Abstract
Actin assembly at the leading edge of the cell is believed to drive protrusion, whereas membrane resistance and contractile forces result in retrograde flow of the assembled actin network away from the edge. Thus, cell motion and shape changes are expected to depend on the balance of actin assembly and retrograde flow. This idea, however, has been undermined by the reported absence of flow in one of the most spectacular models of cell locomotion, fish epidermal keratocytes. Here, we use enhanced phase contrast and fluorescent speckle microscopy and particle tracking to analyze the motion of the actin network in keratocyte lamellipodia. We have detected retrograde flow throughout the lamellipodium at velocities of 1–3 μm/min and analyzed its organization and relation to the cell motion during both unobstructed, persistent migration and events of cell collision. Freely moving cells exhibited a graded flow velocity increasing toward the sides of the lamellipodium. In colliding cells, the velocity decreased markedly at the site of collision, with striking alteration of flow in other lamellipodium regions. Our findings support the universality of the flow phenomenon and indicate that the maintenance of keratocyte shape during locomotion depends on the regulation of both retrograde flow and actin polymerization.
INTRODUCTION
The lamellipodium of motile cells is formed of a dense network of branched actin filaments (F-actin) polymerizing in the direction of cell movement. Network assembly at the leading edge is thought to generate the forces that push the plasma membrane forward (Theriot and Mitchison, 1991; Mogilner and Oster, 1996; Carlier et al., 2003; Pollard and Borisy, 2003). In most motile cells, the network is simultaneously transported away from the leading edge in a process known as retrograde flow (Cramer, 1997). The flow may be the result of forces of membrane tension counteracting actin polymerization at the edge of the cell (Raucher and Sheetz, 2000) and/or contractile forces of myosin motors (Lin et al., 1996). The balance between forward movement of the cell and backward movement of the actin network is believed to depend on a clutch-like substrate-adhesion mechanism (Smilenov et al., 1999; Jay, 2000). When the actin network is tightly coupled to the substrate (clutch fully engaged), actin polymerization effectively pushes the plasma membrane forward, and myosin-based contraction pulls the cell body in the direction of the leading edge. Conversely, when the clutch is loose, polymerization against the membrane pushes the network backward in concert with contraction pulling the actin network away from the leading edge. Thus, a possible mechanism for the cell to control its shape and net movement is by shifting the balance between protrusion and retrograde flow, supplementing the more widespread notion of spatial variation of actin assembly being the key regulator of cell motility (Pollard et al., 2000). Indeed, several studies report a relationship between retrograde flow and cell motion: the rate of retrograde flow in neuronal growth cones has been found to be inversely proportional to the rate of advance (Lin and Forscher, 1995); the local attenuation of flow was reported to precede local protrusion events (Danuser and Oldenbourg, 2000); the sites of substrate adhesions were found to be stationary in moving fibroblasts but to move retrogradely in stalled ones (Smilenov et al., 1999); and periodic fluctuations of protrusion during fibroblast spreading were correlated with the rearward actin waves (Giannone et al., 2004).
In other studies, however, no correlation between the rates of cell advance and retrograde flow has been observed (Theriot and Mitchison, 1992), suggesting that retrograde flow may not be an intrinsic property of the motile machinery. Another reason to question the generality and the importance of the flow phenomenon is that it was not detected in the cells representing one of the most spectacular and “pure” examples of fast and persistent locomotion: fish epidermal keratocytes (Lee et al., 1993a).
Keratocytes consist of a lamellipodium and a cell body coupled by a contractile belt rich in myosin II (Svitkina et al., 1997). Marking the lamellipodial cytoskeleton with a photoactivated actin probe as well as with a fluorescent myosin II probe did not display any movement in most of the lamellipodial network (Theriot and Mitchison, 1991; Svitkina et al., 1997), suggesting a tight coupling of the cytoskeleton to the substrate. Consistent with this conclusion, integrin-mediated close adhesions were found under the leading edge (Lee and Jacobson, 1997), and dynamics of the focal adhesion component vinculin revealed no movement under the central part of the lamellipodium (Anderson and Cross, 2000). Paradoxically, significant centripetal movement of both the adhesions and the actin network was detected in the lateral wings of the lamellipodia (Lee et al., 1993b; Anderson and Cross, 2000), where the biggest vinculin-containing adhesions were found. Thus, the presence of adhesions is not sufficient to explain the absence of flow. Flow in the wings was suggested to result from the contraction of the actomyosin bundle at the lamellipodium/cell body transition zone. However, it was not clear what mechanisms preclude flow in the central part of keratocyte lamellipodium and how this part couples to the contractile wings.
During locomotion, the keratocyte lamellipodium maintains a crescent shape, which was explained by graded radial extension, i.e., a monotonically decreasing protrusion rate from the cell center toward the sides (Lee et al., 1993b). Given the apparent absence of a retrograde flow, efforts toward modeling keratocyte dynamics have relied on the assumption that graded protrusion is entirely regulated by factors controlling actin polymerization (Mogilner and Edelstein-Keshet, 2002; Grimm et al., 2003).
In this article, we revisit the question of the existence of retrograde flow and challenge the polymerization-centered models. We exploit fluorescent speckle microscopy (FSM) to probe actin network flow with hundreds of fluorescent marks, producing sensitive and detailed maps of the flow pattern (Danuser and Waterman-Storer, 2003). A second technique providing similar possibilities for tracking network movement is an enhanced phase contrast microscopy (Verkhovsky et al., 2003). This method capitalizes on the low noise and high dynamic range of cooled charge-coupled device (CCD) detectors to visualize subtle variations in actin network density, resulting in a field of traceable marks. In contrast to fluorescent speckles, the phase contrast features are photostable and thus permit a tracking of the network over extended periods. We used computer vision tracking as developed for FSM to follow actin motion in both FSM and phase contrast images and mapped significant retrograde flow throughout the entire lamellipodium of a keratocyte. We analyzed the organization of flow in detail and found it persistent in freely locomoting cells but dramatically changing when cells collided with an obstacle.
MATERIALS AND METHODS
Cell Culture and Imaging
Keratocyte culture (Verkhovsky et al., 2003), FSM using the microinjected rhodamine-actin probe (Verkhovsky et al., 1999b), and enhanced phase contrast microscopy (Verkhovsky et al., 2003) were performed as described previously. Images were recorded at a rate of 1 frame per 4 s with a spatial sampling of 80 nm per 1 pixel. The rhodamine-phalloidin probe was prepared for microinjection by dissolving rhodamine-phalloidin (Sigma-Aldrich, St. Louis, MO) in dimethyl sulfoxide (DMSO) and then diluting the solution into the injection buffer (10 mM PIPES, pH 7.2) to obtain 0.3 mg/ml phalloidin solution with 15% DMSO. Injected cells exhibiting normal motile behavior were selected for the observation. Simultaneous imaging in the fluorescence and the phase contrast modes was performed using the 1024BFT cooled CCD camera (Roper Scientific, Trenton, NJ) at a rate of 1 frame per 2 seconds (1 frame per 4 s in each mode and the two sequences interleaved with the 2-s interval) with a spatial sampling of 65 nm per 1 pixel.
Tracking the Actin Flow
FSM images (Figure 1A) contain bright features (speckles) on a dark background, serving as fiduciary marks for the tracking of flow. Phase contrast images (Figure 1F), however, do not present such features. The images had to be preprocessed to extract what we refer to as pseudospeckles. To achieve this, the images were first inverted in contrast and then subjected to the “Flatten Background” filter (size parameter 4 pixels) available in MetaMorph (Universal Imaging, Downingtown, PA). The contrast inversion and background homogenization expose local maxima in actin density, which served as targets for particle tracking (Figure 1G).
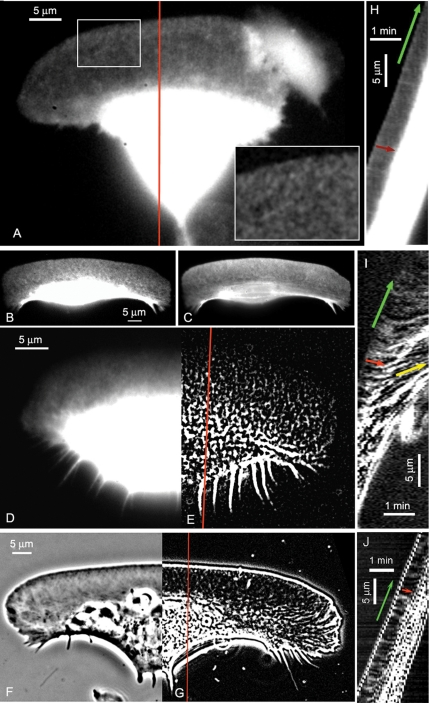
Figure 1.
Imaging actin network flow in keratocytes by FSM and phase contrast microscopy. FSM images of keratocyte injected with low concentration of rhodamine-actin (A) and rhodamine-phalloidin (B). Inset in A shows enlarged region of the lamellipodium to display speckled pattern of rhodamine-actin incorporation. (C) The same cell as in B fixed and stained with excess rhodamine-phalloidin. Note that the contrast setting for B and C are not equal. Speckle intensity in B is low at the front of the cell and increases toward the cell body. Continuous staining of total F-actin in C reveals high actin density at the front of the lamellipodium, indicating that actin distribution was not affected by injection of phalloidin. (D and E) Processing of the image of a phalloidin-injected cell (D) with the Flatten Background filter generates speckles throughout the cell (E), including the cell body. (F and G) Generation of pseudospeckles from digitally enhanced phase contrast image of keratocytes. Enhanced image in direct contrast (F). After contrast inversion and processing with the Flatten Background filter the image of the lamellipodium contains traceable local intensity maxima referred to as “pseudospeckles” (G). (H–J) Kymographs of the image sequences of the cells shown in (A, E, and G), respectively; red profile lines indicate the kymograph location). Green arrows in kymographs indicate the forward movement of the cell edge, red arrows indicate the retrograde flow of the lamellipodium network, and yellow arrow indicates the anterograde flow of phalloidin speckles in the cell body. Supplemental videos show the phalloidin-actin FSM sequence (Video 1) and the phase contrast pseudospeckle sequence (Video 2).
Speckle movements were then analyzed with our particle tracking software (Vallotton et al., 2003): Speckles were extracted from the images as the statistically significant local intensity maxima not related to noise (Figures 2A and 3A). They were subsequently tracked by first generating all candidate displacement vectors between successive frames whose magnitude is below a threshold defining the maximum interframe displacement (4 pixels, i.e., 320 nm, in most cases, but in some sequences [Figure 3], this threshold was increased to 10 pixels to detect fast flow at the wings of the cell). The optimal subset of displacement vectors is then selected globally by a graph search algorithm maximizing the smoothness of speckle trajectories and eliminating topological conflicts, i.e., each speckle in source and target frame can be linked once at most (Figures 2B and 3B). The resulting speckle trajectories are a superposition of flow and random movements associated with thermal fluctuations, local meshwork contraction and image noise. The component corresponding to network flow is estimated on a regular grid (Figures 2C and 3, C and D), by using a distance-weighted sum of the raw speckle displacement vectors in the neighborhood of the grid nodes, where the correlation length of the filter kernel is set to 4 μm, in accord with the persistence length of actin filaments (Gittes et al., 1993).
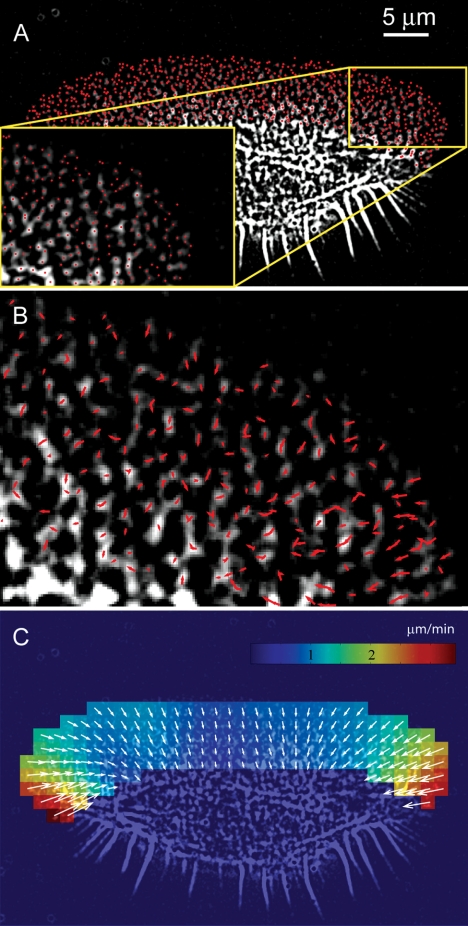
Figure 2.
F-actin network flow in keratocyte lamellipodia measured by particle tracking of fluorescent speckles in time-lapse image sequences of rhodamine-phalloidin–injected cells. (A) Detection of speckles as local intensity maxima in the first frame of the movie. Local intensity maxima whose intensity differences to the surrounding background are in a statistical sense significantly larger than image noise are extracted from the low-pass filtered image and indicated with red dots. Inset, enlarged portion of the image indicated by yellow box. (B) Speckle displacements tracked over two subsequent frame pairs are indicated by red arrows for the region shown in the inset in A. The assignment of corresponding speckles between frames is accomplished by a graph search algorithm using a displacement threshold of 4 pixels/frame (320 nm in object space; sf. Materials and Methods). The majority of the vectors are directed away from the cell edge indicating retrograde flow. (C) Flow field calculated on a regular grid from the speckle displacements. Colors encode the local flow speed.
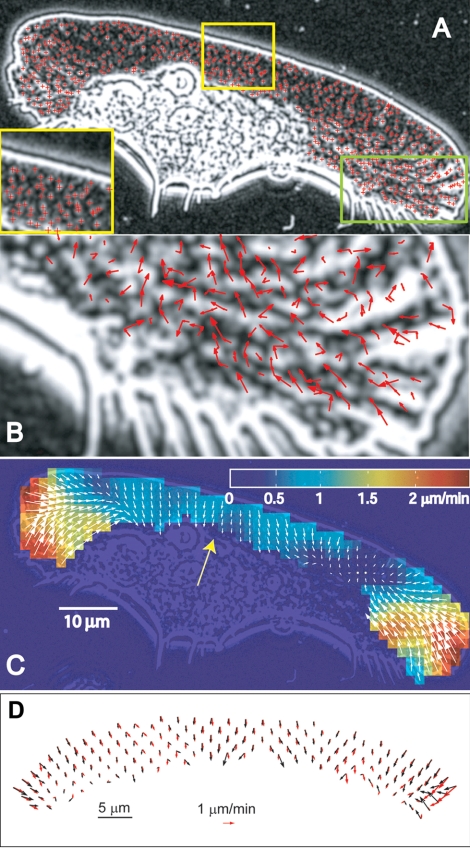
Figure 3.
F-actin network flow measured in enhanced phase contrast image sequences and compared with FSM data. (A) Detection of pseudospeckles as local intensity maxima (indicated with red crosses) in the inverted phase contrast image, which is additionally processed with the Flatten Background filter to extract intensity variations across the image. Inset shows enlarged portion of the image indicated with yellow box. (B) Speckle displacements tracked over two subsequent frame pairs by using a displacement threshold of 10 pixels (800 nm in object space) are indicated by red arrows for the region shown in the inset in A. (C) Flow field calculated on a regular grid from the raw speckle displacements. Colors encode the local flow speed. (D) Comparison of the displacement vector fields obtained by FSM and phase contrast images of the same cell. The keratocyte was injected with a low concentration of rhodamine phalloidin and imaged in the two modes with a 2-s interval between fluorescence and phase contrast images. Flow fields calculated on a regular grid are shown with red arrows for the fluorescence images and black arrows for the phase contrast images. The two flow fields are largely similar, with the differences being likely due to the time interval between the images, image noise, and ruffling at the wings of the cell, which interfere with the tracking of the phase contrast image.
RESULTS
Imaging F-Actin Movement in Migrating Keratocytes
Tracking of F-actin flow in keratocyte lamellipodia was performed in the time-lapse sequences of images of three different types: FSM images obtained by microinjection of low concentration of either rhodamine-actin (Figure 1A) or rhodamine-phalloidin (Figure 1, B and D, and Supplemental Video 1) and enhanced phase contrast images (Figure 1F and Supplemental Video 2). Of the two types of FSM images, those obtained using rhodamine-phalloidin presented higher contrast. This may be explained by the specific binding of phalloidin to actin polymer, whereas microinjected rhodamine-actin contributed to both the polymer and the monomer pool, thus increasing the diffuse background fluorescence level (especially in the relatively thick cell body region). Nevertheless, in both types of FSM images the contrast was sufficient to detect fiduciary marks visualizing the flow of the network
Rhodamine-phalloidin speckles increased in intensity from the cell front to the back of the lamellipodium and the cell body (Figure 1, B and D). When scaling the image contrast to reveal speckles at the cell front the latter two zones seemed saturated, lacking any useful information. This intensity distribution was in apparent discrepancy with the opposite gradient of F-actin density in keratocytes (Small et al., 1995; Svitkina et al., 1997). To explain this effect, it has to be noted that the speckle intensity depends not only on the actin density but also on the local concentration of phalloidin available for binding to actin polymer. The concentration of phalloidin used for FSM imaging is low and may be the rate-limiting component in the binding step. Therefore, distribution gradients throughout the cell will result in significant spatial variation of the fluorescence signal. We tested this by fixation and staining of previously injected cells with excess rhodamine-phalloidin to reveal the full F-actin distribution in a continuous stain (Figure 1C). Here, F-actin revealed the well established decrease of density from the front to the base of the lamellipodium, indicating that phalloidin injection in living cells did not alter the overall structure of the F-actin network. This conclusion was further supported by our observation that injected cells migrated indistinguishably from noninjected cells.
A possible explanation of the low rhodamine-phalloidin speckle intensity at the cell front and its relative enrichment at the base of the lamellipodium and in the cell body is that, while the cell moved forward, the labeling at the front was determined by the efficiency of diffusive and/or convective transport of free phalloidin into this region. Most of the phalloidin is tightly bound to F-actin and any phalloidin that became available upon actin filament disassembly was likely to rebind to the remaining, unsaturated F-actin in the same locality rather than to diffuse to the front. Consequently, the front of the lamellipodium, where the new actin filaments assembled, became depleted of phalloidin. The lateral flanks of the frontal region of the lamellipodium may be the least accessible for diffusion of available phalloidin, explaining the even lower speckle intensity observed there compared with the central part. Another cause for reduced rhodamine-phalloidin label in the vicinity of the leading edge may be the competition for actin binding between phalloidin and ADF/cofilin (Bobkov et al., 2004), which is abundant in this region of the cell (Svitkina and Borisy, 1999).
To equalize the intensity of phalloidin speckles throughout the cell, the images were processed with the Flatten Background filter in MetaMorph software (size parameter 4 pixels). Also, this procedure largely reduced diffuse out-of-focus fluorescence in the cell body, revealing bright features in the substrate plane. The speckles were thus extracted throughout the lamellipodium as well as at the lamellipodium/cell body junction and in the cell body (Figure 1E and Supplemental Video 1 showing the raw and processed sequences). Speckle patterns often presented parallel rows of speckles at the base of the lamellipodium and in the cell body, consistent with the previously described actin bundles in these regions (Anderson et al., 1996; Svitkina et al., 1997).
Phase contrast images of keratocyte lamellipodia displayed a criss-cross meshwork pattern of diagonal linear features (Figure 1F). This pattern reflected the variation of filament density in the actin network, with darker features corresponding to higher polymer density (Verkhovsky et al., 2003). The natural variation of network density is useful to track network movement in the same way as statistical variations of fluorescent label are exploited as fiduciary marks in FSM. Notice that whereas phase contrast images represent the actin network structure in lamellipodia, the image contrast in the cell body is mostly due to the organelles and does not reflect the distribution of actin. To facilitate tracking of features in lamellipodia, phase contrast images were inverted in contrast and processed with the Flatten Background filter in MetaMorph (size parameter 4 pixels). This exposed local maxima of actin density in the form of bright spots, similar to fluorescent speckles (Figure 1G and Supplemental Video 2). Accordingly, we refer to these features as pseudospeckles.
Characterization of Actin Flow
We first made an estimation of the actin network flow by kymograph analysis. In kymographs, a single line or a narrow rectangular region of the image is cropped in successive images, and the bands are pasted side by side in a time montage. If a movement takes place along the long axis of the cropped region, the kymograph displays diagonal streaks whose slopes indicate the velocity of the movement. The most obvious movement recognizable in kymographs of keratocyte image sequences was the forward movement of the whole cell (Figure 1, H–J, green arrows). Inside the lamellipodial region, streaks running opposite to the direction of cell movement also were apparent (red arrows), indicating a retrograde flow of actin features relative to the substratum. This backward flow was detectable in kymographs of all three types of keratocyte images (Figure 1, H–J). The phalloidin-injected cells displayed distinct actin features not only in the lamellipodium but also in the cell body (see previous section), offering a possibility to track actin movement throughout the cell. Kymographs indicated anterograde movement of actin in the cell body (Figure 1I, yellow arrow), converging with the lamellipodial retrograde flow at the lamellipodium/cell body junction, where myosin motors are believed to generate strong contractile forces (Verkhovsky et al., 1999a). The existence of such pronounced convergence zone also has been described in other, less motile cell types (Salmon et al., 2002; Vallotton et al., 2004).
The information obtained in kymographs is, however, limited. Kymographs show the movement within the selected region and along the selected direction only. Moreover, in our data, the measurement of the flow velocity was not reliable because the streaks were short and displayed variable slopes. One of the reasons for the difficulty of this analysis was the fast movement of the keratocytes. The advance of the leading edge (Figure 1, H–J, green arrows) was >10 times faster than the speed of retrograde flow (red arrows). Conversely, the time it took the cell to traverse a distance of the width of the lamellipodium was very short (in our case, 5 frames = 20 s), rendering the measurement of the relatively small retrograde flow speeds susceptible to noise. The high ratio between the speeds of cell advance and flow was likely the reason why the F-actin movement in keratocytes went hitherto unnoticed, including in our own recent report (Verkhovsky et al., 2003).
To obtain robust velocity data in the form of a two-dimensional vector map, we applied single particle tracking (Vallotton et al., 2003; see Materials and Methods), which increases the sensitivity of flow measurements by analyzing a large number of speckle trajectories. We focused on the lamellipodial region because the information for this region was available from three independent imaging techniques. The lamellipodia of keratocytes were of special interest also because the actin network in this region was previously believed to be stationary with respect to the substratum. Tracking the flow in FSM images of phalloidin-injected cells and enhanced phase contrast images is shown in Figures 2 and 3. The illustrated steps are the selection of particles (Figures 2A and 3A), the tracking of their movement between consecutive images (Figures 2B and 3B), and the filtering of noisy single particle tracks to obtain vector maps of the coherent flow field (Figures 2C and 3C) (see Materials and Methods). FSM images of cells injected with rhodamine-actin were analyzed in the same way. Several cells injected with rhodamine-phalloidin also were imaged in a double fluorescence/phase contrast mode, allowing us to track fluorescent speckles and phase contrast pseudospeckles nearly simultaneously in the same cell. Figure 3D shows that the resulting vector fields were nearly identical, supporting the validity of the enhanced phase contrast microscopy for tracking actin network flow. The results obtained using two types of FSM images and phase contrast images in different cells were all remarkably similar in both flow structure and velocity magnitudes. We measured retrograde flow velocities in the lamellipodia in the range 1–3 μm/min, comparable with those reported for other cell types (Salmon et al., 2002; Vallotton et al., 2003). In all cells analyzed, flow maps revealed a flow approximately perpendicular to the edge of the cell. Thus, the flow at the lateral flanks of the lamellipodia was oriented toward the center of the cell rather than backward. Flow velocity varied significantly depending on the position along the edge. The highest velocities were always observed at the sides of the cell and the lowest in the middle. These differences were especially pronounced in wide cells, like the one shown in Figure 3, A–C, where the velocities at the sides were ∼4 times higher than in the center.
Flow Dynamics and the Protrusion Rate
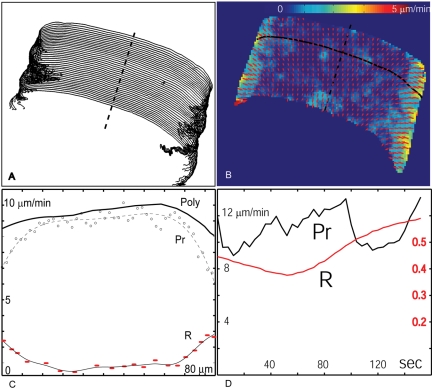
Freely Moving Cells. To maintain their ellipsoidal shape while progressing on surfaces, keratocytes need to protrude more on their main symmetry axis than on their sides (Lee et al., 1993b). This has been generally attributed to the difference in actin polymerization rates (Mogilner and Edelstein-Keshet, 2002; Grimm et al., 2003); however, our finding that keratocyte lamellipodia display a retrograde flow even in their center requires this issue to be reconsidered. We analyzed the relationship between retrograde flow and protrusion rate along the leading edge and estimated the actual polymerization rate as the sum of the two. The protrusion rate was evaluated by measuring the distance between successive positions of the cell leading edge (Figure 4A), whereas the flow rate was measured using our tracking procedure (Figure 4B, where the velocity maps of 40 frames are merged in a time montage). The results suggest that although the retrograde flow velocity increases from the center toward the wings (Figure 4C), thereby contributing to the decrease of the protrusion rate, the gradient is not sufficient to explain the differences in protrusion rate between center and wings. We conclude that both polymerization rate has to be decreased, and retrograde flow rate has to be increased to decrease the protrusion rate at the side sufficiently to maintain the shape of the cell.
Figure 4.
Correlation between leading edge protrusion and retrograde flow. (A) Leading edge positions for 40 frames as tracked with Sobel edge detector (Matlab, Mathwork, Natick, MA). The spacing between adjacent outlines indicates the instantaneous protrusive activity. (B) Time montage of the retrograde flow field. For the time montage, speckle displacements of all 40 time points were collected in a spatial map and subjected to spatial averaging as used for flow extraction in a single time point. (C) Retrograde flow speed (R), and protrusion (Pr) measured along the edge at one time point (solid black curve in B). Protrusion measured as the distance to the next edge position along the normal line. The sum of protrusion and retrograde flow equals the polymerization rate (Poly). (D) Temporal dynamics of protrusion (black line) and retrograde flow (red line), measured at the center of the lamellipodium (position indicated by a dashed line in B).
We also have analyzed the evolution of retrograde flow and protrusion rate at the cell edge over time. No correlation between the protrusion and flow velocities was apparent (Figure 4D). Consistent with the absence of visually pronounced correlation, the correlation coefficients between protrusion and flow rates measured along five different lines parallel to the direction of cell migration varied between -0.22 and 0.43. Systematic variation of the time lag between protrusion and flow did not augment this correlation. These results suggest that the retrograde flow dynamics do not play a significant role in temporal variation of keratocyte speed.
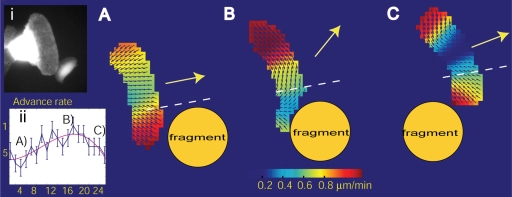
Flow Dynamics during Cell Collision. Some of our movies exhibited events of cell collision, during which the cell motion was transiently altered. We found that retrograde flow undergoes dramatic changes during these events, which could be summarized as a reduction of flow speed at the site of collision and a general reorientation of flow toward this site. During the side collision of a cell with a cell fragment (Figure 5 and Supplemental Video 3), flow aligned parallel to the leading edge toward the collision site, giving the impression of a new lamellipodium forming away from the collision (repolarization). It seemed that the cell moved mostly laterally and away from the cell fragment. In the case of a frontal collision between two cells (Supplemental Video 4), the flow eventually reoriented toward the collision site, resulting in complete reversal of the flow from retrograde to anterograde direction. When the obstacle was passed, the flow resumed its original organization, an expression of the stability of keratocyte dynamics.
Figure 5.
Dynamic reorganization of retrograde flow during collision between a keratocyte and a keratocyte cytoplasmic fragment (cf. inset i). (A) Organization of flow before collision. (B) On collision, the flow immediately behind the contact area is slower. The flow is reoriented mostly parallel to the leading edge and the cell starts to move sideways to by-pass the obstacle. (C) After passing, flow and cell movement resume their normal behavior. Inset ii shows the protrusion dynamics during collision. Maximum protrusion is reached when the retrograde flow is nearly parallel to the leading edge. See also Supplemental Video 3 (side collision with the fragment shown in this figure) and Video 4 (frontal collision of the two cells) for a full time-resolved analysis of the flow organization.
DISCUSSION
In this study, we report the detection of backward movement (retrograde flow) of the actin network relative to the substratum in the lamellipodia of migrating fish epidermal keratocytes. Previously, the dynamics of the keratocyte lamellipodia was investigated using photoactivation of the fluorescent label in narrow bands of the actin network (Theriot and Mitchison, 1991), but no retrograde flow was detected. Also, no flow was found when observing the network dynamics indirectly with a fluorescent myosin II probe (Svitkina et al., 1997). We attribute the detection of retrograde flow to the advance of the new fluorescent and phase contrast speckle imaging as well as image processing and computer tracking techniques. The flow was measured in three different types of images by using both kymograph analysis and single particle tracking. The results obtained with the two imaging modalities, FSM and the enhanced phase contrast microscopy, are complementary and mutually supportive. Although the fluorescent probes in FSM ensure specific imaging of actin dynamics, the enhanced phase contrast technique provides the possibility to look at the “natural” movement of the lamellipodial network in the unperturbed cell without photobleaching and photodamage. The similarity of the results obtained by the two techniques suggests that, on one hand, enhanced phase contrast microscopy is a reliable tool to study actin network movement in lamellipodia, and that, on the other hand, microinjection and the intensive illumination required in FSM do not significantly alter the actin dynamics. After our manuscript was submitted, Jurado et al. (2004) reported on retrograde flow in keratocytes by using FSM of expressed green fluorescent protein-actin and kymograph technique. Our finding are mostly consistent with and complementary to the study of Jurado et al. (2004).
The discovery of retrograde flow in keratocytes is important in two respects. First, it highlights the similarity of keratocytes to other crawling cells, validating the use of keratocytes as a general model for cell motility. Second, our findings speak for the generality of the flow phenomenon, supporting the point of view that flow is the expression of essential forces involved in motility and thus may be intrinsic to all motile cells.
The implications of our findings for the motility mechanism are that the actin network is subjected to significant forces and that its coupling to the underlying substrate is transient even in the central region of keratocyte lamellipodia. The transient coupling between lamellipodium network and the substrate generates forces that are expected to be detected at the substrate level as traction forces with the same direction as the flow field. Well documented pinching forces at the lamellipodium flanks (Oliver et al., 1999) are consistent with the fast flow in these regions. The existence of traction forces under the central lamellipodial region was controversial. No forces were detected in this domain with the elastic substrate assay, leading to the hypothesis that forward traction is generated entirely at the lamellipodial flanks (Oliver et al., 1999). However, the reportedly more sensitive assay by using micromachined substrate detected relatively weak backward directed forces under the middle part of the lamellipodia and similar forward directed forces under the cell body (Galbraith and Sheetz, 1999), consistent with our flow data. The forces under the middle part of the lamellipodium were ∼4 times weaker than under its flanks, in remarkable agreement with the ratio we observe for the flow rates at these two sites. These observations suggest that the dynamics of the central part of the lamellipodia could be described in the same terms as the dynamics at its flanks, with the differences being quantitative rather than qualitative.
The forces driving the flow in keratocyte lamellipodia likely originate from the contraction of the actin/myosin II network at the boundary between the lamellipodium and the cell body, as stipulated by the dynamic network contraction model (Svitkina et al., 1997; Verkhovsky et al., 1999a). In this model, the anterior-posterior component of the contractile force was suggested to drive the forward translocation of the cell body, but the counterforce could be expected to produce retrograde motion of the lamellipodium network. The finding of flow throughout the lamellipodia is consistent with the force being generated along the entire lamellipodium/cell body junction rather than just at the lateral wings of the cell as speculated in other models (Anderson et al., 1996; Oliver et al., 1999). The relative velocity of the retrograde flow and cell body movement and forces at the substrate level are expected to depend both on the internal forces and on the distribution and relative strength of adhesion in different cell domains. Substrate adhesions have been detected under the front of the lamellipodium and under the lateral wings of the keratocyte but not under the bulk of the cell body (Lee and Jacobson, 1997). Stronger adhesion of the lamellipodium than that of the cell body is consistent with the retrograde flow velocity being small in comparison with the cell body translocation rate. However, in contrast to the retrograde flow of actin network that we report here, the adhesions originating under the central part of the lamellipodium were reported to be stationary with the substrate and to dissolve under the cell body (Anderson and Cross, 2000). One possibility is that the actin network is somehow coupled to the adhesion in a dynamical manner, so that it can move with respect to the stationary adhesions. Alternatively, slight motion of the adhesions may have as yet escaped detection similar to how the retrograde actin flow has been previously not detected. In contrast to the adhesions under the central part of the cell, the adhesions under lateral wings were reported to slide toward the cell center and to be eventually ripped off from the substrate. This is consistent with the relatively fast actin flow in the lamellipodium wings and strong forces detected at the substrate level in these regions. These forces are likely caused by the longitudinal tension in the contractile actin/myosin II bundle at the lamellipodium/cell body junction.
Another possible force inducing retrograde flow is generated by actin polymerization against the plasma membrane. However, experiments with inhibitors of actomyosin contractility demonstrated that the membrane tension alone could not keep the lamellipodium from running away from the cell body (Verkhovsky et al., 1999b), suggesting that the membrane tension is small compared with the motor-induced forces.
Our results suggest that the gradient of retrograde flow may contribute to the maintenance of the keratocyte shape during locomotion and that the dynamics of the flow may play an important role in cell collision events. Reduction of the flow at the contact site during collision may reflect a very general and important phenomenon of contact inhibition of cell motility. One could imagine that if actin polymerization is inhibited by increased countertension at the site of cell contact and if the flow is caused by polymerization pressure at the membrane, this will reduce the flow rate. However, this mechanism cannot explain the forward reorientation of the flow upon frontal collision. Also, the absence of a positive correlation between polymerization and flow rate (see above) argues against the possibility that flow is mostly driven by membrane resistance to polymerization. Another possibility for explaining the flow breakdown is that at the contact site transmembrane links mediated by, e.g., cadherins mutually stabilize the actin networks in both cells (Perez-Moreno et al., 2003). The contact area would then act as a pinning point for the lamellipodium actin network, and dynamic network contraction would produce the reorganization of flow consistent with this new geometry. The reorganization of the flow during keratocyte collision may thus be similar to the events during homologous growth cone interaction in Aplysia (Lin and Forscher, 1995) and contact inhibition in epithelial cells (Gloushankova et al., 1997).
If the polymerization rate stayed constant during the collision, one could expect that the changes in retrograde flow would translate into changes in protrusion rate. The protrusion measurement showed that the rate indeed increased to its maximum value simultaneously with the reorientation of flow parallel to the leading edge, and decreased again when the flow resumed its original pattern after the collision, suggesting that network polymerization is retained during collision to push the cell past the obstacle (Figure 5, inset ii). Although both the relatively stable pattern of the flow during persistent movement and the dramatic reorganization of the flow during collision suggest that the flow is regulated in the migrating cell, further studies are necessary to elucidate the mechanisms of the cellular control over the flow.
In summary, we tracked the movement of actin in migrating fish epidermal keratocytes and found that these cells, like most other crawling cells, exhibit significant retrograde flow of their lamellipodium F-actin network. Quantitative characterization of the actin network movement in the relatively simple keratocyte model is essential for the understanding of forces involved in motility and represents one of the initial steps toward the comprehensive biophysical model of cell migration.
Supplementary Material
Acknowledgments
This study was supported by Swiss Science Foundation grants 31-61589 (to A.B.V.) and 21-59452.99 (to G. D.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-07-0615) on January 5, 2005.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Anderson, K. I., and Cross, R. (2000). Contact dynamics during keratocyte motility. Curr. Biol. 10, 253-260. [DOI] [PubMed] [Google Scholar]
- Anderson, K. I., Wang, Y. L., and Small, J. V. (1996). Coordination of protrusion and translocation of the keratocyte involves rolling of the cell body. J. Cell Biol. 134, 1209-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobkov, A. A., Muhlrad, A., Shvetsov, A., Benchaar, S., Scoville, D., Almo, S. C., and Reisler, E. (2004). Cofilin (ADF) affects lateral contacts in F-actin. J. Mol. Biol. 337, 93-104. [DOI] [PubMed] [Google Scholar]
- Carlier, M. F., Le Clainche, C., Wiesner, S., and Pantaloni, D. (2003). Actin-based motility: from molecules to movement. Bioessays 25, 336-345. [DOI] [PubMed] [Google Scholar]
- Cramer, L. P. (1997). Molecular mechanism of actin-dependent retrograde flow in lamellipodia of motile cells. Front. Biosci. 2, d260-270. [DOI] [PubMed] [Google Scholar]
- Danuser, G., and Oldenbourg, R. (2000). Probing f-actin flow by tracking shape fluctuations of radial bundles in lamellipodia of motile cells. Biophys. J. 79, 191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danuser, G., and Waterman-Storer, C. M. (2003). Fluorescent speckle microscopy: where it came from and where it is going. J. Microsc. 211, 191-207. [DOI] [PubMed] [Google Scholar]
- Galbraith, C. G., and Sheetz, M. P. (1999). Keratocytes pull with similar forces on their dorsal and ventral surfaces. J. Cell Biol. 147, 1313-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannone, G., Dubin-Thaler, J., Döbereiner, H.-G., Kieffer, N., Bresnick, A., and Sheetz, M. (2004). Periodic lamellipodial contractions correlate with rearward actin waves. Cell 116, 431-443. [DOI] [PubMed] [Google Scholar]
- Gittes, F., Mickey, B., Nettleton, J., and Howard, J. (1993). Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell Biol. 120, 923-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloushankova, N. A., Alieva, N. A., Krendel, M. F., Bonder, E. M., Feder, H. H., Vasiliev, J. M., and Gelfand, I. M. (1997). Cell-cell contact changes the dynamics of lamellar activity in nontransformed epitheliocytes but not in their ras-transformed descendants. Proc. Natl. Acad. Sci. USA 94, 879-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm, H. P., Verkhovsky, A. B., Mogilner, A., and Meister, J. J. (2003). Analysis of actin dynamics at the leading edge of crawling cells: implications for the shape of keratocyte lamellipodia. Eur. Biophys. J. 32, 563-577. [DOI] [PubMed] [Google Scholar]
- Jay, D. G. (2000). The clutch hypothesis revisited: ascribing the roles of actin-associated proteins in filopodial protrusion in the nerve growth cone. J. Neurobiol. 44, 114-125. [DOI] [PubMed] [Google Scholar]
- Jurado, C., Haserick, J. R., and Lee, J. (2004). Slipping or gripping? Fluorescent speckle microscopy in fish keratocytes reveals two different mechanisms for generating a retrograde flow of actin. Mol. Biol. Cell (in press). [DOI] [PMC free article] [PubMed]
- Lee, J., Ishihara, A., and Jacobson, K. (1993a). The fish epidermal keratocyte as a model system for the study of cell locomotion. In: Cell Behavior: Adhesion and Motility, ed. G. Jones, C. Wigley, and R. Warn, Cambridge, United Kingdom: The Company of Biologists Ltd., 73-89. [PubMed]
- Lee, J., Ishihara, A., Theriot, J. A., and Jacobson, K. (1993b). Principles of locomotion for simple-shaped cells. Nature 362, 167-171. [DOI] [PubMed] [Google Scholar]
- Lee, J., and Jacobson, K. (1997). The composition and dynamics of cell-substratum adhesions in locomoting fish keratocytes. J. Cell Sci. 110, 2833-2844. [DOI] [PubMed] [Google Scholar]
- Lin, C. H., Espreafico, E. M., Mooseker, M. S., and Forscher, P. (1996). Myosin drives retrograde F-actin flow in neuronal growth cones. Neuron 16, 769-782. [DOI] [PubMed] [Google Scholar]
- Lin, C. H., and Forscher, P. (1995). Growth cone advance is inversely proportional to retrograde f-actin flow. Neuron 14, 763-771. [DOI] [PubMed] [Google Scholar]
- Mogilner, A., and Edelstein-Keshet, L. (2002). Regulation of actin dynamics in rapidly moving cells: a quantitative analysis. Biophys. J. 83, 1237-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilner, A., and Oster, G. (1996). Cell motility driven by actin polymerization. Biophys. J. 71, 3030-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, T., Dembo, M., and Jacobson, K. (1999). Separation of propulsive and adhesive traction stresses in locomoting keratocytes. J. Cell Biol. 145, 589-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno, M., Jamora, C., and Fuchs, E. (2003). Sticky business: orchestrating cellular signals at adherens junctions. Cell 112, 535-548. [DOI] [PubMed] [Google Scholar]
- Pollard, T. D., Blanchoin, L., and Mullins, R. D. (2000). Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 29, 545-576. [DOI] [PubMed] [Google Scholar]
- Pollard, T. D., and Borisy, G. G. (2003). Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453-465. [DOI] [PubMed] [Google Scholar]
- Raucher, D., and Sheetz, M. P. (2000). Cell spreading and lamellipodial extension rate is regulated by membrane tension. J. Cell Biol. 148, 127-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon, W. C., Adams, M. C., and Waterman-Storer, C. M. (2002). Dual-wavelength fluorescent speckle microscopy reveals coupling of microtubule and actin movements in migrating cells. J. Cell Biol. 158, 31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, J. V., Herzog, M., and Anderson, K. (1995). Actin filament organization in fish keratocyte lamellipodium. J. Cell Biol. 129, 1275-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilenov, L. B., Mikhailov, A., Pelham, R. J., Marcantonio, E. E., and Gundersen, G. G. (1999). Focal adhesion motility revealed in stationary fibroblasts. Science 286, 1172-1174. [DOI] [PubMed] [Google Scholar]
- Svitkina, T. M., and Borisy, G. G. (1999). Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 145, 1009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina, T. M., Verkhovsky, A. B., McQuade, K. M., and Borisy, G. G. (1997). Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J. Cell Biol. 139, 397-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot, J. A., and Mitchison, T. J. (1991). Actin microfilament dynamics in locomoting cells. Nature 352, 126-131. [DOI] [PubMed] [Google Scholar]
- Theriot, J. A., and Mitchison, T. J. (1992). Comparison of actin and cell surface dynamics in motile fibroblasts. J. Cell Biol. 119, 367-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallotton, P., Ponti, A., Waterman-Storer, C. M., Salmon, E. D., and Danuser, G. (2003). Recovery, visualization, and analysis of actin and tubulin polymer flow in live cells: a fluorescence speckle microscopy study. Biophys. J. 85, 1289-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallotton, P., Gupton, S. L., Waterman-Storer, C. M., and Danuser, G. (2004). Simultaneous mapping of F-actin flow and turnover in migrating cells by Quantitative Fluorescent Speckle Microscopy. Proc. Natl. Acad. Sci. USA 101, 9660-9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhovsky, A. B., Chaga, O. Y., Schaub, S., Svitkina, T. M., Meister, J., and Borisy, G. G. (2003). Orientational order of the lamellipodial actin network as demonstrated in living motile cells. Mol. Biol. Cell 14, 4667-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhovsky, A. B., Svitkina, T. M., and Borisy, G. G. (1999a). Network contraction model for cell translocation and retrograde flow. Biochem. Soc. Symp. 65, 207-222. [PubMed] [Google Scholar]
- Verkhovsky, A. B., Svitkina, T. M., and Borisy, G. G. (1999b). Self-polarization and directional motility of cytoplasm. Curr. Biol. 9, 11-20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.