Abstract
The Aurora family kinases contribute to accurate progression through several mitotic events. ZM447439 (“ZM”), the first Aurora family kinase inhibitor to be developed and characterized, was previously found to interfere with the mitotic spindle integrity checkpoint and chromosome segregation. Here, we have used extracts of Xenopus eggs, which normally proceed through the early embryonic cell cycles in the absence of functional checkpoints, to distinguish between ZM's effects on the basic cell cycle machinery and its effects on checkpoints. ZM clearly had no effect on either the kinetics or amplitude in the oscillations of activity of several key cell cycle regulators. It did, however, have striking effects on chromosome morphology. In the presence of ZM, chromosome condensation began on schedule but then failed to progress properly; instead, the chromosomes underwent premature decondensation during mid-mitosis. ZM strongly interfered with mitotic spindle assembly by inhibiting the formation of microtubules that are nucleated/stabilized by chromatin. By contrast, ZM had little effect on the assembly of microtubules by centrosomes at the spindle poles. Finally, under conditions where the spindle integrity checkpoint was experimentally induced, ZM blocked the establishment, but not the maintenance, of the checkpoint, at a point upstream of the checkpoint protein Mad2. These results show that Aurora kinase activity is required to ensure the maintenance of condensed chromosomes, the generation of chromosome-induced spindle microtubules, and activation of the spindle integrity checkpoint.
INTRODUCTION
Aurora family kinases play roles in several mitotic processes, including the G2/M transition, mitotic spindle organization, chromosome segregation, and cytokinesis (reviewed in Andrews et al., 2003; Katayama et al., 2003; Crane et al., 2004; Meraldi et al., 2004). Aurora A is found in the cytoplasm and at centrosomes during interphase; during mitosis, it also localizes to microtubules near the spindle poles. Aurora A interacts with several different proteins that are required for proper centrosome maturation and spindle function. Aurora B is found at the centromeric regions of chromosomes as part of a “chromosomal passenger protein complex,” where it appears to promote correct bipolar microtubule-kinetochore attachments. After anaphase onset, Aurora B relocalizes to the central microtubules of the anaphase spindle and then to the midbody during the completion of cytokinesis. Little is known about the localization pattern or function of Aurora C.
Insights into the molecular functions of individual Aurora kinases have come from several different approaches including genetics, overexpression of wild-type and mutant forms, and reduction of endogenous kinase levels using RNAi or immunodepletion. The founding Aurora family member, now known as Aurora A, was discovered in Drosophila as an allelic series of mutations at the aurora locus that interfered with mitosis (Glover et al., 1995). Although usually described as producing monopolar spindles, the type of spindle defects seen varies among different alleles and cell types. The most widely cited phenotype is that seen with auroraAe209, where some of the larval neuroblasts accumulate monopolar spindles in which centrioles failed to separate. Other neuroblasts display bipolar-type spindles, in which most centrosomal markers are found only at one pole. The auroraAe209 gene contains two point mutations, one of which is in the kinase domain and is thus predicted to block kinase activity (Glover et al., 1995; Giet et al., 2002). Further genetic studies now suggest that at least some of the auroraAe209 effects could be due to an increase in the dose of catalytically inactive protein, rather than simply to lack of kinase activity (Giet et al., 2002). Support for this idea comes from studies in which addition of recombinant kinase-dead Aurora A to Xenopus egg extracts leads to an increase in the number of monopolar and multipolar types of spindles (Giet and Prigent, 2000), and overexpression of either wild-type or kinase-dead Aurora A in mammalian somatic cells causes defects in spindle morphology and interferes with chromosome segregation and cytokinesis (Littlepage and Ruderman, 2002; Meraldi et al., 2002; Anand et al., 2003). RNAi studies in Caenorhabditis elegans further argue that Aurora A is important for normal spindle structure (Schumacher et al., 1998a; Hannak et al., 2001). Aurora A also plays a role in mitotic entry as well: overexpression of Aurora A accelerates the G2/Meiosis I transition in Xenopus oocytes, and RNAi-mediated reduction Aurora A delays the G2/M transition in mammalian tissue culture cells (Andresson and Ruderman, 1998; Hirota et al., 2003).
No Aurora B mutants have been described so far, but investigations using RNAi or injection of neutralizing antibodies indicate that Aurora B is involved in several mitotic process, including phosphorylation of histone H3 (which is required for chromosome condensation in Tetrahymena thermophila and perhaps higher organisms; Hans and Dimitrov, 2001), chromosome alignment, kinetochore disjunction, the spindle integrity checkpoint, and cytokinesis (Schumacher et al., 1998b; Hsu et al., 2000; Speliotes et al., 2000; Adams et al., 2001; Giet and Glover, 2001; Hagstrom et al., 2002; Kallio et al., 2002; Ditchfield et al., 2003; Hauf et al., 2003). A recently identified Aurora B substrate, the motor protein MCAK (mitotic centromere-associated kinesin; Andrews et al., 2004; Lan et al., 2004; Ohi et al., 2004), acts as a microtubule depolymerase that is required for the changes in microtubule dynamics that lead to the formation of the meiotic spindle in Xenopus egg extracts (Walczak et al., 1996; Ohi et al., 2004).
Investigations using RNAi to reduce endogenous Aurora A and Aurora B proteins have led to significant new information about the localization and functions of their interacting partners. However, in this approach it is difficult to distinguish between effects due to lack of the protein itself, where Aurora-containing complexes and subcomplexes do not form, and those due simply to lack of kinase activity, where substrate phosphorylation is the initial defect. Thus, the development of specific small molecule inhibitors could help determine the importance of Aurora kinase activity on different mitotic processes. This distinction may be especially important in view of the fact that the Aurora family kinases are frequently amplified and/or overexpressed in human cancers (reviewed in Katayama et al., 2003) and overexpression of one of them, Aurora A, is oncogenic (Bischoff et al., 1998; Zhou et al., 1998; Littlepage et al., 2002). Although overexpression of either active and kinase-dead versions of Aurora A in tissue culture cells interferes with chromosome segregation and cytokinesis (Littlepage and Ruderman, 2002; Meraldi et al., 2002; Anand et al., 2003), only kinase active forms of Aurora A have been able to transform cells and generate tumors in mice to date (Bischoff et al., 1998; Littlepage et al., 2002). In view of the roles of Aurora A and Aurora B in checkpoint efficacy and chromosome segregation, and growing links with tumor formation, considerable effort has been given to identifying small molecules that can act as selective inhibitors of Aurora family kinases. Four such inhibitors are now available, ZM447439 (Ditchfield et al., 2003), AKI, a ZM447439 synthetic intermediate (Lampson et al., 2004), hesperadin (Hauf et al., 2003), and VX680 (Harrington et al., 2004).
ZM447439 (“ZM”) was the first such inhibitor to be characterized (Ditchfield et al., 2003). When ZM was added to mammalian somatic tissue culture cells, the cells entered mitosis and formed a mitotic spindle, but phosphorylation of histone H3 was reduced, the spindle was disorganized, chromosomes did not align properly, and cytokinesis was blocked. Despite the presence of misaligned chromosomes, cyclin B was degraded (a marker for anaphase onset in normal cells), sister chromatid cohesion was lost, and cells exited mitosis, clearly indicating that ZM had compromised the spindle integrity checkpoint in some way.
In somatic cells, it can be difficult to generate cell cultures that proceed through G2 and mitosis with high synchrony, and almost impossible to obtain cultures that synchronously go through more than one cell cycle. Furthermore, somatic cells rapidly activate the spindle integrity checkpoint in response to chromosome misalignment, making it difficult to study the effects of inhibitors such as ZM on the basic cell cycle regulatory machinery separately from their effects on the spindle checkpoint. Here, we used Xenopus egg cycling extracts to investigate which specific cell cycle events are affected by ZM. These extracts go through highly synchronous cell cycles in vitro. Normally, checkpoint pathways do not operate during the Xenopus early embryonic cell cycles, making it possible to study the effects of ZM on individual basic events of cell cycle progression free from the complications of checkpoint effects. However, eggs and extracts derived from them do in fact contain all of the spindle checkpoint components needed to arrest cell cycle progression when chromosome alignment is incomplete or mitotic spindles are damaged, and the spindle assembly checkpoint can be activated experimentally (Minshull et al., 1994). Thus, it is possible to use cycling egg extracts to examine how ZM affects the spindle checkpoint as well.
We find that ZM clearly has no effect on either the kinetics or amplitude in the oscillations of the activities of several key cell cycle regulators. ZM does, however, have dramatic effects on chromosome morphology and spindle dynamics. In the presence of ZM, chromosome condensation begins on schedule but then fails to progress properly; instead, chromosomes undergo premature decondensation during mid-mitosis. Unlike somatic cells, where disorganized spindles form in the presence of the drug, ZM almost completely blocks spindle assembly in the egg extracts. Strikingly, ZM selectively inhibits the formation of microtubules nucleated/stabilized by chromatin while having little effect on assembly of microtubules at centrosomes (spindle poles). Finally, under conditions where the spindle integrity checkpoint is experimentally induced, ZM blocks establishment of the checkpoint; however, once the checkpoint has been established, ZM does not interfere with its maintenance. Taken together, these results indicate that i) ZM inhibits several different mitotic processes in the normal unperturbed cell cycle, ii) Aurora kinase activity is required for the completion and maintenance of chromosome condensation, iii) Aurora kinase activity is required for the generation of chromosome-induced microtubules, and iv) in this system, Aurora kinase activity is required for establishment, but not maintenance, of the spindle integrity checkpoint.
MATERIALS AND METHODS
Xenopus Egg Collection
Female adult Xenopus laevis were obtained from the colony in the Cell Biology Department at Harvard Medical School (Boston, MA). Frogs were primed with 50 U of pregnant mare serum gonadotropin (PMSG; Sigma, St. Louis, MO) at least 3 d before egg collection. Frogs were induced to ovulate by injection of 150 U of human chorionic gonadotropin (HCG) and then placed in 1× MMR (100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 0.1 mM EDTA, 5 mM HEPES, pH 7.8) and incubated at 16°C. About 16 h after HCG injection, laid eggs were collected, washed in 1× MMR, and dejellied (100 mM KCl, 0.1 mM CaCl2, 1 mM MgCl2, 2% cysteine, free base, pH 7.8).
Egg Extracts
Cycling Extracts. Concentrated cycling extracts were prepared from activated Xenopus eggs as previously described (Murray, 1991). Briefly, dejellied eggs were washed in MMR and then activated at 16°C using calcium inophore A231187 (Fisher Scientific, Pittsburgh, PA) for 5 min and incubated for an additional 25 min in XB buffer (10 mM HEPES [KOH]) pH 7.7, 50 mM sucrose, 100 mM KCl, 1 mM MgCl2, 0.1 mM CaCl2) to ensure complete meiotic exit. Eggs were transfer to ice and packed by centrifugation at 4°C for 45 s at 2000 rpm using a Sorvall HB-6 swinging bucket rotor. Excess liquid was removed and eggs were crushed by centrifugation at 4°C for 15 min at 10,000 rpm. The cytosolic fraction was then collected on ice. To assay DNA morphology, demembrenated sperm nuclei were prepared as previously described (Murray, 1991), and nuclei were added to extracts on ice to a final concentration of 500 nuclei/μl extract. To assay spindle assembly, rhodamine-labeled tubulin (Cytoskeleton, Denver, CO; TL331M-A) was added to extracts on ice to a final concentration of 50 ng/μl. Cycling extracts were induced to resume cycling by warming them to 21°C. At the indicated times, slides were prepared by fixing 1 μl of extract and squashing it underneath a coverslip with 4 μl of fixative solution (11% formaldehyde, 48% glycerol, 2.5 μg/ml Hoechst 33342, and 1× MMR). Chromosomes and mitotic spindles were imaged by fluorescence microscopy using an E800 upright microscope (Nikon, Garden City, NY) at the Nikon Imaging Center at Harvard Medical School.
Visualization of Individual Chromosomes in Cycling Extracts. Individual chromosomes were visualized as described in Funabiki and Murray (2000). Briefly, 1 μl of extract was diluted in 4 μl of chromosome dilution buffer (10 mM HEPES [KOH], pH 7.7, 200 mM KCl, 0.5 mM MgCl2, 0.5 mM EGTA, 250 mM sucrose) that was supplemented with either dimethyl sulfoxide (DMSO) or 20 μM ZM. Diluted extracts were then incubated at 21°C for 15 min. Slides were prepared by fixing 1 μl of diluted extract and squashing it underneath a coverslip with 3 μl of fixative solution. Chromosomes were imaged by fluorescence microscopy using a Nikon Eclipse 80i upright microscope at the Nikon Imaging Center at Harvard Medical School.
CSF Extracts
Cytostatic factor (CSF) extracts were made from laid metaphase II–arrested eggs as previously described (Murray, 1991). Briefly, dejellied eggs were washed in XB, CSF-XB (10 mM HEPES [KOH], pH 7.7, 50 mM sucrose, 100 mM KCl, 2 mM MgCl2, 0.1 mM CaCl2, 5 mM EGTA, pH 7.7), CSF-XB containing protease inhibitors (10 μg/ml leupeptin, chymostatin, and pepstatin A), and CSF-XB containing protease inhibitors and 10 μg/ml cytochalasin B. Eggs were packed by centrifugation at 16°C for 45 s at 2000 rpm using a Sorvall HB-6 swinging bucket rotor. Excess liquid was removed, and eggs were crushed by centrifugation at 16°C for 15 min at 10,000 rpm. The cytosolic fraction was then collected and placed on ice.
Spindle Assembly Checkpoint. To activate the spindle assembly checkpoint in cycling extracts, sperm nuclei (final 10,000 nuclei/μl extract) and nocodazole (final 10 μg/ml) were added to extracts on ice for 10 min. Extracts were then warmed to 21°C to resume cycling. At various times, samples were taken and processed for histone H1 kinase assays or fixed as above.
To activate the spindle assembly checkpoint in CSF extracts, sperm nuclei (final 10,000 nuclei/μl extract) and nocodazole (final 10 μg/ml) were added. Extracts were then warmed to 21°C and incubated for 30 min (Minshull et al., 1994). To inactivate the CSF arrest, calcium (5 mM HEPES [KOH], pH 7.7, 10 mM CaCl2, 1 mM MgCl2, 100 mM KCl, 150 mM sucrose) was added to extracts to give a final concentration of 0.5 mM. Samples were taken at various times and processed for histone H1 kinase assays or fixed as above. Recombinant Mad2 protein was a gift from Aaron Straight (Stanford University, Stanford, CA) and was added to extracts to a final concentration of 100 ng/μl extract.
Spindle Assembly Assays in M phase–arrested Extracts
CSF extracts were prepared and supplemented on ice with rhodamine-labeled tubulin (final 50 ng/μl) and sperm nuclei (final 600 nuclei/μl extract). Extracts were then warmed to 21°C, and metaphase exit was induced by the addition of calcium. Sixty minutes after calcium addition, DMSO (final 0.2%) or 20 μM ZM was added to extracts. Eighty minutes after calcium addition, extracts were driven into M phase by the addition of CSF extract or nondegradable cyclin B protein. To drive extracts into M phase using CSF extract, a half volume of CSF extract containing DMSO (0.2%) or 20 μM ZM was added. Extract samples were fixed in 20-min intervals until 80 min. Spindle structures were visualized and counted at 80 min after M phase induction. To drive extracts into M phase using nondegradable cyclin B protein, MBP-cyclin B Δ90 protein was added. Extracts were fixed in 20-min intervals until 60 min and spindle structures were visualized by fluorescence microscopy as described above. Recombinant MBP-cyclin B Δ90 protein was a gift from Randall King (Harvard Medical School).
Chromatin Beads. CSF extracts were supplemented on ice with DNA-coated magnetic beads and cycloheximide (final 10 mg/ml) and then warmed to 21°C. Extracts were then driven into interphase by the addition of calcium and incubated for 2 h at 21°C. To drive extracts into M phase, half a volume of CSF extract (containing DMSO [0.2%] or 20 μM ZM) was added and extracts were incubated for 30 min. Extracts were transferred to ice and beads were retrieved and mixed with 30 μl of CSF extract containing rhodamine-labeled tubulin (final 50 ng/μl) and either DMSO (0.2%) or 20 μM ZM. Extracts were warmed to 21°C and spindle assembly was monitored in 10-min intervals until 60 min. DNA-coated beads were a gift from Aaron Groen (Tim Mitchison lab, Harvard Medical School).
Pelleting of Microtubules from Extracts. To pellet microtubule structures from extracts, 20 μl of extract sample was diluted in 200 μl of BRB80 (80 mM PIPES, pH 6.8, 1 mM EGTA, 1 mM MgCl2) containing 30% glycerol. The diluted extract was centrifuged >1 ml of BRB80/40% glycerol at 9000 rpm for 20 min at room temperature. The supernatant was removed and the pellet was resuspended in SDS sample buffer. Equivalent amounts of the supernatant and pellet fractions were separated by 15% SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was blocked for 15 min using 3% nonfat dry milk diluted in TBST and incubated overnight at 4°C with monoclonal anti-α-tubulin DM1A antibodies (Sigma; T 9026). The anti-α-tubulin antibody was diluted 1:2000 in 2% bovine serum albumin (BSA) in TBST and the secondary was sheep-α-mouse (Amersham, Piscataway, NJ) diluted 1:5000 in 3% nonfat milk.
H1 Kinase Assays
One microliter of extract was frozen on liquid nitrogen and stored at -80°C. Samples were transferred to dry ice and thawed on ice by adding 9 μl of histone H1 cocktail mix (80 mM β-glycerophosphate, pH 7.4, 12 mM MgCl2, 16 mM EGTA, 3 μg histone H1 protein (Boehringer Mannheim, Indianapolis, IN), 50 μM ATP, 10 μCi [γ-32P]ATP, and 10 μM PKI). Samples were incubated at 30°C for 10 min and the reactions were terminated by the addition of 10 μl of SDS sample buffer. Samples were separated by SDS-PAGE and gels were dried on chromatography paper (Whatman, Clifton, NJ). Phosphorylation of histone H1 was visualized by autoradiography.
Immunoblotting
One microliter of extract was diluted into 20 μl of SDS sample buffer. Samples were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were blocked with the indicated blocking solutions for 15–30 min and incubated overnight at 4°C with the specified antibodies. Blots were washed at room temperature three times in TBST (10 mM Tris, pH 7.4, 150 mM NaCl, 0.1% Tween), incubated for 1 h with the specified secondary antibody, and washed three more times in TBST; bound antibodies were detected using chemiluminescence (Amersham). All antibody and blocking solutions were diluted in TBST. For α-Aurora A antibodies (Littlepage and Ruderman, 2002), blots were blocked with 3% nonfat milk, the Aurora A antibody was diluted 1:2000 in 1% BSA, and the secondary antibody was donkey-α-rabbit (Amersham) diluted 1:5000 in 3% milk. For α-phospho-MAPK antibodies (Cell Signaling, Beverly, MA; 9101S), blots were blocked with 3% nonfat milk, the phosho-MAPK antibody was diluted 1:5000 in 2% BSA, and the secondary antibody was donkey-α-rabbit diluted 1:5000. For α-cdc2 (PSTARE) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA; sc-53), blots were blocked with 3% nonfat milk, the cdc2 antibody was diluted 1:200 in 2% BSA, and the secondary antibody was donkey-α-rabbit diluted 1:5000 in 3% milk. For α-phospho-histone H3 (S10) antibodies (Upstate, Lake Placid, NY; 06–570), blots were blocked with 3% nonfat milk, the histone H3 antibody was diluted 1:2000 in 2% BSA, and the secondary antibody was donkey-α-rabbit diluted 1:5000 in 3% milk. For α-phospho-cdc25 (S287) antibodies (Duckworth et al., 2002), blots were blocked with 2% BSA, the cdc25 antibody was diluted 1:1000 in 2% BSA, and the secondary antibody was donkey-α-rabbit diluted 1:5000 in 2% BSA. For α-cyclin B1 antibodies (Taieb et al., 2001), gift from Jim Maller (University of Colorado, Colorado Springs, CO), blots were blocked with 3% milk, the cyclin B1 antibody was diluted 1:10,000 in 2% BSA, and the secondary antibody was donkey-α-sheep (Jackson ImmunoResearch, West Grove, PA) diluted 1:50,000 in 2.5% donkey serum (Jackson ImmunoResearch).
RESULTS
ZM Does Not Block Oscillations in cdc2, cdc25, APC/C, or MAPK Activities
To investigate ZM's effects on individual events of the early embryonic cell cycles, we used cycling extracts of Xenopus eggs that reproduce many morphological and biochemical events of cell cycle progression. Briefly, eggs, which are naturally arrested in metaphase of meiosis II, were activated by the addition of calcium ionophore and incubated for 30 min at 16°C. During this time, the eggs exited from metaphase II and entered early interphase of the first mitotic cell cycle. Eggs were transferred to 4°C to stop cell cycle progression and crushed by centrifugation. The cytoplasmic layer was collected and incubated with either DMSO (control) or 20 μM ZM in DMSO for 10 min on ice. Nuclei were added to a final concentration of 500/μl; this concentration is well below the level required for extracts to become responsive to any of the known checkpoint pathways previously studied in this system (Dasso and Newport, 1990; Minshull et al., 1994; Kumagai et al., 1998). Extracts were warmed to 21°C to reinitiate resumption of the cell cycle; morphological and biochemical markers of cell cycle progression were then monitored. In most experiments, the “30-min” time point refers to samples that were removed just before warming the extracts to 21°C.
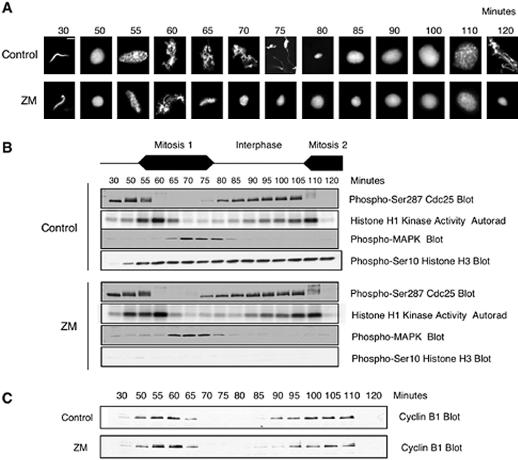
In the control extract (Figure 1A), highly condensed sperm nuclei (30 min) had decondensed by 50 min, indicating that the extract had entered interphase of the first mitotic cycle by this time. Chromatin condensation became detectable between 50 and 55 min, indicating that the extract had entered first mitosis, and chromatin condensation continued until ∼75 min. By 80 min, the extract had entered interphase of the second cycle and then entered second mitosis between 100 and 110 min. At 120 min, the last time point taken in this experiment, the extract was still in mitosis.
Figure 1.
Addition of ZM to Xenopus cycling egg extracts blocks phosphorylation of endogenous histone H3 and induces premature chromosome decondensation but does not interfere with entry into mitosis or the oscillations in the activities of cdc2, cdc25, MAPK, or APC/C activity toward cyclin B. (A) Xenopus cycling egg extracts were prepared as described in the text. Extracts were supplemented with nuclei (500/μl) and warmed to 21°C for resumption of cell cycle progression. Samples were taken at the indicated times and analyzed for histone H1 kinase activity or by SDS-PAGE followed by immunoblotting with antibodies directed against phospho-Ser287 of cdc25 (an inhibitory phosphorylation), phospho-Thr202/Tyr204 of MAPK (an activating phosphorylation), and phospho-Ser10 of histone H3. (B) Samples from the same experiment were fixed, stained with Hoechst 33042, and visualized by fluorescence microscopy. (C) In a separate experiment, cycling extracts were treated with DMSO or ZM as above and blotted with cyclin B1 antibodies. (A) Magnification, ×100. Bar, 10 μm.
Figure 1B shows the status of some useful biochemical markers of cell cycle progression. As described previously for intact embryos (Duckworth et al., 2002) and more recently for cycling extracts (Stanford and Ruderman, unpublished results), cdc25, the phosphatase responsible for activating cdc2 during mitotic entry, displayed the characteristic inhibitory phosphorylation on Ser287 during interphase. As the extract went through the G2/M transition, cdc25 underwent two obvious modifications: the initial electrophoretic retardations that reflect phosphorylations that occur as the extract enters mitosis, and the dephosphorylation of Ser287. Around the same time, increases in cdc2 activation began, as judged by histone H1 (H1) kinase assays. H1 kinase activity dropped sharply between 60 and 65 min; in vivo, this drop is a marker for the metaphase/anaphase transition. Coincident with the drop in cdc2 activity was the rise in MAPK activity, as monitored using a phospho-specific antibody against Thr202/Tyr204, whose phosphorylation is widely used as an excellent surrogate for MAPK activity (Anderson et al., 1990; Bhatt and Ferrell, 1999; Gross et al., 2000). MAPK remained active during the latter stages of mitosis, where previous work has shown that MAPK activity is required for maintenance of the mitotic state after cdc2 inactivation (Minshull et al., 1994; Takenaka et al., 1997; Guadagno and Ferrell, 1998; Bhatt and Ferrell, 1999). The drop in MAPK activity at the end of the first cycle was followed by rephosphorylation of cdc25 Ser287 during exit into interphase of the second cycle and subsequently by dephosphorylation of cdc25 Ser287 and activation of cdc2 as the extract went into mitosis of the second cell cycle. By 120 min, H1 kinase activity had dropped again, but MAPK phosphorylation had not yet appeared.
On blots of total extract protein, phosphorylation of histone H3 at Ser10, a previously identified target of Aurora kinases, began around 50 min (mid-late interphase of the first mitotic cycle), increased over the next 10 min and then remained constant (Figure 1B). This result is intriguing in view that phosphorylation of chromatin-bound histone H3 oscillates across the cell cycle, being high in metaphase and low throughout the rest of the cell cycle (Arnaoutov and Dasso, 2003). This difference between the phosphorylation status of chromatin-bound and whole extract histone H3 suggests that in cycling egg extracts histone H3 phosphorylation and dephosphorylation are tightly regulated at chromosomes, whereas little regulation occurs in the whole cytoplasm.
In the presence of 20 μM ZM, phosphorylation of histone H3 was dramatically reduced (Figure 1B), as has been seen previously in somatic cells (Ditchfield et al., 2003). Strikingly, ZM had little effect on either the timing or amplitude of oscillations in cdc2, cdc25, and MAPK activities. In the presence of ZM, dephosphorylation of cdc25 Ser287, the rise and fall of cdc2 and MAPK activities occurred on schedule. In a separate experiment using an extract that cycled with similar kinetics (Figure 1C), ZM also had no obvious effects on rise and fall in cyclin B protein, events that are regulated by the periodic inactivation and activation, respectively, of the ubiquitin ligase APC/C. Thus, the addition of 20 μM ZM, which almost completely blocked phosphorylation of histone H3, had no detectable effect on oscillations in the activities of several cell cycle markers. Given that each of the biochemical events monitored here depend on one or more upstream kinase activities, these results argue that ZM, which was previously found to be a selective inhibitor of purified Aurora kinases in vitro, is a highly selective inhibitor in concentrated whole cell extracts.
In the Presence of ZM, Chromosome Condensation Begins on Schedule but then Fails to Progress, and Chromosomes Undergo Premature Decondensation
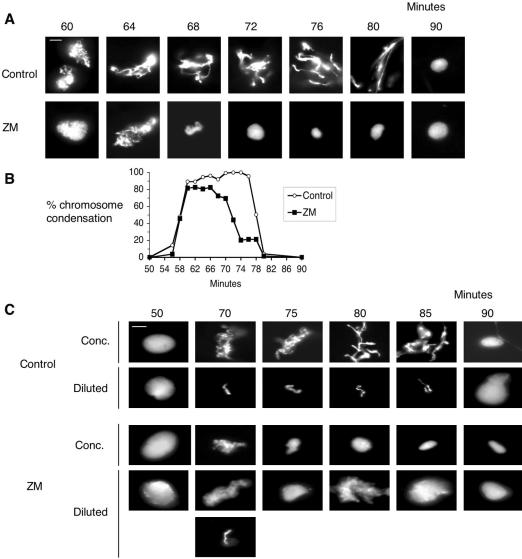
ZM did not interfere with nuclear envelope breakdown (unpublished data) or the initial stages of chromosome condensation (Figure 1A). ZM did, however, have an obvious and dramatic effect on latter stages of mitosis: between 60 and 65 min, when chromatin threads were clearly visible in the control extract, chromatin in the ZM-treated extract collapsed into a tight cluster. By the next time point, 70 min, most of the chromatin in the ZM-treated extract had started to decondense, well ahead of the controls. A more detailed time course (Figure 2) confirmed that although it did not prevent the initial stages of chromatin condensation, ZM did block full condensation, the appearance of individual chromatin threads, and maintenance of the condensed state.
Figure 2.
Addition of ZM to cycling egg extracts prevents the completion of mitotic chromosomal changes and results in premature chromosome decondensation. (A) Cycling egg extracts were incubated on ice for 10 min with either DMSO (control) or 20 μM ZM as in Figure 1. Extracts were supplemented with nuclei (500/μl) and then warmed to 21°C to resume cycling. Samples were taken every 2 min, fixed, stained with Hoechst 33042, and observed by fluorescence microscopy. Magnification, ×100. Bar, 10 μm. (B) At the indicated times, the percent of nuclei with condensed chromosomes was determined. More than 150 nuclei were counted for each time point. (C) In a separate experiment, extracts were treated as in A except that at the indicated times samples were either directly fixed (concentrated) or diluted in chromosome dilution buffer. Diluted extracts were then incubated for 15 min at 21°C. Samples from diluted extracts were fixed and stained with Hoechst 33042, and DNA morphology was observed by fluorescence microscopy. The images shown represent the most abundant DNA structures observed in concentrated and diluted extracts for one of three independent experiments. Bar, 10 μm.
To further characterize the chromosome morphology defects observed in ZM-treated extracts, cycling extracts were diluted in chromosome dilution buffer to physically resolve individual chromosomes (Funabiki and Murray, 2000). In the control extract, visible chromosome threads were observed by 70 min and individual chromosomes were clearly visible after dilution (Figure 2C). Chromosome threads and individual chromosomes persisted until 85 min and chromosomes decondensed between 85 and 90 min. The ZM-treated extract began chromosome condensation with similar kinetics as the control extract. Some individual chromosomes were visible after diluting the extract; many uncondensed nuclei were also present. By 75 min, few individual chromosomes were observed after dilution and large clusters of chromatin were observed, indicating premature decondensation of chromosomes. These results demonstrate that ZM selectively affects one or more of the steps that are required to complete and/or maintain chromosome condensation during the latter part of mitosis.
ZM Inhibits Mitotic Spindle Assembly in Egg Extracts
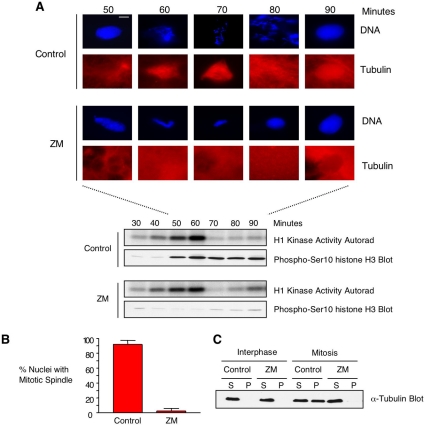
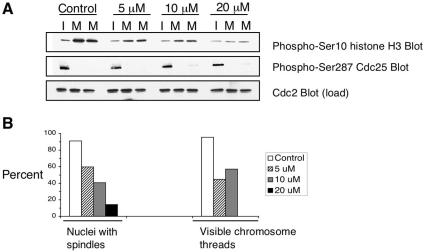
In mammalian tissue culture cells, ZM treatment did not prevent the formation of mitotic spindles (Ditchfield et al., 2003). Thus, we were surprised to see that ZM treatment did inhibit spindle formation in cycling egg extracts (Figure 3). In control extracts, microtubule polymerization around condensing chromosomes was evident by 60 min, when H1 kinase activity was near its peak (Figure 3A). Depolymerization had occurred by 80 min, after H1 kinase activity had dropped and chromosomes were decondensing. The addition of ZM almost completely blocked mitotic spindle formation (Figure 3A). When quantified, <10% of chromatin masses present in ZM-treated extracts contained any detectable microtubule staining (Figure 3B). In a separate experiment (Figure 3C), microtubule pelleting assays showed that ZM almost completely blocked microtubule polymerization. ZM's inhibitory effects on phosphorylation of histone H3 and mitotic spindle formation were dose dependent, with around 90% inhibition of spindle formation achieved with 20 μM ZM (Figure 4). No reduction in histone H3 phosphorylation was observed at a ZM concentration of 2 μM, the concentration previously used in media for human somatic cells (Ditchfield et al., 2003). This indicates that higher ZM concentrations are required when added directly to highly concentrated egg extracts.
Figure 3.
The addition of ZM to cycling egg extracts blocks the formation of mitotic spindles. (A) ZM-treated extracts fail to form mitotic spindles. Cycling egg extracts were incubated on ice for 10 min with either DMSO or ZM. Extracts were supplemented with nuclei (500/μl) and rhodamine-labeled tubulin and then warmed to 21°C to resume cycling. Samples were taken at the indicated times and processed as follows. For morphology, samples were fixed and stained with Hoechst 33342. DNA (blue) and microtubules (red) were analyzed by fluorescence microscopy at ×60 magnification; bar, 15 μm. For biochemical markers of cell cycle progression, portions of the extract were analyzed for cdc2 (H1 kinase) activity, and by blotting for phospho-Ser10 of histone H3. (B) Quantitation of mitotic spindles in ZM-versus DMSO-treated extracts. The number of mitotic spindles in DMSO-(control) or ZM-treated extracts was determined in three separate experiments. More than 100 structures were counted in each experiment. The graph shows the average, expressed as a percentage. (C) ZM-treated extracts exhibit less microtubule polymerization than control extracts. In a separate experiment, extracts with interphase nuclei (interphase) or condensed chromatin (mitosis) were taken and centrifuged to separate polymerized tubulin (pellet, P) from soluble tubulin (supernatant, S). Equivalent portions of the supernatant and pellet were analyzed by SDS-PAGE followed by blotting with anti-α-tubulin antibodies.
Figure 4.
Dose-dependent ZM inhibition of histone H3 phosphorylation, mitotic spindle assembly, and the formation of normal mitotic chromosomes in cycling egg extracts. (A) ZM inhibits phosphorylation of histone H3. Cycling egg extract was prepared as in Figure 1 and supplemented with nuclei (500/μl) and rhodamine-labeled tubulin. The extract was incubated on ice with DMSO or ZM to the indicated final concentration (5, 10, and 20 μM). Extracts were warmed to 21°C to resume cycling. Samples were taken at 40 min, when nuclei were in an interphase state (I) and at 80 and 90 min, when chromosomes were in the mitotic state (M80, M90). Samples in interphase (I) and mitosis (M) were taken and immunoblotted with the indicated antibodies. Histone H3 Ser10 phosphorylation diminished with increasing ZM concentration, and 20 μM ZM had the strongest effect. Cdc25 dephosphorylation on serine-287 was used as a marker for mitosis and cdc2 protein levels were used as loading controls. (B) ZM inhibits spindle formation and chromosome morphology. Samples from the same experiment were fixed at 80 min and spindle and DNA morphology were analyzed by fluorescence microscopy. The number of nuclei that associated with microtubules (left panel) and that formed discrete chromosome threads was determined (right panel). Graph shows the percentage of mitotic spindles (left panel) and condensed, threadlike chromosomal clusters (right panel) in DMSO- and ZM-treated extracts. More than 150 structures were counted.
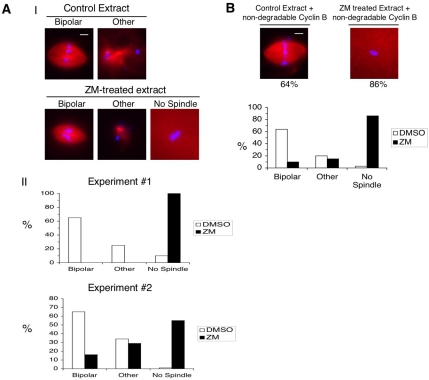
In cycling extracts, the fraction of nuclei associated with bipolar spindles is usually low, and spindle morphology is irregular, with microtubules polymerized mainly around chromatin (Murray, 1991). Thus, in cycling extracts, it can be difficult to distinguish between a delay versus a complete block in spindle assembly and hard to investigate effects of compounds on spindle morphology. We thus used an approach that is more suitable for addressing these questions (Sawin and Mitchison, 1991). Briefly, when metaphase II–arrested eggs are crushed, the resulting extract remains arrested in metaphase; such extracts are referred to as CSF (cytostatic factor–arrested) extracts. The addition of calcium breaks the arrest and induces exit into interphase. Subsequent addition of a half-volume of CSF extract drives the interphase extract into mitosis, where it then arrests in metaphase. Nondegradable cyclin B can also drive the interphase extract into a metaphase arrest (Desai et al., 1999). In both cases, bipolar spindles form with very high efficiency. In the experiment shown in Figure 5A, CSF extracts were first supplemented with sperm nuclei, activated with calcium, and incubated for 60 min. The resulting interphase extract was treated with either DMSO or ZM for 30 min and then induced to enter M phase by the addition of CSF extract that had been pretreated with DMSO or ZM, respectively. Spindle morphology was monitored 80 min later. Representative images are shown in panel I and quantification of the results in two separate experiments are shown in panel II. The majority of spindle structures in control extracts were bipolar; a smaller percentage of nuclei were associated with microtubules that were not organized into a bipolar spindle (referred to as “other” in Figure 5). In the presence of ZM, spindle formation was inhibited. The degree of inhibition varied among extracts (e.g., Figure 5A, experiment 2). ZM also inhibited spindle formation in extracts driven into mitosis by nondegradable cyclin B (Figure 5B).
Figure 5.
ZM reduces spindle assembly in extracts driven into M phase by CSF extract or nondegradable cyclin B protein. (A) Extracts were prepared from metaphase II-arrested eggs (CSF extract) and supplemented with rhodamine-labeled tubulin and sperm nuclei (500/μl). Extracts were driven into interphase by the addition of calcium chloride. Sixty minutes after calcium addition, DMSO (control) or ZM was added. Twenty minutes later, control and ZM-treated extracts were driven into M phase by the addition of CSF extract containing either DMSO or ZM, respectively. Samples were fixed at 80 min after M phase initiation and analyzed by fluorescence microscopy. The number of bipolar-type spindles (Bipolar), spindles that were not obviously bipolar (Other), or nuclei that lacked detectable microtubule staining (No Spindle) were determined. The images in panel I represent the different categories that were quantified in panel II. The inhibitory effect of ZM on spindle assembly varied between experiments. The quantification of spindle structures from two independent experiments is shown in panel II. More than 100 structures were counted for each experiment. Bar, 15 μm. (B) CSF egg extract was treated as in A except that nondegradable cyclin B protein was added to drive the extract into mitosis instead of CSF extract. Spindle structures were counted as in A. Bar, 15 μm. More than 100 structures were counted in this experiment. The graph showing the percentage of spindle structures observed in DMSO- and ZM-treated extracts is a representative of three independent experiments.
ZM Does Not Block the Initial Nucleation of Microtubules from Sperm Centrosomes, but Strongly Reduces the Ability to Form Half Spindles
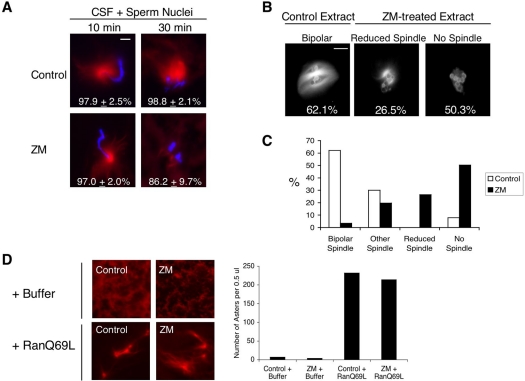
When demembranated sperm nuclei are added to CSF extracts, sperm centrioles quickly assemble into functional centrosomes. These centrosomes nucleate microtubule asters, which later form “half spindles.” In many cases, two half spindles go on to fuse and form a single bipolar spindle (Sawin and Mitchison, 1991). To determine whether ZM inhibits centrosome-induced microtubule nucleation, CSF extracts were incubated with ZM on ice for 60 min; demembranated sperm nuclei and rhodamine-tubulin were then added, and extracts were warmed to 21°C. After 10 min, asters associated with sperm centrosomes were seen in both control and ZM-treated extracts (Figure 6A). However by 30 min, when control extracts had formed half-spindles around condensed chromatin, the chromatin in ZM-treated extracts had little or no associated microtubule structures (Figure 6A, ZM 30′). These results suggest that ZM does not inhibit the ability of sperm centrosomes to initiate microtubule nucleation but, instead, interferes in some way with the stabilization of microtubules nucleated from these centrosomes and/or chromosomes.
Figure 6.
Addition of ZM to egg extracts does not prevent the formation microtubule asters induced from sperm centrosomes or by Ran-GTP, but does reduce chromatin-induced spindle assembly. (A) ZM does not inhibit microtubule nucleation from sperm centrosomes. CSF extracts were supplemented with sperm nuclei (400/μl) and rhodamine-labeled tubulin, and then incubated on ice for 60 min in the presence of either DMSO or 20 μM ZM. Extracts were warmed to 21°C and samples were taken at 10-min intervals and fixed. Microtubules (red) were visualized with rhodamine-tubulin and DNA (blue) with Hoechst 33042 by fluorescence microscopy. Magnification, ×60; bar, 15 μm. The images shown represent the most abundant structures observed in DMSO- and ZM-treated extracts from three independent experiments. (B) ZM reduces the formation of spindles around chromatin-coated beads. Chromatin-coated beads were incubated in CSF extract containing rhodamine-labeled tubulin and either DMSO or 20 μM ZM. Samples were fixed at 60 min, and the status of spindle formation was determined. Magnification, ×60; bar, 15 μm. The images shown represent the most abundant spindle structures observed in DMSO- and ZM-treated extracts. (C) Quantification of spindle structures around chromatin-beads in DMSO- and ZM-treated extracts from B. Samples were fixed 60 min after the initiation of spindle assembly. The type of spindle structures associated with bead clusters that contained at least six beads were assigned to four different categories: “Bipolar Spindles” contained microtubules organized into bipolar structures; “Other” contained microtubules that were not organized into obvious bipolar structures; “Reduced Spindle” contained weak microtubule staining; “No Spindle”, contained no microtubule staining. The graph shown represents one of three independent experiments. More than 150 bead clusters were counted in this experiment. (D) ZM does not inhibit RanQ69L-induced microtubule asters. CSF extracts were supplemented rhodamine-labeled tubulin and then incubated on ice for 60 min in the presence of either DMSO (Control) or 20 μM ZM. Extracts were warmed to 21°C and incubated for an additional 15 min. Buffer or RanQ69L (0.5 mg/ml) was added and samples were taken at 60 min. The total number of microtubule asters was counted.
ZM Inhibits Assembly around Chromatin-coated Beads
Xenopus eggs can also nucleate microtubules from chromatin, even in the absence of kinetochore structures and centrosomes. For example, when plasmid DNA lacking centromeric sequences is microinjected into eggs (Karsenti et al., 1984) or DNA-coated beads are added to egg extracts (Heald et al., 1996), each induces spindle assembly. In both cases, egg proteins are recruited onto the exogenous DNA, resulting in chromatin that nucleates microtubule assembly (Heald et al., 1996; Budde et al., 2001). To test whether ZM inhibits spindle assembly induced by chromatin, DNA-coated beads were added to CSF extracts containing DMSO or ZM, and spindle assembly was monitored 60 min later. In control extracts, microtubule staining was evident around clusters of beads (Figure 6B) and more than 60% of these clusters were associated with bipolar spindles (Figure 6C). By contrast, the majority of bead clusters in ZM-treated extracts were not associated with any detectable microtubules or had very reduced microtubule staining. Thus, ZM reduces spindle assembly in extracts lacking both kinetochores and centrosomes. These results indicate that ZM blocks spindle assembly in egg extracts by interfering with both i) the stabilization of microtubules nucleated by centrosomes and ii) the nucleation and/or stabilization of microtubules nucleated from chromatin.
ZM Does Not Inhibit Microtubule Asters Induced by Ran-GTP
In Xenopus eggs, spindle assembly requires a high local concentration of the GTP-bound form of the small GTPase Ran around chromatin (reviewed in Karsenti and Vernos, 2001). Addition of a hydrolysis-deficient mutant of Ran (RanQ69L) to egg extract induces the formation of microtubule asters in the absence of chromatin and centrosomes (Carazo-Salas et al., 1999). To determine whether ZM blocks a step downstream of Ran-GTP, CSF extract was first incubated on ice with DMSO or ZM for 60 min. Extracts were then warmed to 21°C and RanQ69L was added. Aster formation was monitored 60 min later. As shown on Figure 6D, both control-and ZM-treated extracts contained a similar amount of RanQ69L-induced asters. This suggests that ZM does not block a step downstream of Ran-GTP. However, it does not exclude the possibility that ZM prevents the establishment of the Ran-GTP gradient around chromosomes.
The Premature Decondensation of Chromosomes Seen in ZM-treated Extracts Is Not Due Simply to the Absence of a Mitotic Spindle
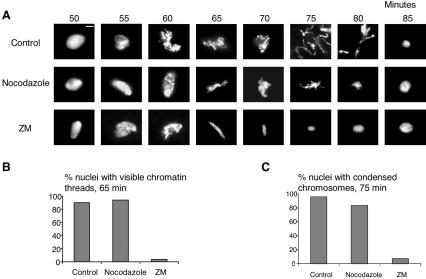
To determine whether the chromosome morphology defects observed in ZM-treated extracts were due solely to problems in spindle assembly, cycling extracts containing a low number of nuclei (500/μl, well below the concentration required to make the extracts responsive to the spindle assembly checkpoint) were incubated with the microtubule-depolymerizing agent nocodazole. In nocodazole-treated extracts, chromosome condensation began on schedule and discrete chromosome threads formed by 65 min, just as in controls extracts lacking ZM (Figure 7, A and B). Although chromosomes in nocodazole-treated extracts aggregated to some extent during mitosis, they remained condensed for the same amount of time as in control extracts, and individual chromosomal threads were evident. This contrasts with the appearance of chromosomes in the ZM-treated extracts, which did not go on to form discrete chromosomal threads but, instead, underwent premature chromosome decondensation (Figure 7, A and C). These results suggest that the defects in chromosome morphology and the premature chromosome decondensation observed in ZM-treated extracts are not simply a consequence of the absence of mitotic spindles.
Figure 7.
ZM blocks the formation of normal chromatin structures independently of spindle assembly. (A) Cycling egg extracts were incubated with DMSO (control), nocodazole (10 μg/ml), or 20 μM ZM on ice. Extracts were supplemented with nuclei (500/μl) and then warmed to 21°C to resume cycling. At the indicated times, samples were fixed and chromosomes were visualized by staining with Hoechst 33042. The images shown are representative of the most abundant chromatin structures observed at the indicated times. Magnification, 100×; bar, 10 μm. (B) Quantification of chromatin structures from A. The number of chromatin groups that appeared to be fully condensed into discrete chromosome threads at 65 min was determined for DMSO-, nocodazole-, or ZM-treated extracts. More than 100 nuclei were counted for each sample. (C) Quantification of the number of condensed chromosomes from A. The number of chromatin groups that contained condensed chromosomes at 75 min was determined for DMSO-, nocodazole-, or ZM-treated extracts. More than 100 nuclei were counted for each sample.
ZM Inhibits the Establishment of the Spindle Integrity Checkpoint, But Not Its Maintenance
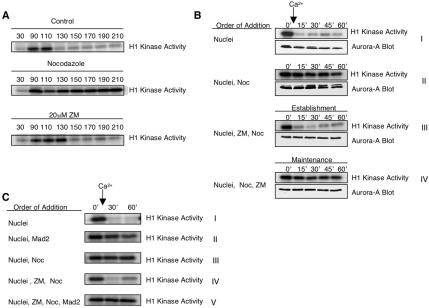
When the concentration of nuclei in extracts exceeds ∼9000/μl, extracts become “spindle checkpoint-responsive:” in the presence of nocodazole or other agents that prevent microtubule polymerization, cyclin B/cdc2 activity stays high and chromosomes remain condensed (Minshull et al., 1994). To ask whether ZM interferes with the spindle integrity checkpoint, 10,000 nuclei/μl and rhodamine-tubulin were added to cycling extracts at the 30-min time point (early interphase) on ice. Portions of the extract were supplemented with either DMSO, nocodazole, or ZM, and after 10 min were warmed to 21°C to allow the resumption of cell cycle progression. In the control extract, histone H1 kinase activation was seen by 90 min and dropped after 110 min (Figure 8A). As expected for the nocodazole-treated extract, histone H1 kinase activity rose and then remained high, demonstrating that the extract was spindle checkpoint-responsive. Despite the lack of mitotic spindles, ZM-treated extracts failed to arrest in mitosis, cdc2 activity dropped (Figure 8A) and chromosomes decondensed (unpublished data). In this particular experiment, the timing of the drop in histone H1 kinase activity in the ZM-treated extract was delayed relative to that seen in the control extract; however, this delay was not consistently seen. These results indicate that ZM prevents either establishment of the spindle assembly checkpoint, its maintenance, or both.
Figure 8.
ZM blocks the establishment, but not the maintenance, of the spindle assembly checkpoint in Xenopus egg extracts. (A) Cycling extracts that contain 10,000 nuclei/μl, a concentration that allows them to arrest when spindle formation is prevented by nocodazole, do not arrest in the presence of ZM. Cycling egg extracts were supplemented with rhodamine-labeled tubulin and a high concentration of nuclei (10,000 nuclei/μl) and incubated with DMSO (control), 10 μg/ml nocodazole, or 20 μM ZM on ice. Extracts were then warmed to 21°C to resume cycling and samples were taken at the indicated times. Samples were taken and used to assay cdc2 (histone H1 kinase) activity. (B) ZM overrides the establishment, but not the maintenance, of the spindle checkpoint. Metaphase II-arrested (CSF) extracts were supplemented with 10,000 nuclei/μl and incubated for 30 min with DMSO (I) or nocodazole (II) at 21°C. Calcium was then added to trigger mitotic exit. Samples were taken before (time 0) and after (15′, 30′, 45′, and 60′) calcium addition and used to determine cdc2 H1 kinase activity or blotted with Aurora A antibodies. For checkpoint establishment experiments (III), 20 μM ZM was added to Metaphase-II extracts on ice and incubated for 30 min. Extracts were then warmed to 21°C, supplemented with 10,000 nuclei/μl and nocodazole, and incubated for an additional 30 min. Calcium was then added and samples were taken and analyzed as above. For checkpoint maintenance experiments (IV), Metaphase-II extracts were supplemented with 10,000 nuclei/μl and incubated with nocodazole for 30 min at 21°C to induce checkpoint activation. ZM was then added to a final concentration of 20 μM and extracts were incubated for an additional 30 min. Calcium was added and samples were taken and analyzed as above. (C) Excess Mad2 causes mitotic arrest in ZM-treated extracts. Metaphase II-arrested extracts were supplemented with 10,000 nuclei/μl and incubated for 30 min at 21°C in either the absence (I) or presence (II) of excess Mad2 protein, or in the presence of nocodazole (III). At the indicated times, samples were taken before (time 0) and after (30′ and 60′) calcium addition and used to do determine cdc2 H1 kinase activity. Metaphase II extracts were also incubated with 20 μM ZM on ice for 30 min and then warmed to 21°C. These extracts were then supplemented with nuclei and nocodozole and were incubated in the absence (IV) or presence (V) of excess Mad2 protein for 30 min. Calcium was added and samples were taken and used to determine cdc2 H1 kinase activity.
To address whether ZM affects establishment of the checkpoint, CSF extract was supplemented with 10,000 nuclei/μl. As described previously (Minshull et al., 1994), addition of calcium breaks the arrest, inducing inactivation of cdc2 kinase, (Figure 8B, panel I) and chromosome decondensation (unpublished data). When CSF extract was incubated first with nocodazole, and then with calcium, histone H1 kinase activity remained high (panel II) and chromosomes decondensed (unpublished data), indicating that the extract was checkpoint-responsive. When CSF extract was incubated first with ZM, next with nocodazole, and calcium was then added, histone H1 kinase activity dropped (panel III), and chromosomes decondensed (unpublished data). In other experiments, cycling extracts that were treated in interphase first with ZM, and then with nocodazole also failed to arrest in mitosis (unpublished data). These results indicate that ZM interferes with establishment of the spindle integrity checkpoint.
To test whether ZM affects maintenance of the checkpoint, nocodazole was added first, and then ZM. After calcium addition, histone H1 kinase activity remained high (Figure 8B, panel IV) and chromosomes remained condensed (unpublished data) for the duration of the experiment. Similarly, when ZM was added to cycling extracts that were subsequently arrested in mitosis by nocodazole treatment, extracts remained arrested with high H1 kinase activity and condensed chromosomes (unpublished data). Thus, once the spindle checkpoint arrest had been established, ZM did not override that arrest. In some experiments, ZM did inhibit the maintenance of the checkpoint, but only at concentrations higher than 80 μM. Taken together, these results indicate that ZM blocks establishment of the spindle integrity checkpoint arrest, but not its maintenance.
In contrast to our finding that ZM inhibits the establishment but not the maintenance of the spindle integrity checkpoint, Kallio et al. (2002) reported that addition of Aurora B–neutralizing antibodies to Xenopus egg extracts inhibited both the establishment and maintenance of the checkpoint (Kallio et al., 2002). One possible explanation for the different outcomes is that some Aurora B protein resides in a complex that is essential for the establishment and maintenance of the spindle checkpoint, but only kinase activity is required to establish the checkpoint. Thus, the addition of Aurora B antibodies might disrupt or otherwise interfere with protein-protein interactions that are required for checkpoint maintenance, whereas inhibition of kinase activity by ZM might allow these interactions to persist.
ZM Interferes with the Spindle Integrity Checkpoint Arrest at a Point Upstream of mad2
Unattached kinetochores are believed to generate the activated form of Mad2, one of the components in the spindle assembly checkpoint pathway. Activated Mad2 inhibits the APC/C activator Cdc20, an event that is thought to be the last step in the spindle checkpoint pathway (Li et al., 1997; Fang et al., 1998; Kallio et al., 1998). Addition of large amounts of Mad2 protein to Xenopus extracts inhibits the APC/C independently of kinetochore attachment and signaling (Chen et al., 1998). As expected, addition of excess Mad2 protein or nocodazole to metaphase II arrested extracts containing high numbers of nuclei prevented mitotic exit (Figure 8C, panels II and III). As above, extracts incubated with first with ZM and then with nocodazole failed to establish the checkpoint arrest (Figure 8C, panel IV). However, when extracts were incubated first with ZM and then with Mad2, the extract remained arrested in mitosis (Figure 8C, panel V). Thus, ZM appears to inhibit a step that occurs upstream of Mad2.
DISCUSSION
The main points to come out of this work are the following. i) In the early embryonic cell cycles, which lack checkpoints, ZM has no detectable effects on either the frequency or amplitude of oscillations in cdc2, cdc25, and MAPK activities, or with APC/C activity toward cyclin B. ii) In the presence of ZM, chromosome condensation begins on schedule. However, changes in chromosome architecture fail to progress further, and, instead, chromosomes decondense prematurely. iii) Unlike somatic tissue culture cells, where mitotic spindles formed in the presence of ZM but were disorganized (Ditchfield et al., 2003), spindle formation in egg extracts is almost completely blocked by ZM. This difference probably reflects the fact that, in somatic cells, the bulk of spindle formation is driven by centrosomes at the spindle poles, whereas spindle formation in eggs relies more on nucleation and stabilization of microtubules by the chromosomes. iv) When egg extracts are prepared under checkpoint-responsive conditions, ZM inhibits establishment of the spindle integrity checkpoint, but not maintenance of the checkpoint.
ZM Specificity
In the first report of ZM's specificity and effects on cells, Ditchfield et al. (2003) found that ZM inhibited the in vitro kinase activities of purified human Aurora A and Aurora B proteins equally well, with IC50 values in the 100-nM range. The amounts of ZM required to inhibit a panel of other purified kinases were 10- to >100-fold higher. In vivo, ZM treatment of human somatic tissue culture cells did not interfere with their ability to enter mitosis, form mitotic spindles, degrade cyclin B, or exit from mitosis. ZM treatment did, however, reduce phosphorylation of histone H3, a physiologically relevant target of Aurora B, and caused defects in chromosome alignment, chromosome segregation, and cytokinesis, most likely by interfering with the spindle integrity checkpoint.
Our work significantly extends those findings. In Xenopus cycling egg extracts, concentrations of ZM that completely inhibited phosphorylation of endogenous histone H3 had no detectable effects on either the frequency or amplitude of oscillations in cdc2, cdc25, or MAPK activities, or the periodic destruction of cyclin B that is mediated by the APC/C ubiquitin ligase. The activation and inactivation of each of those regulators requires the activities of other kinases. For example, cdc2 activity requires phosphorylation of the T loop residue Thr161 by CAK (Fesquet et al., 1993; Poon et al., 1993; Solomon et al., 1993) and is inhibited during interphase through phosphorylation of Tyr15 by the kinase wee1 (Parker et al., 1991, 1992; Parker and Piwnica-Worms, 1992; McGowan and Russell, 1993). Full activation of cdc25, the phosphatase that dephosphorylates and activates cdc2, depends on phosphorylation by Plk (polo-like kinase) and cdc2 itself (Kumagai and Dunphy, 1992; Izumi and Maller, 1993; Qian et al., 1998, 2001). MAPK requires activation by a MAP kinase kinase, which, in turn, is dependent on activation by a MAP kinase kinase kinase. Activation of the APC/C requires the activities of cyclin B/cdc2 and other kinases (Hershko et al., 1994; Lahav-Baratz et al., 1995; Peters et al., 1996; Yamada et al., 1997; Descombes and Nigg, 1998; Kotani et al., 1998). Thus, the fact that ZM completely blocks at least one known Aurora kinase–dependent event, phosphorylation of histone H3, without interfering with oscillations in the activities of many basic cell cycle regulators attests to the high selectivity of ZM in the context of whole cells.
ZM Specificity: Aurora A, Aurora B, or Both?
As noted in the Introduction, the formation of monopolar spindles, almost universally cited as the diagnostic for a selective effect on Aurora A, is now known to be an unreliable criterion (Giet et al., 2002). Thus, previous observations that treatment with ZM or other inhibitors results in bipolar-like spindles, rather than monopolar spindles (Ditchfield et al., 2003; Hauf et al., 2003; Lampson et al., 2004), should not by themselves be interpreted to mean that those inhibitors are specific for Aurora B. Immunodepletion of Aurora B, but not Aurora A, blocks phosphorylation of histone H3 in Xenopus egg extracts (Sumara et al., 2002), arguing that Aurora B is the main histone H3 kinase in that system. RNAi studies in a variety of systems also suggest that histone H3 is a physiological target of Aurora B (Hsu et al., 2000; Speliotes et al., 2000; Adams et al., 2001; Giet and Glover, 2001; Hauf et al., 2003). The ability of ZM to block histone H3 phosphorylation in tissue culture cells indicates that ZM effectively inhibits Aurora B (Ditchfield et al., 2003), but says nothing about ZM's ability to inhibit Aurora A in vivo. So far, the main evidence consistent with the idea that Aurora B is a better in vivo target of ZM than Aurora A comes from studies on the G2/M transition. In Xenopus oocytes, overexpression of Aurora A accelerates the G2/Meiosis I transition, suggesting that Aurora A participates in meiotic entry in these cells (Andresson and Ruderman, 1998). In egg extracts, Aurora A also affects the timing of the G2/M transition (Wu, Liu, and Ruderman, unpublished results). In tissue culture cells, RNAi-mediated reduction of Aurora A delays mitotic entry (Hirota et al., 2003). Taken together with the findings that ZM treatment of whole cells (Ditchfield et al., 2003) and egg extracts (this study) does not affect timing of mitotic entry. This suggests that ZM is considerably more selective for Aurora B than Aurora A in vivo.
ZM Interferes with Mitotic Changes in Chromosome Architecture and Induces Premature Chromosome Decondensation
Addition of ZM to cycling egg extracts did not block the initial stages of chromosome condensation that are characteristic of mitotic entry. However, ZM had dramatic effects on later mitotic changes: discrete chromosome threads failed to form, chromosomes decondensed prematurely, and interphase-like nuclei appeared in mid-mitosis. Thus, in egg extracts, ZM inhibits completion of mitotic chromosome changes and/or the maintenance of the condensed state. So far, no studies using Aurora A RNAi have noted defects in chromosome condensation (Hannak et al., 2001; Kufer et al., 2002; Ditchfield et al., 2003; Marumoto et al., 2003), although it is hard to make firm conclusions about the role of Aurora A in this process since in most cases significant levels of Aurora A protein still remained. With regard to Aurora B depletion by RNAi, reported effects on chromosome morphology range from none (Ditchfield et al., 2003; Hauf et al., 2003) to the appearance of “dumpy” chromosomes (Adams et al., 2001) to the formation of “incompletely” condensed chromosomes that fail to recruit condensin (Giet and Glover, 2001). Differences in cell type, the amounts of remaining Aurora B protein, and experimental details could each contribute to the variation in results.
In budding yeast, there is a single Aurora kinase, Ipl1. After the completion of our experiments, it was reported that rDNA chromatin condensation is normal when Ipl1 mutants are arrested in metaphase, but resemble G1-like chromatin when the mutants are arrested in anaphase (Lavoie et al., 2004). Those and other results now suggest that two pathways regulate condensation in budding yeast, one requiring the cohesin complex in metaphase and a second pathway dependent on Ipl1 activity in anaphase. Taken together with our finding that ZM results in incomplete condensation and premature chromosome decondensation, it is likely that Aurora B kinase activity is required to maintain chromosome condensation during late mitosis.
ZM Effects on the Dynamics of Spindle Formation
ZM treatment of somatic human (DLD-1 and HeLa) cells did not block formation mitotic spindles, even though the concentrations of ZM used clearly interfered with chromosome alignment, chromosome segregation, and cytokinesis (Ditchfield et al., 2003). By contrast, ZM did strongly inhibit spindle assembly in Xenopus egg extracts. One possibility is that ZM inhibits Aurora C or another target that plays an essential role in this process in the early embryonic cell cycles, but is lacking or relatively unimportant in the somatic cell cycle. Another draws on differences in the ways that microtubules are nucleated in somatic cells versus eggs. There is increasing evidence that in somatic cells, centrosomes are the dominant microtubule nucleating centers, whereas in eggs, microtubules are mainly nucleated from chromatin. For example, in Xenopus egg extracts, beads coated with chromatin can support spindle assembly in the absence of kinetochores and centrosomes (Heald et al., 1996). Our finding that ZM strongly interferes with the assembly of microtubules from chromatin beads suggests that Aurora kinase activity contributes to the assembly of noncentrosomal spindles in egg extracts. With regard to ZM's effect on centrosome-mediated microtubule assembly, aster microtubules did begin to form from sperm centrosomes in the presence of ZM, but those microtubules were not stable. These results suggest that ZM does not significantly inhibit centrosome-induced microtubule nucleation, but, instead, prevents microtubule nucleation and/or stabilization around chromosomes.
In Xenopus egg extracts, the microtubule-destabilizing kinesin MCAK (mitotic centromere-associated kinesin) is important for spindle assembly. Immunodepletion of MCAK results in the formation of large microtubule asters and inhibition of MCAK activity by addition of anti-MCAK antibodies reduces the mitotic catastrophe frequency (Walczak et al., 1996; Tournebize et al., 2000). Those results indicate that MCAK is the major microtubule-destabilizing protein in eggs. Recent studies show that phosphorylation of MCAK by Aurora B inhibits the ability of MCAK to depolymerize microtubules in vitro (Andrews et al., 2004; Lan et al., 2004; Ohi et al., 2004). Furthermore, in studies that appeared after the completion of our work, immunodepletion of INCENP (which also removes Aurora B, Survivin, and other interacting proteins) was found to inhibit spindle formation in egg extracts; this effect was rescued by the codepletion of MCAK (Sampath et al., 2004). Because Aurora B is a negative regulator of MCAK, one obvious idea is that ZM blocks spindle assembly in egg extracts by inhibiting Aurora B kinase activity, thus generating an increase in MCAK activity and an increase microtubule destabilization around chromosomes.
ZM and the Spindle Integrity Checkpoint
In human DLD-1 cells, the addition of ZM interferes with the spindle integrity checkpoint. Mechanistically, ZM treatment reduces the kinetochore localization of the checkpoint proteins Mad2, BubRI and CENP-E, suggesting that Aurora kinase activity is needed for their recruitment to kinetochores (Ditchfield et al., 2003). It is likely that ZM's effect on recruitment of those proteins is due mainly to inhibition of Aurora B, which localizes close to the kinetochores and is thus in the right position to affect this process. RNAi-mediated ablation of Aurora B blocks HeLa cells from arresting in response to taxol and nocodazole, agents that prevent spindle tension and microtubule-kinetochore attachments, respectively (Ditchfield et al., 2003). Similar results were obtained using the Aurora inhibitor hesperadin (Hauf et al., 2003). Our work extends those findings: in eggs, where the dominant microtubule nucleating activity comes from chromatin, ZM clearly blocks establishment of the spindle integrity checkpoint. Furthermore, excess Mad2 overrides the ability of ZM to inhibit establishment of the checkpoint, arguing that Aurora activity is required upstream of Mad2. Mad2 protein regulates checkpoint activation and maintenance by binding to cdc20 and preventing it from activating the APC/C and initiating anaphase onset. The fact that Mad2 still arrests extracts in mitosis in the presence of ZM, suggests that Aurora activity does not regulate checkpoint activation by directly inhibiting cdc20. Although the precise target of Aurora B is not known, one obvious candidate is BubRI, because ZM prevents its mitotic phosphorylation and reduces its kinetochore localization (Ditchfield et al., 2003). With the identification of additional targets of the Aurora kinases and the development of phospho-specific antibodies against regulatory sites phosphorylated by the Aurora kinases, it should be possible to make further headway into the roles of these kinases in the cell cycle, in development, and in cancer.
Acknowledgments
We are extremely grateful to Nicholas Keen and AstraZeneca Pharmaceuticals for providing ZM447439. We thank Jim Maller (University of Colorado) for cyclin B1 antibody, Randall King (Harvard Medical School) for recombinant MBP-cyclin B Δ90 protein, Aaron Groen (Mitchison Lab, Harvard Med-ical School) for DNA-coated beads and recombinant RanQ69L, and Aaron Straight (Stanford University) for recombinant Mad2 protein. We are grateful to Puck Ohi (Mitchison Lab, Harvard Medical School, Boston, MA) for helpful discussions and advice, Quentin Liu and Jennifer Stanford (Ruderman lab, Harvard Medical School) for interesting discussions and help with cycling extracts, and Jennifer Waters Schuler and Lara J. Petrakin in the Harvard Medical School Nikon Imaging Center. All the members of the Ruderman lab provided enthusiastic interest and feedback. B.B.G. was supported by National Cancer Institute Training grant T32CA09361. This work was funded by National Institutes of Health grant HD23696 to J.V.R. The authors declare that they have no competing financial interests.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-10-0891) on December 22, 2004.
References
- Adams, R.R., Maiato, H., Earnshaw, W.C., and Carmena, M. (2001). Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell Biol. 153, 865-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand, S., Penrhyn-Lowe, S., and Venkitaraman, A. R. (2003). AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell 3, 51-62. [DOI] [PubMed] [Google Scholar]
- Anderson, N. G., Maller, J. L., Tonks, N. K., and Sturgill, T. W. (1990). Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature 343, 651-653. [DOI] [PubMed] [Google Scholar]
- Andresson, T., and Ruderman, J. V. (1998). The kinase Eg2 is a component of the Xenopus oocyte progesterone-activated signaling pathway. EMBO J. 17, 5627-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews, P. D., Knatko, E., Moore, W. J., and Swedlow, J. R. (2003). Mitotic mechanics: the auroras come into view. Curr. Opin. Cell Biol. 15, 672-683. [DOI] [PubMed] [Google Scholar]
- Andrews, P. D., Ovechkina, Y., Morrice, N., Wagenbach, M., Duncan, K., Wordeman, L., and Swedlow, J. R. (2004). Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 6, 253-268. [DOI] [PubMed] [Google Scholar]
- Arnaoutov, A., and Dasso, M. (2003). The Ran GTPase regulates kinetochore function. Dev. Cell 5, 99-111. [DOI] [PubMed] [Google Scholar]
- Bhatt, R. R., and Ferrell, J. E., Jr. (1999). The protein kinase p90 rsk as an essential mediator of cytostatic factor activity. Science 286, 1362-1365. [DOI] [PubMed] [Google Scholar]
- Bischoff, J. R. et al. (1998). A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 17, 3052-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde, P. P., Kumagai, A., Dunphy, W. G., and Heald, R. (2001). Regulation of Op18 during spindle assembly in Xenopus egg extracts. J. Cell Biol. 153, 149-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carazo-Salas, R. E., Guarguaglini, G., Gruss, O. J., Segref, A., Karsenti, E., and Mattaj, I. W. (1999). Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature 400, 178-181. [DOI] [PubMed] [Google Scholar]
- Chen, R. H., Shevchenko, A., Mann, M., and Murray, A. W. (1998). Spindle checkpoint protein Xmad1 recruits Xmad2 to unattached kinetochores. J. Cell Biol. 143, 283-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane, R., Gadea, B., Littlepage, L., Wu, H., and Ruderman, J. V. (2004). Aurora A, meiosis and mitosis. Biol. Cell 96, 215-229. [DOI] [PubMed] [Google Scholar]
- Dasso, M., and Newport, J. W. (1990). Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell 61, 811-823. [DOI] [PubMed] [Google Scholar]
- Desai, A., Murray, A., Mitchison, T. J., and Walczak, C. E. (1999). The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 61, 385-412. [DOI] [PubMed] [Google Scholar]
- Descombes, P., and Nigg, E. A. (1998). The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO J. 17, 1328-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchfield, C., Johnson, V. L., Tighe, A., Ellston, R., Haworth, C., Johnson, T., Mortlock, A., Keen, N., and Taylor, S. S. (2003). Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161, 267-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth, B. C., Weaver, J. S., and Ruderman, J. V. (2002). G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc. Natl. Acad. Sci. USA 99, 16794-16799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, G., Yu, H., and Kirschner, M. W. (1998). The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 12, 1871-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesquet, D., Labbe, J. C., Derancourt, J., Capony, J. P., Galas, S., Girard, F., Lorca, T., Shuttleworth, J., Doree, M., and Cavadore, J.C. (1993). The MO15 gene encodes the catalytic subunit of a protein kinase that activates cdc2 and other cyclin-dependent kinases (CDKs) through phosphorylation of Thr161 and its homologues. EMBO J. 12, 3111-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki, H., and Murray, A. W. (2000). The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell 102, 411-424. [DOI] [PubMed] [Google Scholar]
- Giet, R., and Glover, D. M. (2001). Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 152, 669-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet, R., McLean, D., Descamps, S., Lee, M. J., Raff, J. W., Prigent, C., and Glover, D. M. (2002). Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J. Cell Biol. 156, 437-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet, R., and Prigent, C. (2000). The Xenopus laevis aurora/Ip11p-related kinase pEg2 participates in the stability of the bipolar mitotic spindle. Exp. Cell Res. 258, 145-151. [DOI] [PubMed] [Google Scholar]
- Glover, D. M., Leibowitz, M. H., McLean, D. A., and Parry, H. (1995). Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell 81, 95-105. [DOI] [PubMed] [Google Scholar]
- Gross, S. D., Schwab, M. S., Taieb, F. E., Lewellyn, A. L., Qian, Y. W., and Maller, J. L. (2000). The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90(Rsk). Curr. Biol. 10, 430-438. [DOI] [PubMed] [Google Scholar]
- Guadagno, T. M., and Ferrell, J. E., Jr. (1998). Requirement for MAPK activation for normal mitotic progression in Xenopus egg extracts. Science 282, 1312-1315. [DOI] [PubMed] [Google Scholar]
- Hagstrom, K. A., Holmes, V. F., Cozzarelli, N. R., and Meyer, B. J. (2002). C. elegans condensin promotes mitotic chromosome architecture, centromere organization, and sister chromatid segregation during mitosis and meiosis. Genes Dev. 16, 729-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannak, E., Kirkham, M., Hyman, A. A., and Oegema, K. (2001). Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J. Cell Biol. 155, 1109-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans, F., and Dimitrov, S. (2001). Histone H3 phosphorylation and cell division. Oncogene 20, 3021-3027. [DOI] [PubMed] [Google Scholar]
- Harrington, E. A. et al. (2004). VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat. Med. 10, 262-267. [DOI] [PubMed] [Google Scholar]
- Hauf, S. et al. (2003). The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 161, 281-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald, R., Tournebize, R., Blank, T., Sandaltzopoulos, R., Becker, P., Hyman, A., and Karsenti, E. (1996). Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382, 420-425. [DOI] [PubMed] [Google Scholar]
- Hershko, A., Ganoth, D., Sudakin, V., Dahan, A., Cohen, L. H., Luca, F. C., Ruderman, J. V., and Eytan, E. (1994). Components of a system that ligates cyclin to ubiquitin and their regulation by the protein kinase cdc2. J. Biol. Chem. 269, 4940-4946. [PubMed] [Google Scholar]
- Hirota, T., Kunitoku, N., Sasayama, T., Marumoto, T., Zhang, D., Nitta, M., Hatakeyama, K., and Saya, H. (2003). Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell 114, 585-598. [DOI] [PubMed] [Google Scholar]
- Hsu, J. Y. et al. (2000). Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell 102, 279-291. [DOI] [PubMed] [Google Scholar]
- Izumi, T., and Maller, J. L. (1993). Elimination of cdc2 phosphorylation sites in the cdc25 phosphatase blocks initiation of M-phase. Mol. Biol. Cell 4, 1337-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio, M., Weinstein, J., Daum, J. R., Burke, D. J., and Gorbsky, G. J. (1998). Mammalian p55CDC mediates association of the spindle checkpoint protein Mad2 with the cyclosome/anaphase-promoting complex, and is involved in regulating anaphase onset and late mitotic events. J. Cell Biol. 141, 1393-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio, M. J., McCleland, M. L., Stukenberg, P. T., and Gorbsky, G. J. (2002). Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr. Biol. 12, 900-905. [DOI] [PubMed] [Google Scholar]
- Karsenti, E., Newport, J., and Kirschner, M. (1984). Respective roles of centrosomes and chromatin in the conversion of microtubule arrays from interphase to metaphase. J. Cell Biol. 99, 47s-54s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenti, E., and Vernos, I. (2001). The mitotic spindle: a self-made machine. Science 294, 543-547. [DOI] [PubMed] [Google Scholar]
- Katayama, H., Brinkley, W. R., and Sen, S. (2003). The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev. 22, 451-464. [DOI] [PubMed] [Google Scholar]
- Kotani, S., Tugendreich, S., Fujii, M., Jorgensen, P. M., Watanabe, N., Hoog, C., Hieter, P., and Todokoro, K. (1998). PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol. Cell 1, 371-380. [DOI] [PubMed] [Google Scholar]
- Kufer, T. A., Sillje, H. H., Korner, R., Gruss, O. J., Meraldi, P., and Nigg, E. A. (2002). Human TPX2 is required for targeting Aurora-A kinase to the spindle. J. Cell Biol. 158, 617-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai, A., and Dunphy, W. G. (1992). Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell 70, 139-151. [DOI] [PubMed] [Google Scholar]
- Kumagai, A., Yakowec, P. S., and Dunphy, W. G. (1998). 14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol. Biol. Cell 9, 345-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav-Baratz, S., Sudakin, V., Ruderman, J. V., and Hershko, A. (1995). Reversible phosphorylation controls the activity of cyclosome-associated cyclin-ubiquitin ligase. Proc. Natl. Acad. Sci. USA 92, 9303-9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson, M. A., Renduchitala, K., Khodjakov, A., and Kapoor, T. M. (2004). Correcting improper chromosome-spindle attachments during cell division. Nat. Cell Biol. 6, 232-237. [DOI] [PubMed] [Google Scholar]
- Lan, W., Zhang, X., Kline-Smith, S. L., Rosasco, S. E., Barrett-Wilt, G. A., Shabanowitz, J., Hunt, D. F., Walczak, C. E., and Stukenberg, P. T. (2004). Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 14, 273-286. [DOI] [PubMed] [Google Scholar]
- Lavoie, B. D., Hogan, E., and Koshland, D. (2004). In vivo requirements for rDNA chromosome condensation reveal two cell-cycle-regulated pathways for mitotic chromosome folding. Genes Dev. 18, 76-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Gorbea, C., Mahaffey, D., Rechsteiner, M., and Benezra, R. (1997). MAD2 associates with the cyclosome/anaphase-promoting complex and inhibits its activity. Proc. Natl. Acad. Sci. USA 94, 12431-12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlepage, L. E., and Ruderman, J. V. (2002). Identification of a new APC/C recognition domain, the A box, which is required for the Cdh1-dependent destruction of the kinase Aurora-A during mitotic exit. Genes Dev. 16, 2274-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlepage, L. E., Wu, H., Andresson, T., Deanehan, J. K., Amundadottir, L. T., and Ruderman, J. V. (2002). Identification of phosphorylated residues that affect the activity of the mitotic kinase Aurora-A. Proc. Natl. Acad. Sci. USA 99, 15440-15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumoto, T., Honda, S., Hara, T., Nitta, M., Hirota, T., Kohmura, E., and Saya, H. (2003). Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J. Biol. Chem. 278, 51786-51795. [DOI] [PubMed] [Google Scholar]
- McGowan, C. H., and Russell, P. (1993). Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. EMBO J. 12, 75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi, P., Honda, R., and Nigg, E. A. (2002). Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53(-/-) cells. EMBO J. 21, 483-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi, P., Honda, R., and Nigg, E. A. (2004). Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr. Opin. Genet. Dev. 14, 29-36. [DOI] [PubMed] [Google Scholar]
- Minshull, J., Sun, H., Tonks, N. K., and Murray, A. W. (1994). A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell 79, 475-486. [DOI] [PubMed] [Google Scholar]
- Murray, A. W. (1991). Cell cycle extracts. Methods Cell Biol. 36, 581-605. [PubMed] [Google Scholar]
- Ohi, R., Sapra, T., Howard, J., and Mitchison, T. J. (2004). Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol. Biol. Cell 15, 2895-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, L. L., Atherton-Fessler, S., Lee, M. S., Ogg, S., Falk, J. L., Swenson, K. I., and Piwnica-Worms, H. (1991). Cyclin promotes the tyrosine phosphorylation of p34cdc2 in a wee1+ dependent manner. EMBO J. 10, 1255-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, L. L., Atherton-Fessler, S., and Piwnica-Worms, H. (1992). p107wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc. Natl. Acad. Sci. USA 89, 2917-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, L. L., and Piwnica-Worms, H. (1992). Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science 257, 1955-1957. [DOI] [PubMed] [Google Scholar]
- Peters, J. M., King, R. W., Hoog, C., and Kirschner, M. W. (1996). Identification of BIME as a subunit of the anaphase-promoting complex. Science 274, 1199-1201. [DOI] [PubMed] [Google Scholar]
- Poon, R. Y., Yamashita, K., Adamczewski, J. P., Hunt, T., and Shuttleworth, J. (1993). The cdc2-related protein p40MO15 is the catalytic subunit of a protein kinase that can activate p33cdk2 and p34cdc2. EMBO J. 12, 3123-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, Y. W., Erikson, E., Li, C., and Maller, J. L. (1998). Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol. Cell. Biol. 18, 4262-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, Y. W., Erikson, E., Taieb, F. E., and Maller, J. L. (2001). The polo-like kinase Plx1 is required for activation of the phosphatase Cdc25C and cyclin B-Cdc2 in Xenopus oocytes. Mol. Biol. Cell 12, 1791-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath, S. C., Ohi, R., Leismann, O., Salic, A., Pozniakovski, A., and Funabiki, H. (2004). The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell 118, 187-202. [DOI] [PubMed] [Google Scholar]
- Sawin, K. E., and Mitchison, T. J. (1991). Mitotic spindle assembly by two different pathways in vitro. J. Cell Biol. 112, 925-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, J. M., Ashcroft, N., Donovan, P. J., and Golden, A. (1998a). A highly conserved centrosomal kinase, AIR-1, is required for accurate cell cycle progression and segregation of developmental factors in Caenorhabditis elegans embryos. Development 125, 4391-4402. [DOI] [PubMed] [Google Scholar]
- Schumacher, J. M., Golden, A., and Donovan, P. J. (1998b). AIR-2, an Aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos. J. Cell Biol. 143, 1635-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon, M. J., Harper, J. W., and Shuttleworth, J. (1993). CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 12, 3133-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speliotes, E. K., Uren, A., Vaux, D., and Horvitz, H. R. (2000). The survivin-like C. elegans BIR-1 protein acts with the Aurora-like kinase AIR-2 to affect chromosomes and the spindle midzone. Mol. Cell 6, 211-223. [DOI] [PubMed] [Google Scholar]
- Sumara, I., Vorlaufer, E., Stukenberg, P. T., Kelm, O., Redemann, N., Nigg, E. A., and Peters, J. M. (2002). The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol. Cell 9, 515-525. [DOI] [PubMed] [Google Scholar]
- Taieb, F. E., Gross, S. D., Lewellyn, A. L., and Maller, J. L. (2001). Activation of the anaphase-promoting complex and degradation of cyclin B is not required for progression from Meiosis I to II in Xenopus oocytes. Curr. Biol. 11, 508-513. [DOI] [PubMed] [Google Scholar]
- Takenaka, K., Gotoh, Y., and Nishida, E. (1997). MAP kinase is required for the spindle assembly checkpoint but is dispensable for the normal M phase entry and exit in Xenopus egg cell cycle extracts. J. Cell Biol. 136, 1091-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournebize, R., Popov, A., Kinoshita, K., Ashford, A. J., Rybina, S., Pozniakovsky, A., Mayer, T. U., Walczak, C. E., Karsenti, E., and Hyman, A. A. (2000). Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Nat. Cell Biol. 2, 13-19. [DOI] [PubMed] [Google Scholar]
- Walczak, C. E., Mitchison, T.J., and Desai, A. (1996). XKCM1, a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell 84, 37-47. [DOI] [PubMed] [Google Scholar]
- Yamada, H., Kumada, K., and Yanagida, M. (1997). Distinct subunit functions and cell cycle regulated phosphorylation of 20S APC/cyclosome required for anaphase in fission yeast. J. Cell Sci. 110(Pt 15), 1793-1804. [DOI] [PubMed] [Google Scholar]
- Zhou, H., Kuang, J., Zhong, L., Kuo, W.L., Gray, J. W., Sahin, A., Brinkley, B. R., and Sen, S. (1998). Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat. Genet. 20, 189-193. [DOI] [PubMed] [Google Scholar]