Abstract
Knockdown of growth factor receptor binding protein 2 (Grb2) by RNA interference strongly inhibits clathrin-mediated endocytosis of the epidermal growth factor receptor (EGFR). To gain insights into the function of Grb2 in EGFR endocytosis, we have generated cell lines in which endogenous Grb2 was replaced by yellow fluorescent protein (YFP)-tagged Grb2 expressed at the physiological level. In these cells, Grb2-YFP fully reversed the inhibitory effect of Grb2 knockdown on EGFR endocytosis and, moreover, trafficked together with EGFR during endocytosis. Overexpression of Grb2-binding protein c-Cbl did not restore endocytosis in Grb2-depleted cells. However, EGFR endocytosis was rescued in Grb2-depleted cells by chimeric proteins consisting of the Src homology (SH) 2 domain of Grb2 fused to c-Cbl. The “knockdown and rescue” analysis revealed that the expression of Cbl-Grb2/SH2 fusions containing RING finger domain of Cbl restores normal ubiquitylation and internalization of the EGFR in the absence of Grb2, consistent with the important role of the RING domain in EGFR endocytosis. In contrast, the carboxy-terminal domain of Cbl, when attached to Grb2 SH2 domain, had 4 times smaller endocytosis-rescue effect compared with the RING-containing chimeras. Together, the data suggest that the interaction of Cbl carboxy terminus with CIN85 has a minor and a redundant role in EGFR internalization. We concluded that Grb2-mediated recruitment of the functional RING domain of Cbl to the EGFR is essential and sufficient to support receptor endocytosis.
INTRODUCTION
Activation of the epidermal growth factor (EGF) receptor (EGFR) triggers multiple signal transduction events and accelerates endocytosis of EGFR through clathrin-coated pits. Clathrin-mediated endocytosis and subsequent targeting of internalized EGF–receptor complexes to lysosomes result in down-regulation of EGFR protein levels and attenuation of receptor signaling. Endocytosis also controls subcellular localization of activated receptors and their signaling complexes. However, the molecular mechanisms of EGFR endocytosis are not fully understood.
Growth factor receptor binding protein 2 (Grb2) is an essential component of EGFR signaling to Ras (Li et al., 1993; Rozakis-Adcock et al., 1993). The Src homology (SH) 2 domain of Grb2 can bind directly to phosphotyrosines 1068 and 1086 of the activated EGFR or indirectly through the tyrosine-phosphorylated adaptor protein Shc (Batzer et al., 1994; Okutani et al., 1994). The SH3 domains of Grb2 are constitutively associated with Son of Sevenless (SOS), an exchange factor of Ras GTPase (Li et al., 1993; Rozakis-Adcock et al., 1993). Binding of the Grb2–SOS complex to the EGFR places SOS in proximity to Ras, thus leading to GTP-loading of Ras and subsequent activation of Ras effectors, such as Raf kinases and phosphatidylinositol 3-kinase.
Grb2 also is involved in EGF-induced signaling to the actin cytoskeleton (She et al., 1997) and EGFR endocytosis (Wang and Moran, 1996; Yamazaki et al., 2002; Jiang et al., 2003). Although the pathway of Grb2 signaling through Ras is well understood, the molecular mechanisms by which Grb2 controls clathrin-mediated endocytosis of the EGFR remain to be elucidated. The knockdown of Grb2 by small interfering RNA (siRNA) results in dramatic inhibition of the initial steps of EGFR endocytosis (Jiang et al., 2003; Huang et al., 2004). Grb2 was found to move to coated pits together with activated EGFR (Jiang et al., 2003; Stang et al., 2004). However, how Grb2 links EGFR to coated pits is unknown. Most likely, Grb2 function is mediated by a protein that binds to the SH3 domains of Grb2. Besides interaction with SOS, Grb2 SH3 domains are capable of association with several proteins, including dynamin and Cbl (c-Cbl, Cbl-b, and Cbl-3) (Meisner et al., 1995; Kranenburg et al., 1999; Courbard et al., 2002), both implicated in the regulation of EGFR endocytosis.
Dynamin is a component of the general clathrin-coated pit machinery and is necessary for endocytosis of all types of cargo internalized through coated pits. Recently, however, it has been proposed that dynamin can be phosphorylated in response to EGF (Ahn et al., 2002). The exact role of this phosphorylation and how this dynamin modification contributes specifically to EGFR endocytosis are unclear. Cbl is an E3 ubiquitin ligase that has been implicated in EGFR endocytosis and postendocytic trafficking based on the effects of Cbl mutant overexpression (Levkowitz et al., 1999; Thien and Langdon, 2001; Dikic and Giordano, 2003; Marmor and Yarden, 2004). However, whereas the role of Cbl in intracellular sorting of EGFR is well established, Cbl participation in the first step of endocytosis, internalization through coated pits, is a subject of debate. The demonstration of Cbl localization in coated pits (de Melker et al., 2001; Jiang and Sorkin, 2003; Stang et al., 2004) and inhibition of EGFR internalization by c-Cbl mutant overexpression suggested that Cbl is involved in this process (Thien et al., 2001; Jiang and Sorkin, 2003). However, the observation of normal EGFR internalization in cells derived from c-Cbl knockout mouse argues against the role of Cbl proteins in this step of EGFR trafficking (Duan et al., 2003).
c-Cbl and Cbl-b may function in EGFR endocytosis either by 1) ubiquitylation of the EGFR (Levkowitz et al., 1999; Thien et al., 2001; Jiang and Sorkin, 2003) or 2) linking EGFR to the CIN85/endophilin complex (Soubeyran et al., 2002). The relative contribution of these two pathways in EGFR endocytosis is unknown. The analysis of Cbl role in endocytosis is complicated due to the presence of three Cbl proteins that may have redundant functions.
Previous studies analyzed the spatial and temporal control of Grb2 function in signaling and endocytosis by using overexpression of tagged Grb2 and its interactors. Hence, we developed a new approach to analyze Grb2 function. In this approach, endogenous Grb2 is knocked down by siRNA and replaced by either wild-type Grb2 tagged with yellow fluorescent protein (YFP) or a chimeric protein consisting of the SH2 domain of Grb2 linked to a Grb2-interacting protein, thus bypassing the SH3 domain interactions of Grb2. Our analysis using the “knockdown and rescue” approach was focused on Cbl proteins and revealed the key role of Grb2-mediated recruitment of Cbl to the EGFR in the physiological pathway of receptor internalization via coated pits.
MATERIALS AND METHODS
Reagents
Pfu polymerase and the QuikChange site-directed mutagenesis kit were from Stratagene (La Jolla, CA). EGF conjugated with rhodamine (EGF-Rh) was purchased from Molecular Probes (Eugene, OR). Monoclonal antibodies specific to early endosomal antigen (EEA.1), c-Cbl, and PP1 were obtained from BD Transduction Laboratories (San Diego, CA). Monoclonal antibodies to green fluorescent protein (GFP) and EGFR (Ab528) were from Zymed Laboratories (South San Francisco, CA) and American Type Culture Collection (Manassas, VA), respectively. Polyclonal antibody to Grb2 and monoclonal antibody (mAb) P4D1 to ubiquitin were from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal antibodies to total and phosphorylated mitogen-activated protein kinase kinase (MEK)1/2 were from Cell Signaling Technology (Beverly, MA). Rabbit serum Ab2913 specific to the intracellular domain of EGFR was kindly provided by Dr. L. Beguinot (DIBIT Rafaele, Milan, Italy).
Plasmid Constructs and Point Mutations
To generate a Grb2 RNA interference (RNAi) construct (U6/Grb2), a DNA oligonucleotide was inserted into the pSilencer1.0-U6 vector (Ambion, Austin, TX) between ApaI (blunted) and EcoRI sites. The oligonucleotide, which contained the sense strand of human Grb2 DNA fragment 609–627 followed by a 6-nt loop and then antisense strand of the Grb2 fragment and five Ts, is 5′-G CATGTTTCCCCGCAATTAT CAAGAC ATAATTGCGGGGAAACATG CC TTTTT-3′ (forward) and 5′-AATT AAAAA GG CATGTTTCCCCGCAATTAT GTCTTG ATAATTGCGGGGAAACATG C-3′ (reverse).
The Grb2-YFP construct was described previously (Sorkin et al., 2000). Six silent mutations without changing the amino acid sequence of Grb2 were generated in the construct by using the QuikChange mutagenesis kit to make Grb2-YFP resistant to the Grb2 siRNA (609–627) silencing. The DNA primer for the mutagenesis is 5′-C GGG CAG ACC GGA ATG TTC CCA CGT AAC TAC GTC ACC CCC GTG-3′ (forward) and 5′-CAC GGG GGT GAC GTA GTT ACG TGG GAA CAT TCC GGT CTG CCC G-3′ (reverse) (underlined are mutated).
To generate YFP-Cbl, full-length c-Cbl was cut from Cbl-YFP (Jiang and Sorkin, 2003) and cloned into pEYFP-C3 by using KpnI and BamHI sites. Cbl-GFP construct was kindly provided by Drs. G. Levkowitz and Y. Yarden (Weizmann Institute of Science, Rehovot, Israel). DNA encoding SH2 domain of human Grb2 (amino acids 53–164) with the stop codon was amplified using Pfu polymerase and inserted into pEYFP-C3 vector (BD Biosciences Clontech, Palo Alto, CA) between SacII and BamHI restriction sites, which generated YFP-SH2. To generate YFP-Cbl-SH2 chimeric proteins, DNAs amplified by polymerase chain reaction encoding full-length c-Cbl or c-Cbl fragments were then inserted between XhoI and KpnI sites of YFP-SH2 construct. The chimeras of the c-Cbl N terminus with SH2 domains of rat phospholipase Cγ1 (PLCγ1) (provided by Dr. G. Carpenter, Vanderbilt University, Nashville, TN) were made by cloning DNAs encoding N-terminal domain (corresponding to residues 548–661) or both SH2 domains (residues 548–759) of PLCγ1 with the stop codon into pEYFP-C3 between SacII and BamHI restriction sites followed by inserting c-Cbl fragment between XhoI and KpnI sites of YFP-PLC/SH2 constructs. All point mutations in the constructs were generated using the QuikChange mutagenesis kit according to the manufacturer's protocol. All constructs and point mutations were verified by automatic dideoxynucleotide sequencing.
Cell Culture and Transfections
HeLa cells were grown in DMEM containing 10% fetal bovine serum, antibiotics, and glutamine. Grb2 siRNA3 duplex (Jiang et al., 2003) was resuspended in 1× siRNA universal buffer provided by Dharmacon (Lafayette, CO), to 20 μM before transfection. To knock down Grb2, HeLa cells in 12-well plates were transfected twice with 4 μl of siRNA3 duplex in 3 μl of LipofectAMINE 2000 reagent (Invitrogen, Carlsbad, CA) at 24-h intervals. For mock-transfections control Cy5-siRNA duplex verified for the absence of off-site effects (Dharmacon) was used. siRNA to μ2 subunit of adaptor protein-2 (AP-2) was prepared and transfected precisely as described previously (Motley et al., 2003). For the purpose of expressing Cbl-SH2 chimeric proteins in the Grb2-depleted cells, Cbl constructs were cotransfected with siRNA3 in the second siRNA transfection. Cells were plated to new 12-well plates or glass coverslips 24 h before experiments, which were performed 3 or 4 d after the first transfection.
The HeLa/Grb2-YFP cell lines were obtained by cotransfection of cells with U6/Grb2 and silent Grb2-YFP plasmids followed by single cell clone selection with 0.4 mg/ml G418 (Invitrogen).
Immunoprecipitation and Western Blotting
To assay ubiquitylation of EGFR, HeLa cells transiently transfected with Grb2 siRNA and YFP-Cbl-SH2 in 60-mm dishes were pretreated with 20 ng/ml EGF for 2 min at 37°C and washed with Ca2+, Mg2+-free phosphate-buffered saline (CMF-phosphate-buffered saline). Cells were lysed by scraping with a rubber policeman in Triton X-100/glycerol solubilization buffer (TGH) as described previously (Huang et al., 2003, 2004) supplemented with 10 mM N-ethyl-maleimide and by incubating further for 10 min at 4°C. The lysates were then cleared by centrifugation for 10 min at 14,000 × g. EGFR was immunoprecipitated with antibody Ab528. The precipitates were washed twice with TGH buffer supplemented with 100 mM NaCl, and once without NaCl, and then denatured by heating in sample buffer. Immunoprecipitates and supernatants after immunoprecipitation were resolved on 7.5% SDS-PAGE followed by transfer to nitrocellulose membrane and Western blotting with various antibodies followed by species-specific secondary antibodies or protein A (Zymed Laboratories) conjugated with horseradish peroxidase. The enhanced chemiluminescence kit was from Pierce Chemical (Rockford, IL).
To probe for active MEK1/2, cells in 12-well plates were starved overnight (12–16 h) in DMEM supplemented with 1% conditioned medium, treated with 1 ng/ml EGF at 37°C for 0–30 min, lysed with TGH buffer, and resolved on 10% SDS-PAGE followed by transfer to the nitrocellulose membrane and Western blotting.
125I-EGF Internalization
Mouse receptor-grade EGF (Collaborative Research, Bedford, MA) was iodinated using a modified chloramine T method as described previously (Jiang et al., 2003). 125I-EGF internalization was measured using 1 ng/ml 125I-EGF and the specific rate constant for internalization, ke, was calculated as described previously (Jiang et al., 2003). A low concentration of 125I-EGF was used to avoid saturation of the internalization machinery.
Fluorescence Microscopy
HeLa cells on glass coverslips were treated with 2 ng/ml EGF-Rh at 37°C for 5 min or with 10 ng/ml EGF-Rh at 4°C for 1 h, washed with ice-cold phosphate buffer saline, and fixed with freshly prepared 4% paraformaldehyde (Electron Microscopy Sciences, Ft. Washington, PA) for 30 min on ice. In some experiments, cells incubated with EGF-Rh were treated with cold 0.2 M acetic acid, pH 4.5, containing 0.5 M NaCl for 2 min before fixation to remove surface-bound EGF-Rh. In other experiments, to estimate colocalization of EGF-Rh with EEA.1, fixed cells were mildly permeabilized using a 30-min incubation in CMF-phosphate-buffered saline containing 0.02% saponin and 0.1% bovine serum albumin (saponin solution) at room temperature, incubated in saponin solution at room temperature for 1 h with mAb to EEA.1, and then incubated for 30 min with the secondary donkey antimouse IgG labeled with Cy5 (Jackson ImmunoResearch Laboratories, West Grove, PA). Both primary and secondary antibody solutions were precleared by centrifugation at 100,000 × g for 10 min. The coverslips were mounted in Fluoromount-G (Southern Biotechnology, Birmingham, AL) containing 1 mg/ml para-phenylenediamine.
Images were acquired using a Marianas imaging workstation (Intelligent Imaging Innovation, Denver, CO). Twelve serial two-dimensional images were recorded at 300-nm intervals. A Z-stack of images obtained was deconvoluted using a nearest neighbor method. The quantitations of EGF-Rh were performed only in experiments where cells were not permeabilized with the detergent because the latter procedure results in a loss of cell-associated EGF-Rh.
The amount of internalized EGF-Rh was quantitated as follows. The background was subtracted from deconvoluted images. The integrated intensity of rhodamine fluorescence in all voxels corresponding to the z-stack of two-dimensional images was calculated for each individual cells. These measurements produced consistently similar values of rhodamine integrated intensity for Grb2-depleted or intact cells in all independent experiments, thus validating the method. The single-cell assay differs from the conventional internalization assay by using 125I-EGF in that the microscopy assay emphasizes the punctate EGF-Rh fluorescence (coated pits, vesicles, and other receptor clusters), whereas it underestimates the diffuse fluorescence of the plasma membrane-bound EGF-Rh due to insufficient sensitivity of imaging systems within the linear range of cooled charged-coupled detectors.
Statistical significance (p value) was assessed using the two-tailed Student's t test for unpaired samples.
Time-Lapse Imaging of Grb2-YFP
HeLa/Grb2-YFP cells (Cl-1) were grown on coverslips and assembled into the closed perfusion chamber (Harvard Apparatus, Holliston, MA). The chamber was mounted onto the microscope stage. The time-lapse image acquisition was performed at 33°C through the narrow GFP (excitation 492/10 nm) channel. Typically, 30 images (150-ms integration time) were acquired with 15-s intervals. Binning 2 × 2 mode was used. QuickTime movies were created from the original SlideBook time-lapse images.
RESULTS
Stably Expressed Grb2-YFP Restores EGFR Endocytosis and Signaling in Grb2-depleted Cells
Our previous studies in HeLa and porcine aortic endothelial (PAE) cells demonstrated that knockdown of Grb2 by siRNA results in dramatic inhibition of EGFR internalization (Jiang et al., 2003; Huang et al., 2004). Hence, we analyzed the mechanisms by which Grb2 regulates EGFR endocytosis. First, we tested whether expression of a Grb2 fusion protein, such as Grb2-YFP, can reverse the effects of Grb2 depletion. To this end, we have chosen a strategy to stably express Grb2-YFP in cells depleted of endogenous Grb2 by siRNA. In this approach, cells constitutively expressing Grb2-YFP at physiological levels can be isolated.
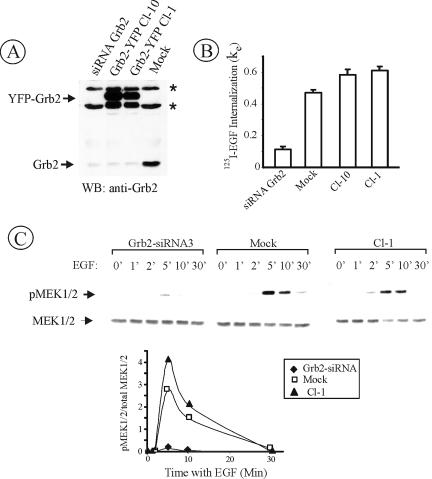
To generate HeLa cells stably expressing Grb2-YFP, endogenous Grb2 was knocked down using vector-based short hairpin RNA (shRNA) with simultaneous expression of Grb2-YFP that has silencing mutations rendering this construct insensitive to shRNA. From the selected clones, two single cell clones (Cl-1 and Cl-10) of HeLa/Grb2-YFP cells with minimal expression of endogenous Grb2 and moderate expression of Grb2-YFP were chosen (Figure 1A). Cl-1 expressed very low amount of Grb2 (<5% of Grb2 level in mock-transfected cells) and a nearly physiological level of Grb2-YFP (∼40% higher than the Grb2 level in the parent HeLa cells). This clone was used in most of the subsequent experiments.
Figure 1.
Expression of Grb2-YFP restores EGFR internalization and mitogen-activated protein kinase signaling in Grb2-depleted HeLa cells. (A) Western blot detection of Grb2 in HeLa cells transiently transfected with Grb2 siRNA (siRNA Grb2), transiently mock-transfected (Mock), and HeLa cell lines Cl-10 and Cl-1 stably expressing Grb2-YFP and U6/Grb2-siRNA plasmids. Asterisks show nonspecific bands. (B) 125I-EGF (1 ng/ml) internalization rate constant (ke) measured in cells described in A. The data represent mean values from three experiments (±SEM). (C) Cells described in A were serum starved overnight and treated with 1 ng/ml EGF at 37°C for the indicated times. Cell lysates were resolved by electrophoresis. Activated MEK1/2 was detected by blotting with antibodies to phosphorylated MEK1/2. The same blots were then reprobed with the antibody to total MEK1/2. The extent of MEK1/2 phosphorylation is expressed as a ratio of phosphorylated to total cellular MEK1/2.
Both Cl-1 and Cl-10 displayed high rates of 125I-EGF internalization, indicating that Grb2-YFP fully rescued the inhibitory effects of Grb2 knockdown on EGFR endocytosis (Figure 1B). Depletion of Grb2 by siRNA also caused dramatic inhibition of EGF-induced MEK1/2 activation (Figure 1C). MEK1/2 activity was, however, fully restored in Cl-1 cells (Figure 1C). Interestingly, ERK1/2 activity was inhibited only by 50% in cells depleted of Grb2 and also fully rescued by Grb2-YFP (our unpublished data). The significant activation of ERK1/2 in cells with very low MEK1/2 activity can be due to substantial molar excess of MEK1/2 over ERK1/2 in the cells and a 1000-fold increase of ERK kinase activity by MEK1/2, leading to dramatic signal amplification at this step of the ERK activation cascade (reviewed in Pearson et al., 2001). In fact, binding of EGF to a very small pool of EGFR was sufficient for the full activation of ERK1/2 in several cell types (Soler et al., 1994a; Johannessen et al., 2000).
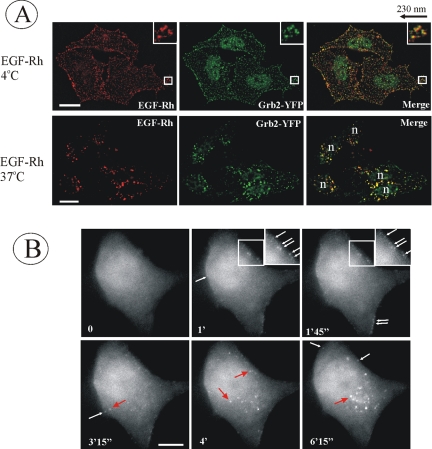
The effective endocytosis of EGFR also could be observed in HeLa/Grb2-YFP cells by fluorescence microscopy by using an EGF-Rh conjugate. When Cl-1 cells were incubated with EGF-Rh at 4°C, conditions that allow recruitment of the EGFR into coated pits but restrict endocytosis, Grb2-YFP dots were highly colocalized with EGF-Rh dots at the plasma membrane (Figure 2A). Previous studies demonstrated that these dots represent clathrin-coated pits (Jiang et al., 2003). When cells were incubated with EGF-Rh at 37°C, EGF-Rh and Grb2-YFP were accumulated in the peripheral and perinuclear endosomes (Figure 2A).
Figure 2.
Visualization of Grb2-YFP in HeLa/Grb2-YFP Cl-1 cells. (A) Cl-1 cells were treated with 10 ng/ml EGF-Rh at 4°C for 1 h or with 2 ng/ml EGF-Rh at 37°C for 5 min and fixed. A z-stack of images was acquired through the Cy3 (red) and YFP (green) channels and deconvoluted. YFP and Cy3 images representing individual optical sections were merged after adjustment of both fluorescence signals to similar levels (Merge). Insets represent high-magnification images of the regions shown by white rectangles. In the merged inset image, YFP image was shifted approximately by 230 nm to the left relative to rhodamine images to clearly assess the colocalization of Grb2-YFP with EGF-Rh. n, cell nuclei. Bars, 10 μm. (B) Cells were incubated with 100 ng/ml EGF at 33°C, and images were acquired at 15-s intervals. This high concentration of EGF was used because of the significant dilution of EGF during perfusion of the microscope chamber. Times after EGF addition are indicated in the left bottom corner of the images. White arrows show Grb2-YFP localized to endocytic coats and forming vesicles at cell edges, whereas red arrows show Grb2-YFP localized in endosomes at some distance from cell edges or in the perinuclear area of the cell. The corresponding Quicktime movie is presented in Supplemental Materials. Bar, 10 μm.
To examine localization of Grb2-YFP in living cells, time-lapse imaging of EGF-stimulated cells was performed (Figure 2B) (see Quicktime movie in Supplemental Materials). In unstimulated cells, Grb2-YFP was diffusely distributed in the cytosol and nucleus. Similar pattern of localization was observed for endogenous Grb2 by using immunofluorescence staining (Sorkin et al., 2000). Rapidly after EGF stimulation, Grb2-YFP began to concentrate in small dots at the edges of the cell. Further incubation at 37°C led to accumulation of Grb2-YFP in endosomes located mostly in the perinuclear area of the cell. Together, microscopy analyses of fixed and living cells demonstrated that Grb2-YFP accompanies EGFR at the plasma membrane and during the passage of receptors through endocytic compartments. These data also illustrate that the dynamic localization of Grb2-YFP can be analyzed in cells, in which this fusion protein is expressed at physiological levels.
Cbl Overexpression Does Not Rescue EGF Endocytosis in Grb2-depleted Cells
Stemming from our findings that Grb2 is essential for EGFR internalization in HeLa cells and that Grb2-YFP can functionally replace endogenous Grb2, we further investigated the mechanisms by which Grb2 controls EGFR internalization. Because Cbl proteins have been implicated in EGFR internalization (Levkowitz et al., 1999; Thien et al., 2001; Waterman et al., 2002; Jiang and Sorkin, 2003), we tested whether overexpression of c-Cbl can overcome the inhibition of endocytosis imposed by Grb2 knockdown. In these experiments, cells were cotransfected with Grb2-targeted siRNA and a YFP-Cbl construct (Figure 3A). The endocytosis of EGFR was analyzed using a fluorescence microscopy assay by comparing EGF-Rh endocytosis in Grb2-depleted cells expressing or not expressing YFP-Cbl. The cells were incubated with 2 ng/ml EGF-Rh for 5 min at 37°C, acid washed (pH 4.5) to remove surface EGF-Rh, and analyzed by three-dimensional (3-D) deconvolution microscopy as described in Materials and Methods. These conditions are optimized for monitoring the clathrin-mediated pathway of EGFR internalization. For instance, endocytosis of EGF-Rh measured using an identical assay was severely inhibited by siRNA knockdown of clathrin heavy chain (Huang et al., 2004).
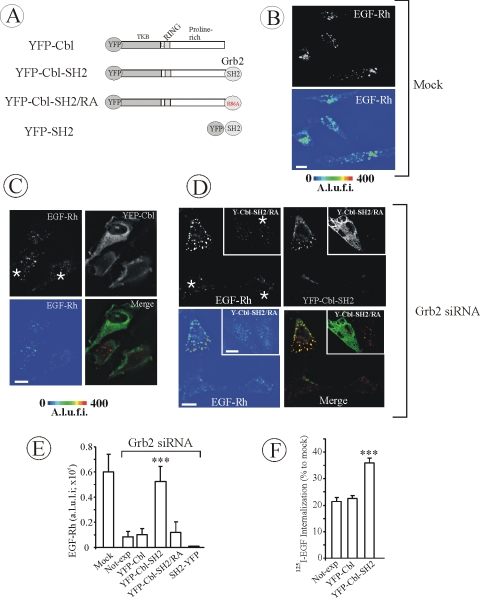
Figure 3.
YFP-Cbl-SH2 chimera rescues EGFR internalization in HeLa cells depleted of Grb2. (A) Schematic representation of Cbl-Grb2 chimeric proteins fused to YFP. The YFP-Cbl-SH2 chimera consists of YFP, residues 1–906 of c-Cbl, and the SH2 domain of Grb2. YFP-Cbl-SH2/RA is a mutant YFP-Cbl-SH2 chimera with the substitution of Arg86 by alanine in the Grb2 SH2 domain. YFP-SH2 is the SH2 domain of Grb2 fused to YFP. (B–D) HeLa cells were mock transfected (B) or transiently transfected (C and D) with Grb2 siRNA. The cells also were transfected with YFP-Cbl (C), YFP-Cbl-SH2 (D), or YFP-Cbl-SH2/RA (D, insets). The cells were incubated with 2 ng/ml EGF-Rh for 5 min at 37°C and fixed. A z-stack of optical sections was acquired through the Cy3 (red) and YFP (green) channels and deconvoluted. YFP and Cy3 images representing individual optical sections were merged after adjustment of the YFP signal (Merge). EGF-Rh images also are displayed in quantitative pseudocolor using the same scale for all images. A.l.u.f.i., arbitrary linear units of fluorescence intensity. White asterisks show positions of Grb2-depleted cells that do not express YFP fusion proteins (nonexp). Some of these cells can be seen by their autofluorescence in the YFP filter channel. Bars, 10 μm. (E) Quantification of the amount of internalized EGF-Rh from experiments performed as described in B–D. In experiments with all YFP-tagged proteins, the values of integrated intensity did not depend on the expression levels of these fusion proteins. The error bars represent SEs (n = 20). ***p < 0.0001 compared with cells depleted of Grb2 and not expressing YFP-tagged constructs (nonexp). (F) HeLa cells were transfected with control siRNA (mock) or Grb2 siRNA. The cells were also transfected with YFP-Cbl or YFP-Cbl-SH2 as described in D. Internalization rates of 125I-EGF were measured as in Figure 1B.
As shown in Figure 3B, EGF-Rh was rapidly internalized and accumulated in early/intermediate endosomes in the perinuclear area of control HeLa cells (processed with all siRNA transfection reagents). In Grb2-depleted cells, a small amount of EGF-Rh was present in the perinuclear vesicular structures (presumably, endosomes) (see cells not expressing YFP marked by asterisks in Figure 3, C and D), and it was virtually impossible to find cells with the significant amount of internalized EGF-Rh. The residual internalization in Grb2-depleted cells was probably due to either Grb2-independent pathways of internalization or a small pool of Grb2 remaining in the cells. The pattern of localization and the intensity of EGF-Rh fluorescence was essentially the same in cells expressing or not expressing YFP-Cbl (Figure 3C) or Cbl-GFP fusion proteins (our unpublished data). These data suggest that c-Cbl overexpression did not functionally compensate for the absence of Grb2.
Recruitment of Cbl to the EGFR through the SH2 Domain of Grb2 Restores EGFR Endocytosis in Grb2-depleted Cells
Our previous study suggested the importance of Cbl binding to the EGFR through the interaction of its carboxy terminus with the SH3-containing proteins, such as Grb2 (Jiang and Sorkin, 2003). Thus, we reasoned that to functionally rescue the effects of Grb2 knockdown, Cbl must be situated on the receptor molecule in a way that would mimic Cbl position when it is bound to Grb2. Therefore, a chimeric protein containing full-length c-Cbl fused at the carboxy terminus to the SH2 domain of Grb2 (YFP-Cbl-SH2) was constructed (Figure 3A). Such a chimera was expected to bind to Grb2 binding sites of the EGFR.
Surprisingly, when expressed in Grb2-depleted cells, YFP-Cbl-SH2 caused significantly larger accumulation of EGF-Rh in endosomes compared with Grb2-depleted cells that did not express the chimeric protein (Figure 3D). YFP-Cbl-SH2 was highly colocalized with EGF-Rh, suggesting that the chimera efficiently binds to the EGFR. Mutational inactivation of the Grb2 SH2 domain in the YFP-Cbl-SH2 construct (R86A mutation) resulted in a chimeric protein that failed to restore EGF-Rh endocytosis in Grb2-depleted cells (Figure 3D, insets). This observation established that the rescue effect of YFP-Cbl-SH2 chimera requires the functional SH2 domain of Grb2.
Quantitative analysis revealed that the amount of internalized EGF-Rh in Grb2-depleted, YFP-Cbl-SH2–expressing cells was significantly higher than the amount of EGF-Rh in neighboring cells that did not express the YFP-Cbl-SH2 chimera or in independent cultures of Grb2-depleted, DNA mock-transfected cells (Figure 3E). Although the extent of Grb2 depletion in each individual cell could not be controlled by immunostaining because of the loss of EGF-Rh due to cell permeabilization, very small variation in the amount of EGF-Rh per cell within the population of randomly chosen Grb2-depleted cells (Figure 3E) suggested that EGF-Rh endocytosis was substantially inhibited in all cells in the population.
The extent of EGF-Rh endocytosis in Grb2-depleted and YFP-Cbl-SH2–expressing cells was similar to that in cells that were not depleted of Grb2 (Figure 3E). Interestingly, control experiments, in which Grb2 SH2 domain tagged with YFP (Figure 3A) (Jiang et al., 2003) was expressed in Grb2-depleted cells, revealed that this fusion protein not only failed to rescue EGFR endocytosis but caused complete disappearance of detectable internalized EGF-Rh (Figure 3E). This observation implies that the residual endocytosis in Grb2-depleted cells is likely to be dependent on Grb2 binding sites in the EGFR, which are blocked by overexpression of SH2-YFP.
In addition to the single-cell internalization assay, 125I-EGF endocytosis was measured in total cell populations of Grb2-depleted cells as described in Figure 1B. As shown in Figure 3F, the apparent rate of internalization was increased by ∼65% in cells transfected with YFP-Cbl-SH2, whereas it was not increased by expression of YFP-Cbl compared with Grb2-depleted cells. Such partial increase was expected because the efficiency of DNA plasmid transfection under conditions of siRNA/DNA cotransfection was significantly lower (15–20%) compared with that of siRNA transfection (essentially 100%). This resulted in YFP-Cbl-SH2 expression and therefore rescue of endocytosis in only a small pool of Grb2-depleted cells. Because the data obtained in two internalization assays correlated well and because the conventional biochemical assay is less reliable due to difficulties in matching expression levels and transfection efficiencies of different Cbl-SH2 fusion constructs, subsequent experiments relied on the quantitative single-cell assay.
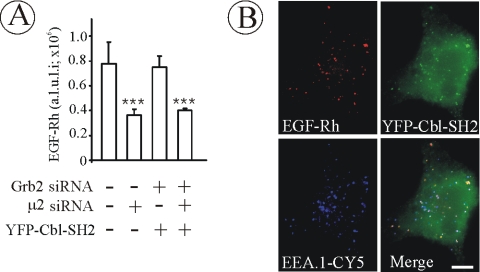
The data in Figure 3 demonstrated that the YFP-Cbl-SH2 chimera that is capable of binding to Grb2 interaction sites of the EGFR restores EGFR endocytosis in Grb2-depleted cells. To test whether this restored endocytosis is mediated by clathrin-associated machinery, the effect of depletion of clathrin adaptor complex AP-2 on EGFR endocytosis in Grb2-depleted/YFP-Grb2-SH2 chimera-rescued cells was tested using siRNA targeting μ2 subunit of AP-2 (Motley et al., 2003). As shown in Figure 4A, μ2 siRNA inhibited EGF-Rh endocytosis to the same extent in mock-transfected (control siRNA and DNA) and Grb2-depleted/YFP-Cbl-SH2–rescued cells. Furthermore, EGF-Rh was well colocalized with EEA.1 in YFP-Cbl-SH2–expressing cells (Figure 4B), confirming that the vesicular-shape compartments containing EGF-Rh represent early/sorting endosomes. These data imply that the pathways of EGFR endocytosis in YFP-Cbl-SH2–rescued cells are similar to those pathways normally utilized by EGFR in intact cells.
Figure 4.
EGF-Rh is internalized in AP-2–dependent manner and accumulates in early endosomes in cells depleted of Grb2 and expressing YFP-Cbl-SH2 chimera. (A) HeLa cells were mock transfected or transfected with Grb2 siRNA or μ2 siRNA. The cells were also transfected with YFP-Cbl-SH2 as described in Figure 3D. The incubations with EGF-Rh and quantitations of the amount of EGF-Rh in endosomes were performed as described in Figure 3, B–E. The error bars represent SEs (n = 10). ***p < 0.0001 compared with cells depleted of μ2. (B) HeLa cells were transfected with Grb2 siRNA and YFP-Cbl-SH2, incubated with EGF-Rh as described in D, fixed, permeabilized, and stained with a mAb to EEA.1 followed by secondary donkey anti-mouse IgG conjugated with Cy5. A z-stack of optical sections was acquired through the Cy3, Cy5, and YFP channels and deconvoluted. Cy5, YFP, and Cy3 images representing individual optical sections cells were merged (Merge). Yellow signifies colocalization of rhodamine and YFP labels; cyan of rhodamine and Cy5, and white of all three labels. Bar, 10 μm.
Cbl RING Finger Domain Is Critical for the Rescue Effects of the Cbl-Grb2 Chimera
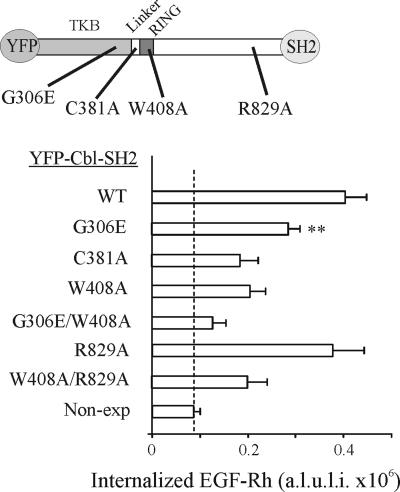
Figure 3 shows that the recruitment of c-Cbl to the EGFR via receptor Grb2 binding sites is sufficient to compensate for the inhibitory effect of Grb2 knockdown on receptor endocytosis. To examine which functions of YFP-Cbl-SH2 chimera are responsible for the rescue effects, several point mutations in the SH2-like/phosphotyrosine binding (PTB) domain (G306E), linker (C381), RING domain (W408A) (Levkowitz et al., 1999; Thien et al., 2001), and CIN85 binding site (R829A) (Kowanetz et al., 2003) were made in the chimera, and the resulting mutants were tested for the ability to rescue EGF-Rh endocytosis in Grb2-depleted cells as described in Figure 3. The quantitative analysis of 3-D images revealed that both mutations that abolish the activity of RING domain (C381A and W408A) resulted in a significantly reduced ability to support EGF-Rh endocytosis compared with “wild-type” Cbl-SH2 chimera (Figure 5). Very minimal, if any, colocalization of EGF-Rh with EEA.1 was detected in cells expressing YFP-Cbl/C381A-SH2 or YFP-Cbl/W408A-SH2 fusion proteins (our unpublished data). In contrast, mutation of CIN85 binding site did not affect the internalization rescue capacity of YFP-Cbl-SH2. Interestingly, a mutant with defective SH2-like domain (G306E) displayed intermediate rescue capacity (Figure 5). These data indicate that the RING domain is critical for the Cbl function in endocytosis. However, inactivation of the RING domain did not abolish EGFR endocytosis to the level observed in Grb2-depleted cells, suggesting that interactions of other parts of Cbl molecule may support endocytosis in the absence of RING function.
Figure 5.
Point mutations in the YFP-Cbl-SH2 chimera affect endocytosis rescue capacity of the chimera in cells depleted of Grb2. HeLa cells were transiently transfected with Grb2 siRNA and either with YFP-Cbl-SH2 (WT) or its mutants schematically shown on the top. The cells were incubated with EGF-Rh, fixed, and imaged as described in Figure 3. The quantification of the amount of internalized EGF-Rh from experiments was performed as in Figure 3E. Cells transfected with Grb2 siRNA and not expressing YFP fusion proteins are designated as nonexp. In experiments with all YFP-tagged proteins, the values of integrated intensity did not depend on the expression levels of these fusion proteins. The error bars represent SEs (n = 20). **p < 0.002 compared with cells depleted of Grb2 and expressing YFP-Cbl-SH2 (WT). A.l.u.f.i., arbitrary linear units of fluorescence intensity.
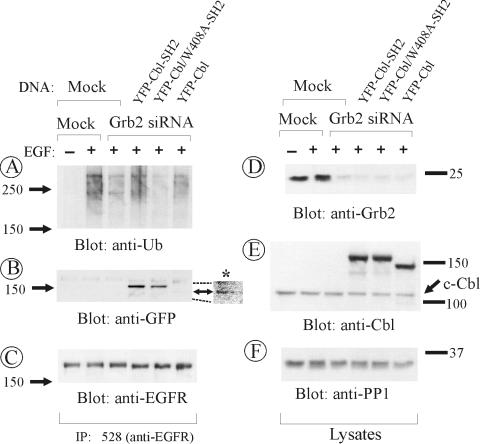
To test whether expression of YFP-Cbl-SH2 chimera results in ubiquitylation of EGFR, EGFR was immunoprecipitated from Grb2-depleted cells that were DNA mock transfected or transfected with YFP-Cbl, YFP-Cbl-SH2, or YFP-Cbl/W408A-SH2. EGFR ubiquitylation was not detected in unstimulated cells regardless of whether cells were transfected or not with YFP constructs. EGF stimulation of mock-transfected cells resulted in the appearance of a characteristic smear of high-molecular-weight ubiquitin immunoreactivity in EGFR immunoprecipitates (Figure 6A). The ubiquitylation of EGFR was, however, dramatically inhibited in cells depleted of Grb2. These data demonstrate that Grb2 is essential for Cbl-mediated ubiquitylation of EGFR.
Figure 6.
YFP-Cbl-SH2 chimeras bind EGFR and rescues ubiquitination of EGFR in Grb2-depleted cells. HeLa cells were transiently mock siRNA transfected or transfected with Grb2 siRNA. The cells also were mock DNA transfected or transfected with YFP-Cbl-SH2 chimera, mutant W408A of this chimera (see Figure 4), or YFP-Cbl. The cells were incubated with 20 ng/ml EGF for 2 min at 37°C and lysed. EGFR was immunoprecipitated (IP) with antibody 528. Immunoprecipitates and lysates were resolved on 7.5% SDS-PAGE and probed by Western blotting with antibody P4D1 to ubiquitin (A), GFP (B), antibody 2913 to EGFR (C), Grb2 (D), c-Cbl (E), and PP1 (F, nonspecific control). Asterisk in B marks a portion of the blot that was overexposed to demonstrate the presence of a small amount of YFP-Cbl in EGFR immunoprecipitates.
Expression of YFP-Cbl-SH2 chimera in Grb2-depleted cells recovered EGFR ubiquitylation (Figure 6A). Consistently, Cbl-Grb2 chimeric proteins were effectively coprecipitated with EGFR due to the interaction of the Grb2 SH2 domain with the receptor (Figure 6B). The ubiquitylation-rescue effect was absolutely dependent on the presence of the functional RING domain because W408A (Figure 6A) or C381A mutants (our unpublished data) of the chimera did not restore EGFR ubiquitylation in Grb2-depleted cells. In contrast, EGFR ubiquitylation in Grb2-depleted cells was not rescued by overexpression of YFP-Cbl as assessed by quantitations of the amounts of ubiquitin immunoreactivity normalized to the EGFR immunoreactivity in EGFR immunoprecipitates (Figure 6, A and C). This conclusion is supported by the observation of negligible coimmunoprecipitation of YFP-Cbl with EGFR in the absence of Grb2 (Figure 6B).
Cbl Amino-Terminal Domain Is Sufficient for Mediating Grb2-dependent Endocytosis of EGFR
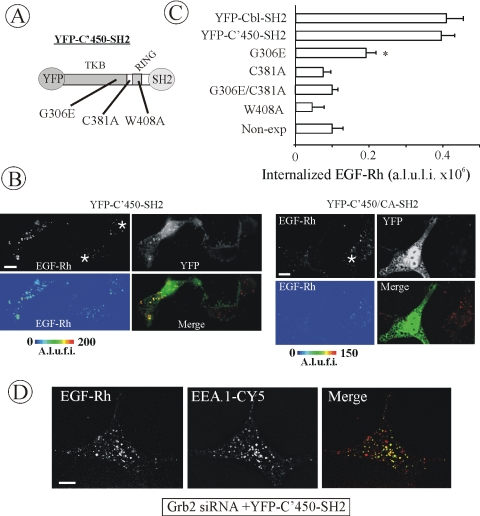
The data of Figures 5 and 6 demonstrated the importance of RING domain function in the endocytosis rescue capacity of Cbl-Grb2 chimeras. To test whether the functional RING domain is sufficient to recover EGFR endocytosis in Grb2-depleted cells, a fusion of the Grb2 SH2 domain and the amino terminus of c-Cbl (residues 1–450) encompassing the tyrosine kinase binding (TKB) linker and RING domains was generated (YFP-C'450-SH2; Figure 7A). Several amino acid residues in the TKB domain are engaged in the intramolecular interactions with the linker and RING domains ensuring proper folding of the RING domain (Zheng et al., 2000). Figure 7B shows that EGF-Rh was efficiently internalized in Grb2-depleted cells expressing this chimeric protein. The pattern of EGF-Rh localization was indistinguishable from that observed in cells expressing a chimeric protein containing the full-length c-Cbl. EGF-Rh was well colocalized with EEA.1 in endosomes of cells expressing YFP-C'450-SH2 (Figure 7D). The amounts of endosomal EGF-Rh were similar in cells expressing YFP-C'450-SH2 and YFP-Cbl-SH2 proteins (Figure 7C). Measurements of 125I-EGF uptake performed as described in Figure 3F demonstrated essentially the same rescue effect (∼65%) of YFP-C'450-SH2 and YFP-Cbl-SH2 proteins (our unpublished data). Together, the data in Figure 7 suggested that the amino terminus of Cbl recruited to the Grb2 binding sites of the EGFR is sufficient to reverse the inhibition of EGFR internalization caused by Grb2 knockdown.
Figure 7.
Grb2 chimera with amino terminus of Cbl restores EGFR internalization in Grb2-depleted cells. (A) Schematic representation of YFP-C'450-SH2 chimeric protein. YFP-C'450-SH2 contains c-Cbl fragment encompassing residues 1–450 flanked with YFP and the SH2 domain of Grb2. Mutations made in the construct are indicated. For example, YFP-C'450/CA-SH2 is a mutant of YFP-C'450-SH2 with the substitution of Cys381 by alanine. (B) HeLa cells were transfected with Grb2 siRNA and YFP-C'450-SH2, YFP-C'450/CA-SH2 or other mutants (our unpublished data). The cells were treated with EGF-Rh, fixed, and imaged as described in Figure 3. YFP and Cy3 images representing individual optical sections were merged after adjustment of the YFP signal (Merge). EGF-Rh images also are displayed in quantitative pseudocolor by using the same scale for both images. A.l.u.f.i., arbitrary linear units of fluorescence intensity. White asterisks show positions of Grb2-depleted cells that do not express YFP fusion proteins (nonexp). Bars, 10 μm. (C) Quantification of the amount of internalized EGF-Rh from experiments performed as described in B. In experiments with all YFP-tagged proteins, the values of integrated intensity did not depend on the expression levels of these fusion proteins. The error bars represent SEs (n = 15–20). *p < 0.05 compared with cells depleted of Grb2 and not expressing YFP-tagged constructs (nonexp). (D) HeLa cells transfected with Grb2 siRNA and YFP-C'450-SH2 were incubated with EGF-Rh, fixed, and processed for immunostaining with EEA.1 antibody as in Figure 3F. The z-stack of optical sections was acquired through the Cy3 (red), Cy5 (green), and YFP (our unpublished data) channels and deconvoluted. Cy5 and Cy3 images representing individual optical sections were merged (Merge). Yellow signifies colocalization of rhodamine and Cy5 labels. Bar, 10 μm.
To test whether SH2-like, linker, and RING domains of Cbl are important for the rescue ability of the Cbl amino terminus, corresponding mutants of YFP-C'450-SH2 were prepared (Figure 7A). Expression of YFP-C'450-SH2 with mutations inactivating RING domain failed to restore EGFR endocytosis (Figure 7, B and C). As in experiments with the full-length Cbl-Grb2 chimeras (Figure 5), G306E mutation in the YFP-C'450-SH2 partially abolished the rescue effect of this chimera (Figure 7C).
Thus, the data in Figure 7 demonstrated that the association of the amino-terminal domain of c-Cbl containing functional RING domain with the EGFR is necessary and sufficient to restore EGFR endocytosis in cells depleted of Grb2. The SH2/-like domain of Cbl may be necessary for the full effectiveness of the RING domain.
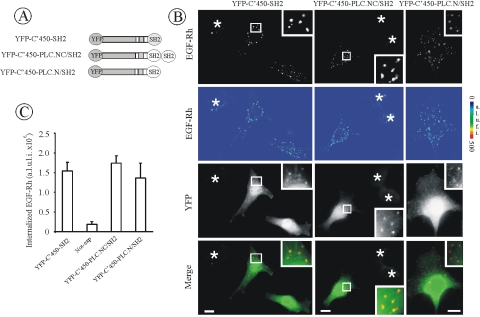
To test whether recruitment of the Cbl amino-terminal domain via an SH2 domain of EGFR-binding proteins other than Grb2 also can restore endocytosis in Grb2-depleted cells, we took advantage from the observation of the robust binding of the N-terminal SH2 domain of PLCγ1 to the EGFR, which is maintained during EGFR endocytosis (Chattopadhyay et al., 1999; Matsuda et al., 2001; Wang et al., 2001; Wang and Wang, 2003). The SH2 domain of Grb2 in YFP-C'450-SH2 was replaced by either single N-SH2 or a tandem of two SH2 domains of PLCγ1 to generate YFP-C'450-PLC.N/SH2 and C'450-PLC.NC/SH2, respectively (Figure 8A). Expression of these chimeric proteins restored EGF-Rh endocytosis in Grb2-depleted cells (Figure 8, B and C). Both Cbl-PLCγ1 chimeras were colocalized with endosomal EGF-Rh, indicative of high affinity and stable association with activated EGFR (Figure 8B). These data suggest that the strength and longevity of association with EGFR are important for the endocytosis-rescue activity of the Cbl amino terminus. Because SH2 domains of PLCγ1 can bind to a number of phosphotyrosines of EGFR, including Tyr1068 (Soler et al., 1994b; Chattopadhyay et al., 1999), it is difficult to determine whether a particular architecture of the EGFR:chimera complex is beneficial for the endocytosis-rescue effects.
Figure 8.
Chimera consisting of PLCγ1 SH2 domains and the amino terminus of Cbl restores EGFR internalization in Grb2-depleted cells. (A) Schematic representation of YFP-C'450-SH2 chimeric proteins. YFP-C'450-PLC.N/SH2 and YFP-C'450-PLC.NC/SH2 contain c-Cbl fragment encompassing residues 1–450 flanked with YFP and, respectively, the single N-SH2 domain or a tandem of two SH2 domains of PLCγ1. (B) HeLa cells were cotransfected with Grb2 siRNA and either YFP-C'450-SH2, YFP-C'450-PLC.N/SH2, or YFP-C'450-PLC.NC/SH2. The cells were treated with EGF-Rh, fixed, and imaged as described in Figure 3. YFP and Cy3 images representing individual optical sections were merged after adjustment of the YFP signal (Merge). EGF-Rh images also are displayed in quantitative pseudocolor by using the same scale for both images. Insets represent high-magnification images of the regions shown by white rectangles and demonstrate localization of the chimeric proteins in endosomes containing EGF-Rh. A.l.u.f.i., arbitrary linear units of fluorescence intensity. Bars, 10 μm. (C) Quantification of the amount of internalized EGF-Rh from experiments similar to those presented in B. The error bars represent SEs (n = 10).
The Interaction of Cbl with CIN85 Has a Redundant Role in EGFR Endocytosis
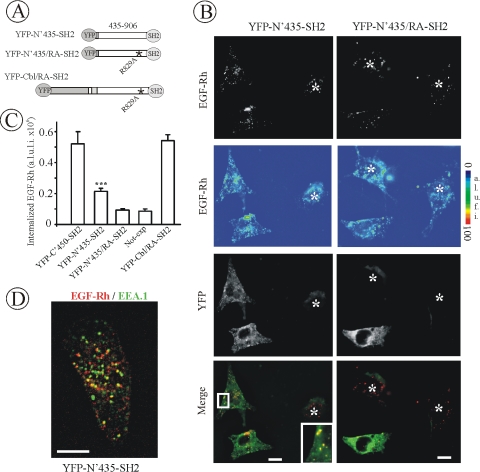
To test whether Cbl interaction with CIN85 has a role in Grb2-mediated EGFR endocytosis, we tested whether the carboxy-terminal domain of Cbl, responsible for interaction with CIN85, is capable of restoring EGF-Rh endocytosis in Grb2-depleted cells. To this end, a YFP-tagged fusion of the Cbl fragment encompassing residues 435–906 with the Grb2 SH2 domain was generated (Figure 9A). The expression of this chimera in Grb2-depleted cells resulted in a slightly larger accumulation of EGF-Rh in endosomes as compared with non-YFP–expressing cells, suggesting a partial rescue effect. However, the extent of the rescue of EGFR endocytosis was 4 times smaller than that of the rescue achieved in cells expressing a RING-containing chimeric protein (YFP-C'450-SH2) (Figure 9, B and C). The reduced rate of endocytosis also was suggested by the observation that EGF-Rh was accumulated in EEA.1-containing endosomes in cells expressing the YFP-N′435-SH2 chimera only after 15 min of continuous endocytosis (Figure 9D). In contrast, 5 min of internalization was sufficient for EGF-Rh to reach large perinuclear EEA.1-positive endosomes in cells rescued by expression of RING-containing chimeras (Figure 7C).
Figure 9.
CIN85 interaction with Cbl is dispensable for EGFR internalization. (A) Schematic representation of Cbl-Grb2 chimeric proteins fused to YFP. The YFP-N′435-SH2 chimera consists of YFP, residues 435–906 of c-Cbl, and the SH2 domain of Grb2. YFP-N′435/RA-SH2 is a mutant YFP-N′435-SH2 chimera with the substitution of Arg829 (essential for CIN85 binding) to alanine in the c-Cbl fragment. YFP-Cbl/RA-SH2 contains YFP, full-length c-Cbl with the R829A mutation, and the SH2 domain of Grb2. (B) HeLa cells were transfected with Grb2 siRNA and either YFP-N′435-SH2 or YFP-N′435/RA-SH2. Cells were incubated with EGF-Rh, fixed, and imaged as described in Figure 3. YFP and Cy3 images representing individual optical sections were merged (Merge). EGF-Rh images also are displayed in quantitative pseudocolor using the same scale for both images. A.l.u.f.i., arbitrary linear units of fluorescence intensity. White asterisks show positions of Grb2-depleted cells that do not express YFP fusion proteins (nonexp). Inset represents high-magnification image of the region shown by white rectangles and demonstrate localization of the chimeric protein in endosomes containing EGF-Rh. Bars, 10 μm. (C) Quantification of the amount of internalized EGF-Rh from experiments similar to those presented in Figures 7B and 9B. In experiments with all YFP-tagged proteins, the values of integrated intensity did not depend on the expression levels of these fusion proteins. The error bars represent SEs (n = 20). ***p < 0.0001 compared with cells depleted of Grb2 and not expressing YFP-tagged constructs (nonexp). (D) HeLa cells transfected with Grb2 siRNA and YFP-N′435-SH2 were incubated with EGF-Rh for 15 min at 37°C, fixed, and processed for immunostaining with EEA.1 antibody. The z-stack of optical sections was acquired through the Cy3 (red), Cy5 (green) and YFP (our unpublished data) channels and deconvoluted. Cy5 and Cy3 images representing individual optical sections were merged (Merge). Yellow signifies colocalization of rhodamine and Cy5 labels. Bar, 10 μm.
The partial rescue of EGFR endocytosis by YFP-N′435-SH2 was dependent on the interaction of this chimeric protein with the SH3 domain of CIN85 because mutation of Arg829, a key residue in the CIN85 interaction motif in c-Cbl (Kowanetz et al., 2003), abolished the rescue effect of the YFP-N′435-SH2 chimera (Figure 9, B and C). However, in agreement with Figure 4, R829A mutation had no influence on the endocytosis rescue capacity of the full-length Cbl chimera in Grb2-depleted cells (Figures 9C). These data suggest that Arg829-mediated interactions are not essential for Cbl function in EGF-Rh endocytosis but may participate in endocytosis in the absence of RING domain activity of Cbl proteins associated with the EGFR.
DISCUSSION
In this study, we exploited the siRNA knockdown and rescue approach to confirm the role of Grb2 in EGFR internalization and to further investigate the mechanisms by which Grb2 participates in this process. At least in two types of cells, HeLa and PAE cells, Grb2 is essential for clathrin-mediated pathway of EGFR internalization (Jiang et al., 2003; Huang et al., 2004; this study). In both cell types, this conclusion was reached under conditions of moderate EGFR expression and by using low physiological concentrations of EGF. In NIH 3T3 and B82 mouse fibroblast cell lines, expressing transfected EGFR, Grb2 depletion by siRNA resulted in 65 and 40% reduction of EGF internalization rates, respectively (our unpublished data). Elimination of Grb2 binding sites in the EGFR by point mutations or large deletions did not affect receptor internalization in NIH 3T3 and B82 cells (Chen et al., 1989; Chang et al., 1993; Sorkina et al., 2002). Therefore, there might be other physiological and/or compensatory mechanisms of EGFR endocytosis that do not rely on Grb2. These alternative pathways can be clathrin dependent and independent. For example, it has not been tested whether endocytosis of EGFR mutants lacking Grb2 binding sites in NIH 3T3 and B82 cells is mediated by clathrin-coated pits. For some truncated EGFR mutants, such as C'1022, the kinetics and EGFR kinase dependence of internalization is different from that of wild-type EGFR, which indicates that the mechanism of endocytosis of such truncated mutants may not be physiological (Chen et al., 1989; our unpublished data). In addition, in the absence of Grb2 binding sites in EGFR mutants, other phosphotyrosines in the EGFR may bind Grb2 and support endocytosis, especially in cells overexpressing these EGFR mutants. Finally, the relative contribution of Grb2-dependent and other pathways of clathrin-dependent and -independent endocytosis may be cell specific.
The mechanisms of Grb2-dependent EGFR endocytosis have been difficult to analyze because Grb2 is indispensable for cell growth and survival. The importance of Grb2 is illustrated by early embryonic lethality of Grb2 knockout mice that made it impossible to generate mouse embryonic fibroblast lines from Grb2-/- animals (Cheng et al., 1998). Likewise, we were unable to develop stable cell lines depleted of Grb2 or expressing Grb2-YFP or its mutants. The cells constitutively expressing Grb2-YFP could be generated only when endogenous Grb2 was eliminated by shRNA, and Grb2-YFP was expressed at physiological levels (Figure 1). The development of cell lines expressing fluorescently tagged Grb2, in which the effects of siRNA Grb2 knockdown on endocytosis and signaling were fully reversed, allowed demonstration the specificity of Grb2 siRNA effects.
The data obtained with HeLa/Grb2-YFP cells have additional implications. Over the years, numerous studies, including from our laboratory, have analyzed localization and activities of various proteins that were tagged with green fluorescent protein or its variants and transiently or stably expressed in mammalian cells. However, the caveats of these types of experiments are the presence of an endogenous protein and overexpression of tagged proteins, which often leads to their mistargeting and altered activities. Our work offers a simple approach of creating a human cell model system that allows visualization and analysis of the behavior and function of fluorescently labeled proteins expressed at physiological levels without the interference of the endogenous protein. Even initial comparison of Grb2 trafficking in this system and in cells overexpressing Grb2 revealed significant differences. Whereas overexpressed Grb2-YFP was robustly recruited to the plasma membrane ruffles upon EGF treatment (Sorkin et al., 2000), no ruffle targeting of Grb2-YFP was observed in cells expressing physiological levels of Grb2-YFP (Figure 2B).
The knockdown and rescue approach also has enabled us to dissect the mechanisms by which Grb2 interaction with Cbl regulates EGFR endocytosis. Because of the controversial literature on the function of Cbl in EGFR internalization, we initially attempted to use siRNA approach to directly test for the role of Cbl proteins in EGFR endocytosis. After examination of 15 different siRNA duplexes targeted to Cbl-b and c-Cbl, including those recently published (Mitra et al., 2004), we have not been able to identify siRNAs that produced >90% decrease in the combined immunoreactivity of c-Cbl and Cbl-b. Under these conditions EGFR internalization rates were reduced by 50%. It is possible that the residual pool of c-Cbl/Cbl-b proteins is sufficient to support endocytosis of a small number of activated EGFR under conditions of our internalization assay. It is also possible that Cbl-3 (Cbl-c), which can interact with Grb2 (Courbard et al., 2002), promotes EGFR ubiquitylation and endocytosis in the absence of c-Cbl and Cbl-b. Similarly, residual Cbl-b and Cbl-3 in c-Cbl-/- mouse embryonic fibroblasts could adequately support EGFR endocytosis in these cells (Duan et al., 2003). Therefore, the functional redundancy in Cbl family prompted us to examine the role of Grb2–Cbl interactions by using Grb2-Cbl chimeric proteins in the context of knockdown and rescue assay.
The experiments with Cbl-Grb2 chimeras demonstrated that the recruitment of Cbl to Grb2 binding sites of the EGFR is sufficient to completely restore receptor internalization in the absence of Grb2 (Figures 3 and 4). This finding suggests that Grb2 interactions with dynamin, SOS, or other SH3 binding proteins, such as POB1 (Nakashima et al., 1999), are dispensable, if not important at all, for EGFR endocytosis. In fact, Grb2 SH2 chimeras with SOS1 or dynamin II that were constructed analogously to the Cbl-Grb2 chimeras did not rescue EGFR endocytosis in Grb2-depleted cells (our unpublished data). On the other hand, we were able to generate stable HeLa cell lines, in which Grb2 was down-regulated by shRNA, whereas the SOS-Grb2 chimera was stably expressed (our unpublished data). The growth compensation effect of the SOS-Grb2 chimera is reminiscent to the mouse knockin studies, in which a similar Grb2-SOS chimera rescued the embryonic lethality caused by Grb2 knockout (Cheng et al., 1998). In contrast, growth defects due to Grb2 depletion could not be compensated by expression of the YFP-Cbl-SH2 chimera (our unpublished data).
Our data emphasized the importance of indirect, Grb2-mediated binding of Cbl to the EGFR for the regulation of receptor internalization. Cbl contains a variant of SH2 domain. However, this SH2-like domain alone cannot bind phosphotyrosine-containing motifs but requires the cooperation of the entire amino-terminal TKB domain (Meng et al., 1999). The direct binding of Cbl TKB domain to phosphorylated Tyr1045 (pTyr1045) was demonstrated using in vitro competition experiments with v-Cbl, a truncated variant of c-Cbl consisting of the TKB domain and a pTyr1045-containing peptide (Levkowitz et al., 1999). However, the same peptide did not interfere with the interaction of the full-length c-Cbl and EGFR, suggesting the prevailing role of other Cbl-EGFR association determinants (Levkowitz et al., 1999). In our experiments overexpression of c-Cbl, capable of direct binding to the EGFR, did not lead to stable association of c-Cbl with EGFR in the absence of Grb2, as judged by the absence of coimmunoprecipitation, and did not functionally compensate for Grb2 knockdown (Figures 3, 5, and 6). Thus, we propose that within the receptor dimer or monomer, the association of the carboxy-terminal proline-rich sequences of Cbl with Grb2 bound to EGFR allows Cbl TKB domain to be stably associated with EGFR pTyr1045, whereas the affinity of the latter interaction is not sufficient to sustain the association without the cooperation of the SH3-mediated interaction. Our findings are consistent with the model of Cbl interaction with the c-Met receptor, although unlike EGFR, the site of direct Cbl interaction with c-Met is in the juxtamembrane portion of the receptor (Peschard et al., 2001). Whereas Grb2-mediated association is the main mechanism of Cbl recruitment to the EGFR and is sufficient for EGFR internalization, interaction of Cbl TKB domain with pTyr1045 is essential for the maximal ubiquitylation and lysosomal targeting of the receptor (Levkowitz et al., 1999; Waterman et al., 2002; Jiang et al., 2003; Jiang and Sorkin, 2003).
In light of this model, it is surprising that functional SH2-like domain of Cbl was necessary for the full internalization-rescue activity of Cbl amino terminus (Figures 4 and 6). These data disagree with the observations that overexpression of c-Cbl G306E mutant did not affect EGFR internalization (Jiang and Sorkin, 2003). This discrepancy emphasizes the importance of the knockdown and rescue approach as opposed to an approach of a plain overexpression of the dominant-negative mutants that often suffers from the insufficient levels of mutant expression and presence of endogenous proteins. Because Cbl binding site in the EGFR (Tyr1045) is not necessary for internalization (Jiang and Sorkin, 2003; Oksvold et al., 2003; Grovdal et al., 2004), it is possible that TKB domain of Cbl has a function(s) additional to interaction with phosphotyrosine residues, as suggested previously (Meng et al., 1999).
The amino-terminal domain of c-Cbl, containing functional RING domain, fully restored EGFR endocytosis when recruited to the receptor by the SH2 domain of Grb2 (Figure 7). Furthermore, other SH2 domains, such as PLCγ1 N-SH2, capable of high-affinity interaction with EGFR, could replace Grb2 SH2 domain in the context of the RING-containing chimeric proteins in their capacity to restore EGFR endocytosis in Grb2-depleted cells (Figure 8). Although c-Cbl is capable of interaction with PLCγ1 SH3 domain (Tvorogov and Carpenter, 2002; Choi et al., 2003), PLCγ1-/- fibroblasts display normal EGFR trafficking (Ji et al., 1998). This suggests that Grb2-mediated binding of Cbl to EGFR is the main physiological mechanism of Cbl recruitment to the EGFR; however, in the absence of Grb2, association of Cbl through PLCγ1and other proteins can be a compensatory mechanism. These data also suggest that sustained association with EGFR is critical for the rescue activity of the Cbl amino terminus. Because SH2 domains of PLCγ1 and other proteins, when overexpressed, can bind to various phosphotyrosines of EGFR (Soler et al., 1994b; Chattopadhyay et al., 1999), it is difficult to determine whether a particular positioning of Cbl on the EGFR is most effective to support EGFR internalization.
Analysis of various mutant Cbl-Grb2 chimeras strongly indicated that the function of the RING domain is essential for the rescue effect of these chimeras on EGFR endocytosis (Figures 3, 4, 5, 6, 7). These data are consistent with the observations of the dominant-negative effects of overexpression of RING domain mutants of c-Cbl (Thien et al., 2001; Jiang and Sorkin, 2003) and Sprouty 2 that binds to the RING domain of Cbl (Stang et al., 2004) on EGFR internalization. However, the effect of Sprouty 2 cannot be unequivocally interpreted because Sprouty also directly binds to Grb2 and, when overexpressed, reduces Cbl binding to the EGFR.
The major and a well recognized function of the RING domain of Cbl is to recruit the ubiquitin ligase complex that mediates ubiquitylation of the EGFR. Alternatively, binding of proteins other than E2 ubiquitin ligases to the RING domain may link the EGFR–Grb2–Cbl complex to the clathrin coat. An example of RING-binding protein is Sprouty; however, this protein is thought to play a negative role in EGFR endocytosis (Wong et al., 2002; Stang et al., 2004). Two observations made in our study support the notion that the ubiquitylation function of Cbl is important for EGFR endocytosis mediated by Cbl-Grb2 chimeras. First, two different mutations in Cbl, both known to inhibit RING domain function through distinct mechanisms, have inactivated this chimera (Figures 5 and 7). One of the mutated residues, Trp408, directly participates in the interaction of the RING with the E2 ligase (Zheng et al., 2000). Second, expression of YFP-Cbl-SH2 protein in Grb2-depleted cells restored EGFR ubiquitylation in a manner absolutely dependent on the intact RING domain (Figure 6). On the other hand, dramatic inhibition of EGFR ubiquitylation in EGFR Y1045F mutant did not result in significant inhibition of its internalization (Jiang and Sorkin, 2003; Grovdal et al., 2004). This suggests that if EGFR ubiquitylation is indeed involved in internalization, very small number of ubiquitination sites in the EGFR is sufficient to support this process.
Although our experiments established the essential role of the RING domain, they also confirmed that at least in HeLa cells, binding to CIN85 to the carboxy terminus of Cbl can function as an internalization mechanism, although this mechanism is significantly less effective than the RING-dependent mechanism. Because mutation of CIN85 binding site in full-length Cbl-Grb2 chimera did not affect the rescue capacity of this chimera, we suggest that CIN85-mediated mechanism is not normally involved in Grb2- and Cbl-mediated endocytosis unless the function of the RING domain is unavailable. This is consistent with our previous observations that overexpression of dominant-negative mutants of CIN85 did not have a specific inhibitory effect on EGFR internalization in HeLa cells (Jiang and Sorkin, 2003).
Assuming that EGFR ubiquitylation is indeed important for receptor endocytosis, it remains unknown how exactly this ubiquitylation participates in the clathrin-dependent process. Three coated pit proteins, epsin, Eps15, and Eps15R, contain ubiquitin-interacting domains. It is therefore tempting to suggest that ubiquitinated EGFR can bind to one of these proteins. For instance, in some cells Eps15 can be recruited to the plasma membrane in an EGF-dependent manner and positioned in the same location at the rim of coated pits as the EGFR–Grb2–Cbl complex (Stang et al., 2004). However, siRNA knockdown analysis of epsin 1, Eps15, and Eps15R did not provide support for the notion of the specific role of these proteins in clathrin-mediated internalization of EGFR (Huang et al., 2004). Mapping the ubiquitylation sites in the EGFR that are specifically responsible for the internalization step of endocytic trafficking of the receptor will be necessary to unequivocally establish the role of ubiquitylation and may allow identification of the binding partners of these ubiquitins in the clathrin-coated pits.
Supplementary Material
Acknowledgments
We thank Dr. G. Carpenter for PLCγ1 DNA, Dr. E. Galperin for YFP-Cbl construct, and members of Sorkin laboratory for critical reading of the manuscript. This work was supported by grants from the National Cancer Institute and the American Cancer Society.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-09-0832) on January 5, 2005.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Ahn, S., Kim, J., Lucaveche, C. L., Reedy, M. C., Luttrell, L. M., Lefkowitz, R. J., and Daaka, Y. (2002). Src-dependent tyrosine phosphorylation regulates dynamin self-assembly and ligand-induced endocytosis of the EGF receptor. J. Biol. Chem. 277, 26642-26651. [DOI] [PubMed] [Google Scholar]
- Batzer, A. G., Rotin, D., Urena, J. M., Skolnik, E. Y., and Schlessinger, J. (1994). Hierarchy of binding sites for Grb2 and Shc on the EGF receptor. Mol. Cell. Biol. 14, 5192-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C.-P., et al. (1993). Ligand-induced internalization of the EGF receptor is mediated by multiple endocytic codes analogous to the tyrosine motif found in constitutively internalized receptors. J. Biol. Chem. 268, 19312-19320. [PubMed] [Google Scholar]
- Chattopadhyay, A., Vecchi, M., Ji, Q., Mernaugh, R., and Carpenter, G. (1999). The role of individual SH2 domains in mediating association of phospholipase C-gamma1 with the activated EGF receptor. J. Biol. Chem. 274, 26091-26097. [DOI] [PubMed] [Google Scholar]
- Chen, W. S., Lazar, C. S., Lund, K. A., Welsh, J. B., Chang, C. P., Walton, G. M., Der, C. J., Wiley, H. S., Gill, G. N., and Rosenfeld, M. G. (1989). Functional independence of the epidermal growth factor receptor from a domain required for ligand-induced internalization and calcium regulation. Cell 59, 33-43. [DOI] [PubMed] [Google Scholar]
- Cheng, A. M., et al. (1998). Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell 95, 793-803. [DOI] [PubMed] [Google Scholar]
- Choi, J. H., Bae, S. S., Park, J. B., Ha, S. H., Song, H., Kim, J. H., Cocco, L., Ryu, S. H., and Suh, P. G. (2003). Cbl competitively inhibits EGF-induced activation of phospholipase C-gamma1. Mol. Cells 15, 245-255. [PubMed] [Google Scholar]
- Courbard, J. R., Fiore, F., Adelaide, J., Borg, J. P., Birnbaum, D., and Ollendorff, V. (2002). Interaction between two ubiquitin-protein isopeptide ligases of different classes, CBLC and AIP4/ITCH. J. Biol. Chem. 277, 45267-45275. [DOI] [PubMed] [Google Scholar]
- de Melker, A. A., van der Horst, G., Calafat, J., Jansen, H., and Borst, J. (2001). c-Cbl ubiquitinates the EGF receptor at the plasma membrane and remains receptor associated throughout the endocytic route. J. Cell Sci. 114, 2167-2178. [DOI] [PubMed] [Google Scholar]
- Dikic, I., and Giordano, S. (2003). Negative receptor signalling. Curr. Opin. Cell Biol. 15, 128-135. [DOI] [PubMed] [Google Scholar]
- Duan, L., et al. (2003). Cbl-mediated ubiquitinylation is required for lysosomal sorting of EGF receptor but is dispensable for endocytosis. J. Biol. Chem. 278, 28950-28960. [DOI] [PubMed] [Google Scholar]
- Grovdal, L. M., Stang, E., Sorkin, A., and Madshus, I. H. (2004). Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp. Cell Res. 300, 388-395. [DOI] [PubMed] [Google Scholar]
- Huang, F., Jiang, X., and Sorkin, A. (2003). Tyrosine phosphorylation of the beta2 subunit of clathrin adaptor complex AP-2 reveals the role of a di-leucine motif in the EGF receptor trafficking. J. Biol. Chem. 278, 43411-43417. [DOI] [PubMed] [Google Scholar]
- Huang, F., Khvorova, A., Marshall, W., and Sorkin, A. (2004). Analysis of clatyhrin-mediated internalizaiton of EGF receptor by RNA interference. J. Biol. Chem. In press. [DOI] [PubMed]
- Ji, Q. S., Ermini, S., Baulida, J., Sun, F. L., and Carpenter, G. (1998). EGF signaling and mitogenesis in Plcg1 null mouse embryonic fibroblasts. Mol. Biol. Cell 9, 749-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X., Huang, F., Marusyk, A., and Sorkin, A. (2003). Grb2 Regulates Internalization of EGF Receptors through Clathrin-coated Pits. Mol. Biol. Cell 14, 858-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X., and Sorkin, A. (2003). EGF receptor internalization through clathrin-coated pits requires Cbl RING finger and proline-rich domains but not receptor polyubiquitylation. Traffic 4, 529-543. [DOI] [PubMed] [Google Scholar]
- Johannessen, L. E., Ringerike, T., Molnes, J., and Madshus, I. H. (2000). EGF receptor efficiently activates mitogen-activated protein kinase in HeLa cells and hep2 cells conditionally defective in clathrin-dependent endocytosis. Exp. Cell Res. 260, 136-145. [DOI] [PubMed] [Google Scholar]
- Kowanetz, K., Szymkiewicz, I., Haglund, K., Kowanetz, M., Husnjak, K., Taylor, J. D., Soubeyran, P., Engstrom, U., Ladbury, J. E., and Dikic, I. (2003). Identification of a novel proline-arginine motif involved in CIN85-dependent clustering of Cbl and down-regulation of EGF receptors. J. Biol. Chem. 278, 39735-39746. [DOI] [PubMed] [Google Scholar]
- Kranenburg, O., Verlaan, I., and Moolenaar, W. H. (1999). Gi-mediated tyrosine phosphorylation of Grb2 (growth-factor-receptor-bound protein 2)-bound dynamin-II by lysophosphatidic acid. Biochem. J. 339, 11-14. [PMC free article] [PubMed] [Google Scholar]
- Levkowitz, G., et al. (1999). Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1 [In Process Citation]. Mol. Cell 4, 1029-1040. [DOI] [PubMed] [Google Scholar]
- Li, N., Batzer, A., Daly, R., Yajnik, V., Skolnik, E., Chardin, P., Bar-Sagi, D., Margolis, B., and Schlessinger, J. (1993). Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling [see comments]. Nature 363, 85-88. [DOI] [PubMed] [Google Scholar]
- Marmor, M. D., and Yarden, Y. (2004). Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene 23, 2057-2070. [DOI] [PubMed] [Google Scholar]
- Matsuda, M., Paterson, H. F., Rodriguez, R., Fensome, A. C., Ellis, M. V., Swann, K., and Katan, M. (2001). Real time fluorescence imaging of PLC gamma translocation and its interaction with the EGF receptor. J. Cell Biol. 153, 599-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisner, H., Conway, B. R., Hartley, D., and Czech, M. P. (1995). Interactions of Cbl with Grb2 and phosphatidylinositol 3′-kinase in activated Jurkat cells. Mol. Cell. Biol. 15, 3571-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, W., Sawasdikosol, S., Burakoff, S. J., and Eck, M. J. (1999). Structure of the amino-terminal domain of Cbl complexed to its binding site on ZAP-70 kinase. Nature 398, 84-90. [DOI] [PubMed] [Google Scholar]
- Mitra, P., Zheng, X., and Czech, M. P. (2004). RNAi-based analysis of CAP, Cbl, and CrkII function in the regulation of GLUT4 by insulin. J. Biol. Chem. 279, 37431-37435. [DOI] [PubMed] [Google Scholar]
- Motley, A., Bright, N. A., Seaman, M. N., and Robinson, M. S. (2003). Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162, 909-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima, S., Morinaka, K., Koyama, S., Ikeda, M., Kishida, M., Okawa, K., Iwamatsu, A., Kishida, S., and Kikuchi, A. (1999). Small G protein Ral and its downstream molecules regulate endocytosis of EGF and insulin receptors. EMBO J. 18, 3629-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksvold, M. P., Thien, C. B., Widerberg, J., Chantry, A., Huitfeldt, H. S., and Langdon, W. Y. (2003). Serine mutations that abrogate ligand-induced ubiquitination and internalization of the EGF receptor do not affect c-Cbl association with the receptor. Oncogene. 22, 8509-8518. [DOI] [PubMed] [Google Scholar]
- Okutani, T., Okabayashi, Y., Kido, Y., Sugimoto, Y., Sakaguchi, K., Matuoka, K., Takenawa, T., and Kasuga, M. (1994). Grb2/Ash binds directly to tyrosines 1068 and 1086 and indirectly to tyrosine 1148 of activated human EGF receptors in intact cells. J. Biol. Chem. 269, 31310-31314. [PubMed] [Google Scholar]
- Pearson, G., Robinson, F., Beers Gibson, T., Xu, B. E., Karandikar, M., Berman, K., and Cobb, M. H. (2001). Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22, 153-183. [DOI] [PubMed] [Google Scholar]
- Peschard, P., Fournier, T. M., Lamorte, L., Naujokas, M. A., Band, H., Langdon, W. Y., and Park, M. (2001). Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol. Cell 8, 995-1004. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock, M., Fernley, R., Wade, J., Pawson, T., and Bowtell, D. (1993). The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1 [see comments]. Nature 363, 83-85. [DOI] [PubMed] [Google Scholar]
- She, H. Y., Rockow, S., Tang, J., Nishimura, R., Skolnik, E. Y., Chen, M., Margolis, B., and Li, W. (1997). Wiskott-Aldrich syndrome protein is associated with the adapter protein Grb2 and the EGF receptor in living cells. Mol. Biol. Cell 8, 1709-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler, C., Alvarez, C. V., Beguinot, L., and Carpenter, G. (1994a). Potent SHC tyrosine phosphorylation by EGF at low receptor density or in the absence of receptor autophosphorylation sites. Oncogene 9, 2207-2215. [PubMed] [Google Scholar]
- Soler, C., Beguinot, L., and Carpenter, G. (1994b). Individual EGF receptor autophosphorylation sites do not stringently define association motifs for several SH2-containing proteins. J. Biol. Chem. 269, 12320-12324. [PubMed] [Google Scholar]
- Sorkin, A., McClure, M., Huang, F., and Carter, R. (2000). Interaction of EGF receptor and grb2 in living cells visualized by fluorescence resonance energy transfer (FRET) microscopy. Curr. Biol. 10, 1395-1398. [DOI] [PubMed] [Google Scholar]
- Sorkina, T., Huang, F., Beguinot, L., and Sorkin, A. (2002). Effect of tyrosine kinase inhibitors on clathrin-coated pit recruitment and internalization of EGF receptor. J. Biol. Chem. 277, 27433-27441. [DOI] [PubMed] [Google Scholar]
- Soubeyran, P., Kowanetz, K., Szymkiewicz, I., Langdon, W. Y., and Dikic, I. (2002). Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature 416, 183-187. [DOI] [PubMed] [Google Scholar]
- Stang, E., Blystad, F. D., Kazazic, M., Bertelsen, V., Brodahl, T., Raiborg, C., Stenmark, H., and Madshus, I. H. (2004). Cbl-dependent ubiquitination is required for progression of EGF receptors into clathrin-coated pits. Mol. Biol. Cell 15, 3591-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thien, C. B., and Langdon, W. Y. (2001). Cbl: many adaptations to regulate protein tyrosine kinases. Nat. Rev. Mol. Cell. Biol. 2, 294-307. [DOI] [PubMed] [Google Scholar]
- Thien, C. B., Walker, F., and Langdon, W. Y. (2001). RING finger mutations that abolish c-Cbl-directed polyubiquitination and downregulation of the epidermal growth factor receptor are insufficient for cell transformation. Mol. Cell 7, 355-365. [DOI] [PubMed] [Google Scholar]
- Tvorogov, D., and Carpenter, G. (2002). Epidermal growth factor-dependent association of phospholipase C-gamma1 with c-Cbl. Exp. Cell Res. 277, 86-94. [DOI] [PubMed] [Google Scholar]
- Wang, X. J., Liao, H. J., Chattopadhyay, A., and Carpenter, G. (2001). epidermal growth factor-dependent translocation of green fluorescent protein-tagged PLC-gamma1 to the plasma membrane and endosomes. Exp. Cell Res. 267, 28-36. [DOI] [PubMed] [Google Scholar]
- Wang, Y., and Wang, Z. (2003). Regulation of epidermal growth factor-induced phospholipase C-gamma1 translocation and activation by its SH2 and PH domains. Traffic 4, 618-630. [DOI] [PubMed] [Google Scholar]
- Wang, Z., and Moran, M. F. (1996). Requirement for the adapter protein GRB2 in epidermal growth factor receptor endocytosis. Science 272, 1935-1939. [DOI] [PubMed] [Google Scholar]
- Waterman, H., Katz, M., Rubin, C., Shtiegman, K., Lavi, S., Elson, A., Jovin, T., and Yarden, Y. (2002). A mutant epidermal growth factor-receptor defective in ubiquitylation and endocytosis unveils a role for Grb2 in negative signaling. EMBO J. 21, 303-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, E. S., Fong, C. W., Lim, J., Yusoff, P., Low, B. C., Langdon, W. Y., and Guy, G. R. (2002). Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling. EMBO J. 21, 4796-4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki, T., Zaal, K., Hailey, D., Presley, J., Lippincott-Schwartz, J., and Samelson, L. E. (2002). Role of Grb2 in epidermal growth factor-stimulated EGFR internalization. J. Cell Sci. 115, 1791-1802. [DOI] [PubMed] [Google Scholar]
- Zheng, N., Wang, P., Jeffrey, P. D., and Pavletich, N. P. (2000). Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell 102, 533-539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.