Abstract
Versican is a large chondroitin sulfate proteoglycan belonging to the lectican family. Alternative splicing of versican generates at least four isoforms named V0, V1, V2, and V3. We have shown that the versican V1 isoform not only enhanced cell proliferation, but also modulated cell cycle progression and protected the cells from apoptosis. Futhermore, the V1 isoform was able to not only activate proto-oncogene EGFR expression and modulate its downstream signaling pathway, but also induce p27 degradation and enhance CDK2 kinase activity. As well, the V1 isoform down-regulated the expression of the proapoptotic protein Bad. By contrast, the V2 isoform exhibited opposite biological activities by inhibiting cell proliferation and down-regulated the expression of EGFR and cyclin A. Furthermore, V2 did not contribute apoptotic resistance to the cells. In light of these results, we are reporting opposite functions for the two versican isoforms whose expression is differentially regulated. Our studies suggest that the roles of these two isoforms are associated with the subdomains CSβ and CSα, respectively. These results were confirmed by silencing the expression of versican V1 with small interfering RNA (siRNA), which abolished V1-enhanced cell proliferation and V1-induced reduction of apoptosis.
INTRODUCTION
The extracellular matrix (ECM) not only acts as a physical framework, but also exerts a profound effect on cell shape and behavior, including cell adhesion, spreading, migration, proliferation, and differentiation (Ingber and Folkman, 1989). The major components of the ECM are proteoglycans, glycoproteins, and collagens. Versican is a member of the large aggregating chondroitin sulfate proteoglycan family. It is distributed in a wide variety of tissues during development and is also associated with tissue injury (Landolt et al., 1995). Our data and those of others have shown that versican plays a role in cell adhesion, migration, proliferation, and differentiation (Landolt et al., 1995; Henderson et al., 1997; Zhang et al., 1998a, 1998b; Ang et al., 1999; Cattaruzza et al., 2002, 2004).
Structurally, versican possesses two globular domains, the N-terminal G1 domain and the C-terminal G3 domain, as well as a chondroitin sulfate (CS) attachment sequence. The CS sequence is divided into two alternatively spliced domains, termed CSα and CSβ. As a result of the splicing, four isoforms may be generated, namely V0, V1, V2, and V3 (Ito et al., 1995; Lemire et al., 1999). V0 contains both CSα and CSβ; V1 and V2 possess only CSβ and CSα, respectively; and V3 contains neither CSα nor CSβ. The G3 domain is composed of two epidermal growth factor (EGF)-like repeats, a lectin-like motif, and a complement binding protein-like motif (Shinomura et al., 1993).
Although the versican V1/V0 isoforms are mainly expressed in the late stages of embryonic development (Landolt et al., 1995), versican V2 is a major chondroitin sulfate proteoglycan in the mature brain (Schmalfeldt et al., 1998). The different expression patterns suggest that versican isoforms may play different functions. Indeed, versican V2 inhibits axon and neurite growth (Schmalfeldt et al., 2000), whereas versican V1 promotes neurite outgrowth (Wu et al., 2004b). These distinct functions may be a result of the different CS domain. Recently, we have successfully cloned full-length chicken versican isoforms V1 and V2 and have studied their physiological functions after transfection in NIH3T3 cells. In this article we report that the V1 isoform is able to enhance cell proliferation. Moreover, this versican isoform contributes apoptotic resistance in fibroblasts. In contrast, the V2 isoform inhibits cell proliferation and has no effect on cell apoptosis.
MATERIALS AND METHODS
Materials
DMEM, fetal bovine serum (FBS), Hanks' balanced salt solution (HBSS), trypsin/EDTA, Lipofectamine, and Geneticin (G418) were purchased from Invitrogen (Burlington, Ontario, Canada). Tissue culture plates were purchased from Nunc. NIH3T3 fibroblasts were from the American Type Culture Collection (Rockville, MD). The ECL Western blot detection kit was from Amersham Life Science (Baie d'Urf'e, Quebec, Canada). Monoclonal antibodies against horseradish peroxidase–conjugated goat anti-mouse IgG secondary antibody, oligonucleotides, GW8510, HA14–1, C2-ceramide, and all chemicals were purchased from Sigma (St. Louis, MO). Antibodies used are as follows: anti-ERK (D-2), antiphospho-Erk (p44/p42; Tyr204) (E-4), anti-EGFR (1005), anticyclin E (M-20), anticyclin D (R-124), anticyclin A (H-432), anti-CDK2 (D-12), anti-CDK4 (C-22) and antiphospho-p27 (Thr187) (all from Santa Cruz Biotechnology, Santa Cruz, CA), anti-p27 (Transduction Laboratories, Lexington, KY), antiubiquitin (Zymed Laboratories, Markham, Ontario, Canada), and anti-Bad (New England Biolabs, Pickering, Ontario, Canada).
Construct Generation and Expression
To study the effect of versican on mediating cell activities, we used four constructs: versican V1 and V1 mutant (V1mut), and versican V2 and V2 mutant (V2mut). Both mutants contain deletions in their CS domains. V2mut (also called mini-versican) was constructed and used by this laboratory previously (Zhang et al., 1998b; Yang et al., 2000). To generate the versican V2 construct, the CS fragment in mini-versican was replaced with the full-length CSα domain, which was generated by PCR using two primers, pCS1NMluI (aaa acg cgt cgt aaa aaa att gta tca gag cct aca) and pCS1CXhoI (ggg ctc gag tgt atc att gcc tgt gat), and chicken genomic DNA as the template. The PCR product was digested with restriction endonucleases MluI and XhoI. The PCR product, combined with EcoRI-MluI–digested G1 domain (from the mini-versican), was inserted into an EcoRI-XhoI–digested G3 domain–containing vector (pcDNA3, obtained from the mini-versican construct) to generate the V2 construct.
Generation of the V1 construct was performed as follows: The G1 domain was amplified with two primers, LPNKozEcoRI (aaa gaa ttc gcc gcc acc atg gca agt) and pG1CSalI (aaa gtc gac ttc gta gca gta ggc atc), using mini-versican as a template. Because the CSβ domain is 5.2 kb, we generated this domain by PCR as four smaller fragments, namely Fragments A, B, C, and D, using the genomic DNA as template. The primers for Fragment A were pCS2NSalI (ccc gtc gac gat cat ccg gtg aat gag ttt) and pCS2BstEIIR (aag ctt ctc tct caa gaa agg agt ggc); for Fragment B, pCS2BstEIIF (ggt acc atg cat tct gaa atc aaa aag) and pCS2MRSphI (atg aac cac agc gga tgg cat gct gac); for Fragment C, pCS2MFSphI (ggc aga aaa act gtc agc atg cca tcc) and pCS2 EcoRI R (cca gat cca aat gaa ttc tct tca act gta g); and for Fragment D, pCS2 EcoRI F (ata ctt aat ttt tct aca gtt gaa gag) and pCS2C XhoI (ccc ctc gag aat ttg cac tgc tgt gcc ttc aac cga acc). The PCR products were digested with appropriate restriction enzymes. Fragments were then cloned into Bluescript plasmid that was predigested with the appropriate restriction enzymes. The G3 domain was obtained from mini-versican by digestion with XhoI and ApaI. Fragments A, B, and C were linked together and cloned into SalI-EcoRI–digested Bluescript to yield Plasmid ABC. Because the 5′ region of Fragment B also contains a restriction site for XbaI, Fragment BC was obtained by digestion with XbaI and EcoRI. Fragment BC was linked together with EcoRI-XhoI–digested Fragment D and XhoI-ApaI–digested G3 domain and cloned into XbaI-ApaI–digested Bluescript to produce Plasmid BCDG3. Finally, the G1 domain was obtained by digestion with EcoRI and SalI. Fragment A and half of Fragment B (from the 5′ end to an NcoI site in the middle of Fragment B) was generated by digestion with SalI and NcoI. Half of Fragment B, and Fragments C and D, and the G3 domain were obtained by digestion of Plasmid BCDG3 with NcoI and ApaI. These fragments were linked together and cloned into pcR3.1 plasmid to obtain the versican V1 construct (Figure 1A).
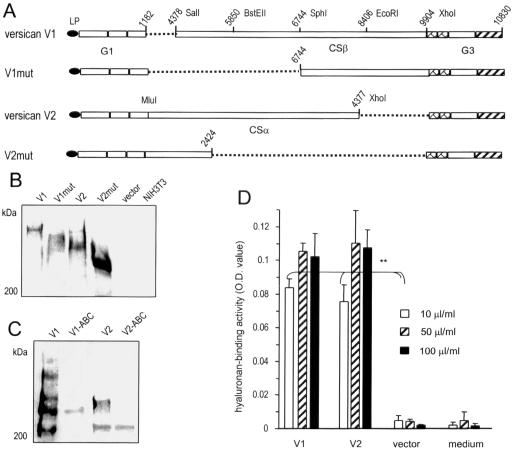
Figure 1.
Generation and expression of versican constructs. (A) Full-length versican V1 and V2 isoforms and two mutants (V1mut and V2mut; derived from V1 and V2 isoforms, respectively) were generated as shown in the figure. (B) Stable expression of V1, V2, V1mut, and V2mut in NIH3T3 fibroblasts was assessed by Western blot probed with the monoclonal antibody 4B6 that recognizes an epitope on the leading peptide (LP) of each construct. (C) The products of V1 and V2 were digested with chondroitinase ABC followed by Western blot analysis to confirm the removal of GAG chains from the product. (D) To test the binding activity of the secreted proteoglycan, hyaluronan, which binds the G1 domain of versican, was coated on ELISA plates at different concentrations followed by incubation with fresh medium or culture medium collected from cells transfected with V1, V2, or the control vector. Bound products were detected with a polyclonal antibody that recognizes the G3 domain of versican. The V1 and V2 products exhibited hyaluronan-binding activity (n = 3, **p < 0.01).
To generate V1mut, the G1 domain containing a small sequence of Fragment A was amplified with primers LPNKozEcoRI and verCS2AccSphI (ccc ggg cat gct tat gaa ggt gtt tgt ttc), with the V1 construct as template. The PCR product was digested with EcoRI and SphI. Plasmid-BCDG3 was digested with SphI and XhoI to yield Fragment CD. The G1 domain and Fragment CD were linked together and inserted into G3-containing pcR3.1 vector, which was derived from the V1 construct by digestion with EcoRI and XhoI, to obtain the V1mut construct.
These constructs were confirmed by restriction digestion and sequencing. They were also expressed in COS-7 cells, and stably expressed in NIH3T3 fibroblasts using Lipofectamine according to the manufacturer's instructions. Gene expression was confirmed by Western blotting.
To generate a small interfering RNA, a sequence (nucleotides 5337–5355, gcc tga cat gac tgc ttc t of chicken versican) was inserted into the pSuper plasmid according to the manufacturer's instructions. After DNA sequencing, a construct containing the correct insert, named siRNA-ver5337, was obtained. To examine the efficiency of this siRNA, a recombinant construct harboring this siRNA target sequence was generated. This was performed by PCR using two primers pCS2N XhoI (ggg ctc gag gat cat ccg gtg aat gag) and pCSβaCApaI (5′ ttt ggg ccc tct caa gaa agg agt ggc) to obtain Fragment A. The PCR product was doubly digested with XhoI and ApaI and ligated together with the fragment encoding the leading peptide (LP) in pcR3.1, producing the recombinant construct CSA. The silencing efficiency of siRNA-ver5337 was performed by cotransfection of COS-7 cells with CSA and pSuper (control) or CSA and siRNA-ver5337. Cell lysate was prepared and analyzed by Western blot.
Product Analysis
To confirm the modification of glycosaminoglycan (GAG) chains to the V1 and V2 core proteins, the products were purified by anion-exchange chromatography followed by chondroitinase treatment. Briefly, the cell-associated V1- and V2-containing fractions were mixed with Q-Sepharose (Amersham Pharmacia Biotech, Baie d'Urf'e, Quebec, Canada) preequilibrated with a buffer containing 50 mM sodium acetate, pH 6.0, 4 M Urea, 0.2 M NaCl, and 0.5% Triton X-100. The mixture was packed into columns, followed by extensive washes. The proteoglycans were eluted stepwise using the same buffer with increasing ionic strength. Eluted proteins were concentrated using Amicon Ultra Centrifugal Filters (Fisher Scientific, Ottawa, Ontario, Canada). Proteoglycan-enriched fractions (10 μg) were digested with 0.02 U of protease-free chondroitinase ABC (Sigma) at 37°C for 2 h in a buffer containing 50 mM Tris-Cl, pH 8.0, 30 mM sodium acetate, 20 mM EDTA, 10 mM NEM, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), and 0.02 mM sodium azide. Proteoglycans with or without the treatment were subjected to Western blot analysis.
Western blotting of proteins was performed as previously described (Chen et al., 2003; Zheng et al., 2004a). RT-PCR was carried out using the same method routinely used in our laboratory (Zheng et al., 2004b).
Cell Proliferation and Flow Cytometry
NIH3T3 fibroblasts transfected with versican constructs or a control vector, as well as parental cells, were seeded on 100-mm tissue culture dishes at a density of 5 × 105 cells/dish in DMEM containing 10% FBS and maintained at 37°C for 4 d. Cells were harvested daily, and the cell number was assessed with a Coulter counter. For cell cycle analysis, cells were pulse labeled with 10 μM/L bromodeoxyuridine (BrdU) and processed for flow cytometry analysis.
Cell Survival and Apoptotic Assays
To study cell survival, 5 × 105 cells were cultured in 100-mm Petri dishes for 24 h in DMEM containing 10% FBS. The medium was then replaced with serum-free DMEM. The medium was changed every 4 d for 16 d. The number of viable cells was assessed every 4 d using trypan blue exclusion. Alternatively, cells (2 × 105 cells/well) were cultured in 6-well plates. The cultures were also subjected to Annexin V-FITC staining (15 min in the dark) using an Annexin V Apoptosis Detection Kit (from Santa Cruz Biotechnology, Santa Cruz, CA) to monitor apoptosis as done previously (Wu et al., 2004a). For experiments using the Bcl-2 inhibitor HA14–1 and C2-ceramide, another apoptotic inducer, cells were incubated with growth media containing 25 μM HA14–1 or a combination of 7 or 10 μM HA14–1 and 100 μM C2-ceramide. Two hours later, detached and adherent cells were harvested and pooled. The pooled cells were subjected to Annexin V staining according to the manufacturer's instructions, followed by flow cytometry analysis.
Pulse-Chase Experiments
To examine p27 degradation, transfected cells were serum starved for 24 h and pulse labeled for 4 h with 150 μCi/ml [35S]methionine. The cells were then chased for the times indicated in DMEM containing 10% FBS and excess methionine. After washing with phosphate-buffered saline (PBS), cell lysates were prepared and subjected to immunoprecipitation with anti-p27 antibody. Immune complexes were washed extensively and the precipitated proteins were analyzed by SDS-PAGE on 15% acrylamide gels. The protein levels of p27 were detected by fluorography.
Immunoprecipitation
Cell lysate was prepared in lysis buffer (20 mM Tris-Cl, pH 7.4, 150 mM NaCl, 2 mM PMSF, 10 μg/ml leupeptin, 2 mM EDTA, 5 μg/ml bovine serum albumin (BSA), and 1.5% Triton X-100) followed by centrifugation at high speed (12000 × g for 20 min) to recover proteins in supernatant. Protein G beads were saturated with primary antibodies and blocked with 5% BSA. Supernatant was added to these beads and incubated at 4°C overnight. After washing, the beads were boiled in 1× protein loading dye for 5 min and analyzed on Western blot probed with the appropriate antibodies.
Hyaluronan Binding Assay
Maxisorp 96-well microtiter plates were coated with human umbilical cord hyaluronan at 10, 50, and 100 μg/ml in 200 mM sodium carbonate buffer, pH 9.6, at 4°C overnight. The plates were washed with 0.5% Tween-20 in PBS and blocked with 1% BSA. Versican-transfected cells were transferred into fresh medium and incubated for 20–24 h. Overnight culture media was harvested and centrifuged to remove cellular material or particulates. Cell culture supernatant was applied to the plates and incubated at room temperature for 1 h. After washing with 0.5% Tween-20, rabbit polyclonal antibody against chicken versican G3 domain, which was prepared by this laboratory, was added to the wells, and incubated for 1 h, after which the wells were washed with 0.5% Tween-20. Biotinylated secondary antibody (anti-rabbit) was added to the wells and incubated for 1 h, and the wells were washed again. The plates were then incubated with peroxidase-conjugated streptavidin (Sigma) for 1 h and washed. Color was developed using substrate-chromogen solution (Zymed Laboratories). The reaction was stopped with 2 N H2SO4 and absorbance was read at 630 nm.
Phosphatase Treatment
Cell lysate was immunoprecipitated with anti-p27 antibody. The precipitates were washed twice with phosphatase buffer (50 mM Tris, pH 8.0, and 10% glycerol) and were then incubated in the presence or absence of 10 U per 10-μl reaction of calf intestinal alkaline phosphatase (Roche, Laval, Quebec, Canada) at 37°C for 3 h. After removal of the incubation buffer, samples were processed for Western blot probed with anti-p27 antibody.
Cyclin E1-associated Kinase Assay
Cyclin E1–associated kinase assay was performed using anticyclin E antibody E172 (a gift from E. Harlow, Massachusetts General Hospital, Boston) for immunoprecipitation (Slingerland et al., 1994) and histone H1 as the substrate as described (Sandhu et al., 1997). For the control, a nonspecific mouse immunoglobulin (Ig) G (IgG) was used for immunoprecipitation.
RESULTS
Versican V1 Isoform Enhances whereas V2 Inhibits Cell Proliferation
After versican isoforms V1 and V2 and their mutants V1mut and V2mut (for constructs see Figure 1A) were stably expressed in NIH3T3 fibroblasts, at least 15 cell lines were selected for protein expression and functional analysis. Expression of all isoforms and mutants was detected using the antibody 4B6, which recognizes an epitope in the leading peptide engineered into the N-terminus of each construct (Figure 1B). The proteoglycan mass of the V1 isoform was around 900 kDa, whereas that of the V2 isoform was ∼600 kDa. The expression levels of V1 and V1mut were much lower than V2 and V2mut, perhaps because of insufficient GAG modification, because GAG modification is essential for product synthesis and secretion (Yang et al., 2000; Kiani et al., 2003), or/and proteolytic activity, because cleavage in the CSβ domain has been reported (Sandy et al., 2001). It should be noted that smaller fragments were detected after longer period of exposure (unpublished data). When the products of V1 and V2 were treated with chondroitinase ABC (Zhang et al., 1998b), smaller fragment of products, presumably the core proteins, was detected (Figure 1C). To ensure that the intact proteoglycans (V1 and V2) were active in the culture medium, hyaluronan-binding assays were performed, as the native G1 domain of versican binds hyaluronan. Detection was further confirmed by the polyclonal antibody against the G3 domain of versican (Figure 1D). Furthermore in an immunoprecipitation assay, both V1 and V2 were shown to interact with β1-integrin (unpublished data), suggesting the presence of a native G3 domain, as we have previously demonstrated that the native G3 domain interacts with β1-integrin (Wu et al., 2002).
We observed a remarkable alteration in cell growth in V1-transfected cells: by day 4, a significant increase in cell numbers had occurred, suggesting enhanced cell proliferation. In the V1mut-transfected cells, no significant increase was observed (Figure 2A). In contrast, V2 expression exerted an inhibitory effect on cell growth, whereas V2mut (mini-versican) expression reversed V2's effect by enhancing cell proliferation, consistent with our previous data (Zhang et al., 1998b). Analysis with flow cytometry revealed that V1-transfected cell lines had the highest proportion of cells detected in S phase (Figure 2B), whereas V1mut-transfected cells displayed a cell cycle profile similar to that of the controls. The V2-transfected population had the lowest proportion of cells detected in S phase. The V2mut-transfected cells showed a higher percentage in S phase than control cell lines. Based on the differences in structure between versican isoforms V1 and V2, our results indicate that the CSβ domain of the V1 isoform is likely involved in cell proliferation enhancement and that a full-length CSα domain is probably responsible for the inhibitory effect on cell proliferation.
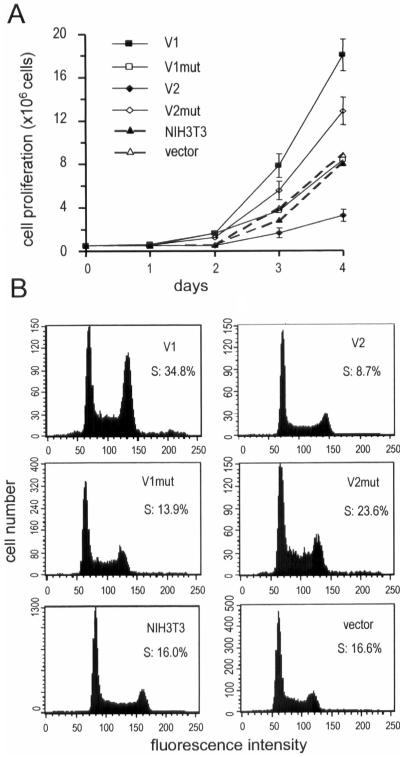
Figure 2.
The versican V1 isoform promotes cell proliferation and isoform V2 inhibits cell proliferation. (A) To measure cell proliferation, 5 × 105 cells were seeded in DMEM containing 10% FBS. Cell numbers were counted by trypan blue staining at the indicated time points. Each point represents the mean of values obtained from three distinct subclones compared with vector-transfected and wild-type NIH3T3 fibroblasts. Isoform V1 and V2mut enhanced cell proliferation; isoform V2 inhibited cell proliferation; V1mut and vector transfection had no effect on cell proliferation compared with the parental NIH3T3 cells. (B) Cell lines expressing V1, V2, V1mut, V2mut, and vector-transfected cells, as well as wild-type NIH3T3 cells, were cultured in DMEM containing 10% FBS to subconfluence. Cell cycle progression was analyzed by flow cytometry. V1 expression facilitated the highest proportion of cell entry into S phase, whereas V2-expressing cells had the lowest proportion of cells detected in S phase compared with the other cell lines.
Versican V1 Isoform Contributes to Apoptotic Resistance in Fibroblasts
We next examined whether versican affected cell proliferation in serum-free conditions. V1-, V2-, and vector-transfected fibroblasts, as well as wild-type NIH3T3 cells were cultured sparsely in serum-free medium. Flow cytometry analysis showed that apoptosis was induced in wild-type, V2-, and vector-transfected NIH3T3 cells, but not in V1-transfected cells (Figure 3A). The V1-transfected cells continued to grow under serum starvation, but wild-type, V2-, and vector-transfected NIH3T3 cells died (Figure 3B). Apoptotic cell death was also measured by counting viable cells at regular intervals following serum removal. Our results showed that V1-transfected cells escaped cell death and the viable cell number reached 23 × 106 at day 16. In contrast, the viable cell number progressively decreased in V2- and vector-transfected cells and wild-type cells. Our experiments demonstrated that V1 expression contributed to apoptotic resistance. We then examined expression of antiapoptotic and proapoptotic proteins and observed that Bad expression was down-regulated in V1-transfected cells compared with that of vector-transfected and parental NIH3T3 fibroblasts, whereas V2-transfection had no effect (Figure 3C).
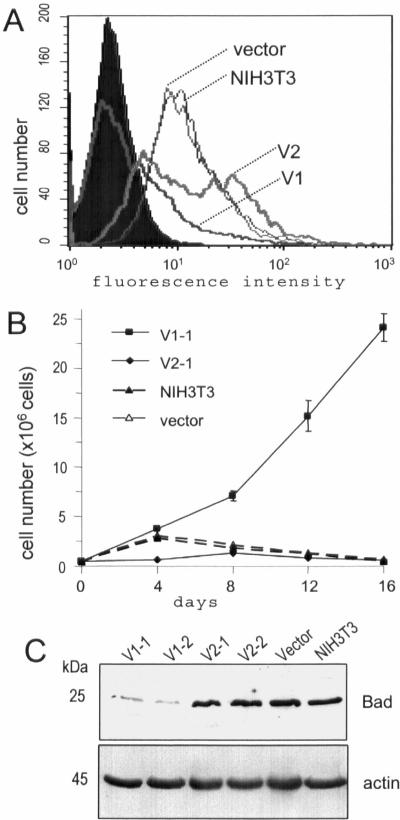
Figure 3.
Expression of the versican V1 isoform protects cells from apoptosis. (A) Cells (5 × 105) were cultured in serum-free DMEM. Cell apoptosis induced by serum starvation was detected by flow cytometry in wild-type, V2-, and vector-transfected cells, but not in V1-transfected cells. (B) Cells cultured in serum-free DMEM were fed every 4 d with serum-free medium until day 16. Viable cells were counted by trypan blue staining at the indicated time points. Each point represents the mean of three individual cell lines. Only V1-transfected cell lines survived and continued to grow. Cultures of the other cell lines detached from the plates and died. (C) Expression of the apoptosis-associated protein Bad was analyzed on Western blot probed with anti-Bad antibody. Reduced levels of Bad proteins were detected in versican V1-transfected cell lines, but not in the other cell lines.
It has been shown that HA14–1 induces apoptosis in NIH3T3 cells through inhibition of the antiapoptotic effect of Bcl-2 and regulation of Bax localization (Chen et al., 2002a). We then investigated whether HA14–1 could induce apoptosis in versican-transfected NIH3T3 cells. When the cells were treated with the apoptosis-inducing agent HA14–1 (25 μM) for 2 h, only the V1-transfected cells exhibited apoptotic resistance (Figure 4A). All of the other cells started to detach from the plates, while the V1-transfected cells remained attached to the plates after overnight treatment (unpublished data).
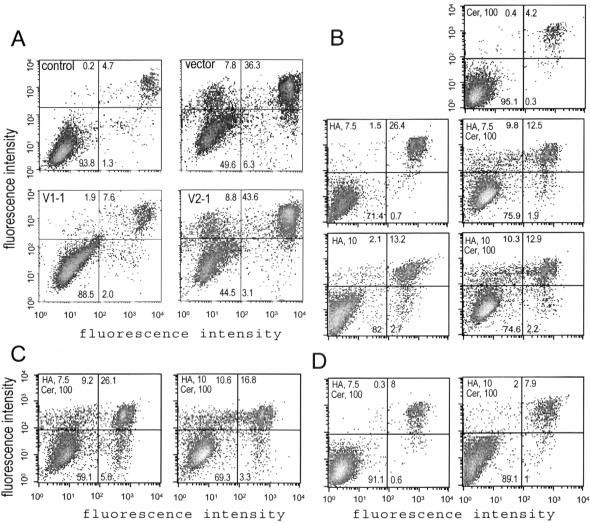
Figure 4.
The V1-transfected cells are resistant to chemical-induced apoptosis. (A) Cells stably transfected with V1, V2, or the control vector were treated with the apoptosis-inducing agent HA14-1 (25 μM) for 2 h, followed by cell harvesting, annexin V staining, and FACS analysis. HA14-1 induced greater rates of apoptosis in V2- (8.8%) and vector- (7.8%) transfected cells than in the V1-transfected cells (1.9%). (B) The vector-transfected cells were treated with HA14-1 (7.5 and 10 μM), C2-ceramide (100 μM), or combinations of HA14-1 and C2-ceramide (7.5 and 100 μM, or 10 and 100 μM, respectively) for 2 h, followed by apoptosis analysis. Few apoptotic cells were detected when treated with HA14-1 or C2-ceramide alone. However, much greater apoptosis was observed when the cells were treated with combinations of HA14-1 and C2-ceramide. (C) The V2-transfected cells were treated with combinations of HA14-1 and C2-ceramide (7.5 and 100 μM, or 10 and 100 μM, respectively) for 2 h, followed by apoptosis analysis. The combinations induced high levels of cell apoptosis (9.2 and 10.6%, respectively). (D) The V1-transfected cells were treated with combinations of HA14-1 and C2-ceramide (7.5 and 100 μM, or 10 and 100 μM, respectively) for 2 h, followed by apoptosis analysis. Few apoptotic cells were detected.
Cells were also treated with another apoptosis-inducing agent, C2-ceramide. Our experiments indicated that apoptosis was not detected in the vector-transfected cells treated with C2-ceramide alone or HA14–1 at low concentrations. However, combinations of C2-ceramide with low concentrations of HA14–1 induced cell apoptosis (Figure 4B). Similar results were obtained when the V2-transfected cells were treated with these combinations (Figure 4C). Interestingly, the V1-transfected cells were resistant to the chemical-induced apoptosis at the same combinations (Figure 4D).
The Effects of V1 and V2 Isoforms on the EGFR-associated Signaling Pathway
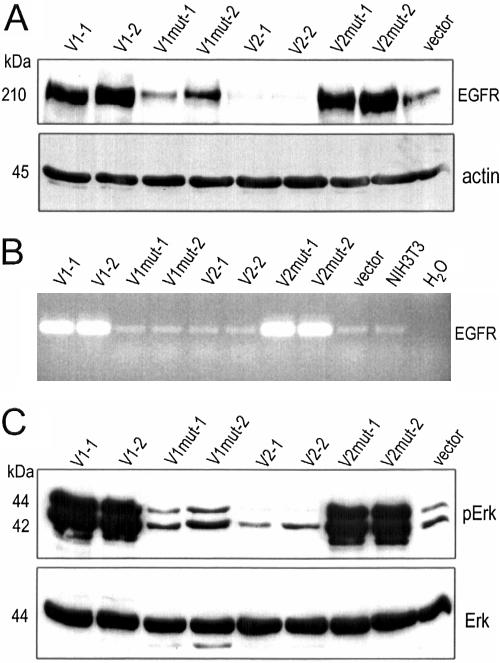
Because the EGFR-associated signaling pathway plays a key role in cell proliferation, we explored the possibility that versican might affect cell proliferation through regulation of EGFR and its downstream signaling pathway. The levels of EGFR in all transfectants were analyzed by Western blotting. We observed a remarkable upregulation of EGFR in V1-transfected cells, but not in the V1mut-transfected cells. On the other hand, V2-transfection strongly down-regulated EGFR expression, whereas V2mut exerted an opposite effect (Figure 5A). It appeared that the complete CSβ domain is necessary for EGFR activation by the V1 isoform, and that the intact CSα domain is required for EGFR down-regulation by the V2 isoform. RT-PCR analysis indicated that the V1-, and V2mut-transfected cells expressed higher levels of EGFR mRNA as compared with the other cells (Figure 5B), which is in agreement with the protein analysis.
Figure 5.
Versican expression stimulates EGFR expression and Erk phosphorylation. (A) Equal amounts of cell lysate were separated on SDS-PAGE and subjected to Western blot analysis probed with anti-EGFR and antiactin (for loading control) antibodies. Cells transfected with V1 and V2mut expressed higher levels of EGFR compared with the other cell lines. (B) Total mRNA was prepared from the cells and subjected to RT-PCR using two primers (5′ cga ccc tca ggg acc gcg aga acc and 5′ gcc acc tcc tgg atg gtc ttt aag) generating a fragment (480 base pairs) of EGFR, followed by agarose gel electrophoresis. The V1- and V2mut-transfected cells produced a higher level of EGFR mRNA. (C) Phosphorylation of Erk (pErk) was analyzed by Western blotting using an antibody against phosphorylated Tyr204 of Erk. Consistent with the activation of EGFR, high levels of Erk phosphorylation were detected in V1- and V2mut-transfected cells. Low levels of Erk phosphorylation were observed in V2-transfected cells, and the phosphorylation level was not altered in V1mut-transfected cells. Erk protein levels were analyzed by Western blotting using Erk-specific antibody. Little difference was detected between the cell lines tested.
EGFR has been documented as a prototypic receptor tyrosine kinase (RTK) which mediates downstream signaling. The classic signaling cascade downstream of EGFR is the extracellular regulated kinase (Erk) pathway (Maruta and Burgess, 1994). P42/p44 Erk is directly activated by dual phosphorylation on a Thr and a Tyr residue by MEK (Fukuda et al., 1997). To examine whether versican isoforms were able to regulate the EGFR downstream signaling pathway, we analyzed p42/p44 Erk phosphorylation by using an antibody that specifically recognizes the phosphorylated form of p42/p44 Erk. We detected strong signals from p42/p44 Erk phosphorylation in V1- and V2mut-transfected cells. The phosphorylation state of p42/p44 Erk in V1mut-transfected cells was comparable to that of vector-transfected cells, and a reduced signal was observed in V2-transfected cells (Figure 5C, top panel). Expression of the p42/p44 Erk protein was similar in all cell lines analyzed (Figure 5C, bottom panel). These results correlated with EGFR expression, suggesting that different versican isoforms and mutants were able to regulate EGFR expression and modulate its downstream signal transduction.
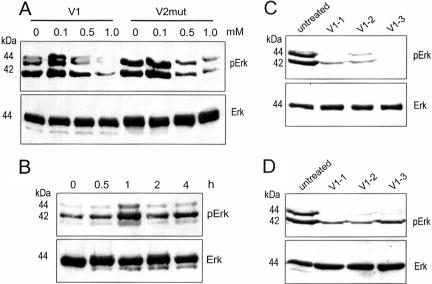
We then used Erk phosphorylation as an indicator of versican activity to test whether versican functioned inside or outside the cell. Because GAG modification is a prerequisite for secretion of the chondroitin sulfate proteoglycans (Yang et al., 2000; Chen et al., 2002b; Kiani et al., 2003), β-xyloside was used to inhibit GAG modification thus product secretion as we reported previously (Lee et al., 2000). Treatment with β-xyloside reduced Erk phosphorylation of the V1- and V2mut-transfected cells (Figure 6A), but had little effect on the V2- and vector-transfected cells (unpublished data). This result suggests that secretion of versican was required for Erk activation. Culture medium obtained from the V2mut-transfected cells was also incubated with NIH3T3 fibroblasts, followed by analysis of Erk phosphorylation. Erk phosphorylation reached the highest level when the cells were incubated with the V2mut-containing medium for 1 h (Figure 6B). Three V1-transfected cell lines were also subjected to treatment with brefeldin A (to target Golgi transport system) and monensin (to inhibit protein secretion). Erk phosphorylation was decreased when treated by brefeldin A (Figure 6C) and monensin (Figure 6D), compared with the untreated cells.
Figure 6.
(A) The cells were treated with β-xyloside at concentrations of 0, 0.1, 0.5, and 1.0 mM for 24 h. Cell lysate was prepared and analyzed for Erk phosphorylation on Western blot. Only the V1- and V2mut-transfected cells exhibited decreased levels of Erk phosphorylation by β-xyloside treatment. (B) Culture medium from the V2mut-transfected cells was incubated with NIH3T3 fibroblasts for different time intervals followed by analysis of Erk phosphorylation. Erk phosphorylation reached the highest level when the cells were incubated with V2mut-containing medium for 1 h. (C and D) Three V1-transfected cell lines were treated with brefeldin A at a concentration of 5 mg/ml (C) or monensin at a concentration of 5 μM (D) for 24 h. Untreated cells served as a control. Cell lysate was prepared and analyzed for Erk phosphorylation. Only the chemical-treated cells exhibited decreased levels of Erk phosphorylation. Expression of Erk protein was not affected.
V1 Induces p27kip1 Phosphorylation, Ubiquitination, and Degradation
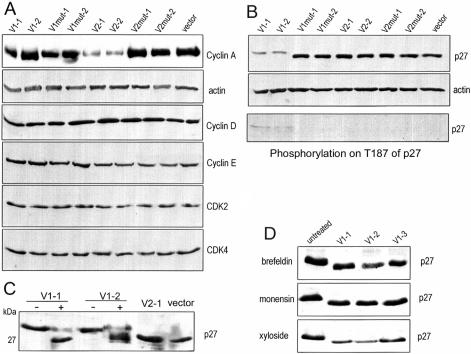
Because different versican isoforms have different effects on cell proliferation, expression levels of cell cycle–related proteins were analyzed in transfected cell lines. In all samples tested, similar expression levels of cyclin D, cyclin E, CDK2, and CDK4 were detected (Figure 7A). However, a noticeable down-regulation of cyclin A was observed in V2-transfected cells. This result supports our observations of the inhibitory effect that the V2 isoform had on cell proliferation.
Figure 7.
Expression of the versican V1 isoform induces p27 phosphorylation. (A) The expression of cell cycle-related proteins was analyzed by Western blot probed with appropriate antibodies as indicated. β-actin expression was used as a loading control. Although expression of cyclin A decreased in V2-transfected cells, little difference could be detected in the other cell types. Expression of cyclin D, cyclin E, CDK2, and CDK4 was similar in all cell lines analyzed. (B) Equal amounts of cell lysate were analyzed on Western blot probed with anti-p27 and antiactin antibodies. Cells transfected with V1 expressed p27, which migrated more slowly than samples from the other cell lines. Probing with an antibody against p27 phosphorylated on T187 showed that p27 phosphorylation was exclusively detected in V1-transfected cells. (C) The p27 protein was immunoprecipitated from lysate of different cell lines as shown. The precipitated proteins were treated with (+) or without phosphatase (-), followed by Western blot probed with anti-p27 antibody. After phosphatase treatment, the p27 bands from V1-transfected cells shifted to locations equivalent to that seen in p27 bands from V2- and vector-transfected cells. (D) Three V1-transfected cell lines were treated with brefeldin A (5 mg/ml), monensin (5 μM), or β-xyloside (0.5 mM) for 24 h. Untreated cells served as a control. Cell lysate was prepared and analyzed for p27 migration on Western blot. The chemical-treated cells exhibited a shift of p27 protein bands.
The expression of p27kip1 (hereafter p27), a cyclin-dependent kinase (CDK) inhibitor, was analyzed in all cell lines. Our results showed that the levels of p27 protein were lower in V1-transfected cells, and its apparent molecular mass was slightly larger than p27 expressed in the other cell lines (∼29 kDa, Figure 7B). These data suggest that V1 expression triggered p27 modification. To test if this mobility shift occurred due to p27 phosphorylation, V1-containing samples were treated with phosphatase after immunoprecipitation. Our results show that part of p27 reverted to normal mobility after phosphatase treatment, indicating abolishment of p27 phosphorylation (Figure 7C). To confirm this, we analyzed p27 phosphorylation by using an antibody that specifically recognizes the phosphorylated threonine187 of p27 (Thr187). The experiments showed that Thr187 phosphorylation of p27 was detected in V1-transfected cells (Figure 7B). Treated with brefeldin A, monensin, and β-xyloside, all V1-transfected cell lines exhibited a shift in p27 migration (Figure 7D), suggesting decreased p27 phosphorylation.
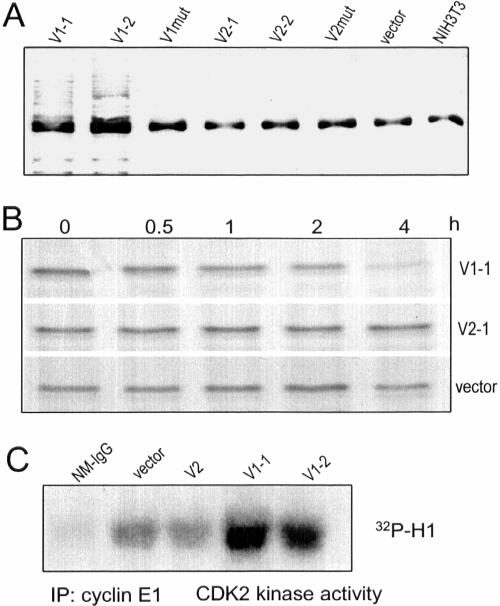
Phosphorylation of p27 at Thr187 is necessary for p27 degradation through the ubiquitination pathway. The ubiquitin ligase for p27 is p45skp2 (SKP2; Carrano et al., 1999), which tags target proteins with ubiquitin, allowing the proteasome to recognize and degrade it. Because V1 was able to induce p27 phosphorylation at the Thr187 residue, we analyzed p27 ubiquitination using an antibody against ubiquitin. Ubiquitination of p27 was found only in V1-transfected cells and not in the other cell lines (Figure 8A). Pulsechase experiments were conducted to verify p27 degradation, which was again observed only in V1-transfected cells (Figure 8B). These results demonstrate that V1 expression induced ubiquitination and degradation of p27. This data are consistent with the immunoblot results that show that p27 protein levels are lower in V1-transfected cells than in other cell lines.
Figure 8.
V1 expression induces p27 ubiquitination and degradation. (A) After immunoprecipitation of p27 from different cell lysate with anti-p27 antibody, the precipitated proteins were analyzed on Western blot probed with antiubiquitin antibody. Ubiquitination of p27 was observed only in V1-transfected cells. The common bands might be the light chain of IgG which was recognized by the secondary antibody used in the Western blot. (B) NIH3T3 fibroblasts, transfected with V1, V2, or the control vector, were incubated with 35S for 1 h. The medium was removed and the cells were washed with DMEM, followed by incubation of the cells in DMEM containing 10% FBS for 0, 0.5, 1, 2, and 4 h. Cell lysate was prepared and immunoprecipitated with anti-p27 antibody. The precipitated proteins were separated by SDS-PAGE, followed by gel-drying and exposure to x-ray film. V1 expression enhanced p27 degradation, but this was not detected in V2- or vector-transfected cells. (C) Cell lysate was immunoprecipitated with anticyclin E antibody E172. The kinase activity of the coprecipitated protein CDK2 was assessed by Western blot incubated with CDK2's substrate histone H1 and 32P. As a control, nonspecific mouse Ig G (NM-IgG) was used for immunoprecipitation. Only the V1-transfected cells produced higher CDK2 activity.
It has been shown that the cyclin E/CDK2 complex mediates p27 phosphorylation at Thr187 (Sheaff et al., 1997). Cyclin E–dependent kinase activity was assayed in V1-, V2-, and vector-transfected cells. Our results show that cyclin E–dependent kinase activity was substantially increased in cells transfected with V1, but not in cells transfected with V2 or the control vector (Figure 8C). These data indicate that CDK2 kinase activity was specifically activated in V1-transfected cells. To directly test CDK2 involvement in p27 phosphorylation, we blocked CDK2 kinase activity in V1-transfected cells using the CDK2 specific inhibitor GW8510. The mobility shift of p27 was not abolished after CDK2 inhibition, indicating that it is not attributable to Thr187 phosphorylation but must be due to a novel mechanism (unpublished data).
Silencing V1 Expression Reversed Its Effects on Proliferation and Apoptosis
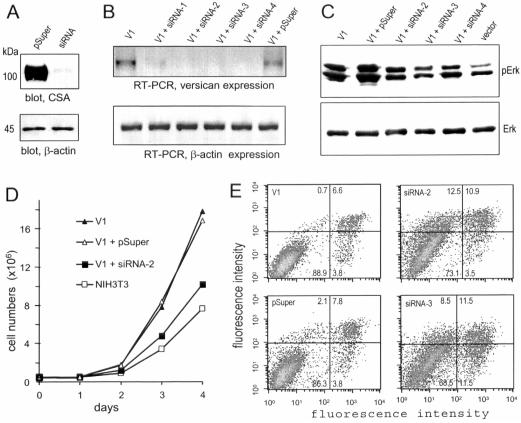
To further elucidate the direct involvement of the versican V1 isoform in cell proliferation and apoptosis, we used small interfering RNA to reduce V1 expression. One construct (siRNA-ver5337), containing a target sequence against the CSβ domain, was found to greatly silence expression of the recombinant construct CSA (Figure 9A). V1-expressing NIH3T3 cells were cotransfected with siRNA-ver5337 and pcDNA3.1/Hygro or pSuper and pcDNA3.1/Hygro. Cell lines stably transfected with siRNA-ver5337 or pSuper were selected with hygromycin. Expression of V1 in the selected cell lines was analyzed by RT-PCR, and cell lines exhibiting reduced V1 expression were used for functional studies. The experiments indicate that the siRNA vector (pSuper) had no effect while siRNA-ver5337 transfection reduced V1 expression (Figure 9B).
Figure 9.
Reduction in V1 function by siRNA. (A) COS-7 cells were cotransfected with recombinant constructs CSA and siRNA-ver5337 or CSA and a control vector pSuper. Cell lysate was analyzed on Western blot probed with 4B6 and the same blot was reprobed with anti-actin antibody. Cotransfection with siRNA-ver5337 reduced CSA expression greatly. (B) V1-expressing cells were stably cotransfected with pcDNA3.1/Hygro and siRNA-ver5337 or pSuper. Hygromycin-resistant cell lines were selected. Reduction in V1 expression was analyzed by RT-PCR using two primers 5′ gag caa gac aca gag act and 5′ tgt tcc ttt ctt gca ggt (complementary to the CRD motif of versican). The control actin primers were 5′ ccg gca tgt gca aag ccg gct tcg and 5′ gct cat tgt aga agg tgt ggt gcc (complementary to actin nucleotides 121–360). Four siRNA-ver5337–transfected cell lines (siRNA-1, -2, -3, and -4) exhibited reduced versican expression. (C) Erk phosphorylation (pErk) was analyzed in the siRNA-ver5337– and pSuper-transfected cell lines. The V1- and vector-transfected NIH3T3 cell lines served as controls. The levels of pErk decreased in the siRNA-ver5337-transfected cells compared with pSuper-transfected cells. (D) In proliferation assays, cells stably transfected with siRNA-ver5337 exhibited a reduced rate of proliferation. (E) Cells were treated with HA14–1 (25 μM) for 2 h, followed by apoptosis analysis. This chemical had little effect on V1- and pSuper-transfected cells, but induced apoptosis in the siRNA-ver5337–transfected cells (siRNA-2 and siRNA-3).
Because V1 enhanced NIH3T3 cell proliferation and activated Erk phosphorylation, it was necessary to examine whether the cell lines stably transfected with the siRNA-ver5337 construct exhibited reduced levels of Erk phosphorylation. Analysis of cell lysate indicated that Erk phosphorylation was not affected by pSuper transfection but was clearly decreased by siRNA-ver5337 transfection, whereas the protein levels of Erk were not affected (Figure 9C).
In cell proliferation assays, siRNA-transfected cells were seeded on 100-mm dishes, and the cell number was counted daily. The experiments indicated that stable transfection of the siRNA-ver5337 construct abolished V1-enhanced cell proliferation (Figure 9D). Finally, we examined the sensitivity of versican silencing in apoptosis stimulated with HA14–1. Although V1-transfected cells and pSuper-transfected V1-expressing cells were resistant to HA14–1–induced apoptosis, the V1-expressing cells stably transfected with the siRNA-ver5337 construct became sensitive to HA14–1 induced apoptosis (Figure 9E). These results indicate that the versican V1 product was responsible for the enhanced proliferation and reduced apoptosis in the V1-transfected cells.
DISCUSSION
We have generated and expressed two differentially distributed versican isoforms, namely the V1 and V2 isoforms, in NIH3T3 fibroblasts. Analysis of the individual isoforms have revealed different biological functions for these two proteoglycans: the V1 isoform displayed an ability to enhance cell growth, whereas the V2 isoform exerted an inhibitory effect. V1mut, possessing a 2367-base pair deletion in its CSβ domain, abolished V1's proliferative activity, indicating that the presence of the full CSβ domain is essential for the promoting effect of V1 on cell proliferation. V2mut, containing a 1953-base pair deletion in the CSα domain, was able to enhance cell growth, suggesting that the full CSα domain is important for the inhibitory activity of V2.
Molecular analysis of the cell surface growth factor-receptor EGFR and its downstream pathway showed that upregulation of EGFR expression and activation of Erk were evident in V1-transfected cells, but down-regulation of EGFR expression and inactivation of Erk were detected in V2-transfected cells. EGFR and its downstream signaling is a critical pathway in cell proliferation. Normal cells require mitogenic growth signals to move into an active proliferative state from a quiescent state. EGFR serves as a transmembrane receptor that transmits the extracellular signal into the cell, leading to different cell functions such as motility, survival, and proliferation. We have found that EGFR expression and its signaling pathway was regulated by V1 and V2, which correlated with V1 promotion and V2 inhibition of cell proliferation. This suggests that regulation of EGFR and its downstream signaling pathway were involved in V1- and V2-mediated cell proliferation.
In recent studies using the PC12 cell line, we have demonstrated that V1 and V2 are able to up-regulate the expression and activation of EGFR (Wu et al., 2004b). V1 exerts a stronger effect on EGFR activation and is able to induce PC12 cell differentiation and generate fully developed neurites, whereas V2 activates EGFR at a lower level and can only induce the initiation of cell differentiation followed by cell death. It thus appears that V1 and V2 can play different functional roles in different cell types. Furthermore, we have demonstrated that the C-terminal domain of versican is able to modulate the association of EGFR with β1-integrin and regulate cell proliferation (Wu et al., 2004a). These data are consistent with our previous report that EGFR is involved in versican's promotion of cell growth (Zhang et al., 1998b). Our studies have thus demonstrated the importance of EGFR in versican function.
The involvement of V1 and V2 in cell proliferation was also corroborated by the activity of the mutants: V1mut lost the ability to activate EGFR and its downstream signaling pathway and could no longer enhance cell growth, whereas V2mut acquired activation of EGFR and its downstream signaling pathway and promoted cell growth. These results provide evidence that the CSβ domain is responsible for the activation of EGFR expression and its downstream signal pathway and that the CSα domain plays a role in the suppression of EGFR expression and its downstream signaling pathway. The inhibitory effect of V2 on cell proliferation was also demonstrated by the observation that cyclin A expression was down-regulated in V2-transfected cells.
The functions of the CSα and CSβ domains could also be distinguished by their role in regulating cell growth in conditions of serum starvation. V2-transfected cells died through apoptosis under serum deprivation, but V1-transfected cells escaped cell death. The contribution of V1 to apoptosis resistance was further tested in parental and transfected NIH3T3 cells in response to different proapoptotic stimuli. In these studies, V1 rendered cells resistant to apoptosis induced by HA14–1 and C2-ceramide. Our experiments thereafter provide evidence that V1 was able to down-regulate Bad expression. Bad is a proapoptotic Bcl-2 family member. It binds to Bcl-xL and Bcl-2, resulting in a proapoptotic state. Our results imply that the CSα and CSβ domains play different roles in cell proliferation and survival, ascribing distinct biological activities to the V1 and V2 isoforms.
The opposing effects of V1 and V2 on cell proliferation suggest that a dynamically balanced expression pattern of these two isoforms may provide a suitable extracellular environment for normal proliferation and survival of cells. This balanced extracellular environment might be altered by modifying the distribution of various versican isoforms. The extracellular environment may become favorable for cell proliferation and survival when V1 expression is increased, as in the case of tissue development and tumor formation (Landolt et al., 1995; Cattaruzza et al., 2002; Touab et al., 2002). By contrast, a V2-predominant environment may suppress cell proliferation, as in the case of maintenance of mature tissues. How V2mut caused an opposite effect is not clear.
Our results also indicate that V1 and V2 alter cell proliferation in NHI3T3 cells differently, not only through the EGFR pathway but also through cell cycle–related proteins. Expression of p27 was detected at a lower level in V1-transfected cells, and this protein appeared to be phosphorylated. Furthermore, V1 expression promoted p27 degradation through ubiquitin-mediated proteolysis. p27 has been identified as a member of the Cip/Kip family of cdk inhibitors, whose principle role in cell cycle regulation is to inhibit the cyclin E/cdk2 and cyclin A/cdk2 kinases (Di Fiore et al., 1987). The amount of p27 increases in quiescent cells, and p27 knockout animals develop hyperplasia in multiple organs (Blain et al., 2003), indicating an important antiproliferative role for this protein. Mitogens can promote p27 degradation, allowing quiescent cells to reenter the cell cycle. The lower level of p27 in the V1-transfected cells might cause an insufficient suppression on cdk2 kinase. This was confirmed by observing an increased activity of cdk2 in the V1-transfected cells.
Unbalanced expression of versican isoforms may be associated with pathological conditions. Increased expression of the V2 isoform in CNS injury inhibits axon regeneration, whereas expression of the V1 and V0 isoforms increases in tumors (Touab et al., 2002), suggesting that these isoforms may be involved in tumor development. The presence of the V1 isoform might be explained by its ability to regulate EGFR expression and its downstream signal transduction pathway as well as p27 expression. Increased EGFR expression is widely observed in a variety of human tumors (Merlino et al., 1985). Overexpression of growth factor receptors may increase the sensitivity of tumors to ambient levels of growth factor that normally would not trigger proliferation. Moreover, overexpression of growth factor receptor can generate ligand-independent signaling (Di Fiore et al., 1987). Reduced p27 expression is common in human tumors; p27 mRNA is not usually down-regulated; rather, lower p27 expression has been shown to result from enhanced proteolysis (Chiarle et al., 2000). Overexpression of EGFR and decreased expression of p27 are prognostic of poor survival in many human cancers (Catzavelos et al., 1997). The expression of versican in human tumors has also been shown to be associated with tumor relapse (Ricciardelli et al., 2002). The ability of the V1 isoform to regulate EGFR expression and p27 degradation implies that V1 might be involved in the poor survival. However, how the versican V1 isoform regulates its expression remains to be determined.
Finally, to confirm the roles of the versican V1 isoform in cell proliferation and apoptosis, we used the siRNA approach. After confirming the reduction of V1 expression in the siRNA-ver5337–transfected cells, we demonstrated that the V1-enhanced cell proliferation and reduced apoptosis were abolished by silencing versican V1 expression. These results demonstrate that the V1 product was necessary for the effects observed. It was neither due to the transfection selection of the V1 construct, nor due to insertion of the V1 construct to the genome.
In conclusion, the CSβ domain–containing V1 isoform is implicated not only in cell proliferation enhancement, but also in cell cycle progression and apoptosis resistance. The CSβ domain may cause activation of the EGFR signaling pathway and p27 degradation, as well as Bad down-regulation. The CSα domain-containing V2 isoform is associated with suppression of cell proliferation and causes inhibition of the EGFR signaling pathway. The different activities of versican isoforms V1 and V2 are therefore mediated by the CSβ and CSα domain, respectively.
Acknowledgments
This work was supported by grant NA-5127 from Heart and Stroke Foundation of Ontario and grant MOP-62729 from Canadian Institutes of Health Research to B.B.Y. who is a New Investigator of CIHR.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-04-0295) on January 5, 2005.
Abbreviations used: EGF, epidermal growth factor; EGFR, EGF receptor; G3, selectin-like domain; CRD, carbohydrate recognition domain; CBP, complement binding protein; FBS, fetal bovine serum; HBSS, Hanks' balanced salt solution; siRNA, small interfering RNA.
References
- Ang, L. C., Zhang, Y., Cao, L., Yang, B. L., Young, B., Kiani, C., Lee, V., Allan, K., and Yang, B. B. (1999). Versican enhances locomotion of astrocytoma cells and reduces cell adhesion through its G1 domain. J. Neuropathol. Exp. Neurol. 58, 597-605. [DOI] [PubMed] [Google Scholar]
- Blain, S. W., Scher, H. I., Cordon-Cardo, C., and Koff, A. (2003). p27 as a target for cancer therapeutics. Cancer Cell 3, 111-115. [DOI] [PubMed] [Google Scholar]
- Carrano, A. C., Eytan, E., Hershko, A., and Pagano, M. (1999). SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1, 193-199. [DOI] [PubMed] [Google Scholar]
- Cattaruzza, S., Schiappacassi, M., Kimata, K., Colombatti, A., and Perris, R. (2004). The globular domains of PG-M/versican modulate the proliferation-apoptosis equilibrium and invasive capabilities of tumor cells. FASEB J. 18, 779-781. [DOI] [PubMed] [Google Scholar]
- Cattaruzza, S., Schiappacassi, M., Ljungberg-Rose, A., Spessotto, P., Perissinotto, D., Morgelin, M., Mucignat, M. T., Colombatti, A., and Perris, R. (2002). Distribution of PG-M/versican variants in human tissues and de novo expression of isoform V3 upon endothelial cell activation, migration, and neoangiogenesis in vitro. J. Biol. Chem. 277, 47626-47635. [DOI] [PubMed] [Google Scholar]
- Catzavelos, C. et al. (1997). Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat. Med. 3, 227-230. [DOI] [PubMed] [Google Scholar]
- Chen, J., Freeman, A., Liu, J., Dai, Q., and Lee, R. M. (2002a). The apoptotic effect of HA14–1, a Bcl-2-interacting small molecular compound, requires Bax translocation and is enhanced by PK11195. Mol. Cancer Ther. 1, 961-967. [PubMed] [Google Scholar]
- Chen, L., Wu, Y., Lee, V., Kiani, C., Adams, M. E., Yao, Y., and Yang, B. B. (2002b). The folded modules of aggrecan G3 domain exert two separable functions in glycosaminoglycan modification and product secretion. J. Biol. Chem. 277, 2657-2665. [DOI] [PubMed] [Google Scholar]
- Chen, L., Yang, B. L., Wu, Y., Yee, A., and Yang, B. B. (2003). G3 domains of aggrecan and PG-M/versican form intermolecular disulfide bonds that stabilize cell-matrix interaction. Biochemistry 42, 8332-8341. [DOI] [PubMed] [Google Scholar]
- Chiarle, R. et al. (2000). Increased proteasome degradation of cyclin-dependent kinase inhibitor p27 is associated with a decreased overall survival in mantle cell lymphoma. Blood 95, 619-626. [PubMed] [Google Scholar]
- Di Fiore, P. P., Pierce, J. H., Kraus, M. H., Segatto, O., King, C. R., and Aaronson, S. A. (1987). erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science 237, 178-182. [DOI] [PubMed] [Google Scholar]
- Fukuda, M., Gotoh, I., Adachi, M., Gotoh, Y., and Nishida, E. (1997). A novel regulatory mechanism in the mitogen-activated protein (MAP) kinase cascade. Role of nuclear export signal of MAP kinase kinase. J. Biol. Chem. 272, 32642-32648. [DOI] [PubMed] [Google Scholar]
- Henderson, D. J., Ybot-Gonzalez, P., and Copp, A. J. (1997). Over-expression of the chondroitin sulphate proteoglycan versican is associated with defective neural crest migration in the Pax3 mutant mouse (splotch). Mech. Dev. 69, 39-51. [DOI] [PubMed] [Google Scholar]
- Ingber, D. E., and Folkman, J. (1989). Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J. Cell Biol. 109, 317-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, K., Shinomura, T., Zako, M., Ujita, M., and Kimata, K. (1995). Multiple forms of mouse PG-M, a large chondroitin sulfate proteoglycan generated by alternative splicing. J. Biol. Chem. 270, 958-965. [DOI] [PubMed] [Google Scholar]
- Kiani, C., Chen, L., Lee, V., Zheng, P. S., Wu, Y., Wen, J., Cao, L., Adams, M. E., Sheng, W., and Yang, B. B. (2003). Identification of the motifs and amino acids in aggrecan G1 and G2 domains involved in product secretion. Biochemistry 42, 7226-7237. [DOI] [PubMed] [Google Scholar]
- Landolt, R. M., Vaughan, L., Winterhalter, K. H., and Zimmermann, D. R. (1995). Versican is selectively expressed in embryonic tissues that act as barriers to neural crest cell migration and axon outgrowth. Development 121, 2303-2312. [DOI] [PubMed] [Google Scholar]
- Lee, V., Cao, L., Zhang, Y., Kiani, C., Adams, M.E., and Yang, B.B. (2000). The roles of matrix molecules in mediating chondrocyte aggregation, attachment, and spreading. J. Cell. Biochem. 79, 322-333. [DOI] [PubMed] [Google Scholar]
- Lemire, J. M., Braun, K. R., Maurel, P., Kaplan, E. D., Schwartz, S. M., and Wight, T. N. (1999). Versican/PG-M isoforms in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 19, 1630-1639. [DOI] [PubMed] [Google Scholar]
- Maruta, H., and Burgess, A. W. (1994). Regulation of the Ras signalling network. Bioessays 16, 489-496. [DOI] [PubMed] [Google Scholar]
- Merlino, G. T., Xu, Y. H., Richert, N., Clark, A. J., Ishii, S., Banks-Schlegel, S., and Pastan, I. (1985). Elevated EGF receptor gene copy number and expression in a squamous carcinoma cell line. J. Clin. Invest. 75, 1077-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardelli, C., Brooks, J. H., Suwiwat, S., Sakko, A. J., Mayne, K., Raymond, W. A., Seshadri, R., LeBaron, R. G., and Horsfall, D. J. (2002). Regulation of stromal versican expression by breast cancer cells and importance to relapse-free survival in patients with node-negative primary breast cancer. Clin. Cancer Res. 8, 1054-1060. [PubMed] [Google Scholar]
- Sandhu, C., Garbe, J., Bhattacharya, N., Daksis, J., Pan, C. H., Yaswen, P., Koh, J., Slingerland, J. M., and Stampfer, M. R. (1997). Transforming growth factor beta stabilizes p15INK4B protein, increases p15INK4B-cdk4 complexes, and inhibits cyclin D1-cdk4 association in human mammary epithelial cells. Mol. Cell. Biol. 17, 2458-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy, J. D. et al. (2001). Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J. Biol. Chem. 276, 13372-13378. [DOI] [PubMed] [Google Scholar]
- Schmalfeldt, M., Bandtlow, C. E., Dours-Zimmermann, M. T., Winterhalter, K. H., and Zimmermann, D. R. (2000). Brain derived versican V2 is a potent inhibitor of axonal growth. J. Cell Sci. 113, 807-816. [DOI] [PubMed] [Google Scholar]
- Schmalfeldt, M., Dours-Zimmermann, M. T., Winterhalter, K. H., and Zimmermann, D. R. (1998). Versican V2 is a major extracellular matrix component of the mature bovine brain. J. Biol. Chem. 273, 15758-15764. [DOI] [PubMed] [Google Scholar]
- Sheaff, R. J., Groudine, M., Gordon, M., Roberts, J. M., and Clurman, B. E. (1997). Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 11, 1464-1478. [DOI] [PubMed] [Google Scholar]
- Shinomura, T., Nishida, Y., Ito, K., and Kimata, K. (1993). cDNA cloning of PG-M, a large chondroitin sulfate proteoglycan expressed during chondrogenesis in chick limb buds. Alternative spliced multiforms of PG-M and their relationships to versican. J. Biol. Chem. 268, 14461-14469. [PubMed] [Google Scholar]
- Slingerland, J. M., Hengst, L., Pan, C. H., Alexander, D., Stampfer, M. R., and Reed, S. I. (1994). A novel inhibitor of cyclin-Cdk activity detected in transforming growth factor beta-arrested epithelial cells. Mol. Cell. Biol. 14, 3683-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touab, M., Villena, J., Barranco, C., Arumi-Uria, M., and Bassols, A. (2002). Versican is differentially expressed in human melanoma and may play a role in tumor development. Am. J. Pathol. 160, 549-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y., Chen, L., Cao, L., Sheng, W., and Yang, B. B. (2004a). Overexpression of the C-terminal PG-M/versican domain impairs growth of tumor cells by intervening in the interaction between EGF receptor and {beta}1-integrin. J. Cell Sci. 117, 2227-2237. [DOI] [PubMed] [Google Scholar]
- Wu, Y., Chen, L., Zheng, P. S., and Yang, B. B. (2002). beta 1-Integrin-mediated glioma cell adhesion and free radical-induced apoptosis are regulated by binding to a C-terminal domain of PG-M/versican. J. Biol. Chem. 277, 12294-12301. [DOI] [PubMed] [Google Scholar]
- Wu, Y., Sheng, W., Chen, L., Dong, H., Lee, V., Lu, F., Wong, C. S., Lu, W. Y., and Yang, B. B. (2004b). Versican V1 isoform induces neuronal differentiation and promotes neurite outgrowth. Mol. Biol. Cell 15, 2093-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B. L., Cao, L., Kiani, C., Lee, V., Zhang, Y., Adams, M. E., and Yang, B. B. (2000). Tandem repeats are involved in G1 domain inhibition of versican expression and secretion and the G3 domain enhances glycosaminoglycan modification and product secretion via the complement-binding protein-like motif. J. Biol. Chem. 275, 21255-21261. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Cao, L., Kiani, C. G., Yang, B. L., and Yang, B. B. (1998a). The G3 domain of versican inhibits mesenchymal chondrogenesis via the EGF-like motifs. J. Biol. Chem. 273, 33054-33063. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Cao, L., Yang, B. L., and Yang, B. B. (1998b). The G3 domain of versican enhances cell proliferation via epidermal growth factor-like motifs. J. Biol. Chem. 273, 21342-21351. [DOI] [PubMed] [Google Scholar]
- Zheng, P. S., Vais, D., Lapierre, D., Liang, Y. Y., Lee, V., Yang, B. L., and Yang, B. B. (2004a). PG-M/versican binds to P-selectin glycoprotein ligand-1 and mediates leukocyte aggregation. J. Cell Sci. 117, 5887-5895. [DOI] [PubMed] [Google Scholar]
- Zheng, P. S., Wen, J., Ang, L. C., Sheng, W., Viloria-Petit, A., Wang, Y., Wu, Y., Kerbel, R. S., and Yang, B. B. (2004b). Versican/PG-M G3 domain promotes tumor growth and angiogenesis. FASEB J. 18, 754-756. [DOI] [PubMed] [Google Scholar]