Abstract
Turmeric or Curcuma is a natural product that has anti-inflammatory, antioxidant and anti-apoptotic pharmacological properties. It can be used in the control of the aging process that involves oxidative stress, inflammation, and apoptosis. Aging is a physiological process that affects higher cortical and cognitive functions with a reduction in learning and memory, limited judgment and deficits in emotional control and social behavior. Moreover, aging is a major risk factor for the appearance of several disorders such as cerebrovascular disease, diabetes mellitus, and hypertension. At the brain level, the aging process alters the synaptic intercommunication by a reduction in the dendritic arbor as well as the number of the dendritic spine in the pyramidal neurons of the prefrontal cortex, hippocampus and basolateral amygdala, consequently reducing the size of these regions. The present review discusses the synaptic changes caused by the aging process and the neuroprotective role the Curcuma has through its anti-inflammatory, antioxidant and anti-apoptotic actions
Keywords: Curcuma, prefrontal cortex, hippocampus, amygdala, dendrites, aging
Introduction
Recently, various reports have suggested that there are several nutraceuticals which have an effect on aging, such as Ginkgo Biloba, Resveratrol, and Curcuma. In addition, it is known that all these products also possess antioxidant effects (for review see Chainani-Wn, 2003; Scapagnini et al., 2011; Ghosh et al., 2015; Mazzantiet al., 2016). At the neuronal level, these food supplements have also been shown to have neuroprotective and neuroreparative effects (for review see Scapagnini et al., 2011; Motaghinejad et al., 2017). One of these products, in various cultures, is used as a condiment of several foods, the extract of Curcuma longa L. or turmeric, best known as Curcuma.
Curcuma longa L., anative plant of India, is used as a remedy for several health disorders for at least 2,500 years (Gupta et al., 2013). The main components of this ancestral remedy are aromatic-turmerone (21.4%), alpha-santalene (7.2%) and aromatic-curcumene (6.1%) (Singh et al., 2010; Calapai et al., 2014). However, several reports suggest that the most active component of Curcuma are the curcuminoids and essential oils (Chainani-Wn et al., 2003; Hassan et al., 2016). Curcuminoids contain curcumin (diferuloylmethane), desmethoxycurcumin and bisdemethoxycurcumin (Chainani-Wn et al., 2003; Hassan et al., 2016), but curcumin is the most studied component of the Curcuma for its pharmacological properties and yellow color (Gupta et al., 2013; Chin, 2016; Hassan et al., 2016).
For its anti-inflammatory and antioxidant properties, Curcuma is used to treat an amazing number of diseases (for review see Kumar et al., 2011; Gupta et al., 2013; Chin, 2016). Oxidative stress and inflammatory processes have been involved in the pathogenesis of several disorders such as dementia, and therefore this extract is considered a medicine. Recent studies have been reported the effect of Curcuma on the aging process (for review see Trujillo et al., 2014), with a special focus on the brain. The neural aging process produces a reduction in memory and learning due to anatomical changes such as reduction in the size of the prefrontal cortex (PFC) and hippocampus (for review see Flores et al., 2016a, c). However, recent reports in animal models have been demonstrated that Curcuma and curcumin, drastically improve the memory process in aging animals (Pyrzanowska et al., 2010; Belviranli et al., 2013; Vidal al et al., 2017).
Considering the previous information, the present review revises the most relevant reports on the effects of the Curcuma on aging with a special focus on neural connectivity between brain regions implicated in the process of memory and learning.
Main Regions Involved in Cerebral Aging
There are several regions that are related to cognitive changes that occur with aging. However, aging studies are focused on understanding the role of the cortical structures such as the PFC, the hippocampus and basolateral amygdala (BLA), learning and memory process, and knowing what changes these structures undergo with aging (for review see Flores et al., 2016a, c). Moreover, the intercommunication among these three structures seems to be critical in processes of both spatial memory and object recognition, which declines with brain aging (for review see Flores et al., 2016a, c). The PFC receives aglutamatergic projection from hippocampus and BLA and sends glutamatergic projection to thalamus and nucleus accumbens (NAcc) (for review see Flores et al., 2016b). The interaction among the PFC, the dorsal hippocampus and amygdale change throughout life (for review see Flores et al., 2016a, c). In the aged brain, these structures undergo remodeling which reduces the volume of the hippocampus and amygdala, and reduces the thickness of the PFC (for review see Tabatabeei-Jafari et al., 2015; Gorbach et al., 2017; Tian et al., 2017). Anatomical and histological studies have demonstrated that the aging hippocampus reduced in size due to neuron loss, especially the CA1 region (for review see Miller and O’Callaghan, 2005). The reduction in hippocampal size may start during adulthood, but aging accelerates this process and reaches a loss of 0.3 to 2.1% per year (for review see Miller and O’Callaghan, 2005). The PFC is the region of the brain which is more vulnerable to a decrease in volume with age (for review see Juraska and Lowry, 2012). These morphological changes are correlated with a decline of the memory process that reduces the life span during old aging (for review see Gorbach et al., 2017).
Curcuma as a Natural Product
Natural products have been criticized a lot, and it has been suggested that these products may be used for the treatment of a great variety of diseases, which has caused them to be publicized as miracle products. It is important to understand that many natural products exert a well-established action on common processes in various diseases. A wide variety of diseases are known to have inflammatory processes, so all natural products with the anti-inflammatory pharmacological properties may be useful. In relation to Curcuma or turmeric, several reports mentioned its antidiabetic, antihypertensive, anti-fibrotic, anti-ulcer, anti-lupus, and pharmacological properties (Kumar et al., 2011; Hasssan et al., 2016). However, the beneficial effect of Curcuma is due to its anti-inflammatory, antioxidant and anti-apoptotic properties, by means of which it acts on the complications of various diseases. For example, in the case of diabetes mellitus, there is an increase in blood glucose due to low or null production and release of insulin, which produces a deterioration in all the cells of the body. Turmeric does not modify insulin levels and glucose levels, but through well-demonstrated mechanisms, it reduces inflammatory processes and oxidative stress (Ghosh et al., 2015), which are present in diabetic patients and are a consequence of the high blood glucose levels. In this sense, it is known that chronic high levels of glucose in blood can lead to alterations at the vascular level, which leads to processes of local ischemia and a production of free radicals and inflammation (for review see Ghosh et al., 2015; Luppi and Drain, 2016; Turkmen, 2017). Curcuma reduces the complication due to the chronic high level of glycemia in diabetic patients by reducing oxidative stress and/or inflammation and apoptosis in certain tissues of the human body, such as the brain (for review see Patel and Udayabanu, 2017). As a consequence, Curcuma may be used in the treatment of diabetes mellitus complications (for a review see Rivera-Mancia et al., 2015).
In a similar vein, Curcuma and other polyphenols such as resveratrol are also used in the treatment of dementia and brain aging process (for review see Flores et al., 2016c; Hernandez-Hernandez et al., 2016; Mazzanti and Di Giocomo, 2016). Both polyphenols showed antioxidants and anti-inflammatory properties (for review see Suh et al., 2001; Murakami and Ohigashi, 2007; Howes and Simmonds, 2014).
Neural Changes during the Aging Process
Age changes the structure of the brain, specifically in size, connectivity, and the number of neurons per region. However, the changes are not the same in all regions. There are regions where the changes are very subtle but critical. Many of these changes have been linked to local inflammatory processes, often related to oxidative stress, since with age the cellular mechanisms that attenuate the effects of stress are reduced (for review see Biragyn et al., 2017). These reasons may suggest that neural aging is a complex biological process due to differences in the stress sensitivity of different regions of the brain. In addition, brain aging is a natural process, not a disease, however, structural change with age increases the risk factor for neurodegenerative diseases such as Alzheimer's or vascular dementia (for review see Flores et al., 2016a; Bitagyn et al., 2017). An increasing number of reports support the association between inflammatory process and neurodegenerative progress (for review see Biragyn et al., 2017). In the elderly brain, a common denominator is the presence of the inflammation processes. The main reason for this neural disorder is due to the brain having its own immune system (for review see Streit and Xue, 2010). Interestingly, microglia cells are the main component of the brain immune system. The microglial cells play a critical role in neuroprotection and immunologically competent cells at the same time (for review see Streit and Xue, 2010). With age, microglial cells also suffer aging, which reduces the function of microglia cells and causes the increase in the microglial cell number (for review see Strait and Xue, 2010). In addition, the inflammatory process causes an overactivation of the immune system with the increasing number of microglia cells, resulting in a production increase of proinflammatory cytokines that increased susceptibility to neurodegeneration (for review see Strait and Xue, 2010; Deleidi et al., 2015; Rawji et al., 2016). Moreover, with the age, anti-inflammatory mediators are reduced (Ye and Johnson, 1999; Corbi et al., 2016).
The oxidative stress process appears when the system is unable to establish a balance between how many oxidizing molecules are produced, and how many are used (for review see Ham and Raju, 2016). This balance is lost with age, and this allows the accumulation of free radicals such as hydrogen peroxide, hydroxyl radicals, and superoxide anions (for review see Ham and Raju, 2016). Also, the mitochondrial function is reduced with age, which allows the accumulation of free radicals (for review see Grimm et al., 2016). When the accumulation of free radicals is higher enough to damage proteins and deoxyribonucleic acid (DNA) and induce lipid peroxidation (for review see Grimm et al., 2016). This leads to neurodegeneration.
The after effects of oxidative stress, and inflammatory processes, and progressive neurodegeneration lead to loss of synaptic connections in several interconnected brain regions such as the PFC and hippocampus (for review see Kim et al., 2016). These regions are involved in learning and memory processes, which is mentioned in several neuroimaging studies (for review see Grimm et al., 2016; Benedetto et al., 2017). In addition, in the hippocampus, chronic stress causes shrinkage of the dendritic arborin the CA3 and DFG neurons and reduces the number of dendritic spines in CA1 neurons (McEwen, 1999a, b; McEwen et al., 2016). Additionally, stress also increases cortical levels and overwhelms the cortical receptors in the hippocampus. The interaction between glucocorticoids and mineralocorticoids with hippocampal neurons has been well documented (for review see McEwen et al., 2016). Interestingly, these hormones may mediate biphasic effects on long-term potentiation and long-term depression (Pavlides et al., 1995; McEwen et al., 2016), both processes are related to the memory process.
In a similar vein, neuroimaging studies have been clearly demonstrated that the aging process causes an enlarged ventricular system (Scahill et al., 2003; Acabchuk et al., 2015; Liu et al., 2017; Madan and Kensinger, 2017), reduced global cortical thickness (Salat et al., 2004; Espeseth et al., 2008; Liu et al., 2017; Pink et al., 2017) and smaller brain volume (for review see Liu et al., 2017). Interestingly, the most affected cortical regions are the frontal and temporal cortex (Pink et al., 2017).
Curcuma Effects on Aging
Considering the aforementioned, it could be postulated that Curcuma, a polyphenol due to its anti-inflammatory and antioxidative properties, has beneficial effects on the aging process, but the pharmacological mechanism that this polyphenol has is still unclear. However, several reports suggest that the anti-inflammatory effect of the Curcuma is because of its antioxidant action. Interestingly, several in vitro studies have demonstrated that curcumin, a component of the Curcuma, may reduce the monocyte chemoattractant / chemotactic protein-1 (MCP-1) production in different cell lines (for review see Kiriman et al., 2016). Moreover, in vivo studies also suggested that this polyphenol may decrease mRNA expression of MCP-1, interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) and ameliorate the enhanced expression of glial fibrillary acidic protein (GFAP) and ionized calcium binding adaptor molecule 1 (Iba-1) in the hippocampus or cerebral cortex in an animal model of epilepsy (an animal model of the brain inflammation) (Kaur et al., 2015). Considering this information, it is possible to suggest that Curcuma has direct anti-inflammatory effects by reducing glial activation with curcumin as the active ingredient (Kaur et al., 2015).
In the same vein, several reports also suggested that curcumin may regulate other molecules such as heme-oxygenase-1 (HO-1) and brain-derived neurotrophic factor (BDNF) in the hippocampus (for review see Scapagnini et al., 2011; Corbi et al., 2016). For example, curcumin causes an increased expression of the neurotrophin, BDNF, in the hippocampus (Xu et al., 2007). In addition, curcumin plays a critical role in activating the antioxidant enzymes such as glutathione peroxidase (GSH-Px) and glutathione-S-transferase (GST) by targeting the common transcription factor called nuclear factor erythroid 2-related factor 2 (NrF2) (Liby et al., 2007; Tosetti et al., 2009; Wu et al., 2015; Corbi et al., 2016). In addition, a recent report suggests that curcumin strongly induces HO-1 expression and activity in different brain regions by the activation of heterodimers of the Nrf2/antioxidant responsive element (ARE) pathway (Scapagnini et al., 2011). Moreover, Wu et al. (2015) demonstrated that curcumin also increases thioredoxin (Trx) protein expression and Trx enzyme activity in rat cortical neurons. Both proteins, Nrf2 and Trx, have an antioxidant action (Das and Das, 2000; Scapagnini et al., 2011; Ma, 2013; Wu et al., 2015).
Curcuma Improved Synaptic Connections
Recent reports from our group have shown that aged rats showed a reduced dendritic length and a reduced number of dendritic spine density in limbic regions such as the PFC, hippocampus and BLA with deficits in the memory process (Alcantara-Gonzalez et al., 2010; Juárez et al., 2011; Sanchez et al., 2011; Alcantara-Gonzalez et al., 2012; Hernandez-Hernandez et al., 2016; Solis-Gaspar et al., 2016). However, the chronic administration of Curcuma improves the dendritic arborization and the number of dendritic spines of the PFC, hippocampus, and BLA in aged animals. These data suggest that Curcuma improved local communication among pyramidal neurons, which was analyzed by measuring the dendritic length in the aforementioned regions. In addition, long distance communication was also evaluated through the analysis of distal dendritic spine density and the results suggested that Curcuma also improved the communication among the limbic regions, especially between the PFC and hippocampus. Therefore, it is possible to suggest that the chronic effect of Curcuma produces a rearrangement at the level of dendritic communication that is reflected with a better memory process, especially long-term memory, as our recent report suggested (Vidal et al., 2017). According to our Curcuma results, resveratrol, another polyphenol, also causes increases in the dendritic length and dendritic spine density of the pyramidal neurons of layers 3 and 5 of the PFC and CA1 and CA3 of the dorsal hippocampus (Hernandez-Hernandez et al., 2016; Flores et al., 2016c). Although the mechanism involved in the maintenance of synaptic communication during aging is not clearly described, aforementioned reports show that during aging there is a reduction in neurotrophic factor such as BDNF, and that curcumin has the ability to increase these factors in the hippocampus (Zhang et al., 2015; Choi et al., 2017; Motaghinejad et al., 2017, Xu et al., 2017). Moreover, recent reports have demonstrated that curcumin not only increases levels of BDNF in the hippocampus and prefrontal cortex (Xu et al., 2006; Liu et al., 2014), but also increases the level of extracellular signal-regulated kinase (ERK) protein in the hippocampus (Liu et al., 2014) and reduces serum corticosterone levels (Xu et al., 2006) in the animal model of chronic stress. High levels of corticosterone in the hippocampus and the PFC with chronic stress cause atrophy of the dendrites of the pyramidal neurons (for review see McEwen, 1999a; McEwen et al., 2016). Consequently, corticosterone in the rat and cortisol in the human also regulates the dendritic structural plasticity in the hippocampus and the PFC. Another candidate is nitric oxide (NO), and several reports suggest that NO may regulate the dendritic spine number and arborization in the pyramidal neurons of the PFC and hippocampus (for review see Yoshihara et al., 2009). For example, increased level of NO by application of a NO donor leads to the formation of dendritic spines (Nikonenko et al., 2008; Yoshihara et al., 2009). On the contrary, reduced levels of NO caused by a blockade of neural nitric oxide synthase (nNOS) in neonatal rats by L-NNA result in a decrease in dendritic spine density (Morales-Medina et al., 2007). However, high levels of NO due to chronic stress by its neurotoxicity may cause a dendritic atrophy (for review see McEwen et al., 2016). Consequently, NO functional levels are necessary to regulate dendritic morphology, especially, dendritic spine density. Interestingly, curcumin reduced the effects of chronic stress by reducing NO levels in cortical regions (Murakami and Ohigashi, 2007; Choi et al., 2017).
In response to inflammatory cytokines such as IL-1β and TNF, the levels of ciclooxigenase-2 (COX-2) protein suffer from an increase. However, this protein is an enzyme that transforms arachidonic acid in prostaglandins (PGD, PGI2, and PGE2) (Choi et al., 2017). The anti-inflammatory effects of curcumin reduce the COX-2 levels through inhibiting the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (Surth et al., 2001; Maher et al., 2010; Shen et al., 2015). More, curcumin prevents the onset of inflammation also by inhibiting the production of TNF-α and interferon gamma (IFN-γ) (Brouet and Ohlshima, 1995; He et al., 2010). In addition, chronic stress also increases the COX-2 levels in the cortex and hippocampus (Choi et al., 2017). However, curcumin ameliorates the effects of chronic stress by reducing the COX-2 levels (Murakami and Ohigashi, 2007; Choi et al., 2017). In addition, recent reports also suggest that curcumin also reduces hippocampus cell death by reducing the reaction of the astrocytes. This effect is due to a reduction in the upregulation of caspase-3, GFAP, and endothelial constitutive nitric oxide synthase (eNOS) (Shin et al., 2007). Consequently, Curcuma exerts beneficial effects on the aging brain main through its anti-inflammatory, antioxidant and antiapoptotic properties (Figure 1).
Figure 1.
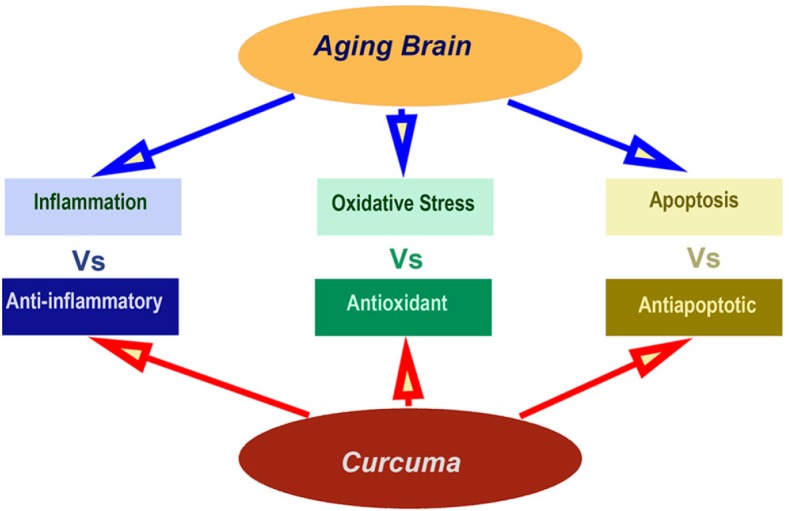
Main pharmacological properties of curcuma.
The cognitive deficits in the brain aging process are due to inflammatory, oxidative stress and the apoptotic process. Interestingly, the beneficial effects of Curcuma on the aging brain are caused by anti-inflammatory, antioxidant and antiapoptotic pharmacological properties.
Conclusions
Several reports suggest that the anti-inflammatory, antioxidant and antiapoptotic pharmacological properties of Curcuma may be beneficial in the brain aging process. Animal models of aging have roundly demonstrated the biochemical and morphological effects of the Curcuma on the PFC and hippocampus which are the regions involved in the memory and learning process. Consequentially, the effects of Curcuma on the processes of memory involve an improvement in the interneuronal communication of both regions. Accordingly, Curcuma should be considered as a therapeutic alternative in the control of the aging process with the main aim of improving the quality of life of elderly people.
Acknowledgments
GF acknowledges the “Sistema Nacional de Investigadores” of Mexico for membership. Thanks to Professor Robert Simpson for editing the English language text. None of the funding institutions had any further role in the study design, the collection of data, analyses, interpretation of data, writing of the report or in the decision to submit the paper for publication.
Footnotes
Funding: This study was supported by grants from the VIEP-BUAP grant (No. FLAG-2017), ProDES (CA-BUAP-120) and CONACYT grant (No. 252808) to GF.
Conflicts of interest: The author has no conflicts of interest to declare.
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check: This paper has been checked twice with duplication-checking software iThenticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
References
- Acabchuk RL, Sun Y, Wolferz R, Jr, Eastman MB, Lennington JB, Shook BA, Wu Q, Conover JC. 3D modeling of the lateral ventricles and histological characterization of periventricular tissue in humans and mouse. J Vis Exp. 2015:e52328. doi: 10.3791/52328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara-Gonzalez F, Mendoza-Perez CR, Zaragoza N, Juarez I, Arroyo-García LE, Gamboa C, De La Cruz F, Zamudio S, Garcia-Dolores F, Flores G. Combined administration of cerebrolysin and donepezil induces plastic changes in prefrontal cortex in aged mice. Synapse. 2012;66:938–349. doi: 10.1002/syn.21588. [DOI] [PubMed] [Google Scholar]
- Alcantara-Gonzalez F, Juarez I, Solis O, Martinez-Tellez I, Camacho-Abrego I, Masliah E, Mena R, Flores G. Enhanced dendritic spine number of neurons of the prefrontal cortex, hippocampus, and nucleus accumbens in old rats after chronic donepezil administration. Synapse. 2010;64:786–793. doi: 10.1002/syn.20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belviranlı M, Okudan N, Atalık KE, Öz M. Curcumin improves spatial memory and decreases oxidative damage in aged female rats. Biogerontology. 2013;14:187–196. doi: 10.1007/s10522-013-9422-y. [DOI] [PubMed] [Google Scholar]
- Biragyn A, Aliseychik M, Rogaev E. Potential importance of B cells in aging and aging-associated neurodegenerative diseases. Semin Immunopathol. 2017;39:283–294. doi: 10.1007/s00281-016-0615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouet I, Ohshima H. Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem Biophys Res Commun. 1995;206:533–540. doi: 10.1006/bbrc.1995.1076. [DOI] [PubMed] [Google Scholar]
- Calapai G, Miroddi M, Minciullo PL, Caputi AP, Gangemi S, Schmidt RJ. Contact dermatitis as an adverse reaction to some topically used European herbal medicinal products - part 1: Achillea millefolium-Curcuma longa. Contact Dermatitis. 2014;71:1–12. doi: 10.1111/cod.12222. Calapai G, Miroddi M, Minciullo PL, Caputi AP, Gangemi S, Schmidt RJ. [DOI] [PubMed] [Google Scholar]
- Chainani-Wn N. Safety and anti-inflammatory activity of curcumin: A compound of turmeric (Curcuma Longa) J Akltern Complement Med. 2003;9:161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- Chin KY. The spice for joint inflammation: anti-inflammatory role of curcumin in treating osteoarthritis. Drug Des Devel Ther. 2016;10:3029–3042. doi: 10.2147/DDDT.S117432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GY, Kim HB, Hwang ES, Lee S, Kim MJ, Choi JY, Lee SO, Kim SS, Park JH. Curcumin alters neural plasticity and viability of intact hippocampal circuits and attenuates behavioral despair and COX-2 expression in chronically stressed rats. Mediators Inflamm 2017. 2017:6280925. doi: 10.1155/2017/6280925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbi G, Conti V, Davinelli S, Scapagnini G, Filippelli A, Ferrara N. Dietary phytochemicals in neuroimmunoaging: a new therapeutic possibility for humans? Front Pharmacol. 2016;7:364. doi: 10.3389/fphar.2016.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleidi M, Jäggle M, Rubino G. Immune aging, dysmetabolism, and inflammation in neurological diseases. Front Neurosci. 2015;9:172. doi: 10.3389/fnins.2015.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto S, Müller L, Wenger E, Düzel S, Pawelec G. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci Biobehav Rev. 2017;75:114–128. doi: 10.1016/j.neubiorev.2017.01.044. [DOI] [PubMed] [Google Scholar]
- Espeseth T, Westlye LT, Fjell AM, Walhovd KB, Rootwelt H, Reinvang I. Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E epsilon 4. Neurobiol Aging. 2008;29:329–340. doi: 10.1016/j.neurobiolaging.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Flores G, Flores-Gómez GD, de Jesús Gomez-Villalobos M. Neuronal changes after chronic high blood pressure in animal models and its implication for vascular dementia. Synapse. 2016a;70:198–205. doi: 10.1002/syn.21887. [DOI] [PubMed] [Google Scholar]
- Flores G, Morales-Medina JC, Diaz A. Neuronal and brain morphological changes in animal models of schizophrenia. Behav Brain Res. 2016b;301:190–203. doi: 10.1016/j.bbr.2015.12.034. [DOI] [PubMed] [Google Scholar]
- Flores G, Vázquez-Roque RA, Diaz A. Resveratrol effects on neural connectivity during aging. Neural Regen Res. 2016c;11:1067–1068. doi: 10.4103/1673-5374.187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Atzori M. The potential of cerebrolysin in the treatment of schizophrenia. Pharmacol Pharmacy. 2014;5:691–704. [Google Scholar]
- Ghosh S, Banerjee S, Sil PC. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem Toxicol. 2015;83:111–124. doi: 10.1016/j.fct.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Grimm A, Mensah-Nyagan AG, Eckert A. Alzheimer, mitochondria and gender. Neurosci Biobehav Rev. 2016;67:89–101. doi: 10.1016/j.neubiorev.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Sung B, Kim JH, Prasad S, Li S, Aggarwal BB. Multitargeting by turmeric the golden spice: From kitchen to clinic. Mol Nutr Food Res. 2013;57:1510–1528. doi: 10.1002/mnfr.201100741. [DOI] [PubMed] [Google Scholar]
- Ham PB, 3rd, Raju R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog Neurobiol. 2016 doi: 10.1016/j.pneurobio.2016.06.006. doi:10.1016/j.pneurobio.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan W, Gul S, Rehman S, Kanwal F, Afridi MS, Fazal H, Shah Z, Rahman A, da Rocha JB. Gas chromatography coupled with mass spectrometric characterization of Curcuma longa: Protection against pathogenic microbes and lipid peroxidation in rat's tissue homogenate. Pak J Pharm Sci. 2016;29:615–621. [PubMed] [Google Scholar]
- He LF, Chen HJ, Qian LH, Chen GY, Buzby JS. Curcumin protects pre-oligodendrocytes from activated microglia in vitro and in vivo. Brain Res. 2010;1339:60–69. doi: 10.1016/j.brainres.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Hernández-Hernández EM, Serrano-García C, Vázquez-Roque RA, Díaz A, Monroy E, Rodríguez-Moreno A, Florán B, Flores G. Chronic administration of resveratrol prevents morphological changes in prefrontal cortex and hippocampus of aged rats. Synapse. 2016;70:206–217. doi: 10.1002/syn.21888. [DOI] [PubMed] [Google Scholar]
- Howes MJ, Simmonds MS. The role of phytochemicals as micronutrients in health and disease. Curr Opin Clin Nutr Metab Care. 2014;17:558–566. doi: 10.1097/MCO.0000000000000115. [DOI] [PubMed] [Google Scholar]
- Juárez I, González DJ, Mena R, Flores G. The chronic administration of cerebrolysin induces plastic changes in the prefrontal cortex and dentate gyrus in aged mice. Synapse. 2011;65:1128–1135. doi: 10.1002/syn.20950. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Lowry NC. Neuroanatomical changes associated with cognitive aging. Curr Top Behav Neurosci. 2012;10:137–162. doi: 10.1007/7854_2011_137. [DOI] [PubMed] [Google Scholar]
- Kaur H, Patro I, Tikoo K, Sandhir R. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem Int. 2015;89:40–50. doi: 10.1016/j.neuint.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Karimian MS, Pirro M, Majeed M, Sahebkar A. Curcumin as a natural regulator of monocyte chemoattractant protein-1. Cytokine Growth Factor Rev. 2016;33:55–63. doi: 10.1016/j.cytogfr.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Kim HK, Nunes PV, Oliveira KC, Young LT, Lafer B. Neuropathological relationship between major depression and dementia: A hypothetical model and review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;67:51–57. doi: 10.1016/j.pnpbp.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Kumar P, Mishra S, Malik A, Satya S. Repellent, larvicidal and pupicidal properties of essential oils and their formulations against the housefly, Musca domestica. Med Vet Entomol. 2011;25:302–310. doi: 10.1111/j.1365-2915.2011.00945.x. [DOI] [PubMed] [Google Scholar]
- Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- Liu D, Wang Z, Gao Z, Xie K, Zhang Q, Jiang H, Pang Q. Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav Brain Res. 2014;271:116–121. doi: 10.1016/j.bbr.2014.05.068. [DOI] [PubMed] [Google Scholar]
- Liu H, Yang Y, Xia Y, Zhu W, Leak RK, Wei Z, Wang J, Hu X. Aging of cerebral white matter. Ageing Res Rev. 2017;34:64–76. doi: 10.1016/j.arr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi P, Drain P. C-peptide antioxidant adaptive pathways in β cells and diabetes. J Intern Med. 2017;281:7–24. doi: 10.1111/joim.12522. [DOI] [PubMed] [Google Scholar]
- Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan CR, Kensinger EA. Age-related differences in the structural complexity of subcortical and ventricular structures. Neurobiol Aging. 2017;50:87–95. doi: 10.1016/j.neurobiolaging.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P, Akaishi T, Schubert D, Abe K. A pyrazole derivative of curcumin enhances memory. Neurobiol Aging. 2010;31:706–709. doi: 10.1016/j.neurobiolaging.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Mazzanti G, Di Giacomo S. Curcumin and resveratrol in the management of cognitive disorders: what is the clinical evidence? Molecules. 2016;21:E1243. doi: 10.3390/molecules21091243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999a;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and the aging hippocampus. Front Neuroendocrinol. 1999b;20:49–70. doi: 10.1006/frne.1998.0173. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Medina JC, Mejorada A, Romero-Curiel A, Flores G. Alterations in dendritic morphology of hippocampal neurons in adult rats after neonatal administration of N-omega-nitro-L-arginine. Synapse. 2007;61:785–789. doi: 10.1002/syn.20406. [DOI] [PubMed] [Google Scholar]
- Motaghinejad M, Motevalian M, Fatima S, Hashemi H, Gholami M. Curcumin confers neuroprotection against alcohol-induced hippocampal neurodegeneration via CREB-BDNF pathway in rats. Biomed Pharmacother. 2017;87:721–740. doi: 10.1016/j.biopha.2016.12.020. [DOI] [PubMed] [Google Scholar]
- Murakami A, Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int J Cancer. 2007;121:2357–2363. doi: 10.1002/ijc.23161. [DOI] [PubMed] [Google Scholar]
- Nikonenko I, Boda B, Steen S, Knott G, Welker E, Muller D. PSD-95 promotes synaptogenesis and multiinnervated spine formation through nitric oxide signaling. J Cell Biol. 2008;183:1115–1127. doi: 10.1083/jcb.200805132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SS, Udayabanu M. Effect of natural products on diabetes associated neurological disorders. Rev Neurosci. 2017;28:271–293. doi: 10.1515/revneuro-2016-0038. [DOI] [PubMed] [Google Scholar]
- Pink A, Przybelski SA, Krell-Roesch J, Stokin GB, Roberts RO, Mielke MM, Spangehl KA, Knopman DS, Jack CR, Jr, Petersen RC, Geda YE. Cortical thickness and anxiety symptoms among cognitively normal elderly persons: The Mayo Clinic Study of Aging. J Neuropsychiatry Clin Neurosci. 2017;29:60–66. doi: 10.1176/appi.neuropsych.15100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrzanowska J, Piechal A, Blecharz-Klin K, Lehner M, Skórzewska A, Turzyńska D, Sobolewska A, Plaznik A, Widy-Tyszkiewicz E. The influence of the long-term administration of Curcuma longa extract on learning and spatial memory as well as the concentration of brain neurotransmitters and level of plasma corticosterone in aged rats. Pharmacol Biochem Behav. 2010;95:351–358. doi: 10.1016/j.pbb.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Rawji KS, Mishra MK, Michaels NJ, Rivest S, Stys PK, Yong VW. Immunosenescence of microglia and macrophages: impact on the aging central nervous system. Brain. 2016;139:653–661. doi: 10.1093/brain/awv395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Mancía S, Lozada-García MC, Pedraza-Chaverri J. Experimental evidence for curcumin and its analogs for management of diabetes mellitus and its associated complications. Eur J Pharmacol. 2015;756:30–37. doi: 10.1016/j.ejphar.2015.02.045. [DOI] [PubMed] [Google Scholar]
- Sánchez F, Gómez-Villalobos Mde J, Juarez I, Quevedo L, Flores G. Dendritic morphology of neurons in medial prefrontal cortex, hippocampus, and nucleus accumbens in adult SH rats. Synapse. 2011;65:198–206. doi: 10.1002/syn.20837. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, Fabio G. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol. 2011;44:192–201. doi: 10.1007/s12035-011-8181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LL, Jiang ML, Liu SS, Cai MC, Hong ZQ, Lin LQ, Xing YY, Chen GL, Pan R, Yang LJ, Xu Y, Dong J. Curcumin improves synaptic plasticity impairment induced by HIV-1gp120 V3 loop. Neural Regen Res. 2015;10:925–931. doi: 10.4103/1673-5374.158358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, Kapoor IP, Singh P, de Heluani CS, de Lampasona MP, Catalan CA. Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma longa. Linn) Food Chem Toxicol. 2010;48:1026–1031. doi: 10.1016/j.fct.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Solis-Gaspar C, Vazquez-Roque RA, De Jesús Gómez-Villalobos M, Flores G. Cerebrolysin improves memory and ameliorates neuronal atrophy in spontaneously hypertensive, aged rats. Synapse. 2016;70:378–389. doi: 10.1002/syn.21912. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2991:480–481. doi: 10.1016/s0027-5107(01)00183-x. 243-268. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Xue QS. The brain's aging immune system. Aging Dis. 2010;1:254–261. [PMC free article] [PubMed] [Google Scholar]
- Tosetti F, Noonan DM, Albini A. Metabolic regulation and redox activity as mechanisms for angioprevention by dietary phytochemicals. Int J Cancer. 2009;125:1997–2003. doi: 10.1002/ijc.24677. [DOI] [PubMed] [Google Scholar]
- Trujillo J, Granados-Castro LF, Zazueta C, Andérica-Romero AC, Chirino YI, Pedraza-Chaverrí J. Mitochondria as a target in the therapeutic properties of curcumin. Arch Pharm (Weinheim) 2014;347:873–884. doi: 10.1002/ardp.201400266. [DOI] [PubMed] [Google Scholar]
- Turkmen K. Inflammation, oxidative stress, apoptosis, and autophagy in diabetes mellitus and diabetic kidney disease: the Four Horsemen of the Apocalypse. Int Urol Nephrol. 2017;49:837–844. doi: 10.1007/s11255-016-1488-4. [DOI] [PubMed] [Google Scholar]
- Vidal B, Vázquez-Roque RA, Gnecco D, Enríquez RG, Floran B, Díaz A, Flores G. Curcuma treatment prevents cognitive deficit and alteration of neuronal morphology in the limbic system of aging rats. Synapse. 2017;71:e21952. doi: 10.1002/syn.21952. [DOI] [PubMed] [Google Scholar]
- Wu JX, Zhang LY, Chen YL, Yu SS, Zhao Y, Zhao J. Curcumin pretreatment and post-treatment both improve the antioxidative ability of neurons with oxygen-glucose deprivation. Neural Regen Res. 2015;10:481–489. doi: 10.4103/1673-5374.153700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Chen H, Huang C, Gu X, Wang J, Xu D, Yu X, Shuai C, Chen L, Li S, Xu Y, Gao T, Ye M, Su W, Liu H, Zhang J, Wang C, Chen J, Wang Q, Cui W. Curcumin attenuates surgery-induced cognitive dysfunction in aged mice. Metab Brain Dis. 2017;32:789–798. doi: 10.1007/s11011-017-9970-y. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ku B, Tie L, Yao H, Jiang W, Ma X, Li X. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res. 2006;1122:56–64. doi: 10.1016/j.brainres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, De Roo M, Muller D. Dendritic spine formation and stabilization. Curr Opin Neurobiol. 2009;19:146–153. doi: 10.1016/j.conb.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Zhang L, Fang Y, Xu Y, Lian Y, Xie N, Wu T, Zhang H, Sun L, Zhang R, Wang Z. Curcumin improves amyloid β-peptide (1-42) induced spatial memory deficits through BDNF-ERK signaling pathway. PLoS One. 2015;10:e0131525. doi: 10.1371/journal.pone.0131525. [DOI] [PMC free article] [PubMed] [Google Scholar]


