Keywords: nerve regeneration, cortical electrical stimulation, afterdischarges, intractable epilepsy, functional brain mapping, high frequency stimulation, low frequency stimulation, neuromodulation, neural regeneration
Abstract
Brief-pulse stimulation at 50 Hz has been shown to terminate afterdischarges observed in epilepsy patients. However, the optimal pulse stimulation parameters for terminating cortical electrical stimulation-induced afterdischarges remain unclear. In the present study, we examined the effects of different brief-pulse stimulation frequencies (5, 50 and 100 Hz) on cortical electrical stimulation-induced afterdischarges in 10 patients with refractory epilepsy. Results demonstrated that brief-pulse stimulation could terminate cortical electrical stimulation-induced afterdischarges in refractory epilepsy patients. In conclusion, (1) a brief-pulse stimulation was more effective when the afterdischarge did not extend to the surrounding brain area. (2) A higher brief-pulse stimulation frequency (especially 100 Hz) was more likely to terminate an afterdischarge. (3) A low current intensity of brief-pulse stimulation was more likely to terminate an afterdischarge
Introduction
Resective surgery is used to treat patients with intractable partial epilepsy, although some patients with multifocal epilepsy or epileptic foci and eloquent cortex overlap are not optimal surgical candidates (Fong et al., 2011; Forcadas-Berdusan et al., 2011). Moreover, some patients with non-lesional neocortical epilepsy may not have the same seizure-free result after surgery as patients with mesial temporal sclerosis or lesional epilepsy (Asadi-Pooya et al., 2016; Goldenholz et al., 2016). Neurostimulation (especially cortical stimulation) for epilepsy treatment offers distinct benefits, while avoiding the potential toxic, cognitive and idiosyncratic side effects of medication. The responsive neurostimulation closed-loop system is designed to stimulate the brain shortly after seizure onset, and terminate a seizure before it evolves into an uncontrollable seizure. Although responsive neurostimulation has benefits, the optimal stimulation parameters remain unclear (Sun and Morrell, 2014).
Functional mapping studies in patients with subdural grids indicate that stimulation may produce afterdischarges, which are an inevitable side effect of electrical cortical stimulation, while a subsequent stimulation can terminate the afterdischarges (Lesser et al., 1999; Motamedi et al., 2002). The majority of human studies on afterdischarges have investigated their termination using a 50 Hz stimulation frequency. Further, a lower risk of inducing afterdischarges was reported when using 50 Hz stimulation compared with 100 Hz (Motamedi et al., 2007). However, to our knowledge, detailed testing of 5 Hz to 100 Hz frequencies for terminating afterdischarges has not been reported, and there are only a few studies describing the effects of varying frequencies in humans. Thus, in the present study, we examined the effects of low and high frequency pulse stimulation of the human cortex on inhibition of afterdischarges induced by cortical electrical stimulation.
Subjects and Methods
Subjects
We selected 10 sequential patients who received treatment from November 2013 to March 2014 in Beijing Institute of Functional Neurosurgery of China based on the following inclusion criteria: (1) diagnosed with medically intractable epilepsy (Berg, 2006; Go and Snead, 2008); (2) underwent subdural electrode placement for an invasive evaluation; (3) was able to cooperate during cortical electrical stimulation for functional brain mapping; and (4) afterdischarges were observed during cortical stimulation. Other patients were excluded because of low cortical excitability resulting in no identifiable afterdischarges, or because of high cortical excitability resulting in recurrent epilepsy seizures during cortical stimulation (Figure 1).
Figure 1.
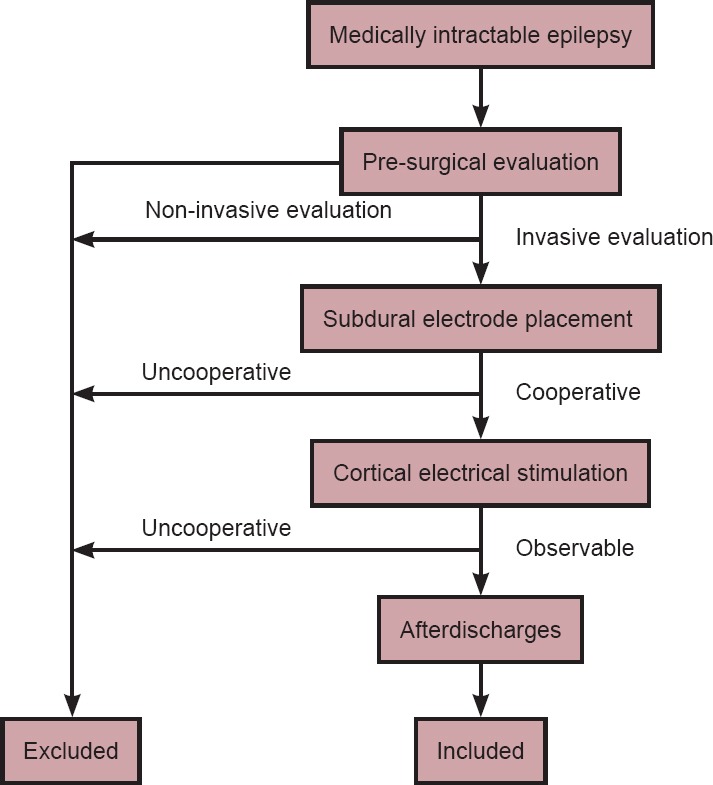
Flow chart of the study.
To estimate the group size, we first measured the terminating rate for the three different frequency groups in 10 patients. The terminating rates of the different groups were 19.4% (5 Hz), 41.9% (50 Hz) and 53.3% (100 Hz). Power analysis using α = 0.05, two-tailed and a power of 80% indicated that 30 trials per group were required to show group differences. In the 10 enrolled patients, the trial numbers of different groups were 31 (5 Hz), 43 (50 Hz) and 30 (100 Hz).
This study followed the Declaration of Helsinki and the relevant set of ethical principles. All patients (or parent/guardian where appropriate) provided written informed consent. The Ethics Review Committee of the Xuanwu Hospital, Capital Medical University and the Ministry of Health, China approved this study (approval number: [2015]001). All measurements performed in the present study were in compliance with the current laws and regulations in China.
Pre-surgical evaluation
All patients underwent an individualized presurgical evaluation, including a history interview, seizure semiology, 3.0-T magnetic resonance imaging (Siemens Verio, Erlangen, Germany) and scalp video-electroencephalogram (EEG) monitoring, which captured at least three spontaneous seizures. Visual and neuropsychological examinations were conducted if necessary. An invasive subdural electrode or stereotactic deep electrode implantation was performed if there were inconsistent findings or the epileptogenic zone was closely related to the eloquent cortex. Electrode placement was guided by non-invasive exams and intraoperative electrocorticography. If the patients’ epileptogenic zones were near the eloquent cortex, we performed preoperative cortical electrical stimulation for functional mapping.
Intracranial electrodes
The subdural electrodes were 1.5-mm-thick soft silastic sheets embedded with platinum-iridium disc electrodes (3 mm total diameter, 2.5 mm diameter exposed to the cortical surface, 90% platinum and 10% iridium) that were equally spaced with 10 mm center-to-center distances in a rectangular or linear array (HKHS, Beijing, China). The electrodes were arranged in either a grid (4 × 8 to 8 × 8) or a strip (1 × 8 to 2 × 8).
Intracranial video-EEG monitoring and functional mapping
Interictal and ictal EEGs were recorded using a video-EEG monitoring system (BRAIN QUICK, Micromed; Treviso, Italy) that could simultaneously record up to 128 channels, with 1,024 samples per second per channel. Electrical stimulation for functional mapping was conducted to localize the sensory, motor and language areas after EEGs were recorded at least twice to capture the clinical seizures, which usually occurred 3–4 days after electrode implantation. For electrical stimulation, 0.2-ms bipolar monophasic square wave pulses were repeated at 50 Hz and presented in trains lasting 3 seconds, with a 20-second interval (SD LTM STIM; Micromed, Treviso, Italy). Stimuli at each electrode (using the adjacent electrode as a reference) started at 1.0 mA and increased in 0.5–1.0 mA increments subsequently until a functional alteration was achieved and an afterdischarge was recorded or 12 mA was reached (Guojun et al., 2014).
EEG analysis and afterdischarge observation
We used previous afterdischarge definitions, descriptions and classifications (Lesser et al., 1999; Blume et al., 2004), and we revised the morphological classification as repetitive spikes, rhythmic waves, spike-waves or polyspike bursts. We used a viewer (SystemPLUS; Micromed) to review EEGs that simultaneously displayed up to 128 channels, which allowed us to precisely mark the afterdischarge location and other events. Data were reviewed using the following settings: 400–800 μV/cm sensitivity, 100 Hz low pass filter, 0.5 Hz high pass filter and speeds of 1–10 second/screen. Only epileptiform discharges that were clearly distinguishable from the underlying EEG activity after cortical stimulation were considered as afterdischarges. The afterdischarge frequency was calculated based on the mean interpeak distance.
Several individuals performed the preliminary assessments of portions of the recordings, but one board certified electroencephalographer (Yuanyuan Piao, Beijing Institute of Functional Neurosurgery) performed the final markings of all recordings, and marked the entire data set twice. The kappa coefficient for the two reviews was good (κ = 0.95).
Brief-pulse stimulation (BPS)
Occasionally, we used a BPS to terminate afterdischarges during cortical electrical stimulation. When afterdischarges occurred, we often stimulated the same pair again using the same parameters. In the present study, we assessed whether a BPS was more effective at a higher frequency or lower frequency. To study the effects of different BPS parameters, especially the effect of different frequencies on afterdischarge termination, we repeated the pulse stimulations at three frequencies (5 Hz, 50 Hz and 100 Hz) at one electrode pair. For example, we repeated stimulations three times at one electrode pair, and if each trial induced an afterdischarge, then the BPS was applied at three different frequencies (from 5 Hz to 100 Hz sequentially). However, if a trial did not induce an afterdischarge, then we moved to the next frequency. After the functional mapping, we repeated the electrical stimulation on the electrode pairs that previously generated afterdischarges using a slightly lower intensity, and then continued to increase the stimulation intensity until the afterdischarges recurred or 12 mA was reached. Once afterdischarges recurred, we used a BPS at the same electrode pair.
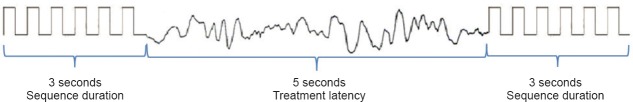
To avoid side effects, we did not repeatedly stimulate after seizure onset or repeatedly stimulate the electrode pairs that were located at the eloquent cortex. For analytical convenience, we used the same pulse type, pulse width and sequence duration, with a treatment latency of 5 seconds (Figures 2, 3). In addition, we used a low frequency (5 Hz), intermediate frequency (50 Hz) and a high frequency (100 Hz), and then recorded and evaluated the afterdischarge response to a BPS. When an afterdischarge was not observed after a BPS, or when what appeared to be afterdischarge termination was observed within the first 2 seconds after a BPS, we considered that the BPS was effective (Lesser et al., 1999).
Figure 2.
Schematic diagram of the effect of brief-pulse stimulation on cortical electrical stimulation-induced afterdischarge.
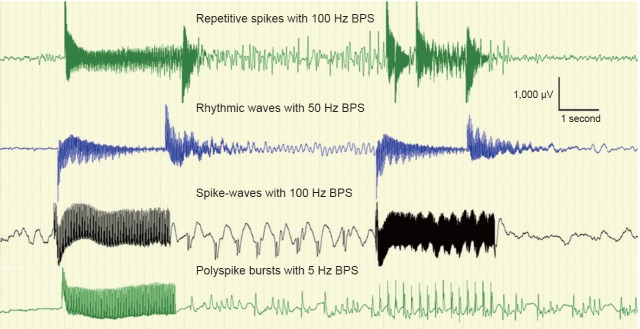
Figure 3.
Different afterdischarge types with different frequencies of brief-pulse stimulation (BPS).
Statistical analysis
All statistical analyses were performed with statistical software (SPSS 19.0; IBM Corporation, Armonk, NY, USA). We calculated the number and percentage of electrodes that placed, stimulated and produced afterdischarges via the electrode location in the frontal lobe, central lobe, parietal-occipital lobe and temporal lobe. The efficacy of different BPS frequency groups on afterdischarges was analyzed using chi-square tests. The mean threshold and afterdischarge extension were analyzed by one-way analysis of variance on the effective and ineffective groups. A logistic regression was used to examine whether there was an increase or decrease in the overall probability of afterdischarges terminated by BPSs for some characteristics of afterdischarges and BPSs, including afterdischarge extension, afterdischarge frequency, BPS frequency and BPS intensity.
A kappa (κ) statistic was calculated to evaluate the consistency of the afterdischarges observed between the two reviews performed by the board-certified electroencephalographer. The consistency was poor if κ < 0.2, fair if κ = 0.21–0.40, moderate if κ = 0.41–0.60, good if κ = 0.61–0.80 and excellent if κ ≥ 0.81. The kappa coefficient for the two reviews (κ = 0.95) was excellent.
A P-value of < 0.05 was considered statistically significant. Based on the logistic regression model analysis, we reported odds ratios, 95% confidence intervals and P-values.
Results
General characteristics and cortical electrical stimulation outcome
For the 10 patients, seven were men, three were women, the mean age at surgery was 11.4 years (range, 9–31 years), seizure duration was 10.1 years (range, 3.5–22 years) and the mean number of intracranial electrodes was 72 (range, 64–96). The number of trials per patient ranged from 77 to 424; a total of 2,662 trials were included in the analysis, and 375 trials (14.1%) elicited afterdischarges. A total of 720 electrodes were placed in the subdural space, 132 electrodes (18.3%) evoked afterdischarges and the number of electrodes that covered the frontal, central, parietal-occipital and temporal lobes were 163, 149, 234 and 174, respectively. We stimulated 79, 148, 116 and 42 sites in the frontal, central, parietal-occipital and temporal lobes, respectively, of which 36, 35, 50 and 12 sites evoked afterdischarges, respectively. The basic patient data and the distribution of afterdischarges are listed in Table 1.
Table 1.
Basic patient information

Characteristics of afterdischarges
Afterdischarge frequency ranged from 1 Hz to 12 Hz, with δ-band (0.3–3.5 Hz) frequencies for 50% of the afterdischarges, θ-band (4–7.5 Hz) frequencies for 45% of the afterdischarges and α-band (8–13 Hz) frequencies for 5% of the afterdischarges. The duration of the afterdischarges ranged from 3 to 140 seconds (mean: 24.3 ± 23.9), the threshold ranged from 4 mA to 12 mA (mean: 8.7 ± 2.1) and the extension ranged from 1 electrode to 12 positions (mean: 3.9 ± 2.2). Stimuli evoked a wide variety of afterdischarge morphologies and repetitive spikes being the most common (73.1%). Borders between category sets were occasionally blurred, such as for repetitive spikes with pauses and polyspike bursts, and polyspike bursts and spike-waves. We used the initial afterdischarge morphologies and extents as one afterdischarge may evolve into another morphology and spread to adjacent electrode positions.
Afterdischarge characteristics and afterdischarge termination
Within the 132 electrode pairs that could elicit afterdischarges, we repeated the stimulation at the same parameter as prior to the afterdischarge for 63 pairs, and 51 pairs showed re-induced afterdischarges. Among the 51 electrode pairs, BPSs were applied and 47 pairs had stable afterdischarges in 104 trials. In 40 trials (38.5%), afterdischarges were terminated immediately or within the first 2 seconds. The afterdischarge extension was smaller in the BPS effective group (range, 1–7; mean: 3.0 ± 1.5) compared with the BPS ineffective group (range, 1–12; mean: 4.4 ± 2.3; F = 6.37, P = 0.019; Figure 4A). Of the four types of afterdischarge waveforms, rhythmic waves were the most likely (75%) to be terminated by the BPS, while polyspikes were the least likely (0%; Table 2). Among the three different afterdischarge frequency bands, the δ bands were the most likely (54.1%) to be terminated by the BPS, while the θ bands were the least likely (26.2%; Table 2). A significant difference was observed in the termination efficiencies among three band groups (χ2 = 6.537, P = 0.038).
Figure 4.

Effect of BPS on AD extension and BPS intensity.
(A) Box plot of AD extension (number of electrodes displaying AD waveforms) for the BPS effective group and ineffective group (P = 0.019, one-way analysis of variance), indicating the smaller the AD, the easier the AD to terminate. Thus, BPS was more effective when the AD did not extend into the surrounding brain area. (B) Box plot of BPS intensity for the BPS effective and ineffective groups (P = 0.021, one-way analysis of variance), indicating that the BPS intensity in the BPS effective group was lower than that in the BPS ineffective group. AD: Afterdischarge; BPS: brief-pulse stimulation.
Table 2.
Effect of BPS on terminating different afterdischarge waveforms and frequencies, and different BPS frequencies on terminating afterdischarge

BPS characteristics and afterdischarge termination
The pulse stimulation intensity was smaller in the BPS effective group (range, 2–12; mean: 6.5 ± 2.2) compared with the BPS ineffective group (range, 2–12; mean 7.2 ± 2.1; F = 5.50, P = 0.021; Figure 4B). A high frequency was more effective in terminating an afterdischarge than a low frequency (Table 2); the 100-Hz frequency had the highest effective rate of 53.3%, and was significantly different from the effective rate of the 5-Hz frequency (χ2 = 7.80, P = 0.020).
Predictor variables in a logistic regression model
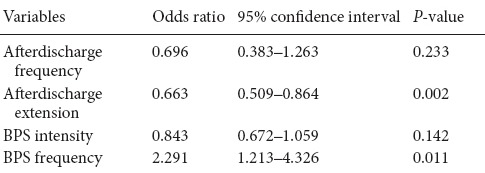
Next, we performed a logistic regression to identify the factors that determined the BPS effect. Five variables were included in regression model, and afterdischarge extension and BPS frequency were found as predictive variables (P < 0.05; Table 3).
Table 3.
Predictor variables in the logistic regression model
Discussion
The results of the present study confirmed those of a preliminary study showing that a stimulation pulse can terminate cortical-stimulated afterdischarges (Lesser et al., 1999; Motamedi et al., 2002). A higher frequency stimulation of 100 Hz was particularly effective at afterdischarge termination during functional mapping in epilepsy patients, even though the stimulation pulse was not administered shortly after seizure onset, as with the responsive neurostimulation system (Bergey, 2008; Sun and Morrell, 2014). The present study was designed to investigate the optimal stimulation frequency, and to explore the underlying mechanisms of BPS efficacy. Our main findings were: (1) a BPS was more effective when the afterdischarge did not extend into the surrounding brain area; (2) a higher BPS frequency was more likely to terminate an afterdischarge; (3) a BPS was more likely to terminate the afterdischarge when administered at a low current intensity; and (4) a BPS was more effective when the afterdischarge frequency was in the δ-band range. With respect to the capacity of an electrical stimulus to terminate an afterdischarge, we found significant differences for afterdischarge extension, afterdischarge frequency, BPS intensity and BPS frequency, although logistic regression model analysis revealed a predictive value for only afterdischarge extension and BPS frequency.
Our data suggest that use of a BPS to control an afterdischarge following a BPS is more effective if the afterdischarge does not evolve over an extensive area or far from the primary site; i.e., seizure control is easier by pulse stimulation when the epileptic activity is localized into a small area. In support, the responsive neurostimulation system can stimulate the brain shortly after a seizure onset, and terminate one seizure before it evolves into an uncontrollable seizure (Bergey, 2008; Sun and Morrell, 2014).
A preliminary study in human subjects used only a 50 Hz stimulation pulse (Lesser et al., 1999), while in the present study we used three different BPS frequencies (5 Hz, 50 Hz and 100 Hz) and compared their efficacy in afterdischarge suppression. Our findings are consistent with those in studies using deep brain stimulation and spinal cord stimulation, where high frequency stimulation had an inhibitory effect, mimicking the effects of making a lesion (Lee, 2009). By contrast, low frequency stimulation was reported to have an excitatory effect (Linderoth, 2009), although the underlying mechanism remains unclear.
For deep brain stimulation of the subthalamic nucleus in patients with Parkinson's disease, a stimulation frequency of 130 Hz or higher is generally used (Benabid, 2003). By contrast, deep brain stimulation in the pedunculopontine tegmentum uses a stimulation frequency of 30 Hz (Mazzone et al., 2005). In many regions of the brain, including the amygdala, hippocampus and cerebral cortex, rapid kindling using high frequency (50 Hz) stimulation as a model of epileptogenesis allows for the acceleration of epilepsy induction (Sankar et al., 2010). Therefore, a number of studies have applied low frequency stimulation to those regions to suppress epileptic activity (Zhong et al., 2012). Indeed, low frequency stimulation (1 Hz for 15 minutes) after kindling of the amygdala and hippocampus inhibited the development and expression of amygdala-kindled seizures (Weiss et al., 1995, 1998), suppressed the afterdischarge duration (Velisek et al., 2002) and increased the afterdischarge threshold (Bragin et al., 2002). However, in a rat model of genetic absence epilepsy, very high frequencies (500–1,000 Hz) were more effective at shortening seizure durations (Nelson et al., 2011), while a comparison of low (1 Hz) versus high (100 Hz) frequency stimulation in rat hippocampal brain slices found that both frequency stimulations suppressed epileptiform activity (Albensi et al., 2004). Human studies have also shown that electrical cortical stimulation of the seizure onset zone using both low (0.9 Hz) and high (50 Hz) frequencies suppressed epileptogenesis (Kinoshita et al., 2005). Our data indicate that high frequency stimulation may play an important role in acute seizure termination in humans.
The BPS at a low current intensity showed a trend towards higher efficacy, although this was not significant in a logistic regression model, indicating that the relationship with the effect of BPS intensity is not linear. This difference was expected as the average afterdischarge threshold in our study was approximately 8.7 mA, while a stimulation pulse over 8 mA was more likely to induce an afterdischarge. However, this finding does not indicate that a lower BPS intensity is better for afterdischarge suppression, as a sufficient stimulation pulse energy level is still required to induce a neuronal response. We suggest that a stimulation pulse of 1–7 mA (the mean intensity for the BPS effective group was 6.5 mA) is appropriate to terminate an afterdischarge, and if possible to abort the spontaneous seizure immediately after seizure onset.
A potential limitation of our study is that we only investigated the effects of three stimulation frequencies that were less than 100 Hz, while we did not investigate the effects of higher frequencies (> 300 Hz) or other parameters, such as pulse width and waveform type, duration and pattern.
In conclusion, high frequency and low intensity (<7 mA) stimulation pulses can effectively terminate afterdischarges during cortical stimulation.
Footnotes
Funding: This work was supported by the Capital Health Research and Development Special Funds of China, No. 2016-1-2011.
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by the Ethics Review Committee of the Xuanwu Hospital, Capital Medical University, China (approval number [2015]001). Patients and/or their family members volunteered to participate in the study.
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the form the patient have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check: This paper has been checked twice with duplication-checking software iThenticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M
References
- Albensi BC, Ata G, Schmidt E, Waterman JD, Janigro D. Activation of long-term synaptic plasticity causes suppression of epileptiform activity in rat hippocampal slices. Brain Res. 2004;998:56–64. doi: 10.1016/j.brainres.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Asadi-Pooya AA, Nei M, Sharan A, Sperling MR. Historical risk factors associated with seizure outcome after surgery for drug-resistant mesial temporal lobe epilepsy. World Neurosurg. 2016;89:78–83. doi: 10.1016/j.wneu.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Benabid AL. Deep brain stimulation for Parkinson's disease. Curr Opin Neurobiol. 2003;13:696–706. doi: 10.1016/j.conb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Berg AT. Defining intractable epilepsy. Adv Neurol. 2006;97:5–10. [PubMed] [Google Scholar]
- Bergey GK. Responsive neurostimulation for the treatment of epileptic seizures. In: Schelter B, Timmer J, Schulze-Bonhage A, editors. Seizure Prediction in Epilepsy. Weinheim: Wiley-VCH Press; 2008. pp. 299–306. [Google Scholar]
- Blume WT, Jones DC, Pathak P. Properties of after-discharges from cortical electrical stimulation in focal epilepsies. Clin Neurophysiol. 2004;115:982–989. doi: 10.1016/j.clinph.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J., Jr Increased afterdischarge threshold during kindling in epileptic rats. Exp Brain Res. 2002;144:30–37. doi: 10.1007/s00221-002-1023-y. [DOI] [PubMed] [Google Scholar]
- Fong JS, Jehi L, Najm I, Prayson RA, Busch R, Bingaman W. Seizure outcome and its predictors after temporal lobe epilepsy surgery in patients with normal MRI. Epilepsia. 2011;52:1393–1401. doi: 10.1111/j.1528-1167.2011.03091.x. [DOI] [PubMed] [Google Scholar]
- Forcadas-Berdusan MI, Bustos-Sanchez JL, Valle-Quevedo E, Aurrecoechea Obieta J, Mateos Goni B, Martinez-Indart L, Molano Salazar A, Gomez-Esteban JC, Garamendi-Ruiz I. Predictive factors for a good prognosis following surgery for temporal lobe epilepsy: a cohort study in Spain. Epileptic Disord. 2011;13:36–46. doi: 10.1684/epd.2011.0413. [DOI] [PubMed] [Google Scholar]
- Go C, Snead OC., 3rd Pharmacologically intractable epilepsy in children: diagnosis and preoperative evaluation. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC/2008/25/9/E2. [DOI] [PubMed] [Google Scholar]
- Goldenholz DM, Jow A, Khan OI, Bagic A, Sato S, Auh S, Kufta C, Inati S, Theodore WH. Preoperative prediction of temporal lobe epilepsy surgery outcome. Epilepsy Res. 2016;127:331–338. doi: 10.1016/j.eplepsyres.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guojun Z, Duanyu N, Fu P, Lixin C, Tao Y, Wei D, Liang Q, Zhiwei R. The threshold of cortical electrical stimulation for mapping sensory and motor functional areas. J Clin Neurosci. 2014;21:263–267. doi: 10.1016/j.jocn.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Ikeda A, Matsuhashi M, Matsumoto R, Hitomi T, Begum T, Usui K, Takayama M, Mikuni N, Miyamoto S, Hashimoto N, Shibasaki H. Electric cortical stimulation suppresses epileptic and background activities in neocortical epilepsy and mesial temporal lobe epilepsy. Clin Neurophysiol. 2005;116:1291–1299. doi: 10.1016/j.clinph.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Lee KH. Mechanisms of action of deep brain stimulation: a review. In: Krames ES, Peckham PH, Rezai A, editors. Neuromodulation. Oxford: Academic Press; 2009. pp. 157–169. [Google Scholar]
- Lesser RP, Kim SH, Beyderman L, Miglioretti DL, Webber WR, Bare M, Cysyk B, Krauss G, Gordon B. Brief bursts of pulse stimulation terminate afterdischarges caused by cortical stimulation. Neurology. 1999;53:2073–2081. doi: 10.1212/wnl.53.9.2073. [DOI] [PubMed] [Google Scholar]
- Linderoth B. Mechanisms of spinal cord stimulation in neuropathic and ischemic pain syndromes. In: Krames ES, Peckham PH, Rezai A, editors. Neuromodulation. Oxford: Academic Press; 2009. pp. 345–354. [Google Scholar]
- Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, Stefani A. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson's disease. Neuroreport. 2005;16:1877–1881. doi: 10.1097/01.wnr.0000187629.38010.12. [DOI] [PubMed] [Google Scholar]
- Motamedi GK, Okunola O, Kalhorn CG, Mostofi N, Mizuno-Matsumoto Y, Cho YW, Meador KJ. Afterdischarges during cortical stimulation at different frequencies and intensities. Epilepsy Res. 2007;77:65–69. doi: 10.1016/j.eplepsyres.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Motamedi GK, Lesser RP, Miglioretti DL, Mizuno-Matsumoto Y, Gordon B, Webber WR, Jackson DC, Sepkuty JP, Crone NE. Optimizing parameters for terminating cortical afterdischarges with pulse stimulation. Epilepsia. 2002;43:836–846. doi: 10.1046/j.1528-1157.2002.24901.x. [DOI] [PubMed] [Google Scholar]
- Nelson TS, Suhr CL, Freestone DR, Lai A, Halliday AJ, McLean KJ, Burkitt AN, Cook MJ. Closed-loop seizure control with very high frequency electrical stimulation at seizure onset in the GAERS model of absence epilepsy. Int J Neural Syst. 2011;21:163–173. doi: 10.1142/S0129065711002717. [DOI] [PubMed] [Google Scholar]
- Sankar R, Auvin S, Kwon YS, Pineda E, Shin D, Mazarati A. Evaluation of development-specific targets for antiepileptogenic therapy using rapid kindling. Epilepsia 51 Suppl. 2010;3:39–42. doi: 10.1111/j.1528-1167.2010.02607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FT, Morrell MJ. The RNS System: responsive cortical stimulation for the treatment of refractory partial epilepsy. Expert Rev Med Devices. 2014;11:563–572. doi: 10.1586/17434440.2014.947274. [DOI] [PubMed] [Google Scholar]
- Velisek L, Veliskova J, Stanton PK. Low-frequency stimulation of the kindling focus delays basolateral amygdala kindling in immature rats. Neurosci Lett. 2002;326:61–63. doi: 10.1016/s0304-3940(02)00294-x. [DOI] [PubMed] [Google Scholar]
- Weiss SR, Eidsath A, Li XL, Heynen T, Post RM. Quenching revisited: low level direct current inhibits amygdala-kindled seizures. Exp Neurol. 1998;154:185–192. doi: 10.1006/exnr.1998.6932. [DOI] [PubMed] [Google Scholar]
- Weiss SR, Li XL, Rosen JB, Li H, Heynen T, Post RM. Quenching: inhibition of development and expression of amygdala kindled seizures with low frequency stimulation. Neuroreport. 1995;6:2171–2176. [PubMed] [Google Scholar]
- Zhong K, Wu DC, Jin MM, Xu ZH, Wang Y, Hou WW, Li XM, Zhang SH, Chen Z. Wide therapeutic time-window of low-frequency stimulation at the subiculum for temporal lobe epilepsy treatment in rats. Neurobiol Dis. 2012;48:20–26. doi: 10.1016/j.nbd.2012.05.011. [DOI] [PubMed] [Google Scholar]