Abstract
Adenosine modulates diverse physiological and pathological processes in the brain, including neuronal activities, blood flow, and inflammation. However, the mechanisms underlying the dynamics of extracellular adenosine are not fully understood. We have recently developed a novel biosensor, called an adenosine sensor cell, and we have characterized the neuronal and astrocytic pathways for elevating extracellular adenosine. In this review, the physiological implications and therapeutic potential of the pathways revealed by the adenosine sensor cells are discussed. We propose that the multiple pathways regulating extracellular adenosine allow for the diverse functions of this neuromodulator, and their malfunctions cause various neurological and psychiatric disorders.
Keywords: adenosine, aquaporin, astrocyte, BDNF, calcium channel, epilepsy, psychiatric disorder
Adenosine Signaling in the Brain
Adenosine is a major neuromodulator that does not induce neuronal activities by itself but influences the efficacy of synaptic transmission and spike frequency. Four G protein-coupled receptors, namely Gi/o-coupled A1 and A3 receptors and Gs-coupled A2A and A2B receptors, mediate adenosine responses with marked differences in their affinity to adenosine (70 nM for A1 receptors, 150 nM for A2A, 5,100 nM for A2B, and 6,500 nM for A3) (Dunwiddie and Masino, 2001). A1 and A2A receptors are broadly distributed in the brain and largely localize at synapses in both pre- and post-synaptic structures (Sebastiao and Ribeiro, 2015). The A1 receptor is one of the most abundant G protein-coupled receptors in the brain, and it downregulates neuronal activity and protects neuron from excitotoxicity by suppressing glutamate release, as well as subsequent neuronal firing (Chen et al., 2013). The A2A receptor, which is highly expressed in the striatum, upregulates excitatory synaptic transmission (Ciruela et al., 2006) and is essential for some forms of synaptic plasticity, especially those depending on brain-derived neurotrophic factor (BDNF) (Rebola et al., 2008; Jeronimo-Santos et al., 2014).
Adenosine affects the signaling of other neurotransmitters, because adenosine receptors form heterodimers and competitively interact with other neurotransmitter receptors. One notable example is the interaction between the adenosine A2A receptor and dopamine D2 receptor in the striatum; the upregulation of D2 receptor signaling by A2A antagonists is considered a potential therapeutic strategy for Parkinson's disease (Cieślak et al., 2008). Vasculature, blood cells, glial cells, and neural stem cells are also under the influence of adenosine in the brain (Tabrizchi and Bedi, 2001). Adenosine is a potent vasodilator in many organs, including the brain (Ralevic and Dunn, 2015), as well as an opener of the blood brain barrier (Bynoe et al., 2015). Inflammatory responses of the resident immune cells of the brain, microglia (Orr et al., 2009), as well as peripheral neutrophils (Barletta et al., 2012) and monocytes (Hasko and Pacher, 2012), are modulated in an anti-inflammatory direction by adenosine. Astrocyte function (Orr et al., 2015) and pathological activation (Brambilla et al., 2003) are also modulated by adenosine. Neural stem cells strongly express NTPDase2, which increases extracellular adenosine by hydrolyzing extracellular adenosine triphosphate (ATP) and adenosine diphosphate (ADP) (Gampe et al., 2015), and their proliferation is upregulated by adenosine (Migita et al., 2008). To the best of our knowledge, adenosine interacts with all cell types residing and circulating in the brain, except for mature oligodendrocytes, and it modulates diverse brain functions and pathologies.
Dynamics of Extracellular Adenosine Measured by Adenosine Sensor Cells
Cerebral adenosine fluctuates on a daily cycle, and it promotes sleep in a manner that is blocked by caffeine, whereas a ketogenic diet for epilepsy treatment boosts cerebral adenosine for days or even weeks (Masino et al., 2009). Meanwhile, brief electrical stimulations to brain slices elevate adenosine for only a few seconds (Nguyen and Venton, 2015). These temporally distinct fluctuations of extracellular adenosine are essential for diverse brain functions, and they are likely enabled by multiple pathways for releasing, uptaking, producing, and inactivating adenosine. Thus, the malfunction or disruption of these pathways should cause neurological and psychiatric disorders.
Extracellular adenosine has been studied using biochemical and physiological methods. In early days, cellular ATP was labelled by incubating with radiolabelled adenine, and the release of ATP metabolites, including adenosine, was analyzed by high-performance liquid chromatography (HPLC) (Lloyd et al., 1993). Current HPLC techniques can be used to detect adenosine in in vivo samples collected by a microdialysis probe every 5–10 minutes (Haink and Deussen, 2003), however, the temporal resolution of these biochemical methods is insufficient to analyze the fast dynamics (less than 50 ms) of extracellular adenosine, as suggested by the indirect pharmacological measurement of A1 receptor-mediated synaptic inhibition in brain slices (Cunha et al., 1998). Thus, two electrochemical methods have been developed for measuring the fast adenosine dynamics. One method is the enzymatic electrode, in which a series of enzymatic reactions are used to degrade adenosine to urea, and its byproduct, hydrogen peroxides, are measured using a redox electrode (Dale and Frenguelli, 2012). The other is cyclic voltammetry, in which the oxidation of adenosine on the surface of a carbon fiber electrode is measured (Nguyen and Venton, 2015). The temporal resolution of the cyclic voltammetry method (100 ms) is better than that of the enzymatic electrode (2 s for rise time). On the other hand, only the enzymatic electrode enables long-term measurement; cyclic voltammetry measurements are stable for no more than 90 seconds. These methods allowed accurate descriptions of adenosine dynamics in brain tissue under physiological and pathological conditions, and they suggest various forms of elevations of extracellular adenosine (Nguyen and Venton, 2015).
For a detailed analysis of the mechanisms underlying adenosine dynamics, we have developed a biosensor called an adenosine sensor cell that allows for the imaging of adenosine by conventional calcium imaging (Yamashiro et al., 2017). The adenosine sensor cell is a cell line that stably expresses the A1 receptor and Gqi5 (Conklin et al., 1993), which is an artificial G protein mutant capable of mediating between Gi-coupled receptor and phospholipase C. Adenosine above 0.1 μM elevates intracellular calcium in the adenosine sensor cells, and this calcium response is successfully used to detect the elevation of extracellular adenosine in a brain slice placed on top of these cells, as illustrated in Figure 1. This novel method revealed new aspects of extracellular adenosine dynamics, as summarized in Figure 2, and their implications are discussed below.
Figure 1.
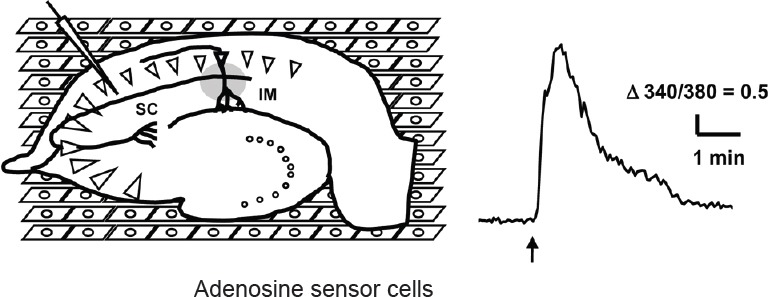
Measurement of adenosine release in hippocampal slice following electrical stimulation by adenosine sensor cells.
A hippocampal slice was placed on the top of the adenosine sensor cells (human embryonic kidney 293 (HEK293) cells expressing A1 receptor and Gqi5) loaded with Fura-2AM and a high-frequency electrical stimulation was delivered to schaffer collateral (SC) (left). Calcium response of an adenosine sensor cell imaged by an inverted microscope (IM) following electrical stimulation (arrow) (right).
Figure 2.
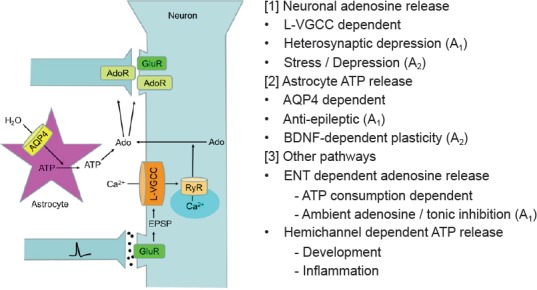
Pathways for elevating extracellular adenosine.
AdoR: Adenosine receptors; AQP4: aquaporin 4; ATP: adenosine triphosphate; BDNF: brain-derived neurotrophic factor; ENT: equilibrative nucleoside transporter; EPSP: excitatory postsynaptic potential; GluR: glutamate receptors; L-VGCC: L-type voltage gated calcium channel; RyR: ryanodine receptor.
Neuronal Mechanism for Elevating Extracellular Adenosine and Psychiatric Disorders
The adenosine sensor cell detected the adenosine release in the hippocampal CA1 region following high-frequency electrical stimulation (HFS, 30 Hz for 5 seconds) of the presynaptic or postsynaptic pathways to pyramidal neurons, and this release was blocked by the pharmacological inhibitions of the L-type voltage gated calcium channel (L-VGCC) or calcium-induced calcium release (CICR) via the ryanodine receptor (Yamashiro et al., 2017). The spatiotemporal distribution of the evoked adenosine released was well correlated with that of the heterosynaptic depression, which is a broadly-distributed suppression of excitatory synaptic transmission due to A1 receptor activation lasting for a few minutes after HFS (Manzoni et al., 1994). Thus, the neuronal adenosine release depending on L-VGCC most likely underlies this classical form of synaptic plasticity. Activity-dependent presynaptic ATP release had been demonstrated in the parasympathetic nerve terminal (Ralevic and Dunn, 2015) and was suggested in a biochemical analysis of extracellular fluid collected from hippocampal slices electrically stimulated for 3 minutes (Cunha et al., 1996). However, ATP release was not detected in hippocampal slices after HFS by adenosine sensor cells, as in a previous study using an enzymatic electrode (Wall and Dale, 2013). Thus, the evoked ATP release in the hippocampus likely reached detectable levels after accumulation by continuous stimulation for a couple of minutes, and the contribution of presynaptic ATP release to the rapid dynamics of extracellular adenosine involved in synaptic plasticity in the hippocampus is limited. Equilibrative nucleoside transporter (ENT) blockers had been reported to inhibit the evoked adenosine release measured by enzymatic electrode (Wall and Dale, 2013), but they did not affect the release measured by adenosine sensor cells. ENT transports nucleoside, depending on the concentration gradient across the plasma membrane, and thus, it may release adenosine if intracellular adenosine is elevated by ATP consumption. However, pharmacological inhibition or genetic ablation of ENT were reported to elevate, rather than decrease, extracellular adenosine as measured by biochemical methods and to protect brain and cardiac tissue (Van Belle et al., 1987; Fredholm et al., 1994; Rose et al., 2010). The elevation of adenosine by ENT blockers was interpreted as the fact that the inhibition of adenosine uptake into adenosine degrading cells, rather than adenosine release, dominates the effects of ENT inhibition on extracellular nucleoside in a simulation study (Newby, 1986). Thus, ENT blockers likely suppress the degradation of adenosine by astrocytes, rather than the release of adenosine by neurons after electrical stimulation. Indeed, ENT blockers reduce extracellular inosine and hypoxanthine, which are the degradation products of adenosine and are contained in interstitial fluid at a higher concentration than adenosine, in the heart or hippocampus under metabolic stress (Van Belle et al., 1987; Fredholm et al., 1994; Rose et al., 2010). The reduction in adenosine release measured by using an electrode (Wall and Dale, 2013) likely reflects the reduction in inosine, rather than adenosine, because the enzymatic electrode is incapable of discriminating inosine from adenosine.
Because the evoked elevation of extracellular adenosine is detected only within a few seconds by cyclic voltammetry, the adenosine release following neuronal activities is mediated by a rapid process, such as exocytosis, rather than by the metabolic production of adenosine and subsequent passive release via ENT. The back-propagating action potential was recently shown to induce calcium release and lysosomal exocytosis in dendrites (Padamsey et al., 2017). The activation of L-VGCC and CICR, which was suggested to mediate dendritic adenosine release in the study using adenosine sensor cells, was also reported to induce dendritic BDNF release (Kolarow et al., 2007). Thus, adenosine is likely released as part of the activity-dependent membrane dynamics in dendrite, which has been proposed to be essential for synaptic plasticity (Padamsey et al., 2017).
Both L-VGCC and adenosine, especially A2A receptor signaling, are linked to psychiatric disorders, in particular depression; thus, the L-VGCC-mediated neuronal adenosine release is presumably a pathway involved in developing psychiatric disorders. Caffeine improves the mental condition in stressful environments, and the blockade of A2A signaling alleviates the depression from chronic stress (Kaster et al., 2015). Meanwhile, the polymorphism of an L-VGCC gene, CACNA1A, is associated with bipolar disease (Gonzalez et al., 2013), and dihydropyridine compounds, which are L-VGCC selective calcium channel blockers, possess antidepressant-like effects as well as synergistically enhancing the effects of tricyclic antidepressants (Casamassima et al., 2010). These findings suggest that chronic stress induces excessive brain activities, which have already been imaged by functional magnetic resonance imaging (fMRI) during psychological as well as physiological stresses (Kogler et al., 2015), and this causes pathological A2A receptor activation leading to depression via L-VGCC-mediated adenosine release
Astrocytic Mechanism for Elevating Adenosine and Synaptic Plasticity
The study using adenosine sensor cells also revealed an astrocytic pathway for elevating extracellular adenosine. Because astrocytes are equipped with a number of mechanisms, including exocytosis, gap junction hemichannel, P2X7 receptor, and anion channels for ATP release (Butt, 2011), we tested which conditions known to induce ATP release from cultured astrocytes elevate adenosine detected by adenosine sensor cells in hippocampal slices. Treatments with hypoosmotic condition or potassium channel blockers, both of which are known to swell cultured astrocytes and induce ATP release via the volume-regulated anion channel (Liu et al., 2008), were found to elevate adenosine (Yamashiro et al., 2017). These adenosine elevations are distinct from those produced by electrical stimulation, because they were not affected by the pharmacological inhibitions of L-VGCC or CICR via the ryanodine receptor. Meanwhile, the hypoosmotically-induced adenosine elevation was suppressed by the pharmacological inhibitions of aquaporin 4 (AQP4), a water channel subtype strongly expressed in astrocytes or extracellular nucleotidases, which convert extracellular ATP to adenosine, suggesting the involvement of astrocyte ATP release following water influx via AQP4 and subsequent conversion to adenosine by extracellular nucleotidases.
A study using an enzymatic electrode also reported adenosine elevation owing to astrocyte ATP release in hippocampal slices; however, it attributed the ATP release to astrocyte exocytosis following neuronal activities (Wall and Dale, 2013). In this study, adenosine elevation following electrical stimulation was reduced by the inhibitions of extracellular nucleotidases, and this component was eliminated in a transgenic mouse expressing dominant negative SNARE (dnSNARE) under the control of the astrocyte-selective GFAP promoter. This mouse line was widely used to inhibit astrocyte exocytosis, but the conclusions of these studies are recently reconsidered because it has been shown to express dnSNARE not only in astrocytes but also in neurons (Fujita et al., 2014). Thus, the ATP exocytosis characterized by the enzymatic electrode can be at least partly attributed to neurons.
Astrocyte swellings are also induced by epileptic neuronal activity or glutamate treatment in slices, and they were eliminated in slices of AQP4 ko mice (Binder et al., 2004). Thus, astrocyte is supposed to elevate extracellular adenosine during epileptic neuronal activities by AQP4-mediated swelling. This possibility is supported by the fact that the duration of chemically or electrically induced seizure is longer in AQP4 ko mice (Binder et al., 2006), but it still needs to be tested by measuring the elevation of extracellular adenosine following epilepsy. AQP4 ko mice show normal excitatory post-synaptic potential and paired-pulse facilitation, indicating that the A1 receptor-mediated presynaptic inhibition reflecting the ambient adenosine level is not affected in this mouse line (Fan et al., 2013). Meanwhile, synaptic plasticity is affected in AQP4 ko mice, and adenosine is likely involved in the altered plasticity. The long-term potentiation (LTP) induced by HFS is normal; however, the BDNF-dependent late phase of theta burst-induced LTP is impaired in AQP4 ko mice (Skucas et al., 2011). Since the activation of the A2A receptor is essential for BDNF signaling as well as for normal expression of BDNF (Tebano et al., 2008), the lack of AQP4 is assumed to reduce the expression or signaling of BDNF and to impair LTP by suppressing astrocyte ATP release and subsequent A2A receptor activation. The normal HFS-induced LTP in AQP4 ko mice is consistent with the lack of AQP4-mediated adenosine elevation following similar HSF in our study using adenosine sensor cells (Yamashiro et al., 2017), and the theta burst, as well as epileptic neuronal activities, may be a more potent way to induce astrocyte ATP release after swelling, than HFS.
Future Directions
Pharmacological characterizations using adenosine sensor cells revealed the novel aspects of neuronal and astrocytic pathways for elevating extracellular adenosine. The implications of these pathways in brain functions and pathology are discussed in this review, and they will be addressed by using mice with modifications of related genes (i.e., L-VGCC or AQP4). In addition to these rapid changes in extracellular adenosine following the neuronal activities or alterations of the extracellular ionic environment, the slow fluctuations of extracellular adenosine play important roles in the brain. Neural activities are broadly suppressed by tonic A1 receptor activation by ambient adenosine, which is presumably derived from the cellular ATP metabolism but not necessarily as a consequence of neuronal electrical activities. Increasing temperature elevates the ambient adenosine released via ENT (Dunwiddie and Diao, 2000), which passively releases intracellularly increased adenosine (Brundege and Dunwiddie, 1996). The passive diffusion of nucleoside via ENT in the brain likely determines the level of ambient adenosine by a complicated interaction between adenosine-releasing neurons and adenosine-degrading astrocytes. The astrocyte expression of enzymes degrading adenosine, especially adenosine kinase, affect ambient adenosine via this interaction and play crucial roles in neuroprotection and the development of epilepsy (Fedele et al., 2005). However, the regulation of ambient adenosine remains unclear. The ATP release via connexin and pannexin hemichannels is another pathway for elevating adenosine; however, these channels are also not yet fully characterized. A couple of astrocyte pathways for releasing ATP play important roles in brain development and pathology, presumably via extracellular adenosine. Astrocyte calcium waves are accompanied by ATP release, which is essential for brain development (Weissman et al., 2004). This ATP is assumed to increase extracellular adenosine and activate adenosine receptors, including A2B and A3 receptors, which are minor in the adult brain; however, the ablation of these genes cause significant alterations in brain development (Chen et al., 2013). ATP release via the connexin hemichannel, which is activated by oxidative stress (Retamal et al., 2016), and subsequent extracellular production of adenosine also play crucial roles in the brain pathology, especially inflammation (Ribeiro et al., 2002). The extracellular dynamics of adenosine under developmental and pathological conditions, which are poorly characterized so far, constitute the next important issue to be analyzed by adenosine sensor cells. Collectively, multiple pathways including nucleotide release and uptake as well as intracellular and extracellular metabolism of nucleotides allow the involvement of extracellular adenosine in diverse physiological and pathological processes, which are spatiotemporally distinct in the brain. Further characterizations of these pathways are expected to provide novel therapeutic targets of brain disorders.
Footnotes
Conflicts of interest: None declared.
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check: This paper has been checked twice with duplication-checking software iThenticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
References
- Barletta KE, Ley K, Mehrad B. Regulation of neutrophil function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32:856–864. doi: 10.1161/ATVBAHA.111.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Papadopoulos MC, Haggie PM, Verkman AS. In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J Neurosci. 2004;24:8049–8056. doi: 10.1523/JNEUROSCI.2294-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Yao X, Zador Z, Sick TJ, Verkman AS, Manley GT. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006;53:631–636. doi: 10.1002/glia.20318. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Cottini L, Fumagalli M, Ceruti S, Abbracchio MP. Blockade of A2A adenosine receptors prevents basic fibroblast growth factor-induced reactive astrogliosis in rat striatal primary astrocytes. Glia. 2003;43:190–194. doi: 10.1002/glia.10243. [DOI] [PubMed] [Google Scholar]
- Brundege JM, Dunwiddie TV. Modulation of excitatory synaptic transmission by adenosine released from single hippocampal pyramidal neurons. J Neurosci. 1996;16:5603–5612. doi: 10.1523/JNEUROSCI.16-18-05603.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM. ATP: a ubiquitous gliotransmitter integrating neuron-glial networks. Semin Cell Dev Biol. 2011;22:205–213. doi: 10.1016/j.semcdb.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Bynoe MS, Viret C, Yan A, Kim DG. Adenosine receptor signaling: a key to opening the blood-brain door. Fluids Barriers CNS. 2015;12:20. doi: 10.1186/s12987-015-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamassima F, Hay AC, Benedetti A, Lattanzi L, Cassano GB, Perlis RH. L-type calcium channels and psychiatric disorders: A brief review. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1373–1390. doi: 10.1002/ajmg.b.31122. [DOI] [PubMed] [Google Scholar]
- Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Dis. 2013;12:265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieślak M, Komoszyński M, Wojtczak A. Adenosine A(2A) receptors in Parkinson's disease treatment. Purinergic Signal. 2008;4:305–312. doi: 10.1007/s11302-008-9100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortés A, Canela EI, López-Giménez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1–A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin BR, Farfel Z, Lustig KD, Julius D, Bourne HR. Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature. 1993;363:274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Sebastiao AM, Ribeiro JA. Inhibition by ATP of hippocampal synaptic transmission requires localized extracellular catabolism by ecto-nucleotidases into adenosine and channeling to adenosine A1 receptors. J Neurosci. 1998;18:1987–1995. doi: 10.1523/JNEUROSCI.18-06-01987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, Vizi ES, Ribeiro JA, Sebastiao AM. Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high- but not low-frequency stimulation of rat hippocampal slices. J Neurochem. 1996;67:2180–2187. doi: 10.1046/j.1471-4159.1996.67052180.x. [DOI] [PubMed] [Google Scholar]
- Dale N, Frenguelli BG. Measurement of purine release with microelectrode biosensors. Purinergic Signal. 2012;8:27–40. doi: 10.1007/s11302-011-9273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L. Regulation of extracellular adenosine in rat hippocampal slices is temperature dependent: role of adenosine transporters. Neuroscience. 2000;95:81–88. doi: 10.1016/s0306-4522(99)00404-2. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Ann Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Fan Y, Liu M, Wu X, Wang F, Ding J, Chen J, Hu G. Aquaporin-4 promotes memory consolidation in Morris water maze. Brain Struct Funct. 2013;218:39–50. doi: 10.1007/s00429-011-0373-2. [DOI] [PubMed] [Google Scholar]
- Fedele DE, Gouder N, Guttinger M, Gabernet L, Scheurer L, Rulicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Lindstrom K, Wallman-Johansson A. Propentofylline and other adenosine transport inhibitors increase the efflux of adenosine following electrical or metabolic stimulation of rat hippocampal slices. J Neurochem. 1994;62:563–573. doi: 10.1046/j.1471-4159.1994.62020563.x. [DOI] [PubMed] [Google Scholar]
- Fujita T, Chen MJ, Li B, Smith NA, Peng W, Sun W, Toner MJ, Kress BT, Wang L, Benraiss A, Takano T, Wang S, Nedergaard M. Neuronal transgene expression in dominant-negative SNARE mice. J Neurosci. 2014;34:16594–16604. doi: 10.1523/JNEUROSCI.2585-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampe K, Stefani J, Hammer K, Brendel P, Potzsch A, Enikolopov G, Enjyoji K, Acker-Palmer A, Robson SC, Zimmermann H. NTPDase2 and purinergic signaling control progenitor cell proliferation in neurogenic niches of the adult mouse brain. Stem cells (Dayton, Ohio) 2015;33:253–264. doi: 10.1002/stem.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Xu C, Ramirez M, Zavala J, Armas R, Contreras SA, Contreras J, Dassori A, Leach RJ, Flores D, Jerez A, Raventos H, Ontiveros A, Nicolini H, Escamilla M. Suggestive evidence for association between L-type voltage-gated calcium channel (CACNA1C) gene haplotypes and bipolar disorder in Latinos: a family-based association study. Bipolar Disord. 2013;15:206–214. doi: 10.1111/bdi.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haink G, Deussen A. Liquid chromatography method for the analysis of adenosine compounds. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;784:189–193. doi: 10.1016/s1570-0232(02)00752-3. [DOI] [PubMed] [Google Scholar]
- Hasko G, Pacher P. Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32:865–869. doi: 10.1161/ATVBAHA.111.226852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimo-Santos A, Batalha VL, Muller CE, Baqi Y, Sebastiao AM, Lopes LV, Diogenes MJ. Impact of in vivo chronic blockade of adenosine A2A receptors on the BDNF-mediated facilitation of LTP. Neuropharmacology. 2014;83:99–106. doi: 10.1016/j.neuropharm.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Kaster MP, Machado NJ, Silva HB, Nunes A, Ardais AP, Santana M, Baqi Y, Muller CE, Rodrigues AL, Porciuncula LO, Chen JF, Tome AR, Agostinho P, Canas PM, Cunha RA. Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Natl Acad Sci U S A. 2015;112:7833–7838. doi: 10.1073/pnas.1423088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L, Muller VI, Chang A, Eickhoff SB, Fox PT, Gur RC, Derntl B. Psychosocial versus physiological stress - Meta-analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage. 2015;119:235–251. doi: 10.1016/j.neuroimage.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarow R, Brigadski T, Lessmann V. Postsynaptic secretion of BDNF and NT-3 from hippocampal neurons depends on calcium calmodulin kinase II signaling and proceeds via delayed fusion pore opening. J Neurosci. 2007;27:10350–10364. doi: 10.1523/JNEUROSCI.0692-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Toychiev AH, Takahashi N, Sabirov RZ, Okada Y. Maxi-anion channel as a candidate pathway for osmosensitive ATP release from mouse astrocytes in primary culture. Cell Res. 2008;18:558–565. doi: 10.1038/cr.2008.49. [DOI] [PubMed] [Google Scholar]
- Lloyd HG, Lindstrom K, Fredholm BB. Intracellular formation and release of adenosine from rat hippocampal slices evoked by electrical stimulation or energy depletion. Neurochem Int. 1993;23:173–185. doi: 10.1016/0197-0186(93)90095-m. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Manabe T, Nicoll RA. Release of adenosine by activation of NMDA receptors in the hippocampus. Science. 1994;265:2098–2101. doi: 10.1126/science.7916485. [DOI] [PubMed] [Google Scholar]
- Masino S, Kawamura M, Wasser C, Pomeroy L, Ruskin D. Adenosine, ketogenic diet and epilepsy: the emerging therapeutic relationship between metabolism and brain activity. Curr Neuropharmacol. 2009;7:257–268. doi: 10.2174/157015909789152164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migita H, Kominami K, Higashida M, Maruyama R, Tuchida N, McDonald F, Shimada F, Sakurada K. Activation of adenosine A1 receptor-induced neural stem cell proliferation via MEK/ERK and Akt signaling pathways. J Neurosci Res. 2008;86:2820–2828. doi: 10.1002/jnr.21742. [DOI] [PubMed] [Google Scholar]
- Newby AC. How does dipyridamole elevate extracellular adenosine concentration? Predictions from a three-compartment model of adenosine formation and inactivation. Biochem J. 1986;237:845–851. doi: 10.1042/bj2370845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Venton BJ. Fast-scan cyclic voltammetry for the characterization of rapid adenosine release. Comput Struct Biotechnol J. 2015;13:47–54. doi: 10.1016/j.csbj.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AG, Orr AL, Li XJ, Gross RE, Traynelis SF. Adenosine A(2A) receptor mediates microglial process retraction. Nat Neurosci. 2009;12:872–878. doi: 10.1038/nn.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AG, Hsiao EC, Wang MM, Ho K, Kim DH, Wang X, Guo W, Kang J, Yu GQ, Adame A, Devidze N, Dubal DB, Masliah E, Conklin BR, Mucke L. Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat Neurosci. 2015;18:423–434. doi: 10.1038/nn.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padamsey Z, McGuinness L, Bardo SJ, Reinhart M, Tong R, Hedegaard A, Hart ML, Emptage NJ. Activity-dependent exocytosis of lysosomes regulates the structural plasticity of dendritic spines. Neuron. 2017;93:132–146. doi: 10.1016/j.neuron.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Dunn WR. Purinergic transmission in blood vessels. Auton Neurosci. 2015;191:48–66. doi: 10.1016/j.autneu.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Retamal MA, Garcia IE, Pinto BI, Pupo A, Baez D, Stehberg J, Del Rio R, Gonzalez C. Extracellular cysteine in connexins: role as redox sensors. Front Physiol. 2016;7:1. doi: 10.3389/fphys.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastião AM, de Mendonça A. Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol. 2002;68:377–392. doi: 10.1016/s0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- Rose JB, Naydenova Z, Bang A, Eguchi M, Sweeney G, Choi DS, Hammond JR, Coe IR. Equilibrative nucleoside transporter 1 plays an essential role in cardioprotection. Am J Physiol Heart Circ Physiol. 2010;298:H771–777. doi: 10.1152/ajpheart.00711.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiao AM, Ribeiro JA. Neuromodulation and metamodulation by adenosine: Impact and subtleties upon synaptic plasticity regulation. Brain Res. 2015;1621:102–113. doi: 10.1016/j.brainres.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Skucas VA, Mathews IB, Yang J, Cheng Q, Treister A, Duffy AM, Verkman AS, Hempstead BL, Wood MA, Binder DK, Scharfman HE. Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4. J Neurosci. 2011;31:6392–6397. doi: 10.1523/JNEUROSCI.6249-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizchi R, Bedi S. Pharmacology of adenosine receptors in the vasculature. Pharmacol Ther. 2001;91:133–147. doi: 10.1016/s0163-7258(01)00152-8. [DOI] [PubMed] [Google Scholar]
- Tebano MT, Martire A, Potenza RL, Grò C, Pepponi R, Armida M, Domenici MR, Schwarzschild MA, Chen JF, Popoli P. Adenosine A2A receptors are required for normal BDNF levels and BDNF-induced potentiation of synaptic transmission in the mouse hippocampus. J Neurochem. 2008;104:279–286. doi: 10.1111/j.1471-4159.2007.05046.x. [DOI] [PubMed] [Google Scholar]
- Van Belle H, Goossens F, Wynants J. Formation and release of purine catabolites during hypoperfusion, anoxia, and ischemia. Am J Physiol. 1987;252:H886–893. doi: 10.1152/ajpheart.1987.252.5.H886. [DOI] [PubMed] [Google Scholar]
- Wall MJ, Dale N. Neuronal transporter and astrocytic ATP exocytosis underlie activity-dependent adenosine release in the hippocampus. J Physiol. 2013;591:3853–3871. doi: 10.1113/jphysiol.2013.253450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Yamashiro K, Fujii Y, Maekawa S, Morita M. Multiple pathways for elevating extracellular adenosine in the rat hippocampal CA1 region characterized by adenosine sensor cells. J Neurochem. 2017;140:24–36. doi: 10.1111/jnc.13888. [DOI] [PMC free article] [PubMed] [Google Scholar]


