Abstract
We investigated the role of phospholipase D (PLD) and its product phosphatidic acid (PA) in myogenic differentiation of cultured L6 rat skeletal myoblasts. Arginine-vasopressin (AVP), a differentiation inducer, rapidly activated PLD in a Rho-dependent way, as shown by almost total suppression of activation by C3 exotoxin pretreatment. Addition of 1-butanol, which selectively inhibits PA production by PLD, markedly decreased AVP-induced myogenesis. Conversely, myogenesis was potentiated by PLD1b isoform overexpression but not by PLD2 overexpression, establishing that PLD1 is involved in this process. The expression of the PLD isoforms was differentially regulated during differentiation. AVP stimulation of myoblasts induced the rapid formation of stress fiber-like actin structures (SFLSs). 1-Butanol selectively inhibited this response, whereas PLD1b overexpression induced SFLS formation, showing that it was PLD dependent. Endogenous PLD1 was located at the level of SFLSs, and by means of an intracellularly expressed fluorescent probe, PA was shown to be accumulated along these structures in response to AVP. In addition, AVP induced a PLD-dependent neosynthesis of phosphatidylinositol 4,5-bisphosphate (PIP2), which also was accumulated along actin fibers. These data support the hypothesis that PLD participates in myogenesis through PA- and PIP2-dependent actin fiber formation.
INTRODUCTION
Phospholipase D (PLD) hydrolyzes phosphatidylcholine of cell membranes in response to agonist stimulation, to give rise to phosphatidic acid (PA). PLD and PA seem to play important roles in cell signaling, although the mechanisms through which they participate in the regulation of cell functions are incompletely elucidated. Among the pathways known to involve PLD activation, the triggering of mitogen-activated protein kinase cascade through PA-mediated translocation of Raf-1 has been particularly well characterized. In HIRcB cells, PLD activation by insulin and the resulting PA accumulation in plasma membrane drive the translocation of cytosolic Raf-1 to this compartment, where its activation by its membrane-bound regulator Ras initiates the signaling cascade (Rizzo et al., 1999, 2000). Raf-1 translocation is made possible by the existence on the kinase of a lipid binding domain displaying selective affinity for PA (Ghosh et al., 1996; Rizzo et al., 1999, 2000; Hekman et al., 2002). In other respects, we reported that PA can stimulate cAMP hydrolysis by binding to a specific domain on cAMP-phosphodiesterase PDE4D3 and thus participates in the regulation of intracellular cAMP levels (Grange et al., 2000). Recently, PA has been shown to be involved in signaling devices as diverse as translocation and activation of protein kinase C (PKC)ε (Jose Lopez-Andreo et al., 2003) or function of acetylcholine receptors (Poveda et al., 2002). Interestingly, it has been recognized that PA can activate the phosphatidylinositol 4-phosphate 5-kinases (PI-4P 5-kinases) of type I in vitro (Jenkins et al., 1994) and thereby regulate phosphatidylinositol 4,5-bisphosphate (PIP2) synthesis. This regulation has been confirmed to occur at the whole cell level (Jones et al., 2000; Divecha et al., 2000; Skippen et al., 2002) and is expected to have functional consequences in both vesicle trafficking and actin reorganization (Anderson et al., 1999). A role of PLD in cell differentiation also has been proposed, in view of the sustained activation of the enzyme and/or increase in the expression of PLD isoforms that correlate with the progress of differentiation in keratinocytes (Jung et al., 1999) and in glioma and pheochromocytoma cells (Nakashima and Nozawa, 1999). However, neither the existence of a causal link between PLD activity and these differentiation processes nor the mechanisms involved have been addressed in these studies.
Skeletal myogenesis is a highly ordered process that allows the differentiation of proliferating myoblasts into multinucleated myotubes expressing the contractile apparatus. A coordinate induction of muscle-specific gene products occurs concomitantly with the cell morphological changes. Transcription factors of the basic helix-loop-helix muscle regulatory factors family, such as myogenin, which are activated during myogenesis, play a crucial role in these processes (Perry and Rudnick, 2000). Myogenesis takes place during histogenesis in the embryo and in regenerating adult muscle after tissue damage. It can be induced in culture, by depriving cycling myoblasts of serum, and/or by stimulating myoblasts with certain hormonal factors such as the neurohypophyseal hormone arginine-vasopressin (AVP). AVP is a potent inducer of myogenesis in different myogenic cell lines, including the fetal rat muscle-derived L6 line and in primary satellite cells (Teti et al., 1993; Nervi et al., 1995; Minotti et al., 1998). AVP is known to rapidly stimulate PLD activity in L6 myoblasts (Thompson et al., 1994; Naro et al., 1997). Furthermore, it has been demonstrated that AVP can induce myogenesis at nanomolar concentrations, allowing the activation of PLD without a concomitant activation of phospholipase C, which suggested a predominant role of the PLD signaling pathway in the first steps of the myogenic response (Naro et al., 1997). However, the requirement of PLD for myogenesis had not been directly demonstrated, and no data were available about the mechanism linking PLD activity with myogenic differentiation.
We thus set out to investigate the role of PLD in myogenic differentiation by using the L6 line and first sought to characterize the PLD system in these cells. We determined the pathways leading to PLD activation by AVP and the changes in PLD isoform pattern of expression accompanying cell differentiation. We studied in parallel the influence of alterations in PLD activity, induced by pharmacological inhibition or by ectopic PLD overexpression, on the differentiation of L6 cells. Because these studies supported the conclusion that PLD is required for myogenesis, we attempted to delineate a mechanism by which PLD activity and PA could intervene in induction of the differentiated phenotype. Different approaches, including F-actin labeling and imaging of PA with an intracellularly expressed fluorescent probe, led us to propose that the myogenesis promoting effects of PLD rely, at least in part, on cytoskeleton rearrangements directly involving PA–actin fibers interaction.
MATERIALS AND METHODS
Cell Culture
L6 cells of the C5 subclone (Teti et al., 1993, Minotti et al., 1998) were seeded at the density of 10,000/cm2 in DMEM supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated fetal bovine serum (FBS). Twenty-four hours after plating, cultures were washed and shifted to serum-free medium supplemented with 1% bovine serum albumin (BSA) (reference no. 652237; Roche Diagnostics, Mannheim, Germany). For differentiation studies, 10-7 M arg8-vasopressin (AVP) (V0377; Sigma-Aldrich, St. Louis, MO) was added, and the cells were cultured for various times as stated. In experiments involving an inhibition of phosphatidic acid formation, 0.5% 1- or 2-butanol was added before AVP, and the medium was changed every day to prevent alcohol evaporation. Alternatively, the cells could be differentiated by culture in 1% FBS-containing DMEM. Creatine kinase activity of the cells was assayed as described previously (Nervi et al., 1995).
PLD Activity Assay
PLD was evaluated on the basis of its transphosphatidylation activity, by quantitating phosphatidylbutanol accumulated in intact cells. The cells cultured in 10% FBS-containing medium (15 × 104 cells/35-mm dish) were shifted to serum-free medium and incubated for 2 h in the presence of 2 μCi/ml [3H]palmitic acid (PerkinElmer Life and Analytical Sciences). After two washes, the labeled cells were shifted to 1% BSA-containing medium and treated or not for 30 min (except when stated otherwise) by 10-7 M AVP in the presence of 1% 1-butanol. When required, 10-7 M 12-O-tetradecanoylphorbol-13-acetate (TPA) was added 24 h before labeling. In experiments using C3 exoenzyme from Clostridium botulinum (a generous gift of Dr. P. Boquet, Institut National de la Santé et de la Recherche Médicale Unit 452, Nice, France), the cells were washed by 2 ml of 114 mM KCl, 1 mM NaCl, 5.5 mM MgCl2, 10 mM Tris-HCl, pH 7.5, and gently scraped off in 0.5 ml of the same buffer containing 5 μg/ml C3 exoenzyme, so as to allow an efficient penetration of the toxin. The cells were then washed and plated in 10% FBS-medium. The attached cells showed no signs of toxicity. After 24 h, they were challenged with 10-7 M AVP, and PLD activity was assayed. PLD assay incubations were terminated by washing with ice-cold phosphate-buffered saline (PBS) and adding 0.5 ml of 0.1 N HCl in PBS. The cells were scraped and lipids were extracted according to Bligh and Dyer (1959), in the presence of 50 μM butylhydroxytoluene. Phosphatidylbutanol was separated by bidimensional thin layer chromatography (TLC) on silica gel G60 plates (Merck, Darmstadt, Germany) by using chloroform/methanol/28% ammonia (65:35: 5.5 by volume) as a solvent for the first migration and ethyl acetate/isooctane/acetic acid (90:50:20 by volume) for migration in the second dimension. Spots stained by Coomassie Brilliant Blue R were scraped off, and radioactivity was measured by liquid scintillation counting. The radioactivity associated with phosphatidylbutanol was expressed in percentage of total phospholipid radioactivity.
Immunoblotting Experiments
RhoA. The cells were kept in 1% BSA-containing medium for 3 h before being challenged by 10-7 M AVP. They were then homogenized in ice-cold 20 mM Tris-HCl, pH 7.6, containing a protease inhibitor cocktail (P2714; Sigma-Aldrich) diluted 1:4 in a Dounce homogenizer (30 strokes). The extracts were ultracentrifuged for 1 h at 105,000 × g. The pellet and supernatant fractions were analyzed by SDS-PAGE on a 15% polyacrylamide gel and immunoblotted with anti-RhoA polyclonal antibody (sc-179; Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:400.
Myogenin. After 48 h of culture in BSA medium in the presence of appropriate agents, the cells were homogenized as described above. The homogenates were analyzed on a 15% polyacrylamide gel and immunoblotted with F5D anti-myogenin monoclonal antibody (mAb) (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) diluted 1:50.
PLD1 and PLD2. After 8 d of culture in the specified medium, cell homogenates prepared in a 20 mM Tris-HCl, 100 mM NaCl, 1% Triton X-100, pH 7.6, buffer, including protease inhibitor cocktail, were mixed with Laemmli buffer supplemented with 2 M urea, boiled for precisely 1 min, and fractionated on a 8% SDS-polyacrylamide gel, including 4 M urea. The blots were probed with PLD1- or PLD2-specific polyclonal antibodies kindly provided by Dr. S. Bourgoin (Université Laval, Laval, Québec, Canada), diluted 1:2000. Immunoblotting with the anti-PLD1 antibody has been shown to induce the labeling of a protein band comigrating with recombinant PLD1, which was suppressed by neutralization of the antibody with the immunogenic peptides (Marcil et al., 1997). In our hands, both anti-PLD1 and anti-PLD2 antibodies labeled a major band comigrating with the corresponding recombinant PLD, in the above-described conditions (inclusion of urea and short heating of the samples were critical). Immunoblots were revealed with the enhanced chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ). After stripping, the membranes were reprobed for normalization, with an anti-α-tubulin mAb (T5168; Sigma-Aldrich). Videodensitometric quantitation of the bands was performed by using a charge-coupled device camera system (ImageMaster VSD-CL; Amersham Biosciences) and ImageQuant software. Proteins were assayed by the Bradford method.
Cytofluorescence Experiments
Myogenin Immunofluorescence. After 48 h of culture in BSA medium in the presence of appropriate agents, the cells were fixed by 3.7% formaldehyde for 10 min at 4°C, permeabilized with 0.1% Triton for 15 min at 4°C, and aspecific labeling was blocked in 1% BSA for 20 min. Anti-myogenin F5D mAb was added undiluted and incubated overnight at room temperature (RT). After extensive washing by 1% BSA, fluorescein- or rhodamine-conjugated (Jackson ImmunoResearch Laboratories, West Grove, PA) anti-mouse IgG antibody was added diluted 1:200 in 1% BSA, for 30 min.
Myosin Immunofluorescence. After 9 d of culture, the cells were treated as described above, with anti-sarcomeric myosin heavy chain MF20 mAb (kindly given by Dr. D. Fischman, Cornell University Medical College, New York, NY) diluted 1:50 and incubated for 1 h at RT, as a primary antibody. The nuclei were stained for 5 min with 1 μg/ml 4,6-diamidino-2-phenylindole (DAPI) (Molecular Probes, Eugene, OR).
Actin Labeling. The cells were kept in 1% BSA-containing medium for 3 h before being challenged by 10-7 M AVP for the time indicated. In experiments involving an inhibition of phosphatidic acid formation by butanol transphosphatidylation, the alcohol was added to the culture medium 10 min before the hormone. The cells were fixed and permeabilized as described above and stained for 5 min by rhodamine-conjugated phalloidin (Molecular Probes) diluted 1:400.
PLD1 Immunofluorescence. The cells were fixed with 3.7% formaldehyde for 30 min at RT, treated for 10 min with 50 mM NH4Cl, and with 0.1% BSA for 20 min. The anti-PLD1 polyclonal antibody described above, diluted 1:1000 in a 0.05% saponin, 0.1% BSA solution in PBS, was incubated overnight at 4°C. The secondary antibody was Alexa Fluor 546- or Alexa Fluor 488-conjugated anti-rabbit IgG antibody (Molecular Probes), diluted 1:1000.
PIP2 Immunofluorescence. The cells were fixed and treated as for PLD1. The primary antibody was a mouse PIP2-specific antibody (reference no. 90541; Assay Design, Ann Arbor, MI), diluted 1:500. The secondary antibody was fluorescein- or rhodamine-conjugated anti-mouse IgG antibody diluted 1:200. The cells were examined by fluorescence microscopy with a Zeiss Axiovert 200 microscope, an objective LD A-plan, 20×/0.30 PHI ∞/40, a Zeiss Axiocam MRm camera, and Axiovision 4.1 image acquisition software.
Transient Transfections
The hPLD1b- and hPLD2-carrying pCDNA3 plasmids were kindly provided by Dr. M. Record (Institut National de la Santé et de la Recherche Médicale Unit 563, Toulouse, France). The green fluorescent protein (GFP-PLD) constructs were prepared by inserting the hPLD1b and hPLD2 coding sequences at the SalI site, and Eco R I and SalI sites, respectively, of the pEGFP-C1 vector polylinker (BD Biosciences Clontech, Palo Alto, CA). The plasmid carrying the PA-specific fluorescent probe was constructed as described in Rizzo et al., 2000. Briefly, the sequence corresponding to the Raf-1 PA-binding domain (residues 390–426) was amplified by polymerase chain reaction (PCR), with pCDNA3-HA1-cRaf-1 plasmid (a gift from Dr. A. Eychene, Centre National de la Recherche Scientifique Unit 146, Orsay, France) used as template and inserted into pEGFP-C1 vector. The mutated probe was prepared by introducing a double arginine-to-alanine mutation, corresponding to Raf residues 398 and 401, into this construct with the QuikChange mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. The correctness of the obtained plasmids was verified by restriction analysis and sequencing. Plasmid DNA (2 μg for pCDNA3 constructs, 3 μg for pEGFP-C1 constructs) was mixed with diluted FuGENE 6 (Roche Diagnostics) (94 μl of serum-free medium + 6 μl of FuGENE) and left in contact for 30 min. The mix was then added dropwise to 200,000 L6 cells in suspension in 1 ml of 10% FBS-medium. The cells were plated and cultured for 18 h in the same medium. pCDNA3-PLD–transfected cells were then switched to 1% BSA-medium and cultured for additional 24 h in the presence or absence of AVP, and myogenin nuclear accumulation was assessed by immunofluorescence. Cells transfected with pEGFP-Raf-1-PA binding domain constructs were switched to 1% BSA-medium for 3 h and then treated or not for 10 min with 10-7 M AVP or for 15 min with 1 nM TPA, before fixation, actin staining with rhodamine-conjugated phalloidin and fluorescence microscopy examination. Cells transfected with the pEGFP-PLD constructs were submitted to myogenin immunofluorescence assessment immediately after the 18 h culture in 10% FBS-medium.
Reverse Transcriptase (RT)-PCR
Total RNA was isolated from L6 cells by using TriReagent (Sigma-Aldrich), as indicated by the manufacturer. RNA samples (5 μg) were reverse transcribed using Moloney murine leukemia virus reverse transcriptase and oligo-dT (Promega, Madison, WI). Specific primers for the amplification of rat PLD1 transcripts designed to discriminate between rPLD1a and rPLD1b splicing variants were 5′-AGGACAGTCTCTGGGCTCTC-3′ (sense) and 5′-TGCCTTTCCGTGAACCACAG-3′ (antisense). Primers designed for the amplification of rat PLD2 transcripts were 5′-TGAACAGGGGCAGTGTTTCC-3′ (sense) and 5′-AGGTCTGGCCAGGTATTTGC-3′ (antisense). β-Actin transcripts were amplified using primers 5′-TCATGAAGTGTGACGTTGACATCCGT-3′ (sense) and 5′-CCTAGAAGCATTTGCGGTGCACGATG-3′ (antisense). PCRs were carried out with 2 U/sample of Taq polymerase (Roche Diagnostics), by performing 40 cycles of 94°C for 45 s, 54°C for 45 s, and 72°C for 45 s for PLD1 amplification; 35 cycles of 94°C for 45 s, 56°C for 45 s, and 72°C for 30 s for PLD2 amplification; and 35 cycles of 94°C for 45 s, 65°C for 45 s, and 72°C for 30 s for β-actin amplification. The PCR products were analyzed on a 2% agarose gel, by ethidium bromide staining.
Assay of Newly Synthesized PIP2 in Response to AVP Stimulation
All procedures were performed at 37°C, in six-well plaques, in phosphate-free and serum-free medium. The cells were labeled by addition of 50 μCi/ml [33P]Pi (Amersham Biosciences). Ten minutes later, they were treated or not with 1% 1-butanol or 2-butanol for an additional 10 min. AVP (1 nM) was then added for 5 min. This AVP concentration is known to trigger PLD activation but not PIP2 hydrolysis by PLC in L6 cells (Naro et al., 1997). The reaction was stopped by addition of 1 ml of methanol per well, and lipids were extracted according to Bligh and Dyer (1959) and analyzed by TLC as described in Apgar (1995). The plaques were autoradiographed in a Storm PhosphorImager, and PIP2 spots were quantified by ImageQuant software.
RESULTS
PLD Is Activated in L6 Cells by AVP, an Inducer of Myogenic Differentiation
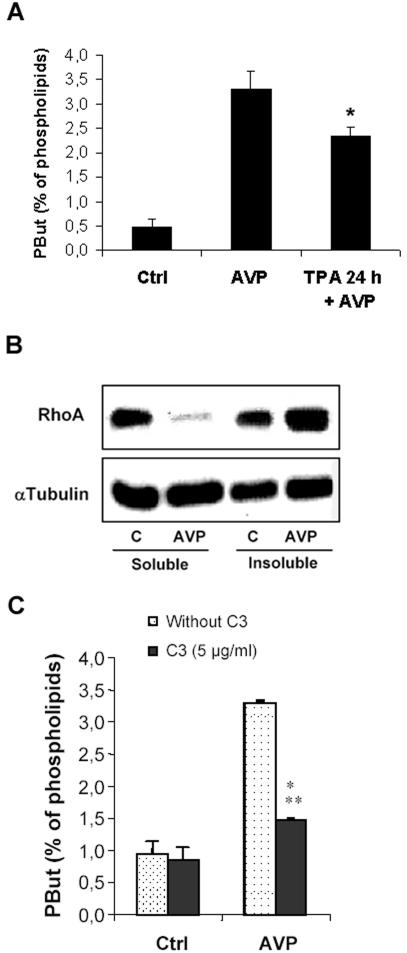
As it has been previously reported, AVP, a well-characterized inducer of L6 cell myogenic differentiation in vitro, was able to markedly activate PLD. The activity measured by quantitation of phosphatidylbutanol accumulated in intact cells was already increased after 1 min of treatment by the hormone, reached a plateau at 5 min, and remained stable for up to 30 min (our unpublished data). It has been established in a number of cell systems that PLD can be activated through various pathways, including activation of monomeric G proteins and PKCs (Exton, 2002). Down-regulating PKCs by a 24-h cell pretreatment with 10-7 M TPA had only a limited effect on AVP-stimulated activity of intact cells (-29%) (Figure 1A), indicating that PKCs only take a minor part in this stimulation. We then investigated the possible involvement of the monomeric G protein Rho. As a preliminary step, we looked for an effect of AVP on the subcellular localization of Rho. We observed that the hormone induced the translocation of RhoA from the soluble to the insoluble fraction, an event reflecting the conversion of Rho to the activated GTP-bound state (Seasholtz et al., 1999), thus establishing that AVP actually activates RhoA in these cells (Figure 1B). The effects of C. botulinum C3 exoenzyme, which specifically inactivates Rho, were then tested. As shown in Figure 1C, C3 exoenzyme treatment strongly decreased (-74%) the activation of PLD induced by AVP, suggesting that PLD in this system is preponderently under the control of the Rho pathway.
Figure 1.
AVP activates PLD in L6 cells mainly through Rho pathway. (A) PLD activity in L6 cells, as evaluated by phosphatidylbutanol formation in intact cells. The cells were labeled with [3H]palmitate, treated or not (control) for 30 min with 10-7 M AVP, in the presence of 1% butanol, before lipid extraction and analysis. When required, the cells were pretreated by 10-7 M TPA for 24 h. *, different from treatment by AVP alone, p < 0.05 (n = 3). (B) Immunoblotting of RhoA protein in soluble versus insoluble cell fractions. The cells were treated or not (control) by 10-7 M AVP for 1 min before harvesting, homogenizing, and fractionating the extracts by ultracentrifugation. Equal amounts of proteins from each fraction (30 μg) were deposited on SDS gels. The blots were probed with a RhoA-specific antibody, stripped, and reprobed with a tubulin-specific antibody to assess equal loadings. (C) Effect of C3 exoenzyme on AVP-induced PLD stimulation. The cells were scraped off and treated or not in suspension by C3 exoenzyme. After plating, they were challenged or not (control) with 10-7 M AVP and PLD activity was assayed. ***, different from C3-untreated cells, p < 0.0001, n = 3.
PLD Activity Is Required for Myogenic Differentiation of L6 Cells
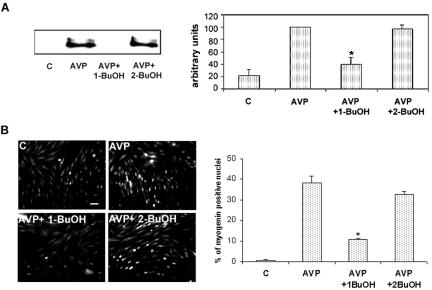
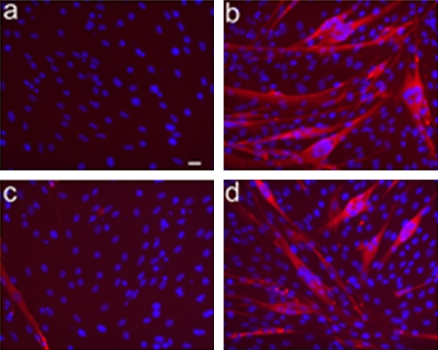
To investigate whether PLD activity is required for the differentiation of L6 cells, we prevented the formation of the natural PLD product PA by addition of 1-butanol to the medium, which reroutes enzyme activity toward the formation of phosphatidylalcohol. This treatment markedly inhibited AVP-induced differentiation, as evaluated by the expression of the muscle-specific transcription factor myogenin, an early marker of myogenesis. Immunoblotting experiments showed that 1-butanol reduced by 60% myogenin expression induced by AVP after 48 h of culture (Figure 2A). Control treatment by 2-butanol, an isomer that is not recognized by PLD, had no effect on myogenin expression, which rules out aspecific effects of alcohol treatment (Figure 2A). Commitment of L6 cells into myogenesis also was assessed by the extent of nuclear accumulation of this transcription factor, a crucial early step of the differentiation process. The percentage of myogenin-positive nuclei in AVP-treated cells was thus evaluated by immunofluorescence (Figure 2B). 1-Butanol strongly reduced the percentage of myogenin positive nuclei by 72%, whereas 2-butanol exhibited little effect (-14%). To verify that the blockade of PA production also affected terminal differentiation, AVP-treated cells were cultured for 9 d in the absence or presence of alcohols (Figure 3). The control cells fused, giving rise to large differentiated myotubes expressing sarcomeric myosin, as evidenced by immunofluorescence. 1-Butanol addition totally prevented myotube formation, even though a small percentage of cells still accumulated myosin, whereas 2-butanol had no notable effects. Similar observations were made with myoblasts of the mouse satellite cell line C2C12 induced to differentiate by culture in 2% horse serum-containing medium (our unpublished data). Collectively, these results show that a reduction in the production of PA strongly inhibited the early phases of the differentiation process and prevented myotube formation, suggesting an essential role of this signaling pathway.
Figure 2.
AVP induces myogenin expression and nuclear accumulation in a PLD-dependent way. (A) Immunoblots of myogenin from L6 cells treated for 48 h by AVP alone or in the presence of 0.5% 1-butanol or 0.5% 2-butanol. Videodensitometric quantitation of myogenin protein: the blots were reprobed for tubulin, and myogenin amounts were normalized by tubulin. The average of three different experiments is shown in the diagram. *, different from AVP alone, p < 0.01. (B) Immunofluorescence of myogenin in L6 cells treated for 48 h by AVP alone, or in the presence of 0.5% 1-butanol or 0.5% 2-butanol. Nuclear myogenin was revealed by using a monoclonal antimyogenin antibody and a fluorescein-conjugated secondary antibody. The total number of nuclei was evaluated on the phase contrast image. The average percentages of myogenin-positive nuclei counted in five to nine different fields (∼70 cells per field) in one experiment are shown in the diagram. *, different from AVP alone, p < 0.01. Three experiments gave similar results. Full reversibility of the effects of a 15-min treatment by 1-butanol on myogenin nuclear accumulation was verified (our unpublished data). Bar, 40 μm.
Figure 3.
1-Butanol suppresses AVP-induced myotube formation. The cells were cultured for 9 d in 1% BSA medium, in the absence (a) or presence (b–d) of AVP, with the addition of 0.5% 1-butanol (c) or 2-butanol (d). Sarcomeric myosin immunofluorescence was then examined, by using a specific mAb and a rhodamine-conjugated secondary antibody, to assess the formation of terminally differentiated myotubes. The nuclei were labeled by DAPI staining. No signs of toxicity were apparent in the presence of alcohols. Bar, 20 μm.
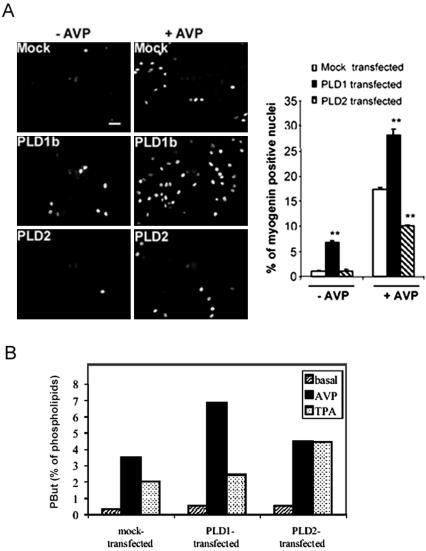
To further support the hypothesis of an involvement of PLD in myogenic differentiation, L6 cells were transiently transfected with PLD1b and PLD2 isoforms, and their ability to accumulate nuclear myogenin was evaluated after 24 h (Figure 4A). In the absence of AVP in the culture medium, control cells transfected with the empty vector showed very few signs of differentiation, whereas a substantial percentage of the PLD1b-transfected cells started to differentiate, as shown by nuclear myogenin labeling. AVP treatment induced an appreciable fraction of control cells to differentiate, and this was markedly potentiated by PLD1b overexpression, which increased by 62% the fraction of myogenin-positive cells. By contrast, overexpression of PLD2 had a negative effect, decreasing myogenin-positive cells by 42%. Thus, it seems that PLD1b is positively involved in differentiation, whereas PLD2 rather hampers this process. As a control of the efficiency of overexpression, PLD activity was evaluated in transfected L6 cells (Figure 4B). Both PLD1b- and PLD2-transfections induced a substantial increase in basal PLD activity (+83 and +63%, respectively). AVP-stimulated PLD activity was markedly higher in PLD1b-overexpressing cells than in control (mock-transfected) cells (+95%), whereas it was only weakly increased in PLD2-overexpressing cells (+27.5%), suggesting that AVP preferentially activated PLD1. Conversely, TPA-stimulated PLD activity was increased to a higher extent in PLD2-overexpressing cells than in PLD1b-overexpressing cells, with respect to control cells (+120 and + 21%, respectively), suggesting that TPA activated more efficiently PLD2 than PLD1. To confirm the ability of PLD1 overexpression to enhance differentiation, L6 cells were transfected with GFP-tagged PLD, and myogenin nuclear accumulation was assessed in the sole subset of cells expressing the green fluorescent construct, in the absence of AVP. 42 ± 3% of cells expressing GFP-PLD1b were myogenin positive versus 0% of cells expressing unconjugated GFP or GFP-PLD2 (mean ± SE of 3 experiments).
Figure 4.
Effects of overexpression of the PLD isoforms on myogenin nuclear accumulation. L6 cells were transfected by either the empty pCDNA3 vector (Mock) or PLD1b- or PLD2-carrying vector. (A) Cells were treated or not by AVP for 24 h, and myogenin was detected by immunofluorescence, by using a rhodamine-conjugated secondary antibody. The average percentages of myogenin-positive nuclei counted in 10 different fields (∼140 cells/field) are shown. **, different from mock-transfected cells, p < 0.0001. Similar efficiencies of transfections were verified by cotransfecting the pEGFP-C1 plasmid and evaluating the percentages of green fluorescent cells. Three independent experiments gave similar results. Bar, 40 μm. (B) As a control, PLD activity was assayed in transfected cells, in the absence (basal) or presence of 10-7 M AVP or 1 nM TPA (n = 3, SE ≤ 10%).
PLD1 Is Selectively Down-Regulated in the Course of Myogenic Differentiation
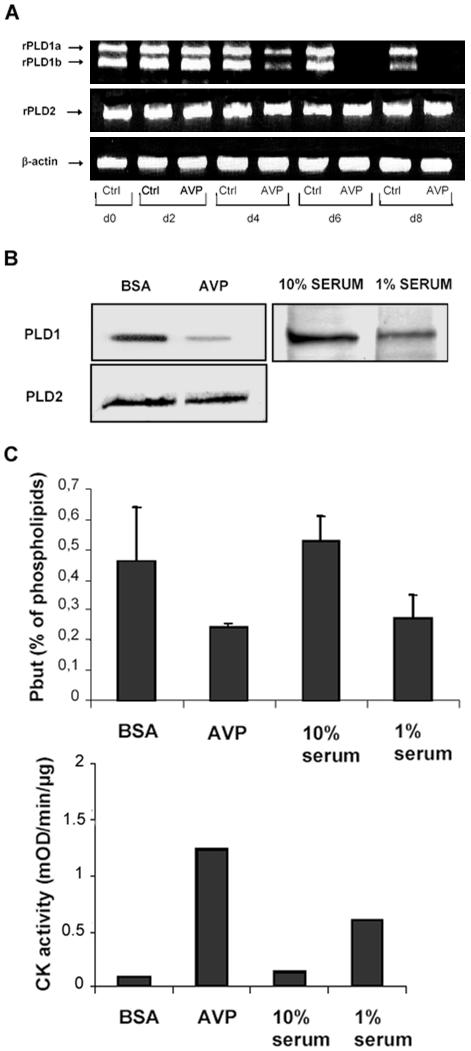
Because the above-mentioned experiments suggested that the PLD isoforms differently affect myogenic differentiation, we determined their patterns of expression in undifferentiated myoblasts and in cells undergoing differentiation. As shown in Figure 5A, RT-PCR experiments showed that undifferentiated myoblasts, either proliferating or maintained quiescent in serum-free medium, expressed two different mRNA species issuing from the expression of the PLD1 gene. The sizes of the amplified fragments were the ones expected for PLD1a and PLD1b splicing variants. The cells also expressed one mRNA species deriving from PLD2 gene. Both PLD1a and PLD1b mRNAs were down-regulated starting from day 4 of AVP treatment, and they became hardly detectable at days 6–8, when the cells were fully differentiated into multinucleated myotubes. In contrast, PLD2 mRNA was expressed at a constant level during differentiation. Furthermore, immunodetection experiments showed that in cells treated for 8 d with AVP and expressing the terminal differentiation marker creatine kinase, the amount of PLD1 protein was reduced by 60% compared with resting cells cultured in serum-free medium (Figure 5B). Similarly, cells differentiated by culture in a low-serum medium had only 42% of the PLD1 protein amount detected in proliferating myoblasts (cultured in high-serum medium), showing that down-regulation of PLD1 was linked to differentiation itself, regardless of the method of its induction and not restricted to AVP effects (Figure 5B). In contrast, no significant changes in the amount of PLD2 protein were detected during cell differentiation.
Figure 5.
PLD1 is selectively down-regulated in the course of myogenic differentiation. (A) RT-PCR was performed with total RNA from cells cultured for 0–8 d in 1% BSA medium, in the presence or absence (control) of AVP. Primers specific for either PLD1, PLD2, or β-actin (for normalization) were used. The result shown is representative of three independent experiments. (B) Immunoblots of PLD1 and PLD2 from L6 cells differentiated by 8-d culture in either 1% BSA-containing medium in the presence of AVP (control, cells cultured without AVP) or in 1% serum-containing medium (control, cells cultured in 10% serum-containing medium). Blots were reprobed for tubulin, for normalization (our unpublished data). Videodensitometry quantitation of PLD proteins, after normalization by tubulin amounts, showed that the amount of PLD1 protein in AVP-treated cells was 40.0 ± 10.0% of that in the control cells (n = 6); in 1% serum-cultured cells, it was 41.8% of that in the 10% serum-cultured cells (n = 2). (C) Basal PLD activity of quiescent cells (BSA), proliferating cells (10% serum), and cells differentiated by either a 8-d AVP treatment or 8-d culture in 1% serum medium. The means of four independent experiments are shown. As a control for cell differentiation, the activity of the differentiation marker creatine kinase was assayed after the different treatments.
To evaluate the consequences of the decrease in PLD1 expression accompanying differentiation on the PLD activity of L6 cells, we compared the basal activity of cells submitted or not to a 8-d differentiation treatment. We observed an ∼50% decrease in activity for cells differentiating in the presence of AVP, compared with cells maintained quiescent in serum-free medium, as well as for cells differentiating in low-serum medium, compared with proliferating cells maintained in high-serum medium (Figure 5C).
Myogenic Stimulation Induces the Formation of Stress Fiber-like Actin Structures in a PLD-dependent Way
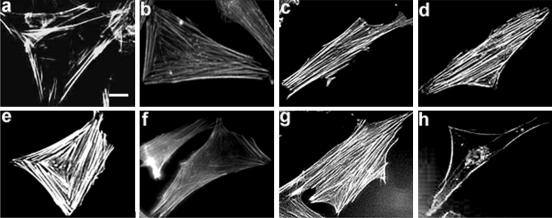
It has been reported that AVP induces the formation of actin stress fibers in fibroblasts expressing heterologous vasopressin V1a receptor (Gohla et al., 1999), and PLD activity is known to be involved in stress fiber setting up in endothelial cells or in fibroblasts stimulated by lysophosphatidic acid (Cross et al., 1996; Kam and Exton, 2001). On the other hand, cultured myogenic cells (Holtzer et al., 1985), or human muscle satellite cells induced to differentiate by switching to low-nutrition medium (van der Ven et al., 1993), display SFLSs constituted by actin filaments, which are considered as scaffolds directing the assembly of nascent striated myofibrils in the course of myogenesis. We thus examined whether AVP affected actin fiber formation in L6 cells, and were it the case, whether PLD was involved in this response. Labeling with phalloidin-rhodamine conjugate showed that AVP treatment induced the rapid appearance of actin SFLSs. The fibers started to be detectable at 30 s, becoming denser during the next 10 min, and then remaining stable for at least 1 h (Figure 6, a–e). Their kinetics of formation was thus plainly compatible with an involvement of PLD. To investigate the role of PLD in this effect, the cells were treated by AVP in the presence of either 1-butanol or 2-butanol. The primary alcohol totally suppressed SFLS formation (Figure 6f), whereas the secondary alcohol had little effects (Figure 6g). In further support to the hypothesis of PLD involvement in SFLS formation in L6 cells, we observed that TPA, which activates PLD in these cells (Figure 4B), also efficiently promoted SFLS formation (our unpublished data). To confirm PLD involvement in SFLS formation, the effect of PLD1b overexpression was examined; 31.5 ± 2.1% of L6 cells transiently transfected with pCDNA3-PLD1b formed SFLSs versus 2.5 ± 1.0% of mock-transfected cells. This response was significantly amplified by AVP stimulation (61.1 ± 3.7% of SFLS-forming cells). 1-Butanol suppressed the formation of SFLSs induced by PLD1b overexpression and AVP treatment (4.8 ± 1.3% of SFLS-forming cells; means ± SE of 10 fields). It can be concluded that PLD1 participates in the mechanism leading to SFLS formation in myoblasts induced to differentiate. Consistent with this notion, we observed that C3 exoenzyme pretreatment, which strongly counteracts PLD stimulation by AVP, totally suppressed SFLS formation (Figure 6h). However, because C3 exoenzyme also suppresses the Rho-mediated activation of other effectors that are necessary, together with PLD, to the organization of actin fibers (Kam and Exton, 2001), it cannot be concluded that PLD is the only factor set in motion by AVP and responsible for SFLS formation.
Figure 6.
Stress fiber-like actin structures are formed in a PLD-dependent way in L6 cells stimulated by AVP. Cells treated for 0–60 min by AVP, were fixed, labeled with rhodamine-phalloidin, and observed by fluorescence microscopy (a–e, times 0, 30 s, 1 min, 10 min, and 60 min). The effects of a cotreatment by 1% 1-butanol (f) or 1% 2-butanol (g), or of a pretreatment by C3 exoenzyme (h) on the SFLS formation induced by a 10-min AVP treatment are shown. SFLSs were present in 56 ± 6% of AVP-treated cells after 10 min versus 2.0 ± 0.9% of control cells. In the presence of 1-butanol, only 1.6 ± 0.6% of AVP-treated cells displayed SFLSs versus 40 ± 3.3% for 2-butanol + AVP-treated cells (means ± SE of 10 fields, ∼300 cells). The inhibition of SFLS formation by butanol was reversed by washing out the alcohol (our unpublished data). Bar, 5 μm.
PLD1 Is Present at the Level of SFLSs
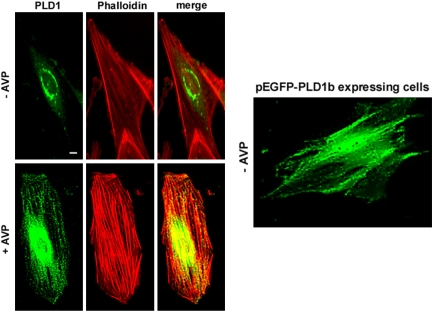
In the light of the data suggesting an involvement of PLD in SFLS formation, we wondered whether PLD localizes to these structures. We thus performed immunofluorescence experiments to detect endogenous PLD1 by using a PLD1-specific antibody, before and after stimulation of the cells. In control cells, PLD1 was localized in a vesicular perinuclear compartment (Figure 7, top left). AVP stimulation of L6 cells induced a partial relocalization of PLD1, which, in addition to the perinuclear region, was distributed according to a pattern suggestive of filamentous structures, coinciding with actin fibers (Figure 7, bottom left). As a confirmation, the localization of GFP-tagged PLD1b was examined in AVP-stimulated live cells. Similarly to endogenous PLD1, the GFP-PLD1b fusion protein distribution was found to follow fibrous structures parallel to the axis of the cell (Figure 7, right). By contrast, immunofluorescence of PLD2 showed a localization in a diffuse perinuclear area, without any labeling of actin fibers, in resting cells as well as in AVP-stimulated cells (our unpublished data).
Figure 7.
Localization of endogenous and overexpressed PLD1 in L6 cells. Quiescent (top left) or 10 min AVP-stimulated cells (bottom left) were fixed, and PLD1 was detected by immunofluorescence by using a specific antibody. Colabeling of actin was performed with phalloidin, and the images were merged. Fluorescence of GFP-PLD1b expressed in live cells stimulated for 10 min by AVP was examined (right). Bar, 5 μm.
PA Selectively Accumulates at the Level of SFLSs in Response to Myogenic Stimulation
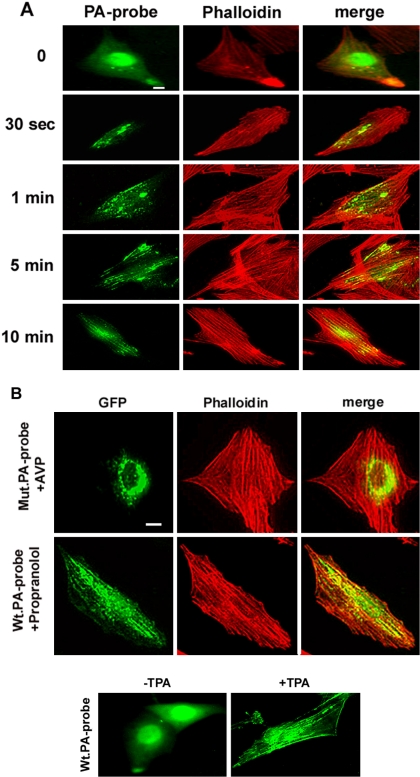
To further delineate the role of the PLD product PA in the response of myoblasts to an inducer of differentiation, a fluorescent PA-specific probe consisting in the PA binding domain of Raf-1 kinase (Ghosh et al., 1996) linked to GFP was expressed in myoblasts. This probe allows to monitor the spatiotemporal changes in PA production at the single-cell level (Rizzo et al., 2000). As shown in Figure 8 A, fluorescence was diffuse in the cytosol and nuclei of resting cell, whereas AVP stimulation induced a dramatic translocation of the probe, concomitant with SFLS appearance. Indeed, from 0.5–1 min of hormone treatment fluorescently labeled vesicles started to form and line up. After 5–10 min, actin fibers were clearly labeled: the green fluorescence followed dotted lines suggestive of an alignment of small vesicules coinciding with the fibers (see merging). As expected from the experiment of Figure 6, when PA probe-expressing cells were pretreated by 1-butanol, AVP induced neither SFLSs, as evidenced by phalloidin labeling, nor green fluorescent labeling of fibrous structures (our unpublished data).
Figure 8.
Labeling of PA with an endogenously expressed PA-specific fluorescent probe. (A) L6 cells expressing the Raf1 PA-binding domain fused to GFP (PA-probe) were examined for green fluorescence and for rhodamine-phalloidin–stained actin, after treatment by AVP for the time indicated. The images were merged. (B) A probe mutated for arginine residues essential for PA binding (mutPA-probe) was expressed in the cells. After a 10-min AVP stimulation, the cells were examined as described above (top). L6 cells expressing the unmutated PA-probe were treated by 100 μM propranolol for 15 min, before being examined as above (middle). In the presence of propranolol, 24 ± 2.0% of the cells expressed SFLSs versus 3.5 ± 1.1% of untreated cells (means ± SE of 10 fields, ∼200 cells). L6 cells expressing the unmutated PA-probe were treated or not (control) by 1 nM TPA for 15 min, before being examined for probe fluorescence (bottom). Bar, 5 μm.
As a control for the specificity of PA labeling, a mutated probe, in which two arginine residues essential for PA binding were switched to alanine (Rizzo et al., 2000), was expressed in the cells. Under AVP treatment, this mutated probe led to a labeling pattern clearly different from the one induced by expression of the “wild-type probe,” because it labeled vesicular structures in the perinuclear region, without coincidence with actin fibers (Figure 8B, top). To confirm the specificity of the probe for PA, cells overexpressing the “wild-type probe” were treated with propranolol, an inhibitor of PA-phosphatase able to induce accumulation of PA by blocking its metabolism, even in the absence of cell stimulation (Naro et al., 1997, Grange et al., 2000). Interestingly, propranolol by itself was able to stimulate the formation of SFLSs in a substantial percentage of cells and to induce the localization of the probe to these structures (Figure 8B, middle), further demonstrating that the probe specifically recognized PA at the level of SFLSs. Moreover, it suggested that PA, by itself, was able to trigger actin fiber assembly. In agreement with specific labeling of PA accumulated at the level of SFLSs, TPA treatment, which induces both PLD activation and SFLS formation, also provoked the localization of the probe along parallel fibrous structures. In this case, an additional labeling of cell edges could be noticed, suggesting that PA also was accumulated at the plasma membrane level (Figure 8B, bottom).
PIP2 Is Accumulated at the Level of SFLSs after AVP Stimulation
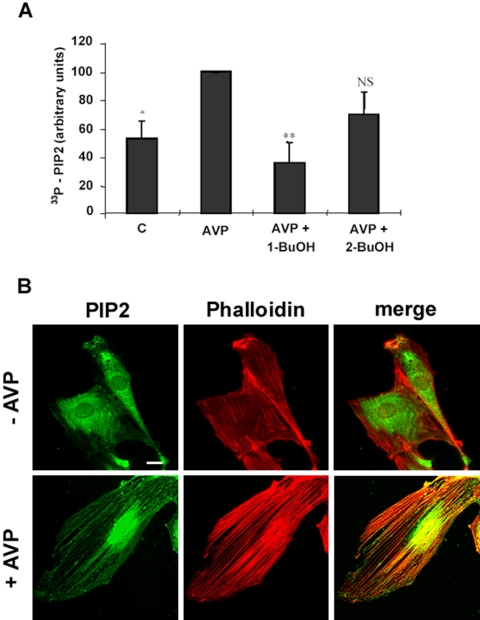
Because the existence of close relationships between PA production and the synthesis of the major signaling phospholipid PIP2 is well documented, we measured the amount of newly synthesized PIP2 formed in response to AVP stimulation. As shown in Figure 9A, AVP induced a marked increase of PIP2 synthesis, which was totally counteracted by 1-butanol, but not by 2-butanol, and thus involved the mediation of PLD activity. We also investigated the localization of PIP2 in L6 cells, by using a specific antibody. In AVP-stimulated cells, PIP2 seemed to be located in a densely labeled area around the nucleus and also in vesicles aligned along the actin fibers according to a pattern similar to what observed for PA (Figure 9B). This shows that PIP2 was locally accumulated in regions where PA itself was formed and raises the possibility that PA induced PIP2 synthesis at the level of SFLSs, through PI-4-P 5-kinase stimulation.
Figure 9.
PIP2 is synthesized and accumulated at the level of SFLSs after AVP stimulation. (A) Newly synthesized PIP2 was assayed by short-term metabolic labeling with [33P]Pi before AVP stimulation, in the absence or presence of 1% 1- or 2-butanol. The amount of PIP2 is expressed in percentage of the +AVP value. The average of three independent experiments is shown. *, different from the +AVP value, p < 0.05; **, p < 0.01; NS, not significantly different. (B) Quiescent (top) or 10-min AVP-stimulated cells (bottom) were fixed, and PIP2 was detected by immunofluorescence by using a specific antibody. Colabeling of actin was performed with phalloidin, and the images were merged. Bar, 5 μm.
DISCUSSION
We have considered in the present work the hypothesis of an involvement of PLD in the triggering of the myogenic program. Several results strongly support this hypothesis. First, AVP, a potent inducer of in vitro myogenic differentiation (Nervi et al., 1995; Minotti et al., 1998), rapidly stimulates L6 cell PLD activity, through G protein-coupled V1a receptor activation (Naro et al., 1997; this study). Furthermore, we observed that the reduction of the output of the normal PLD product PA in the presence of 1-butanol efficiently inhibited myogenesis, whereas control treatment by 2-butanol had only minor effects. Finally, we showed that overexpression of the PLD1b isoform significantly promoted differentiation. It thus seems that PLD, and more specifically PLD1, plays an essential role in the process of myogenic differentiation. In agreement with this conclusion, we have observed that TPA, which strongly activates PLD in L6 myoblasts, is able to promote complete myogenic differentiation at appropriate concentrations (our unpublished data).
Surprisingly, in contrast to PLD1, forced expression of the PLD2 isoform exerted negative effects on differentiation. This constitutes another example of the differences in functions of PLD1 and PLD2 in a given cell type. In mast cells, PLD2 and not PLD1 is involved in membrane ruffling after antigen stimulation (O'Luanaigh et al., 2001). PLD2 is also the isoform involved in extracellular signal-regulated kinase activation by insulin in HIRcB cells (Rizzo et al., 1999). Conversely, PLD1 participates in stress fiber formation in fibroblasts, whereas PLD2 does not (Kam and Exton, 2001). The differences in physiological roles of the products of the two PLD genes may be connected with their distinct subcellular localizations, which have been repeatedly reported (Colley et al., 1997, Diaz et al., 2002). It is thus possible that PLD2 induces, unlike PLD1, PA accumulation in a compartment unrelated with the molecular actors of myogenesis.
We found that, like in many other cell types, both PLD1 and PLD2 genes were expressed in proliferative or quiescent myoblasts, PLD1 giving rise to the two splicing variants PLD1a and PLD1b. In addition, we observed that the PLD1 isoforms, but not the PLD2 isoform, were down-regulated during differentiation, both at the level of mRNAs and proteins. This regulation was reflected by an ∼50% decrease in basal PLD activity in differentiated myotubes. The residual activity might be attributed in large part to PLD2, because this isoform is reported to have a high constitutive activity in the absence of stimulation (Colley et al., 1997). This differential behavior of PLD1 and PLD2 during differentiation is in agreement with the proposed distinct roles for the two enzyme forms. It might seem contradictory to assume that PLD1 plays an essential role in the differentiation process, in view of the progressive disappearance of this enzyme form. However, considering that PLD is activated very rapidly (within minutes) in AVP-treated cells, that the early responses to differentiative signals are already apparent in a short span of time (0.5–10 min for the SFLS formation, 24 h for the expression and nuclear accumulation of myogenin), and that PLD1 mRNA down-regulation only starts around day 4 of treatment, an involvement of PLD1 in the early steps of myogenesis is plainly compatible. By contrast, the later steps of differentiation occurring after day 4, such as expression of contractile proteins and cell fusion, might not directly depend on the presence of active PLD1. In fact, the decreased expression of PLD1 in differentiated myotubes is in line with the lack of detectable PLD1 transcripts in adult rat muscle (Katayama et al., 1998).
We also determined that PLD activation by AVP in L6 myoblasts largely depends on the mediation of Rho, because it was strongly reduced by the botulinic toxin C3. PKCs also were involved, although to a lesser extent. That Rho is an upstream effector of PLD in AVP-stimulated L6 myoblasts is consistent with our observation of AVP-induced Rho translocation in these cells. The involvement of Rho in PLD activation has been reported to occur in a variety of cell systems (Exton, 2002) and in particular in insulin-dependent PLD activation in L6 myotubes (Standaert et al., 1996). Rho directly activates purified PLD1 in in vitro reconstitution assays (Hammond et al., 1997), and requirement for a direct contact between Rho and PLD1 for the activation of the enzyme during agonist signaling has been confirmed in intact cells by the use of overexpressed interaction-deficient point mutants of PLD1 (Du et al., 2000). It is well known that Rho and PLD can be activated by various seven-transmembrane domain receptors. Various studies demonstrate that the activation of Rho by G protein-coupled receptors is in most cases mediated by Gq/11, G12, or G13 heterotrimeric G proteins (Seasholtz et al., 1999). Interestingly, AVP induces stress fiber formation, a Rho-dependent phenomenon, through the mediation of Gα12 in fibroblasts (Gohla et al., 1999). It is thus likely that Rho-dependent PLD stimulation by AVP in L6 myoblasts involves G proteins of the G12 type, but this point has still to be clarified. RhoA has been shown to play an essential role in myogenic differentiation (Takano et al., 1998; Carnac et al., 1998; Wei et al., 1998). Furthermore, it has been proposed that Rho activation is a determining factor of the fate of precursor cells toward the myogenic phenotype. Indeed, embryonic fibroblasts undergo either adipogenic or myogenic differentiation, according to the activation status of Rho, a high level of activation directing the differentiation toward myogenesis (Sordella et al., 2003). On the basis of our observations, we hypothesize that the myogenic effects of Rho depend in part on its ability to activate PLD.
Although PLD has been reported to play a role in the differentiation of several cell types, the delineation of the mechanisms by which PLD activity influences the differentiation processes has not been achieved. Concerning the involvement of PLD in myogenesis, an intervention at different levels can be considered. First, PLD could act through cross-talk with other signaling pathways. We have reported that a cAMP-specific phosphodiesterase isoform, PDE4D3, is activated by PA binding and that PA can thus regulate cellular cAMP levels (Grange et al., 2000). This enzyme is expressed in L6 cells, and PA accumulated after PLD activation might thus be responsible, in part, for the rapid stimulation of phosphodiesterase observed upon AVP addition (Naro et al., 1999). Because the myogenic differentiation process requires a decrease in cAMP levels (Naro et al., 1999), PA activation of PDE4D3 might participate in the early steps of myogenic response.
Another level at which PLD might influence the differentiation process is muscle-specific gene expression, because inhibition of PA production by 1-butanol efficiently decreased the expression of myogenin, an essential transcription factor in myogenesis (Perry and Rudnick, 2000). Interestingly, it has recently been shown in L6 cells that AVP up-regulates myogenin expression through enhancement of the expression of the MEF2 transcription factor acting upstream (Scicchitano et al., 2002). In other respects, RhoA itself has been shown to be involved in transcriptional activation, through the regulation of serum response factor (SRF) (Hill et al., 1995). Active RhoA is required for the expression of both muscle transcription factors MEF2 and myogenin (Takano et al., 1998), and the induction by RhoA of muscle-specific genes, including myogenin, involves the activation and increased expression of SRF (Wei et al., 1998). The hypothesis of an involvement of PLD in the mechanism linking RhoA and SRF activation should be considered, in view of the reported existence of a direct link between actin cytoskeleton reorganization and SRF-promoted gene transcription (Sotiropoulos et al., 1999). This study demonstrates that signals affecting actin polymerization, including RhoA activation, regulate SRF transcriptional activity, probably through the sensing of G-actin cellular levels. The mechanism of regulation of SRF transcriptional activity involves coactivators, the nuclear import of which seems to depend on G-actin, and thus on Rho effects on actin treadmilling (Miralles et al., 2003). The involvement of PLD in actin rearrangements is well accepted (Cross et al., 1996; Kam and Exton, 2001; O'Luanaigh et al., 2002). Furthermore, we observed in the present study that 1) AVP simultaneously activated PLD and the formation of stress fiber-like actin structures in L6 cells; 2) this effect was mimicked by propranolol, a pharmacological agent that induces PA accumulation independently of PLD, and by TPA, an activator of PLD; 3) AVP effect was counteracted by 1-butanol but not 2-butanol; 4) overexpression of PLD1b induced the formation of SFLSs; 5) part of PLD1 was localized to actin fibers in AVP-stimulated cells; and 6) PA was selectively accumulated at the level of these structures. On the whole, these data strongly suggest that SFLSs are formed in a PLD-dependent manner. We can thus propose the hypothesis that PLD is involved in muscle-specific gene transcription through the control of actin cytoskeleton remodeling and the resulting SRF activation.
The PLD-dependent remodeling of actin cytoskeleton also could participate in promoting myogenic differentiation in another way. In fact, unlike nonmuscle cells in which stress fibers might primarily control cytoarchitecture, the stress fiber-like actin structures play a peculiar role in differentiating myogenic cells, because they are involved in the very early stages of myofibrillogenesis. The existence of striking topographical relationships between SFLSs and nascent myofibrils have led investigators to the conclusion that SFLSs serve as transitory scaffolds for the assembly of sarcomeric proteins, giving rise to myofibrils in mature myocytes (Dlugosz et al., 1984; Holtzer et al., 1985; van der Ven et al., 1993). Because our results indicate that SFLS formation in myoblasts depends on PLD, we can infer that PLD could promote myogenesis by enhancing myofibrillogenesis.
The role of the Rho family of monomeric G proteins in actin cytoskeleton rearrangements is well established. In particular, RhoA has been shown to be a key player in stress fiber formation (Ridley, 1999; Mackay and Hall, 1998). The mechanisms linking RhoA activation and actin cytoskeleton changes are still incompletely defined. Among the effectors of RhoA suspected to be involved, there is strong evidence in favor of the Rho-dependent protein kinase ROCK that regulates myosin light chain phosphorylation and could thus influence actin filaments cross-linking and bundling. However, stress fiber formation in response to RhoA clearly involves other effectors (Mackay and Hall, 1998; Sahai et al., 1998; Ridley, 1999). The protein p140mDia, which interacts with the actin monomer binding protein profiling, is a likely candidate. PLD is another RhoA effector that has been shown to play a role in stress fiber assembly induced by thrombin (Ha and Exton, 1993), or by lysophosphatidic acid (Ha et al., 1994) in fibroblasts, and in endothelial cells (Cross et al., 1996). Further studies (Kam and Exton, 2001) have demonstrated that active PLD1 is specifically required for stress fiber formation induced by lysophosphatidic acid, other RhoA effectors being also necessary to this response. How PLD1 activity could contribute to the formation of actin fibers remains hypothetical. Evidences suggest that PA is a regulator of PIP2 levels, due to its ability to promote the synthesis of PIP2 by the PA-activatable type I PI-4-P 5-kinase, a regulation that has been shown to occur in cell-free conditions (Jenkin et al., 1994), in intact cells (Jones et al., 2000), and in permeabilized cells (Skippen et al., 2002). PIP2 itself is an essential cofactor for PLD activity, and concomitant activation of both enzymes is thus expected to trigger an amplification loop, resulting in a burst of local accumulation of both phospholipid products (Divecha et al., 2000; Kam and Exton, 2001; O'Luanaigh et al., 2002). PIP2 is known to be involved in stress fiber assembly via interactions with various proteins linked with actin cytoskeleton (Janmey et al., 1999). In particular, PIP2 could promote actin polymerization by dissociating the capping protein gelsolin from the barbed ends of actin filaments and by releasing actin monomers from binding proteins such as profilin (Tapon and Hall, 1997; Ridley, 1999; Yamamoto et al., 2001). In agreement with our hypothesis that PLD acts on myoblast cytoskeleton by triggering local PIP2 accumulation, we observed that AVP induced a 1-butanol-inhibitable increase in PIP2 synthesis and that a substantial fraction of the phosphoinositide was accumulated at the level of SFLSs, i.e., at the same site as PA.
Our experiments using a PA-specific probe bring new evidence that PLD has a functional link with actin fibers: they show for the first time that PA is accumulated at the level of stress fiber-like actin structures. This finding is in agreement with the reported association of PLD activity with actin cytoskeleton, as it was concluded from subcellular fractionation studies (Hodgkin et al., 1999; Iyer and Kusner, 1999) and from in vitro interaction studies of purified components (Lee et al., 2001; Kusner et al., 2002). However, PLD fluorescence imaging experiments indicated a colocalization of both ectopically expressed PLD1 (Lee et al., 2001; Powner et al., 2002) and PLD2 (Honda et al., 1999; Lee et al., 2001; O'Luanaigh et al., 2002) with plasma membrane-bound actin in RBL mast cells, PC12 pheocromocytoma cells, and COS-7 cells. In contrast, in L6 myoblasts, we observed that endogenous PLD1 and PA were clearly present in stress fiber-like structures and not at the level of cortical actin, showing that PLD has a different physiological function in muscle cells. Visualization of PA also allowed to reveal a temporal coincidence between actin fiber formation, PLD activation, and PA accumulation along actin fibers. Therefore, our observations support a direct involvement of PA in actin fibers assembly and add new insights into the relationships between PLD activity and cytoskeletal reorganization.
On the basis of our conclusions that PLD is involved in myogenesis and directly implicated in actin fiber formation, we can propose a model that allows to integrate events that had not been previously shown to be related. According to this model, myogenesis-promoting agents such as AVP activate, in addition to other pathways, the Rho proteins in myoblasts. Rho then promotes the activation of PLD1 (and other effectors as well) at the level of actin filaments, the association of PLD1 with actin being facilitated by the reported affinity of the enzyme for this cytoskeletal component. It is noteworthy that a sustained vesicular traffic involving phosphatidylcholine is triggered by AVP-stimulation in L6 cells (Coletti et al., 2000), suggesting that this PLD substrate may be available at the level of intracellular structures to be hydrolyzed to PA. This spatially restricted PA accumulation triggers in turn a local production of PIP2 by stimulating PI-4-P 5-kinase. PIP2 would then stimulate actin polymerization and bundling, thus participating in SFLS assembly. The resulting changes in actin status in the cells could influence SRF transcriptional activity and thereby activate muscle-specific gene expression. In particular, the enhanced expression of myogenin could direct the myogenic differentiation process. In addition, the effect of PLD on SFLS formation might contribute to myogenesis by supplying the structural basis for the assembly of nascent myofibrils, thus facilitating the progress of cell maturation toward the myocyte phenotype.
Acknowledgments
This work was supported in part by funds of the University of Rome-La Sapienza (Progetti di Ateneo), by the Italian Ministry of University and Research (COFIN program 2003060328-003), and by the Centro di Eccellenza Molecular Biology and Medicine, La Sapienza University. We are indebted to Dr. T. Kobayashi (Riken Institute, Saitama, Japan, and Institut National de la Santé et de la Recherche Médicale Unit 585, Villeurbanne, France) for fruitful discussions and helpful advice, and to Dr. E. Marchetti for expert assistance with microscopy. We acknowledge Drs. P. Boquet for the gift of C3 exotoxin, S. Bourgoin for the gift of PLD-specific antibodies, and M. Record and A. Eychene for sharing plasmids carrying PLD and Raf1 cDNAs, respectively.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-06-0459) on December 22, 2004.
Abbreviations used: AVP, Arg8-vasopressin; PA, phosphatidic acid; PIP2, phosphatidylinositol 4,5-bisphosphate; PLD, phospholipase D; SFLS, stress fiber like structure; SRF, serum response factor.
References
- Anderson, R. A., Boronenkov, I. V., Doughman, S. D., Kunz, J., and Loijens, J. C. (1999). Phosphatidylinositol phosphate kinases, a multifaceted family of signaling enzymes. J. Biol. Chem. 274, 9907-9910. [DOI] [PubMed] [Google Scholar]
- Apgar, J. R. (1995). Activation of PKC in rat basophilic leukemia cells stimulates increased production of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: correlation with actin polymerization. Mol. Biol. Cell 1, 97-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh, E. G., and Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Med. Sci. 37, 911-917. [DOI] [PubMed] [Google Scholar]
- Carnac, G., Primig, M., Kitzmann, M., Chafey, P., Tuil, D., Lamb, N., and Fernandez, A. (1998). RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol. Biol. Cell 9, 1891-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletti, D., Silvestroni, L., Naro, F., Molinaro, M., Adamo, S., and Palleschi, S. (2000). Vesicle-mediated phosphatidylcholine reapposition to the plasma membrane following hormone-induced phospholipase D activation. Exp. Cell. Res. 256, 94-104. [DOI] [PubMed] [Google Scholar]
- Colley, W. C., Sung, T. C., Roll, R., Jenco, J., Hammond, S. M., Altshuller, Y., Bar-Sagi, D., Morris, A. J., and Frohman, M. A. (1997). Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr. Biol. 7, 191-201. [DOI] [PubMed] [Google Scholar]
- Cross, M. J., Roberts, S., Ridley, A. J., Hodgkin, M. N., Stewart, A., Claesson-Welsh, L., and Wakelam, M. J. (1996). Stimulation of actin stress fibre formation mediated by activation of phospholipase D. Curr. Biol. 6, 588-597. [DOI] [PubMed] [Google Scholar]
- Diaz, O., Berquand, A., Dubois, M., Di Agostino, S., Sette, C., Bourgoin, S., Lagarde, M., Nemoz, G., and Prigent, A. F. (2002). The mechanism of docosahexaenoic acid-induced phospholipase D activation in human lymphocytes involves exclusion of the enzyme from lipid rafts. J. Biol. Chem. 277, 39368-39378. [DOI] [PubMed] [Google Scholar]
- Divecha, N., Roefs, M., Halstead, J. R., D'Andrea, S., Fernandez-Borga, M., Oomen, L., Saqib, K. M., Wakelam, M. J., and D'Santos, C. (2000). Interaction of the type Ialpha PIPkinase with phospholipase D: a role for the local generation of phosphatidylinositol 4, 5-bisphosphate in the regulation of PLD2 activity. EMBO J. 19, 5440-5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugosz, A. A., Antin, P. B., Nachmias, V. T., and Holtzer, H. (1984). The relationship between stress fiber-like structures and nascent myofibrils in cultured cardiac myocytes. J. Cell. Biol. 99, 2268-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, G., Altshuller, Y. M., Kim, Y., Han, J. M., Ryu, S. H., Morris, A. J., and Frohman, M. A. (2000). Dual requirement for rho and PKC in direct activation of phospholipase D1 through G protein-coupled receptor signaling. Mol. Biol. Cell 11, 4359-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton, J. H. (2002). Regulation of phospholipase D. FEBS Lett. 531, 58-61. [DOI] [PubMed] [Google Scholar]
- Ghosh, S., Strum, J. C., Sciorra, V. A., Daniel, L., and Bell, R. M. (1996). Raf-1 kinase possesses distinct binding domains for phosphatidylserine and phosphatidic acid. Phosphatidic acid regulates the translocation of Raf-1 in 12-O-tetradecanoylphorbol-13-acetate-stimulated Madin-Darby canine kidney cells. J. Biol. Chem. 271, 8472-8480. [DOI] [PubMed] [Google Scholar]
- Gohla, A., Offermanns, S., Wilkie, T. M., and Schultz, G. (1999). Differential involvement of Galpha12 and Galpha13 in receptor-mediated stress fiber formation. J. Biol. Chem. 274, 17901-17907. [DOI] [PubMed] [Google Scholar]
- Grange, M., Sette, C., Cuomo, M., Conti, M., Lagarde, M., Prigent, A. F., and Nemoz, G. (2000). The cAMP-specific phosphodiesterase PDE4D3 is regulated by phosphatidic acid binding. Consequences for cAMP signaling pathway and characterization of a phosphatidic acid binding site. J. Biol. Chem. 275, 33379-33387. [DOI] [PubMed] [Google Scholar]
- Ha, K. S., and Exton, J. H. (1993). Activation of actin polymerization by phosphatidic acid derived from phosphatidylcholine in IIC9 fibroblasts. J. Cell. Biol. 123, 1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha, K. S., Yeo, E. J., and Exton, J. H. (1994). Lysophosphatidic acid activation of phosphatidylcholine-hydrolysing phospholipase D and actin polymerization by a pertussis toxin-sensitive mechanism. Biochem. J. 303, 55-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond, S. M., Jenco, J. M., Nakashima, S., Cadwallader, K., Gu, Q., Cook, S., Nozawa, Y., Prestwich, G. D., Frohman, M. A., and Morris, A. J. (1997). Characterization of two alternately spliced forms of phospholipase D1. Activation of the purified enzymes by phosphatidylinositol 4,5-bisphosphate, ADP-ribosylation factor, and Rho family monomeric GTP-binding proteins and PKC-alpha. J. Biol. Chem. 272, 3860-3868. [DOI] [PubMed] [Google Scholar]
- Hekman, M., Hamm, H., Villar, A. V., Bader, B., Kuhlmann, J., Nickel, J., and Rapp, U. R. (2002). Associations of B- and C-Raf with cholesterol, phosphatidylserine, and lipid second messengers. Preferential binding of Raf to artificial lipid rafts. J. Biol. Chem. 277, 24090-24102. [DOI] [PubMed] [Google Scholar]
- Hill, C. S., Wynne, J., and Treisman, R. (1995). The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell 81, 1159-1170. [DOI] [PubMed] [Google Scholar]
- Hodgkin, M. N., Clark, J. M., Rose, S., Saqib, K., and Wakelam, M. J. (1999). Characterization of the regulation of phospholipase D activity in the detergent-insoluble fraction of HL60 cells by PKC and small G-proteins. Biochem. J. 339, 87-93. [PMC free article] [PubMed] [Google Scholar]
- Holtzer, H., Forry-Schaudies, S., Dlugosz, A., Antin, P., and Dubyak, G. (1985). Interactions between IFs, microtubules, and myofibrils in fibrogenic and myogenic cells. Ann. N.Y. Acad. Sci. 455, 106-125. [DOI] [PubMed] [Google Scholar]
- Honda, A., Nogami, M., Yokozeki, T., Yamazaki, M., Nakamura, H., Watanabe, H., Kawamoto, K., Nakayama, K., Morris, A. J., Frohman, M. A., and Kanaho, Y. (1999). Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell 99, 521-532. [DOI] [PubMed] [Google Scholar]
- Iyer, S. S., and Kusner, D. J. (1999). Association of phospholipase D activity with the detergent-insoluble cytoskeleton of U937 promonocytic leukocytes. J. Biol. Chem. 274, 2350-2359. [DOI] [PubMed] [Google Scholar]
- Janmey, P. A., Xian, W., and Flanagan, L. A. (1999). Controlling cytoskeleton structure by phosphoinositide-protein interactions: phosphoinositide binding protein domains and effects of lipid packing. Chem. Phys. Lipids 101, 93-107. [DOI] [PubMed] [Google Scholar]
- Jenkins, J. H., Fisette, P. L., and Anderson, R. A. (1994). Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J. Biol. Chem. 269, 11547-11554. [PubMed] [Google Scholar]
- Jones, D. R., Sanjuan, M. A., and Merida, I. (2000). Type Ialpha phosphatidylinositol 4-phosphate 5-kinase is a putative target for increased intracellular phosphatidic acid. FEBS Lett. 476, 160-165. [DOI] [PubMed] [Google Scholar]
- Jose Lopez-Andreo, M., Gomez-Fernandez, J. C., and Corbalan-Garcia, S. (2003). The simultaneous production of phosphatidic acid and diacylglycerol is essential for the translocation of protein kinase Cepsilon to the plasma membrane in RBL-2H3 cells. Mol. Biol. Cell. 14, 4885-4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, E. M., Betancourt-Calle, S., Mann-Blakeney, R., Griner, R. D., and Bollinger Bollag, W. (1999). Sustained phospholipase D activation is associated with keratinocyte differentiation. Carcinogenesis 20, 569-576. [DOI] [PubMed] [Google Scholar]
- Kam, Y., and Exton, J. H. (2001). Phospholipase D activity is required for actin stress fiber formation in fibroblasts. Mol. Cell. Biol. 21, 4055-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama, K., Kodaki, T., Nagamachi, Y., and Yamashita, S. (1998). Cloning, differential regulation and tissue distribution of alternatively spliced isoforms of ADP-ribosylation-factor-dependent phospholipase D from rat liver. Biochem. J. 329, 647-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusner, D. J., Barton, J. A., Wen, K. K., Wang, X., Rubenstein, P. A., and Iyer, S. S. (2002). Regulation of phospholipase D activity by actin. Actin exerts bidirectional modulation of mammalian phospholipase D activity in a polymerization-dependent, isoform-specific manner. J. Biol. Chem. 277, 50683-50692. [DOI] [PubMed] [Google Scholar]
- Lee, S., Park, J. B., Kim, J. H., Kim, Y., Kim, J. H., Shin, K. J., Lee, J. S., Ha, S. H., Suh, P. G., and Ryu, S. H. (2001). Actin directly interacts with phospholipase D, inhibiting its activity. J. Biol. Chem. 276, 28252-28260. [DOI] [PubMed] [Google Scholar]
- Mackay, D. J., and Hall, A. (1998). Rho GTPases. J. Biol. Chem. 273, 20685-20688. [DOI] [PubMed] [Google Scholar]
- Marcil, J., Harbour, D., Naccache, P. H., and Bourgoin, S. (1997). Human phospholipase D1 can be tyrosine-phosphorylated in HL-60 granulocytes. J. Biol. Chem. 272, 20660-20664. [DOI] [PubMed] [Google Scholar]
- Minotti, S., Scicchitano, B. M., Nervi, C., Scarpa, S., Lucarelli, M., Molinaro, M., and Adamo, S. (1998). Vasopressin and insulin-like growth factors synergistically induce myogenesis in serum-free medium. Cell Growth Differ. 9, 155-163. [PubMed] [Google Scholar]
- Miralles, F., Posern, G., Zaromytidou, A. I., and Treisman, R. (2003). Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113, 329-342. [DOI] [PubMed] [Google Scholar]
- Nakashima, S., and Nozawa, Y. (1999). Possible role of phospholipase D in cellular differentiation and apoptosis. Chem Phys. Lipids 98, 153-164. [DOI] [PubMed] [Google Scholar]
- Naro, F., Donchenko, V., Minotti, S., Zolla, L., Molinaro, M., and Adamo, S. (1997). Role of phospholipase C and D signalling pathways in vasopressin-dependent myogenic differentiation. J. Cell. Physiol. 171, 34-42. [DOI] [PubMed] [Google Scholar]
- Naro, F., Sette, C., Vicini, E., De Arcangelis, V., Grange, M., Conti, M., Lagarde, M., Molinaro, M., Adamo, S., and Némoz, G. (1999). Involvement of type 4 cAMP-phosphodiesterase in the myogenic differentiation of L6 cells. Mol. Biol. Cell 10, 4355-4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nervi, C., Benedetti, L., Minasi, A., Molinaro, M., and Adamo, S. (1995). Arginine-vasopressin induces differentiation of skeletal myogenic cells and up-regulation of myogenin and Myf-5. Cell Growth Differ. 6, 81-89. [PubMed] [Google Scholar]
- O'Luanaigh, N., Pardo, R., Fensome, A., Allen-Baume, V., Jones, D., Holt, M. R., and Cockcroft, S. (2002). Continual production of phosphatidic acid by phospholipase D is essential for antigen-stimulated membrane ruffling in cultured mast cells. Mol. Biol. Cell 13, 3730-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, L., and Rudnick, M. A. (2000). Molecular mechanisms regulating myogenic determination and differentiation. Front. Biosci. 5, D750-D767. [DOI] [PubMed] [Google Scholar]
- Poveda, J. A., Encinar, J. A., Fernandez, A. M., Mateo, C. R., Ferragut, J. A., and Gonzalez-Ros, J. M. (2002). Segregation of phosphatidic acid-rich domains in reconstituted ACh receptor membranes. Biochemistry 41, 12253-12262. [DOI] [PubMed] [Google Scholar]
- Powner, D. J., Hodgkin, M. N., and Wakelam, M. J. (2002). Antigen-stimulated activation of phospholipase D1b by Rac1, ARF6, and PKCalpha in RBL-2H3 cells. Mol. Biol. Cell 13, 1252-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A. J. (1999). Stress fibres take shape. Nat. Cell. Biol. 1, E64-E66. [DOI] [PubMed] [Google Scholar]
- Rizzo, M. A., Shome, K., Vasudevan, C., Stolz, D. B., Sung, T. C., Frohman, M. A., Watkins, S. C., and Romero, G. (1999). Phospholipase D and its product, phosphatidic acid, mediate agonist-dependent raf-1 translocation to the plasma membrane and the activation of the mitogen-activated protein kinase pathway. J. Biol. Chem. 274, 1131-1139. [DOI] [PubMed] [Google Scholar]
- Rizzo, M. A., Shome, K., Watkins, S. C., and Romero, G. (2000). The recruitment of Raf-1 to membranes is mediated by direct interaction with phosphatidic acid and is independent of association with Ras. J. Biol. Chem. 275, 23911-23918. [DOI] [PubMed] [Google Scholar]
- Sahai, E., Alberts, A. S., and Treisman, R. (1998). RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J. 17, 1350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicchitano, B. M., Spath, L., Musaro, A., Molinaro, M., Adamo, S., and Nervi, C. (2002). AVP induces myogenesis through the transcriptional activation of the myocyte enhancer factor 2. Mol. Endocrinol. 16, 1407-1416. [DOI] [PubMed] [Google Scholar]
- Seasholtz, T. M., Majumdar, M., and Brown, J. H. (1999). Rho as a mediator of G protein-coupled receptor signaling. Mol. Pharmacol. 55, 949-956. [DOI] [PubMed] [Google Scholar]
- Skippen, A., Jones, D. H., Morgan, C. P., Li, M., and Cockcroft, S. (2002). Mechanism of ADP ribosylation factor-stimulated phosphatidylinositol 4,5-bisphosphate synthesis in HL60 cells. J. Biol. Chem. 277, 5823-5831. [DOI] [PubMed] [Google Scholar]
- Sordella, R., Jiang, W., Chen, G. C., Curto, M., and Settleman, J. (2003). Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell 113, 147-158. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos, A., Gineitis, D., Copeland, J., and Treisman, R. (1999). Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell 98, 159-169. [DOI] [PubMed] [Google Scholar]
- Standaert, M. L., Bandyopadhyay, G., Zhou, X., Galloway, L., and Farese, R. V. (1996). Insulin stimulates phospholipase D-dependent phosphatidylcholine hydrolysis, Rho translocation, de novo phospholipid synthesis, and diacylglycerol/protein kinase C signaling in L6 myotubes. Endocrinology 137, 3014-3020. [DOI] [PubMed] [Google Scholar]
- Takano, H., Komuro, I., Oka, T., Shiojima, I., Hiroi, Y., Mizuno, T., and Yazaki, Y. (1998). The Rho family G proteins play a critical role in muscle differentiation. Mol. Cell. Biol. 18, 1580-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon, N., and Hall, A. (1997). Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr. Opin. Cell. Biol. 9, 86-92. [DOI] [PubMed] [Google Scholar]
- Teti, A., Naro, F., Molinaro, M., and Adamo, S. (1993). Transduction of arginine vasopressin signal in skeletal myogenic cells. Am. J. Physiol. 265, C113-C121. [DOI] [PubMed] [Google Scholar]
- Thompson, M. G., Mackie, S. C., Morrison, K. S., Thom, A., and Palmer, R. M. (1994). Stimulation of protein synthesis and phospholipase D activity by vasopressin and phorbol ester in L6 myoblasts. Biochim. Biophys. Acta 1224, 198-204. [DOI] [PubMed] [Google Scholar]
- van der Ven, P. F., Schaart, G., Croes, H. J., Jap, P. H., Ginsel, L. A., and Ramaekers, F. C. (1993). Titin aggregates associated with intermediate filaments align along stress fiber-like structures during human skeletal muscle cell differentiation. J. Cell. Sci. 106, 749-759. [DOI] [PubMed] [Google Scholar]
- Wei, L., Zhou, W., Croissant, J. D., Johansen, F. E., Prywes, R., Balasubramanyam, A., and Schwartz, R. J. (1998). RhoA signaling via serum response factor plays an obligatory role in myogenic differentiation. J. Biol. Chem. 273, 30287-30294. [DOI] [PubMed] [Google Scholar]
- Yamamoto, M., Hilgemann, D. H., Feng, S., Bito, H., Ishihara, H., Shibasaki, Y., and Yin, H. L. (2001). Phosphatidylinositol 4,5-bisphosphate induces actin stress-fiber formation and inhibits membrane ruffling in CV1 cells. J. Cell. Biol. 152, 867-876. [DOI] [PMC free article] [PubMed] [Google Scholar]