Abstract
For 50 years ago was introduced L-3,4-dihydroxyphenylalanine (L-dopa) in Parkinson's disease treatment and during this significant advances has been done but what trigger the degeneration of the nigrostriatal system remain unknown. There is a general agreement in the scientific community that mitochondrial dysfunction, protein degradation dysfunction, alpha-synuclein aggregation to neurotoxic oligomers, neuroinflammation, oxidative and endoplasmic reticulum stress are involved in the loss of dopaminergic neurons containing neuromelanin in Parkinson's disease. The question is what triggers these mechanisms. The age of normal onset in idiopathic Parkinson's disease suggests that environmental factors such as metals, pollutants or genetic mutations cannot be involved because these factors are related to early onset of Parkinsonism. Therefore, we have to search for endogenous neurotoxins and neuroprotection in order to understand what trigger the loss of dopaminergic neurons. One important feature of Parkinson's disease is the rate of the degenerative process before the motor symptoms are evident and during the disease progression. The extremely slow rate of Parkinson's disease suggests that the neurotoxins and the neuroprotection have to be related to dopamine metabolism. Possible candidates for endogenous neurotoxins are alpha-synuclein neurotoxic oligomers, 4-dihydroxyphenylacetaldehyde and ortho-quinones formed during dopamine oxidation to neuromelanin. Vesicular monoamine transporter-2, DT-diaphorase and glutathione transferase M2-2 seems to be the most important neuroprotective mechanism to prevent neurotoxic mechanism during dopamine oxidation.
Keywords: VMAT-2; monoamine oxidase; 3,4-Dihydroxyphenylacetaldehyde; 3,4-dihydroxyphenylacetic acid; dopamine; L-dopa; aminochrome; neuromelanin
Endogenous Neurotoxins
The role of environmental factors and genetic predisposition in Parkinson's disease has been discussed for a long time. The average time of normal onset in idiopathic Parkinson's disease is around 60 years old. However, one of the most relevant features of Parkinsonism induced by environmental factors such as manganese, copper and pesticides (paraquat), is the early onset observed in young people exposed to these contaminants. The familial form of the disease induced by a gene mutation (alpha-synuclein, parkin, DJ-1, PINK-1, LRRK-2, ATP13A2, PINK-1 and others) also has early onset in young people. The exposure of humans to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced an extremely rapid Parkinsonism in just 3 days, with the subjects developing severe motor symptoms, suggesting that exogenous neurotoxins cannot play a role in the idiopathic form of the disease. The degeneration of dopaminergic neurons containing neuromelanin in the nigrostriatal system initiates years before the motor symptoms are evident. The rate of the degenerative process in both Parkinsonism induced by contaminants, and familial Parkinson's disease is significantly more rapid, explaining its early onset in young people (Segura-Aguilar and Kostrzewa, 2015). Therefore, the degeneration of the nigrostriatal system seems to involve an endogenous neurotoxin. Possible sources of endogenous neurotoxins are.
Neurotoxins generated during dopamine oxidation to neuromelanin
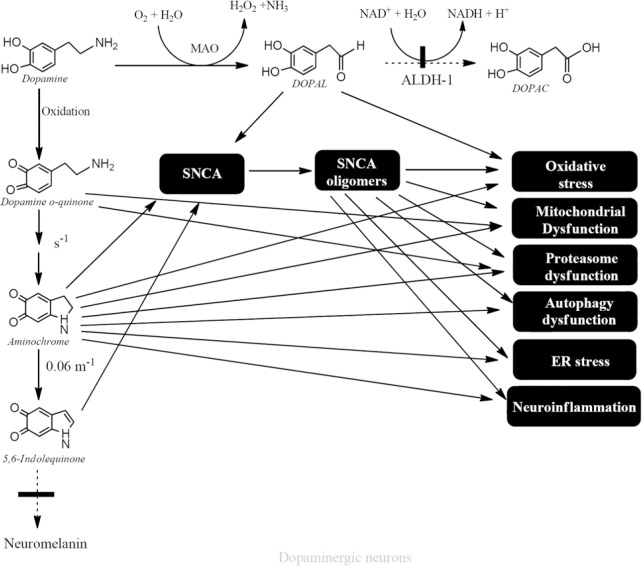
The role of dopamine oxidation to neuromelanin in the loss of dopaminergic neurons containing neuromelanin in the nigrostriatal system in the idiopathic Parkinson's disease seems to play a key role. Dopamine oxidizes to neuromelanin by generating ortho(o)-quinones in a sequential manner that finally polymerize to neuromelanin (dopamine → dopamine o-quinone → aminochrome → 5,6-indolequinone → neuromelanin). However, these o-quinones can be neurotoxic under certain conditions: (i) Dopamine o-quinone: Dopamine o-quinone is stable under pH2 and at physiological pH cyclizes immediately at a rate of s–1. In isolated mitochondria, dopamine o-quinone forms adducts with proteins such as ubiquitin C-terminal hydrolase-L1 (UCH-L1), isocitrate dehydrogenase, complex I, III and V, superoxide dismutase-2, Parkinson protein 7 (DJ-1), actin gamma, mitochondrial creatine kinase, mitochondrial voltage-dependent anion channel 1, heat shock protein 60, mortalin/GRP75/mtHSP70 and other proteins. However, in SH-SY5Y cells dopamine o-quinone forms adducts only with UCH-L1, DJ-1, mortalin/GRP75/mtHSP70 and actin. Dopamine o-quinone forms adducts with the dopamine transporter. Other studies showed that dopamine oxidation's products forms adducts with parkin, and tyrosine hydroxylase and the question is, which o-quinone was involved (dopamine o-quinone or aminochrome). In the case of parkin it seems that dopamine o-quinone was not involved in parkin-dopamine adduct formation in cells, while in tyrosine hydroxylase adducts it is possible that dopamine o-quinone was responsible, because the reaction was performed with purified tyrosine hydroxylase with dopamine and tyrosinase. (ii) The compound 5,6-indolequinone forms adducts with alpha-synuclein. (iii) Dopaminochrome forms adducts with alpha-synuclein, and induces neurotoxicity in cell lines and degeneration in substantia nigra, but the structure of this o-quinone has not been determined. (iv) Aminochrome is the most stable o-quinone formed during dopamine oxidation to neuromelanin and also the most studied. Aminochrome has been reported to be neurotoxic by inducing mitochondria dysfunction, aggregation of alpha-synuclein to neurotoxic oligomers, protein degradation dysfunction, disruption of cytoskeleton architecture, neuroinflammation, and oxidative and endoplasmic reticulum stress in cells (oxidative stress: Segura-Aguilar et al., 1998; Arriagada et al., 2004; Zafar et al., 2006; Fuentes et al., 2007; Paris et al., 2010, 2012; Aguirre et al., 2012; Muñoz et al., 2012a, b, 2015; Huenchuguala et al., 2014; Xiong et al., 2014; Briceño et al., 2015; Muñoz and Segura-Aguilar, 2017b; Santos et al., 2017). Intracerebral injection of aminochrome into the striatum induces a progressive neuronal dysfunction as a consequence of mitochondrial dysfunction, decrease of dopamine release, disruption of monoaminergic vesicles’ axonal transport and increased GABA level (Figure 1; for review see Segura-Aguilar et al. (2014, 2016) and Herrera et al. (2017).
Figure 1.
Neurotoxic mechanism of endogenous neurotoxins.
Free dopamine in the cytosol can (i) oxidize to neurotoxic o-quinones (dopamine o-quinone, aminochrome and 5,6-indolequinone) or (ii) be degraded by monoamine oxidase to DOPAL that can be neurotoxic when it accumulates. ALDH-1: Aldehyde dehydrogenase 1; DOPAL: 3,4-dihydroxyphenylacetaldehyde; ER: endoplasmic reticulum; NADH: nicotinamide adenine dinucleotide.
Another possible endogenous neurotoxin is alpha-synuclein
Alpha-synuclein oligomers: (i) inhibit autophagy, (ii) induce disruption of the endoplasmic reticulum and Golgi traffic, (iii) induce mitochondrial dysfunction, (iv) inhibit proteasomal activity, (v) increase oxidative stress and (vi) induce neuroinflammation by activating microglia and increasing proinflammatory cytokines and nitric oxide (Figure 1; for review see Muñoz and Segura-Aguilar, 2017). However, the question is, what induces alpha-synuclein aggregation to neurotoxic oligomers in the sporadic form of Parkinson's disease? It has been suggested that alpha-synuclein oligomers can be released from the neuron, thus propagating its neurotoxic action. The prion-like hypothesis for alpha-synuclein-induced disease progression is based on the release of many copies of alpha-synuclein oligomers into the synaptic cleft, so that the surrounding neurons and glial cells are able to internalize these alpha-synuclein oligomers. The propagating action of alpha-synuclein should be a rapid process contrasting with the extremely slow progression of Parkinson's disease.
3,4-Dihydroxyphenylacetaldehyde (DOPAL)
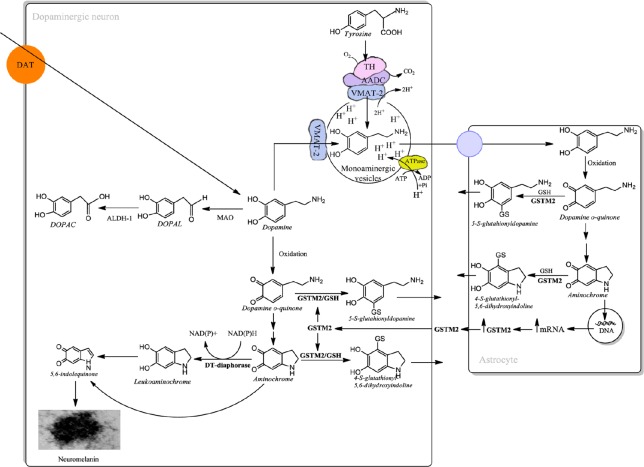
Free cytosolic dopamine oxidizes to neuromelanin generating o-quinones that can be neurotoxic under certain conditions. However, dopamine stored in monoaminergic vesicles is completely stable due to the relatively low pH generated by an ATPase that pumps protons into the monoaminergic vesicles. Dopamine synthesis is performed in the cytosol but the enzymes involved in dopamine synthesis form a kind of complex with the vesicular monoamine transporter-2 (VMAT-2), bound to the monoaminergic vesicles membrane, preventing the existence of free dopamine (Figure 2; for review see Segura-Aguilar et al., 2014). The other source of free dopamine is the reuptake of dopamine released under neurotransmission, but VMAT-2 can take up dopamine into monoaminergic vesicles. However, monoamine oxidase catalyzes the oxidative deamination of dopamine in order to degrade dopamine to DOPAL with the concomitant formation of hydrogen peroxide. DOPAL is oxidized to 3,4-dihydroxyphenylacetic acid (DOPAC) which can be converted to homovanillic acid by catechol ortho-methyltransferase. Dopamine degradation to homovanillic acid depends on the normal level of expression of aldehyde dehydrogenase that catalyzes the oxidation of DOPAL to DOPAC. In the human substantia nigra two genes of aldehyde dehydrogenase 1 and 2 are expressed in cytosol and mitochondria respectively, but only aldehyde dehydrogenase-1 was found to be decreased in Parkinson's disease (Goldstein et al., 2014). Gene expression studies performed with post-mortem material from substantia nigra of Parkinson's disease patients and controls, revealed a significant decrease in aldehyde dehydrogenase-1 (Grünblatt et al., 2004). The low expression of aldehyde dehydrogenase-1 will lead to the accumulation of DOPAL and subsequent toxic effects by inducing oxidative stress and the formation of adducts with proteins (Figure 1; for review see Goldstein et al. (2014)). DOPAL has been reported to induce the formation of alpha-synuclein oligomers suggesting a role in Parkinson's disease (Follmer et al., 2015). However, it is necessary to regard the results obtained with post-mortem material with caution, because the low expression of this enzyme determined in substantia nigra correspond to the surviving neurons and not neurons which have undergone degeneration probably years before. In addition, patients with low expression of aldehyde dehydrogenase-1 should develop the disease with early onset, but we know that the average age for the idiopathic form of Parkinson's disease is 60 years old.
Figure 2.
Cellular protection of dopaminergic neurons containing neuromelanin.
VMAT-2 prevents the existence of free dopamine in the cytosol by taking up dopamine from the reuptake mediated by dopamine transporter and dopamine synthetized from tyrosine. In the case that dopamine is free in cytosol it can be degraded by monoamine oxidase to DOPAL and later to DOPAC by aldehyde deshidrogenase-1. Alternatively, dopamine oxidizes to aminochrome but the enzymes DT-diaphorase and GSTM2 prevent aminochrome-induced neurotoxicity. DOPAC: 3,4-Dihydroxyphenylacetic acid; DOPAL: 3,4-dihydroxyphenylacetaldehyde; GSTM2: glutathione transferase M2-2; VMAT-2: vesicular monoamine transporter-2.
Endogenous Neuroprotection
VMAT-2
The first neuroprotective mechanism is mediated by VMAT-2 located in the membrane of monoaminergic vesicles. VMAT-2 takes up dopamine into the monoaminergic vesicles where dopamine is stable and accumulates to be used for neurotransmission. VMAT-2 prevents the existence of free cytosolic dopamine which participates in oxidizing reactions such as dopamine oxidation during neuromelanin formation with concomitant formation of neurotoxic o-quinones. The level of VMAT-2 expression is inversely correlated with the level of neuromelanin in dopaminergic neurons (Liang et al., 2004). Interestingly, monoaminergic vesicles isolated from Parkinson's disease patients revealed that both dopamine uptake and the binding of VMAT-2 inhibitor were significantly reduced in comparison to control brains (Pifl et al., 2014). VMAT-2 also prevents dopamine oxidative deamination catalyzed by monoamine oxidase to DOPAL.
DT-diaphorase
DT-diaphorase is a flavoenzyme that catalyzes the two-electron reduction of aminochrome to leukoaminochrome. DT-diaphorase prevents aminochrome-induced: (i) formation of neurotoxic α-synuclein oligomers; (ii) cell death; (iii) mitochondria dysfunction; (iv) inhibition of the proteasomal system; (v) inhibition of autophagy/lysosomal system; (vi) inhibition of α- and β-tubulin aggregation and disruption of cytoskeleton architecture; (vi) inhibition of oxidative stress; and (vii) cell shrinkage (Arriagada et al., 2004; Fuentes et al., 2007; Lozano et al., 2010; Paris 2010, 2011; Muñoz et al., 2012a, b, 2015; Segura Aguilar et al., 2014, 2016; Huenchuguala et al., 2016; Herrera et al., 2017; Herrera-Soto et al., 2017; Muñoz and Segura-Aguilar, 2017). The inhibition of DT-diaphorase in vivo induced the loss of dopaminergic neurons in animals intracerebrally injected with aminochrome (Herrera et al., 2017). This enzyme is constitutively expressed both in dopaminergic neurons, where o-quinones are formed during dopamine oxidation to neuromelanin, and in astrocytes that prevent aminochrome-induced toxicity.
Human glutathione transferase M2-2 (GSTM2)
GSTM2 catalyzes glutathione conjugation of both aminochrome and dopamine o-quinone to 4-S-glutathionyl-5,6-dihydroxyindoline and 5-S-glutathionyldopamine respectively (Baez et al., 1997; Segura-Aguilar et al., 1997; Dagnino-Subiabre et al., 2000). The compound 4-S-glutathionyl-5,6-dihydroxyindoline is stable in the presence of biological oxidizing agents such as oxygen, hydrogen peroxide and superoxide radicals, suggesting a protective role for this reaction. The compound 5-S-glutathionyldopamine is finally degraded to 5-S-cysteinyldopamine, which has been detected in the neuromelanin, substantia nigra, putamen, caudate nucleus, globus pallidus, and the cerebrospinal fluid of Parkinson's disease patients, suggesting that this conjugate is an end-product. GSTM2 is expressed only in astrocytes but this enzyme protects both astrocytes and dopaminergic neurons against aminochrome toxicity. Astrocytes protect dopaminergic neurons by secreting GSTM2 which is internalized into dopaminergic neurons (Cuevas et al., 2015; Segura-Aguilar et al., 2015, 2016; Muñoz et al., 2016; Herrera et al., 2017).
Conclusions
The role of environmental factors and genetic predisposition has been discussed for a long time in the idiopathic Parkinson's disease. However, both Parkinsonism induced by metals or pesticides, and the familial form of the disease induced by specific mutations, induce an early-onset form of Parkinson's disease contrasting with the normal onset at 60 years old of the idiopathic form of Parkinson's disease. Therefore, it seems to be plausible that the extremely slow degenerative process before the motor symptoms appear and also under the disease progression, depends on the formation of endogenous neurotoxins during dopamine oxidation to neurotoxic o-quinones, alpha-synuclein neurotoxic oligomers and DOPAL oxidation to an o-quinone. Interestingly, there are endogenous mechanisms to protect dopaminergic neurons which prevent the neurotoxic action of these endogenous neurotoxins, such as VMAT-2, DT-diaphorase and GSTM2.
Footnotes
Funding: This work was supported by FONDECYT 1170033.
Conflicts of interest: None declared.
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check: This paper has been checked twice with duplication-checking software iThenticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Open peer reviewer: Kazunori Adachi, Meikai University School of Dentistry, Japan.
References
- Aguirre P, Urrutia P, Tapia V, Villa M, Paris I, Segura-Aguilar J, Núñez MT. The dopamine metabolite aminochrome inhibits mitochondrial complex I and modifies the expression of iron transporters DMT1 and FPN1. Biometals. 2012;25:795–803. doi: 10.1007/s10534-012-9525-y. [DOI] [PubMed] [Google Scholar]
- Arriagada C, Paris I, Sanchez de las Matas MJ, Martinez-Alvarado P, Cardenas S, Castañeda P, Graumann R, Perez-Pastene C, Olea-Azar C, Couve E, Herrero MT, Caviedes P, Segura-Aguilar J. On the neurotoxicity mechanism of leukoaminochrome o-semiquinone radical derived from dopamine oxidation: mitochondria, damage, necrosis, and hydroxyl radical formation. Neurobiol Dis. 2004;16:468–477. doi: 10.1016/j.nbd.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J. 1997;324:25–28. doi: 10.1042/bj3240025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briceño A, Muñoz P, Brito P, Huenchuguala S, Segura-Aguilar J, Paris IB. Aminochrome toxicity is mediated by inhibition of microtubules polymerization through the formation of adducts with tubulin. Neurotox Res. 2016;29:381–393. doi: 10.1007/s12640-015-9560-x. [DOI] [PubMed] [Google Scholar]
- Cuevas C, Huenchuguala S, Muñoz P, Villa M, Paris I, Mannervik B, Segura-Aguilar J. Glutathione transferase-M2-2 secreted from glioblastoma cell protects SH-SY5Y cells from aminochrome neurotoxicity. Neurotox Res. 2015;27:217–228. doi: 10.1007/s12640-014-9500-1. [DOI] [PubMed] [Google Scholar]
- Dagnino-Subiabre A, Cassels BK, Baez S, Johansson AS, Mannervik B, Segura-Aguilar J. Glutathione transferase M2-2 catalyzes conjugation of dopamine and dopa o-quinones. Biochem Biophys Res Commun. 2000;274:32–36. doi: 10.1006/bbrc.2000.3087. [DOI] [PubMed] [Google Scholar]
- Follmer C, Coelho-Cerqueira E, Yatabe-Franco DY, Araujo GD, Pinheiro AS, Domont GB, Eliezer D. Oligomerization and membrane-binding properties of covalent adducts formed by the interaction of α-synuclein with the toxic dopamine metabolite 3, 4-dihydroxyphenylacetaldehyde (DOPAL) J Biol Chem. 2015;290:27660–27679. doi: 10.1074/jbc.M115.686584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes P, Paris I, Nassif M, Caviedes P, Segura-Aguilar J. Inhibition of VMAT-2 and DT-diaphorase induce cell death in a substantia nigra-derived cell line--an experimental cell model for dopamine toxicity studies. Chem Res Toxicol. 2007;20:776–783. doi: 10.1021/tx600325u. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ, Sharabi Y. Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacol Ther. 2014;144:268–282. doi: 10.1016/j.pharmthera.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünblatt E, Mandel S, Jacob-Hirsch J, Zeligson S, Amariglo N, Rechavi G, Li J, Ravid R, Roggendorf W, Riederer P, Youdim MB. Gene expression profiling of parkinsonian substantia nigra pars compacta; alterations in ubiquitin-proteasome, heat shock protein, iron and oxidative stress regulated proteins, cell adhesion/cellular matrix and vesicle trafficking genes. J Neural Transm. 2004;111:1543–1573. doi: 10.1007/s00702-004-0212-1. [DOI] [PubMed] [Google Scholar]
- Herrera A, Muñoz P, Steinbusch HW, Segura-Aguilar J. Are dopamine oxidation metabolites involved in the loss of dopaminergic neurons in the nigrostriatal system in Parkinson's disease? ACS Chem Neurosci. 2017;8:702–711. doi: 10.1021/acschemneuro.7b00034. [DOI] [PubMed] [Google Scholar]
- Herrera-Soto A, Díaz-Veliz G, Mora S, Muñoz P, Henny P, Steinbusch HW, Segura-Aguilar J. On the role of DT-diaphorase inhibition in aminochrome-induced neurotoxicity in vivo. Neurotox Res. 2017 doi: 10.1007/s12640-017-9719-8. doi:10.1007/s12640-017-9719-8. [DOI] [PubMed] [Google Scholar]
- Huenchuguala S, Muñoz P, Graumann R, Paris I, Segura-Aguilar J. DT-diaphorase protects astrocytes from aminochrome-induced toxicity. Neurotoxicology. 2016;55:10–12. doi: 10.1016/j.neuro.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Huenchuguala S, Muñoz P, Zavala P, Villa M, Cuevas C, Ahumada U, Graumann R, Nore BF, Couve E, Mannervik B, Paris I, Segura-Aguilar J. Glutathione transferase mu 2 protects glioblastoma cells against aminochrome toxicity by preventing autophagy and lysosome dysfunction. Autophagy. 2014;10:618–630. doi: 10.4161/auto.27720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CL, Nelson O, Yazdani U, Pasbakhsh P, German DC. Inverse relationship between the contents of neuromelanin pigment and the vesicular monoamine transporter-2: human midbrain dopamine neurons. J Comp Neurol. 2004;473:97–106. doi: 10.1002/cne.20098. [DOI] [PubMed] [Google Scholar]
- Lozano J, Muñoz P, Nore BF, Ledoux S, Segura-Aguilar J. Stable expression of short interfering RNA for DT-diaphorase induces neurotoxicity. Chem Res Toxicol. 2010;23:1492–1496. doi: 10.1021/tx100182a. [DOI] [PubMed] [Google Scholar]
- Muñoz P, Cardenas S, Huenchuguala S, Briceño A, Couve E, Paris I, Segura-Aguilar J. DT-diaphorase prevents aminochrome-induced alpha-synuclein oligomerformation and neurotoxicity. Toxicol Sci. 2015;145:37–47. doi: 10.1093/toxsci/kfv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz P, Huenchuguala S, Paris I, Segura-Aguilar J. Dopamine oxidation and autophagy. Parkinsons Dis 2012. 2012a:920953. doi: 10.1155/2012/920953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz P, Paris I, Sanders LH, Greenamyre JT, Segura-Aguilar J. Overexpression of VMAT-2 and DT-diaphorase protects substantia nigra-derived cells against aminochrome neurotoxicity. Biochim Biophys Acta. 2012b;1822:1125–1136. doi: 10.1016/j.bbadis.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz P, Paris I, Segura-Aguilar J. Commentary: Evaluation of models of Parkinson's disease. Front Neurosci. 2016;10:320. doi: 10.3389/fnins.2016.00161. Erratum in: Front Neurosci 10:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz PS, Segura-Aguilar J. DT-diaphorase protects against autophagy induced by aminochrome-dependent alpha-synuclein oligomers. Neurotox Res. 2017 doi: 10.1007/s12640-017-9747-4. doi: 10.1007/s12640-017-9747-9754. [DOI] [PubMed] [Google Scholar]
- Paris I, Muñoz P, Huenchuguala S, Couve E, Sanders LH, Greenamyre JT, Caviedes P, Segura-Aguilar J. Autophagy protects against aminochrome-induced cell death in substantia nigra-derived cell line. Toxicol Sci. 2011;121:376–388. doi: 10.1093/toxsci/kfr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris I, Perez-Pastene C, Cardenas S, Iturriaga-Vasquez P, Muñoz P, Couve E, Caviedes P, Segura-Aguilar J. Aminochrome induces disruption of actin, alpha-and beta-tubulin cytoskeleton networks in substantia-nigra-derived cell line. Neurotox Res. 2010;18:82–92. doi: 10.1007/s12640-009-9148-4. [DOI] [PubMed] [Google Scholar]
- Pifl C, Rajput A, Reither H, Blesa J, Cavada C, Obeso JA, Rajput AH, Hornykiewicz O. Is Parkinson's disease a vesicular dopamine storage disorder? Evidence from a study in isolated synaptic vesicles of human and nonhuman primate striatum. J Neurosci. 2014;34:8210–9. doi: 10.1523/JNEUROSCI.5456-13.2014. 8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CC, Araújo FM, Ferreira RS, Silva VB, Silva JHC, Grangeiro MS, Soares ÉN, Pereira ÉPL, Souza CS, Costa SL, Segura-Aguilar J, Silva VDA. Aminochrome induces microglia and astrocyte activation. Toxicol In Vitro. 2017;42:54–60. doi: 10.1016/j.tiv.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J. A new mechanism for protection of dopaminergic neurons mediated by astrocytes. Neural Regen Res. 2015;10:1225–1227. doi: 10.4103/1673-5374.162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Aguilar J, Baez S, Widersten M, Welch CJ, Mannervik B. Human class Mu glutathione transferases, in particular isoenzyme M2-2, catalyze detoxication of the dopamine metabolite aminochrome. J Biol Chem. 1997;272:5727–5731. doi: 10.1074/jbc.272.9.5727. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J, Kostrzewa RM. Neurotoxin mechanisms and processes relevant to Parkinson's disease: an update. Neurotox Res. 2015;27:328–354. doi: 10.1007/s12640-015-9519-y. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J, Muñoz P, Paris I. Aminochrome as New preclinical model to find new pharmacological treatment that stop the development of Parkinson's disease. Curr Med Chem. 2016;23:346–359. doi: 10.2174/0929867323666151223094103. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J, Paris I, Muñoz P, Ferrari E, Zecca L, Zucca FA. Protective and toxic roles of dopamine in Parkinson's disease. J Neurochem. 2014;129:898–915. doi: 10.1111/jnc.12686. [DOI] [PubMed] [Google Scholar]