Keywords: nerve regeneration, brain injury, H2O2, cerebellar granule cells, Bim, Bax, Bcl-2, cytochrome C, caspase-3, neural regeneration
Abstract
Ganoderma lucidum polysaccharides have protective effects against apoptosis in neurons exposed to ischemia/reperfusion injury, but the mechanisms are unclear. The goal of this study was to investigate the underlying mechanisms of the effects of ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis. Hydrogen peroxide (H2O2) was used to induce apoptosis in cultured cerebellar granule cells. In these cells, ganoderma lucidum polysaccharides remarkably suppressed H2O2-induced apoptosis, decreased expression of caspase-3, Bax and Bim and increased that of Bcl-2. These findings suggested that ganoderma lucidum polysaccharides regulate expression of apoptosis-associated proteins, inhibit oxidative stress-induced neuronal apoptosis and, therefore, have significant neuroprotective effects.
Introduction
Oxidative stress-induced brain damage has been implicated in many neurodegenerative disorders, such as Parkinson's disease, Alzheimer's disease, amyotrophic lateral sclerosis, Huntington's disease and stroke (Dawson and Dawson, 2003; Veurink et al., 2003; Malkus et al., 2009). Accumulating evidence has suggested that oxidative stress associated with excessive production of reactive oxygen species profound affects neurodegenerative pathogenesis (de Vries et al., 2008; Zolezzi et al., 2013; Newland et al., 2016). Reactive oxygen species can cause lipid peroxidation, protein denaturation and DNA/RNA damage (Ye et al., 2009; Collado et al., 2012; Zou et al., 2015). Reactive oxygen species induce several signal transduction pathways, including intrinsic and extrinsic caspase activation, which may lead to excessive cell apoptosis and expression of inflammatory genes (Chan, 2001; Allen and Bayraktutan, 2009). Oxidative stress triggers apoptosis through activation of many signaling molecules, including kinases and proteases (Tan et al., 1998; Andersen, 2004; Kaul et al., 2005). Hydrogen peroxide (H2O2) is used as a stressor to induce oxidative stress in experimental models (Brown et al., 2013; Sies, 2014) and to stimulate apoptotic and necrotic pathways (Clement et al., 1998).
Pharmaceutical compounds extracted from mushrooms showed benefits for a variety of conditions such as cancers, immunologic disorders and neurodegenerative diseases (Wang et al., 1997; Wasser and Weis, 1999; Cheung et al., 2000). Ganoderma lucidum (G. lucidum) belongs to the polyporaceae family of Basidiomycota, a type of mushroom widely used as a traditional medicine for thousands of years, especially in Asia (Ji et al., 2007). A variety of bioactive chemicals, such as polysaccharides, triterpenoids and proteins, can be extracted from the fruiting bodies, cultured mycelia and spores of G. lucidum (Mizushina et al., 1999). Clinical trials and other experimental studies indicated that the active compounds isolated from its fruiting body, known as “Lingzhi,” participate in a variety of biological processes, showing anti-inflammatory, antioxidant, anti-tumor and immunomodulatory activities (Lakshmi et al., 2003; Lin and Zhang, 2004; Zhao et al., 2012; Pan et al., 2013; Ferreira et al., 2015). G. lucidum polysaccharides (GLPS) were shown to be neuroprotective, increasing viability in cerebral cortical neurons exposed to ischemia/reperfusion and in models for traumatic spinal cord injury (Zhao et al., 2004; Gokce et al., 2015). This evidence indicated that GLPS is a potentially promising drug candidate. However, the roles of GLPS in modulating oxidative stress-induced neuronal apoptosis have been poorly understood. The aim of our study was to investigate whether GLPS would protect cultured cerebellar granule neurons from apoptosis induced by H2O2.
Materials and Methods
Cell culture
Rat cerebellar granule cells (CGCs) were prepared from 7 or 8-day-old Sprague-Dawley rat pups, as previously described (D’Mello et al., 1993). All experimental procedures were performed in accordance with the Guideline for the Care and Use of Laboratory Animals of the Animal Research Ethics Committee of Peking University Health Science Center (China) and under the principles and guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number and suffering of the animals used in the experiments.
Briefly, neurons were dissociated from freshly dissected cerebella by mechanical disruption in the presence of trypsin (Life Technologies, Carlsbad, CA, USA) and DNase (Life Technologies) and then seeded at a density of 1.5 × 106 cells/mL in basal modified Eagle's media (Life Technologies) containing 10% fetal bovine serum (Life Technologies) and potassium (Sigma-Aldrich, St. Louis, MO, USA) at concentrations causing membrane depolarization (25 mM KCl). The GLPS that was administered (0.5%, 2% or 5% (w/v); at 4, 8 or 12 hours) contained 56.9% carbohydrate and 32.45% protein and was from Lvgu Biotech (Fuzhou, China). After 7 days of culturing in vitro, CGCs were incubated in the presence or absence of 50 μM H2O2 (at 4, 8, 12 hours; Sigma-Aldrich), with or without GLPS, for the indicated time points. More details of specific treatments are in the figure legends. The control for both H2O2 and GLPS was water.
Western blot assay
Western blotting was performed as previously described (Yan et al., 2015). Briefly, neuronal lysates were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and protein bands electrophoretically transferred to a polyvinylidene difluoride membrane. Membranes were blocked in Tris-buffered saline with 5% milk and 0.05% Tween-20 and then probed with primary antibodies at 4°C overnight. The following primary antibodies were used: rabbit anti-cytochrome c, rabbit anti-cleaved caspase-3, mouse anti-Bax, rabbit anti-Bim, mouse anti-Bcl-2 and mouse anti-β-tubulin, each diluted 1:1,000 (all primary antibodies from Cell Signaling Technology, Danvers, MA, USA). Appropriate horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA) were used to detect reactive bands with the enhanced chemiluminescence (ECL) and ECL-plus systems (GE Healthcare, Chalfont St. Giles, UK). To quantify bands on western blots, the average intensity of the pixels in a background-selected region was calculated and subtracted from each pixel in the sample. To correct for deviations, densitometry values obtained within the linear range of detection were normalized to those for β-tubulin.
Quantification of neuronal apoptosis
CGC apoptosis was quantified as previously described (Linseman et al., 2004). In brief, CGCs were cultured in 24-well plates and incubated with various treatments. After incubation for the indicated times, CGCs were stained with the DNA dye Hoechst 33258 (Sigma, 5 μg/mL) to visualize nuclear morphology. Apoptosis was quantified by scoring the percentage of neuronal cells with condensed or fragmented nuclei. Neurons were counted from three randomly chosen fields per well, under a light microscope (BX51WI light microscope; Olympus, Tokyo, Japan). To obtain unbiased results, experiments were performed in a blinded manner and cells were scored by investigators without knowledge of their prior treatments. All experiments were repeated at least three times and over 500 neurons were counted for each treatment group.
Statistical analysis
Statistical analyses were performed using SPSS 16.0 software (SPSS, Chicago, IL, USA). All statistical data are expressed as the mean ± SEM of at least three independent experiments (n ≥ 3). The statistical significance of differences was analyzed using Student's t-test between two groups and one-way analysis of variance with Student-Newman-Keuls post hoc test for comparisons among more than two groups. A value of P < 0.05 was considered statistically significant.
Results
Oxidative stress significantly induced neuronal apoptosis in cultured CGCs
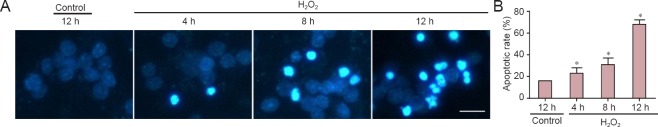
H2O2 is commonly used to induce cell apoptosis, secondary to free radicals (Medina et al., 2002). It is therefore considered a useful agent for generating models of oxidative stress (Chang et al., 2003). Accordingly, we used 50 μM H2O2 for an experimental apoptosis model. First, cultured neurons were treated with H2O2 for 4, 8 or 12 hours, then stained with Hoechst 33258 to view apoptotic cells. H2O2 administration markedly induced neuronal apoptosis, in a time-dependent manner. Apoptotic neurons were those with nuclear shrinkage, chromatin condensation or fragmentation (Figure 1A). Apoptosis began as early as 4 hours after H2O2 treatment and, 12 hours later, the apoptotic rate had reached nearly 70% (Figure 1B). Next, we investigated which proteins were activated during apoptosis. We found that cytosolic cytochrome c levels were increased, and caspase-3 was cleaved (Figure 2A). The statistical data are shown in Figure 2B. These data indicated that H2O2 activated the mitochondrial apoptotic pathway, involving BH3-only family proteins. BH3-only proteins are critical for neuronal apoptosis (Happo et al., 2012; Doerflinger et al., 2015), and can be divided into two classes: (1) pro-apoptotic proteins, such as Bax, Bad and Bim; and (2) anti-apoptotic proteins, such as Bcl-2 and Bcl-xl. As shown in Figure 2C, Bim and Bax levels were increased and those of Bcl-2 were decreased, all changing in a time-dependent manner. The statistical data are shown in Figure 2D. All findings suggested that H2O2 significantly induced neuronal apoptosis in CGCs.
Figure 1.
H2O2 induced apoptosis of cultured CGCs.
(A) Representative images of CGCs incubated in medium containing vehicle control or 50 μM H2O2, for the indicated times. Neurons were stained with Hoechst 33258 to visualize condensed nuclei Scale bar: 10 μm. (B) Apoptotic rates in CGCs were quantified by scoring the percentage of neurons with pyknotic nuclei. The data are expressed as the mean ± SEM (n = 3, analysis of variance and Student-Newman-Keuls post hoc test). *P < 0.05, vs. control. CGCs: Cerebellar granule cells; h: hours.
Figure 2.
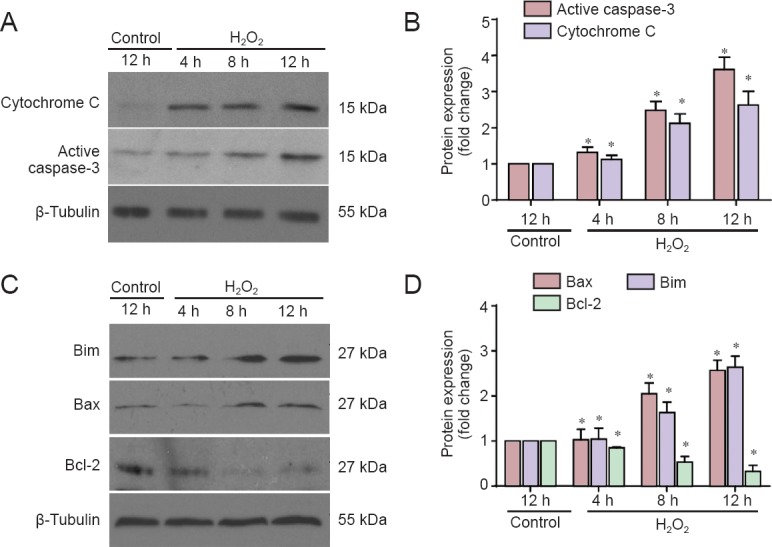
H2O2 treatment altered protein expression in cerebellar granule cells.
(A, C) Western blots for cytochrome c and active caspase-3 (cleaved caspase-3), Bim, Bax, Bcl-2. Cell lysates were subjected to western blotting with antibodies against cytochrome c and active caspase-3 (A) or Bim, Bax, and Bcl-2 (C). (B, D) Quantified grayscale intensities of the bands (A, C), relative to the β-tubulin band in the control group. Data are expressed as the mean ± SEM (n = 4, analysis of variance and Student-Newman-Keuls post hoc test). *P < 0.05, vs. control. h: Hours.
GLPS markedly suppressed oxidative stress-induced apoptosis
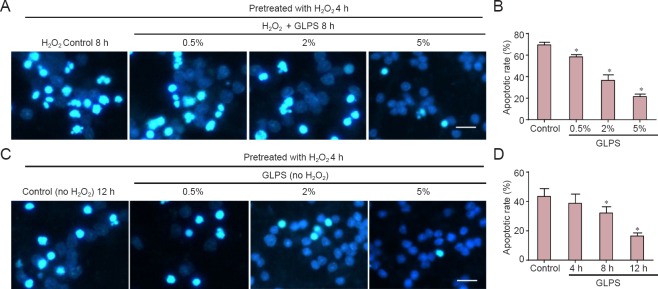
Compounds extracted from G. lucidum showed anti-cancer, antioxidant and liver protective effects. GLPS was neuroprotective against traumatic spinal cord injury in rats (Gokce et al., 2015). However, the role of GLPS in modulating oxidative stress-induced apoptosis in cultured cerebellar granule cells remains unknown. In this study, we found that apoptosis induced by H2O2 was suppressed in a dose-dependent manner by GLPS administration (Figure 3A). The statistical data are shown in Figure 3B. Neurons were pre-treated with H2O2 for 4 hours. Cells were then washed and incubated with GLPS for an additional 4, 8 or 12 hours. The results showed that cells underwent apoptosis, even at 12 hours after H2O2 administration. However, GLPS addition significantly protected neurons from apoptosis, in a time-dependent manner (Figure 3C). The statistical data are shown in Figure 3D. We then examined whether GLPS suppressed apoptosis through inhibition of the mitochondrial pathway. As shown in Figure 4A, levels of activated caspase-3, induced by H2O2, were suppressed by GLPS (Figure 4B). Furthermore, the increased levels of Bax and Bim were also attenuated and the decreased level of Bcl-2 was increased in H2O2 treated cells also receiving GLPS (Figure 4C and D). These results indicated that GLPS suppressed H2O2-induced apoptosis.
Figure 3.
GLPS administration suppressed H2O2-induced apoptosis of cerebellar granule cells (light microscopy).
(A) Neurons were pretreated with H2O2 for 4 h. Control vehicle or GLPS at different concentrations (0.5%, 2% and 5% (w/v)) was added, together with H2O2, for an additional 8 hours. Representative images are shown. Scale bar: 10 μm. (B, D) Quantification of apoptotic rates. (C) Neurons were pretreated with H2O2 for 4 h, then cells were washed with culture medium and incubated with 5% (w/v) GLPS or control vehicle, without H2O2, for the indicated times. Representative images are shown. Scale bar: 10 μm. Data are expressed as the mean ± SEM (n = 3, analysis of variance and Student-Newman-Keuls post hoc test). *P < 0.05, vs. control. GLPS: Ganoderma lucidum polysaccharides; h: hours.
Figure 4.
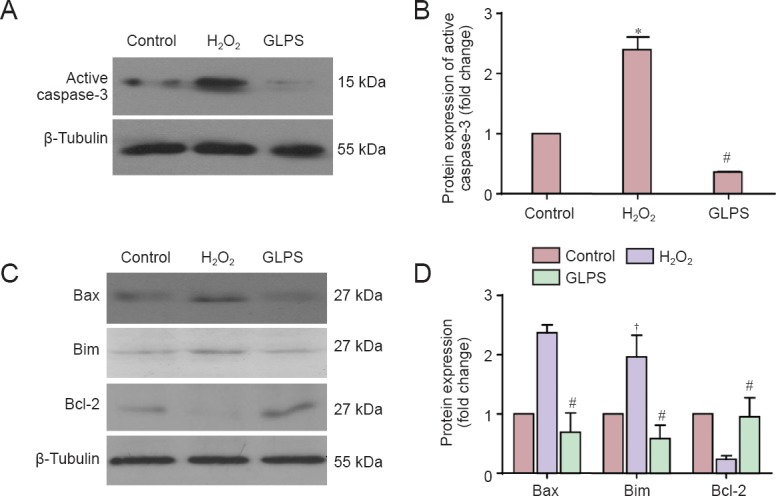
GLPS administration regulated H2O2-induced protein alterations in cerebellar granule cells.
Cerebellar granule cells were treated with vehicle without H2O2 (control group), H2O2 for 8 hours (H2O2 group) or H2O2 together with 5% GLPS for 8 hours (GLPS group). Cell lysates were analyzed by western blotting with antibodies against active caspase-3 (A), Bim, Bax, and Bcl-2 (C). Quantified grayscale intensities (B and D) of bands, relative to that for β-tubulin in the control group, are shown as the mean ± SEM (n = 4). *P < 0.05, vs. control and GLPS groups; †P < 0.05, vs. Bax and Bcl-2 (analysis of variance and Student-Newman-Keuls post hoc test); #P < 0.05, vs. H2O2 group (Student's t-test). GLPS, Ganoderma lucidum polysaccharides.
Discussion
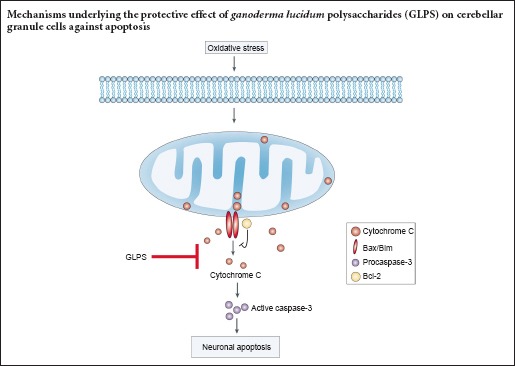
In this study, we demonstrated oxidative stress-induced apoptosis of cultured cerebellar granule cells. H2O2 increased cleavage and, therefore, activation of caspase-3, cytochrome c release, upregulation of the pro-apoptotic proteins Bax and Bim and downregulation of the anti-apoptotic protein Bcl-2, ultimately causing apoptosis in CGCs. GLPS administration significantly suppressed these processes, thus inhibiting H2O2-induced neuronal apoptosis. This elucidation of the neuroprotective mechanisms of GLPS may contribute to clinical use of active compounds isolated from G. lucidum.
G. lucidum has been used as a preventive medicine in Asia for thousands of years (Shiao, 2003). Polysaccharides, isolated from G. lucidum fruiting bodies, have antioxidant (Liu et al., 2010), immunomodulatory (Bao et al., 2001) and antitumor properties (Cao and Lin, 2006). Moreover, polysaccharides were protective against cerebral ischemic injury (Zhou et al., 2010) and traumatic spinal cord injury in rats (Gokce et al., 2015). GLPS induced neuronal differentiation of pheochromocytoma cell cultures and protected PC12 neurons from apoptosis, by the Erk1/2 and the CREB signaling pathways (Cheung et al., 2000). G. lucidum extracts decreased inflammatory mediator production by activated microglia and protected dopaminergic neurons against inflammatory and oxidative damage (Zhang et al., 2011). Furthermore, G. lucidum spores preserved injured spinal motor neurons by modulating expression of proteins important for axonal regeneration (Zhang et al., 2006). These findings suggested that polysaccharides isolated from G. lucidum had both neuroprotective and antioxidant properties. To the best of our knowledge, ours is the first report on the neuroprotective effects of GLPS against oxidative stress-induced apoptosis in cultured cerebellar granule cells.
H2O2 induced apoptosis in neuronal and non-neuronal cells, an effect associated with its cytotoxicity (Mailly et al., 1999; Kumar et al., 2001; Chang et al., 2003). During the late stages of apoptosis, DNA fragmentation occurs, following reactive oxygen species generation, caspase-3 activation and mitochondrial dysfunction (Yang et al., 2004). Moreover, oxidative stress-induced neurotoxicity involves a mitochondria dependent apoptotic pathway, including cytochrome c release, caspase-3 activation and changes in the Bax/Bcl-2 ratio (Brune et al., 2003; Cunha-Oliveira et al., 2007; Lai et al., 2011; Radi et al., 2014), all effects demonstrated in our study. Bcl-2 can counteract the pro-apoptotic effect of Bax/Bim by forming a heterodimer (Kobayashi et al., 1998). During apoptosis, increased Bax translocates to the mitochondria, leading to decreased mitochondrial membrane potential (Linseman et al., 2004; Cunha-Oliveira et al., 2006). Bim forms heterodimers with Bcl-2, releasing Bax from Bcl-2, thus enabling its mitochondrial translocation (Letai et al., 2002) and Bim also directly activates Bax for apoptosis (Gavathiotis et al., 2008; Du et al., 2011). Our results showed that GLPS addition markedly suppressed all signal transduction processes induced by oxidative stress. That is, GLPS inhibited caspase-3 activation, suppressed Bax/Bim upregulation and prevented Bcl-2 downregulation, thus ultimately preventing H2O2-induced apoptosis in CGCs. Among the BH-3-only pro-apoptotic proteins, Bid, Noxa and Puma were reported to mediate apoptosis (Ren et al., 2010). The regulation of these proteins by GLPS, also protecting against oxidative stress-induced neuronal apoptosis, should be investigated in the future.
In summary, our study characterized protection by GLPS against oxidative stress-induced neurotoxicity, contributing new insights into the neuroprotective mechanisms of G. lucidum. These findings provided potential new evidence supporting clinical use of G. lucidum to treat neurodegenerative diseases involving oxidative stress.
Footnotes
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by the Ethics Committee of Peking University Health Science Center, China. The experimental procedure followed the the United States National Institutes of Health Guide for the Care and Use of Laboratory Animal (NIH Publication No. 85-23, revised 1986), and “Consensus Author Guidelines on Animal Ethics and Welfare” produced by the International Association for Veterinary Editors (IAVE). All efforts were made to minimize the number and suffering of the animals used in the experiments. The article was prepared in accordance with the “Animal Research: Reporting of In Vivo Experiments Guidelines” (ARRIVE Guidelines).
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check: This paper has been checked twice with duplication-checking software iThenticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Copyedited by Doctrow SR, Pack M, Wang J, Li CH, Qiu Y, Song LP, Zhao M
References
- Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J stroke. 2009;4:461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- Bao X, Liu C, Fang J, Li X. Structural and immunological studies of a major polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Carbohydr Res. 2001;332:67–74. doi: 10.1016/s0008-6215(01)00075-1. [DOI] [PubMed] [Google Scholar]
- Brown JD, Day AM, Taylor SR, Tomalin LE, Morgan BA, Veal EA. A peroxiredoxin promotes H2O2 signaling and oxidative stress resistance by oxidizing a thioredoxin family protein. Cell Rep. 2013;5:1425–1435. doi: 10.1016/j.celrep.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune B, Zhou J, von Knethen A. Nitric oxide, oxidative stress, and apoptosis. Kidney Int. 2003:S22–24. doi: 10.1046/j.1523-1755.63.s84.6.x. [DOI] [PubMed] [Google Scholar]
- Cao QZ, Lin ZB. Ganoderma lucidum polysaccharides peptide inhibits the growth of vascular endothelial cell and the induction of VEGF in human lung cancer cell. Life Sci. 2006;78:1457–1463. doi: 10.1016/j.lfs.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chang H, Oehrl W, Elsner P, Thiele JJ. The role of H2O2 as a mediator of UVB-induced apoptosis in keratinocytes. Free Radic Res. 2003;37:655–663. doi: 10.1080/1071576031000094907. [DOI] [PubMed] [Google Scholar]
- Cheung WM, Hui WS, Chu PW, Chiu SW, Ip NY. Ganoderma extract activates MAP kinases and induces the neuronal differentiation of rat pheochromocytoma PC12 cells. FEBS Lett. 2000;486:291–296. doi: 10.1016/s0014-5793(00)02317-6. [DOI] [PubMed] [Google Scholar]
- Clement MV, Ponton A, Pervaiz S. Apoptosis induced by hydrogen peroxide is mediated by decreased superoxide anion concentration and reduction of intracellular milieu. FEBS Lett. 1998;440:13–18. doi: 10.1016/s0014-5793(98)01410-0. [DOI] [PubMed] [Google Scholar]
- Collado R, Oliver I, Tormos C, Egea M, Miguel A, Cerda C, Ivars D, Borrego S, Carbonell F, Saez GT. Early ROS-mediated DNA damage and oxidative stress biomarkers in Monoclonal B Lymphocytosis. Cancer Lett. 2012;317:144–149. doi: 10.1016/j.canlet.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Cunha-Oliveira T, Rego AC, Garrido J, Borges F, Macedo T, Oliveira CR. Street heroin induces mitochondrial dysfunction and apoptosis in rat cortical neurons. J Neurochem. 2007;101:543–554. doi: 10.1111/j.1471-4159.2006.04406.x. [DOI] [PubMed] [Google Scholar]
- Cunha-Oliveira T, Rego AC, Cardoso SM, Borges F, Swerdlow RH, Macedo T, de Oliveira CR. Mitochondrial dysfunction and caspase activation in rat cortical neurons treated with cocaine or amphetamine. Brain Res. 2006;1089:44–54. doi: 10.1016/j.brainres.2006.03.061. [DOI] [PubMed] [Google Scholar]
- D’Mello SR, Galli C, Ciotti T, Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci U S A. 1993;90:10989–10993. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J, van Horssen J. Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med. 2008;45:1375–1383. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Doerflinger M, Glab JA, Puthalakath H. BH3-only proteins: a 20-year stock-take. FEBS J. 2015;282:1006–1016. doi: 10.1111/febs.13190. [DOI] [PubMed] [Google Scholar]
- Du H, Wolf J, Schafer B, Moldoveanu T, Chipuk JE, Kuwana T. BH3 domains other than Bim and Bid can directly activate Bax/Bak. J Biol Chem. 2011;286:491–501. doi: 10.1074/jbc.M110.167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira IC, Heleno SA, Reis FS, Stojkovic D, Queiroz MJ, Vasconcelos MH, Sokovic M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry. 2015;114:38–55. doi: 10.1016/j.phytochem.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, Walensky LD. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokce EC, Kahveci R, Atanur OM, Gurer B, Aksoy N, Gokce A, Sargon MF, Cemil B, Erdogan B, Kahveci O. Neuroprotective effects of Ganoderma lucidum polysaccharides against traumatic spinal cord injury in rats. Injury. 2015;46:2146–2155. doi: 10.1016/j.injury.2015.08.017. [DOI] [PubMed] [Google Scholar]
- Happo L, Strasser A, Cory S. BH3-only proteins in apoptosis at a glance. Journal of cell science. 2012;125:1081–1087. doi: 10.1242/jcs.090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z, Tang Q, Zhang J, Yang Y, Jia W, Pan Y. Immunomodulation of RAW264. 7 macrophages by GLIS, a proteopolysaccharide from Ganoderma lucidum. J Ethnopharmacol. 2007;112:445–450. doi: 10.1016/j.jep.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Kaul S, Anantharam V, Yang Y, Choi CJ, Kanthasamy A, Kanthasamy AG. Tyrosine phosphorylation regulates the proteolytic activation of protein kinase Cdelta in dopaminergic neuronal cells. J Biol Chem. 2005;280:28721–28730. doi: 10.1074/jbc.M501092200. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ruan S, Clodi K, Kliche KO, Shiku H, Andreeff M, Zhang W. Overexpression of Bax gene sensitizes K562 erythroleukemia cells to apoptosis induced by selective chemotherapeutic agents. Oncogene. 1998;16:1587–1591. doi: 10.1038/sj.onc.1201681. [DOI] [PubMed] [Google Scholar]
- Kumar S, Bharti A, Mishra NC, Raina D, Kharbanda S, Saxena S, Kufe D. Targeting of the c-Abl tyrosine kinase to mitochondria in the necrotic cell death response to oxidative stress. J Biol Chem. 2001;276:17281–17285. doi: 10.1074/jbc.M101414200. [DOI] [PubMed] [Google Scholar]
- Lai B, Pu H, Cao Q, Jing H, Liu X. Activation of caspase-3 and c-Jun NH2-terminal kinase signaling pathways involving heroin-induced neuronal apoptosis. Neurosci Lett. 2011;502:209–213. doi: 10.1016/j.neulet.2011.07.046. [DOI] [PubMed] [Google Scholar]
- Lakshmi B, Ajith TA, Sheena N, Gunapalan N, Janardhanan KK. Antiperoxidative, anti-inflammatory, and antimutagenic activities of ethanol extract of the mycelium of Ganoderma lucidum occurring in South India. Teratog Carcinog Mutagen. 2003;1:85–97. doi: 10.1002/tcm.10065. [DOI] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Lin ZB, Zhang HN. Anti-tumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta Pharmacol Sinica. 2004;25:1387–1395. [PubMed] [Google Scholar]
- Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA, Bouchard RJ, Florez-McClure ML, Heidenreich KA. Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci. 2004;24:9993–10002. doi: 10.1523/JNEUROSCI.2057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wang H, Pang X, Yao W, Gao X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int J Biol Macromol. 2010;46:451–457. doi: 10.1016/j.ijbiomac.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Mailly F, Marin P, Israel M, Glowinski J, Premont J. Increase in external glutamate and NMDA receptor activation contribute to H2O2-induced neuronal apoptosis. J Neurochem. 1999;73:1181–1188. doi: 10.1046/j.1471-4159.1999.0731181.x. [DOI] [PubMed] [Google Scholar]
- Malkus KA, Tsika E, Ischiropoulos H. Oxidative modifications, mitochondrial dysfunction, and impaired protein degradation in Parkinson's disease: how neurons are lost in the Bermuda triangle. Mol Neurodegener. 2009;4:24. doi: 10.1186/1750-1326-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina S, Martinez M, Hernanz A. Antioxidants inhibit the human cortical neuron apoptosis induced by hydrogen peroxide, tumor necrosis factor alpha, dopamine and beta-amyloid peptide 1-42. Free Radic Res. 2002;36:1179–1184. doi: 10.1080/107157602100006445. [DOI] [PubMed] [Google Scholar]
- Mizushina Y, Takahashi N, Hanashima L, Koshino H, Esumi Y, Uzawa J, Sugawara F, Sakaguchi K. Lucidenic acid O and lactone, new terpene inhibitors of eukaryotic DNA polymerases from a basidiomycete, Ganoderma lucidum. Bioorg Med Chem. 1999;7:2047–2052. doi: 10.1016/s0968-0896(99)00121-2. [DOI] [PubMed] [Google Scholar]
- Newland B, Wolff P, Zhou D, Wang W, Zhang H, Rosser A, Wang W, Werner C. Synthesis of ROS scavenging microspheres from a dopamine containing poly(beta-amino ester) for applications for neurodegenerative disorders. Biomater Sci. 2016;4:400–404. doi: 10.1039/c5bm00542f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan K, Jiang Q, Liu G, Miao X, Zhong D. Optimization extraction of Ganoderma lucidum polysaccharides and its immunity and antioxidant activities. Int J Biol Macromol. 2013;55:301–306. doi: 10.1016/j.ijbiomac.2013.01.022. [DOI] [PubMed] [Google Scholar]
- Radi E, Formichi P, Battisti C, Federico A. Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheimers Dis. 2014;42(Suppl 3):S125–152. doi: 10.3233/JAD-132738. [DOI] [PubMed] [Google Scholar]
- Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiao MS. Natural products of the medicinal fungus Ganoderma lucidum: occurrence, biological activities, and pharmacological functions. Chem Rec. 2003;3:172–180. doi: 10.1002/tcr.10058. [DOI] [PubMed] [Google Scholar]
- Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem. 2014;289:8735–8741. doi: 10.1074/jbc.R113.544635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Wood M, Maher P. Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells. J Neurochem. 1998;71:95–105. doi: 10.1046/j.1471-4159.1998.71010095.x. [DOI] [PubMed] [Google Scholar]
- Veurink G, Fuller SJ, Atwood CS, Martins RN. Genetics, lifestyle and the roles of amyloid beta and oxidative stress in Alzheimer's disease. Ann human Biol. 2003;30:639–667. doi: 10.1080/03014460310001620144. [DOI] [PubMed] [Google Scholar]
- Wang SY, Hsu ML, Hsu HC, Tzeng CH, Lee SS, Shiao MS, Ho CK. The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int J Cancer. 1997;70:699–705. doi: 10.1002/(sici)1097-0215(19970317)70:6<699::aid-ijc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Wasser SP, Weis AL. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: a modern perspective. Crit Rev Immunol. 1999;19:65–96. [PubMed] [Google Scholar]
- Yan YY, Wang XM, Jiang Y, Chen H, He JT, Mang J, Shao YK, Xu ZX. The role of Rho/Rho-kinase pathway and the neuroprotective effects of fasudil in chronic cerebral ischemia. Neural Regen Res. 2015;10:1441–1449. doi: 10.4103/1673-5374.165512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Kaul S, Zhang D, Anantharam V, Kanthasamy AG. Suppression of caspase-3-dependent proteolytic activation of protein kinase C delta by small interfering RNA prevents MPP+-induced dopaminergic degeneration. Mol Cell Neurosci. 2004;25:406–421. doi: 10.1016/j.mcn.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Ye J, Han Y, Wang C, Yu W. Cytoprotective effect of polypeptide from Chlamys farreri on neuroblastoma (SH-SY5Y) cells following HO exposure involves scavenging ROS and inhibition JNK phosphorylation. J Neurochem. 2009;111:441–451. doi: 10.1111/j.1471-4159.2009.06328.x. [DOI] [PubMed] [Google Scholar]
- Zhang R, Xu S, Cai Y, Zhou M, Zuo X, Chan P. Ganoderma lucidum Protects Dopaminergic Neuron Degeneration through Inhibition of Microglial Activation. Evid Based Complement Alternat Med 2011. 2011:156810. doi: 10.1093/ecam/nep075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zeng YS, Wang Y, Liu W, Cheng JJ, Chen SJ. [Primary study on proteomics about Ganoderma lucidium spores promoting survival and axon regeneration of injured spinal motor neurons in rats. Zhongxiyi Jiehe Xuebao. 2006;4:298–302. doi: 10.3736/jcim20060316. [DOI] [PubMed] [Google Scholar]
- Zhao HB, Lin SQ, Liu JH, Lin ZB. Polysaccharide extract isolated from ganoderma lucidum protects rat cerebral cortical neurons from hypoxia/reoxygenation injury. J Pharmacol Sci. 2004;95:294–298. doi: 10.1254/jphs.sc0040011. [DOI] [PubMed] [Google Scholar]
- Zhao W, Jiang X, Deng W, Lai Y, Wu M, Zhang Z. Antioxidant activities of Ganoderma lucidum polysaccharides and their role on DNA damage in mice induced by cobalt-60 gamma-irradiation. Food Chem Toxicol. 2012;50:303–309. doi: 10.1016/j.fct.2011.10.071. [DOI] [PubMed] [Google Scholar]
- Zhou ZY, Tang YP, Xiang J, Wua P, Jin HM, Wang Z, Mori M, Cai DF. Neuroprotective effects of water-soluble Ganoderma lucidum polysaccharides on cerebral ischemic injury in rats. J Ethnopharmacol. 2010;131:154–164. doi: 10.1016/j.jep.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Zolezzi JM, Silva-Alvarez C, Ordenes D, Godoy JA, Carvajal FJ, Santos MJ, Inestrosa NC. Peroxisome proliferator-activated receptor (PPAR) gamma and PPARalpha agonists modulate mitochondrial fusion-fission dynamics: relevance to reactive oxygen species (ROS)-related neurodegenerative disorders? PLoS One. 2013;8:e64019. doi: 10.1371/journal.pone.0064019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Li YJ, Yang S, Zhang QW. Knowledge base, research front and hot spot analysis of Cu-Zn superoxide dismutase. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;19:6861–6867. [Google Scholar]