Abstract
Most of all strokes are ischemic due to occlusion of a vessel, and comprise two main types, thrombotic and embolic. Inflammation and immune response play an important role in the outcome of ischemic stroke. Pharmaceutical and cell-based therapies with immunomodulatory properties could be of benefit in treating ischemic stroke. Possible changes in microRNAs brought about by immunomodulatory treatments may be important. The pharmaceutical studies described in this review have identified several differentially regulated miRNAs associated with disregulation of mRNA targets or the upregulation of several neuroprotective genes, thereby highlighting the potential neuroprotective roles of specific miRNAs such as miR-762, -1892, -200a, -145. MiR-124, -711, -145 are the strongly associated miRNAs predicted to mediate anti-inflammatory pathways and microglia/macrophage M2-like activation phenotype. The cell-based therapy studies reviewed have mainly utilized mesenchymal stem cells or human umbilical cord blood cells and shown to improve functional and neurological outcomes in stroke animals. MiR-145 and miR-133b were implicated in nerve cell remodeling and functional recovery after stroke. Human umbilical cord blood cells decreased proinflammatory factors and promoted M2 macrophage polarization in stroke diabetic animals.
Keywords: ischemic stroke, immunomodulators, pharmaceutical therapies, cell-based therapies, microRNAs, animal models
Introduction
Stroke is a potentially fatal or highly debilitating condition with multiple consequences for patients and their families, as well as healthcare providers and society. It is the second most common cause of death worldwide and the leading cause of disability in adults. Approximately 80% of all strokes are ischemic due to occlusion of a vessel, and comprise two main types, thrombotic and embolic. Thrombotic stroke accounts for almost 50% of all strokes. Thrombolysis and/or mechanical thromboectomy may be considered in patients with severe neurological symptoms and early presentation within 3–6 hours of symptom onset. Many stroke patients are not treated by intravenous thrombolysis because of the narrow window of time for treatment. In patients with minor stroke, while the immediate brain damage and associated symptoms are limited, there is a significant risk of a major stroke within 90 days (Emberson, 2014) even when taking aspirin antiplatelet therapy (Wang et al., 2013). Early intervention in stroke is desirable to limit the serious consequence of a major stroke and also to lower the risk of a minor stroke progressing to a seriously disabling stroke. Also extending the window for therapeutic intervention would enable a greater number of stroke patients to be treated.
Evidence indicates that inflammation and immune response play an important role in the outcome of ischemic stroke (Famakin, 2014). Inflammation after stroke involves leukocyte infiltration in brain parenchyma that contributes to cerebral damage. Peripherally derived mononuclear phagocytes, T lymphocytes, natural killer (NK) cells, and polymorphonuclear leukocytes, which produce and secrete cytokines, can all contribute to central nervous system (CNS) inflammation and gliosis (Brea et al., 2009). Blood-derived leukocytes and resident microglia are the more activated inflammatory cells, accumulating in the brain tissue after cerebral ischemia, leading to inflammatory injury (Akopov et al., 1996). Microglia, the major source of cytokines and other immune molecules of the CNS, are the first non-neuronal cells that respond to CNS injury, becoming phagocytic when fully activated by neuronal death. As cerebral inflammation is one of the earliest events in stroke, early intervention to modify the immune response may have a beneficial effect. We have searched the PubMed database for studies of pharmaceutical and cell-based therapies with immunomodulatory properties that have been used for treating ischemic stroke. We have also examined for possible changes in microRNAs brought about by immunomodulatory treatments on account of a possible role of microRNAs in ischemic stroke (Martinez and Peplow, 2015).
Immunomodulatory Therapies for Ischemic Stroke
Pharmaceutical therapies
The pharmaceutical therapies were with niacin, cytosine-phosphate-guanine (CpG), thymosin beta 4 (Tβ4), lipopolysaccharide (LPS), interleukin-4 (IL-4), MK801 (an NMDA antagonist), and microRNA-146a (miR-146a). These have all been shown to have immunomodulatory properties (niacin: Ganji et al., 2009; CpG: Tan et al., 2009; Tβ4: Badamchian et al., 2003; LPS: Jacobs et al., 1981; IL-4: Splawski et al., 1989; MK801: Esposito et al., 2011; miR-146a: Li et al., 2010). The seven animal studies utilizing these pharmaceutical agents are summarized in Table 1.
Table 1.
Studies of pharmacological therapies with immunomodulatory properties to treat stroke
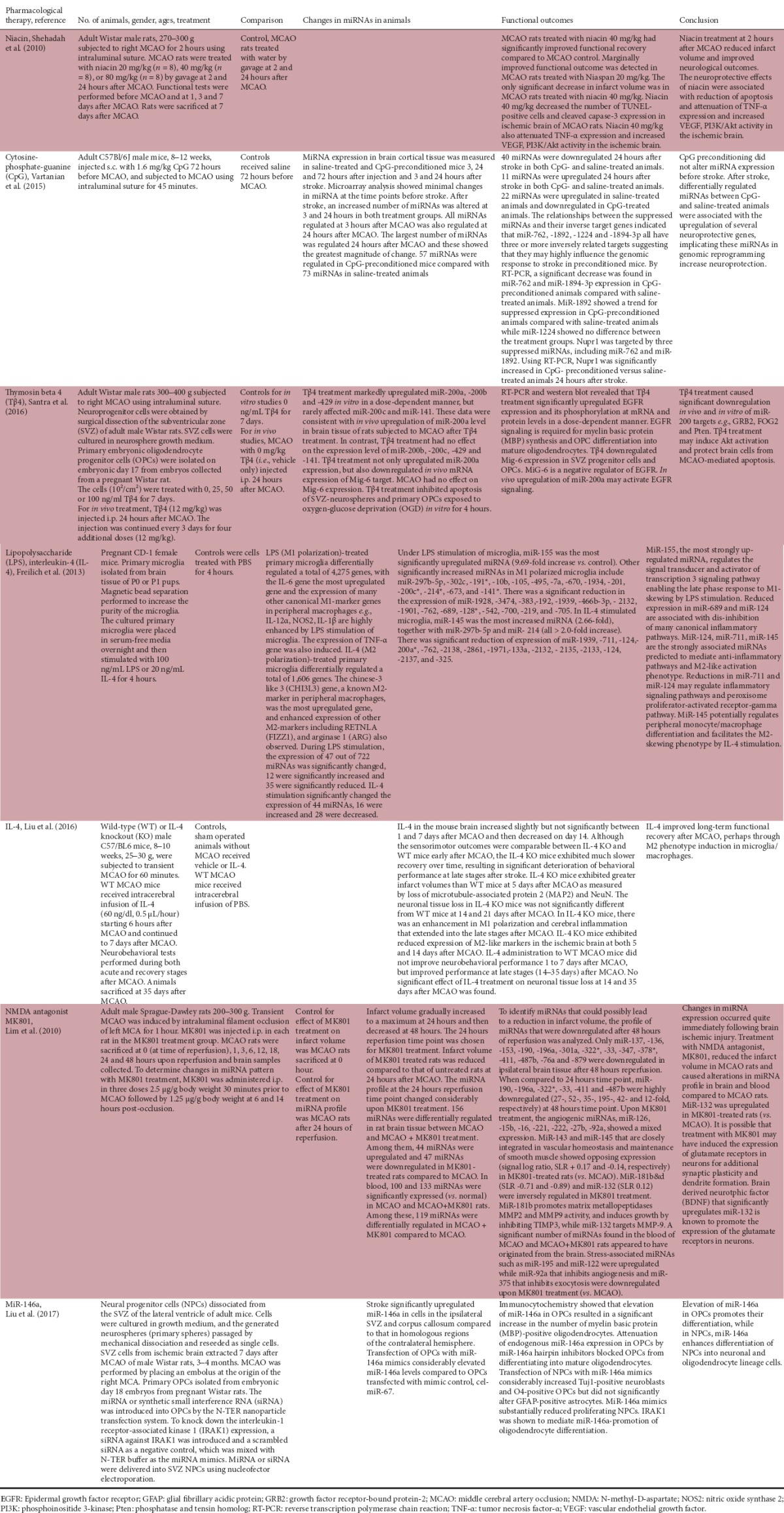
Niacin treatment of middle cerebral artery occlusion (MCAO) normal rats reduced infarct volume and improved neurological outcomes. It decreased apoptosis, attenuated tumor necrosis factor-α (TNF-α) expression, and increased vascular endothelial growth factor (VEGF), phosphoinositide 3-kinase (PI3K)/Akt activity in the ischemic brain (Shehadah, 2010). Conditioning with CpG prior to stroke in mice differentially regulated several miRNAs that were associated with neuroprotective genes (Vartanian et al., 2015). Tβ4 upregulated miR-200a level in the rat ischemic brain and may induce Akt activation and protect brain cells from brain ischemia-mediated apoptosis (Santra et al., 2016). Under LPS stimulation of mouse microglia, miR-155 was the most significantly upregulated miRNA and regulates the signal transducer and activator of transcription 3 signaling pathway enabling the late phase response to M1-skewing by LPS stimulation (Freilich et al., 2013; Figure 1). In IL-4 stimulated microglia, miR-145 was the most increased miRNA (Freilich et al., 2013). MiR-145 potentially regulates peripheral monocyte/macrophage differentiation and facilitates the M2 phenotype in microglia/macrophages by IL-4 stimulation (Liu et al., 2016; Figure 1). Treatment with MK801 reduced the infarct volume in rat ischemic brain and caused alterations in miRNA profile in brain and blood compared to control rats. MiR-132 was upregulated in MK801-treated rats (Lim et al., 2010). Elevation of miR-146a in mouse oligodendrocyte precursor cells (OPCs) promoted their differentiation, while in neural progenitor cells (NPCs), miR-146a enhanced differentiation of these cells into neuronal and oligodendrocyte lineage cells (Liu et al, 2017).
Figure 1.
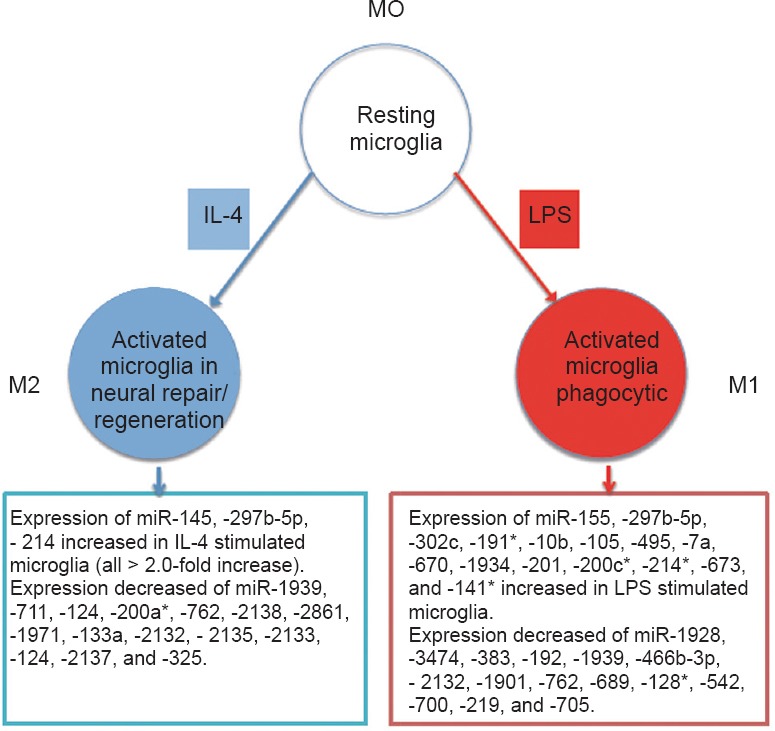
M1- and M2-like activation phenotypes induced by stimulating murine microglia with LPS and IL-4, respectively, and altered miRNA expressions (Freilich et al., 2013).
IL-4: Interleukin-4; LPS: lipopolysaccharide.
Cell-based therapies
The cell-based therapies were with mesenchymal stem cells (MSCs), human umbilical cord blood cells (HUCBCs), and endothelial progenitor cells (EPCs). They have all been associated with immunomodulatory properties (MSCs: Gao et al., 2016; Zhao et al., 2016; HUCBCs: Yu et al., 2009; EPCs: Nuzzolo et al., 2014; Bartaula-Brevik et al., 2016). The studies utilizing these cells are summarized in Table 2. The MSCs were isolated from bone marrow stroma, while HUCBCs and EPCs were obtained commercially. Human cord blood is a source of MSCs (Roura et al, 2012) and EPCs (Lin et al., 2011; Yoder, 2012).
Table 2.
Studies of cell-based therapies with immunomodulatory properties to treat stroke
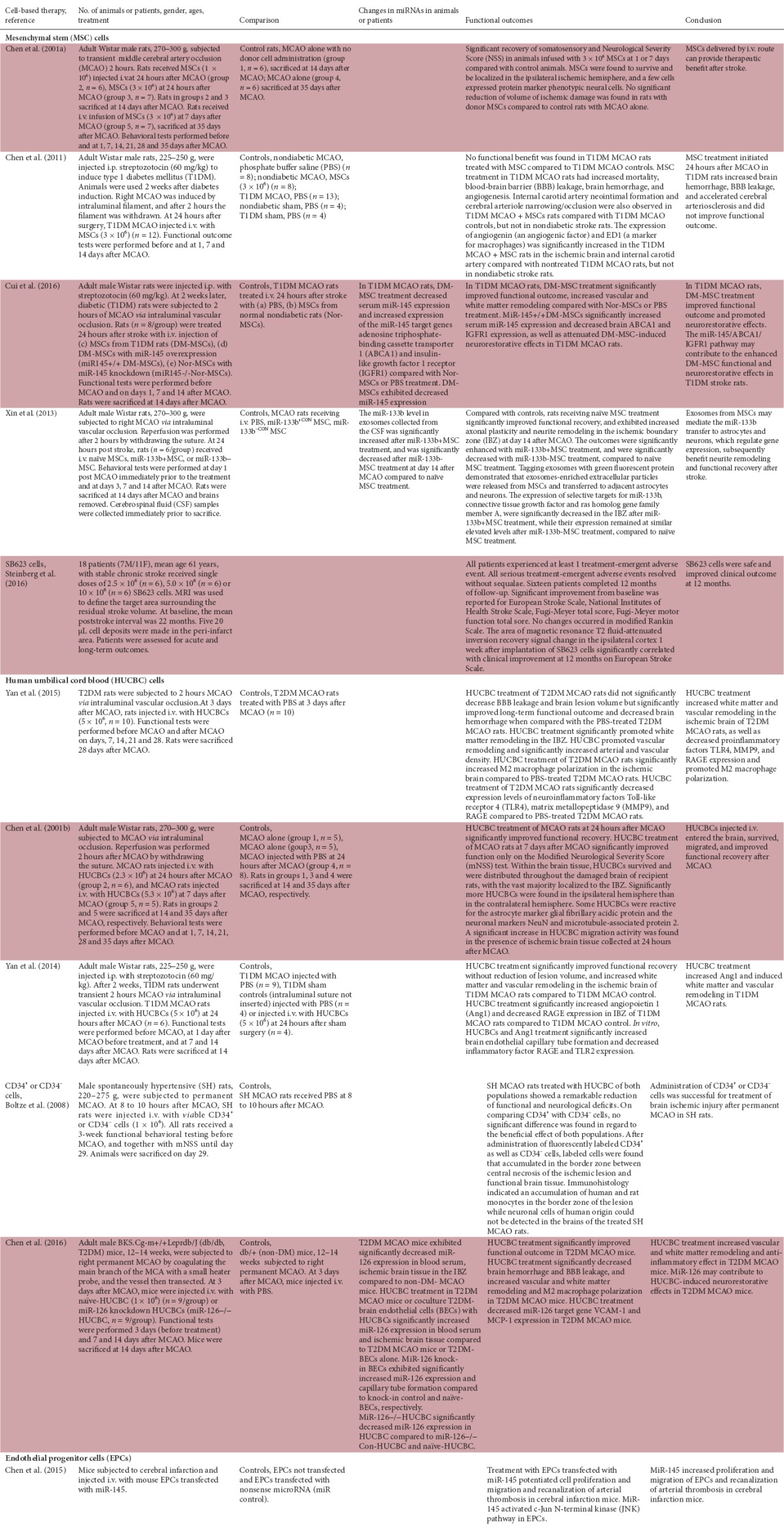
MSC therapy
Four animal studies and one clinical trial were found. Administration of MSCs had positive benefit after stroke in normal rats (Chen et al., 2001a), and transfecting MSCs with miR-133b increased functional outcomes with exosomes-enriched extracellular particles being released from MSCs and transferred to adjacent astrocytes and neurons (Xin et al., 2013). One study showed that MSC treatment after stroke in type 1 diabetes mellitus (T1DM) rats did not improve functional outcomes (Chen et al., 2011). Interestingly treatment with MSCs from T1DM rats improved functional outcome and promoted neurorestorative effects in stroke T1DM rats (Cui et al., 2016). The neurorestorative effects were decreased by administration of MSCs from T1DM rats transfected with miR-145 (Cui et al., 2016). In a phase 1/2a clinical trial, administration of SB623 cells was shown to be safe and improved clinical outcome at 12 months in patients with stable chronic stroke (Steinberg et al., 2016).
HUCBC therapy
Five animal studies were found. HUCBC treatment after stroke of normal rats improved functional recovery, with the HUCBCs surviving and migrating after entering the brain (Chen et al., 2001b). Some HUCBCs were reactive for the astrocyte marker glial fibrillary acidic protein and the neuronal markers NeuN and microtubule-associated protein 2 (Chen et al., 2001b). In stroke rats with type 2 diabetes mellitus (T2DM), HUCBC treatment increased white matter and vascular remodeling, decreased proinflammatory factors Toll-like receptor 4 (TLR4), matrix metallopeptidase 9 (MMP-9), and RAGE expression, and promoted M2 macrophage polarization in the ischemic brain (Yan et al., 2015). Interestingly, HUCBC treatment in T2DM mice after stroke increased miR-126 expression in blood serum and ischemic brain tissue, and miR-126 may contribute to HUCBC-induced neurorestorative effects (Chen et al., 2016). Increased functional recovery occurred in T1DM rats treated with HUCBCs post-stroke, with increased white matter and vascular remodeling in the ischemic brain and increased angiopoietin 1 (Ang1) and decreased RAGE expression in the ischemic boundary zone (IBZ) (Yan et al., 2014). Administration of CD34+ or CD34– cells to spontaneously hypertensive rats after stroke improved functional and neurological outcomes (Boltze et al., 2008).
EPC therapy
One animal study was found. Administration of EPCs transfected with miR-145 promoted cell proliferation and migration and recanalization of arterial thrombosis in normal mice post-stroke (Chen et al., 2015).
Neurorestorative Effects in Stroke of Pharmaceutical and Cell-Based Therapies with Immunomodulatory Properties
Astrocytes and microglia are immune cells of the brain and elicit an inflammatory response by the production of inflammatory mediators (Ransohoff and Brown, 2012). Proinflammatory cytokines are increased during brain ischemia and lowering the levels of these cytokines has been shown to ameliorate ischemic brain injury (Lin et al., 2016; Shu et al., 2016). Cytokines regulate the expression of brain endothelial miRNAs that either promote or inhibit inflammatory pathways to orchestrate neuroinflammation in the brain (Lopez-Ramirez et al., 2016). Proinflammatory cytokines alter the levels of several important miRNA clusters. Members of the miRNA family have been shown to mediate many biological effects including induced cell proliferation and decreased apoptosis (Lopez-Ramirez et al., 2016). MiRNAs play an important role in the regulation of adult neurogenesis, and form an important class of epigenetic regulators that contribute to chronic inflammation in microglia of the brain causing the progression of neurological diseases such as ischemic stroke. Cytokines are likely to be involved in changes in brain capillaries with age and diabetes, as shown for renal capillaries (Bianchi et al., 2016), and increased risk of stroke.
The pharmaceutical studies described in this review have identified several differentially regulated miRNAs associated with disregulation of mRNA targets or the upregulation of several neuroprotective genes, and thereby implicating these miRNAs in neuroprotection. These have included miR-762, -1892, -200a, -145 (Table 1). IL-4 induced M2 polarization in microglia/macrophages (Figure 1). MiR-124, -711, -145 are the strongly associated miRNAs predicted to mediate anti-inflammatory pathways and M2-like activation phenotype. Interestingly, niacin and Tβ4 treatment protected brain cells from stroke-mediated apoptosis, while niacin and the NMDA antagonist MK801 reduced the infarct volume in stroke rats. Niacin treatment attenuated the proinflammatory cytokine TNF-α and increased VEGF, PI3K/Akt activity in the ischemic brain. Transfection of neuroprogenitor cells with miR-146a enhanced their differentiation into neuronal and oligodendrocyte lineage cells. The cell-based therapy studies reviewed have mainly utilized MSCs or HUCBCs and shown to improve functional and neurological outcomes in stroke animals. MiR-145 and miR-133b were implicated in nerve cell remodeling and functional recovery after stroke. HUCBCs decreased proinflammatory factors and promoted M2 macrophage polarization in stroke diabetic animals (Table 2).
Future Perspectives
The in vivo stroke studies were mostly performed with young adult male animals. Future studies need to be made in female animals, and also in aged animals. The possible reasons for MSC therapy not improving functional outcomes in stroke type 1 diabetic rats require scientific clarification. Also the role of miRNAs in pharmaceutical and cell-based therapies in improving functional and neurological outcomes in ischemic stroke needs to be further investigated. Preconditioning of MSCs ex vivo by hypoxia, inflammatory stimuli, or other factors/conditions prior to their use in therapy as an adaptive strategy to prepare such cells to survive in the harsh environment at the site of tissue injury/inflammation should be examined (Saparov et al., 2016). This would also apply to HUCBCs. At the present time, the lack of a complete understanding of the mechanism of action mediating the observed therapeutic benefits of adult stem cell therapy in stroke is a critical limitation (Bang et al., 2016) and restricts their use in clinical studies. Also the role of specific microRNAs in inducing angiogenesis, neurogenesis and oligodendrogenesis could be examined by injection of a vector carrying the microRNA into the brain of normal and stroke animals. MicroRNAs with anti-inflammatory properties include miR-146a, -122, -let-7c (Alexander et al., 2015; Roy et al., 2015; Yu et al., 2016) and these should be trialed.
MiRNAs are major molecular regulators and appear to have pivotal roles in cell-based and possibly pharmacological restorative therapies for stroke. Targeting specific miRNAs may provide major restorative therapies for stroke. This will still hold many challenges due to possible delivery and potential off-target effects.
Additional file (231.9KB, pdf) : Open peer review report 1.
Open peer review report 1 on “Immunomodulators and microRNAs as neurorestorative therapy for ischemic stroke”
Footnotes
Conflicts of interest: None declared.
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check: This paper has been checked twice with duplication-checking software iThenticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Open peer review report:
Reviewer: Pio Conti, University of Chieti, Italy.
Comments to author: Astrocytes are immune cells of the brain that elicit an immune and inflammatory response with production of cytokines, during brain damage. Cytokines regulate the expression of brain endothelial microRNAs that either promote or inhibit inflammatory pathways to orchestrate neuroinflammation in the brain. Proinflammatory cytokines alter the levels of several important microRNA clusters. Members of microRNA family have been shown to mediate many biological effects, including induced cell proliferation and decreased apoptosis, by targeting pro-apoptotic genes. miRNAs play an important role in the regulation of adult neurogenesis. An important class of epigenetic regulators are microRNAs (miRNAs) that contribute to chronic inflammation of microglia in the brain causing the progression of neurological diseases such as ischemic stroke. However, miRNAs are major molecular regulators and appear to have pivotal roles in cell-based, and possibly, pharmacological restorative therapies for stroke. Using miRNAs as therapeutic targets still hold many challenges, due to possible delivery and potential off-target effects, since miRNAs are major molecular regulators and appear to have pivotal roles in cell-based, and possibly, pharmacological restorative therapies for stroke. Targeting microRNA therapeutic strategies to ameliorate CNS disorders and dysfunctions are welcome. See details in the additional file.
References
- Akopov SE, Simonian NA, Grigorian GS. Dynamics of polymorphonuclear leukocyte accumulation in acute cerebral infarction and their correlation with brain tissue damage. Stroke. 1996;27:1739–1743. doi: 10.1161/01.str.27.10.1739. [DOI] [PubMed] [Google Scholar]
- Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, Seabra MC, Round JL, Ward DM, O’Connell RM. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commn. 2015;6:7321. doi: 10.1038/ncomms8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badamchian M, Fagarasan MO, Danner RL, Suffredini AF, Damavandy H, Goldstein AL. Thymosin beta(4) reduces lethality and down-regulates inflammatory mediators in endotoxin-induced septic shock. Int Immunopharmacol. 2003;3:1225–1233. doi: 10.1016/S1567-5769(03)00024-9. [DOI] [PubMed] [Google Scholar]
- Bang OY, Kim EH, Cha JM, Moon GJ. Adult stem cell therapy for stroke: challenges and progress. J Stroke. 2016;18:256–266. doi: 10.5853/jos.2016.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartaula-Brevik, Pederson TO, Finne-Wistrand A, Bolstad AI, Mustafa K. Angiogenic and immunomodulatory properties of endothelial and mesenchymal stem cells. Tissue Eng Part A. 2016;22:244–252. doi: 10.1089/ten.tea.2015.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, Ripandelli G, Taurone S, Feher J, Plateroti R, Kovacs I, Magliulo G, Orlando MP, Micera A, Battaglione E, Artico M. Age and diabetes related changes of the renal capillaries: An ultrastructural and immunohistochemical study. Int J Immunopath Pharmacol. 2016;29:40–53. doi: 10.1177/0394632015615592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltze J, Kowalski I, Geiger K, Reich D, Gunter A, Buhrie C, Egger D, Kamprad M, Emmrich F. Experimental treatment of stoke in spontaneously hypertensive rats by CD34+ and CD34– cord blood cells. Ger Med Sci. 2008;3:Doc09. [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001a;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Liu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001b;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Ye X, Yan T, Zhang C, Yang XP, Cui X, Cui Y, Zacharek A, Roberts C, Liu X, Dai X, Lu M, Chopp M. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke. 2011;42:3551–3558. doi: 10.1161/STROKEAHA.111.627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Venkat P, Zacharek A, Chopp M. Neurorestorative therapy for stroke. Front Hum Neurosci. 2014;8:382. doi: 10.3389/fnhum.2014.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ning R, Zacharek A, Cui C, Cui X, Yan T, Venkat P, Zhang Y, Chopp M. MiR-126 contributes to human umbilical cord blood cell induced neurorestorative effects after stroke in type-2 diabetic mice. Stem Cells. 2016;34:102–113. doi: 10.1002/stem.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Chen S, Liao J, Chen X, Xu X. MiR-145 facilitates proliferation and migration of endothelial progenitor cells and recanalization of arterial thrombus in cerebral infarction mice via JNK signal pathway. Int J Clin Exp Pathol. 2015;8:13770–13776. [PMC free article] [PubMed] [Google Scholar]
- Cui C, Ye X, Chopp M, Venkat P, Zacharek A, Yan T, Ning R, Yu P, Cui G, Chen J. miR-145 regulates diabetes-bone marrow stromal cell-induced neurorestorative effects in diabetes stroke rats. Stem Cells Trans Med. 2016;5:1656–1667. doi: 10.5966/sctm.2015-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg M, Lindley RI, Murray G, Olivot JM, Parsons M, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;284:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito E, Paterniti I, Mazzon E, Genovese T, Galuppo M, Meli R, Bramanti P, Cuzzocrea S. MK801 attenuates secondary injury in a mouse experimental compression model of spinal cord trauma. BMC Neurosci. 2011;12:31. doi: 10.1186/1471-2202-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famakin BM. The immune response to acute focal cerebral ischemia and associated post-stroke immunodepression: a focused review. Aging Dis. 2014;5:307–326. doi: 10.14336/AD.2014.0500307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freilich RW, Woodbury ME, Ikezu T. Integrated expression profiles of mRNA and miRNA in polarized primary murine microglia. PLoS One. 2013;8:e79416. doi: 10.1371/journal.pone.0079416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganji SH, Qin S, Zhang L, Kamanna VS, Kashyap ML. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis. 2009;202:68–75. doi: 10.1016/j.atherosclerosis.2008.04.044. [DOI] [PubMed] [Google Scholar]
- Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, Tse HF, Fu QL, Lian Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062. doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DM. Immunomodulatory effects of bacterial lipopolysaccharide. J Immunopharmacol. 1981;3:119–132. doi: 10.3109/08923978109026423. [DOI] [PubMed] [Google Scholar]
- Li L, Chen XP, Li YJ. MicroRNA-146a and human disease. Scand J Immunol. 2010;71:227–231. doi: 10.1111/j.1365-3083.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- Lim KY, Chua JH, Tan JR, Swaminathan P, Sepramaniam S, Armugam A, Wong PT, Jeyaseelan K. MicroRNAs in cerebral ischemia. Transl Stroke Res. 2010;1:287–303. doi: 10.1007/s12975-010-0035-3. [DOI] [PubMed] [Google Scholar]
- Lin J, Pang L, Liu XL, Xing J. Role of vitacamphore in improving cerebral central pro-inflammatory cytokines following transient global ischemia. J Biol Regul Homeost Agents. 2016;30:1091–1098. [PubMed] [Google Scholar]
- Lin Z, Dreyzin A, Aamodt K, Dudley AC, Melero-Martin JM. Functional endothelial progenitor cells from cryopreserved umbilical cord blood. Cell Transplant. 2011;20:515–522. doi: 10.3727/096368910X532729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu J, Zhao S, Zhang H, Cai W, Cai M, Ji X, Leak RK, Gao Y, Chen J, Hu X. Interleukin-4 is essential for microglia/macrophage M2 polarization and long-term recovery after cerebral ischemia. Stroke. 2016;47:498–504. doi: 10.1161/STROKEAHA.115.012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Chopp M, Pan WL, Wang XL, Fan BY, Zhang Y, Kassis H, Zhang RL, Zhang XM, Zhang ZG. MicroRNA-146a promotes oligodendrogenesis in stroke. Mol Neurobiol. 2017;54:227–237. doi: 10.1007/s12035-015-9655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ramirez MA, Reijerkerk A, de Vries HE, Romero IA. Regulation of brain endothelial barrier function by microRNAs in health and neuroinflammation. FASEB J. 2016;10:2662–2672. doi: 10.1096/fj.201600435RR. [DOI] [PubMed] [Google Scholar]
- Martinez B, Peplow PV. Blood microRNAs as potential diagnostic and prognostic markers in cerebral ischemic injury. Neural Regen Res. 2015;11:1375–1378. doi: 10.4103/1673-5374.191196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzzolo ER, Capodimonti S, Martini M, Iachininoto MG, Bianchi M, Cocomazzi A, Zini G, Leone G, Larocca LM, Teofili L. Adult and cord blood endothelial progenitor cells have different gene expression profiles and immunogenic potential. Blood Transfus. 2014;12(Suppl 1):s367–s374. doi: 10.2450/2013.0042-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura S, Bago JR, Soler-Botija C, Pujal JM, Galvez-Monton C, Prat-Vidal C, Llucia-Valideperas A, Blanco J, Bayes-Genis A. Human umbilical cord blood-derived mesenchymal stem cells promote vascular growth in vivo. PLoS One. 2012;7:e49447. doi: 10.1371/journal.pone.0049447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Benz F, Luedde T, Roderburg C. The role of miRNAs in the regulation of inflammatory processes during hepatofibrogenesis. Hepatobiliary Surg Nutr. 2015;4:24–33. doi: 10.3978/j.issn.2304-3881.2015.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra M, Chopp M, Santra S, Nallani A, Vyas S, Zhang ZG, Morris DC. Thymosin beta 4 up-regulates miR-200a expression and induces differentiation and survival of rat brain progenitor cells. J Neurochem. 2016;136:118–132. doi: 10.1111/jnc.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saparov A, Ogay V, Nuzgozhin T, Jumabay M, Chen WC. Preconditioning of human stem cells to enhance their regulation of the immune response. Stem Cells Internat 2016. 2016:3924858. doi: 10.1155/2016/3924858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehadah A, Chen J, Zacharek A, Cui Y, Ion M, Roberts C, Kapke A, Chopp M. Niaspan treatment induces neuroprotection after stroke. Neurobiol Dis. 2010;40:277–283. doi: 10.1016/j.nbd.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Li Z, Han B. Penehyclidine hydrochloride postconditioning ameliorates cerebral ischemia-reperfusion injury: critical role of mitochondrial ATP sensitive potassium channel. J Biol Regul Homeost Agents. 2016;30:41–53. [PubMed] [Google Scholar]
- Splawski JB, Jelinek DF, Lipsky PE. Immunomodulatory role of IL-4 on the secretion of Ig by human B cells. J Immunol. 1989;142:1569–1575. [PubMed] [Google Scholar]
- Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, Kim AS, Johnson JN, Bates D, King B, Case C, McGrogan M, Yankee EW, Schwartz NE. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke. A phase 1/2a study. Stroke. 2016;47:1817–1824. doi: 10.1161/STROKEAHA.116.012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Rogers TJ, Hatzirodos N, Baker LM, Ooi E, Wormald PJ. Immunomodulatory effect of cytosine-phosphate-guanosine (CpG)-oligonucleotides in nonasthmatic chronic rhinosinusitis: an explant model. Am J Rhinol Allergy. 2009;23:123–129. doi: 10.2500/ajra.2009.23.3279. [DOI] [PubMed] [Google Scholar]
- Vartanian KB, Mitchell HD, Stevens SL, Conrad VK, McDermott JE, Stenzel-Poore MP. CpG preconditioning regulates miRNA expression that modulates genomic reprogramming associated with neuroprotection against ischemic injury. J Cereb Blood Flow Metab. 2015;35:257–266. doi: 10.1038/jcbfm.2014.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, Jia J, Dong Q, Xu A, Zeng J, Li Y, Wang Z, Xia H, Johnston SC. CHANCE Investigators (2013) Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 369:11–19. doi: 10.1056/NEJMoa1215340. [DOI] [PubMed] [Google Scholar]
- Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymsal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Venkat P, Ye X, Chopp M, Zacharek A, Ning R, Cui Y, Roberts C, Kuzmin-Nichols N, Sanberg CD, Chen J. HUCBCs increase angiopoietin 1 and induce neurorestorative effects after stroke in T1DM rats. CNS Neurosci Ther. 2014;20:935–944. doi: 10.1111/cns.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Venkat P, Chopp M, Zacharek A, Ning R, Cui Y, Roberts C, Kuzmin-Nichols N, Sanberg CD, Chen J. Neurorestorative therapy of stroke in type 2 diabetes mellitus rats treated with umbilical cord blood cells. Stroke. 2015;46:2599–2606. doi: 10.1161/STROKEAHA.115.009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med. 2012;2:a006692. doi: 10.1101/cshperspect.a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Borlongan CV, Stahl CE, Hess DC, Ou Y, Kaneko Y, Yu SJ, Yang T, Fang L, Xie X. Systemic delivery of umbilical cord blood cells for stroke therapy: a review. Restor Neurol Neurosci. 2009;27:41–54. doi: 10.3233/RNN-2009-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JH, Long L, Lao ZX, Li LM, You JR. Anti-inflammatory role of micrRNA let-7c in LPS treated alveolar macrophages by targeting STAT3. Asian Pacific J Trop Med. 2016;9:72–75. doi: 10.1016/j.apjtm.2015.12.015. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Ren H, Han Z. Mesenchymal stem cells: immunomodulatory capability and clinical potential in immune diseases. J Cell Immunother. 2016;2:3–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Open peer review report 1 on “Immunomodulators and microRNAs as neurorestorative therapy for ischemic stroke”


