Abstract
Our recent findings have demonstrated that rodent models of closed head traumatic brain injury exhibit comprehensive evidence of progressive and enduring orofacial allodynias, a hypersensitive pain response induced by non-painful stimulation. These allodynias, tested using thermal hyperalgesia, correlated with changes in several known pain signaling receptors and molecules along the trigeminal pain pathway, especially in the trigeminal nucleus caudalis. This study focused to extend our previous work to investigate the changes in monoamine neurotransmitter immunoreactivity changes in spinal trigeminal nucleus oralis, pars interpolaris and nucleus tractus solitaries following mild to moderate closed head traumatic brain injury, which are related to tactile allodynia, touch-pressure sensitivity, and visceral pain. Our results exhibited significant alterations in the excitatory monoamine, serotonin, in spinal trigeminal nucleus oralis and pars interpolaris which usually modulate tactile and mechanical sensitivity in addition to the thermal sensitivity. Moreover, we also detected a robust alteration in the expression of serotonin, and inhibitory molecule norepinephrine in the nucleus tractus solitaries, which might indicate the possibility of an alteration in visceral pain, and existence of other morbidities related to solitary nucleus dysfunction in this rodent model of mild to moderate closed head traumatic brain injury. Collectively, widespread changes in monoamine neurotransmitter may be related to orofacial allodynhias and headache after traumatic brain injury.
Keywords: nerve regeneration, mild to moderate traumatic brain injury, trigeminal sensory system, neuromodulators, facial and somatic allodynia, thermal hyperalgesia, headache, migraine, neural regeneration
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability affecting more than 10 million people globally every year (Hyder et al., 2007). A large number of TBI patients undergo mild to moderate TBI (mTBI) which is often unattended clinically. mTBI causes multiple morbidities and neurological sequela including long-term sensory disabilities. Post-traumatic headache (PTH) is one of the most common and persisting sensory disabilities following TBI (Hoffman et al., 2007, 2011; Hyder et al., 2007; Theeler et al., 2013). Many patients with PTH and migraine also develop increased facial sensitization called facial allodynia (Burstein et al., 2010). The pathophysiology of this PTH or associated facial allodynia is unknown. However, the functional comorbidity of PTH and facial allodynia is consistent with a systemic sensitization affecting the significant anatomical viscerosomatic convergence of the trigeminal vascular afferents and facial cutaneous afferents within the trigeminal sensory system (Burstein et al., 2000; Sandkuhler, 2009; Sokolov et al., 2012; Noseda and Burstein, 2013). The cutaneous allodynia of extra cephalic regions seen in migraine and cluster headache patients (Edelmayer et al., 2009) often includes sensitization to thermal, touch, and pressure modalities.
A significant alteration in facial thermosensitivity/allodynia has been reported recently by us in this rodent model of closed head traumatic brain injury (cTBI) based on an operant avoidance behavior of reward/conflict to a noxious challenge temperature (Mustafa et al., 2016). We reported that cTBI causes significant loss of noradrenergic cells of the locus coeruleus (Bose et al., 2013; Tsuda et al., 2016) and noradrenergic fiber densities in the locus coeruleus, periaqueductal gray, and medial division of central nucleus of amygdala (Tsuda et al., 2016). Physiologically, the noradrenergic inputs are vasoconstrictive through alpha-adrenergic receptor and vasodilator through beta-adrenergic receptor and are essential for maintaining the vasculature tone during normal cerebral blood flow. Accordingly, injury-associated alterations in the multiple roles of noradrenergic input, including modulation of sensory transmission, vascular tone regulation, and upregulation of vasodilators, contribute to PTH development (Figure 1). Based on previous findings from ours (Bose et al., 2013; Mustafa et al., 2016; Tsuda et al., 2016) and others (Uomoto and Esselman, 1993; Ofek and Defrin, 2007; Oshinsky and Gomonchareonsiri, 2007; Edelmayer et al., 2009), we predict that a widespread neurochemical alteration in the trigeminal sensory and associated nuclei accounts for post TBI sensory disabilities that include facial allodynia, headache, and central pain (Figure 2). In this article, we extend our recent findings regarding the alteration of neuromodulators in the trigeminal sensory system after mTBI. Here, we have focused on monoamine neurotransmitter immunoreactivity changes in spinal trigeminal nucleus oralis (Sp5O), pars interpolaris (Sp5I) and nucleus tractus solitaries (NTS) implicated in the pathophysiology of allodynia and headache after mTBI.
Figure 1.
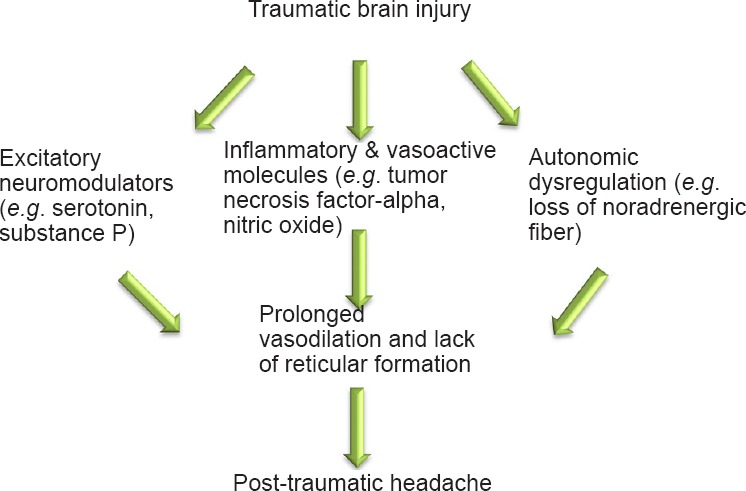
Possible neurological sequelae following traumatic brain injury leading to the development of post-traumatic headache.
Both trigeminal sensory system and trigeminal vascular system have potential to be affected by the inflammatory molecules and altered expression of excitatory and inhibitory neuromodulators.
Figure 2.
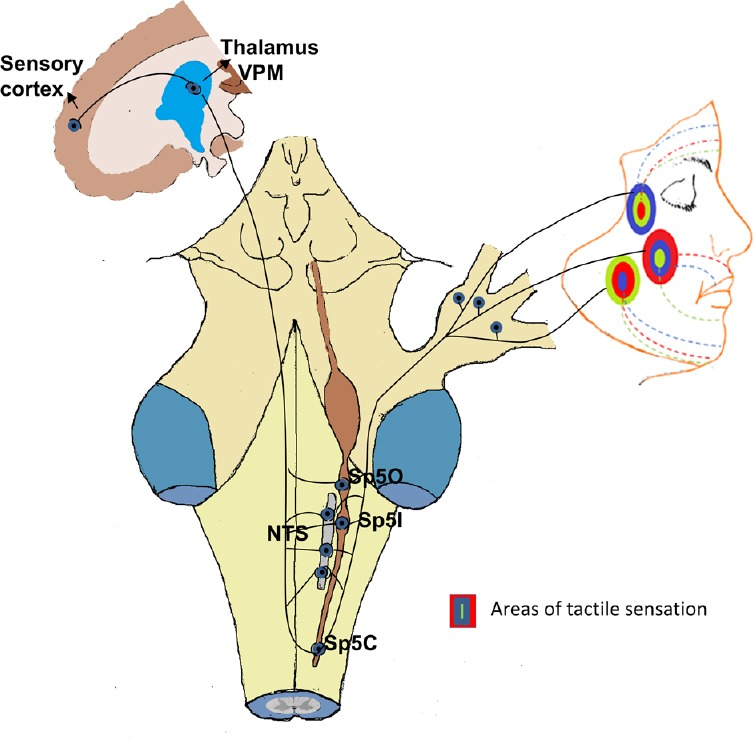
Diagram showing the pathways of neuronal connection between various trigeminal subnuclei [spinal trigeminal nucleus oralis (Sp5O), pars interpolaris (Sp5I), pars caudalis (Sp5C) and nucleus tractus solitaries (NTS)], thalamus and sensory cortex.
The facial areas with different colors show touch (green), pressure (blue) and temperature (red) related allodynia following traumatic brain injury.
Materials and Methods
Animals
Sprauge-Dawley specific pathogen free (SPF) female rats (12 weeks old, weighing 240–270 g at the start of this study; Charles River Laboratory, USA) were used in this study. All procedures were performed in accordance with the U.S Government Principle for the Utilization and Care of Vertebrate Animals, specifically following the National Institutes of Health “Guide for the Care and Use of Laboratory Animals”. Experimental protocols were approved by the Institutional Animal Care & Use Committee (IACUC) at the North Florida/South Georgia Veterans Health System, and the University of Florida, USA.
Surgical approach to create closed head mTBI
For producing closed head mTBI, a standardized 1.25 m/450 g weight-drop method (Marmarou model, Marmarou et al., 1994b) was used using the procedures as previously described in the original reports (Foda and Marmarou, 1994; Marmarou et al., 1994a) and in our recently published reports (Bose et al., 2013; Mustafa et al., 2016; Tsuda et al., 2016).
Tissue collection for immunohistochemistry
Brain tissues were collected 2 weeks following injury, and thus considered as acute. Briefly the animals were transcardially perfused with phosphate-buffered saline (PBS; 0.01 M, pH 7.4), followed by 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4, 4°C) for immunohistochemistry. Briefly, the brain was dissected and post-fixed with fresh 4% paraformaldehyde for 24–48 hours. The whole brain was placed into 30% sucrose for 3 days and then cut serially into 40-μm-thick sections rostrocaudally by Cryostat (Leica CM 1850, Leica Biosystems, Bannockburn, IL, USA) approximately from Bregma: –10.68 mm to –11.04 mm for Sp5O; –12.84 mm to –13.20 mm for Sp5I, and –14.40 mm to –14.76 mm for NTS according to rat stereotaxic atlas of Paxinos and Watson (Watson, 2009).
Immunohistochemical analysis
A total of five sets of serial sections (approximately 80 μm apart from one another) for every specified region (SP50, SP5I and NTS) were collected from each animal (3 normal and 3 mTBI). The sections were incubated with three antibodies for 24 hours at 4°C: rabbit anti-serotonin (5-HT) (1:6,000), mouse anti-dopamine beta-hydroxylase (DβH) for norepinephrine (1:6,000), and rabbit anti-neurokinin 1 receptor (NK1R) (1;1,000). After washing with PBS, sections were then incubated with secondary antibodies (Alexa Fluor 594 goat anti-rabbit immunoglobulin G [IgG], 1:2,000 for 5-HT and NK1R; Alexa Fluor 488 goat anti-mouse IgG, 1: 2,000 for DβH), processed and mounted using procedure as described earlier (Watson, 2009; Bose et al., 2013; Mustafa et al., 2016). For microscopic observation, slides were viewed using a Zeiss Confocal microscope (LSM 710) with Zen software (version 2012; Carl Zeiss, Jena, Germany). The number of immunopositive cells (NK1R) and fibers (5-HT, DβH) in every 3rd equally spaced serial sections for 4 sections was counted in an investigator blinded semi- quantitative approach by using the petrimetric (sine-wave) probe to quantify immunoreactive cells and fibers as the procedure described earlier (Ducros and Wolff, 2016) in Sp5O, Sp5I and NTS. The procedure for acquiring images, and unbiased stereological analysis were the same as we previously reported or reported by others (West and Gundersen, 1990; Bose et al., 2005; Bernstein et al., 2011; Bernstein and O’Malley, 2013; Hou et al., 2014; Mustafa et al., 2016).
Results
Neurochemical alteration in trigeminal subnuclei
In both Sp5O and Sp5l, increased immunoreactive 5-HT expression was observed in closed head mTBI rats than in age- and sex-matched normal control rats (Figure 3A–C). The expression levels of DβH (a surrogate marker for norepinephrine) and NK1R were similar in both closed head mTBI rats and normal control rats (Figure 4A, B). However, the NK1R expression in Sp5O was greater in closed head mTBI rats than in the normal control rats (Figure 4A).
Figure 3.
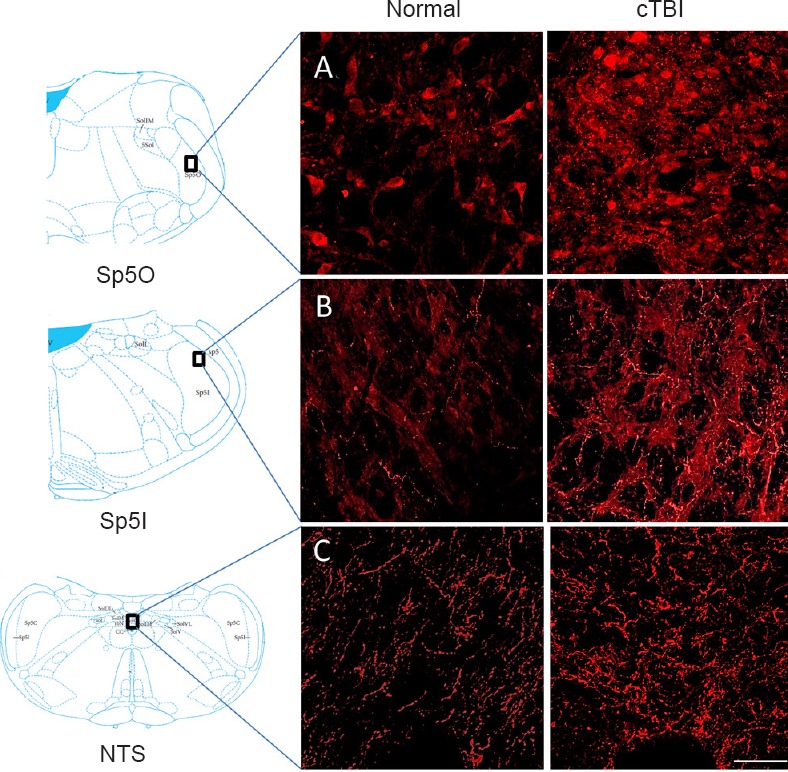
Immunofluorescence staining for 5-HT in the trigeminal subnuclei Sp5O (A), Sp5I (B) and NTS (C).
Mild to moderate cTBI altered the expression of 5-HT (red) in Sp5O (–10.84 mm to Bregma), Sp5I (–13.00 mm to Bregma) and NTS (–14.56 mm to Bregma) compared to the corresponding areas of the normal control animals. Scale bar: 50 μm. The position of Bregma was decided according to the rat brain atlas (Paxinos and Watson, 2007). 5-HT: Serotonin; Sp5O: spinal trigeminal nucleus oralis; Sp5I: spinal trigeminal pars interpolaris; NTS: nucleus tractus solitaries; cTBI: closed head traumatic brain injury.
Figure 4.
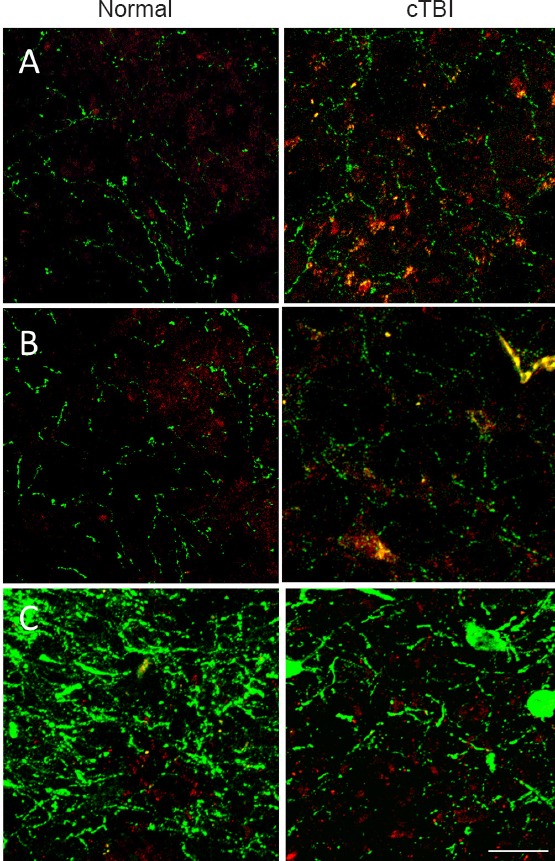
Immunofluorescence labeling of the trigeminal subnuclei Sp5O (A), Sp5I (B) and NTS (C).
Mild to moderate cTBI altered the immunoreactivity of DβH (green) and the expression of NK1R (red) in Sp5O (–10.88 mm to Bregma), Sp5I (–13.04 mm to Bregma) and NTS (–14.60 mm to Bregma) compared to the corresponding areas of control animals. Scale bar: 50 μm in all figures. The position of Bregma was decided according to the rat brain atlas (Paxinos and Watson, 2007). cTBI: Closed head traumatic brain injury; DβH: dopamine beta hydroxylase; NK1R: neurokinin 1 receptor; Sp5O: spinal trigeminal nucleus oralis; Sp5I: spinal trigeminal pars interpolaris; NTS: nucleus tractus solitaries.
Neuromodulatory changes in the NTS
Following TBI, significant changes in the expression of 5-HT and the number of DβH immunoreactive fibers were observed in the caudal NTS. The expression of 5-HT was increased, and DβH immunoreactivity was decreased, in tissues from TBI animals than in normal tissues (Figure 3C, Figure 4C). NK1R expression was similar between closed head mTBI and normal control rats (Figure 4C).
Discussion
mTBI-caused diffuse axonal injury (Bose et al., 2012) results in widespread neurological sequelae in human and experimental animal models. PTH and cephalgic syndrome after TBI may be viewed as expression of surrogate symptomatic markers such as facial allodynia, tactile allodynia, and disturbance in circadian rhythm. PTH is very common in mTBI, so we used an mTBI animal model in our study. However, there are some controversies regarding mTBI models since the pioneer investigators (Marmarou and colleagues) and subsequent users of the Marmarou's closed head injury model compared brain injuries using intensities sustained by 450 g weight drop from 1 m to 2 m; designations: 1 m: mild; 1.5 m: moderate; 2.0 m: severe. This classification was based upon, a) histological changes and b) neurologic deficits (Foda and Marmarou, 1994; Marmarou et al., 1994). However, a more comprehensive characterization of pre-clinical injury severity is needed so that the terms mild to moderate injury models will have more documented relevance to human TBI, for which duration of loss of consciousness and absence of detectable anatomical injury are standard criteria for mTBI. In this regard, it is worth noting that for the original closed head Marmarou TBI model, 1.25 m × 450 g weight drop was not an injury parameter in the original Marmarou report. The 1.25 m/450 g injury parameter was included in our studies to produce an injury that would potentially induce more detectable and enduring pain-like behavior within the setting of a mTBI model. Since this mTBI model exhibits injury patterns and detectable symptoms in pain-like behavior consistent with human mTBI, we extended our recently published work to provide better understanding in the possible pathophysiology of mTBI-induced pain and headache in this report.
We recently reported that several key neuromodulators related to pain perception were observed to be altered in the Sp5C following closed head mTBI (Mustafa et al., 2016). In the present study, we extended our investigation of the extent of neurochemical alteration in the trigeminal nucleus to include the subnuclei, Sp5O and Sp5I. It is known that caudal part of Sp5C receives afferents that transmit pain and temperature from the orofacial region. Whereas, the more rostral Sp5O and Sp5I are involved in the transmission of tactile sensitivity that would be more closely related to tactile allodynia (Sessle et al., 1986). The upregulation of 5-HT in Sp5O and Sp5I subnuclei in the current study is similar to what was observed in the nucleus caudalis in a study from Mustafa et al. (2016). Accordingly, this altered expression of 5-HT may relate to tactile allodynia in the orofacial region. Therefore, these observations suggest that comorbidity of visceral (PTH) and somatic sensitization (facial allodynia) is consistent with TBI-induced neurochemical alterations in both the caudal and more rostral trigeminal subnuclei. The changes in 5-HT expession in these two trigeminal subnuclei may be directly related to the physiological alteration in touch and pressure sensitivity (allodynia) in these closed head mTBI animals. This finding may be related to the changes in touch and pressure sensitivity in the periorbital region of the TBI patients as reported in several case reports (Hines, 1999; Walker et al. 2005; Ofek and Defrin, 2007).
Although the exact mechanism of PTH is unknown, the TBI-associated changes that lead to sensitization of trigeminal system to both vascular afferents (headache) and cutaneous afferents (facial allodynia) are promising candidates for the headache/allodynia related pain. Headache has been considered as a part of a systemic sensitization that includes facial allodynia (Ofek and Defrin, 2007; Bernstein and Burstein, 2012; Defrin, 2014). Accordingly, it is becoming progressively evident that headache (trigeminovascular) and facial allodynia (trigeminosomatic) are companion derivatives of sensitization at multiple levels of the trigeminal system (Ofek and Defrin, 2007; Bernstein and Burstein, 2012; Defrin, 2014). Results from this work exhibited significant alterations in the excitatory molecule, 5-HT, in the trigeminal subnuclei oralis and interpolaris regions, which may be related to the alterations in tactile and mechanical sensitivity from orofacial regions following TBI. Although etiologies associated with trigeminal sensitization are heavily weighting the balance, changes in vessel tone have also been suggested in association with several primary headache disorders (Couturier et al., 1997; Limmroth et al., 2007; Asghar et al., 2011).
We further noticed that closed head mTBI injury caused significant alteration in the immunoreactive expression of some key neuromodulators in NTS. It is known that a group of fibers from the trigeminal nucleus project to the NTS, although the role of NTS in tactile sensation is not clear. However, NTS is well known to subserve afferent projection of general and special visceral afferent modalities including visceral pain, gag reflex, respiratory reflex, and taste sensation, projecting centrally through the cranial nerves VII, IX and X (Goldsmith, 2000; Machado et al., 2000; Cortelli and Pierangeli, 2003; Kitchen et al., 2006; Chen et al., 2008; Deyama et al., 2009). It is also known that general visceral afferent and special visceral (taste) modalities project to the caudal and rostral NTS regions, respectively. Accordingly, these current observations of TBI-induced changes in the caudal solitarius are consistent with the reports that many TBI patients develop neurogenic dysphagia which has been reported to be directly related to dysfunction of NTS (Saito et al., 2006). In patients with serotonergic symptoms, serotonergic hyperactivity at the NTS may inhibit swallowing reflex (Kessler and Jean, 1985). In addition, a direct innervation from mechanoreceptor, and chemoreceptor from carotid body are located near the bifurcation of the carotid artery. Reversible cerebral vasoconstriction syndrome is believed to be related to the use of vasoactive drug in about 50% of cases (Miller et al., 2015a, b). These drugs include selective 5-HT reuptake inhibitors, and other agents or their withdrawal such as nitroglycerin that induce headache (Limmroth et al., 1996; Couturier et al., 1997; Eriksen et al., 2004; Lipton et al., 2004; 2013). We predict that changes in serotonergic neurochemistry in the NTS may play a significant role in the development of PTH by altering the normal integrity of the cerebral blood vessel. In fact, we have recently observed a significant change in the vasculature of the middle cerebral artery following closed head TBI by an MRI-based angiogram study (data not shown). Future studies are needed to detail the changes in the solitary nucleus, which may provide important information regarding the role of solitary nucleus in the PTH, visceral pain, and gastroparesis following TBI.
Conclusion
The changes in immunoreactive markers, especially 5-HT, in the trigeminal subnuclei Sp5O and Sp5I and solitary nuclei are similar to the initial observations made in trigeminal subcaudalis that we reported recently (Mustafa et al., 2016). Collectively, these injury-associated changes contribute to the functional understanding of the widespread facial allodynia, alteration in meningeal and cerebral blood vessels, and visceral pain/allodynia observed following TBI. Although the exact mechanism of PTH is not known, the injury-associated changes that lead to sensitization of trigeminal system are promising candidates for headache related pain. There has been no dependable preclinical model to date to measure the headache related pain. However, facial allodynia, a clinical manifestation of headache-like symptom considered to be manifested in the majority of TBI patients, is here considered as a highly relevant surrogate marker for pain associated with many forms of primary headaches including migraine; and behavioral quantitation of this allodynia is measurable by an operant based technology (Edelmayer et al., 2009). The involvement of trigeminal sensory system and trigeminal vascular system has already been documented by several researchers as key structures for headache related pain (Oshinsky and Gomonchareonsiri, 2007; Noseda and Burstein, 2013). In line to this, our findings show significant changes in several neuromodulators in the spinal trigeminal nuclei, and in the solitary nucleus. Accordingly, widespread changes in the trigeminal nucleus may significantly contribute to the tactile allodynia and facial pain in TBI patients. It is important to note that the level of 5-HT was altered in all three parts of the spinal nucleus of TSS. Accordingly, we predict that the changes in NTS after mTBI may be related to PTH development, and other posttraumatic complications like visceral pain. 5-HT is an excitatory neurotransmitter that is directly related to exacerbation of pain. Clinical data suggest that in addition to PTH, patients with mTBI report problems in gag reflex and in blood pressure, which are directly related to dysfunction of the NTS.
Footnotes
Funding: This work was supported by Merit Review Awards (No. B6570R, B78071, and B1005-R) from the United States (U.S.) Department of Veterans Affairs Rehabilitation Research and Development Service.
Conflicts of interest: There is no financial, personal, or other form of conflict of interest among all the authors or the organizations they are affiliated with.
Research ethics: All procedures were performed in accordance with the U.S Government Principle for the Utilization and Care of Vertebrate Animals, specifically following the National Institutes of Health “Guide for the Care and Use of Laboratory Animals”. Experimental protocols were approved by the Institutional Animal Care & Use Committee (IACUC) at the North Florida/South Georgia Veterans Health System, and the University of Florida, USA.
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check: This paper has been checked twice with duplication-checking software iThenticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Copyedited by Li CH, Song LP, Zhao M
References
- The International Classification of Headache Disorders. 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning P, Larsson HB, Olesen J, Ashina M. Evidence for a vascular factor in migraine. Ann Neurol. 2011;69:635–645. doi: 10.1002/ana.22292. [DOI] [PubMed] [Google Scholar]
- Bernstein AI, O’Malley KL. MPP+-induces PUMA- and p53-dependent, but ATF3-independent cell death. Toxicol Lett. 2013;219:93–98. doi: 10.1016/j.toxlet.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein AI, Garrison SP, Zambetti GP, O’Malley KL. 6-OHDA generated ROS induces DNA damage and p53- and PUMA-dependent cell death. Mol Neurodegener. 2011;6:2. doi: 10.1186/1750-1326-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein C, Burstein R. Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J Clin Neurol. 2012;8:89–99. doi: 10.3988/jcn.2012.8.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose P, Parmer R, Reier PJ, Thompson FJ. Morphological changes of the soleus motoneuron pool in chronic midthoracic contused rats. Exp Neurol. 2005;191:13–23. doi: 10.1016/j.expneurol.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Bose P, Hou J, Nelson R, Nissim N, Parmer R, Keener J, Wacnik PW, Thompson FJ. Effects of acute intrathecal baclofen in an animal model of TBI-induced spasticity, cognitive, and balance disabilities. J Neurotrauma. 2013;30:1177–1191. doi: 10.1089/neu.2012.2740. [DOI] [PubMed] [Google Scholar]
- Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–624. [PubMed] [Google Scholar]
- Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, Becerra L, Borsook D. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010;68:81–91. doi: 10.1002/ana.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Wu XY, Cao ZJ, Fan J, Wang M, Owyang C, Li Y. Subdiaphragmatic vagal afferent nerves modulate visceral pain. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1441–1449. doi: 10.1152/ajpgi.00588.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortelli P, Pierangeli G. Chronic pain-autonomic interactions. Neurol Sci. 2003;24(Suppl 2):S68–70. doi: 10.1007/s100720300045. [DOI] [PubMed] [Google Scholar]
- Couturier EG, Laman DM, van Duijn MA, van Duijn H. Influence of caffeine and caffeine withdrawal on headache and cerebral blood flow velocities. Cephalalgia. 1997;17:188–190. doi: 10.1046/j.1468-2982.1997.1703188.x. [DOI] [PubMed] [Google Scholar]
- Defrin R. Chronic post-traumatic headache: clinical findings and possible mechanisms. J Man Manip Ther. 2014;22:36–44. doi: 10.1179/2042618613Y.0000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyama S, Katayama T, Kondoh N, Nakagawa T, Kaneko S, Yamaguchi T, Yoshioka M, Minami M. Role of enhanced noradrenergic transmission within the ventral bed nucleus of the stria terminalis in visceral pain-induced aversion in rats. Behav Brain Res. 2009;197:279–283. doi: 10.1016/j.bbr.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Ducros A, Wolff V. The typical thunderclap headache of reversible cerebral vasoconstriction syndrome and its various triggers. Headache. 2016;56:657–673. doi: 10.1111/head.12797. [DOI] [PubMed] [Google Scholar]
- Edelmayer RM, Vanderah TW, Majuta L, Zhang E-T, Fioravanti B, De Felice M, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol. 2009;65:184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen MK, Thomsen LL, Olesen J. New international classification of migraine with aura (ICHD-2) applied to 362 migraine patients. Eur J Neurol. 2004;11:583–591. doi: 10.1111/j.1468-1331.2004.00890.x. [DOI] [PubMed] [Google Scholar]
- Foda MA, Marmarou A. A new model of diffuse brain injury in rats. Part II: Morphological characterization. J Neurosurg. 1994;80:301–313. doi: 10.3171/jns.1994.80.2.0301. [DOI] [PubMed] [Google Scholar]
- Goldsmith T. Evaluation and treatment of swallowing disorders following endotracheal intubation and tracheostomy. Int Anesthesiol Clin. 2000;38:219–242. doi: 10.1097/00004311-200007000-00013. [DOI] [PubMed] [Google Scholar]
- Hines ME. Posttraumatic headaches. In: Varney NR, Roberts RJ, editors. The evaluation and treatment of mild traumatic brain injury. Psychology Press: 1999. pp. 375–410. [Google Scholar]
- Hoffman JM, Pagulayan KF, Zawaideh N, Dikmen S, Temkin N, Bell KR. Understanding pain after traumatic brain injury: impact on community participation. Am J Phys Med Rehabil. 2007;86:962–969. doi: 10.1097/PHM.0b013e31815b5ee5. [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Lucas S, Dikmen S, Braden CA, Brown AW, Brunner R, Diaz-Arrastia R, Walker WC, Watanabe TK, Bell KR. Natural history of headache after traumatic brain injury. J Neurotrauma. 2011;28:1719–1725. doi: 10.1089/neu.2011.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Nelson R, Nissim N, Parmer R, Thompson FJ, Bose P. Effect of combined treadmill training and magnetic stimulation on spasticity and gait impairments after cervical spinal cord injury. J Neurotrauma. 2014;31:1088–1106. doi: 10.1089/neu.2013.3096. [DOI] [PubMed] [Google Scholar]
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22:341–353. [PubMed] [Google Scholar]
- Kessler JP, Jean A. Inhibition of the swallowing reflex by local application of serotonergic agents into the nucleus of the solitary tract. Eur J Pharmacol. 1985;118:77–85. doi: 10.1016/0014-2999(85)90665-x. [DOI] [PubMed] [Google Scholar]
- Kitchen AM, O’Leary DS, Scislo TJ. Sympathetic and parasympathetic component of bradycardia triggered by stimulation of NTS P2X receptors. Am J Physiol Heart Circ Physiol. 2006;290:H807–812. doi: 10.1152/ajpheart.00889.2005. [DOI] [PubMed] [Google Scholar]
- Limmroth V, Biondi D, Pfeil J, Schwalen S. Topiramate in patients with episodic migraine: reducing the risk for chronic forms of headache. Headache. 2007;47:13–21. doi: 10.1111/j.1526-4610.2007.00648.x. [DOI] [PubMed] [Google Scholar]
- Limmroth V, May A, Auerbach P, Wosnitza G, Eppe T, Diener HC. Changes in cerebral blood flow velocity after treatment with sumatriptan or placebo and implications for the pathophysiology of migraine. J Neurol Sci. 1996;138:60–65. doi: 10.1016/0022-510x(95)00344-2. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Bigal ME, Steiner TJ, Silberstein SD, Olesen J. Classification of primary headaches. Neurology. 2004;63:427–435. doi: 10.1212/01.wnl.0000133301.66364.9b. [DOI] [PubMed] [Google Scholar]
- Machado BH, Castania JA, Bonagamba LG, Salgado HC. Neurotransmission of autonomic components of aortic baroreceptor afferents in the NTS of awake rats. Am J Physiol Heart Circ Physiol. 2000;279:H67–75. doi: 10.1152/ajpheart.2000.279.1.H67. [DOI] [PubMed] [Google Scholar]
- Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- Miller TR, Shivashankar R, Mossa-Basha M, Gandhi D. Reversible Cerebral Vasoconstriction Syndrome, Part 1: Epidemiology, Pathogenesis, and Clinical Course. AJNR Am J Neuroradiol. 2015a;36:1392–1399. doi: 10.3174/ajnr.A4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TR, Shivashankar R, Mossa-Basha M, Gandhi D. Reversible Cerebral Vasoconstriction Syndrome, Part 2: Diagnostic Work-Up, Imaging Evaluation, and Differential Diagnosis. AJNR Am J Neuroradiol. 2015b;36:1580–1588. doi: 10.3174/ajnr.A4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa G, Hou J, Tsuda S, Nelson R, Sinharoy A, Wilkie Z, Pandey R, Caudle RM, Neubert JK, Thompson FJ, Bose P. Trigeminal neuroplasticity underlies allodynia in a preclinical model of mild closed head traumatic brain injury (cTBI) Neuropharmacology. 2016;107:27–39. doi: 10.1016/j.neuropharm.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain. 2013;154(Suppl 1) doi: 10.1016/j.pain.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek H, Defrin R. The characteristics of chronic central pain after traumatic brain injury. Pain. 2007;131:330–340. doi: 10.1016/j.pain.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47:1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic. Coordinates. 6th ed. New York, USA: Elsevier/Academic Press; 2007. [Google Scholar]
- Saito Y, Kawashima Y, Kondo A, Chikumaru Y, Matsui A, Nagata I, Ohno K. Dysphagia-gastroesophageal reflux complex: complications due to dysfunction of solitary tract nucleus-mediated vago-vagal reflex. Neuropediatrics. 2006;37:115–120. doi: 10.1055/s-2006-924428. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Sessle BJ, Hu JW, Amano N, Zhong G. Convergence of cutaneous, tooth pulp, visceral, neck and muscle afferents onto nociceptive and non-nociceptive neurones in trigeminal subnucleus caudalis (medullary dorsal horn) and its implications for referred pain. Pain. 1986;27:219–235. doi: 10.1016/0304-3959(86)90213-7. [DOI] [PubMed] [Google Scholar]
- Sokolov AY, Lyubashina OA, Panteleev SS. Spinal trigeminal neurons demonstrate an increase in responses to dural electrical stimulation in the orofacial formalin test. J Headache Pain. 2012;13:75–82. doi: 10.1007/s10194-011-0404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeler B, Lucas S, Riechers RG, 2nd, Ruff RL. Post-traumatic headaches in civilians and military personnel: a comparative, clinical review. Headache. 2013;53:881–900. doi: 10.1111/head.12123. [DOI] [PubMed] [Google Scholar]
- Tsuda S, Hou J, Nelson R, Mustafa G, Watts J, Thompson FJ, Bose P. Impact of chronic traumatic brain injury on noradrenergic innervation to the major anxiety-related neural pathways in rats. Poster: 322 - Traumatic Brain Injury: Models, Mechanisms, and Treatments 322.12 Society for Neuroscience Abstract, San Diego, Cal. 2016 [Google Scholar]
- Uomoto JM, Esselman PC. Traumatic brain injury and chronic pain: differential types and rates by head injury severity. Arch Phys Med Rehabil. 1993;74:61–64. [PubMed] [Google Scholar]
- Walker WC, Seel RT, Curtiss G, Warden DL. Headache after moderate and severe traumatic brain injury: a longitudinal analysis. Arch Phys Med Rehabil. 2005;86:1793–1800. doi: 10.1016/j.apmr.2004.12.042. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]


