Abstract
Extracellular ATP-induced Ca2+ signalling is critical in regulating diverse physiological and disease processes. Emerging evidence suggests high concentrations of extracellular ATP in tumour tissues. In this study, we examined the P2 receptor for ATP-induced Ca2+ signalling in human hepatocellular carcinoma (HCC) cells. Fura-2-based measurements of the intracellular Ca2+ concentration ([Ca2+]i) showed that extracellular ATP induced an increase in the [Ca2+]i in human HCC Huh-7 and HepG2 cells. NF546, a P2Y11 receptor agonist was equally effective in inducing an increase in the [Ca2+]i. In contrast, agonists for the P2X receptors (αβmeATP and BzATP), P2Y1 receptor (MRS2365) or P2Y2 receptor (MRS2768) were ineffective. In addition, ATP/NF546-induced increases in the [Ca2+]i were strongly inhibited by treatment with NF340, a P2Y11 receptor antagonist. Immunofluorescent confocal imaging and western blotting analysis consistently demonstrated the P2Y11 receptor expression in Huh-7 and HepG2 cells. Transfection with P2Y11-specific siRNA attenuated the P2Y11 receptor protein expression level and also reduced NF546-induced increase in the [Ca2+]i. Importantly, immunohistochemistry revealed that the P2Y11 receptor was expressed at very high level in human HCC tissues and, by contrast, it was barely detected in normal liver tissues. Trans-well cell migration assay demonstrated that ATP and NF546 induced concentration-dependent stimulation of Huh-7 cell migration. Treatment with NF340 prevented ATP-induced stimulation of cell migration. Taken together, our results show carcinoma-specific expression of the P2Y11 receptor and its critical role in mediating ATP-inducing Ca2+ signalling and regulating cell migration in human HCC cells.
Keywords: HCC cells, extracellular ATP, P2Y11 receptor, cytosolic Ca2+, cell migration
INTRODUCTION
Hepatocellular carcinoma (HCC) is the primary liver cancer; poor prognosis and ineffective treatment of HCC with currently available anti-cancer treatments have made it to be one of the leading and most deadly causes of cancer-related mortality, with the 5-year survival rate being less than 15% [1–4]. The global incidence of HCC, while exhibiting noticeable regional variations, has been reported to increase in the recent past and is anticipated to continue to rise in the coming years [3, 4]. While several disease risk factors including aging, genetics, infection and lifestyle such as smoking and alcohol have been identified to contribute to the development of HCC, it is much less well-understood with respect to the underlying molecular mechanisms. A mechanistic understanding of the pathogenesis and progression of HCC is of great value towards identifying disease biomarkers and drug targets for development of new diagnosis and effective treatments.
The microenvironment in tumour tissues is highly hypoxic, a condition that is well-documented to stimulate release of intracellular ATP [5–7]. In vivo imaging provides clear evidence to show that pericellular ATP can reach hundreds of micro-molar concentrations at the tumour sites but remains almost undetectable in normal tissues [6, 7]. It has been well established that extracellular ATP interacts with ligand-gated ion channel P2X receptors and G-protein-coupled P2Y receptors on the cell surface to induce autocrine and paracrine signalling [8–11]. There are seven mammalian P2X receptor proteins or subunits (P2X1-P2X7) that can assemble into homo/hetero-trimeric P2X receptors [12]. ATP activates all P2X receptors, albeit with different potency [13], that form an ion-conducting pathway across the plasma membrane that allows passage of cations including Ca2+. There are eight mammalian P2Y receptors that are activated by various extracellular nucleotides such as ATP, ADP, UTP and UDP [14]. ATP activates the human P2Y1, P2Y2 and P2Y11 receptors that are mainly coupled to Gα,q/11 and thus their activation stimulates phospholipase C (PLC) and subsequent generation of IP3, which in turns activates the IP3 receptor (IP3R) in the endoplasmic reticulum (ER) to mediate ER Ca2+ release [14]. Therefore, ATP can elevate the intracellular Ca2+ concentrations ([Ca2+]i) via the P2X receptor-mediated extracellular Ca2+ influx or the P2Y receptor-PLC-IP3R signalling pathway leading to internal Ca2+ release. Mammalian cells express multiple P2X and P2Y receptors often in a cell type-specific manner [8, 9] that play a role in a diversity of physiological functions and pathological processes, including cancers [15–19]. Extracellular ATP has been reported to influence cancer cell functions, particularly cancer cell metastasis which is a key process responsible for the high mortality [20]. For example, recent studies of various types of cancer cells have shown that ATP-induced purinergic signalling regulates cancer cell migration, proliferation and survival via the P2X7 receptor [21–32] or P2Y2 receptor [33–37]. There is evidence to indicate mRNA and/or protein expression of the P2Y1 and P2Y2 receptors in primary and immortalized human normal hepatocytes, primary human HCC cells and immortal human HCC cells (e.g., Huh-7, HepG2 and BEL-7404) [37–39], and the P2X4 and P2X7 receptors in HepG2 cells, rat and mouse hepatocytes and rat HCC cells [38]. Further studies demonstrated that activation of the P2Y2 receptor leads to ATP-induced increase in the [Ca2+]i in human normal hepatocytes and human HCC cells [37, 38]. In addition, the P2Y2 receptor expression is upregulated in human HCC cells and genetic suppression of the P2Y2 receptor expression inhibits human HCC cell migration [37]. In contrast, a separate study showed functional expression of the P2X4 receptor and possibly the P2X7 receptor in rat and mouse hepatocytes and rat HCC cells [39]. Thus, different P2X and P2Y receptors have been reported in rodent and human hepatocytes and HCC cells. In the present study, we provide pharmacological, functional and genetic evidence to support the P2Y11 receptor in ATP-induced Ca2+ signalling in human HCC cells, reveal strong HCC-specific P2Y11 receptor expression, and propose their involvement in HCC cell migration.
RESULTS
ATP induces an increase in the [Ca2+]i in Huh-7 cells
We began with measuring intracellular Ca2+ responses to ATP in human HCC Huh-7 cells, using fura-2 based ratiometry and FLEX-station. In the extracellular Ca2+-containing solution, ATP applied at 1-300 μM induced increases in the [Ca2+]i in a concentration-dependent manner (Figure 1A). ATP-induced increase in the [Ca2+]i reached the maximum at 100 μM, and slightly reduced at 300 μM ATP (Figure 1A) probably due to receptor desensitization. Fitting the data to Hill equation yielded an EC50 of 11 μM and Hill coefficient of 1.8 (Figure 1A). Pre-treatment with 30 μM PPADS or suramin, two P2 receptor generic antagonists, strongly inhibited ATP-induced Ca2+ responses (Figure 1B and 1C). These results provide the first indication that ATP can increase the [Ca2+]i in Huh-7 cells via the P2 receptor.
Figure 1. ATP induces concentration-dependent increase in the [Ca2+]i in Huh-7 cells.
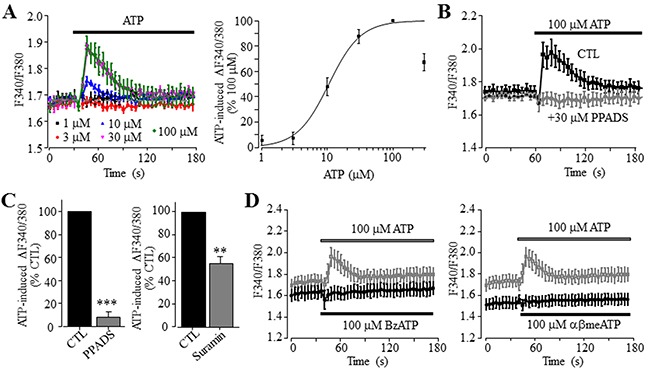
(A) Left, representative recordings of Ca2+ responses induced by 1-100 μM ATP, with six wells of cells for each concentration. Right, ATP concentration-peak Ca2+ response curve, constructed by expressing the responses induced by 1-300 μM ATP as % of that induced by 100 μM ATP. Each data point represents three independent experiments. The smooth line represents the least squared fit to Hill equation with EC50 and Hill coefficient of 11 μM ATP and 1.8, respectively. (B) Representative recordings of Ca2+ responses induced by 100 μM ATP, in control cells or cells pre-treated with 30 μM μM PPADS, with six wells of cells for each case. (C) Summary of ATP-induced peak increase in the [Ca2+]i in control and cells treated with indicated concentrations of 30 μM PPADS (left panel) and 30 μM suramin (right panel), expressed as % of that in control cells, from three independent experiments. **, p < 0.01; ***, p < 0.001. (D) Representative recordings of Ca2+ responses induced by 100 μM BzATP (left panel) or 100 μM αβmeATP (right panel) and 100 μM ATP (in grey), with six wells of cells for each case. Such results were observed in three independent experiments.
As introduced above, activation of the P2X ionotropic receptor can increase the [Ca2+]i as a result of extracellular Ca2+ influx. Protein expression of the P2X7 receptor in Huh-7 cells was previously reported [40] and confirmed using immunocytochemistry (Supplementary Figure 1). However, application of 100 and 300 μM 2’,3’-O-(4-benzoylbenzoyl)-ATP (BzATP), an agonist exhibiting a greater potency than ATP at the human P2X7 receptor and also activating other P2X receptor [18], completely failed to induce any detectable Ca2+ response (Figure 1D). This suggests poor functional expression of the P2X7 receptor. We also examined αβmethyleneATP (αβmeATP), which potently activates the human P2X receptors containing P2X1, P2X3 or P2X5 subunit [11]. There was no discernible Ca2+ response to 100 μM αβmeATP (Figure 1D). A previous study showed functional expression of the P2X4 receptor in rodent HCC cells [40]. Treatment with 10 μM 5-BDBD, a selective P2X4 receptor antagonist with a submicromolar potency [11], however, did not inhibit ATP-induced Ca2+ response (Supplementary Figure 2A), suggesting no major function of the P2X4 receptor at the plasma membrane of Huh-7 cells.
P2Y11 receptor plays a key role in ATP-induced increase in the [Ca2+]i in Huh-7 cells
To examine the role of P2Y receptors in ATP-induced increase in the [Ca2+]i, we determined ATP-induced Ca2+ responses in the extracellular Ca2+-free solution, a widely-used experimental condition to determine Ca2+ release from internal stores. ATP was effective in inducing significant increase in the [Ca2+]i in the extracellular Ca2+-free solution, albeit the amplitude of ATP-induced Ca2+ response was lower than that obtained in the presence of extracellular Ca2+ (Figure 2A). Taken together, these observations clearly support functional expression of ATP-sensitive P2Y receptors. To elaborate which particular P2Y receptor is involved in mediating ATP-induced Ca2+ response, we further examined several P2Y type selective agonists. Exposure to 100 μM ADP, an agonist that preferentially activates the P2Y1 receptor, induced very small but detectable Ca2+ responses in both extracellular Ca2+-free and Ca2+-containing solutions (Figure 2B and Supplementary Figure 2B). Moreover, application of 10 nM MRS2365, a selective P2Y1 receptor agonist, was ineffective in inducing an increase in the [Ca2+]i (Figure 2C). These results consistently indicate no major role for the P2Y1 receptor in ATP-induced Ca2+ response. UTP, an agonist at the P2Y2 receptor as well as at the P2Y4 and P2Y6 receptors, was equally effective as ATP in elevating the [Ca2+]i in the extracellular Ca2+-containing contains (Figure 2D), as previously reported [38]. These results are consistent with the pharmacological properties of the P2Y2 receptor, which was shown to be expressed in both human hepatocytes and HCC cells [37, 38]. Thus, it was surprising to find that exposure to 10 and 30 μM MRS2768, a selective P2Y2 receptor agonist, failed to elevate the [Ca2+]i in the extracellular Ca2+-containing solution (Figure 2E). Such an observation was made using two batches of MRS2768 from different vendors (see Materials and Methods). In contrast, application of 1 and 10 μM NF546, a selective P2Y11 receptor agonist, concentration-dependently increased the [Ca2+]i in the extracellular Ca2+-containing solution, and the Ca2+ response amplitude induced by 10 μM NF546 was virtually the same as that induced by 100 μM ATP (Figure 3A). Moreover, pre-treatment with 10 μM NF340, a selective P2Y11 receptor antagonist, almost completely abolished ATP-induced increase in the [Ca2+]i in the extracellular Ca2+-containing solution (Figure 3B). Immunofluorescent confocal imaging demonstrated expression of the P2Y11 receptor in Huh-7 cells (Figure 3C and Supplementary Figure 1). Similarly, western blotting analysis supports protein expression of the P2Y11 receptor, which was attenuated by transfection with P2Y11-specific siRNA (Figure 3D). Consistently, siRNA-mediated knockdown of the P2Y11 receptor expression resulted in significantly smaller Ca2+ response to NF546 (Figure 3E). Taken together, these pharmacological, biochemical and genetic results provide consistent evidence to support expression of the P2Y11 receptor and its key role in mediating ATP-induced Ca2+ response in Huh-7 cells.
Figure 2. No major role of the P2Y1 and P2Y2 receptors in ATP-induced increase in the [Ca2+]i in Huh-7 cells.
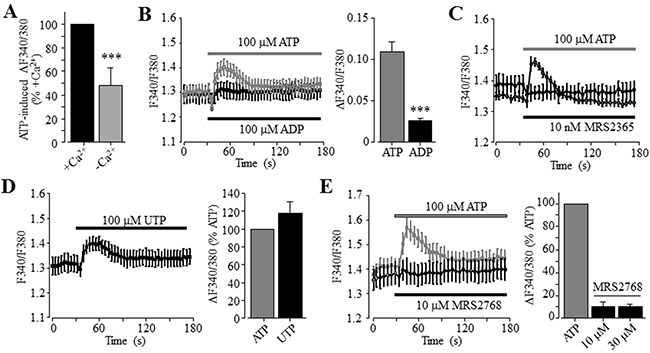
(A) Summary of 100 μM ATP-induced peak increase in the [Ca2+]i in the extracellular Ca2+-containing and Ca2+-free solutions, expressed as % of that induced by ATP in the extracellular Ca2+-containing solution, from three independent experiments. (B) Left, representative recordings of Ca2+ responses induced by 100 μM ATP (grey) and ADP (black) in the extracellular Ca2+-containing solutions, with six wells of cells for each case. Right, summary of the peak increase in the [Ca2+]i.***, p < 0.001 (A and B). (C) Representative recordings of Ca2+ responses induced by 100 μM ATP (grey) and 10 μM MRS2365 (black) in the extracellular Ca2+-containing solution, from six wells of cells for each concentration. (D) Left, representative recordings of Ca2+ responses induced by 100 μM UTP in the extracellular Ca2+-containing solution with six wells of cells for each case. Right, summary of UTP-induced peak increase in the [Ca2+]i, expressed as % of that induced by 100 μM ATP, from three independent experiments. (E) Left, representative recordings of the Ca2+ responses induced by 10 μM MRS2768 (black) and 100 μM ATP (grey) in the extracellular Ca2+-containing solution with six wells of cells for each case. Right, summary of MRS2768-induced peak increase in the [Ca2+]i, expressed as % of that induced by 100 μM ATP, from three independent experiments.
Figure 3. A key role of the P2Y11 receptor in ATP-induced increase in the [Ca2+]i in Huh-7 cells.
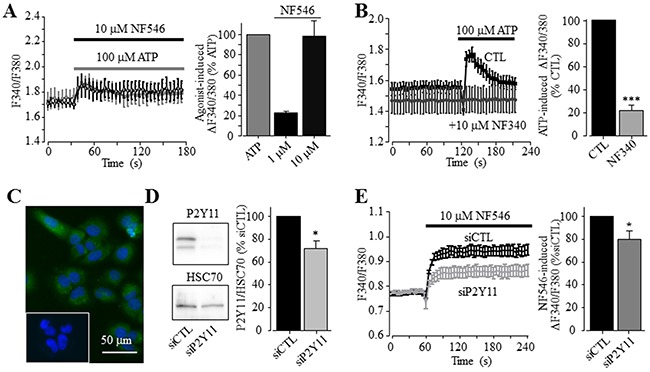
(A) Left, representative recordings of Ca2+ responses induced by 10 μM NF546 (black) and 100 μM ATP (grey) in the extracellular Ca2+-containing solution with six wells of cells for each case. Right, summary of the peak increase in the [Ca2+]i induced by 1 and 10 μM NF546, expressed as % of that induced by 100 μM ATP, from three independent experiments. (B) Left, representative recordings of Ca2+ responses induced by 100 μM ATP in control cells or cells pre-treated with 10 μM NF340, with six wells of cells for each case. Right, summary of ATP-induced peak increases in the [Ca2+]i in control or NF340- treated cells, expressed as % of that in control cells, from three independent experiments. ***, p < 0.001. (C) Representative confocal images showing P2Y11 immunostaining. Cells were countstained with DAPI. The insert shows control cells stained only with the secondary antibody. Similar results were observed in two independent experiments. (D) Left, representative western blot analysing P2Y11 receptor expression in cells transfected with control and P2Y11-specific siRNA (siCTL and siP2Y11). Right, summary of mean P2Y11 protein expression, normalized to the HSC70 protein level and presented as % of the value in cells transfected with siCTL, from six independent experiments. *, p < 0.05. (E) Left, representative recordings of Ca2+ responses induced by 10 μM NF546 in cells transfected with indicate siRNA, with 4 wells of cells for each case. Right, summary of ATP-induced peak increases in the [Ca2+]i in cells transfected with siCTL and siP2Y11, expressed as % of that in cells transfected with siCTL, from six independent experiments.
P2Y11 receptor also contributes in ATP-induced Ca2+ signalling in HepG2 cells
To further investigate whether the P2Y11 receptor plays a more general role in mediating ATP-induced Ca2+ signalling in human HCC cells, we measured Ca2+ responses to ATP and NF546 in HepG2 cells. Like in Huh-7 cells, 10-100 μM ATP induced noticeable increase in the [Ca2+]i in HepG2 cells (Figure 4A). Similarly, 10 μM NF546 was also effective (Figure 4B). Treatment with NF340 significantly attenuated NF546-induced Ca2+ response (Figure 4B). Western blotting showed protein expression of the P2Y11 receptor, with its expression level being strongly reduced in HepG2 cells after transfection with P2Y11-specific siRNA (Figure 4C). Such siRNA-mediated knockdown of the P2Y11 receptor expression significantly decreased NF546-induced Ca2+ response (Figure 4D).
Figure 4. A role of the P2Y11 receptor in ATP-induced increase in the [Ca2+]i in HepG2 cells.
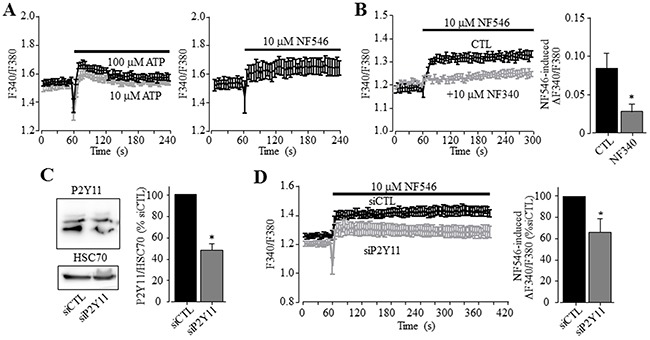
(A) Representative recordings of Ca2+ responses induced by 10 μM (grey) and 100 μM ATP (black) (left panel) and 10 uM NF546 (right panel) in the extracellular Ca2+-containing solution with 4 wells of cells for each case. (B) Left, representative recordings of the Ca2+ responses induced by 10 μM NF546 in control cells or cells pre-treated with 10 μM NF340, with 4 wells of cells for each case. Right, summary of NF546-induced peak increase in the [Ca2+]i in control or NF340-treated cells, from three independent experiments. (C) Left, representative western blot showing P2Y11 receptor expression in cells transfected with control and P2Y11-specific siRNA (siCTL and siP2Y11). Right, summary of the mean P2Y11 protein expression, normalized to HSC70 protein and presented as % of the value in cells transfected with siCTL, from 8 independent experiments. (D) Left, representative recordings of Ca2+ responses induced by 10 μM NF546 in cells transfected with indicate siRNA, with 4 wells of cells for each case. Right, summary of ATP-induced peak increase in the [Ca2+]i in cells transfected with siCTL and siP2Y11, expressed as % of that in cells transfected with siCTL, from three independent experiments. *, p < 0.05.
P2Y11 expression is abundantly expressed in human HCC but not normal live tissues
The findings described above in two human HCC cells prompted us to investigate whether the P2Y11 receptor is expressed in primary human HCC tissues and, furthermore, whether the P2Y11 receptor expression in human HCC tissues differs from that in normal liver tissues. Immunohistochemistry showed abundant expression of the P2Y11 receptor, mainly located closely to the plasma membrane, in human HCC tissues (Figure 5A). In striking contrast, there was no immunostaining of the P2Y11 receptor in normal liver tissues (Figure 5B). These results provide evidence to support the P2Y11 receptor is expressed in human HCC tissues as well as human HCC cells and, furthermore, such P2Y11 receptor expression appears HCC-specific.
Figure 5. Expression of the P2Y11 receptor in human hepatocellular carcinoma and normal live tissues.
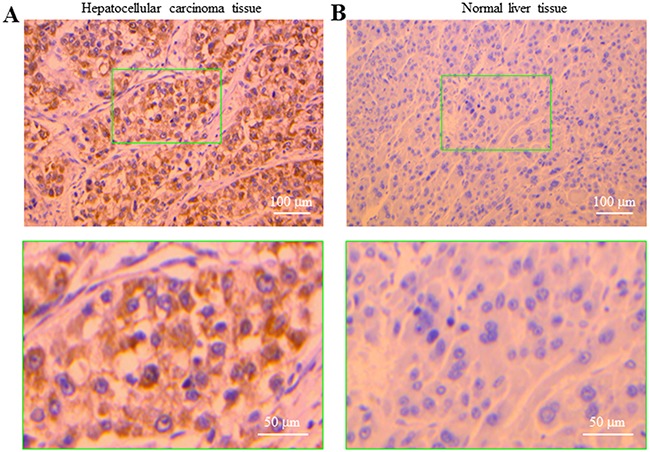
Immunostaining of the P2Y11 receptor in human HCC (A) and normal liver tissues (B), counterstained with haematoxylin. The bottom panels show enlarged images of the areas in the green rectangle in the top panels.
ATP and NF546 stimulate Huh-7 cell migration
In a recent study, ATP is shown to promote cell migration of native human HCC cells and HepG2 and BEL-7404 cells, and activation of the P2Y2 receptor is proposed to be critical in mediating ATP-induced cell migration [37]. This recent study has not examined the expression of the P2Y11 receptor and its role in ATP-induced stimulation of HCC cell migration, and also has not determined ATP-induced effect on Huh-7 cell migration. We thus performed the trans-well chamber assay to investigate whether extracellular ATP stimulated Huh-7 cell migration and if so, whether this could be reproduced using the specific P2Y11 receptor agonist NF546. ATP applied at 1, 10 and 100 μM resulted in concentration-dependent stimulation of cell migration (Figure 6A). The increase in cell migration by 10 and 100 μM ATP reached the significant level (Figure 6C). Treatment with 1, 10 and 100 μM NF546 also stimulated cell migration in a concentration-dependent manner (Figure 6B and 6D). At the same concentrations, NF546 induced slightly greater increase in cell migration than ATP (c.f. Figure 6C and 6D). Treatment with ATP at 100 μM induced no detectable cell death (Supplementary Figure 3). Taken together, these results provide clear evidence to support a critical role for the P2Y11 receptor in driving ATP-induced stimulation of Huh-7 cell migration. We also examined the effect of NF340 on ATP-induced stimulation of Huh-7 cell migration. Treatment with 10 μM NF340 completely blocked ATP-induced stimulation of cell migration with no significant effect on cell migration under the basal condition, providing further evidence to support that P2Y11 receptor activation is critical in stimulating cell migration by ATP (Figure 7A and 7B). It is well known that ATP is metabolically unstable and can be rapidly metabolized to ADP and further to adenosine by ecto-enzymes [40]. Adenosine also acts as an extracellular signalling molecule via activating G-protein-coupled adenosine receptors on the cell surface [8]. Activation of the adenosine receptors has been shown in a very recent study to be significantly involved in ATP-induced regulation of breast cancer cell migration [29]. The role of adenosine receptors in ATP-induced stimulation of HCC cell migration has not been investigated in previous studies. Therefore, we examined the effect of CGS15943, a generic antagonist for adenosine receptors, on ATP-induced stimulation of Huh-7 cell migration. Intriguingly, treatment with 100 nM CGS15943 was also effective in preventing ATP-induced increase in cell migration with no significant effect on cell migration under the basal condition (Figure 7A and 7C), suggesting a substantial role for adenosine receptors in ATP-induced stimulation in HCC cell migration.
Figure 6. ATP stimulates Huh-7 cell migration via activating the P2Y11 receptor.
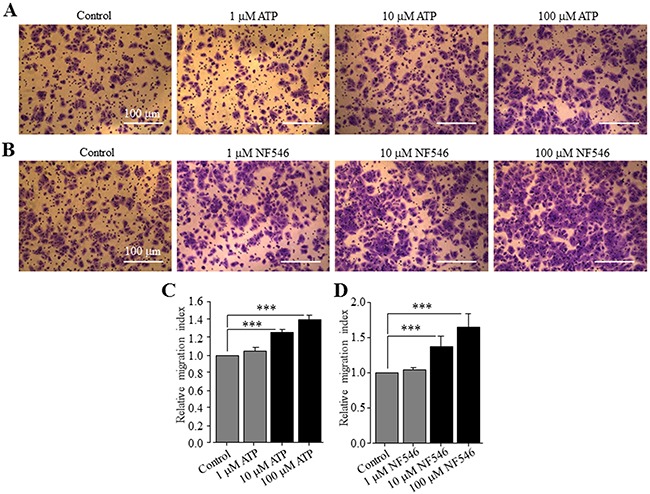
(A-B) Representative crystal violet staining images showing cell migration under basal condition and in the presence of indicated concentrations of ATP (A) and NF546 (B) in trans-well assays. (C-D) Summary of the effects of different concentrations of ATP (C) and NF546 (D) on cell migration in three independent experiments, respectively. ***, p < 0.001.
Figure 7. P2Y11 and adenosine receptors are involved in ATP-stimulated Huh-7 cell migration.
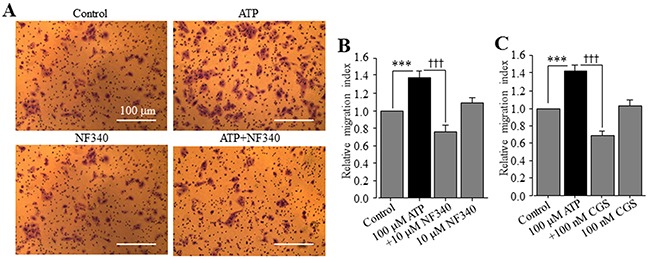
(A) Representative crystal violet staining images showing cell migration under the basal (control) condition and in the presence of 100 μM ATP alone, 10 μM NF340 alone, and 100 μM ATP and 10 μM NF546 (ATP+NF340) in trans-well assays. (B-C) Summary of the effects of treatment with NF340 (B) and 100 nM CGS15943 (C) on ATP-induced increase in cell migration from three independent experiments. ***, †††, p < 0.001.
DISCUSSION
The present study provides pharmacological and genetic evidence that consistently supports functional expression of the P2Y11 receptor as a critical mechanism in mediating ATP-induced Ca2+ signalling and stimulating cell migration in human HCC cells and, importantly, provide evidence to suggest HCC-specific P2Y11 receptor expression.
We first showed that ATP induced robust increases in the [Ca2+]i in Huh-7 cells (Figure 1A). ATP-induced Ca2+ responses were sensitive to inhibition by PPADS and suramin (Figure 1B and 1C). In addition, ADP was much less effective than ATP in elevating the [Ca2+]i (Figure 2B). Overall, these results are consistent with those reported in an early study of Huh-7 cells [38] and also with those in a recent study of native human HCC cells and HepG2 and BEL-7404 cells [37]. In the present study, the P2Y1 receptor agonist MRS2365 was ineffective in inducing an increase in the [Ca2+]i (Figure 2C), largely ruling out a major role of the P2Y1 receptor, despite its mRNA expression being previously reported in Huh-7 cells [38]. In Huh-7 cells, UTP was as potent as ATP in elevating the [Ca2+]i (Figure 2D). Such results could be simply interpreted to indicate involvement of the P2Y2 receptor [38]. Indeed, a recent study shows that shRNA-mediated knockdown of the P2Y2 receptor reduced ATP-induced increase in the [Ca2+]i in HepG2 and BEL-7404 cells, supporting functional expression of the P2Y2 receptor in these cells [37]. However, the present study found that the P2Y2 receptor agonist MRS2768 failed to induce any significant increase in the [Ca2+]i in Huh-7 cells (Figure 2E). We examined expression of the P2Y11 receptor, another ATP-sensitive P2Y receptor and its role in ATP-induced Ca2+ responses in Huh-7 cells, which was not investigated in previous studies. Consistently, P2Y11 receptor agonist NF546 was equally effective as ATP in eliciting robust Ca2+ responses in Huh-7 cells (Figure 3A). Moreover, pre-treatment with P2Y11 receptor specific antagonist NF340 abrogated ATP-induced Ca2+ response (Figure 3B). Huh-7 cells showed positive P2Y11 receptor protein expression as examined by immunofluorescent confocal imaging (Figure 3C) and western blotting (Figure 3D). SiRNA-mediated knockdown of the P2Y11 receptor protein expression (Figure 3D) significantly attenuated NF546-induced increase in the [Ca2+]i in Huh-7 cells (Figure 3E). Similarly, both ATP and NF546 were effective in evoking Ca2+ responses in HepG2 cells (Figure 4A) and NF546-induced increase in the [Ca2+]i was inhibited by NF340 (Figure 4B). The P2Y11 receptor protein in HepG2 cells was also detected by western blotting (Figure 4C). Both the P2Y11 receptor protein expression level and NF546-induced Ca2+ response were significantly reduced by P2Y11-specific siRNA (Figure 4C and 4D). Collectively, our results provide strong evidence to indicate that the P2Y11 receptor is critical in ATP-induced Ca2+ response in human HCC cells.
ATP-induced Ca2+ responses in the extracellular Ca2+-free solution were significantly smaller than those in the extracellular Ca2+-containing solution (Figure 2A). This could result from two distinct molecular mechanisms. The first potential mechanism is that reduction in the ER Ca2+ level, due to Ca2+ release following activation of the P2Y11-Gα,q/11-PLC-IP3R signalling pathway, activates the store-operated Ca2+ entry. Such Ca2+ signalling mechanism has been recently shown to exist in native human HCC cells and HepG2 and BEL-7404 cells [37]. The second and alternative mechanism is the P2X receptor that can also mediates extracellular Ca2+ influx. Previous studies, performed in rat HCC cells, showed that BzATP induced large fast-desensitizing inward currents using patch-clamp recording and a rapid extracellular Ca2+ influx using Ca2+ imaging, and the P2X4 receptor and possibly the P2X7 receptor were thought to mediate such responses [39]. While suramin and PPADS were effective in inhibiting ATP-induced Ca2+ responses (Figure 1B and 1C), the P2X4 receptor selective antagonist 5-BDBD resulted in no inhibition (Supplementary Figure 2A). In the present study, we confirmed protein expression of the P2X7 receptor in Huh-7 cells (Figure 3C and Supplementary Figure 1). However, there was no Ca2+ response to BzATP (Figure 1D), which is known to activate the P2X7 and other P2X receptors including the P2X4 receptor [13]. Exposure to αβmeATP also induced no increase in the [Ca2+]i (Figure 1D), suggesting no expression of functional P2X receptors containing the P2X1, P2X3 or P2X5 subunit. While further studies are required to examine the potential contribution of P2X2 and P2X6 receptors, it is clear from the present study that that the P2X4 and P2X7 receptors are unlikely to have a significant role in mediating ATP-induced Ca2+ responses in Huh-7 cells, thus differing from rat HCC cells [39]. Species difference could be an important factor, and if this is true, cautions need to be exercised in using rodent cells and disease models to elucidate the molecular mechanisms underlying human HCC.
The second interesting finding from the present study is specific expression of the P2Y11 receptor in human HCC tissues. A recent study has reported functional expression of the P2Y2 receptor in hepatocytes, and its expression was elevated in human primary HCC, HepG2 and BEL-7404 cells [37]. Here, our results indicate the P2Y11 receptor is abundantly expressed in human HCC tissues but barely detected in normal liver tissues (Figure 5). This finding brings significant implications. Such HCC-specific P2Y11 receptor expression provides a promising disease biomarker, although it is clearly interesting to examine whether there is close association of the P2Y11 receptor expression with the severity or grade of human HCC. As discussed below, our study suggests a potential role for the P2Y11 receptor in ATP-induced stimulation of cell migration. Further preclinical studies are required to confirm the role of the P2Y11 receptor in the regulation of HCC cell migration and metastasis in vivo, and if this is true, it is interesting to explore the therapeutic promise of targeting the P2Y11 receptor for HCC treatment, as a number of P2Y11 receptor selective antagonists have already developed for clinical uses mainly as anti-platelet drugs [14].
Emerging evidence suggests high micromolar concentrations of extracellular ATP at the tumour sites [6, 7]. Such information, when considered together with HCC-specific expression of the P2Y11 receptor, raises important questions with regard to the relationship of ATP-induced P2Y11-mediated signalling mechanism to the pathogenesis of HCC. In this study, we showed that extracellular ATP up to 100 μM had no effect on Huh-7 cell viability (Supplementary Figure 3) but significantly stimulated cell migration (Figure 6A). As mentioned above, a recent study has reported similar ATP-induced stimulation of cell migration of native human HCC cells and HepG2 and BEL-7404 and attributed the P2Y2 receptor to be critical [37]. In contrast, the present study provides evidence to suggest a distinctive or additional mechanism responsible for ATP-induced stimulation of HCC cell migration. The P2Y11 receptor agonist NF546 was equally potent and even more potent than ATP in stimulating Huh-7 cell migration (Figure 6). Importantly, treatment with the P2Y11 receptor antagonist NF340 completely prevented ATP-induced cell migration (Figure 7A–7B). Therefore, these results support activation of the P2Y11 receptor to be the important molecular mechanism for ATP-induced stimulation of Huh-7 cell migration. Intriguingly, there was no effect of ATP or NF546 on cell migration in scratch-induced wound healing assays (data not shown). Such discrepancy in the results may be related to the difference in the mode of cell migration and the way it is assessed. Cancer cell migration requires important regulations of cell cytoskeleton, volume, morphology, cell-to-matrix and cell-to-cell adhesions. Over the recent years, multiple modes of cancer cell migration have been characterized, from single-cell, when cell-to-cell junctions are absent, to collective migration, when cells move as multicellular groups [41]. Two modes on individual cell migration have been described, the “mesenchymal mode”, in which cytoskeletal protrusions and adhesion capabilities are strong, and cells harbour an elongated fibroblast-like morphology, with a rear-to-front lamellopodial cell polarity, focalized cell-matrix adhesions containing integrin clusters and proteolytic activity towards the extracellular matrix (ECM) [42], and the “amoeboid mode”, in which cancer cells show no obvious polarity but a rounded morphology, a weaker adhesion to the substratum, and display high potentials for motility because of their capacity to deform and squeeze inside tissue gaps [43]. Furthermore, as shown in our recent study, these modes of migration can be further unstable and change as the microenvironmental condition changes, resulting in intermediate or mixed phenotypes [44]. Cell migration, assessed in the trans-well assay, strongly depends on the capacity of cells to deform themselves and go through the pores of the inserts and, by contrast, in the wound-healing assay, the ability of cells to form and recycle focal adhesions is critical. Therefore, one could argue that P2Y11 activation might induce a type of migration that would be related to the amoeboid mode. In addition, in our experimental conditions of trans-well migration, agonists (ATP or NF546) were added into the lower compartment and might act as chemoattractant, which is not the case in the wound-healing assay. The present study indicates contribution of adenosine receptors in ATP-induced stimulation of cell migration (Figure 7C). Evidently, further studies are required to gain a better understanding of these intriguing observations.
In summary, our study provides strong evidence to show human HCC-specific expression of the P2Y11 receptor and its critical role in ATP-induced Ca2+ signalling and cell migration. These findings are important for not only understanding of the pathogenesis of HCC but also for identification of disease biomarkers and drug targets in development of new diagnosis and therapeutic approaches to HCC.
MATERIALS AND METHODS
Reagents and cell culture
All general chemicals were purchased from Sigma-Aldrich, except those indicated specifically. Phosphate-buffered saline (PBS), Dulbecco's modified Eagle's medium (DMEM), foetal bovine serum (FBS), penicillin-streptomycin, trypsin-EDTA, pluronic acid F-127 were from Life Technology, and MRS2768, 5-BDBD, NF546 and NF340 from Tocris Bioscience. MRS2768 was also obtained from Santa Cruz. Huh-7 cells were kindly provided by Prof M Harris (University of Leeds, UK).
Huh-7 and HepG2 cells were maintained in DMEM supplemented with 10% heat inactivated FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C and 5% CO2, and passaged when cells reached 70% confluency. Huh-7 and HepG2 cells were transfected with 15 nM small interfering RNA (siRNA) targeting the P2YR11 gene with the following sequences (siP2Y11): forward 5’-CCUGCUGGGCAGCGUCAUC(TT)-3’ and reverse 5’-GAUGACGCUGCCCAGCAGG(TT)-3’. Irrelevant sequences, not targeting any known gene were used as control sequences (siCTL): forward, 5’-GCCGACCAAUUCACGGCCG(TT)-3’ and reverse, 5’-CGGCCGUGAAUUGGUCGGC(TT)-3’. These sequences were produced by Sigma-Aldrich. Transfection was performed with Lipofectamine RNAi max (Invitrogen) according to the manufacturer's instructions, and cells were used 72 hr after transfection.
[Ca2+]i measurement
Agonist-induced changes in the [Ca2+]i was monitored using Fura-2/AM dye and FLEXstation III (Molecular Device). Cells were seeded onto a 96-well assay plates (Greiner Bio-one) at a density of 2-5x104 cells per well and incubated in culture medium overnight. Cells were loaded with 2 μM Fura-2/AM and 0.4% pluronic acid F-127 (Molecular Probes) in standard buffer solution (SBS: 147 mM NaCl, 2 mM KCl, 1.5 mM CaCl2, 1 mM MgCl2, 10 mM HEPES and 13 mM glucose 13, pH 7.3) at 37°C for 1 hr, and after washing, maintained in SBS at 37°C for 30 min. F340/F380, the ratio of the fluorescence intensity excited alternatively by 340 nm and 380 nm and emitted at 510 nm, was used to indicate the [Ca2+]i. ΔF340/F380 is agonist-induced change in F340/F380. Agonist was added after the baseline was established. Antagonists were added 5 min before addition of agonist. ATP concentration-response curve was constructed by expressing ATP-induced ΔF340/F380 as percentage of ΔF340/F380 induced by 100 μM ATP (Figure 1B) and least squared fit to Hill equation: ΔF340/F380=100/(1+(EC50/[agonist])n), where EC50 is the agonist concentration evoking half of the maximal Ca2+ response, and n is Hill coefficient. Data fit was carried out using Origin software.
Immunocytochemistry
Huh7 cells were seeded on coverslips and incubated in culture medium overnight. Cells were fixed with 4% paraformaldehyde for 10 min and permeabilized with 0.2% Triton X-100 in phosphate buffer saline (PBS-T) for 10 min, and blocked with 5% goat serum or bovine serum albumin in PBS for 1 hr. Cells were then incubated with primary rabbit anti-P2X7 or anti-P2Y11 antibody (Almone) at a dilution of 1: 50 overnight at 4°C and, after extensive washing in PBS-T, were incubated with FITC-conjugated anti-rabbit IgG secondary antibody (Sigma) at a dilution of 1:1000 for 1 hr at room temperature. After washing in PBS-T, coverslips were rinsed in water, dried on tissue papers, and mounted inversely on a glass slide with a small drop of anti-fade mounting medium containing 4’,6-diamidino-2-phenylindole (DAPI). Fluorescent images were captured using an EVOS Cell Imaging System (Thermo Fisher Scientific) and images were merged by Image J software.
Human samples of normal liver and hepatocellular carcinoma were obtained from the tumour biobank of the University-Hospital of Tours, declared to the French Ministry of Research (N°DC2008-308). Briefly, tissues were fixed in formalin, paraffin included, and cut in 5 μm sections. Slides were deparaffinized, rehydrated, and heated in citrate buffer pH 6.0 for antigenic retrieval. Slides were incubated with rabbit anti-P2Y11 antibody at 1: 200 for 1 hr. Immunohistochemistry was performed using the streptavidin-biotin-peroxidase method with diaminobenzidine as the chromogen (Kit LSAB, Dakocytomation). Slides were finally counterstained with haematoxylin. Negative control was obtained after omission of the primary antibody.
Western blotting
Cells were washed with PBS and lysed in presence of a lysis buffer (20 mM Tris, pH 7, 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2), containing 1% Triton X-100 and a cocktail of protease inhibitor (Sigma-Aldrich). Cell lysates were cleared by centrifugation at 10,000 x g for 10 min. Western blotting experiments were performed according to standard protocols. Total protein concentrations were determined using a Pierce® BCA Protein Assay Kit (Fisher Scientific). Protein sample buffer was added and the samples were boiled at 100°C for 3 min. Total protein samples were electrophoretically separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis in 10% gels, and transferred to polyvinylidene fluoride membranes (Millipore). P2Y11 proteins were detected using anti-P2Y11 antibody at a dilution of 1:1,000 and horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody at 1:5,000 (TebuBio). HSC70 protein was detected as a sample loading control using anti-HSC70 mouse antibody at 1:30,000 (TebuBio) and HRP-conjugated anti-mouse-IgG secondary antibodies at 1:2,000 (TebuBio).
Cell migration assay
Trans-well cell migration assays were carried out in 24-well plates with polyethylene terephthalate membrane cell culture inserts containing 8-μm trans-well pores (BD Biosciences) as described in our recent study [45]. The upper compartment was seeded with 1 x 105 cells, and both the upper and lower compartments were filled with DMEM supplemented with 10% FBS, which represent the control conditions. Agonist was added to the medium in the lower chamber, and antagonist was added into the upper chamber at the same time as or 30 min before addition of agonist. After incubation at 37°C and 5% CO2 for 24 hr, cells were fixed with 4% paraformaldehyde and stained with 0.05% crystal violet for 30 min at room temperature. Cells in 9 different areas of each insert were imaged using an ECLIPSE TE2000-U microscope (Nikon) and counted using Image J software. For meaningful comparisons between different experiments, cell migration was presented by expressing the migrated cell number as % of that under control conditions.
Cell death assay
Propidium iodide (PI) staining cell death assays were carried out to examine the effects of ATP on cell viability, cells were seeded on 24-well plated at 1x 104 cells per well and incubated in culture medium without or with supplementation of ATP for 24 hr. Cells were stained with 5 μg/ml PI and 1 μg/ml Hoechst for 30 min. Images were captured using an EVOS Cell Imaging System (Thermo Fisher Scientific).
Data presentation and statistical analysis
All data are presented as mean ± standard error of mean (S.E.M.), where appropriately. Statistical analysis was carried out using Student's t-test to compare two groups one-way ANOVA and Tukey post hoc tests to compare more than two groups using Origin software, with p < 0.05 being indicative of significance.
SUPPLEMENTARY MATERIALS FIGURES
Acknowledgments
We are grateful to Prof M Harris in School of Molecular and Cellular Biology, University of Leeds for kindly providing Huh-7 cells used in this study. The work was supported in part by a travel award from Higher Education Commission Pakistan (MK), the Department of Education of Henan Province and a visiting professorship grant from University François-Rabelais of Tours (L-HJ), the “Ministère de la Recherche et des Technologies”, the Inserm, the “Ligue Nationale Contre le Cancer – Interrégion Grand-Ouest”, the Région Centre (grant “CancerInflamm”, project “ARD2020 Biomédicaments”) and the “Association CANCEN”(SR).
Authors’ contribution
L-HJ, SR and SM conceived the research; MK, LB, MT, RG, GF, YH, SSM and FM performed the experiments; MK, LB, L-HJ and SR analysed the data; L-HJ wrote the manuscript; all authors participated in discussing and revising the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 3.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15:5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 5.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- 6.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3:e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falzoni S, Donvito G, Di Virgilio F. Detecting adenosine triphosphate in the pericellular space. Interface Focus. 2013;3:20120101. doi: 10.1098/rsfs.2012.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 9.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 10.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 11.Jiang LH. P2X receptor-mediated ATP purinergic signaling in health and disease. Cell Health Cytoskelet. 2010;4:83–101. [Google Scholar]
- 12.Browne LE, Jiang LH, North RA. New structure enlivens interest in P2X receptors. Trends Pharmacol Sci. 2010;31:229–237. doi: 10.1016/j.tips.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 14.von Kügelgen I, Harden TK. Molecular pharmacology, physiology, and structure of the P2Y receptors. Adv Pharmacol. 2011;61:373–415. doi: 10.1016/B978-0-12-385526-8.00012-6. [DOI] [PubMed] [Google Scholar]
- 15.Di Virgilio F. Purines, purinergic receptors, and cancer. Cancer Res. 2012;72:5441–5447. doi: 10.1158/0008-5472.CAN-12-1600. [DOI] [PubMed] [Google Scholar]
- 16.Adinolfi E, Amoroso F, Giuliani AL. P2X7 Receptor Function in Bone-Related Cancer. J Osteoporos. 2012;2012:637863. doi: 10.1155/2012/637863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adinolfi E, Capece M, Amoroso F, De Marchi E, Franceschini A. Emerging roles of P2X receptors in cancer. Curr Med Chem. 2015;22:878–890. doi: 10.2174/0929867321666141012172913. [DOI] [PubMed] [Google Scholar]
- 18.Roger S, Jelassi B, Couillin I, Pelegrin P, Besson P, Jiang LH. Understanding the roles of the P2X7 receptor in solid tumour progression and therapeutic perspectives. Biochim Biophys Acta. 2015;1848:2584–2602. doi: 10.1016/j.bbamem.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 19.Jiang JX, Riquelme MA, Zhou JZ. ATP, a double-edged sword in cancer. Oncoscience. 2015;2:673–674. doi: 10.18632/oncoscience.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Adinolfi E, Melchiorri L, Falzoni S, Chiozzi P, Morelli A, Tieghi A, Cuneo A, Castoldi G, Di Virgilio F, Baricordi OR. P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood. 2002;99:706–708. doi: 10.1182/blood.v99.2.706. [DOI] [PubMed] [Google Scholar]
- 22.Adinolfi E, Cirillo M, Woltersdorf R, Falzoni S, Chiozzi P, Pellegatti P, Callegari MG, Sandonà D, Markwardt F, Schmalzing G, Di Virgilio F. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J. 2010;24:3393–3404. doi: 10.1096/fj.09-153601. [DOI] [PubMed] [Google Scholar]
- 23.Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, Di Virgilio F. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72:2957–2969. doi: 10.1158/0008-5472.CAN-11-1947. [DOI] [PubMed] [Google Scholar]
- 24.Jelassi B, Chantôme A, Alcaraz-Pérez F, Baroja-Mazo A, Cayuela ML, Pelegrin P, Surprenant A, Roger S. P2X7 receptor activation enhances SK3 channels- and cysteine cathepsin-dependent cancer cells invasiveness. Oncogene. 2011;30:2108–2122. doi: 10.1038/onc.2010.593. [DOI] [PubMed] [Google Scholar]
- 25.Jelassi B, Anchelin M, Chamouton J, Cayuela ML, Clarysse L, Li J, Goré J, Jiang LH, Roger S. Anthraquinone emodin inhibits human cancer cell invasiveness by antagonizing P2X7 receptors. Carcinogenesis. 2013;34:1487–1496. doi: 10.1093/carcin/bgt099. [DOI] [PubMed] [Google Scholar]
- 26.Giuliani AL, Colognesi D, Ricco T, Roncato C, Capece M, Amoroso F, Wang QG, De Marchi E, Gartland A, Di Virgilio F, Adinolfi E. Trophic activity of human P2X7 receptor isoforms A and B in osteosarcoma. PLoS One. 2014;9:e107224. doi: 10.1371/journal.pone.0107224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu Y, Li WH, Zhang HQ, Liu Y, Tian XX, Fang WG. P2X7 mediates ATP-driven invasiveness in prostate cancer cells. PLoS One. 2014;9:e114371. doi: 10.1371/journal.pone.0114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia J, Yu X, Tang L, Li G, He T. P2X7 receptor stimulates breast cancer cell invasion and migration via the AKT pathway. Oncol Rep. 2015;34:103–110. doi: 10.3892/or.2015.3979. [DOI] [PubMed] [Google Scholar]
- 29.Zhou JZ, Riquelme MA, Gao X, Ellies LG, Sun LZ, Jiang JX. Differential impact of adenosine nucleotides released by osteocytes on breast cancer growth and bone metastasis. Oncogene. 2015;34:1831–1842. doi: 10.1038/onc.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adinolfi E, Capece M, Franceschini A, Falzoni S, Giuliani AL, Rotondo A, Sarti AC, Bonora M, Syberg S, Corigliano D, Pinton P, Jorgensen NR, Abelli L, et al. Accelerated tumor progression in mice lacking the ATP receptor P2X7. Cancer Res. 2015;75:635–644. doi: 10.1158/0008-5472.CAN-14-1259. [DOI] [PubMed] [Google Scholar]
- 31.Amoroso F, Capece M, Rotondo A, Cangelosi D, Ferracin M, Franceschini A, Raffaghello L, Pistoia V, Varesio L, Adinolfi E. The P2X7 receptor is a key modulator of the PI3K/GSK3β/VEGF signaling network: evidence in experimental neuroblastoma. Oncogene. 2015;34:5240–5251. doi: 10.1038/onc.2014.444. [DOI] [PubMed] [Google Scholar]
- 32.Giannuzzo A, Pedersen SF, Novak I. The P2X7 receptor regulates cell survival, migration and invasion of pancreatic ductal adenocarcinoma cells. Mol Cancer. 2015;14:203. doi: 10.1186/s12943-015-0472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li WH, Qiu Y, Zhang HQ, Liu Y, You JF, Tian XX, Fang WG. P2Y2 receptor promotes cell invasion and metastasis in prostate cancer cells. Br J Cancer. 2013;109:1666–1675. doi: 10.1038/bjc.2013.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24:130–137. doi: 10.1016/j.ccr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Jin H, Eun SY, Lee JS, Park SW, Lee JH, Chang KC, Kim HJ. P2Y2 receptor activation by nucleotides released from highly metastatic breast cancer cells increases tumor growth and invasion via crosstalk with endothelial cells. Breast Cancer Res. 2014;16:R77. doi: 10.1186/bcr3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chadet S, Jelassi B, Wannous R, Angoulvant D, Chevalier S, Besson P, Roger S. The activation of P2Y2 receptors increases MCF-7 breast cancer cells migration through the MEK-ERK1/2 signalling pathway. Carcinogenesis. 2014;35:1238–1247. doi: 10.1093/carcin/bgt493. [DOI] [PubMed] [Google Scholar]
- 37.Xie R, Xu J, Wen G, Jin H, Liu X, Yang Y, Ji B, Jiang Y, Song P, Dong H, Tuo B. The P2Y2 nucleotide receptor mediates the proliferation and migration of human hepatocellular carcinoma cells induced by ATP. J Biol Chem. 2014;289:19137–19149. doi: 10.1074/jbc.M113.540047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schöfl C, Ponczek M, Mader T, Waring M, Benecke H, von zur Mühlen A, Mix H, Cornberg M, Böker KH, Manns MP, Wagner S. Regulation of cytosolic free calcium concentration by extracellular nucleotides in human hepatocytes. Am J Physiol. 1999;276:G164–172. doi: 10.1152/ajpgi.1999.276.1.G164. [DOI] [PubMed] [Google Scholar]
- 39.Emmett DS, Feranchak A, Kilic G, Puljak L, Miller B, Dolovcak S, McWilliams R, Doctor RB, Fitz JG. Characterization of ionotrophic purinergic receptors in hepatocytes. Hepatology. 2008;47:698–705. doi: 10.1002/hep.22035. [DOI] [PubMed] [Google Scholar]
- 40.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 41.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 43.Wolf K, Friedl P. Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol. 2011;21:736–44. doi: 10.1016/j.tcb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Bon E, Driffort V, Gradek F, Martinez-Caceres C, Anchelin M, Pelegrin P, Cayuela ML, Marionneau-Lambot S, Oullier T, Guibon R, Fromont G, Gutierrez-Pajares JL, Domingo I, et al. SCN4B acts as a metastasis-suppressor gene preventing hyperactivation of cell migration in breast cancer. Nat Commun. 2016;7:13648. doi: 10.1038/ncomms13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng H, Hao Y, Mousawi F, Roger S, Li J, Sim JA, Ponnambalam S, Yang X, Jiang LH. Purinergic and store-operated Ca2+ signaling mechanisms in mesenchymal stem cells and their roles in ATP-Induced stimulation of cell migration. Stem Cells. 2016;34:2102–2014. doi: 10.1002/stem.2370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


