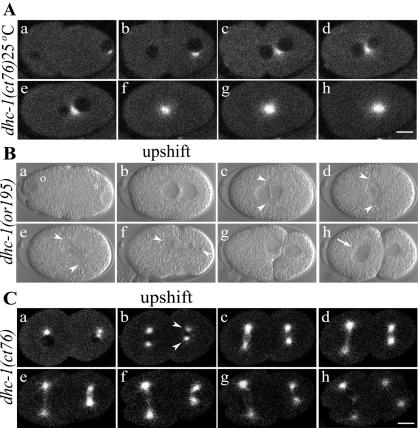
Early development of wild-type, dhc-1(RNAi), and dhc-1 mutant embryos. Each image is a single frame from a time-lapse movie of a live embryo captured using DIC microscopy. Arrowheads indicate centrosomes, and arrows point to micronuclei. Oocyte pronuclei are marked with “o” and sperm pronuclei with “s” in the first frame of each series. In all cases (except C), embryos were dissected from hermaphrodites 24 h after a shift from 16 to 25°C, initiated when the mothers were L4 larvae. (A and Video 1) Wild-type embryo. (A, a and b) Pronuclear migration and meeting. (A, c) Rotation of the centrosome/nucleus complex. (A, d) Anaphase with a shift of the posterior spindle pole toward the posterior cortex. (A, e) Posterior spindle pole flattening. (A, g) P1 centrosome axis alignment with the AP axis. (B and Video 2) dhc-1(RNAi) embryo. (B, a–c) Pronuclei failed to migrate. (B, d and e) Bipolar spindle assembly failed. (B, f and g) Chromosome separation failure produced multiple micronuclei. (B, e–g) Cytokinesis failed, although numerous transient membrane invaginations formed. (C and Video 3) Embryo from a dhc-1(ct76)/+ hermaphrodite raised at permissive temperature (16°C). All events resembled wild type, except that the posterior spindle pole did not flatten (C, e). (D and Video 4) Embryo from a dhc-1(ct76)/+ hermaphrodite. (D, a and b) Pronuclei met more centrally than in wild type. (D, c–e) Centrosome separation failed, and a bipolar spindle failed to form. (D, d and e) Posterior shift of the monopolar spindle. (D, f and g) Chromosome separation and cytokinesis failed. (E and Video 5) Embryo from a dhc-1(or195) hermaphrodite. (E, a and b) A minority case in which pronuclei met at a normal posterior position. (E, b–d) Centrosomes separated, but the centrosome axis failed to rotate and a stunted spindle formed on the transverse axis. (E, d and e) Posterior shift of the spindle. (E, e–g) Chromosome separation and cytokinesis failed. (F and Video 6) Embryo from a dhc-1(ct42)/+ hermaphrodite. (F, a) Occasionally two sperm pronuclei were observed in mutant embryos. (F, b) The pronuclei met near the center. (F, c and d) Centrosomes separated, but the centrosome axis did not rotate fully onto the AP axis. (F, d–f) A small spindle formed and moved toward the posterior cortex, chromosomes separated, and cytokinesis occurred. (F, g) The centrosome axis failed to rotate in P1, and cytokinesis failed in AB. Bar, 10 μm.
Table 2.
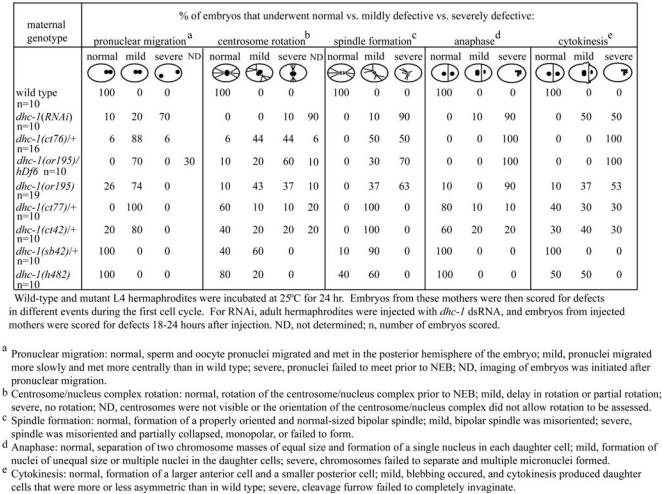
Summary of defects observed in dhc-1 (RNAi) and dhc-1 mutant embryos.
Our analysis of events in dhc-1 mutant embryos was performed with six ts alleles: the dominant ct42, ct76, ct77, and sb42, plus the recessive or195 and h482 (Howell and Rose, 1990; Mains et al., 1990; Mitenko et al., 1997; Hamill et al., 2002). The 19 nonconditional recessive alleles result in larval lethality: homozygous progeny from heterozygous mothers undergo apparently normal embryo development, hatch, and arrest at a midlarval stage (Howell and Rose 1990). The different ts alleles of dhc-1 create different pools of DHC-1 dimer. Early embryos from mothers homozygous for a recessive ts allele contain fully mutant dimers, whereas early embryos from mothers heterozygous for a dominant ts allele are presumed to contain a mix of fully mutant dimers, fully wild-type dimers, and heterodimers with one mutant and one wild-type subunit. To simplify wording below, embryos derived from both types of mothers will be referred to as dhc-1 ts mutant embryos. dhc-1 ts embryos displayed low levels of embryonic lethality at permissive temperature (16°C) and 100% lethality at elevated temperature: 18.4°C in ct76 and 20°C in ct77-ct42 (Supplemental Table s2).
Our initial studies of the conditional mutants involved shifting mutant mothers (as L4 larvae) to a restrictive temperature (25°C) for 24 h and then analyzing their early embryos by time-lapse DIC microscopy at 25°C. The embryonic processes affected by this temperature regime were similar for all the ts alleles, but the severities of defects varied, indicating an allelic series as follows: strongest— ct76, or195, ct77-ct42, sb42, h482—weakest. To test the severity of a strong allele over a null, we examined embryos from worms carrying the recessive allele or195 over a deficiency of the region, hDf6 (Table 2). The phenotypes of embryos from dhc-1(or195)/hDf6 mutants at 25°C were slightly more severe than those of dhc-1(or195)/dhc-1(or195) and similar to those of dhc-1(ct76). This suggests that although neither or195 nor ct76 is a null allele, they do cause a nearly complete loss of function. We saw no evidence of a neomorphic gain of function here or in other tests.
Pronuclear Migration. Although dhc-1 ts mutant embryos at 25°C showed some defects in meiosis (our unpublished data), normal pronuclei usually formed (Figure 4, D and E, and Videos 4 and 5). In ct76 embryos (Figure 4D and Video 4), the female pronucleus seemed to migrate slowly and met the advancing male pronucleus near the midpoint of the embryo rather than in the posterior half (Figure 4D, b). To quantify this effect, the position of the center of the female pronucleus was measured as a function of time. In wild-type embryos (n = 10), an initial “slow phase” of migration (0.035 ± 0.004 μm/s) moved the female pronucleus to ∼37% of embryo length (EL; anterior pole is 0%, posterior pole is 100%); a subsequent “fast phase” (0.26 ± 0.03 μm/s) moved it to the sperm pronucleus at 69 ± 4% EL. In ct76 embryos (n = 10), the slow phase was normal (0.035 ± 0.004 μm/s), but the fast phase was only 0.13 ± 0.07 μm/s and the pronuclei met at 58 ± 3% EL. Thus, the ct76 lesion reduces but does not eliminate the fast phase of migration. Other dhc-1 ts alleles also affect pronuclear migration, causing the pronuclei to meet more centrally than in wild type (Supplemental Table s3)
Centrosome Movements and Spindle Formation. It was evident that restrictive temperature also affected the behavior of centrosomes in dhc-1 ts mutant embryos. In wild type (Figure 4A and Video 1), newly duplicated centrosomes on the male pronucleus move away from one another, separating to opposite sides, usually producing a centrosome-centrosome axis that lies transverse to the AP axis of the embryo. As male and female pronuclei meet, the centrosome/nucleus complex rotates 90° to align the centrosomes on the AP axis (Figure 4A, b–d). In embryos from dhc-1 ts mutant mothers cultured at permissive temperature, these processes looked normal (e.g., Figure 4C and Video 3). Of 16 dhc-1(ct76) embryos grown at restrictive temperature, centrosome separation failed completely in five, leading to formation of a monopolar first spindle (Figures 4D, b–d, and 5A). In the remaining 11 embryos, centrosome separation was delayed until after the pronuclei met. In some cases, delayed separation was transverse to the AP axis, and in other cases it was along the AP axis. Rotation of the centrosome/nucleus complex failed or was partial in most embryos with transversely separated centrosomes (Table 2). In all cases with full separation, centrosomes collapsed toward one another after NEB, and abnormally small spindles formed. In or195, ct77, and ct42 embryos, initial centrosome separation was successful, centrosomes usually collapsed toward one another after NEB, and small spindles formed. Rotation onto the AP axis was often defective (Figure 4E, d and F, d; Videos 5 and 6; and Table 2), especially in or195, which is the strongest of the three alleles. Overall, our analysis of dhc-1 ts mutant and RNAi embryos as well as the RNAi studies of Gönczy et al. (1999) agree that loss of cytoplasmic DHC function inhibits centrosome separation, causes separated centrosomes to collapse toward one another after NEB, and inhibits rotation of the centrosome axis into alignment with the AP axis.
Figure 5.
Rotation of centrosomes fails in dhc-1 ts mutant embryos upshifted just before the rotation period. (A) Time-lapse confocal fluorescence images of an embryo from a dhc-1(ct76)/+ hermaphrodite expressing GFP::β-tubulin. Embryos generated at 25°C (Figure 4D) or shifted to 25°C early, in this case at the beginning of pronuclear migration (a), fail in centrosome separation (b–h). (B and Video 8) Time-lapse DIC microscopy images of a dhc-1(or195) embryo upshifted from 16 to 25°C after centrosome separation and pronuclear migration (b). The centrosome/nucleus complex failed to rotate from the transverse onto the AP axis, and after NEB the mitotic spindle formed transverse to the AP axis (c and d, arrowheads point to centrosomes in c and spindle poles in d) (see Figure 4, A and C, for normal rotation). Subsequent movement of one spindle pole toward the posterior cortex during anaphase eventually resulted in spindle orientation and position that resembled wild type (e and f). Chromosome segregation and cytokinesis occurred but were defective, as evidenced by the aberrant cleavage furrow (g) and the double nucleus in AB (h, arrow). (C and Video 9) Time-lapse confocal images of a two-cell embryo from a dhc-1(ct76)/+; GFP::β-tubulin hermaphrodite. The embryo was upshifted after the centrosomes in P1 (b, arrowheads) had begun to separate. The centrosome/nucleus complex failed to rotate (c and d), and a mitotic spindle formed transverse to the AP axis (d–g). Bar, 10 μm.
Anaphase. Time-lapse DIC movies revealed that anaphase chromosome separation failed in the most severe dhc-1 ts mutants (Figure 4, D and E, and Table 2). In wild type (Figure 4A and Video 1), anaphase consists of spindle elongation (anaphase B) with no chromosome-to-pole movement (anaphase A) (Oegema et al., 2001). The anterior pole moves little, whereas the posterior pole moves toward the posterior cortex and oscillates dramatically from side to side, rocking the spindle. The posterior centrosome subsequently flattens, whereas the anterior centrosome remains spherical. In embryos from dhc-1 ts mutant mothers held at permissive temperature, these processes looked normal, except that posterior centrosome rocking and flattening did not occur in the five strongest ts alleles (Videos 3 and 7; our unpublished data). At restrictive temperature in severe ts mutants (ct76 and or195), stunted bipolar spindles rarely elongated and chromosomes did not separate (Figure 4, D and E; Videos 4 and 5; and Table 2). Notably however, during anaphase the monopolar or small bipolar spindles did migrate toward the posterior cortex, leading to formation of micronuclei in the posterior hemisphere. Subsequently, a cleavage furrow usually formed, but cytokinesis was not successful. Subsequent cell cycles continued and aberrant mitosis/cytokinesis resulted in multinucleate, sometimes multicellular, embryos. The less severe ts mutants often completed the first division cycle successfully and then failed in later cycles (Figure 4F, Video 6, and Table 2).
To investigate whether DHC-1 contributes to the posterior spindle shift that ensures an asymmetric P0 division in normal embryos, we measured changes in the position of the posterior centrosome as a function of time (Supplemental Table s3). After pronuclei met and moved to the center of the embryo, the position of the point of contact between the two pronuclei (the approximate position of both centrosomes) was not distinguishable in wild-type versus dhc-1 mutant embryos (Supplemental Table s3). During the ensuing mitosis in wild type, the posterior centrosome moved at a net rate of 0.067 ± 0.005 μm/s to a position 9.9 ± 0.6 μm from the posterior cortex. In dhc-1 ts mutant embryos, that rate was modestly reduced (0.056 ± 0.010 for ct76; 0.048 ± 0.013 for or195; Supplemental Table s3), but the final position of the posterior centrosome was similar to wild type. Thus, either residual DHC-1 function in dhc-1 ts mutant embryos is sufficient for the posterior spindle shift, or DHC-1 is not the primary motor for that movement.
Fast Temperature-Shift Analysis of DHC-1 Function in Rotation of the Centrosome/Nucleus Complex
Although the preceding tests of DHC-1 function were informative, the time delay between initiation of DHC-1 inhibition and analysis of events in embryos was 24 h. A process of interest might be influenced indirectly by defects in processes that preceded it. As noted above, RNAi depletion of DHC-1 can compromise the maternal germline sufficiently that it stops producing oocytes. The background physiology of embryos analyzed before that shutdown is probably abnormal to some degree. A more obvious problem is that spindle assembly defects preclude meaningful assessment of dynein's involvement in chromosome congression and segregation. In hopes of circumventing these problems, we tested how quickly phenotypes occurred in dhc-1 ts mutants after a temperature upshift. To allow direct observation during a temperature shift, a temperature-controlled microscope stage was built (Supplemental Figure s1). With two solid-state heat pumps controlling the slide temperature, it was possible to drive embryos from 16 to 25°C in ∼30 s during observation with an oil immersion objective. dhc-1 ts mutant embryos (ct76, or195, and ct77-ct42) showed mutant phenotypes (e.g., cortical blebbing and spindle collapse) within 60 s of initiation of such an upshift. In additional tests of ct76, a fast upshift to 25°C that prevented spindle assembly and cytokinesis in P0 followed by a downshift to 16°C allowed mitosis and cytokinesis to occur during the second cell cycle. Thus, with at least one ts allele, thermal inhibition of DHC-1 function could be reversed.
With the thermal stage, we first addressed the question of whether DHC-1 has a direct role in rotation of the centrosome/nucleus complex in the first two cell cycles. In all dhc-1 ts mutant embryos observed at 16°C, centrosomes separated normally and then established a mitotic spindle along the AP axis (n = 11) (Figure 4C and Videos 3 and 7). In ct76 embryos that showed normal transverse centrosome separation (n = 9), an upshift at the moment of pronuclear meeting prevented rotation of the centrosome axis onto the AP axis (our unpublished data). In or195 embryos (n = 10), an upshift at pronuclear meeting prevented rotation in eight cases (Figure 5B, d, and Video 8). In the other two cases, rotation was partial (∼45°). Transverse spindles in or195 embryos eventually did rotate onto the AP axis, when one or the other spindle pole was pulled toward the posterior cortex during anaphase (Figure 5B, e and f). These results show that inhibition of DHC-1 has a direct effect on rotation of the P0 centrosome-centrosome axis, consistent with a mechanism in which dynein-mediated pulling forces on microtubules emanating from one or both centrosomes drive that rotation.
The influence of dynein on rotation of the centrosome axis also was studied in P1. The smaller size of P1 and variations in the geometry of centrosome separation in wild type made observation of centrosomes and the timing of upshifts more difficult with DIC microscopy. Furthermore, in both wild-type and ct76 embryos held at 16°C, P1 centrosome separation sometimes occurred directly along the AP axis. To facilitate well timed upshifts immediately after transverse centrosome separation, we generated a ct76 strain expressing GFP::β-tubulin (Figure 5, A and C). When ct76 embryos were shifted to 25°C immediately after transverse P1 centrosome separation, rotation always failed (n = 12) (Figure 5C, c–e, and Video 9). Because the first mitotic midbody is thought to provide a cue for rotation of the centrosome axis in P1, it is noteworthy that the ct76 embryos described above had completed cytokinesis and had a normally positioned midbody before the temperature shift. These results, along with the transient accumulation of DHC-1 observed along the AB-P1 boundary (Figure 2D), support the hypothesis of Skop and White that dynein-dynactin anchored to the anterior cortex of P1 generates the force for centrosome axis rotation (Skop and White, 1998).
Fast Temperature-Shift Analysis of DHC-1 Function in Mitosis
Cytoplasmic dynein has been proposed to contribute to multiple processes during mitosis, including chromosome congression and silencing of the kinetochore-dependent spindle checkpoint by means of transporting checkpoint proteins from kinetochores to spindle poles (Savoian et al., 2000; Sharp et al., 2000b; Howell et al., 2001; reviewed in McIntosh et al., 2002). To test those hypotheses, ct76 and or195 embryos containing GFP-tagged histone H2B were subjected to fast upshifts immediately after NEB. Although earlier upshifts prevented spindle assembly, post-NEB upshifts allowed formation of what appeared to be normal bipolar spindles (Video 7). After the upshift, chromosomes in ct76 and or195 embryos congressed toward the spindle equator but then failed to form a tight metaphase plate (Figure 6, C and D; compare Videos 10–12). Anaphase commonly resulted in lagging chromosomes (Figure 6C, e and f; Figure 6D, e; and Videos 11 and 12). Even when the upshift was delayed until the initial phase of chromosome congression was mostly completed, metaphase plates were irregular, rather than linear and well focused (Figure 6E, d, and Video 13). These results suggest that minus-end–directed forces generated by cytoplasmic dynein contribute to prometaphase-metaphase chromosome orientation and alignment, which is critical in preventing or correcting merotelic chromosome attachments that result in single chromosomes being stretched between separating spindle poles during anaphase (Powers et al., 2004).
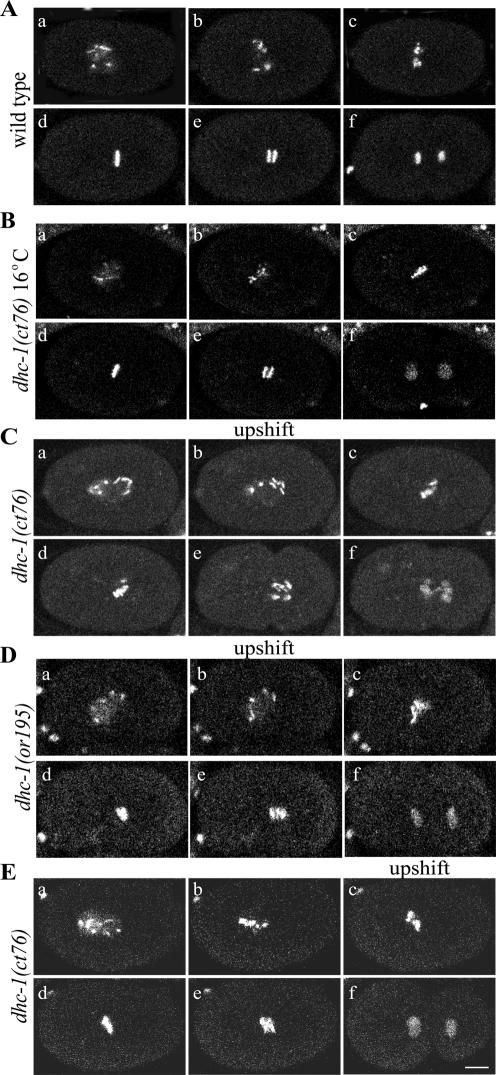
Figure 6.
Defects in chromosome congression in dhc-1 embryos upshifted during prometaphase. Time-lapse confocal images show embryos expressing GFP::histone. (A and Video 10) In wild-type embryos incubated at either 16 or 25°C, chromosomes congressed to form a tight metaphase plate (d) and segregated to form two discrete and well ordered groups (e and f). (B) In dhc-1(ct76) mutant embryos maintained at 16°C, chromosome congression (c and d) and segregation (e and f) looked normal. (C and Video 11) In dhc-1(ct76) mutant embryos shifted from 16 to 25°C at NEB, chromosomes congressed poorly (c and d), chromosomes lagged during anaphase (e), and segregation was defective (e and f). (D and Video 12) In dhc-1(or195) mutant embryos shifted from 16 to 25°C at NEB, chromosome congression was defective (c and d) and some chromosomes lagged in early anaphase (e), but segregation was better than in ct76 embryos (f). (E and Video 13) In dhc-1(ct76) embryos shifted from 16 to 25°C in late prometaphase during congression, chromosomes did not fully congress to a tight metaphase plate (d). Some lagging chromosomes were observed in early anaphase (e), but the subsequent extent of separation and cytokinesis seemed normal (f). Bar, 10 μm.
If dynein contributes to silencing the kinetochore-dependent checkpoint, one would expect dynein inhibition to delay anaphase. At 16°C, the interval between NEB and the beginning of anaphase was slightly shorter for wild-type than for ct76 embryos (238 ± 38 and 284 ± 51 s, respectively; n = 10 for each; Supplemental Table s3). For wild-type embryos at 25°C that interval decreased to 158 ± 37 s (n = 10). For ct76 and or195 embryos upshifted to 25°C at or just after NEB, the interval increased dramatically to 583 ± 174 s (n = 6) and 340 ± 110 s (n = 6), respectively (compare Videos 10–12). It is unlikely that this anaphase delay resulted from the chromosome congression/orientation defects just described, because similar defects caused by KLP-19 inhibition do not cause anaphase delays (Powers et al., 2004). These observations, along with the findings of Encalada et al. (2004), suggest that DHC-1 contributes to silencing of a kinetochore-dependent spindle checkpoint in C. elegans embryos, as has been suggested for other organisms (Howell et al., 2001; McIntosh et al., 2002).
Different experimental systems have provided conflicting results about whether cytoplasmic dynein generates force that moves chromosomes apart during anaphase (see Discussion). In C. elegans embryos, chromosome segregation is accomplished solely by anaphase B pole separation (Oegema et al., 2001), during which the anterior pole remains relatively stationary, while the posterior pole moves toward the posterior cortex. The spindle severing experiments of Grill et al. (2001) suggest that this pole movement is driven by cortical forces that pull most strongly on astral microtubules attached to the posterior spindle pole. Cortically anchored dynein is a good candidate for that cortical force generator. However, our tests with the dhc-1 ts mutants do not provide support for this idea. In ts mutant embryos with spindles oriented along the AP axis, the posterior spindle pole moved to a normal posterior position, although at a somewhat reduced rate (see above; Supplemental Table s3). In ts mutant embryos with bipolar spindles set up transverse to the AP axis, at least one spindle pole moved toward the posterior during anaphase (Figure 5B) (n = 19). In more severe cases, when ts mutant embryos had monopolar or collapsed spindles, the entire spindle moved toward the posterior cortex (Figure 4D) (n = 14). Finally, ct76 and or195 embryos upshifted during prometaphase, well before the onset of anaphase, showed fairly normal anaphase chromosome separation (Figure 6, C and D, and Videos 11 and 12). This was quantified by measuring the final distance between the centers of decondensing chromosome masses during telophase (wild-type = 4.7 ± 0.3 μm [n = 9]; ct76 = 4.8 ± 0.5 μm [n = 9], p = 0.8; or195 = 5.1 ± 0.4 μm [n = 6], p = 0.09). Thus, although these alleles are not completely null, they suggest that cytoplasmic dynein is dispensable during anaphase spindle pole separation in C. elegans embryos. Other possible mechanisms for generating posterior cortical force include minus-end–directed kinesins and tethered microtubule depolymerization.
DISCUSSION
Because cytoplasmic dynein contributes to so many processes, pursuing questions about its specific functions with approaches that cause a gradual loss of function has left gaps in our understanding. The conditional dhc-1 alleles we have characterized allow both long-term and very rapid inhibition of the motor subunit of dynein. Direct observation of cellular/developmental processes of interest during rapid inhibition allows focused analysis of specific dynein functions, minimizing complications due to earlier requirements and the potential for indirect effects.
Insights into Functions of DHC-1 during C. elegans Early Embryonic Development
Spindle Alignment. Because the position and orientation of the mitotic spindle dictate the pattern of cell division and segregation of components to daughter cells (reviewed in White and Strome, 1996), alignment of the spindle is tightly regulated in many developing organisms. Studies in yeast, Drosophila, C. elegans, and mammalian cells have implicated dynein in spindle alignment (Eschel et al., 1993; Li et al., 1993; McGrail and Hays, 1997; Skop and White, 1998; Gönczy et al., 1999; O'Connell and Wang, 2000). In C. elegans, RNAi depletion of DNC-1 or DNC-2, two components of dynactin, or partial RNAi depletion of DHC-1 impaired the 90° rotation of the centrosome/nucleus complex that is required to correctly align the mitotic spindle in the P0 and P1 blastomeres (Skop and White, 1998; Gönczy et al., 1999). Because of the gradual depletion caused by RNAi, the possibility of indirect effects was not eliminated.
Our studies of fast-acting ts dhc-1 mutations and DHC-1 location provide strong support for the direct involvement of dynein in rotation: rotation did not occur in ts mutant embryos upshifted just before rotation in P0 or P1. Hyman and White (Hyman and White, 1987; Hyman, 1989) demonstrated that the rotation in P1 is mediated by an interaction of astral microtubules with a site on the anterior cortex. We observed a transient accumulation of DHC-1 along the anterior cortex of P1 during the period of rotation, similar to that observed for DNC-1 (Skop and White, 1998), consistent with the hypothesis that dynein/dynactin tethered to the anterior cortex captures and reels in microtubules emanating from one centrosome. By analogy to P1, dynein anchored to the anterior cortex of P0 may capture astral microtubules and pull on them to mediate rotation of the centrosome/nucleus complex in that cell as well. An alternative model for P0, suggested by analysis of let-99 mutant embryos (Tsou et al., 2002), is that dynein-generated pulling forces are distributed around the entire cortex but are attenuated at posterior-lateral sites by LET-99 protein. Our results do not discriminate between the “localized dynein” and “broadly distributed dynein with localized attenuation” models. They might be distinguished via spatially restricted thermal inactivation of ts DHC-1 by irradiation of specific cortical regions with a heat-generating microbeam of light.
Chromosome Congression. Dynein has been identified as a kinetochore component in numerous cell types (Pfarr et al., 1990; Steuer et al., 1990; Starr et al., 1998; Sharp et al., 2000b), consistent with a role in mitotic chromosome movement. Previous experiments have produced conflicting views of dynein function during prometaphase. Inhibition of dynein in Drosophila and mammalian cells by a variety of methods, including genetic mutations, RNAi, antibody injection, and overexpression of dynamitin, in some cases caused chromosome congression defects and in other cases did not. In Drosophila, congression defects included a reduced rate of poleward chromosome movement during prometaphase (Savoian et al., 2000) and a failure of kinetochore alignment at metaphase (Sharp et al., 2000b); congression defects were not observed by Starr et al. (1998) or Goshima and Vale (2003). In mammalian systems, Echeverri et al. (1996) observed defects in chromosome alignment in COS-7 cells, whereas Howell et al. (2001) did not observe congression defects in PtK1 cells. In our experiments, DHC-1 inhibition at NEB or later in prometaphase consistently caused disordered metaphase plates, revealing that in C. elegans DHC-1 does contribute to chromosome congression. The congression defects may be due to impaired dynein-dependent force generation at the kinetochore or to altered spindle microtubule dynamics and organization.
Anaphase. Studies of dynein contributions to anaphase in other systems have often been complicated by the requirement for dynein in spindle assembly (Robinson et al., 1999) and have produced conflicting results. For example, injection of dynein inhibitors into Drosophila embryos disrupted both poleward chromosome movement (anaphase A) and spindle pole separation (anaphase B) (Sharp et al., 2000a,b). Also in Drosophila, mutations in zw10, which disrupt dynein localization to kinetochores but do not otherwise impair spindle structure, caused reduced rates of poleward chromosome movement, lagging chromosomes, and segregation defects (Starr et al., 1998; Savoian et al., 2000). In contrast, RNAi depletion of dynein from Drosophila S2 cells caused a delay in the metaphase-to-anaphase transition, but did not impair chromosome segregation (Goshima and Vale, 2003). Similarly, inhibition of dynein function in PtK1 cells affected spindle checkpoint inactivation but did not impair chromosome segregation during anaphase (Howell et al., 2001). Thus, whether dynein contributes directly to anaphase chromosome separation remains controversial.
Two aspects of anaphase in C. elegans are unusual and intriguing. First, chromosome segregation is accomplished solely by spindle pole separation (anaphase B) (Oegema et al., 2001). Thus, a role for dynein in anaphase A chromosome-to-pole movement is not an issue. Second, anaphase B spindle pole separation seems to be driven primarily by cortical pulling forces on astral microtubules (Grill et al., 2001) with little or no contribution from pushing forces by antiparallel microtubules in the spindle interzone. In fact, the C. elegans interzone may actually resist pole separation (Grill et al., 2001; Saunders and Saxton, unpublished data). The cortical pulling forces are stronger toward the posterior than toward the anterior (Grill et al., 2001, 2003). Thus, anaphase B is accomplished primarily by movement of the posterior spindle pole toward the posterior cortex. This movement also positions the spindle asymmetrically, leading to an unequal first division, which is critical for subsequent normal development.
Dynein, tethered at the posterior cortex, has been a prime candidate for the posterior cortical pulling motor. Our studies of spindle movements in upshifted dhc-1 ts mutant embryos do not support such a role for dynein. All upshifted dhc-1 embryos that we analyzed displayed robust posterior movement of the spindle during anaphase. Mutant embryos containing a bipolar spindle displayed posterior migration of one or both spindle poles. Monopolar spindles, too, underwent posterior movement. Furthermore, anaphase chromosome separation was achieved by ts mutant embryos upshifted after they had well-formed bipolar spindles. Thus, either dynein is not the posterior pulling motor, or adequate pulling forces can be generated by residual dynein function in the upshifted ts mutants. The accumulation of DHC-1 on centrosomes and spindle poles in ts mutant embryos suggests that mutant DHC-1 might be capable of translocating along microtubules to their minus ends and fail to release. So, mutant DHC-1 tethered to the cortex could retain the ability to capture and reel in astral microtubules during anaphase. Arguing against retention of the ability to generate cortical pulling forces, at least by the more severe ts mutants, is the fact that they are unable to pull on astral microtubules during rotation of the centrosome/nucleus complex.
In wild-type embryos, anaphase B movement of the posterior spindle pole is accompanied by dramatic lateral oscillations and followed by flattening of the centrosome. Gotta and Ahringer (2001) observed that all three events require G protein function, consistent with the general assumption that posterior-directed movement, oscillations, and flattening of the posterior spindle pole are mechanistically linked, perhaps by a single force-producing mechanism. Our analysis of ts dhc-1 mutants suggests otherwise. The five most severe dhc-1 ts mutations caused a marked reduction or complete absence of oscillations and centrosome flattening at both 16 and 25°C, but posterior-directed anaphase movement of the spindle occurred (e.g., compare Videos 3 and 7 with Video 1). Severson and Bowerman (2003) observed similar results in embryos partially depleted of DHC-1 by RNAi. This demonstrates that the lateral oscillations and flattening are not causally linked to posterior-directed spindle migration. This suggests different force-generating mechanisms; oscillations and centrosome flattening require normal DHC-1 activity, whereas the posterior spindle shift requires little or none.
Lesions in the ts Variants of DHC-1
The DHC motor domain contains six AAA domains (reviewed in King, 2000; Vale, 2000), four that contain a complete P-loop motif (1–4) and two that do not (5 and 6). The current view is that AAA1 is the critical domain for ATP hydrolysis, AAA3 binds but may not hydrolyze ATP, and AAA2 and AAA4 have structural rather than nucleotide-binding roles (King, 2000; Silvanovich et al., 2003; Reck-Peterson and Vale, 2004). The three lesions identified thus far in C. elegans dhc-1 mutants alter amino acids in AAA2, AAA3, and AAA4. The dominant ct76 mutation, which is the most severe allele, causes an Ala-to-Asp change in the Box VI region in AAA4. The recessive or195 mutation, which is the next most severe allele, causes a His-to-Leu change six residues upstream from the AAA2 P-loop (Hamill et al., 2002). Because ATP binding by AAA2 and AAA4 does not occur or is not important for dynein function, it is likely that the or195 and ct76 lesions cause either local defects in folding of their respective AAA domains or longer range defects in structural relationships between domains. The dominant ct42 and ct77 alleles cause a Gly-to-Glu substitution in the fourth residue of the P-loop in AAA3. This confirms the in vivo importance of the AAA3 P-loop and raises the possibility that the long negative side chain of glutamic acid alters ATP binding at nonpermissive temperature. All three dominant alleles behave genetically as gain-of-function poisons with phenotypes sensitive to mutant versus wild-type gene dosage (Mains et al., 1990). This raises the question of how the mutant DHC-1 proteins inactivate wild-type protein. Because the cytoplasmic dynein complex incorporates two DHC subunits, as well as a number of associated proteins, it is possible that the presence of one mutant DHC in a complex is sufficient to dramatically impair force-producing interactions of the motor domains with microtubules, causing binding to be either too weak or too strong. The poison effect could mean that cytoplasmic dynein is an obligate two-headed motor and that the presence of one “motor dead” subunit eliminates critical processivity. However, the or195 ts mutation in AAA2 is recessive. Furthermore, all of the many nonconditional dhc-1/let-354 alleles are recessive. This indicates that the poison effects of the ct76 and ct42-ct77 alleles are allele-specific. Future structural and kinetic investigation will provide insights into the functional relationship between the two heads.
The prospects for using the Gly-to-Glu P-loop mutation to create fast-acting temperature-sensitive mutations in other dyneins are good. The sequence of the AAA3 P-loop is completely conserved in all known DHCs, and engineering the Gly-to-Glu change into S. cerevisiae DHC1 did cause a dominant ts dynein phenotype. It is noteworthy that, when no wild-type gene is present, the ct42-ct77 lesion in worms and yeast causes a nonconditional mutant phenotype. Thus, the ts P-loop mutation does not allow completely normal dynein function at permissive temperature. However, it is tantalizing to consider that creating fast-acting ts dynein mutants in other model systems and cells could help address a number of central questions about mitosis, cytoplasmic transport, and axoneme bending. Furthermore, the Gly-to-Glu or similar mutations also could be effective in kinesins, myosins, G proteins, and a variety of other P-loop proteins, allowing time-resolved function-disruption studies. Issues that will likely impact the success of this approach are temperature range of the organism and oligomerization state of the target protein. Our preliminary tests indicate that the Gly-to-Glu mutation does render a Drosophila dimeric kinesin ts, and we are preparing to test monomeric P-loop proteins.
Supplementary Material
Acknowledgments
We are especially grateful to Paul Mains, Danielle Hamill, Bruce Bowerman, and Ann Rose for sharing dhc-1 alleles and information with us. We thank Alan Bender, Kerry Bloom, Arshad Desai, Margaret Fuller, Pierre Gönczy, Tony Hyman, Yoji Kohara, Chris Malone, John White, and Haining Zhang for reagents; Adam Saunders for help with some strain constructions; Aaron Pilling for the NIH Image tracking macro; Lynda Delph for assistance with statistics; Jon Henry for building the temperature-controlled stage; and members of the Strome and Saxton laboratories for discussions and ideas. This research was supported by National Institutes of Health grants GM58811 (to W.M.S. and S. S.) and GM34059 (to S. S.)
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-06-0523) on December 22, 2004.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Asai, D. J., and Koonce, M. P. (2001). The dynein heavy chain: structure, mechanics and evolution. Trends Cell Biol. 11, 196-202. [DOI] [PubMed] [Google Scholar]
- Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium, T. C. e. S. (1998). Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282, 2012-2018. [DOI] [PubMed] [Google Scholar]
- Deyrup, A. T., Krishnan, S., Cockburn, B. N., and Schwartz, N. B. (1998). Deletion and site-directed mutagenesis of the ATP-binding motif (P-loop) in the bifunctional murine ATP-sulfurylase/adenosine 5′-phosphosulfate kinase enzyme. J. Biol. Chem. 273, 9450-9456. [DOI] [PubMed] [Google Scholar]
- Encalada, S. E., Willis, J., Lyczak, R., and Bowerman, B. (2004). A spindle checkpoint functions during mitosis in the early Caenorhabditis elegans embryo. Mol. Biol. Cell 16, 1056-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel, D., Urrestarazu, L. A., Vissers, S., Jauniaux, J. C., van Vliet-Reedijk, J. C., Planta, R. J., and Gibbons, I. R. (1993). Cytoplasmic dynein is required for normal nuclear segregation in yeast. Proc. Natl. Acad. Sci. USA 90, 11172-11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee, M. A., Heuser, J. E., and Vallee, R. B. (1997). An extended microtubule-binding structure within the dynein motor domain. Nature 390, 636-639. [DOI] [PubMed] [Google Scholar]
- Gepner, J., Li, M., Ludmann, S., Kortas, C., Boylan, K., Iyadurai, S. J., McGrail, M., and Hays, T. S. (1996). Cytoplasmic dynein function is essential in Drosophila melanogaster. Genetics 142, 865-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons, I. R. (1995). Dynein family of motor proteins: present status and future questions. Cell Motil. Cytoskeleton 32, 136-144. [DOI] [PubMed] [Google Scholar]
- Gibbons, I. R., Gibbons, B. H., Mocz, G., and Asai, D. J. (1991). Multiple nucleotide-binding sites in the sequence of dynein beta heavy chain. Nature 352, 640-643. [DOI] [PubMed] [Google Scholar]
- Gönczy, P., Pichler, S., Kirkham, M., and Hyman, A. A. (1999). Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. J. Cell Biol. 147, 135-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima, G., and Vale, R. D. (2003). The roles of microtubule-based motor proteins in mitosis: comprehensive RNAi analysis in the Drosophila S2 cell line. J. Cell Biol. 162, 1003-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotta, M., and Ahringer, J. (2001). Distinct roles for Gα and Gβγ in regulating spindle position and orientation in Caenorhabditis elegans embryos. Nat. Cell Biol. 3, 297-300. [DOI] [PubMed] [Google Scholar]
- Grill, S. W., Gönczy, P., Stelzer, E. H., and Hyman, A. A. (2001). Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature 409, 630-633. [DOI] [PubMed] [Google Scholar]
- Grill, S. W., Howard, J., Schaffer, E., Stelzer, E. H., and Hyman, A. A. (2003). The distribution of active force generators controls mitotic spindle position. Science 301, 518-521. [DOI] [PubMed] [Google Scholar]
- Hamill, D. R., Severson, A. F., Carter, J. C., and Bowerman, B. (2002). Centrosome maturation and mitotic spindle assembly in C. elegans require SPD-5, a protein with multiple coiled-coil domains. Dev. Cell 3, 673-684. [DOI] [PubMed] [Google Scholar]
- Harada, A., Takei, Y., Kanai, Y., Tanaka, Y., Nonaka, S., and Hirokawa, N. (1998). Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J. Cell Biol. 141, 51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, D. B., Pearson, C. G., Yen, T. J., Howell, B. J., and Salmon, E. D. (2001). Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol. Biol. Cell 12, 1995-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, A. M., and Rose, A. M. (1990). Essential genes in the hDf6 region of chromosome I in Caenorhabditis elegans. Genetics 126, 583-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, B. J., McEwen, B. F., Canman, J. C., Hoffman, D. B., Farrar, E. M., Rieder, C. L., and Salmon, E. D. (2001). Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 155, 1159-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman, A. A. (1989). Centrosome movement in the early divisions of Caenorhabditis elegans: a cortical site determining centrosome position. J. Cell Biol. 109, 1185-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman, A. A., and White, J. G. (1987). Determination of cell division axes in the early embryogenesis of Caenorhabditis elegans. J. Cell Biol. 105, 2123-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath, R. S., Martinez-Campos, M., Zipperlen, P., Fraser, A. G., and Ahringer, J. (2001). Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2, RESEARCH0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S. M. (2000). AAA domains and organization of the dynein motor unit. J. Cell Sci. 113, 2521-2526. [DOI] [PubMed] [Google Scholar]
- Koonce, M. P. (1997). Identification of a microtubule-binding domain in a cytoplasmic dynein heavy chain. J. Biol. Chem. 272, 19714-19718. [DOI] [PubMed] [Google Scholar]
- Kull, F. J., Sablin, E. P., Lau, R., Fletterick, R. J., and Vale, R. D. (1996). Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature 380, 550-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. Y., Yeh, E., Hays, T., and Bloom, K. (1993). Disruption of mitotic spindle orientation in a yeast dynein mutant. Proc. Natl. Acad. Sci. USA 90, 10096-10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye, R. J., Wilson, R. K., and Waterston, R. H. (1995). Genomic structure of a cytoplasmic dynein heavy chain gene from the nematode Caenorhabditis elegans. Cell Motil. Cytoskeleton 32, 26-36. [DOI] [PubMed] [Google Scholar]
- Mains, P. E., Sulston, I. A., and Wood, W. B. (1990). Dominant maternal-effect mutations causing embryonic lethality in Caenorhabditis elegans. Genetics 125, 351-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrail, M., and Hays, T. S. (1997). The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation in Drosophila. Development 124, 2409-2419. [DOI] [PubMed] [Google Scholar]
- McIntosh, J. R., Grishchuk, E. L., and West, R. R. (2002). Chromosome-microtubule interactions during mitosis. Annu. Rev. Cell Dev. Biol. 18, 193-219. [DOI] [PubMed] [Google Scholar]
- Milisav, I. (1998). Dynein and dynein-related genes. Cell Motil. Cytoskeleton 39, 261-272. [DOI] [PubMed] [Google Scholar]
- Mitenko, N. L., Eisner, J. R., Swiston, J. R., and Mains, P. E. (1997). A limited number of Caenorhabditis elegans genes are readily mutable to dominant, temperature-sensitive maternal-effect embryonic lethality. Genetics 147, 1665-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell, C. B., and Wang, Y. L. (2000). Mammalian spindle orientation and position respond to changes in cell shape in a dynein-dependent fashion. Mol. Biol. Cell 11, 1765-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema, K., Desai, A., Rybina, S., Kirkham, M., and Hyman, A. A. (2001). Functional analysis of kinetochore assembly in Caenorhabditis elegans. J. Cell Biol. 153, 1209-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, K. (1991). Four ATP-binding sites in the midregion of the beta heavy chain of dynein. Nature 352, 643-645. [DOI] [PubMed] [Google Scholar]
- Ogawa, K., and Mohri, H. (1996). A dynein motor superfamily. Cell Struct. Funct. 21, 343-349. [DOI] [PubMed] [Google Scholar]
- Paschal, B. M., Shpetner, H. S., and Vallee, R. B. (1987). MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J. Cell Biol. 105, 1273-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr, C. M., Coue, M., Grissom, P. M., Hays, T. S., Porter, M. E., and McIntosh, J. R. (1990). Cytoplasmic dynein is localized to kinetochores during mitosis. Nature 345, 263-265. [DOI] [PubMed] [Google Scholar]
- Powers, J., Rose, D. J., Saunders, A., Dunkelbarger, S., Strome, S., and Saxton, W. M. (2004). Loss of KLP-19O polar ejection force causes misorientation and missegregation of holocentric chromosomes. J. Cell Biol. 166, 991-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson, S. L., and Vale, R. D. (2004). Molecular dissection of the roles of nucleotide binding and hydrolysis in dynein's AAA domains in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 101, 1491-1495. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Robinson, J. T., Wojcik, E. J., Sanders, M. A., McGrail, M., and Hays, T. S. (1999). Cytoplasmic dynein is required for the nuclear attachment and migration of centrosomes during mitosis in Drosophila. J. Cell Biol. 146, 597-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salina, D., Bodoor, K., Eckley, D. M., Schroer, T. A., Rattner, J. B., and Burke, B. (2002). Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell 108, 97-107. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E. F., and Maniatis, T. (1982). Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Savoian, M. S., Goldberg, M. L., and Rieder, C. L. (2000). The rate of poleward chromosome motion is attenuated in Drosophila zw10 and rod mutants. Nat. Cell Biol. 2, 948-952. [DOI] [PubMed] [Google Scholar]
- Sawin, K. E., and Scholey, J. M. (1991). Motor proteins in cell division. Trends Cell Biol. 1, 122-129. [DOI] [PubMed] [Google Scholar]
- Severson, A. F., and Bowerman, B. (2003). Myosin and the PAR proteins polarize microfilament-dependent forces that shape and position mitotic spindles in Caenorhabdities elegans. J. Cell Biol. 161, 21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp, D. J., Brown, H. M., Kwon, M., Rogers, G. C., Holland, G., and Scholey, J. M. (2000a). Functional coordination of three mitotic motors in Drosophila embryos. Mol. Biol. Cell 11, 241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp, D. J., Rogers, G. C., and Scholey, J. M. (2000b). Cytoplasmic dynein is required for poleward chromosome movement during mitosis in Drosophila embryos. Nat. Cell Biol. 2, 922-930. [DOI] [PubMed] [Google Scholar]
- Shen, H., Yao, B. Y., and Mueller, D. M. (1994). Primary structural constraints of P-loop of mitochondrial F1-ATPase from yeast. J. Biol. Chem. 269, 9424-9428. [PubMed] [Google Scholar]
- Silvanovich, A., Li, M. G., Serr, M., Mische, S., and Hays, T. S. (2003). The third P-loop domain in cytoplasmic dynein heavy chain is essential for dynein motor function and ATP-sensitive microtubule binding. Mol. Biol. Cell 14, 1355-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skop, A. R., and White, J. G. (1998). The dynactin complex is required for cleavage plane specification in early Caenorhabditis elegans embryos. Curr. Biol. 8, 1110-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr, D. A., Williams, B. C., Hays, T. S., and Goldberg, M. L. (1998). ZW10 helps recruit dynactin and dynein to the kinetochore. J. Cell Biol. 142, 763-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer, E. R., Wordeman, L., Schroer, T. A., and Sheetz, M. P. (1990). Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature 345, 266-268. [DOI] [PubMed] [Google Scholar]
- Strome, S., Powers, J., Dunn, M., Reese, K., Malone, C. J., White, J., Seydoux, G., and Saxton, W. M. (2001). Spindle dynamics and the role of γ-tubulin in early Caenorhabditis elegans embryos. Mol. Biol. Cell 12, 1751-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome, S., and Wood, W. B. (1983). Generation of asymmetry and segregation of germ-line granules in early C. elegans embryos. Cell 35, 15-25. [DOI] [PubMed] [Google Scholar]
- Tsou, M. B., Hayashi, A., DeBella, L. R., McGrath, G., and Rose, L. S. (2002). LET-99 determines spindle position and is asymmetrically enriched in response to PAR polarity cues in C. elegans embryos. Development 129, 4469-4481. [DOI] [PubMed] [Google Scholar]
- Vaisberg, E. A., Koonce, M. P., and McIntosh, J. R. (1993). Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J. Cell Biol. 123, 849-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale, R. D. (2000). AAA proteins. Lords of the ring. J. Cell Biol. 150, F13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale, R. D. (2003). The molecular motor toolbox for intracellular transport. Cell 112, 467-480. [DOI] [PubMed] [Google Scholar]
- Vallee, R. B., and Hook, P. (2003). Molecular motors: a magnificent machine. Nature 421, 701-702. [DOI] [PubMed] [Google Scholar]
- Verdon, G., Albers, S. V., Dijkstra, B. W., Driessen, A. J., and Thunnissen, A. M. (2003). Crystal structures of the ATPase subunit of the glucose ABC transporter from Sulfolobus solfataricus: nucleotide-free and nucleotide-bound conformations. J. Mol. Biol. 330, 343-358. [DOI] [PubMed] [Google Scholar]
- Walhout, A. J., and Vidal, M. (2001). High-throughput yeast two-hybrid assays for large-scale protein interaction mapping. Methods 24, 297-306. [DOI] [PubMed] [Google Scholar]
- Walker, J. E., Saraste, M., Runswick, M. J., and Gay, N. J. (1982). Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1, 945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J., and Strome, S. (1996). Cleavage plane specification in C. elegans: how to divide the spoils. Cell 84, 195-198. [DOI] [PubMed] [Google Scholar]
- Wicks, S. R., de Vries, C. J., van Luenen, H. G., and Plasterk, R. H. (2000). CHE-3, a cytosolic dynein heavy chain, is required for sensory cilia structure and function in Caenorhabditis elegans. Dev. Biol. 221, 295-307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.