Abstract
The diverse functions of noncoding RNAs (ncRNAs) can influence virtually every aspect of the transcriptional process including epigenetic regulation of genes. In the CNS, regulatory RNA networks and epigenetic mechanisms have broad relevance to gene transcription changes involved in long-term memory formation and cognition. Thus, it is becoming increasingly clear that multiple classes of ncRNAs impact neuronal development, neuroplasticity, and cognition. Currently, a large gap exists in our knowledge of how ncRNAs facilitate epigenetic processes, and how this phenomenon affects cognitive function. In this review, we discuss recent findings highlighting a provocative role for ncRNAs including lncRNAs and piRNAs in the control of epigenetic mechanisms involved in cognitive function. Furthermore, we discuss the putative roles for these ncRNAs in cognitive disorders such as schizophrenia and Alzheimer's disease.
Keywords: : chromatin, epigenetics, long noncoding RNA, neuroplasticity, neuroscience, short noncoding RNA
Epigenetic mechanisms have emerged as critical components of the memory formation process and are involved in cognition and cognitive disorders. As related to the nervous system, the term epigenetics refers to long-term, potentially heritable changes in gene expression patterns that do not result from mutations in the DNA sequence. This broad definition encompasses a number of chemical modifications to DNA residues as well as alterations to closely associated molecules such as histone proteins or chromatin-associated RNAs [1]. These epigenetic modifications (also referred to as epigenetic marks) are involved in numerous cellular and molecular functions, ultimately influencing nuclear organization and transcriptional activity at chromatin regions [1]. The semipermanent nature of these epigenetic modifications thus allows genetically identical cells to differentiate into distinct lineages, express unique gene patterns, and perform unique functions in a lineage-dependent fashion [2,3].
As the epigenome plays an important role in driving cellular development, it is not surprising that epigenetic mechanisms critically control the development of the nervous system [4–7]. However, in the past decade it has become increasingly clear that despite the postmitotic state of most neurons, epigenetic mechanisms continue to play a critical role in controlling transcription into adulthood (reviewed in [8]). This is especially evident in the context of transcription-dependent cognitive processes such as long-term memory formation, where altered epigenetic processes can either impair or improve performance in memory tasks.
While the importance of epigenetic regulation in cognition has been well established, the mechanisms by which epigenetic marks are targeted to particular genes or loci remain relatively unexplored. Instead, most studies to date have examined either global changes in the levels of epigenetic marks within specific brain regions or the presence of epigenetic marks at particular genes and promoters with known cognitive function. As a result of these studies, it is becoming increasingly clear that the neuro-epigenetics landscape can differ significantly within and across brain regions, and that dysregulation of chromatin-modifying enzymes (CMEs) can have profound effects on brain function, and thereby, cognition and cognitive disorders [9–11]. Indeed, evidence suggests that dysfunction of epigenetic processes is involved in the etiology of many cognitive disorders, including schizophrenia, bipolar disorder and major depressive disorder [9]. Such studies have advanced our knowledge of the role of epigenetic mechanisms in specific brain regions, and have given rise to the rapidly expanding field of cognitive epigenetics. However, as very few CMEs have demonstrated sequence-specific DNA binding, the question of how CMEs are directed to their target gene regions remains largely unsolved. Some observations have indicated that DNA binding proteins, such as the transcription factors including nuclear factor-ĸB, Nanog, and Oct4, interact with CMEs to direct site-specific epigenetic gene regulation [12–14]. Additionally, recent studies suggest that multiple families of regulatory RNAs also mediate epigenetic targeting.
To date, a majority of the research studies have described regulatory RNAs with little to no protein-coding potential, thus defined as noncoding RNAs (ncRNAs). Although poorly described relative to protein-coding genes, ncRNAs comprise a major portion of the mammalian transcriptome [15]. While estimates differ as to the abundance of ncRNAs, the general consensus of the field is that ncRNAs are quite plentiful, particularly in brain tissues [15–19]. It remains plausible that regulatory RNAs have both translation-independent functions and protein-coding potential, and indeed, translation-independent activity of mRNAs has been identified in well-studied pathways such as p53 signaling [20–22]. However, the field of epigenetics has largely focused on characterizing such functions in ncRNAs, in part as a precaution against experimental confounds.
Common practice in the epigenetics field describes ncRNAs as either long or short, with the division being set at a length of 200 nucleotides. While seemingly arbitrary, this division allows for the useful separation of the many characterized classes of small functional RNAs, including miRNAs, piRNAs (PIWI-interacting RNAs), siRNAs (small interfering RNA), snoRNAs (small nucleolar RNAs) and tRNAs (transfer RNAs) from the less well characterized long noncoding RNAs (lncRNAs) [23]. Among other functions, both short and lncRNAs have attributable roles in neuronal epigenetic mechanisms [24,25] – a finding with exciting implications for cognitive sciences. In this review, we will highlight key findings that are beginning to elucidate a role for ncRNAs in the control of neuronal and cognitive function via epigenetic mechanisms. Our goal for this review is to draw attention to this phenomenon and encourage new investigations into ncRNA-mediated control of the epigenome in cognition and cognitive disorders. Further, while miRNAs are well studied with regard to cognitive disorders, they are poorly studied in the context of epigenetic function. In contrast, while piRNAs and lncRNAs are better characterized in the context of epigenetic function, they are poorly studied with regard to cognitive disorders – even though many such disorders involve dysregulation of the epigenome. Nonetheless, emerging studies are beginning to explore the interesting idea that ncRNAs are involved in the epigenetic process underlying cognition and may be altered in cognitive disorders.
Short ncRNA
Mechanisms of canonical RNA interference
When Lee and colleagues [26] first demonstrated that the small (22-nucleotide) ncRNA dubbed lin–4 represses the translation of several developmental genes in Caenorhabditis elegans, the scientific community failed to recognize this discovery as anything more than a curious feature of the invertebrate model's genetics. As a consequence, few of these ncRNAs were identified or characterized until the discovery of RNA interference (RNAi), a post-transcriptional regulatory process that will be reviewed and discussed in later sections. However, we begin our discussion with small regulatory RNAs initially shown to be highly conserved in plants and animals in the early 2000s [27]. Today, the known roles of miRNAs and their related structures have expanded to encompass the view that as many as 60% of protein-coding RNAs are regulated by miRNA activity [28,29]. Since the days of Ambrose and Lee, tens of thousands of miRNAs have been annotated [30–34], and miRNAs have been shown to regulate such diverse biological processes as developmental pattern formation [27], pluripotency [35], cell signaling [36], cardiovascular disease [37], cancer [38], diabetes [39], neural plasticity and memory [40], among others [41]. This review will largely describe miRNAs as they relate to the epigenome; however, in order to better understand how miRNAs have come to be associated with epigenetic activity, it is first necessary to understand the canonical post-transcriptional pathway by which these miRNAs regulate gene expression.
In the canonical pathway (reviewed in [42]), the nascent miRNA begins as a transcript of intronic or intergenic DNA, a miRNA precursor molecule known as a primary miRNA (pri-miRNA). While still in the nucleus, this pri-miRNA is bound and cleaved by a microprocessor complex composed of Drosha and Dgcr8. Processing of the pri-miRNA by this complex leads to the formation of a shorter hairpin-like structure defined as a precursor miRNA (pre-miRNA) [42,43]. The pre-miRNA is then exported into the cytoplasm via Exportin 5 where it undergoes further cleavage by the RNAase enzyme Dicer, thereby forming a complementary duplex of two miRNA strands. Unwinding of this duplex releases one of the RNA strands, while the remaining strand is bound to an AGO protein in the RNA-induced silencing complex (RISC). The mature miRNA, coupled with RISC (now referred to as miRISC), then functions to detect complementary sequences inside messenger RNAs, usually found in the 3′-untranslated region of the target mRNA [43,44]. The binding of this miRISC complex to a target mRNA results in silencing of the mRNA, which may occur either by degrading the target transcript via the endonuclease activity of AGO2, or by simply preventing translation of the target transcript in cases of less perfect complementarity.
Although studies have generally focused on the regulation of mRNA by the canonical RNAi pathway, there is considerable evidence that interaction between canonical RNAi and other ncRNA signaling pathways occurs and may have broad ramifications in neuroplasticity and cognition (reviewed in [45]). Many miRNAs are expressed in the brain, and a number of studies have examined their role in cognitive disorders (Table 1) [46]. Indeed, roles for miRNA have been attributed to molecular signaling in neurodevelopment [47], neural stem cell differentiation [48,49] and cortical neurodegeneration [50,51], where knockout of the enzyme Dicer results in the dysregulation of these processes. Intriguingly, studies using an inducible Dicer1 knockout mouse model demonstrate improved performance in several memory tasks, suggesting that miRNA signaling may also act to restrict memory formation in some cases [52].
Table 1 . Examples of noncoding RNAs in epigenetically-linked cognitive disorders.
| Name | Type | Putative epigenetic MOA | Disorder | Ref. |
|---|---|---|---|---|
| 17A |
lncRNA |
None indicated |
AD |
[53] |
| 1810014B01Rik |
lncRNA |
None indicated |
AD, PD |
[53] |
| BACE1-AS |
lncRNA |
None indicated |
AD |
[54] |
| GDNFOS |
lncRNA |
None indicated |
AD |
[53] |
| Gomafu |
lncRNA |
Binds to the polycomb repressor complex, PRC1 |
Scz, Anx |
[55] |
| Malat–1 |
lncRNA |
Associates with the EZH2 subunit of the polycomb repressor complex |
Alc |
[56] |
| NAT-Rad18 |
lncRNA |
None indicated |
AD |
[53] |
| Sox2OT |
lncRNA |
None indicated |
AD, PD |
[57] |
| miR-106b |
miRNA |
None indicated |
Scz |
[58] |
| miR-125a |
miRNA |
– |
MDD |
[59] |
| miR-132 |
miRNA |
Targets the DNA methyltransferase DNMT3-α |
Scz, MDD |
[60,61] |
| miR-137 |
miRNA |
Targets the EZH2 subunit of the polycomb repressor complex; targets the histone demethylase KDM1A |
Scz |
[62] |
| miR-16 |
miRNA |
None indicated |
Scz |
[58] |
| miR-182 |
miRNA |
None indicated |
MDD |
[59,61] |
| miR-195 |
miRNA |
None indicated |
Scz |
[63] |
| miR-212 |
miRNA |
None indicated |
Scz |
[64] |
| miR-219-3p |
miRNA |
None indicated |
Scz |
[58] |
| miR-298 |
miRNA |
None indicated |
MDD |
[59] |
| miR-29c | miRNA | None indicated | AD | [65] |
AD: Alzheimers disease; Alc: Alcoholism; Anx: Anxiety; MDD: Major depressive disorder; MOA: Mechanism of Action; PD: Parkinsons disease; Scz: Schizophrenia.
Canonical miRNA signaling in schizophrenia
Among miRNA-related cognitive disorders, schizophrenia is perhaps the most extensively studied. Patients suffering from DiGeorge Syndrome often experience microdeletions affecting the miRNA microprocessing gene Dgcr8, and are 30-times more likely to suffer from schizophrenia or schizoaffective disorders [66]. This observation suggests a critical importance for miRNA signaling in schizophrenia. Postmortem studies in the brains of schizophrenic patients have identified dysregulation of two miRNAs, miR-132 and miR-219, both of which have been linked to schizophrenia-associated cognitive or behavioral impairments [60,67–69]. Both miRNAs are dysregulated in response to NMDA-receptor blockade [42], an interesting finding in light of the hypothesis that hypofunction/downregulation of the NMDA receptor plays a critical role in schizophrenia pathophysiology. A third candidate, miR-195, is increased in the brains of patients with schizophrenia, where it regulates several schizophrenia-related genes, including Bdnf [70], Reln, Vsnl1, Htr2a and Grin3 [71]. Indeed, in silico studies of miRNAs associated with transcription factors suggest that miR-195 plays a central role within a regulatory network of schizophrenia-related genes [63].
Because schizophrenia is a heterogeneous and complex disorder that involves several brain regions and cell types, the role miRNAs in the control of gene transcription must be considered in this context as well. For example, patients with schizophrenia are known to exhibit a wide range of up-regulated miRNAs in the superior temporal gyrus and prefrontal cortex, including miR-107, miR-15a, miR-15b, miR-16, miR-195, miR181b, let-7e, miR-20a, miR-26b, miR-19a [71]. Interestingly, none of the miRNAs described above are specific to neurons: miR-219 regulates oligodendrocyte maturation [72], while miR-195 and miR-132 are expressed in astrocytes [73–75]. Comprehensive studies should further elucidate the role of miRNAs in the nervous system, as well as test the possibility that global changes in miRNA processing may result in the disease's pathogenesis [71].
While the miRNAs described above target schizophrenia related genes directly, there is also evidence for miRNA-mediated epigenetic dysfunction in schizophrenia, and some schizophrenia-related miRNAs are known to be more directly involved in epigenetic regulation via the targeting of CMEs (Figure 1). Such regulatory miRNAs include miR-132, miR-212 and miR-195 [64,71,76–77]. miR-132, which was described above, is downregulated in the prefrontal cortex (PFC) of patients with schizophrenia, and regulates expression of DNA methyltransferase 3 α (DNMT3-α), thereby regulating DNA methylation [60]. This is especially interesting, considering that DNMT3-α is known to be upregulated some brain regions in schizophrenia – including the PFC (see [78] for review). Another promising candidate is miR-137, an miRNA identified as having a SNP strongly associated with schizophrenia in a meta-analysis of genome-wide association studies [79]. miR-137 targets multiple chromatin-modifying genes [80], including the histone-lysine N-methyl-transferase EZH2 [81], as well as the lysine-specific histone demethylase 1A (KDM1A), which governs neuron differentiation and migration [62]. While such epigenetic regulation may occur, future studies are required to determine the exact nature and extent of miRNA-mediated epigenetic regulation in the development and pathophysiology of cognitive disorders such as schizophrenia.
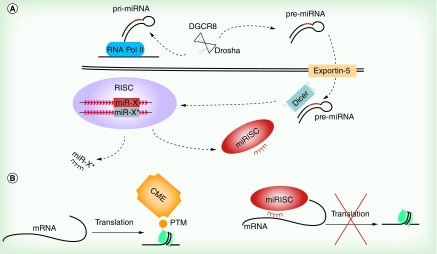
Figure 1. . Canonical mechanism of miRNA generation and epigenetic regulation.
(A) Schematic of canonical miRNA biogenesis. Pri-miRNA are transcribed by RNA Pol II, and stem-loop regions are processed by Drosha and DGCR8. The resulting pre-miRNA is exported through the nuclear membrane into the cytoplasm, where Dicer further cleaves the pre-miRNA into a short double-stranded RNA region. A guide strand is selected and bound by AGO within the RISC complex, while the passenger strand is cleaved and degraded. (B) miRNA and siRNA in conjunction with the cytoplasmic RISC complex target proteins involved in epigenetic regulation at the mRNA level. This post-transcriptional silencing, ultimately results in global alterations in the epigenome and cellular function.
AGO: Argonaute; CME: Chromatin-modifying enzyme; miRISC: miRNA in complex with RISC; PTM: Post-translational modifications; Pri-miRNA: Primary miRNA; Pre-miRNA: Precursor miRNA; RISC: RNA-induced silencing complex.
Canonical function of siRNA-mediated RNA inteference
There are significant functional similarities between miRNA- and siRNA-mediated RNAi. In this section we will highlight some of the more unique aspects of siRNA generation and regulation. Similar to miRNAs, siRNAs are short (˜21 nucleotide), noncoding transcripts that are canonically generated from exogenous dsRNAs. When siRNAs were first discovered in plants by David Baulcombe and colleagues [82], they appeared to function as part of a natural, antiviral immune response. Upon exposure, exogenous, double-stranded RNAs (dsRNAs) – often from viruses – are digested by Dicer in the cytoplasm, generating short RNA duplexes. These RNA duplexes are bound by AGO as part of the RISC complex and guide the complex to a complementary target, in this case, a copy of the viral RNA. Once bound, the endonuclease ‘slicer’ activity of AGO2 is further activated by the complementation of the siRNA-target interaction, thus mediating target destruction [83]. In this fashion, canonical siRNA-mediated RNAi initiation turns viral RNA against itself for destruction. Interestingly, newly discovered noncanonical mechanisms of endogenous siRNA (endo-siRNA) generation and function are gaining increased relevance in cognitive neuroscience. Below, we discuss emerging roles for siRNA-directed epigenetic regulation of gene expression changes.
Emerging mechanisms of siRNA directed epigenetic regulation
siRNAs participate in epigenetic regulation of genes through DNA methylation as well as by histone modification (Figure 2) [84,85]. The precise mechanism of siRNA generation depends on the organism involved. In Schizosaccaromyces pombe, endo-siRNAs are generated by an RNA-directed RNA polymerase complex (RDRC), and epigenetic regulation is carried out by the RNA-induced transcriptional silencing (RITS) complex, with the latter being dependent on siRNAs generated by the former (for review see [86]). In Arabidopsis thaliana, this process involves two plant-specific RNA polymerase II related RNA polymerase enzymes: Pol IV and Pol V [87]. First, transcripts from Pol IV are used as templates by the RNA-dependent RNA polymerase RDR2 to form dsRNA which is reduced into 24-nucleotide duplexes by the Dicer protein DCL3. From the cytoplasm, one strand of a duplex is then loaded onto AGO4, which translocate into the nucleus [88] and binds to complementary, nascent transcripts created by Pol V [89,90]. Once stabilized to a target transcript by Pol V and the Pol V transcript binding protein KTF1, AGO4 associates with the DRM2 DNA methyltransferase, a writer of the 5 mC epigenetic mark at CHH sites [91]. RDM1, a subunit of the final complex responsible for linking AGO4 to Pol V and DRM2, has itself an affinity for methylated DNA, a finding that suggests a predilection of the Pol V-AGO4 complex for pre-existing sites of methylated DNA. While still speculative, these studies suggest a parallel between siRNA-directed histone modification and siRNA-directed DNA methylation insofar as both may be mediated as part of a self-perpetuating feedback loop [92].
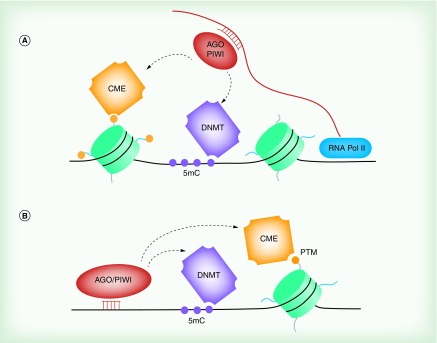
Figure 2. . Epigenetic regulation by nuclear short noncoding RNA.
Proposed mechanisms of sncRNA-mediated epigenetic regulation. (A) Studies have demonstrated short non-coding RNA (sncRNA)-mediated targeting of mRNA cotranscriptionally. This results in the recruitment of AGO or PIWI proteins to the gene locus of nascent transcripts and may result in the recruitment of CMEs and epigenetic regulation via DNA methylation or the post-translational modification of associated proteins, such as histones [93,94]. (B) sncRNAs in complex with AGO/PIWI also associate directly with DNA. This results in the recruitment of CMEs and epigenetic regulation [95]. AGO/PIWI indicates a member of either the argonaute or PIWI family of proteins.
CME: Chromatin-modifying enzyme; DNMT: DNA methyltransferase; PTM: Post-translational modifications; RNA Pol II: RNA polymerase II.
Although endo-siRNA generation and epigenetic function is well-characterized in S. pombe and A. thaliana, other studies speculate on a potential role in endogenous production of siRNAs and their epigenetic function in human cells [96,97]. Mammalian endo-siRNAs are known to be generated from hybridized mRNAs and antisense transcripts [97], which may then regulate the epigenome. An alternative pathway for the generation of such endo-siRNAs has also been identified in which a complex composed of human TERT (hTERT), Brahma-related gene 1 (BRG1) and nucleostemin (NS) – together referred to as the TBN complex-produces dsRNAs. These dsRNAs can then be processed into siRNAs that facilitate the formation of heterochromatic regions [98].
Promisingly, several studies demonstrate endo-siRNA-mediated histone methylation and DNA methylation in cultured mammalian cells [84–85,99]. The mechanistic actions of mammalian endo-siRNA are poorly characterized; moreover, a neurological role for these endo-siRNAs also remains to be established, as little neuroepigenetic research has focused on these mechanisms. Importantly, targeted sequencing studies demonstrate a large number of these RNAs in human somatic cells [100], and recent studies have identified putative endo-siRNA populations in hippocampal tissues [101,102]. Thus, indicating a potential epigenetic role for endo-siRNAs in neuronal alterations.
piRNAs: regulators of the epigenome and neuroplasticity
In exploring the role for ncRNAs in cognition and cognitive sciences, piRNAs have become a topic of some intriguing investigations. piRNAs are distinguished from siRNAs by their size (they are slightly longer at 26–31 nt rather than 20–24 nt), and association with PIWI proteins, a clade of the AGO family [103]. Unlike miRNAs and siRNAs, piRNAs are generated from single-stranded RNA species in a Dicer-independent manner [104]. piRNAs are preeminently expressed and best characterized in the context of germ cell development [105–107]. Indeed, the name ‘PIWI’ has its humorous origin owing to the discovery of the ‘P-element induced wimpy testis’ in the gonadal cells of Drosophila. PIWI proteins translocate into the nucleus in an RNA-dependent manner, guided by piRNAs [107,108] where they serve to silence transposons in the nuclei of germ cells [109], ostensibly for the purpose of genome protection in the vulnerable germline DNA. However, this functionality is not exclusive, as protein-coding genes may also code for piRNAs [110]. Moreover, recent studies provide evidence for numerous piRNAs expressed in adult organ tissues, including in the brain, suggesting new possibilities for epigenome regulation in neurons [111,112].
With regard to epigenetic initiation and targeting, piRNAs have been shown to target heterochromatic regions with the help of bound heterochromatin protein 1a (HP1a) as part of a PIWI-piRNA complex [113]. This complex typically associates with repressive histone lysine methylation marks, but may also facilitate transcription [114], and some evidence suggests that piRNA could form an initiator complex on chromatin that recruits other chromatin-modifying agents [115]. Additionally, Carmell and colleagues provide an interesting set of studies that suggests chromatin regulation by piRNAs. Specifically, Carmell and colleagues demonstrate that knockout of a murine PIWI results in increased transposon expression due to a loss of inhibitory DNA methylation at transposon sites [116]. Further elucidation of this mechanism by Aravin and colleagues revealed that piRNA-mediated silencing of transposons by PIWI orthologs plays a significant role in maintaining the genome integrity of the mouse testis [117,118]. Interestingly, some transposable elements have been identified as sources of dsRNAs, which feed into the endo-siRNA pathway, suggesting a degree of redundancy between endo-siRNA and piRNA pathways [104]. While still largely unexplored in mammalian systems, at least one population of piRNAs has been identified in the murine hippocampus via next-generation sequencing [119].
With regard to the neuroepigenetic function of piRNAs, recent studies suggest an epigenetically-active population of serotonin-induced piRNAs in the CNS of Aplysia. These studies demonstrate (via knockout of PIWI) the necessity of PIWI for serotonin induced long-term facilitation [111] – a synaptic correlate for memory formation. piRNAs have also been demonstrated to silence CREB2 – a suppressor of memory formation- in an activity-dependent manner in Aplysia [111], further supporting the idea that piRNA signaling is necessary for memory formation. Collectively, these results are suggestive of a broader role for piRNAs in epigenetic regulation than was previously expected and future studies will likely uncover additional piRNAs mediating neuroepigenetic regulation.
lncRNA
Discovery & characterization of lncRNAs
Typically, sncRNAs can be separated into distinct classes by clearly defined homologies of structure and function, while lncRNAs represent a heterogeneous and often modular set of transcripts [120–122]. As a result, the most common working nomenclature of lncRNA structure describes transcripts on the basis of their genomic location relative to nearby protein-coding genes [23,120]. Among these subcategories of lncRNAs are antisense, bidirectional, intergenic, intronic and overlapping transcripts (Figure 3).
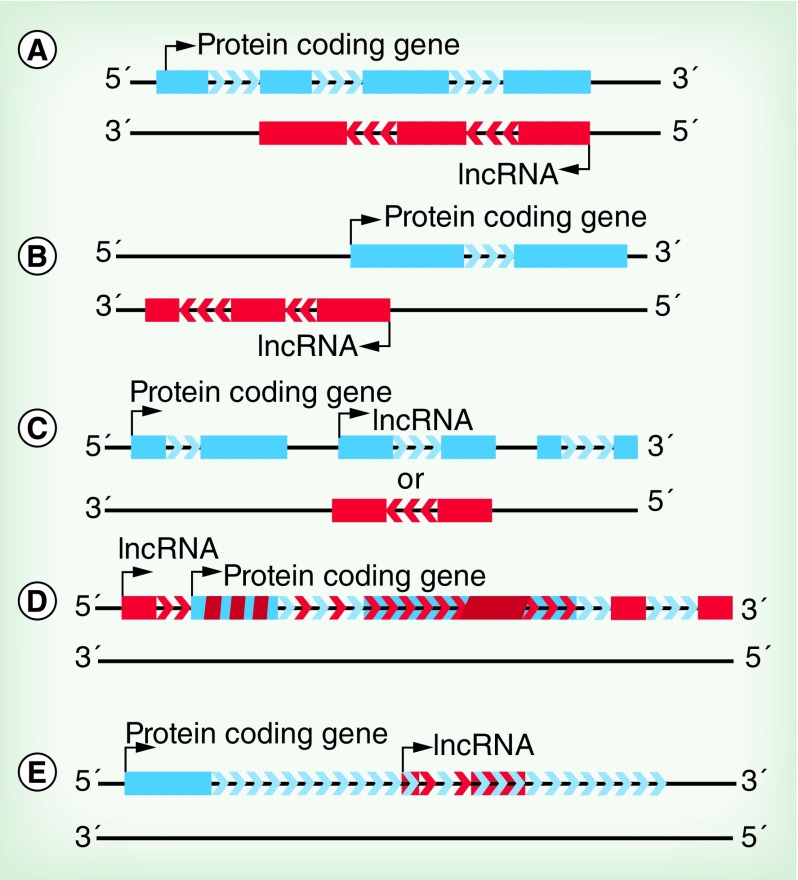
Figure 3. . Origins of long noncoding RNAs.
(A–E) Many lncRNA genomic loci are colocalized with protein coding genes, and they are often described in relation to these genes. A number of common naming conventions have come into general use to describe the various protein coding gene associated lncRNAs. (A) Antisense transcripts overlap protein coding genes, but are transcribed from the antisense strand. (B) Bidirectional transcripts share transcription start sites with protein coding genes, but are transcribed in the opposite direction. (C) Intergenic transcripts do not overlap with protein coding genes. (D) Overlapping transcripts overlap significantly with or encompass protein coding genes on the sense strand. (E) Intronic transcripts are located within a sense-strand intron of a protein coding gene. Solid bars indicate exons of mRNAs (blue), lncRNAs (red). Diagonal stripes indicate overlapping exons. Chevron arrows indicate introns of mRNAs (blue), lncRNAs (red) or overlapping transcripts (alternating red and blue). Curved arrows indicate transcription start sites.
Although the first functional role attributed to a lncRNA was described prior to the discovery of sncRNAs [123], it is only recently that the abundance of lncRNAs in the mammalian transcriptome has been fully recognized. Recent studies have identified thousands of lncRNA genes in the human transcriptome [19,124]. While these studies have expanded our knowledge of the transcriptome, most observations are still limited in scope to cultured cells and resting state expression profiles within tissues. Given the highly specific expression profiles of many known lncRNAs and their comparatively lower expression levels (ten-fold lower than protein coding genes, on average) [125,126], it is likely that many functional lncRNA transcripts are expressed below the power of detection for such studies. Indeed, novel deep-sequencing methodologies demonstrate that the full transcriptome is much larger than current sequencing studies have revealed [127–129]. A thorough investigation of lncRNA abundance will likely require the targeted transcriptional profiling of specific tissues, cell types or perturbations. Moreover, as many as 80% of mammalian protein coding loci express some form of antisense transcript along with their respective mRNAs [130–132]. Antisense transcripts can often impact the regulation of associated protein coding genes [133], though this is not a necessity, nor does it preclude additional in trans effects [23].
In addition to shared genomic loci, lncRNAs and mRNAs share characteristics that distinguish them from other small RNA species. Similar to mRNAs, lncRNAs demonstrate properties such as chromatin structure typical of RNA polymerase II (Pol II) transcription, alternative splicing sites and regulation by transcription factors [134]. Furthermore, many lncRNAs are polyadenylated and capped with 5′-methylguanisine [124]. There have even been reports of lncRNAs associating with ribosomes – although ribosome profiling experiments suggest that such associations are usually inactive [135,136]. Surprisingly, some translation of lncRNAs has been observed, resulting in the expression of small proteins products, though recent studies suggest that global translation of all ncRNAs may occur in a manner resembling pervasive gene transcription [137], though the importance of this mechanism remains to be defined in any cell system.
lncRNAs impact cellular processes via a number of molecular mechanisms. These include regulation of transcription [138–140], epigenetic regulation [141], scaffolding of protein complexes [142,143], guiding of regulatory complexes [143], acting as decoys to regulatory complexes [144] or simply being transcribed [145]. These mechanisms of action often rely on the ability of RNAs to bind both proteins and nucleic acids in a targeted manner. An RNA molecule's primary structure – that is, the linear sequence of nucleotides – allows RNA transcripts to bind homologous DNA regions via canonical or noncanonical base pairing. Recently, tools have been developed for the computational prediction of lncRNA DNA-binding motifs and binding sites [146]. Similar hybridization also allows single stranded RNA to fold into complex secondary and tertiary structures, and to bind with other RNA molecules. It is these structural arrangements, in addition to sequence specificity, that often underlie interactions with RNA-binding proteins (RBPs) [147,148]. Many currently known mechanisms of lncRNA activity rely largely on interaction with RBPs and alterations in localization, activity or association with other proteins. RBPs are a functionally and structurally diverse class of molecules, and recent studies have estimated that 40% of RBPs (out of a cohort of 1542 RBPs) are involved in ncRNA-related processes [149]. Additionally, lncRNAs have been observed to bind and regulate other small RNA molecules such as miRNAs [150,151], and extensive noncoding interactomes have been proposed [152].
In the nucleus, lncRNAs modulate gene expression via regulation of transcription and the epigenetic landscape (Figure 4) [134,153]. Indeed, lncRNAs can bind to a number of CMEs, usually ‘writers’ of epigenetic marks [153]. The extent of such a phenomenon was established in 2009, when it was shown that some 20% of lncRNAs (out of a cohort of 3300) associate with the PRC2, a histone methyltransferase that catalyzes the addition of repressive H3K27 methylation [154]. Additionally, binding of lncRNAs to CMEs can prevent or restrict CME activity, as was recently demonstrated to occur at the CEBPA locus, where an overlapping lncRNA (sometimes described as an extracoding RNA or ecRNA) preferentially binds the DNA methyltransferase DNMT1 [144], ultimately leading to decreased local DNA methylation. Interestingly, this phenomenon is not restricted to the CEBPA locus but occurs at multiple methylation sites across the epigenome [144].
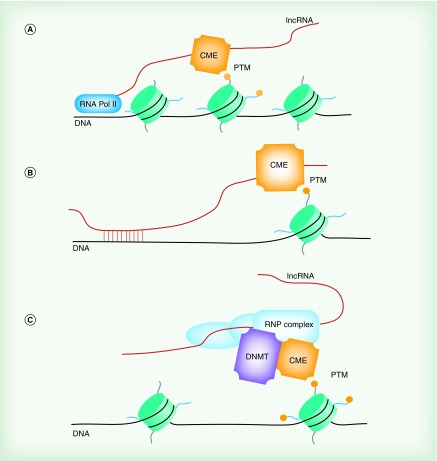
Figure 4. . lncRNA-mediated epigenetic regulation.
lncRNAs possess a number of mechanisms by which they initiate or facilitate epigenetic regulation, and multiple archetypal functions are often utilized within a single lncRNA transcript. (A) lncRNAs often recruit chromatin-modifying enzymes in cis, thereby mediating epigenetic regulation of nearby genes (dimitrova, zhang, redrup). (B) lncRNAs may also act as guides, targeting associated CMEs to target loci in trans, potentially through direct interaction with target regions. (C) lncRNAs may act as scaffolding factors, and mediate the assembly of ribonucleoprotein complexes with multiple regulatory functions. This may occur either in cis, as occurs with the direct CMEs to target loci in trans.
CME: Chromatin-modifying enzyme; DNMT: DNA methyltransferase; PTM: Post-translational modifications; RNA Pol II: RNA polymerase II; RNP complex: Ribonucleoprotein complex.
lncRNAs in cognitive disorders
The importance of epigenetics is become increasingly recognized in neuronal alterations and cognitive function. A recent GWAS study of common cognitive disorders found that epigenetic – specifically, histone methylation – pathways were strongly associated with impaired cognition [9], and a number of screening studies suggest that lncRNA dysregulation is associated with neurodevelopmental and cognitive disorders [155], including Rett syndrome [156], autism [157] and Fragile X syndrome [55]. While the widespread mechanisms of ncRNA-mediated regulation have been established for some time, only in very recent years have these mechanisms been investigated in a neurological or cognitive context. lncRNAs have been found to be co-expressed with genes that are critical for neuronal activity, including C-fos, Arc and BDNF, suggesting an extensive network of protein coding and noncoding genes involved in neuronal plasticity [55,158]. Additionally, lncRNAs are known to play a role in normal brain development [159]. While the majority of lncRNA transcripts have been characterized either in cell culture or during development, efforts to examine the functional roles of neuronal lncRNAs in cognition are still continuing. Here, we will recount some of the better-characterized examples of lncRNAs functioning in the context of the adult brain, and their impact on cognition or cognitive disorders.
Malat1
One lncRNA that has been observed to regulate neuronal activity is Malat1 (also known as Neat2). This highly conserved nuclear lncRNA is expressed in numerous tissues, with a high degree of expression in neurons [160]. Knockdown studies of Malat1 have resulted in decreased synaptic density in cultured hippocampal neurons [160], and postmortem studies have demonstrated that Malat1 is upregulated in multiple brain regions in both human alcoholism as well as rodent models of alcoholism [56]. Malat1 can regulate gene expression in cis, thus controlling the expression of proximally located genes which are involved in nuclear function [138]. Conversely, Malat1 can associate with hundreds of sites in trans, where it preferentially binds the gene body of active genes in a transcription-dependent fashion [161]. Epigenetically, Malat1 has been shown to associate in vivo with EZH2, a subunit of the PRC2 [162]. Interestingly, and despite many functional associations, Malat1 knockout in mice does not affect viability or normal development [138,163].
Gomafu
The lncRNA Gomafu has also been shown to play multiple roles in the adult brain. Gomafu has been observed to govern SZ-related alternative splicing by acting as a splicing factor scaffold for QK1 and SRSF1, and it is known to be dysregulated in postmortem studies of schizophrenia patients [164]. Recently, an additional study has suggested that Gomafu functions in cis to mediate epigenetic regulation of gene expression via the PRC1 complex, and that knockdown of Gomafu in adult mice results in abnormal behavioral phenotypes and increased anxiety [55].
BACE1-AS
Another example of lncRNAs involved in neuronal disorders, is the antisense lncRNA, BACE1-AS that has been implicated in Alzheimer's disease (AD). AD is a progressive neuro-degenerative disorder which has been previously associated with epigenetic dysregulation, particularly in histone acetylation [165,166]. A characteristic marker of AD pathology is the accumulation of β amyloid plaques consisting of oligomerized amyloid β peptides. These plaques form as a result of the processing of amyloid precursor protiens (APP), the rate limiting step of which is the cleavage of APP by the Beta-secretase enzyme (BACE1) [167]. Dysregulation of BACE1 contributes to AD pathology via the overproduction of Aβ [167]. Recent studies have identified an antisense lncRNA at the BACE1 locus (BACE1-AS) which physically associates with and stabilizes BACE1 mRNA, increasing BACE1 expression both in vitro and in vivo, and ultimately resulting in increased generation of Aβ [54]. BACE1 mRNA is targeted by the miR-485–5p, which normally results in BACE1 repression; however, BACE1-AS prevents this repression by competitively binding the miRNA target site [168]. Both the BACE1-AS lncRNA and BACE1 mRNA are overexpressed in the parietal lobe and in the cerebellum of postmortem AD patients, suggesting a relevant mechanistic link between the BACE1-AS lncRNA and the pathophysiology of AD [168]. Interestingly, knockdown of BACE1 or BACE1-AS results in reduction of Alzheimer's pathology in an APP mouse model of AD [169].
While the dysregulation of lncRNAs has been implicated in cognitive disorders, the task of exploring the role of lncRNA-mediated epigenetic regulation in normal cognitive function remains incomplete.
Transgenerational impacts of ncRNA-mediated epigenetic regulation
Since the discovery of epigenetics, there has been much curiosity and speculation as to the transgenerational heritability of epigenetic marks. In mammals, much of the epigenome is erased during the processes of fertilization and generation of primary germ cells (reviewed in [170]); nonetheless, evidence of a transgenerationally altered epigenome has steadily accumulated, including heritable cognitive changes and behavioral phenotypes [171–175]. A simple explanation for this phenomenon would be incomplete erasure of DNA and histone modifications. While there is some evidence in support of this hypothesis (reviewed in [176]), other studies have demonstrated the existence of an indirect mechanism of chromatin regulation via generational transfer of ncRNAs.
Recently developed mammalian epimutation models – in which phenotypes are derived from a heritable change in gene expression, as opposed to an altered genome – have demonstrated the sufficiency of parental RNA to alter the epigenome of treated progeny [177]. Additionally, in a rodent stress model, treatment of fertilized mouse oocytes with ncRNAs from the sperm of stressed males is sufficient to recapitulate heritable stress-related behavioral and metabolic phenotypes [173]. These results indicate that alterations in the transcriptome are sufficient for the transfer of epigenetic information across generations, and play a critical role in cognitive function.
The most direct evidence for a neuronal role in transgenerational epigenetic phenomenon comes from C. elegans, where neuronally expressed RNA species are transported to the cells of the germline. These RNAs then initiate the transgenerational epigenetic silencing of particular genomic loci, thereby impacting gene expression in the germ line and, potentially, in any progeny [178]. It is tempting to speculate that an analogous mechanism could exist in mammals, by which somatic tissues such those of the brain may regulate the epigenome of cells distant in both space and time. Clearly, such a finding would have far-reaching consequences for cognitive science.
Conclusion & future perspective
While numerous studies have found associations between ncRNAs, cognition and cognitive disorders, few have fully investigated and characterized the diverse mechanisms that can be attributed to ncRNAs. In this review, we provide insights for future direction in the investigation of different classes of ncRNAs and discuss regulatory RNAs that have both established roles in cellular and molecular processes and a defined relationship to the epigenome. Thus, we present the provocative research idea that ncRNAs might serve to control epigenetic mechanisms involved in cognition by illustrating the few cases of such phenomena that have been described in the literature.
To date, only a few regulatory RNAs have been discovered to have both epigenetic and cognitive relevance. However, these few examples underscore the extent to which the numerous and heterogeneous classes of regulatory RNAs are still unexplored. Anatomically, these species are expressed primarily within the cognitive centers of the brain, and indeed, their relevance to cognition is well established. However, emerging studies are beginning to explore beyond the canonical pathways of regulatory RNA function established in previous decades. The studies we have reviewed here demonstrate the long-term impact of regulatory RNAs on the epigenome and thereby cognition. Although the canonical functions of ncRNAs involve diverse mechanisms, new insights suggest that several classes of ncRNAs impact the epigenome, a common ground where both protein and RNA species converge to regulate cellular function. Groundbreaking studies are beginning to demonstrate that epigenetic regulation by ncRNAs is – to a yet poorly explored extent – actively influencing neuronal and cognitive function. Therefore, it is likely that future studies will focus on increasing knowledge of ncRNA-mediated epigenetic regulation on well-characterized cognitive functions, such as memory formation. Moreover, we fully expect that further investigations into the role of regulatory RNAs will reveal novel epigenetic roles for this versatile class of molecules in cognitive disorders.
Executive summary.
This review discusses mechanisms of multiple classes of nonoding RNAs (ncRNAs) and how they relate to the epigenome.
Schizophrenia, a cognitive disorder with epigenetic dysfunction, is used to describe a possible role for canonical miRNA species in the regulation of epigenetic mechanisms.
A description of small interfering RNA mechanism of action is provided, and newly defined mechanisms of epigenetic regulation not yet investigated in cognition or cognitive dysfunction.
An introduction to PIWI-interacting RNAs is provided along with studies describing the potential role of PIWI-interacting RNAs as regulators of the epigenome in neuroplasticity.
We present a description of long noncoding RNAs, a newly defined class of ncRNAs, and describe the roles of several long noncoding RNAs in cognition and cognitive disorders. Moreover, we highlight new studies indicating that long ncRNAs impact cognition via their role as epigenetic regulators.
Importantly, we highlight recent studies on the transgenerational impact of ncRNAs and the epigenome on cognitive function.
The key findings presented throughout this review warrant future studies of ncRNAs, as related to the epigenome and cognitive function.
Acknowledgements
The authors apologize to all their colleagues whose important work were not directly cited, or were overlooked in the preparation of this manuscript.
Footnotes
Financial & competing interests disclosure
This work was supported by the National Institute of Mental Health (MH082106, MH097909), the UAB Intellectual and Developmental Disabilities Research Center (P30-HD38985) and the Evelyn F McKnight Brain Research Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Bonasio R, Tu S, Reinberg D. Molecular signals of epigenetic states. Science. 2010;330:612–616. doi: 10.1126/science.1191078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gan Q, Yoshida T, McDonald OG, Owens GK. Concise review: epigenetic mechanisms contribute to pluripotency and cell lineage determination of embryonic stem cells. Stem Cells. 2007;25:2–9. doi: 10.1634/stemcells.2006-0383. [DOI] [PubMed] [Google Scholar]
- 3.Hemberger M, Dean W, Reik W. Epigenetic dynamics of stem cells and cell lineage commitment: digging Waddington's canal. Nat. Rev. Mol. Cell Biol. 2009;10:526–537. doi: 10.1038/nrm2727. [DOI] [PubMed] [Google Scholar]
- 4.Maze I, Noh K-M, Allis CD. Histone regulation in the CNS: basic principles of epigenetic plasticity. Neuropsychopharmacology. 2013;38(1):3–22. doi: 10.1038/npp.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juliandi B, Abematsu M, Nakashima K. Epigenetic regulation in neural stem cell differentiation. Dev. Growth Differ. 2010;52:493–504. doi: 10.1111/j.1440-169X.2010.01175.x. [DOI] [PubMed] [Google Scholar]
- 6.Yu Y, Casaccia P, Richard Lu Q. Shaping the oligodendrocyte identity by epigenetic control. Epigenetics. 2010;5:124–128. doi: 10.4161/epi.5.2.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudenko A, Tsai L-H. Epigenetic modifications in the nervous system and their impact upon cognitive impairments. Neuropharmacology. 2014;80(2014):70–82. doi: 10.1016/j.neuropharm.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 8.Lubin FD, Gupta S, Parrish RR, Grissom NM, Davis RL. Epigenetic mechanisms: critical contributors to long-term memory formation. Neuroscientist. 2011;17(6):616–632. doi: 10.1177/1073858411386967. [DOI] [PubMed] [Google Scholar]
- 9.Network P. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat. Neurosci. 2015;18(2):199–209. doi: 10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta-Agarwal S, Franklin AV, DeRamus T, et al. G9a/GLP histone lysine dimethyltransferase complex activity in the hippocampus and the entorhinal cortex is required for gene activation and silencing during memory consolidation. J. Neurosci. 2012;32:5440–5453. doi: 10.1523/JNEUROSCI.0147-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarome TJ, Lubin FD. Epigenetic mechanisms of memory formation and reconsolidation. Neurobiol. Learn. Mem. 2014;115:116–127. doi: 10.1016/j.nlm.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reviews the considerable body of work on the epigenetics of memory consolidation and reconsolidation. As memory is one of the best studied cognitive phenomenon, this provides an introduction to the wide scope of epigenetic mechanisms in cognition.
- 12.Liang J, Wan M, Zhang Y, et al. Nanog and Oct4 associate with unique transcriptional repression complexes in embryonic stem cells. Nat. Cell Biol. 2008;10(6):731–739. doi: 10.1038/ncb1736. [DOI] [PubMed] [Google Scholar]
- 13.Vento-Tormo R, Rodriguez-Ubreva J, Lisio LD, et al. NF-ĸB directly mediates epigenetic deregulation of common microRNAs in Epstein–Barr virus-mediated transformation of B-cells and in lymphomas. Nucleic Acids Res. 2014;42(17):11025–11039. doi: 10.1093/nar/gku826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-ĸB determines its association with CBP/p300 or HDAC-1. Mol. Cell. 2002;9(3):625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- 15.Harrow J, Denoeud F, Frankish A, et al. GENCODE: producing a reference annotation for ENCODE. Genome Biol. 2006;7(Suppl. 1):S4.1–9. doi: 10.1186/gb-2006-7-s1-s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravasi T, Suzuki H, Pang KC, et al. Experimental validation of the regulated expression of large numbers of non-coding RNAs from the mouse genome. Genome Res. 2006;16:11–19. doi: 10.1101/gr.4200206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercer T, Mercer T, Dinger M, et al. Specific expression of long noncoding RNAs in the mouse brain. Proc. Natl Acad. Sci. USA. 2008;105:716–721. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lunnon K, Smith R, Hannon E, et al. Methylomic profiling implicates cortical deregulation of ANK1 in Alzheimer's disease. Nat. Neurosci. 2014;17(9):1164–1170. doi: 10.1038/nn.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrow J, Frankish A, Gonzalez JM, et al. GENCODE: the reference human genome annotation for the ENCODE project. Genome Res. 2012;22:1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Candeias MM, Malbert-Colas L, Powell DJ, et al. P53 mRNA controls p53 activity by managing Mdm2 functions. Nat. Cell Biol. 2008;10(9):1098–1105. doi: 10.1038/ncb1770. [DOI] [PubMed] [Google Scholar]
- 21.Gajjar M, Candeias MM, Malbert-Colas L, et al. The p53 mRNA-Mdm2 interaction controls Mdm2 nuclear trafficking and is required for p53 activation following dna damage. Cancer Cell. 2012;21(1):25–35. doi: 10.1016/j.ccr.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Naski N, Gajjar M, Bourougaa K, Malbert-Colas L, Fåhraeus R, Candeias MM. The p53 mRNA-Mdm2 interaction. Cell Cycle. 2009;8(1):31–34. doi: 10.4161/cc.8.1.7326. [DOI] [PubMed] [Google Scholar]
- 23.Mattick JS, Rinn JL. Discovery and annotation of long noncoding RNAs. Nat. Publ. Gr. 2015;22(1):5–7. doi: 10.1038/nsmb.2942. [DOI] [PubMed] [Google Scholar]; • This recent review provides a thorough review of lncRNA discovery and annotation, described by two of the most prominent RNA biologists involved.
- 24.Cam HP. Roles of RNAi in chromatin regulation and epigenetic inheritance. Epigenomics. 2010;2:613–626. doi: 10.2217/epi.10.46. [DOI] [PubMed] [Google Scholar]; • This review discusses the RNAi pathway as it relates to heterochromatin assembly – and therefore, transcriptional regulation – in yeast.
- 25.Schaukowitch K, Kim T-K. Emerging epigenetic mechanisms of long non-coding RNAs. Neuroscience. 2014;264:25–38. doi: 10.1016/j.neuroscience.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 27.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 28.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 29.Friedman RC, Farh KKH, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffiths-Jones S. The microRNA Registry. Nucleic Acids Res. 2004;32:D109–D111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffiths-Jones S, Saini HK, Van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36(Suppl. 1):D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozomara A, Griffiths-Jones S. MiRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39(Suppl. 1):D152–D157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozomara A, Griffiths-Jones S. MiRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42(D1):D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonardo TR, Schultheisz HL, Loring JF, Laurent LC. The functions of microRNAs in pluripotency and reprogramming. Nat. Cell Biol. 2012;14(11):1114–1121. doi: 10.1038/ncb2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ichimura A, Ruike Y, Terasawa K, Tsujimoto G. miRNAs and regulation of cell signaling. FEBS J. 2011;278(10):1610–1618. doi: 10.1111/j.1742-4658.2011.08087.x. [DOI] [PubMed] [Google Scholar]
- 37.Ono K, Kuwabara Y, Han J. MicroRNAs and cardiovascular diseases. FEBS J. 2011;278(10):1619–1633. doi: 10.1111/j.1742-4658.2011.08090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Valverde SL, Taft RJ, Mattick JS. MicroRNAs in β-cell biology, insulin resistance, diabetes and its complications. Diabetes. 2011;60(7):1825–1831. doi: 10.2337/db11-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bredy TW, Lin Q, Wei W, Baker-Andresen D, Mattick JS. MicroRNA regulation of neural plasticity and memory. Neurobiol. Learn. Mem. 2011;96(1):89–94. doi: 10.1016/j.nlm.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Park CY, Choi YS, McManus MT. Analysis of microRNA knockouts in mice. Hum. Mol. Genet. 2010;19(R2):R169–R175. doi: 10.1093/hmg/ddq367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 43.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 44.Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17(3):118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Barry G. Integrating the roles of long and small non-coding RNA in brain function and disease. Mol. Psychiatry. 2014;19(4):410–416. doi: 10.1038/mp.2013.196. [DOI] [PubMed] [Google Scholar]
- 46.Kocerha J, Dwivedi Y, Brennand KJ. Noncoding RNAs and neurobehavioral mechanisms in psychiatric disease. Mol. Psychiatry. 2015;20(6):677–684. doi: 10.1038/mp.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Pietri Tonelli D, Pulvers JN, Haffner C, Murchison EP, Hannon GJ, Huttner WB. miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development. 2008;135(23):3911–3921. doi: 10.1242/dev.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang T, Liu Y, Huang M, Zhao X, Cheng L. Wnt1-cre-mediated conditional loss of Dicer results in malformation of the midbrain and cerebellum and failure of neural crest and dopaminergic differentiation in mice. J. Mol. Cell Biol. 2010;2(3):152–163. doi: 10.1093/jmcb/mjq008. [DOI] [PubMed] [Google Scholar]
- 49.Kawase-Koga Y, Low R, Otaegi G, et al. RNAase-III enzyme Dicer maintains signaling pathways for differentiation and survival in mouse cortical neural stem cells. J. Cell Sci. 2010;123(Pt 4):586–594. doi: 10.1242/jcs.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hébert SS, Papadopoulou AS, Smith P, et al. Genetic ablation of Dicer in adult forebrain neurons results in abnormal tau hyperphosphorylation and neurodegeneration. Hum. Mol. Genet. 2010;19(20):3959–3969. doi: 10.1093/hmg/ddq311. [DOI] [PubMed] [Google Scholar]
- 51.Davis TH, Cuellar TL, Koch SM, et al. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J. Neurosci. 2008;28(17):4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konopka W, Kiryk A, Novak M, et al. MicroRNA loss enhances learning and memory in mice. J. Neurosci. 2010;30(44):14835–14842. doi: 10.1523/JNEUROSCI.3030-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu P, Zuo X, Deng H, Liu X, Liu L, Ji A. Roles of long noncoding RNAs in brain development, functional diversification and neurodegenerative diseases. Brain Res. Bull. 2013;97:69–80. doi: 10.1016/j.brainresbull.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Liu T, Huang Y, Chen J, et al. Attenuated ability of BACE1 to cleave the amyloid precursor protein via silencing long noncoding RNA BACE1-AS expression. Mol. Med. Rep. 2014;10(3):1275–1281. doi: 10.3892/mmr.2014.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spadaro PA, Flavell CR, Widagdo J, et al. Long noncoding RNA-directed epigenetic regulation of gene expression is associated with anxiety-like behavior in mice. Biol. Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.02.004. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates lncRNA-mediated epigenetic disruptions accompanied by a perturbed cognitive state.
- 56.Kryger R, Fan L, Wilce PA, Jaquet V. MALAT–1, a non protein-coding RNA is upregulated in the cerebellum, hippocampus and brain stem of human alcoholics. Alcohol. 2012;46(7):629–634. doi: 10.1016/j.alcohol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Fenoglio C, Ridolfi E, Galimberti D, Scarpini E. An emerging role for long non-coding RNA dysregulation in neurological disorders. Int. J. Mol. Sci. 2013;14(10):20427–20442. doi: 10.3390/ijms141020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smalheiser NR, Lugli G, Zhang H, Rizavi H, Cook EH, Dwivedi Y. Expression of micrornas and other small RNAs in prefrontal cortex in schizophrenia, bipolar disorder and depressed subjects. PLoS ONE. 2014;9(1):1–12. doi: 10.1371/journal.pone.0086469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao M-Q, Chen D-H, Zhang C-H, Wu Z-Z. [Screening of specific microRNA in hippocampus of depression model rats and intervention effect of Chaihu Shugan San] Zhongguo Zhong Yao Za Zhi. 2013;38(10):1585–1589. [PubMed] [Google Scholar]
- 60.Miller BH, Zeier Z, Xi L, et al. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc. Natl Acad. Sci. 2012;109(8):3125–3130. doi: 10.1073/pnas.1113793109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li YJ, Xu M, Gao ZH, et al. Alterations of serum levels of BDNF-related miRNAs in patients with depression. PLoS ONE. 2013;8(5):1–7. doi: 10.1371/journal.pone.0063648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun G, Ye P, Murai K, et al. miR-137 forms a regulatory loop with nuclear receptor TLX and LSD1 in neural stem cells. Nat. Commun. 2011;2:529. doi: 10.1038/ncomms1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo A-Y, Sun J, Jia P, Zhao Z. A novel microRNA and transcription factor mediated regulatory network in schizophrenia. BMC Syst. Biol. 2010;4:10. doi: 10.1186/1752-0509-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tognini P, Putignano E, Coatti A, Pizzorusso T. Experience-dependent expression of miR-132 regulates ocular dominance plasticity. Nat. Neurosci. 2011;14(10):1237–1239. doi: 10.1038/nn.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lei X, Lei L, Zhang Z, Zhang Z, Cheng Y. Downregulated miR-29c correlates with increased BACE1 expression in sporadic Alzheimer's disease. Int. J. Clin. Exp. Pathol. 2015;8(2):1565–1574. [PMC free article] [PubMed] [Google Scholar]
- 66.Stark KL, Xu B, Bagchi A, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat. Genet. 2008;40(6):751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- 67.Kocerha J, Faghihi MA, Lopez-Toledano MA, et al. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc. Natl Acad. Sci. USA. 2009;106(9):3507–3512. doi: 10.1073/pnas.0805854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moreau MP, Bruse SE, David-Rus R, Buyske S, Brzustowicz LM. Altered MicroRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol. Psychiatry. 2011;69(2):188–193. doi: 10.1016/j.biopsych.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santarelli DM, Beveridge NJ, Tooney PA, Cairns MJ. Upregulation of Dicer and MicroRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol. Psychiatry. 2011;69(2):180–187. doi: 10.1016/j.biopsych.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 70.Mellios N, Huang HS, Grigorenko A, Rogaev E, Akbarian S. A set of differentially expressed miRNAs, including miR-30a-5p, act as post-transcriptional inhibitors of BDNF in prefrontal cortex. Hum. Mol. Genet. 2008;17(19):3030–3042. doi: 10.1093/hmg/ddn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beveridge NJ, Gardiner E, Carroll AP, Tooney PA, Cairns MJ. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol. Psychiatry. 2010;15(12):1176–1189. doi: 10.1038/mp.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dugas JC, Cuellar TL, Scholze A, et al. Dicer1 and miR-219 are required for normal oligodendrocyte differentiation and myelination. Neuron. 2010;65(5):597–611. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moser JJ, Fritzler MJ. The microRNA and messengerRNA profile of the RNA-induced silencing complex in human primary astrocyte and astrocytoma cells. PLoS ONE. 2010;5(10):e13445. doi: 10.1371/journal.pone.0013445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mor E, Cabilly Y, Goldshmit Y, et al. Species-specific microRNA roles elucidated following astrocyte activation. Nucleic Acids Res. 2011;39(9):3710–3723. doi: 10.1093/nar/gkq1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Numakawa T, Yamamoto N, Chiba S, et al. Growth factors stimulate expression of neuronal and glial miR-132. Neurosci. Lett. 2011;505(3):242–247. doi: 10.1016/j.neulet.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 76.Li Z, Yang C-S, Nakashima K, Rana TM. Small RNA-mediated regulation of iPS cell generation. EMBO J. 2011;30(5):823–834. doi: 10.1038/emboj.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang S, Hao J, Xie F, et al. Downregulation of miR-132 by promoter methylation contributes to pancreatic cancer development. Carcinogenesis. 2011;32(8):1183–1189. doi: 10.1093/carcin/bgr105. [DOI] [PubMed] [Google Scholar]
- 78.Grayson DR, Guidotti A. The dynamics of DNA methylation in schizophrenia and related psychiatric disorders. Neuropsychopharmacology. 2013;38(1):138–166. doi: 10.1038/npp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ripke S, Sanders AR, Kendler KS, et al. Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 2011;43(10):969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Akbarian S. Epigenetics of schizophrenia. Curr. Top. Behav. Neurosci. 2010;2010(4):611–628. doi: 10.1007/7854_2010_38. [DOI] [PubMed] [Google Scholar]
- 81.Szulwach KE, Li X, Smrt RD, et al. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J. Cell Biol. 2010;189(1):127–141. doi: 10.1083/jcb.200908151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hamilton AJ. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286(5441):950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- 83.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20(5):515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 84.Chen L, Dahlstrom JE, Lee SH, Rangasamy D. Naturally occurring endo-siRNA silences LINE–1 retrotransposons in human cells through DNA methylation. Epigenetics. 2012;7(7):758–771. doi: 10.4161/epi.20706. [DOI] [PubMed] [Google Scholar]
- 85.Palanichamy JK, Mehndiratta M, Bhagat M, et al. Silencing of integrated human papillomavirus–16 oncogenes by small interfering RNA-mediated heterochromatization. Mol. Cancer Ther. 2010;9(7):2114–2122. doi: 10.1158/1535-7163.MCT-09-0977. [DOI] [PubMed] [Google Scholar]
- 86.Verdel A, Vavasseur A, Le Gorrec M, Touat-Todeschini L. Common themes in siRNA-mediated epigenetic silencing pathways. Int. J. Dev. Biol. 2009;53(2–3):245–257. doi: 10.1387/ijdb.082691av. [DOI] [PubMed] [Google Scholar]
- 87.Matzke MA, Mosher RA. RNA-directed DNA methylation: an epigenetic pathway of increasing complexity. Nat. Rev. Genet. 2014;15(6):394–408. doi: 10.1038/nrg3683. [DOI] [PubMed] [Google Scholar]
- 88.Ye R, Wang W, Iki T, et al. Cytoplasmic Assembly and selective nuclear import of arabidopsis ARGONAUTE4/siRNA complexes. Mol. Cell. 2012;46(6):859–870. doi: 10.1016/j.molcel.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 89.Wierzbicki AT, Haag JR, Pikaard CS. Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell. 2008;135(4):635–648. doi: 10.1016/j.cell.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He XJ, Hsu YF, Pontes O, et al. NRPD4, a protein related to the RPB4 subunit of RNA polymerase II, is a component of RNA polymerases IV and v and is required for RNA-directed DNA methylation. Genes Dev. 2009;23(3):318–330. doi: 10.1101/gad.1765209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao Z, Liu H-L, Daxinger L, et al. An RNA polymerase II- and AGO4-associated protein acts in RNA-directed DNA methylation. Nature. 2010;465(7294):106–109. doi: 10.1038/nature09025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhong X, Du J, Hale CJ, et al. Molecular mechanism of action of plant DRM de novo DNA methyltransferases. Cell. 2014;157(5):1050–1060. doi: 10.1016/j.cell.2014.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aliya N, Rahman S, Khan ZK, Jain P. Cotranscriptional chromatin remodeling by small RNA species: an HTLV-1perspective. Leuk. Res. Treatment. 2012;2012:1–15. doi: 10.1155/2012/984754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li LC. Chromatin remodeling by the small rna machinery in mammalian cells. Epigenetics. 2014;9(1):45–52. doi: 10.4161/epi.26830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Toscano-Garibay JD, Aquino-Jarquin G. Transcriptional regulation mechanism mediated by miRNA-DNA•DNA triplexstructure stabilized by Argonaute. Biochim. Biophys. Acta. 2014;1839(11):1079–1083. doi: 10.1016/j.bbagrm.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 96.Tam OH, Aravin AA, Stein P, et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature. 2008;453(7194):534–538. doi: 10.1038/nature06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Watanabe T, Totoki Y, Toyoda A, et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 2008;453(7194):539–543. doi: 10.1038/nature06908. [DOI] [PubMed] [Google Scholar]
- 98.Maida Y, Yasukawa M, Okamoto N, et al. Involvement of telomerase reverse transcriptase in heterochromatin maintenance. Mol. Cell. Biol. 2014;34(9):1576–1593. doi: 10.1128/MCB.00093-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Babiarz JE, Ruby JG, Wang Y, Bartel DP, Blelloch R. Mouse ES cells express endogenous shRNAs, siRNAs, and other microprocessor-independent, Dicer-dependent small RNAs. Genes Dev. 2008;22(20):2773–2785. doi: 10.1101/gad.1705308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Castellano L, Stebbing J. Deep sequencing of small RNAs identifies canonical and non-canonical miRNA and endogenous siRNAs in mammalian somatic tissues. Nucleic Acids Res. 2013;41(5):3339–3351. doi: 10.1093/nar/gks1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smalheiser NR. The search for endogenous siRNAs in the mammalian brain. Exp. Neurol. 2012;235(2):455–463. doi: 10.1016/j.expneurol.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smalheiser NR, Lugli G, Thimmapuram J, Cook EH, Larson J. Endogenous siRNAs and noncoding RNA-derived small RNAs are expressed in adult mouse hippocampus and are up-regulated in olfactory discrimination training. RNA. 2011;17(1):166–181. doi: 10.1261/rna.2123811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Le Thomas A, Tóth KF, Aravin AA. To be or not to be a piRNA: genomic origin and processing of piRNAs. Genome Biol. 2014;15(1):204. doi: 10.1186/gb4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han BW, Zamore PD. piRNAs. Curr. Biol. 2014;24(16):R730–R733. doi: 10.1016/j.cub.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 105.Kuramochi-Miyagawa S, Kimura T, Ijiri TW, et al. Mili, a mammalian member of PIWI family gene, is essential for spermatogenesis. Development. 2004;131(4):839–849. doi: 10.1242/dev.00973. [DOI] [PubMed] [Google Scholar]
- 106.Lin H, Spradling AC. A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development. 1997;124(12):2463–2476. doi: 10.1242/dev.124.12.2463. [DOI] [PubMed] [Google Scholar]
- 107.Cox DN, Chao A, Baker J, Chang L, Qiao D, Lin H. A novel class of evolutionarily conserved genes defined by PIWI are essential for stem cell self-renewal. Genes Dev. 1998;12(23):3715–3727. doi: 10.1101/gad.12.23.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grimaud C, Bantignies F, Pal-Bhadra M, Ghana P, Bhadra U, Cavalli G. RNAi components are required for nuclear clustering of polycomb group response elements. Cell. 2006;124(5):957–971. doi: 10.1016/j.cell.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 109.Girard A, Sachidanandam R, Hannon GJ, Carmell MA. A germline-specific class of small RNAs binds mammalian PIWI proteins. Nature. 2006;442(7099):199–202. doi: 10.1038/nature04917. [DOI] [PubMed] [Google Scholar]
- 110.Robine N, Lau NC, Balla S, et al. A broadly conserved pathway generates 3′UTR-directed primary piRNAs. Curr. Biol. 2009;19(24):2066–2076. doi: 10.1016/j.cub.2009.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rajasethupathy P, Antonov I, Sheridan R, et al. A role for neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity. Cell. 2012;149:693–707. doi: 10.1016/j.cell.2012.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study is among the first evidence of neuroepigenetic regulation in memory-related neuronal activity by piRNAs, and drives the expectation that future studies will uncover piRNA-mediated neurocognitive mechanisms.
- 112.Ross RJ, Weiner MM, Lin H. PIWI proteins and PIWI-interacting RNAs in the soma. Nature. 2014;505(7483):353–359. doi: 10.1038/nature12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brower-Toland B, Findley SD, Jiang L, et al. Drosophila PIWI associates with chromatin and interacts directly with HP1a. Genes Dev. 2007;21(18):2300–2311. doi: 10.1101/gad.1564307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Piacentini L, Fanti L, Negri R, et al. Heterochromatin Protein 1 (HP1a) positively regulates euchromatic gene expression through RNA transcript association and interaction with hnRNPs in Drosophila . PLoS Genet. 2009;5(10):e1000670. doi: 10.1371/journal.pgen.1000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yin H, Lin H. An epigenetic activation role of PIWI and a PIWI-associated piRNA in Drosophila melanogaster . Nature. 2007;450(7167):304–308. doi: 10.1038/nature06263. [DOI] [PubMed] [Google Scholar]
- 116.Carmell MA, Girard A, van de Kant HJG, et al. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev. Cell. 2007;12(4):503–514. doi: 10.1016/j.devcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 117.Aravin AA, Sachidanandam R, Bourc'his D, et al. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol. Cell. 2008;31(6):785–799. doi: 10.1016/j.molcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuramochi-Miyagawa S, Watanabe T, Gotoh K, et al. DNA methylation of retrotransposon genes is regulated by PIWI family members MILI and MIWI2 in murine fetal testes. Genes Dev. 2008;22(7):908–917. doi: 10.1101/gad.1640708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee EJ, Banerjee S, Zhou H, et al. Identification of piRNAs in the central nervous system. RNA. 2011;17(6):1090–1099. doi: 10.1261/rna.2565011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wright MW. A short guide to long non-coding RNA gene nomenclature. Hum. Genomics. 2014;8(7) doi: 10.1186/1479-7364-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsai M-C, Manor O, Wan Y, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pachnis V, Brannan CI, Tilghman SM. The structure and expression of a novel gene activated in early mouse embryogenesis. EMBO J. 1988;7:673–681. doi: 10.1002/j.1460-2075.1988.tb02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sanli K, Karlsson FH, Nookaew I, Nielsen J. FANTOM: functional and taxonomic analysis of metagenomes. BMC Bioinformatics. 2013;14(38) doi: 10.1186/1471-2105-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pauli A, Valen E, Lin MF, et al. Systematic identification of long noncoding RNAs expressed during zebrafish embryogenesis. Genome Res. 2012;22:577–591. doi: 10.1101/gr.133009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cabili M, Trapnell C, Goff L, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mercer TR, Clark MB, Crawford J, et al. Targeted sequencing for gene discovery and quantification using RNA CaptureSeq. Nat. Protoc. 2014;9:989–1009. doi: 10.1038/nprot.2014.058. [DOI] [PubMed] [Google Scholar]
- 128.Mercer TR, Gerhardt DJ, Dinger ME, et al. Targeted RNA sequencing reveals the deep complexity of the human transcriptome. Nat. Biotechnol. 2011;30:99–104. doi: 10.1038/nbt.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fu GK, Xu W, Wilhelmy J, et al. Molecular indexing enables quantitative targeted RNA sequencing and reveals poor efficiencies in standard library preparations. Proc. Natl Acad. Sci. USA. 2014;111:1891–1896. doi: 10.1073/pnas.1323732111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Morris K V. Long antisense non-coding RNAs function to direct epigenetic complexes that regulate transcription in human cells. Epigenetics. 2009;4:296–301. doi: 10.4161/epi.4.5.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Klevebring D, Bjursell M, Emanuelsson O, Lundeberg J. In-depth transcriptome analysis reveals novel TARs and prevalent antisense transcription in human cell lines. PLoS ONE. 2010;5(3):e9762. doi: 10.1371/journal.pone.0009762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Katayama S, Tomaru Y, Kasukawa T, et al. Antisense transcription in the mammalian transcriptome. Science. 2005;309:1564–1566. doi: 10.1126/science.1112009. [DOI] [PubMed] [Google Scholar]
- 133.Pelechano V, Steinmetz LM. Gene regulation by antisense transcription. Nat. Rev. Genet. 2013;14:880–893. doi: 10.1038/nrg3594. [DOI] [PubMed] [Google Scholar]
- 134.Morris K V, Mattick JS. The rise of regulatory RNA. Nat. Rev. Genet. 2014;15:423–437. doi: 10.1038/nrg3722. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This excellent review discusses the history of RNA and describes many important mechanisms of regulatory NA function.
- 135.Chew G-L, Pauli A, Rinn JL, Regev A, Schier AF, Valen E. Ribosome profiling reveals resemblance between long non-coding RNAs and 5 leaders of coding RNAs. Development. 2013;140:2828–2834. doi: 10.1242/dev.098343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guttman M, Russell P, Ingolia NT, Weissman JS, Lander ES. Ribosome profiling provides evidence that large noncoding RNAs do not encode proteins. Cell. 2013;154(1):240–251. doi: 10.1016/j.cell.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ingolia NT, Brar GA, Stern-Ginossar N, et al. Ribosome profiling reveals pervasive translation outside of annotated protein-coding genes. Cell Rep. 2014;8(5):1365–1379. doi: 10.1016/j.celrep.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang B, Arun G, Mao YS, et al. The lncRNA malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult. Cell Rep. 2012;2:111–123. doi: 10.1016/j.celrep.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Modarresi F, Faghihi MA, Lopez-Toledano M A, et al. Inhibition of natural antisense transcripts in vivo results in gene-specific transcriptional upregulation. Nat. Biotechnol. 2012;30(5):453–459. doi: 10.1038/nbt.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jiao AL, Slack FJ. RNA-mediated gene activation. Epigenetics. 2014;9:27–36. doi: 10.4161/epi.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schaukowitch K, Kim T-K. Emerging epigenetic mechanisms of long non-coding RNAs. Neuroscience. 2014;264:25–38. doi: 10.1016/j.neuroscience.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zappulla DC, Cech TR. RNA as a flexible scaffold for proteins: yeast telomerase and beyond. Cold Spring Harb. Symp. Quant. Biol. 2006;71:217–224. doi: 10.1101/sqb.2006.71.011. [DOI] [PubMed] [Google Scholar]
- 143.Froberg JE, Yang L, Lee JT. Guided by RNAs: X-inactivation as a model for lncRNA function. J. Mol. Biol. 2013;425:3698–3706. doi: 10.1016/j.jmb.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Di Ruscio A, Ebralidze AK, Benoukraf T, et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nature. 2013;503(7476):371–376. doi: 10.1038/nature12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kornienko AE, Guenzl PM, Barlow DP, Pauler FM. Gene regulation by the act of long non-coding RNA transcription. BMC Biol. 2013;11:59. doi: 10.1186/1741-7007-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.He S, Zhang H, Liu H, Zhu H. LongTarget: a tool to predict lncRNA DNA-binding motifs and binding sites via Hoogsteen base-pairing analysis. Bioinformatics. 2014;31(2):178–186. doi: 10.1093/bioinformatics/btu643. [DOI] [PubMed] [Google Scholar]
- 147.Li X, Kazan H, Lipshitz HD, Morris QD. Finding the target sites of RNA-binding proteins. Wiley Interdiscip. Rev. RNA. 2014;5:111–130. doi: 10.1002/wrna.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li X, Quon G, Lipshitz HD, Morris Q. Predicting in vivo binding sites of RNA-binding proteins using mRNA secondary structure. RNA. 2010;16:1096–1107. doi: 10.1261/rna.2017210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nat. Rev. Genet. 2014;15(12):829–845. doi: 10.1038/nrg3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kartha RV, Subramanian S. Competing endogenous RNAs (ceRNAs): new entrants to the intricacies of gene regulation. Front. Genet. 2014;5:8. doi: 10.3389/fgene.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bosia C, Pagnani A, Zecchina R. Modeling competing endogenous RNAs networks. Arxiv Prepr. Arxiv. 2012;1210:2336. [Google Scholar]
- 152.Jalali S, Bhartiya D, Lalwani MK, Sivasubbu S, Scaria V. Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0053823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nakagawa S, Kageyama Y. Nuclear lncRNAs as epigenetic regulators-beyond skepticism. Biochim. Biophys. Acta. 2014;1839:215–222. doi: 10.1016/j.bbagrm.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 154.Khalil AM, Guttman M, Huarte M, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl Acad. Sci. USA. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Up to 20% of lncRNAs are shown to bind to the PRC2, a chromatin-modifying complex with known activity in the CNS.
- 155.Zhao T, Xu J, Liu L, et al. Computational identification of epigenetically regulated lncRNAs and their associated genes based on integrating genomic data. FEBS Lett. 2015;589(4):521–531. doi: 10.1016/j.febslet.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 156.Petazzi P, Sandoval J, Szczesna K, et al. Dysregulation of the long non-coding RNA transcriptome in a Rett syndrome mouse model. RNA Biol. 2013;10:1197–1203. doi: 10.4161/rna.24286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ziats MN, Rennert OM. Aberrant expression of long noncoding RNAs in autistic brain. J. Mol. Neurosci. 2013;49:589–593. doi: 10.1007/s12031-012-9880-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lipovich L, Dachet F, Cai J, et al. Activity-dependent human brain coding/noncoding gene regulatory networks. Genetics. 2012;192(3):1133–1148. doi: 10.1534/genetics.112.145128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sauvageau M, Goff LA, Lodato S, et al. Multiple knockout mouse models reveal lncRNAs are required for life and brain development. Elife. 2013;2013(2):e01749. doi: 10.7554/eLife.01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bernard D, Prasanth K V, Tripathi V, et al. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 2010;29(18):3082–3093. doi: 10.1038/emboj.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.West JA, Davis Cp, Sunwoo H, et al. The long noncoding RNAs NEAT1 and MALAT1 bind active chromatin sites. Mol. Cell. 2014;55(5):791–802. doi: 10.1016/j.molcel.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guil S, Soler M, Portela A, et al. Intronic RNAs mediate EZH2 regulation of epigenetic targets. Nat. Struct. Mol. Biol. 2012;19(7):664–670. doi: 10.1038/nsmb.2315. [DOI] [PubMed] [Google Scholar]; • Describes a role for noncoding, intronic RNA in controlling gene transcription via the PRC2 This complex containing the methyltransferase activity of its EZH2 subunit is known to influence hippocampal neurogenesis and memory formation.
- 163.Eißmann M, Gutschner T, Hämmerle M, et al. Loss of the abundant nuclear non-coding RNA MALAT1 is compatible with life and development. RNA Biol. 2012;9(8):1076–1087. doi: 10.4161/rna.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Barry G, Briggs J, Vanichkina D. The long non-coding RNA Gomafu is acutely regulated in response to neuronal activation and involved in schizophrenia-associated alternative splicing. Mol. Psychiatry. 2013;19(4):486–494. doi: 10.1038/mp.2013.45. [DOI] [PubMed] [Google Scholar]
- 165.Stilling RM, Fischer A. The role of histone acetylation in age-associated memory impairment and Alzheimer's disease. Neurobiol. Learn. Mem. 2011;96:19–26. doi: 10.1016/j.nlm.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 166.Lu X, Deng Y, Yu D, et al. Histone acetyltransferase p300 mediates histone acetylation of PS1 and BACE1 in a cellular model of Alzheimer's disease. PLoS ONE. 2014;9(7):1–9. doi: 10.1371/journal.pone.0103067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sathya M, Premkumar P, Karthick C, Moorthi P, Jayachandran KS, Anusuyadevi M. BACE1 in Alzheimer's disease. Clin. Chim. Acta. 2012;414:171–178. doi: 10.1016/j.cca.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 168.Faghihi MA, Zhang M, Huang J, et al. Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biol. 2010;11(5):R56. doi: 10.1186/gb-2010-11-5-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Modarresi F, Faghihi MA, Patel NS, Sahagan BG, Wahlestedt C, Lopez-Toledano MA. Knockdown of BACE1-AS nonprotein-coding transcript modulates beta-amyloid-related hippocampal neurogenesis. Int. J. Alzheimers. Dis. 2011;2011:929042. doi: 10.4061/2011/929042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Morgan HD, Santos F, Green K, Dean W, Reik W. Epigenetic reprogramming in mammals. Hum. Mol. Genet. 2005;14(1):R47–R58. doi: 10.1093/hmg/ddi114. [DOI] [PubMed] [Google Scholar]
- 171.Carone BR, Fauquier L, Habib N, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143(7):1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 2014;17(1):89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gapp K, Jawaid A, Sarkies P, et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014;17(5):667–669. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pembrey ME, Bygren LO, Kaati G, et al. Sex-specific, male-line transgenerational responses in humans. Eur. J. Hum. Genet. 2006;14(2):159–166. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- 175.Skinner MK, Mohan M, Haque MM, Zhang B, Savenkova MI. Epigenetic transgenerational inheritance of somatic transcriptomes and epigenetic control regions. Genome Biol. 2012;13(10):R91. doi: 10.1186/gb-2012-13-10-r91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Migicovsky Z, Kovalchuk I. Epigenetic memory in mammals. Front. Genet. 2011;2:28. doi: 10.3389/fgene.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yuan S, Oliver D, Schuster A, Zheng H, Yan W. Breeding scheme and maternal small RNAs affect the efficiency of transgenerational inheritance of a paramutation in mice. Sci. Rep. 2015;5:9266. doi: 10.1038/srep09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Devanapally S, Ravikumar S, Jose AM. Double-stranded RNA made in C. elegans neurons can enter the germline and cause transgenerational gene silencing. Proc. Natl Acad. Sci. USA. 2015;112(7):2133–2138. doi: 10.1073/pnas.1423333112. [DOI] [PMC free article] [PubMed] [Google Scholar]