Abstract
BACKGROUND
There is urgent need for improved staging in patients with metastatic colorectal cancer (mCRC). In this study, we evaluated the prognostic value of circulating endothelial cells (CEC) in comparison with circulating tumor cells (CTC) in patients with mCRC amenable for potentially curative surgery.
METHODS
A total of 140 patients were enrolled prospectively. CTC and CEC were measured with the CellSearch System (Veridex, NJ, USA). Cut-off values were determined using ROC analyses. Prognostic factors were identified by Cox proportional hazards models.
RESULTS
ROC analyses revealed ≥ 21 CEC as cut-off levels for detection, which was present in 68 (49%). CEC detection was associated with female gender (p = 0.03) only, whereas CTC detection was associated with presence of the primary tumor (p = 0.007), metastasis size (p < 0.001), bilobar liver metastases (p = 0.02), CEA (p < 0.001) and CA 19-9 levels (p < 0.001). On multivariate analysis only CEC detection (HR 1.81; p = 0.03) and preoperative CA19-9 levels (HR 2.28, p = 0.005) were revealed as independent predictors of poor survival.
CONCLUSIONS
CEC are of stronger prognostic value than CTC. Further studies are required to validate these results and to evaluate CEC as predictive biomarker for systemic therapy alone as well as in combination with other markers such as CA19-9.
Keywords: circulating endothelial cells, CEC, circulating tumor cells, CTC, colorectal cancer
INTRODUCTION
Solid tumors are critically dependent on angiogenesis to exceed a size of 2-3 mm3 [1, 2]. In addition to vascular injury, which regularly occurs in all solid tumors and may be a result of overshooting angiogenesis, all modes of tumor angiogenesis induce shedding of cells of endothelial origin into the circulation. These circulating endothelial cells (CEC) may therefore be used as surrogate marker for the tumor's angiogenic activity as well as the degree of vascular injury. In addition, endothelial progenitor cells (EPC) from the bone marrow are recruited to tumors by pro-angiogenic cytokines [3].
Numerous different phenotypes have been used to define, quantify and isolate CEC (reviewed in reference [4]). As the methods of CEC detection and the patient populations studied vary vastly [4], comparisons between CEC studies have been difficult.
CRC metastases are highly angiogenic, which may explain the clinical effectiveness of antiangiogenic therapies in this disease [5]. In the past, CEC numbers have been shown to be predictive of the clinical activity of antiangiogenic agents [6–9]. However, the prognostic value of CEC in CRC patients with metastatic disease who are amenable to curative therapy has never been investigated.
It was therefore the aim of the present study to investigate the prognostic value of CEC in patients with CRC liver metastases who underwent potentially curative therapy using the semi-automated and highly reproducible CellSearch System for CEC detection [8,10–12]. As CTC are well-established prognostic markers in primary and metastatic CRC [13–22], we aimed to evaluate the prognostic value of CEC in a ‘head-to-head’ comparison with CTC in the same cohort of patients with metastatic CRC.
RESULTS
Study population
A total of 140 patients were enrolled between September 2009 and August 2012 (Supplementary Table 1). There were 80 (57.1%) men and the median age was 62 (28 – 82) years. The primary tumor was located in the colon in 96 (68.6%) patients and was node-positive in 92 (65.7%). 96 (68.6%) patients had previously undergone resection of the primary tumor. Bilobar metastases were present in 39 (27.9%) patients and extrahepatic disease in 15 (10.8%) patients. 55 (39.3%) patients had received neoadjuvant therapy. Data on the KRAS mutational status was available for 81 patients of whom 47 (58%) had KRAS wild-type tumors. None of the patients required vascular resection/reconstruction.
Preoperative detection of CEC and CTC and association with clinicopathologic variables
ROC analyses revealed a cut-off of ≥ 21 for detection of CEC (AUC 0.672; 95% CI 0.55 – 0.79) with a true positive rate of 64.3% and a true negative rate of 45.5%, respectively (Supplementary Figure 1). Based on previous reports using the CellSearch technology a cut-off of ≥ 2 was used for CTC detection [15, 17, 19, 23]. Using these cut-off values 66 (47.1%) patients were positive for CEC and 28 (20%) patients were positive for CTC. CEC numbers ranged from 0 to 1,120 with a median of 20 and a mean of 82.4 CEC (Figure 1A). Only 12 (8.6%) patients had no detectable CEC. CTC values varied between 0 and 83 with a median and mean value of 0 and 1.9, respectively (Figure 1B). Neither CEC detection rates (p = 0.16), nor CEC counts (p = 0.33) differed significantly in CTC-positive as compared to CTC-negative patients (Figure 1C and 1D). Moreover, CEC detection rate was not significantly different in patients with and without cardiovascular comorbidities (56.2% vs. 45.7%; p = 0.44).
Figure 1. Detection of CEC and CTC in patients with colorectal liver metastases.

(A) Histogram of CEC counts per 4 mL blood. (B) Histogram of CTC counts per 7.5 mL blood. (C) CTC-dependent CEC detection. (D) CTC-dependent CEC count.
To evaluate whether detection of CEC and CTC reflect the extent of tumor burden or might serve as independent prognostic and predictive biomarkers, we next assessed if detection of CEC and CTC is associated with clinicopathologic variables (Table 1). In line with published data on endothelial progenitor cells (which are included by the markers used for CEC detection by the CellSearch system) there was a higher CEC detection rate (p = 0.03) and CEC count (p = 0.009) in women as compared to men [24, 25]. Also, there was a trend toward a lower CEC detection rate (p = 0.08) and CEC count (p = 0.09) in patients who received neoadjuvant chemotherapy together with bevacizumab. CEC detection was not significantly different in patients with and without sinusoidal obstruction syndrome after previous chemotherapy (p = 0.27). There was neither a significant association of a staged vs. simultaneous resection with CEC detection (p = 0.14) nor with CTC detection (p = 0.5). The KRAS mutational status was not associated with detection of CEC (p = 0.39) or CTC (p = 0.59). As reported previously [26], CTC detection in this cohort of metastatic CRC patients was associated with presence of the primary tumor (p = 0.007), metastasis size (p < 0.001), bilobar liver metastases (p = 0.02), CEA (p < 0.001) and CA 19-9 levels (p < 0.001). Similarly, CTC counts were higher in patients without resection of the primary tumor (p = 0.01), metastasis size (p < 0.001), bilobar distribution of metastases, CEA (p < 0.01) and CA 19-9 levels (p < 0.001). Furthermore, CTC detection rate (p < 0.001) and (p = 0.002) count was associated with the patients’ MSKCC risk score.
Table 1. Univariate analyses of clinicopathologic factors associated with detection rate and count of CEC and CTC.
| CEC + | CTC + | |||||||
|---|---|---|---|---|---|---|---|---|
| N (%) | p | Mean (median;range) | p | N (%) | p | Mean (median;range) | p | |
| Gender | ||||||||
| Male | 31 (38.8) | 0.03 | 77.9 (17; 0 – 1120) | 0.009 | 11 (13.8) | 0.05 | 0.7 (0; 0 – 8) | 0.06 |
| Female | 35 (58.3) | 88.3 (24; 0 – 911) | 17 (28.3) | 3.4 (0; 0 – 83) | ||||
| Age [years] | ||||||||
| < 65 | 38 (44.2) | 0.39 | 68 (18.5; 0 – 983) | 0.28 | 17 (19.8) | 0.97 | 2.1 (0; 0 – 83) | 0.82 |
| ≥ 65 | 28 (51.6) | 105.3 (23; 0 – 1120) | 11 (20.4) | 1.5 (0; 0 – 34) | ||||
| Disease-free interval [months] | ||||||||
| < 12 | 44 (47.8) | 0.86 | 94.2 (20; 0 – 1120) | 0.25 | 21 (22.8) | 0.28 | 1.9 (0; 0 – 83) | 0.47 |
| ≥ 12 | 22 (45.8) | 59.8 (18.5; 0 – 655) | 7 (14.6) | 1.8 (0; 0 – 34) | ||||
| Node-positive primary | ||||||||
| Yes | 47 (51.1) | 0.22 | 92.0 (22.5; 0 – 983) | 0.19 | 20 (21.7) | 0.51 | 2.0 (0; 0 – 83) | 0.96 |
| No | 19 (39.6) | 63.9 (17.5; 0 – 1120) | 8 (16.7) | 1.6 (0; 0 – 34) | ||||
| Site of primary tumor | ||||||||
| Colon | 42 (43.8) | 0.28 | 61.3 (19.5; 0 – 1120) | 0.12 | 23 (23.9) | 0.11 | 2.1 (0; 0 – 83) | 0.08 |
| Rectum | 24 (54.6) | 128.4 (23; 0 – 805) | 5 (11.4) | 1.3 (0; 0 – 34) | ||||
| KRAS mutation status1 | ||||||||
| Wild-type KRAS | 19 (40.4) | 0.39 | 68.1 (18; 0 – 1258) | 0.47 | 10 (21.3) | 0.59 | 1.4 (0; 0 – 22) | 0.94 |
| Mutant KRAS | 17 (50) | 126 (20.5; 0 – 983) | 9 (26.7) | 4.4 (0; 0 – 83) | ||||
| Previous resection of primary | ||||||||
| Yes | 48 (50.0) | 0.36 | 60.7 (21.5; 0 – 655) | 0.57 | 13 (13.5) | 0.007 | 1.0 (0; 0 – 34) | 0.01 |
| No | 18 (40.9) | 129.8 (19; 0 – 1120) | 15 (34.1) | 3.8 (0; 0 – 83) | ||||
| Number of metastases | ||||||||
| 1 | 25 (43.9) | 0.61 | 79.6 (18; 0 -1120) | 0.16 | 10 (17.5) | 0.67 | 0.8 (0; 0 – 8) | 0.62 |
| > 1 | 41 (49.4) | 84.3 (21; 0 -983) | 18 (21.7) | 2.6 (0; 0 – 83) | ||||
| Size of largest metastasis [cm] | ||||||||
| < 5 | 47 (45.6) | 0.57 | 64.0 (20; 0 – 1120) | 0.13 | 10 (9.7) | < 0.001 | 0.6 (0; 0 – 8) | < 0.001 |
| ≥ 5 | 19 (51.4) | 133.5 (24; 0 – 983) | 18 (48.6) | 5.5 (1; 0 – 83) | ||||
| Distribution of metastases | ||||||||
| Unilobar | 50 (49.5) | 0.45 | 82.9 (21; 0 – 1120) | 0.93 | 15 (14.9) | 0.02 | 0.7 (0; 0 – 8) | 0.03 |
| Bilobar | 16 (41.0) | 81.2 (19; 0 – 911) | 13 (33.3) | 4.9 (0; 0 – 83) | ||||
| Extrahepatic disease | ||||||||
| Yes | 9 (60.0) | 0.41 | 64.9 (25; 0 – 448) | 0.61 | 1 (6.7) | 0.3 | 2.1 (0; 0 – 83) | 0.07 |
| No | 57 (45.9) | 85.1 (20; 0 – 1120) | 27 (21.8) | 0.2 (0; 0 – 2) | ||||
| CEA level [μg/l] | ||||||||
| < 2.5 | 18 (46.2) | 0.98 | 93.6 (18; 0 – 1120) | 0.92 | 1 (2.6) | <0.001 | 0.2 (0; 0 – 2) | 0.01 |
| ≥ 2.5 | 47 (47.9) | 80.0 (20; 0 – 983) | 26 (26.5) | 2.6 (0; 0 – 83) | ||||
| CA 19-9 level [μg/l] | ||||||||
| < 37 | 42 (45.2) | 0.47 | 88.3 (18; 0 – 1120) | 0.65 | 10 (10.8) | <0.001 | 0.8 (0; 0 – 22) | <0.001 |
| ≥ 37 | 23 (52.3) | 74.5 (23; 0 – 911) | 17 (38.6) | 4.3 (1; 0 – 83) | ||||
| MSKCC risk score | ||||||||
| 0 | 2 (40.0) | 0.08 | 24.2 (21; 0 – 54) | 0.06 | 1 (20.0) | <0.001 | 0.8 (0; 0 – 3) | 0.002 |
| 1 | 7 (28.0) | 89.4 (11; 0 – 1120) | 1 (4.0) | 0.4 (0; 0 -8) | ||||
| 2 | 27 (50.0) | 64.3 (21; 0 – 655) | 6 (11.1) | 0.6 (0; 0 – 8) | ||||
| 3 | 23 (60.5) | 77.9 (31.5; 0 - 805) | 8 (21.1) | 1.9 (0; 0 – 34) | ||||
| 4 | 4 (28.6) | 87.8 (14.5; 0 – 983) | 11 (78.6) | 10.3 (3.5; 0 – 83) | ||||
| 5 | 3 (75.0) | 379.5 (298; 0 – 911) | 1 (25.0) | 0.8 (0; 0 – 3) | ||||
| Neoadjuvant chemotherapy | ||||||||
| Yes | 28 (42.4) | 0.29 | 99.1 (17; 0 – 983) | 0.48 | 10 (15.2) | 0.18 | 0.7 (0; 0 – 10) | 0.12 |
| No | 38 (51.3) | 67.5 (23; 0 – 1120) | 18 (24.3) | 2.9 (0; 0 – 83) | ||||
| Neoadjuvant Bevacizumab | ||||||||
| Yes | 13 (35.1) | 0.08 | 79.2 (15; 0 – 805) | 0.09 | 6 (16.2) | 0.52 | 0.8 (0; 0 – 10) | 0.11 |
| No | 53 (51.5) | 83.6 (23; 0 – 1120) | 22 (21.4) | 2.3 (0; 0 – 83) | ||||
| Neoadjuvant Cetuximab | ||||||||
| Yes | 6 (42.9) | 0.69 | 172.5 (15.5; 0 – 983) | 0.71 | 2 (14.3) | 0.56 | 0.6 (0; 0 – 3) | 0.89 |
| No | 60 (48.4) | 73.4 (20.5; 0 – 1120) | 26 (20.9) | 2.1 (0; 0 – 83) | ||||
Data are presented as n (%) or mean (median; range); CEA, Carcinoembryonic antigen; MSKCC, Memorial Sloan-Kettering Cancer Center
1Data missing for 59 patients.
Prognostic value of CEC and CTC in patients with resectable colorectal liver metastases
The median duration of follow-up was 32 (0.5 – 80) months for the entire study cohort and 41 (0.4 – 80) months for survivors. Some 56 (40%) patients died during the follow-up period. On univariate analyses a significant association with overall survival was found for detection of ≥ 21 CEC (45.2 vs. 58.2 months; p = 0.005) and detection of ≥ 2 CTC (39.2 vs. 55.2 months; p = 0.03) (Figure 2A and 2B). The association of CEC with survival was confirmed using various cut-off levels such as the 30th percentile of CEC counts (48.9 vs. 60.4 months; p = 0.03), the median CEC count (44.7 vs. 60.1 months; p = 0.001) and the 70th percentile of CEC counts (44.1 vs. 55.1 months; p = 0.02). However, there was no or only moderate association of CTC detection with survival using ≥ 1 CTC (46.1 vs. 53.7 months; p = 0.06) and ≥ 3 CTC (33.4 vs. 54.3 months; p = 0.13) as cut-off values (Supplementary Table 2). To further explore the impact of neoadjuvant therapy with and without VEGF-targeted therapy on the prognostic value of CEC in metastatic CRC patients we performed further subgroup survival analyses. These analyses confirmed the prognostic value of CEC in the subset of patients without neoadjuvant chemotherapy (43.6 vs. 59.7 months; p = 0.04) as well as patients without neoadjuvant therapy with bevacizumab (45.8 vs. 61.1 months; p = 0.007). In addition, univariate analyses revealed multiple metastases (46.3 vs. 59.2 months; p = 0.04), CEA levels ≥ 2.5 μg/L (45.2 vs. 63.0 months; p = 0.01), CA 19-9 levels ≥ 37 μg/L (33.3 vs 59.3 months; p < 0.001) and a MSKCC risk score > 2 (41.4 vs. 59.1 months, p = 0.002) to be associated with a poor prognosis (Table 2).
Figure 2. Kaplan-Meier plots for overall survival.
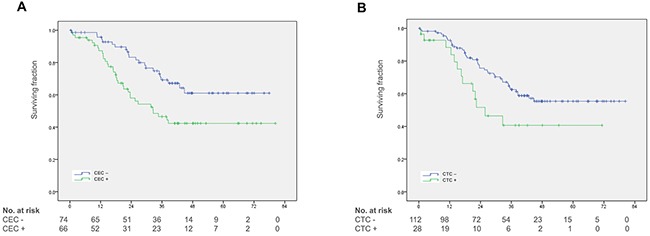
(A) Overall survival in patients with and without CEC detection. (B) Overall survival in patients with and without CTC detection.
Table 2. Univariate analyses of overall survival.
| 3-year survival [%] | Mean survival[months] | P | |
|---|---|---|---|
| Gender | |||
| Male | 58.7 | 52.1 (45.4 – 58.8) | 0.78 |
| Female | 58.5 | 52.4 (43.9 – 61.0) | |
| Age [years] | |||
| < 65 | 56.6 | 51.1 (44.3 – 57.9) | 0.72 |
| ≥ 65 | 61.3 | 53.6 (45.0 – 62.2) | |
| Disease-free interval [months] | |||
| < 12 | 53.6 | 50.6 (43.7 – 57.4) | 0.23 |
| ≥ 12 | 67.4 | 54.9 (46.1 – 63.7) | |
| Node-positive primary | |||
| Yes | 58.2 | 52.7 (43.6 – 61.7) | 0.78 |
| No | 58.7 | 51.9 (45.2 – 58.6) | |
| Site of primary tumor | |||
| Colon | 59.1 | 52.8 (46.0 – 59.6) | 0.98 |
| Rectum | 55.4 | 49.1 (41.0 – 57.2) | |
| Previous resection of primary | |||
| Yes | 62.2 | 54.7 (48.1 – 61.2) | 0.32 |
| No | 51 | 46.3 (37.5 – 55.2) | |
| Number of metastases | |||
| 1 | 71.4 | 59.2 (51.0 – 67.3) | 0.04 |
| > 1 | 49.4 | 46.3 (39.6 – 52.9) | |
| Size of largest metastasis [cm] | |||
| < 5 | 61.9 | 54.5 (48.4 – 60.6) | 0.11 |
| ≥ 5 | 45.4 | 46.4 (35.1 – 57.8) | |
| Distribution of metastases | |||
| Unilobar | 62.1 | 54.6 (48.3 – 60.9) | 0.25 |
| Bilobar | 48.1 | 45.9 (36.6 – 55.8) | |
| Extrahepatic disease | |||
| Yes | 53.3 | 34.1 (24.5 – 43.6) | 0.69 |
| No | 71 | 52.9 (47.2 – 58.6) | |
| CEA level [μg/l] | |||
| < 2.5 | 75.7 | 63.0 (54.3 – 71.7) | 0.01 |
| ≥ 2.5 | 48.3 | 45.2 (39.1 – 51.3) | |
| CA 19-9 level [μg/l] | |||
| < 37 | 69.3 | 59.3 (53.0 – 65.5) | < 0.001 |
| ≥ 37 | 33.3 | 33.3 (26.1 – 40.6) | |
| MSKCC risk score | |||
| ≤ 2 | 70.3 | 59.1 (52.4 – 65.7) | 0.002 |
| > 2 | 40 | 41.4 (33.3 – 49.4) | |
| CEC | |||
| < 21 | 69.3 | 58.2 (51.4 – 64.9) | 0.005 |
| ≥ 21 | 44.5 | 45.2 (47.4 – 58.3) | |
| CTC | |||
| < 2 | 62.6 | 55.2 (49.4 – 61.1) | 0.03 |
| ≥ 2 | 40.6 | 39.2 (27.7 – 50.8) |
Data are presented as n (%) or mean (median; range); Analyses were performed using the log-rank test.
CEA, Carcinoembryonic antigen; MSKCC, Memorial Sloan-Kettering Cancer Center; CEC, Circulating endothelial cells; CTC, Circulating tumor cells.
We next constructed a multivariate Cox proportional hazards model to assess the independent prognostic relevance of CEC and CTC in the context of the identified clinicopathologic factors with prognostic value in this patient cohort. This ‘head-to-head’ comparison confirmed CEC as independent prognostic biomarker (HR 1.81, 95% CI 1.05 – 3.14; p = 0.03), whereas CTC were of no prognostic value (HR 1.34, 95% CI 0.65 – 2.77; p = 0.43). However, this model revealed elevated CA 19-9 levels as strongest prognostic biomarker (HR 2.28, 95% CI 1.28 - 4.06; p = 0.005) (Table 3).
Table 3. Multivariate analysis of factors associated with overall survival.
| Variable | Comparison | Hazard ratio | 95% CI | p Value |
|---|---|---|---|---|
| Number of metastases | 1 vs. ≥ 2 | 1.52 | 0.81 – 2.89 | 0.19 |
| MSKCC clinical risk score | > 2 vs. ≤ 2 | 1.37 | 0.72 – 2.62 | 0.34 |
| CA 19-9 [μg/l] | < 23 vs. ≥ 23 | 2.28 | 1.28 – 4.06 | 0.005 |
| CTC | ≥ 2 vs. < 2 | 1.34 | 0.65 – 2.77 | 0.43 |
| CEC | ≥ 21 vs. <21 | 1.81 | 1.05 – 3.14 | 0.03 |
Metastasis size and CEA were not considered in the model as they are included in the MSKCC risk score.
Memorial Sloan-Kettering Cancer Center; CEC, Circulating endothelial cells; CTC, Circulating tumor cells.
DISCUSSION
We here show that high CEC numbers are an independent prognostic factor of poor survival that outperform CTC as prognostic biomarker in metastatic CRC after adjustment for other clinical variables. In contrast to CTC, CEC are independent of other tumor-related clinical variables assessed in the present study. The potential of CEC to provide biological information in addition to commonly used factors to assess the extent of disease underlines its potential as independent biomarker that may improve staging and precision medicine approaches.
Two studies on patients with non-small cell lung cancer and breast cancer have already demonstrated that CEC detection with the CellSearch system is not associated with other clinicopathologic variables commonly used for staging of patients, even though CEC in these studies were of significant prognostic value [11, 27]. Our results are clearly in line with these data. The currently available data about CEC in CRC as well as other tumor entities is not only very limited but also highly heterogeneous due to differences in patient cohorts and CEC detection assays [28–34]. Most studies involving CEC in CRC used flow cytometry protocols with varying sets of surface markers [4,6,7,28–32], resulting in data which is difficult to compare. To date, only two publications present CEC numbers in CRC patients obtained in a standardized manner using the CellSearch System [8, 9]. Interestingly, the studies had contradictory results, Matsusaka et al. demonstrated a prognostic value of CEC only for patients who received FOLFOX together with bevacizumab [8], whereas Simkens et al. presented no predictive value of CEC in patients treated with chemotherapy and bevacizumab [9]. There are several reasons that might explain the differences between our data and the results of these studies. First, both studies enrolled patients with palliative therapy. This is fundamentally different from our study which is the first to investigate the prognostic significance of CEC in metastatic CRC patients amenable to curative surgery. Second, in both studies patients were treated with systemic chemotherapy, whereas about half of our patients received no preoperative chemotherapy and chemotherapy protocols in the remaining half included irinotecan and EGFR-targeting agents in a significant proportion of patients. Finally, Matsusaka et al. used a cut-off level of 65 CEC / 4 mL of blood, whereas Simkens et al. did not use a cut-off value. We used ROC analyses to determine a cut-off level of 21 CEC / 4 mL blood for our study. Collectively, these differences in study designs render cross-comparisons between our study and the published studies difficult and may explain the observed differences.
CEC must be distinguished from endothelial progenitor cells (EPC), which share many surface markers with CEC, but are a distinct cell population of different origin and function: CEC are thought to be shed from the vasculature and are incapable of colony formation, whereas EPC are bone marrow-derived, immature cells [35]. While CEC may reflect damage of peripheral vessels, there is evidence that EPC contribute to neovascularization [36, 37]; although contradictory data has been published as well [38]. The phenotype of EPC involves endothelial markers, but by definition also includes stem cell markers such as CD133 or CD34 [35]. The phenotype detected by the CellSearch System therefore encompasses both CEC and EPC as it uses the pan-endothelial marker CD146 and does not exclude stem cells [39].
The biological role of CEC remains controversial. There is some consensus that mature CEC may reflect the level of vascular injury whereas circulating endothelial progenitors may reflect the level of vascular repair and neovascularization [32,35–37,40]. CEC originating from damaged tumor vasculature may be responsible for our finding of a therapy-induced reduction of CEC by bevacizumab as the tumor vasculature is also reduced and normalized during VEGF-targeted therapy [41, 42]. Conversely, highly vascularized tumors have better nutrient and oxygen supply as well as better access to the vascular system. Such tumors will shed more CEC and have a worse prognosis, explaining the prognostic value of CEC. However, this study did not investigate mechanistic processes and therefore does not allow conclusions about the actual role of CEC in disease progression of patients with metastatic CRC.
Elevated preoperative CA19-9 levels were found to be the strongest prognostic marker in our survival analyses. This is in agreement with multiple previous studies on patients with primary and metastatic CRC [43–52]. As CA19-9 levels did not correlate with CEC detection in our study, both markers may provide complementary information and thus be of potential use as combined biomarkers in future management of patients with metastatic CRC.
In conclusion, CEC numbers as measured by the CellSearch System are an independent prognostic factor in metastatic colorectal cancer and as such may be superior to CTC. CEC may thus serve as valuable addendum to the currently available panel of prognostic markers is mCRC. Future studies are required to elucidate subgroups of CEC with particularly strong biological and thus prognostic relevance. Furthermore, future studies need to evaluate the predictive value of CEC as a single biomarker as well as in combination with other markers such as CA19-9.
PATIENTS AND METHODS
Patients
Our management of patients with colorectal liver metastases has been reported previously [14, 53]. Briefly, patients with histologically confirmed colorectal liver metastases who underwent curative surgery at the Department of General Surgery, University Hospital Heidelberg between September 2009 and August 2012 were eligible for inclusion in this prospective study. All procedures were performed as open surgery. Exclusion criteria included emergency surgery, unresectable disease, or a history of any other malignancy within the past 5 years. The study was approved by the independent ethics committee of the University of Heidelberg. All patients provided written informed consent prior to surgery.
Blood sampling and quantification of circulating endothelial cells and circulating tumor cells
All blood samples were obtained directly prior to surgery. Blood sampling and cell quantification using the CellSearch System have been described in detail before [23]. Briefly, blood samples for CEC and CTC detection were drawn from a central venous catheter into 7.5 mL cell preservative tubes (CellSave Tubes, Veridex, NJ) prior to the first incision. Samples were maintained at room temperature and processed within 96 hours (CTC) and 72 hours (CEC). The analysis via the CellSearch System (CEC and CTC Kits, Veridex, NJ) was conducted in an operator-blinded fashion by specifically trained and Veridex-certified staff. CTC were defined as EpCAM+CK+DAPI+CD45- and CEC as CD146+CD105+DAPI+CD45- events. Each sample was analyzed by two independent operators. Disagreements were resolved by discussion. Results of CTC and CEC detection were expressed as per 7.5 mL blood (CTC) and per 4 mL blood (CEC).
Statistical analysis
Categorical data were presented as absolute and relative frequencies and compared using the χ2-test. Continuous data were presented as median and range and compared using the Wilcoxon test. In addition, the arithmetic mean was reported for CEC and CTC counts. The primary endpoint of the present study was overall survival defined as the time interval from the date of operation until death. Receiver operating curve (ROC) analyses were used to determine cutoff values with optimal sensitivity and specificity for the association of CEC and CTC detection with mortality [54]. In line with previous studies cut-off levels for CEA and CA19-9 values were chosen based on the reference ranges provided of our hospital laboratory [43, 44, 51]. Survival curves were constructed according to the Kaplan-Meier method and compared using the log-rank test. Variables with significant associations with survival on univariate analyses were included in a multivariate Cox proportional hazards regression analysis together with CEC and CTC as additional factors. A p-value ≤ 0.05 was considered to indicate a statistically significant difference. All p-values were two-sided. Statistical analyses were done with SPSS® software version 23 (SPSS, Chicago, USA) and JMP program version 7 (SAS Institute Inc., Cary, USA).
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Acknowledgments
This work was supported by the German Research foundation (WE 3548/4-1). We cordially thank Melanie Bernhard, Michaela Magenreuther und Maria Thomalla-Starzl for excellent technical assistance.
Footnotes
CONFLICTS OF INTERESTS
The authors have no conflicts of interest.
REFERENCES
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Mancuso P, Calleri A, Bertolini F. Circulating endothelial cells and circulating endothelial progenitors. Recent Results Cancer Res. 2012;195:163–170. doi: 10.1007/978-3-642-28160-0_14. [DOI] [PubMed] [Google Scholar]
- 4.Dome B, Timar J, Ladanyi A, Paku S, Renyi-Vamos F, Klepetko W, Lang G, Dome P, Bogos K, Tovari J. Circulating endothelial cells, bone marrow-derived endothelial progenitor cells and proangiogenic hematopoietic cells in cancer: from biology to therapy. Crit Rev Oncol Hematol. 2009;69:108–124. doi: 10.1016/j.critrevonc.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R, Zaniboni A, Tonini G, Buonadonna A. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609–1618. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 6.Custodio A, Barriuso J, de Castro J, Martínez-Marín V, Moreno V, Rodríguez-Salas N, Feliu J. Molecular markers to predict outcome to antiangiogenic therapies in colorectal cancer: current evidence and future perspectives. Cancer Treat Rev. 2013;39:908–924. doi: 10.1016/j.ctrv.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Malka D, Boige V, Jacques N, Vimond N, Adenis A, Boucher E, Pierga JY, Conroy T, Chauffert B, François E, Guichard P, Galais MP, Cvitkovic F. Clinical value of circulating endothelial cell levels in metastatic colorectal cancer patients treated with first-line chemotherapy and bevacizumab. Ann Oncol. 2012;23:919–927. doi: 10.1093/annonc/mdr365. [DOI] [PubMed] [Google Scholar]
- 8.Matsusaka S, Suenaga M, Mishima Y, Takagi K, Terui Y, Mizunuma N, Hatake K. Circulating endothelial cells predict for response to bevacizumab-based chemotherapy in metastatic colorectal cancer. Cancer Chemother Pharmacol. 2011;68:763–768. doi: 10.1007/s00280-010-1543-2. [DOI] [PubMed] [Google Scholar]
- 9.Simkens LH, Tol J, Terstappen LW, Teerenstra S, Punt CJ, Nagtegaal ID. The predictive and prognostic value of circulating endothelial cells in advanced colorectal cancer patients receiving first-line chemotherapy and bevacizumab. Ann Oncol. 2010;21:2447–2448. doi: 10.1093/annonc/mdq640. [DOI] [PubMed] [Google Scholar]
- 10.Ali AM, Ueno T, Tanaka S, Takada M, Ishiguro H, Abdellah AZ, Toi M. Determining circulating endothelial cells using CellSearch system during preoperative systemic chemotherapy in breast cancer patients. Eur J Cancer. 2011;47:2265–2272. doi: 10.1016/j.ejca.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Bidard FC, Mathiot C, Degeorges A, Etienne-Grimaldi MC, Delva R, Pivot X, Veyret C, Bergougnoux L, de Cremoux P, Milano G, Pierga JY. Clinical value of circulating endothelial cells and circulating tumor cells in metastatic breast cancer patients treated first line with bevacizumab and chemotherapy. Ann Oncol. 2010;21:1765–1771. doi: 10.1093/annonc/mdq052. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez Hernández A, José Juan O, Vidal Martínez J, Blanco R, Maciá S, Esquerdo Galiana G, Aparisi Aparisi F, Garde Noguera J, Catot S, Losa Gaspá F, García-Piñon F. Quantification of circulating endothelial cells as a predictor of response to chemotherapy with platinum and pemetrexed in patients with advanced non-squamous non-small cell lung carcinoma. Clin Transl Oncol. 2015;17:281–288. doi: 10.1007/s12094-014-1223-5. [DOI] [PubMed] [Google Scholar]
- 13.Rahbari NN, Aigner M, Thorlund K, Mollberg N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M, Weitz J. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138:1714–1726. doi: 10.1053/j.gastro.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Rahbari NN, Reissfelder C, Mühlbayer M, Weidmann K, Kahlert C, Büchler MW, Weitz J, Koch M. Correlation of circulating angiogenic factors with circulating tumor cells and disease recurrence in patients undergoing curative resection for colorectal liver metastases. Ann Surg Oncol. 2011;18:2182–2191. doi: 10.1245/s10434-011-1761-9. [DOI] [PubMed] [Google Scholar]
- 15.Schölch S, Bork U, Rahbari NN, García S, Swiersy A, Betzler AM, Weitz J, Koch M. Circulating tumor cells of colorectal cancer. Can Cell Microenviron. 2014;1:10–14800. [Google Scholar]
- 16.Steinert G, Schölch S, Niemietz T, Iwata N, García SA, Behrens B, Voigt A, Kloor M, Benner A, Bork U, Rahbari NN, Büchler MW, Stoecklein NH, et al. Immune Escape and Survival Mechanisms in Circulating Tumor Cells of Colorectal Cancer. Cancer Res. 2014;74:1694–1704. doi: 10.1158/0008-5472.CAN-13-1885. [DOI] [PubMed] [Google Scholar]
- 17.Bork U, Rahbari NN, Schölch S, Reissfelder C, Kahlert C, Büchler MW, Weitz J, Koch M. Circulating tumour cells and outcome in non-metastatic colorectal cancer: a prospective study. Br J Cancer. 2015;112:1306–1313. doi: 10.1038/bjc.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bork U, Grützmann R, Rahbari NN, Schölch S, Distler M, Reissfelder C, Koch M, Weitz J. Prognostic relevance of minimal residual disease in colorectal cancer. World J Gastroenterol. 2014;20:10296–10304. doi: 10.3748/wjg.v20.i30.10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol. 2009;20:1223–1229. doi: 10.1093/annonc/mdn786. [DOI] [PubMed] [Google Scholar]
- 20.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 21.Schölch S, García SA, Iwata N, Niemietz T, Betzler AM, Nanduri LK, Bork U, Kahlert C, Thepkaysone ML, Swiersy A, Bõchler MW, Reissfelder C, Weitz J, et al. Circulating tumor cells exhibit stem cell characteristics in an orthotopic mouse model of colorectal cancer. Oncotarget. 2016(7):27232–27242. doi: 10.18632/oncotarget.8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinert G, Schölch S, Koch M, Weitz J. Biology and significance of circulating and disseminated tumour cells in colorectal cancer. Langenbecks Arch Surg. 2012;397:535–542. doi: 10.1007/s00423-012-0917-9. [DOI] [PubMed] [Google Scholar]
- 23.Rahbari NN, Bork U, Kircher A, Nimitz T, Schölch S, Kahlert C, Schmidt T, Steinert G, Ulrich AB, Reissfelder C, Büchler MW, Koch M, Weitz J. Compartmental differences of circulating tumor cells in colorectal cancer. Ann Surg Oncol. 2012;19:2195–2202. doi: 10.1245/s10434-011-2178-1. [DOI] [PubMed] [Google Scholar]
- 24.Fadini GP, de Kreutzenberg S, Albiero M, Coracina A, Pagnin E, Baesso I, Cignarella A, Bolego C, Plebani M, Nardelli GB, Sartore S, Agostini C, Avogaro A. Gender differences in endothelial progenitor cells and cardiovascular risk profile: the role of female estrogens. Arterioscler Thromb Vasc Biol. 2008;28:997–1004. doi: 10.1161/ATVBAHA.107.159558. [DOI] [PubMed] [Google Scholar]
- 25.Hoetzer GL, MacEneaney OJ, Irmiger HM, Keith R, Van Guilder GP, Stauffer BL, DeSouza CA. Gender differences in circulating endothelial progenitor cell colony-forming capacity and migratory activity in middle-aged adults. Am J Cardiol. 2007;99:46–48. doi: 10.1016/j.amjcard.2006.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahbari NN, Bork U, Schölch S, Reissfelder C, Thorlund K, Betzler A, Kahlert C, Schneider M, Ulrich AB, Büchler MW, Weitz J, Koch M. Metastatic spread emerging from liver metastases of colorectal cancer: does the seed leave the soil again? Ann Surg. 2016;263:345–352. doi: 10.1097/SLA.0000000000001341. [DOI] [PubMed] [Google Scholar]
- 27.Ilie M, Long E, Hofman V, Selva E, Bonnetaud C, Boyer J, Vénissac N, Sanfiorenzo C, Ferrua B, Marquette CH, Mouroux J, Hofman P. Clinical value of circulating endothelial cells and of soluble CD146 levels in patients undergoing surgery for non-small cell lung cancer. Br J Cancer. 2014;110:1236–1243. doi: 10.1038/bjc.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ronzoni M, Manzoni M, Mariucci S, Loupakis F, Brugnatelli S, Bencardino K, Rovati B, Tinelli C, Falcone A, Villa E, Danova M. Circulating endothelial cells and endothelial progenitors as predictive markers of clinical response to bevacizumab-based first-line treatment in advanced colorectal cancer patients. Ann Oncol. 2010;21:2382–2389. doi: 10.1093/annonc/mdq261. [DOI] [PubMed] [Google Scholar]
- 29.Ramcharan KS, Lip GY, Stonelake PS, Blann AD. Effect of standard chemotherapy and antiangiogenic therapy on plasma markers and endothelial cells in colorectal cancer. Br J Cancer. 2014;111:1742–1749. doi: 10.1038/bjc.2014.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin CC, Liu CY, Chen MJ, Wang TE, Chu CH, Wang HY, Shih SC, Hsu ML, Hsu TC, Chen YJ. Profiles of circulating endothelial cells and serum cytokines during adjuvant chemoradiation in rectal cancer patients. Clin Transl Oncol. 2013;15:855–860. doi: 10.1007/s12094-013-1004-6. [DOI] [PubMed] [Google Scholar]
- 31.Manzoni M, Mariucci S, Delfanti S, Rovati B, Ronzoni M, Loupakis F, Brugnatelli S, Tinelli C, Villa E, Falcone A, Danova M. Circulating endothelial cells and their apoptotic fraction are mutually independent predictive biomarkers in Bevacizumab-based treatment for advanced colorectal cancer. J Cancer Res Clin Oncol. 2012;138:1187–1196. doi: 10.1007/s00432-012-1190-6. [DOI] [PubMed] [Google Scholar]
- 32.Kraan J, Sleijfer S, Foekens JA, Gratama JW. Clinical value of circulating endothelial cell detection in oncology. Drug Discov Today. 2012;17:710–717. doi: 10.1016/j.drudis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Salvador J, Manso L, de la Haba J, Jaen A, Ciruelos E, de Villena MC, Gil M, Murias A, Galan A, Jara C, Bayo J, Baena JM, Casal J, et al. Final results of a phase II study of paclitaxel, bevacizumab, and gemcitabine as first-line therapy for patients with HER2-negative metastatic breast cancer. Clin Transl Oncol. 2015;17:160–166. doi: 10.1007/s12094-014-1210-x. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Yuan D, Ye W, Lv T, Song Y. Prognostic value of circulating endothelial cells in non-small cell lung cancer patients: a systematic review and meta-analysis. Transl Lung Cancer Res. 2015;4:610–618. doi: 10.3978/j.issn.2218-6751.2015.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blann AD, Woywodt A, Bertolini F, Bull TM, Buyon JP, Clancy RM, Haubitz M, Hebbel RP, Lip GY, Mancuso P, Sampol J, Solovey A, Dignat-George F. Circulating endothelial cells. Biomarker of vascular disease. Thrombosis and Haemostasis. 2005 doi: 10.1160/TH04-09-0578. [zitiert 13. Januar 2015] Verfügbar unter: http://th.schattauer.de/en/contents/archive/issue/760/manuscript/4005.html. [DOI] [PubMed] [Google Scholar]
- 36.Stoelting S, Heinze G, Nadrowitz R, Wagner T, Peters SO. Bone marrow-derived endothelial cells contribute to angiogenesis in murine WEHI and JC tumors. Anticancer Res. 2008;28:771–777. [PubMed] [Google Scholar]
- 37.Reddy K, Zhou Z, Schadler K, Jia SF, Kleinerman ES. Bone marrow subsets differentiate into endothelial cells and pericytes contributing to Ewing's tumor vessels. Mol Cancer Res. 2008;6:929–936. doi: 10.1158/1541-7786.MCR-07-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wickersheim A, Kerber M, de Miguel LS, Plate KH, Machein MR. Endothelial progenitor cells do not contribute to tumor endothelium in primary and metastatic tumors. Int J Cancer. 2009;125:1771–1777. doi: 10.1002/ijc.24605. [DOI] [PubMed] [Google Scholar]
- 39.Strijbos MH, Verhoef C, Gratama JW, Sleijfer S. On the origin of (CD105+) circulating endothelial cells. Thromb Haemost. 2009;102:347–351. doi: 10.1160/TH08-11-0762. [DOI] [PubMed] [Google Scholar]
- 40.Hunting CB, Noort WA, Zwaginga JJ. Circulating endothelial (progenitor) cells reflect the state of the endothelium: vascular injury, repair and neovascularization. Vox Sang. 2005;88:1–9. doi: 10.1111/j.1423-0410.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- 41.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 42.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 43.Chen CC, Yang SH, Lin JK, Lin TC, Chen WS, Jiang JK, Wang HS, Chang SC. Is it reasonable to add preoperative serum level of CEA and CA19-9 to staging for colorectal cancer? J Surg Res. 2005;124:169–174. doi: 10.1016/j.jss.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 44.Dexiang Z, Li R, Ye W, Haifu W, Yunshi Z, Qinghai Y, Shenyong Z, Bo X, Li L, Xiangou P, Haohao L, Lechi Y, Tianshu L, et al. Outcome of patients with colorectal liver metastasis: analysis of 1,613 consecutive cases. Ann Surg Oncol. 2012;19:2860–2868. doi: 10.1245/s10434-012-2356-9. [DOI] [PubMed] [Google Scholar]
- 45.Katoh H, Yamashita K, Kokuba Y, Satoh T, Ozawa H, Hatate K, Ihara A, Nakamura T, Onosato W, Watanabe M. Surgical resection of stage IV colorectal cancer and prognosis. World J Surg. 2008;32:1130–1137. doi: 10.1007/s00268-008-9535-7. [DOI] [PubMed] [Google Scholar]
- 46.Ko AH, Dito E, Schillinger B, Venook AP, Xu Z, Bergsland EK, Wong D, Scott J, Hwang J, Tempero MA. A phase II study evaluating bevacizumab in combination with fixed-dose rate gemcitabine and low-dose cisplatin for metastatic pancreatic cancer: is an anti-VEGF strategy still applicable? Invest New Drugs. 2008;26:463–471. doi: 10.1007/s10637-008-9127-2. [DOI] [PubMed] [Google Scholar]
- 47.Levy M, Visokai V, Lipska L, Topolcan O. Tumor markers in staging and prognosis of colorectal carcinoma. Neoplasma. 2008;55:138–142. [PubMed] [Google Scholar]
- 48.Lin PC, Lin JK, Lin CC, Wang HS, Yang SH, Jiang JK, Lan YT, Lin TC, Li AF, Chen WS, Chang SC. Carbohydrate antigen 19-9 is a valuable prognostic factor in colorectal cancer patients with normal levels of carcinoembryonic antigen and may help predict lung metastasis. Int J Colorectal Dis. 2012;27:1333–1338. doi: 10.1007/s00384-012-1447-1. [DOI] [PubMed] [Google Scholar]
- 49.Narita Y, Taniguchi H, Komori A, Nitta S, Yamaguchi K, Kondo C, Nomura M, Kadowaki S, Takahari D, Ura T, Andoh M, Muro K. CA19-9 level as a prognostic and predictive factor of bevacizumab efficacy in metastatic colorectal cancer patients undergoing oxaliplatin-based chemotherapy. Cancer Chemother Pharmacol. 2014;73:409–416. doi: 10.1007/s00280-013-2367-7. [DOI] [PubMed] [Google Scholar]
- 50.Sakamoto Y, Miyamoto Y, Beppu T, Nitta H, Imai K, Hayashi H, Baba Y, Yoshida N, Chikamoto A, Baba H. Post-chemotherapeutic CEA and CA19-9 are prognostic factors in patients with colorectal liver metastases treated with hepatic resection after oxaliplatin-based chemotherapy. Anticancer Res. 2015;35:2359–2368. [PubMed] [Google Scholar]
- 51.Shibutani M, Maeda K, Nagahara H, Ohtani H, Sakurai K, Toyokawa T, Kubo N, Tanaka H, Muguruma K, Ohira M, Hirakawa K. Significance of CEA and CA19-9 combination as a prognostic indicator and for recurrence monitoring in patients with stage II colorectal cancer. Anticancer Res. 2014;34:3753–3758. [PubMed] [Google Scholar]
- 52.Takakura Y, Ikeda S, Imaoka Y, Urushihara T, Itamoto T. An elevated preoperative serum carbohydrate antigen 19-9 level is a significant predictor for peritoneal dissemination and poor survival in colorectal cancer. Colorectal Dis. 2015;17:417–425. doi: 10.1111/codi.12865. [DOI] [PubMed] [Google Scholar]
- 53.Reissfelder C, Rahbari NN, Koch M, Ulrich A, Pfeilschifter I, Waltert A, Müller SA, Schemmer P, Büchler MW, Weitz J. Validation of prognostic scoring systems for patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16:3279–3288. doi: 10.1245/s10434-009-0654-7. [DOI] [PubMed] [Google Scholar]
- 54.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


