Keywords: nerve regeneration, Wallerian degeneration, sciatic nerve transection, peripheral nerve regeneration, microarray, bioinformatic analysis, Kyoto Enrichment of Genes and Genomes, signaling pathway, cytokine-cytokine receptor interaction, neuroactive ligand-receptor interaction, axon guidance, neural regeneration
Abstract
Wallerian degeneration is a critical biological process that occurs in distal nerve stumps after nerve injury. To systematically investigate molecular changes underlying Wallerian degeneration, we used a rat sciatic nerve transection model to examine microarray analysis outcomes and investigate significantly involved Kyoto Enrichment of Genes and Genomes (KEGG) pathways in injured distal nerve stumps at 0, 0.5, 1, 6, 12, and 24 hours, 4 days, 1, 2, 3, and 4 weeks after peripheral nerve injury. Bioinformatic analysis showed that only one KEGG pathway (cytokine-cytokine receptor interaction) was significantly enriched at an early time point (1 hour post-sciatic nerve transection). At later time points, the number of enriched KEGG pathways initially increased and then decreased. Three KEGG pathways were studied in further detail: cytokine-cytokine receptor interaction, neuroactive ligand-receptor interaction, and axon guidance. Moreover, temporal expression patterns of representative differentially expressed genes in these KEGG pathways were validated by real time-polymerase chain reaction. Taken together, the above three signaling pathways are important after sciatic nerve injury, and may increase our understanding of the molecular mechanisms underlying Wallerian degeneration
Introduction
Wallerian degeneration is an important degenerative process that occurs in distal nerve stumps in response to nerve fiber injury (Coleman, 2005; Coleman and Freeman, 2010). It occurs in both the central nervous system and peripheral nervous system, although it normally occurs within 1 to 2 weeks after injury in the peripheral nervous system but does not occur until a few months or even years after injury to the central nervous system (Griffin et al., 1992; George and Griffin, 1994). Timely occurrence of Wallerian degeneration in the peripheral nervous system may contribute to axonal regeneration due to clearance of myelin debris and growth inhibitors, and subsequent establishment of a regenerative microenvironment (Avellino et al., 1995; Vargas and Barres, 2007). However, delayed Wallerian degeneration in the central nervous system may hinder axonal regeneration. Accordingly, it is believed that Wallerian degeneration plays a key role in nerve regeneration (Lunn et al., 1989; Brown et al., 1991, 1992).
Considering the importance of Wallerian degeneration, numerous studies have been performed to identify underlying biological changes. These studies show that macrophages, monocytes, and Schwann cells work together to remove axon and myelin debris, and clear a path for subsequent axonal regrowth and nerve regeneration (Geuna et al., 2009; Sta et al., 2014). These morphological and genetic studies have identified many central factors, including nicotinamide mononucleotide adenylyltransferase 2 (Coleman and Freeman, 2010; Gilley and Coleman, 2010; Gilley et al., 2013). Furthermore, emerging high-throughput studies have been performed to decipher global molecular changes. For example, in our previous study, we jointly re-annotated and re-analyzed microarray data (Yao et al., 2012, 2013) using bioinformatic tools including Euclidean distance calculation, hierarchical clustering, principle component analysis, gene ontology analysis, Kyoto Enrichment of Genes and Genomes (KEGG) analysis, and Ingenuity Pathway Analysis. Altogether, we obtained an integrated global view of genetic changes in injured distal nerve stumps (Yu et al., 2016; Yi et al., 2017). In particular, KEGG analysis outcomes identified pathways with P-values less than 0.05, indicating they may play critical roles in Wallerian degeneration (Yi et al., 2017).
Taking the importance of signaling pathways into account, in the current study, we examined in detail these significantly enriched pathways in distal nerve stumps at various time points following sciatic nerve transection. Our aim was to achieve greater insight into dynamic molecular changes underlying Wallerian degeneration and identify critical biological processes for treatment of peripheral nerve repair and regeneration.
Materials and Methods
Rat sciatic nerve transection
A total of 66 adult, 2-month-old, male Sprague-Dawley rats weighing 180 to 220 g were obtained from the Experimental Animal Center, Nantong University, China (animal license No. SCXK [Su] 2014-0001 and SYXK [Su] 2012-0031). All animal surgery procedures were performed in accordance with Institutional Animal Care guidelines of Nantong University, and ethically approved by the Administration Committee of Experimental Animals, Jiangsu Province, China (No. 20160229-007). The experiment followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978), and “Consensus Author Guidelines on Animal Ethics and Welfare” by the International Association for Veterinary Editors (IAVE).
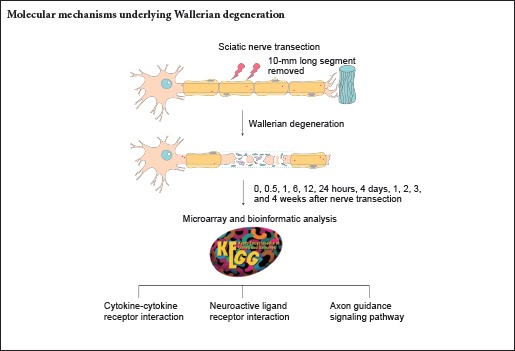
Rat sciatic nerve transection was performed, as previously described (Yu et al., 2016; Yi et al., 2017). Briefly, Sprague-Dawley rats were equally and randomly divided into eleven groups with 6 rats in each group. Following anesthetization, rat hair was shaved and the surgical area cleansed with 75% ethanol. An incision was made on the lateral aspect of the mid-thigh of the rat left hind limb, the sciatic nerve was lifted, and a 10-mm long segment was removed from the middle of the femur. At 0.5, 1, 6, 12, and 24 hours, 4 days, and 1, 2, 3, and 4 weeks post-sciatic nerve transection, rats were sacrificed by decapitation and distal nerve stumps were collected. Sham-operated rats (rats with sciatic nerves exposed but uninjured) were used as controls and designated as 0 hour post-sciatic nerve transection.
RNA extraction and microarray analysis
RNA samples were extracted from distal nerve stumps using Trizol reagent (Life Technology, Carlsbad, CA, USA). Remaining DNA was removed using RNeasy spin columns (Qiagen, Valencia, CA, USA). Purified RNA samples were then quantified using a NanoDrop ND-1000 spectrophotometer (Infinigen Biotechnology Inc., City of Industry, CA, USA).
Microarray analysis was performed in accordance with previous studies (Yao et al., 2012, 2013). Briefly, an Affymetrix GeneChip Oven 640 and Gene Array Scanner 3000 (Affymetrix, Santa Clara, CA, USA) were used to obtain microarray outcomes. These outcomes were then analyzed by the R software platform (v.2.13.0) and limma (linear regression model) package (Ritchie et al., 2015).
Bioinformatic analysis
Expression levels of mRNAs at 0.5, 1, 6, 12, and 24 hours, 4 days, and 1, 2, 3, and 4 weeks post-sciatic nerve transection were compared with those at 0 hour post-nerve transection. Genes with fold changes > 2 or < −2 (absolute value of log2 fold change > 1) and adjusted P-values < 0.05 were considered to be differentially expressed. Differentially expressed genes were then systematically analyzed using Database for Annotation, Visualization, and Integrated Discovery to enrich significant involved KEGG pathways.
Quantitative real time-polymerase chain reaction
RNA samples (0.5 μg) were reverse transcribed to cDNA using the Prime-Script Reagent Kit (TaKaRa, Dalian, China) for subsequent amplification. Quantitative real time-polymerase chain reaction (RT-PCR) was then performed using SYBR Green Premix Ex Taq (TaKaRa) with specific primer pairs on an Applied Biosystems Stepone real-time PCR System (Applied Biosystems, Foster City, CA, USA). Primer pair sequences were: Eph receptor A4 (Epha4), (forward) 5’-CGC CGT AGT ATC AGT GGG TG-3’ and (reverse) 5’-GTC TGT TCG GTA CTG GCT CA-3’; met proto-oncogene (Met), (forward) 5’-CGC TGC AGG CTG TGG ATT TA-3’ and (reverse) 5’-GGT GAA ATG TGC TGT GCG AG-3’; interleukin 11 (Il11), (forward) 5’-CCG ACT GGA ACG GCT ACT TC-3’ and (reverse) 5’-GAC GAT GTC GAT GGT GGC TT-3’; prostaglandin E receptor 2 (Ptger2), (forward) 5’-TTC TAT GGC GGA GAC GG-3’ and (reverse) 5’-GGT CCC ACT TTT CCT TTC GGG-3’; and glyceraldehyde 3-phosphate dehydrogenase (Gapdh), (forward) 5’-CCT TCA TTG ACC TCA ACT ACA TG-3’ and (reverse) 5’-CTT CTC CAT GGT GAA GAC-3’. Ct values were obtained for mRNA quantification using the ΔΔCt method and Gapdh as the reference gene.
Statistical analysis
Data were presented as the mean ± SEM. Differences between groups were tested by one-way analysis of variance using GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA). P-values < 0.05 were considered statistically significant.
Results
Overview of significantly involved KEGG pathways during Wallerian degeneration
Our previous study showed that many KEGG pathways are significantly involved in Wallerian degeneration (Yi et al., 2017). To further study these activated KEGG pathways, we analyzed enriched KEGG pathways at various time points post-sciatic nerve transection. KEGG pathways with P-values less than 0.05 are listed in Table 1.
Table 1.
List of significantly involved Kyoto Enrichment of Genes and Genomes at various time points during Wallerian degeneration identified by bioinformatic analysis
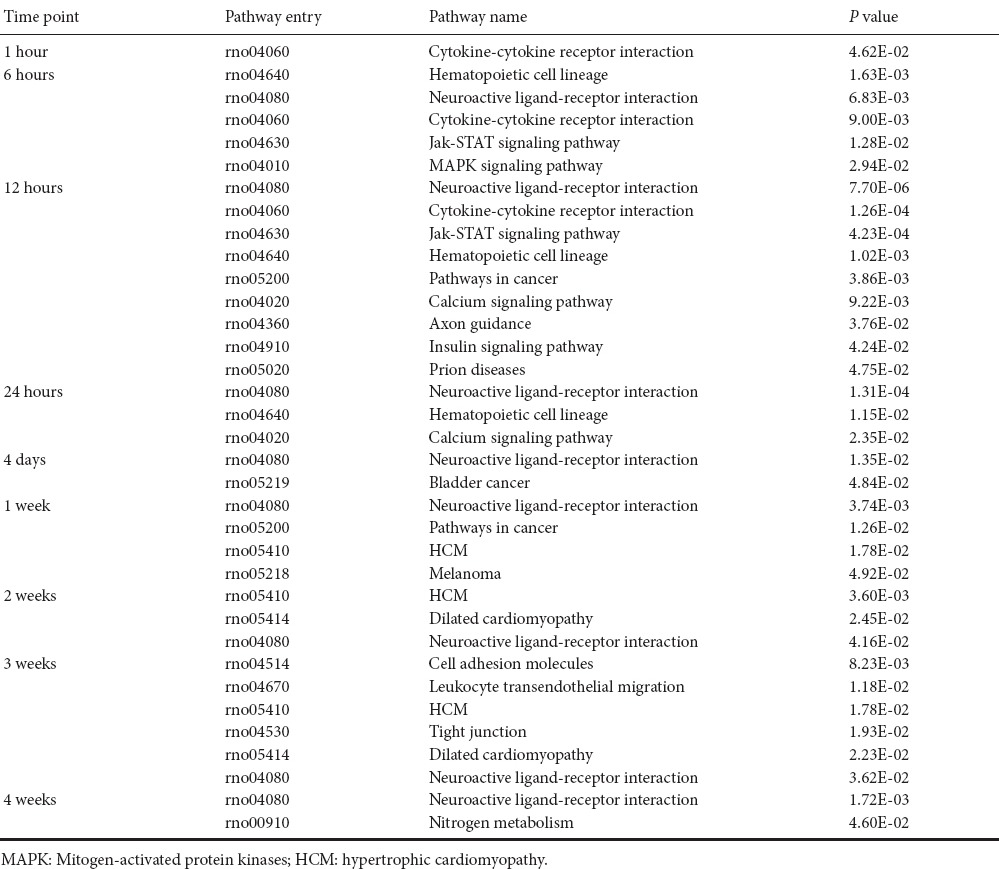
At 0.5 hour post-sciatic nerve transection, no KEGG pathway was significantly involved. At 1 hour post-nerve transection, only one KEGG pathway, cytokine-cytokine receptor interaction was significantly activated. At 6 hours post-nerve transection, hematopoietic cell lineage, neuroactive ligand-receptor interaction, Jak-STAT signaling pathway, and mitogen-activated protein kinases signaling pathway were also activated. At 12 hours post-nerve transection, more KEGG pathways were involved including those involved in cancer, calcium signaling pathway, axon guidance, insulin signaling pathway, and Prion diseases. At later time points, the number of significantly involved KEGG pathways decreased. Some of the above-mentioned pathways (e.g., neuroactive ligand-receptor interaction) remained highly activated. Some novel KEGG pathways emerged at longer time points post-nerve transection, such as cell adhesion molecules (CAMs) and tight junctions.
Cytokine-cytokine receptor interaction
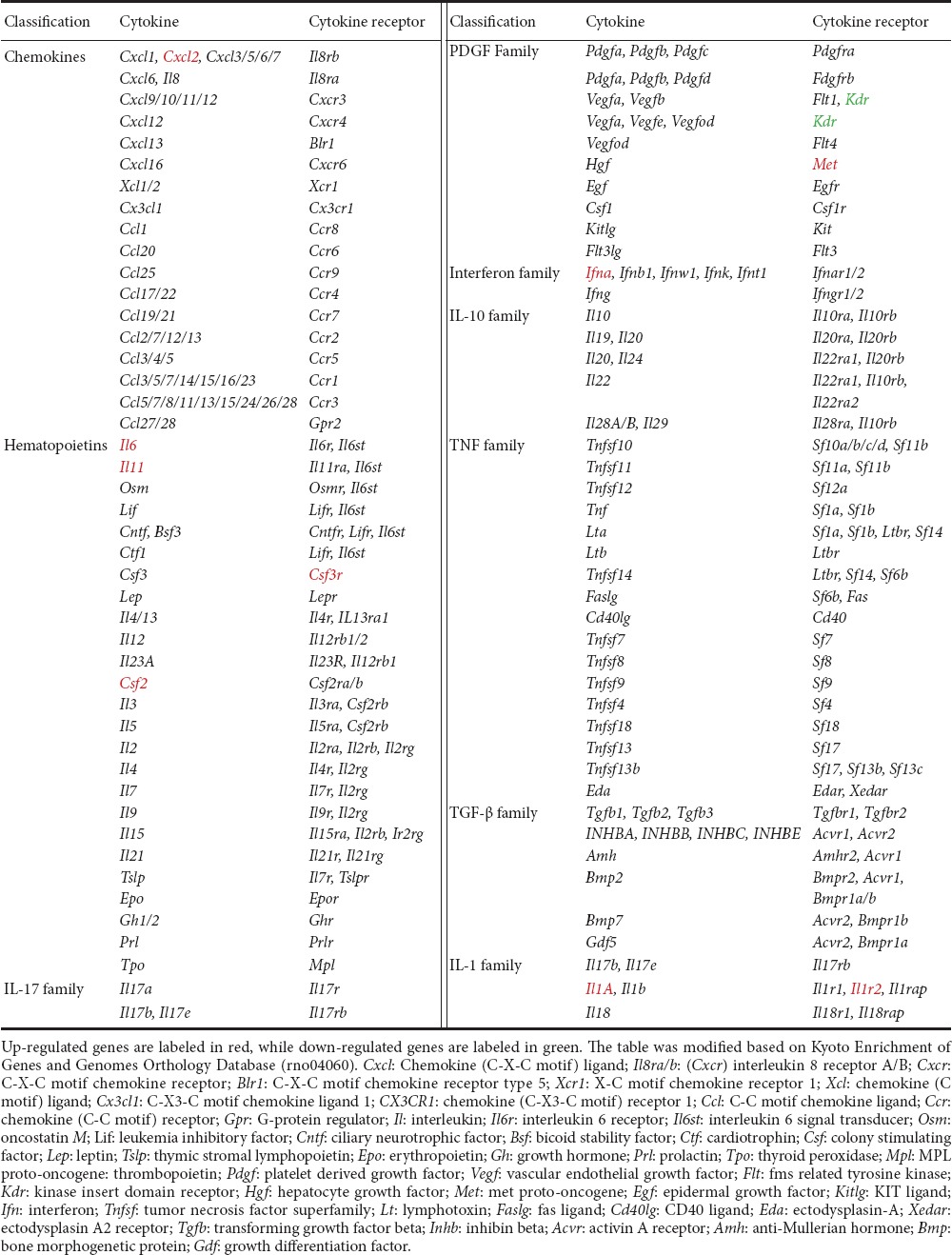
Cytokine-cytokine receptor interaction (rno04060) was the only KEGG pathway activated immediately post-sciatic nerve transection, and strongly activated thereafter at early stages (1, 6, and 12 hours) following nerve transection. A schematic network of the cytokine-cytokine receptor interaction was built based on the KEGG Orthology Database (http://www.genome.jp/kegg/ko.html), with differentially expressed genes labeled (Table 2). Many chemokines, cytokines, and their corresponding receptors, including C-X-C motif chemokine ligand 2 (Cxcl2), interleukin 6 (Il6), interleukin-11 (Il11), colony stimulating factor 3 receptor (Csf3r), colony stimulating factor 2 (Csf2), MET proto-oncogene, receptor tyrosine kinase (Met), interferon A (Ifna), interleukin 1A (Il1a), and interleukin 1R2 (Il1r2) were significantly up-regulated in injured distal nerve stumps. Only one gene, kinase insert domain receptor (Kdr), a gene that encodes vascular endothelial growth factor receptor, was down-regulated post-sciatic nerve transection.
Table 2.
Significant differentially expressed genes in cytokine-cytokine receptor interaction
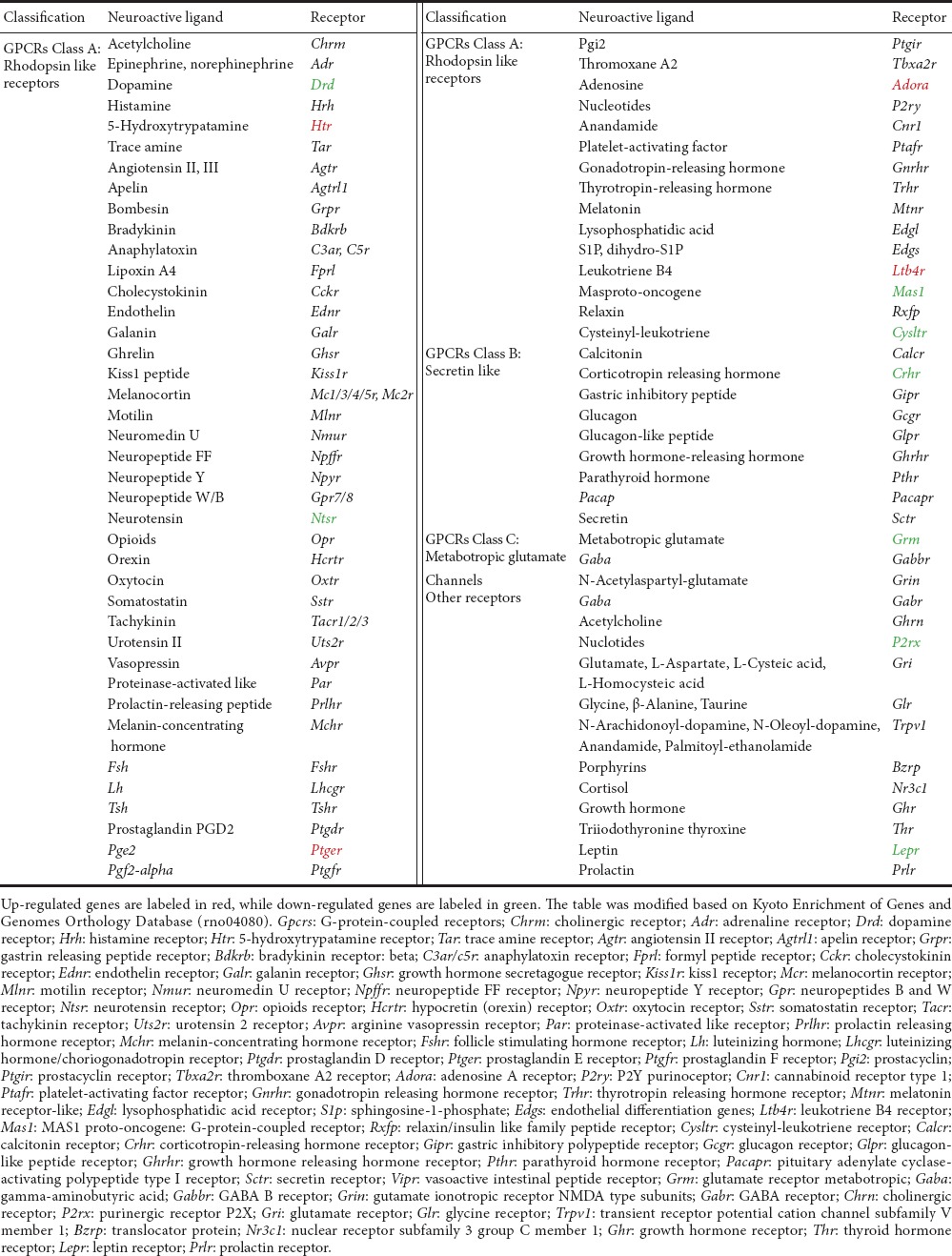
Neuroactive ligand-receptor interaction
Neuroactive ligand-receptor interaction (rno04080) was another KEGG pathway activated at a relatively early time point (6 hours post-sciatic nerve transection). Further, this KEGG pathway was activated at all time points from 6 hours to 4 weeks post-nerve transection, suggesting that it is critical for Wallerian degeneration and subsequent nerve regeneration. Receptors of numerous neurotransmitters and mediators, such as histamine receptor (Htr), prostaglandin E2 receptor 1 (Ptger), adenosine receptor (Adora), and leukotriene B4 receptor (Ltb4r) were up-regulated. In contrast, dopamine receptor (Drd), neurotensin receptor (Ntsr), MAS proto-oncogene or mas-related G-protein coupled receptor A (Mas1), cysteinyl-leukotriene receptor (Cysltr), corticotropin-releasing hormone receptor (Crhr), metabotropic glutamate receptor (Grm), purinergic receptor P2X (P2rx), and leptin receptor (Lepr) were down-regulated (Table 3).
Table 3.
Significant differentially expressed genes in neuroactive ligand-receptor interaction
Axon guidance
Besides cytokine-cytokine receptor interaction and neuroactive ligand-receptor interaction, two KEGG pathways significantly enriched at various time points during Wallerian degeneration, axon guidance signaling pathway (rno04360) was a KEGG pathway activated at 12 hours post-nerve transection, and also studied in detail based on its importance for successful nerve regeneration and functional reconstruction. Accordingly, Met, a previously identified activated gene in the cytokine-cytokine receptor interaction pathway, was also involved in the axon guidance pathway. In addition, the ephrin receptor, Epha, was also up-regulated while the netrin receptor, Unc-5, was down-regulated (Figure 1).
Figure 1.
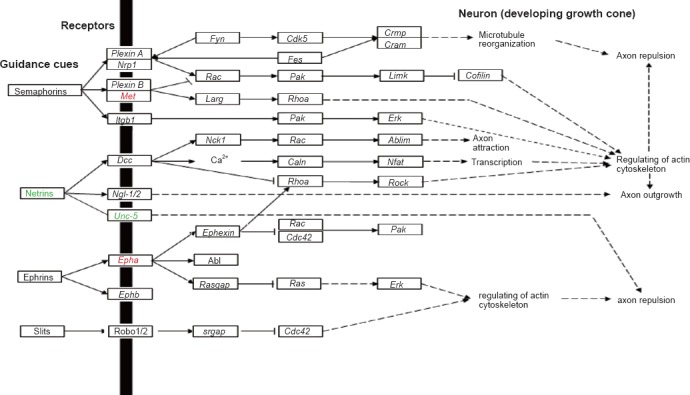
Significant differentially expressed genes in axon guidance signaling.
Up-regulated genes are labeled in red, while down-regulated genes are labeled in green. The figure was modified based on Kyoto Enrichment of Genes and Genomes Orthology Database (rno04360). Fyn: fyn proto-oncogene; Cdk5: cyclin dependent kinase 5; Crmp: collapsing response mediator protein; Cram: dihydropyrimidinase like 5; Nrp1: neuropilin 1; Fes: feline sarcoma oncogene; Rac: Rac protein; Pak: p21-activated kinase; Limk: LIM domain kinase; Met: met proto-oncogene; Larg: rho guanine nucleotide exchange factor 12; Rhoa: ras homolog family member A; Itgb1: integrin subunit beta 1; Erk: extracellular regulated MAP kinase; Dcc: deleted in colorectal carcinoma netrin 1 receptor; Nck1: non-catalytic region of tyrosine kinase adaptor protein 1; Ablim: actin binding LIM protein; Caln: protein phosphatase 3 catalytic subunit alpha; Nfat: nuclear factor of activated T-cells; Rock: rho kinase; Ngl-1/2: netrin-G1 ligand 1/2; Unc-5: netrin receptor unc-5; Epha: Eph receptor A; Cdc42: cell division cycle 42; Abl: Abelson murine leukemia viral oncogene homolog; Rasgap: RAS p21 protein activator 1; Ephb: Eph receptor B; Robo1/2: roundabout homolog 1/2; srgap: SLIT-ROBO rho GTPase activating protein.
RT-PCR verification of microarray results
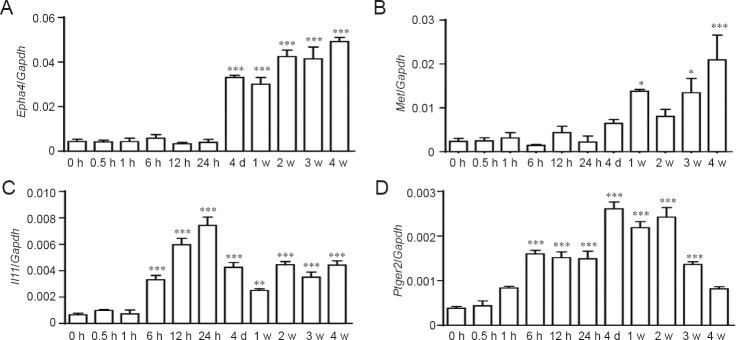
RT-PCR experiments were performed to validate temporal expression patterns of representative differentially expressed genes involved in the KEGG pathways: cytokine-cytokine receptor interaction (Il11), neuroactive ligand-receptor interaction (Ptger), and axon guidance (Epha and Met). Consistent with our microarray analysis outcomes, RT-PCR confirmed that Epha4, Met, Il11, and Ptger2 expression levels were robustly increased, especially at longer time points post-sciatic nerve transection (Figure 2).
Figure 2.
Quantitative real time-polymerase chain reaction for mRNA expression of Epha4 (A), Met (B), Il11 (C), and Ptger2 (D).
Relative levels were normalized to Gapdh. Summarized data are from three independent experiments. Values are shown as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, vs. 0 hour (one-way analysis of variance). Epha4: Eph receptor A4; Met: met proto-oncogene; Il11: interleukin 11; Ptger2: prostaglandin E receptor 2; Gapdh: glyceraldehyde 3-phosphate dehydrogenase; h: hour(s); d: days; w: week(s).
Discussion
Wallerian degeneration helps form a penetrable pathway for axon regrowth, and consequently, is very important for subsequent nerve repair and regeneration (Bittner et al., 2016). Until now, many critical factors for Wallerian degeneration have been identified. However, Wallerian degeneration is a complex biological process mediated by a group of molecules instead of a single gene or protein. Thus, obtaining a global view of molecular changes during Wallerian degeneration is of utmost importance. Accordingly here, by combined use of microarray and bioinformatic analysis, we synthetically analyzed highly activated KEGG signaling pathways (cytokine-cytokine receptor interaction, neuroactive ligand-receptor interaction, and axon guidance signaling pathway) following sciatic nerve transection.
Consistent with previous observations (Li et al., 2013, 2014; Yao et al., 2013; Yi et al., 2017), the KEGG pathway, cytokine-cytokine receptor interaction, was significantly involved from an early stage post-sciatic nerve injury. Detailed study of the cytokine-cytokine receptor interaction KEGG pathway suggests that most differentially expressed genes are related to immunoregulatory, inflammatory, and host defense processes such as Cxcl2, Il6, Il11, Csf3r, Csf2, Ifna, Il1a, and Il1r2. Indeed, Wallerian degeneration has been thought of as an innate-immune response to external injuries (Rotshenker, 2011). Our outcomes demonstrate that immune reactions and inflammatory responses are initiated at an acute phase post-nerve injury, and suggest that innate immune and inflammatory responses in Wallerian degeneration might be critical for successful nerve regeneration and functional reconstitution.
The KEGG pathway, neuroactive ligand-receptor interaction, was significantly enriched at later time points (6 hours to 4 weeks post-nerve injury). Differentially expressed genes in this KEGG pathway are mainly neurotransmitter receptors (e.g., Drd, Htr, Ntsr, Ptger, Adora, Grm, and P2rx). It has been demonstrated that certain neurotransmitters can affect Wallerian degeneration, namely adenosine, guanosine, adenosine triphosphate, and adenosine (Press and Milbrandt, 2009; Shin et al., 2014). From the genetic aspect, our outcomes show that neurotransmitters and their receptors might be involved in Wallerian degeneration.
The axon guidance signaling pathway was also investigated. We found that receptors of semaphorins (Met), ephrins (Epha), and netrins (Unc-5) are differentially expressed following sciatic nerve transection. Semaphorins, ephrins, and netrins are proteins highly related to axon guidance. They play chemotropic roles during axon growth and attract a growing axon to move towards or away from higher concentrated regions. Interestingly, Met and Epha show differential temporal expression patterns compared with Unc-5, suggesting their roles in axon guidance and nerve regeneration might be different. Further studies will be performed to clarify their specific effects.
However, it is worth noting that although we obtained some knowledge of dynamic molecular changes and essential signaling pathways during Wallerian degeneration, it remains unclear which cell types mediate these dynamic changes. Schwann cells and macrophages are important cells in distal nerve stumps, and consequently these dynamic changes may occur in these cell types. In our future studies, single cell sequencing will be performed to further decipher temporal expression patterns of genes in each cell type, and the specific role of each cell type during Wallerian degeneration.
Taken together, we systematically investigated significantly enriched KEGG pathways in distal nerve stumps following sciatic nerve transection, and identified critical KEGG pathways for peripheral nerve repair and regeneration. Our results may help elucidate critical genes, signaling pathways, and biological processes during Wallerian degeneration, and might contribute to identification of potential treatments for peripheral nerve repair and regeneration.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 81501058; the Natural Science Foundation of Jiangsu Province of China, No. BK20150409; the Natural Science Foundation of Jiangsu Higher Education Institutions of China, No. 15KJB180013, 15KJB310014; and a grant from the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by the Administration Committee of Experimental Animals, Jiangsu Province, China (No. 20160229-007). The experiment followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978), and “Consensus Author Guidelines on Animal Ethics and Welfare” produced by the International Association for Veterinary Editors (IAVE). The article was prepared in accordance with the “Animal Research: Reporting of In Vivo Experiments Guidelines” (ARRIVE Guidelines).
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check: This paper has been checked twice with duplication-checking software iThenticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Open peer reviewer: Bogdan K Beirowski, State University of New York, USA.
Copyedited by James R, de Souza M, Yu J, Li CH, Qiu Y, Song LP, Zhao M
References
- Avellino AM, Hart D, Dailey AT, MacKinnon M, Ellegala D, Kliot M. Differential macrophage responses in the peripheral and central nervous system during wallerian degeneration of axons. Exp Neurol. 1995;136:183–198. doi: 10.1006/exnr.1995.1095. [DOI] [PubMed] [Google Scholar]
- Brown MC, Lunn ER, Perry VH. Poor growth of Mammalian motor and sensory axons into intact proximal nerve stumps. Eur J Neurosci. 1991;3:1366–1369. doi: 10.1111/j.1460-9568.1991.tb00069.x. [DOI] [PubMed] [Google Scholar]
- Brown MC, Lunn ER, Perry VH. Consequences of slow Wallerian degeneration for regenerating motor and sensory axons. J Neurobiol. 1992;23:521–536. doi: 10.1002/neu.480230507. [DOI] [PubMed] [Google Scholar]
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Freeman MR. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R, Griffin JW. Delayed macrophage responses and myelin clearance during Wallerian degeneration in the central nervous system: the dorsal radiculotomy model. Exp Neurol. 1994;129:225–236. doi: 10.1006/exnr.1994.1164. [DOI] [PubMed] [Google Scholar]
- Geuna S, Raimondo S, Ronchi G, Di Scipio F, Tos P, Czaja K, Fornaro M. Chapter 3: Histology of the peripheral nerve and changes occurring during nerve regeneration. Int Rev Neurobiol. 2009;87:27–46. doi: 10.1016/S0074-7742(09)87003-7. [DOI] [PubMed] [Google Scholar]
- Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010;8:e1000300. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley J, Adalbert R, Yu G, Coleman MP. Rescue of peripheral and CNS axon defects in mice lacking NMNAT2. J Neurosci. 2013;33:13410–13424. doi: 10.1523/JNEUROSCI.1534-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JW, George R, Lobato C, Tyor WR, Yan LC, Glass JD. Macrophage responses and myelin clearance during Wallerian degeneration: relevance to immune-mediated demyelination. J Neuroimmunol. 1992;40:153–165. doi: 10.1016/0165-5728(92)90129-9. [DOI] [PubMed] [Google Scholar]
- Li M, Guo W, Zhang P, Li H, Gu X, Yao D. Signal flow and pathways in response to early Wallerian degeneration after rat sciatic nerve injury. Neurosci Lett. 2013;536:56–63. doi: 10.1016/j.neulet.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Li M, Zhang P, Guo W, Li H, Gu X, Yao D. Protein expression profiling during wallerian degeneration after rat sciatic nerve injury. Muscle Nerve. 2014;50:73–78. doi: 10.1002/mus.24082. [DOI] [PubMed] [Google Scholar]
- Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S. Absence of wallerian degeneration does not hinder regeneration in peripheral nerve. Eur J Neurosci. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Press C, Milbrandt J. The purine nucleosides adenosine and guanosine delay axonal degeneration in vitro. J Neurochem. 2009;109:595–602. doi: 10.1111/j.1471-4159.2009.06002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker S. Wallerian degeneration: the innate-immune response to traumatic nerve injury. J Neuroinflammation. 2011;8:109. doi: 10.1186/1742-2094-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YH, Chung HJ, Park C, Jung J, Jeong NY. Adenosine 5’-triphosphate (ATP) inhibits schwann cell demyelination during Wallerian degeneration. Cell Mol Neurobiol. 2014;34:361–368. doi: 10.1007/s10571-013-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sta M, Cappaert NL, Ramekers D, Baas F, Wadman WJ. The functional and morphological characteristics of sciatic nerve degeneration and regeneration after crush injury in rats. J Neurosci Methods. 2014;222:189–198. doi: 10.1016/j.jneumeth.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- Yao D, Li M, Shen D, Ding F, Lu S, Zhao Q, Gu X. Gene expression profiling of the rat sciatic nerve in early Wallerian degeneration after injury. Neural Regen Res. 2012;7:1285–1292. doi: 10.3969/j.issn.1673-5374.2012.17.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D, Li M, Shen D, Ding F, Lu S, Zhao Q, Gu X. Expression changes and bioinformatic analysis of Wallerian degeneration after sciatic nerve injury in rat. Neurosci Bull. 2013;29:321–332. doi: 10.1007/s12264-013-1340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S, Tang X, Yu J, Liu J, Ding F, Gu X. Microarray and qPCR analyses of wallerian degeneration in rat sciatic nerves. Front Cell Neurosci. 2017;11:22. doi: 10.3389/fncel.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Gu X, Yi S. Ingenuity pathway analysis of gene expression profiles in distal nerve stump following nerve injury: Insights into Wallerian Degeneration. Front Cell Neurosci. 2016;10:274. doi: 10.3389/fncel.2016.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]