Abstract
Background/Objectives:
Brain-derived neurotrophic factor (BDNF) and its receptor (tropomyosin-related kinase B: TrkB, also known as Ntrk2) have a key role in central regulation of the energy balance. BDNF and TrkB are also expressed in the peripheral tissues, including adipose tissue, but their peripheral role has been unclear. Here we report on the functional significance of the adipose tissue BDNF/TrkB axis in metabolic homeostasis.
Materials and Methods:
To examine the role of the BDNF/TrkB axis in the central nervous system and in adipose tissue, we generated adipocyte-specific or neuron-specific BDNF/TrkB conditional knockout (CKO) mice. Then we compared the feeding behavior and metabolic profile between each type of CKO mouse and their littermates.
Results:
Bdnf expression was significantly increased in the adipose tissue of mice receiving a high-calorie diet, whereas Ntrk2 expression was decreased. The Bdnf/Ntrk2 expression ratio of adipose tissue was higher in female mice than male mice. Fabp4-Cre mice are widely used to establish adipocyte-specific CKO mice. However, we found that Fabp4-Cre-induced deletion of Bdnf or Ntrk2 led to hyperphagia, obesity, and aggressiveness, presumably due to ectopic Fabp4-Cre mediated gene recombination in the brain. Next, we attempted to more specifically delete Bdnf or Ntrk2 in adipocytes using Adipoq-Cre mice. Expression of Ntrk2, but not Bdnf, in the adipose tissue was reduced by Adipoq-Cre mediated gene recombination, indicating that adipocytes only expressed TrkB. No phenotypic changes were detected when Adipoq-Cre TrkB CKO mice were fed a normal diet, whereas female CKO mice receiving a high-calorie diet showed a decrease in food intake and resistance to obesity.
Conclusions:
The adipose tissue BDNF/TrkB axis has a substantial influence on the feeding behavior and obesity in female mice.
Introduction
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophic factor family that shows high-affinity binding to its receptor (tropomyosin-related kinase B: TrkB, also known as Ntrk2) and has a key role in regulating neuronal survival, neuronal differentiation, and synaptic plasticity.1–4 BDNF and the TrkB receptor are expressed in various brain regions, including energy homeostasis centers within the hypothalamus and the hindbrain in adult animals.5–9 Consistent with this pattern of expression, BDNF has an essential role in regulating the body weight and energy homeostasis. Chronic infusion of BDNF into the cerebral ventricles has been shown to reduce food intake and body weight,10,11 whereas heterozygous Bdnf-deficient mice display increased locomotor activity, aggressiveness, hyperphagia, and obesity.12,13 In addition, mice with approximately 75% reduction of TrkB expression exhibit hyperphagia and obesity,5 while mice lacking neuronal Bdnf develop obesity and anxiety.14 It is thought that the anorexic actions of BDNF and TrkB are mediated by the leptin-proopiomelanocortin (POMC) signaling pathway.5 In humans, genome-wide association studies15–18 and rare reports on genetic variants of BDNF19 and NTRK220 have shown an association between the BDNF/TrkB axis and obesity in both adults and children.
A number of studies have demonstrated that BDNF and TrkB are also expressed in non-neural tissues and have an influence on the eating behavior and metabolic homeostasis. The circulating level of BDNF is associated with obesity and diabetes mellitus in humans,21 while systemic administration of BDNF improves glycemic control in db/db mice.22 The liver and pancreatic α-cells might be involved in the underlying mechanism.23,24 In addition, mice with hepatocyte-specific Bdnf deficiency show normal food intake and body weight, but are protected against dietary metabolic abnormalities through enhanced expression of peroxisome proliferator-activated receptor α and fibroblast growth factor 21 (ref. 25). It was reported that BDNF expression is induced in the skeletal muscles by exercise and enhances muscle fat oxidation via activation of AMP-activated protein kinase.26 It has also been demonstrated that BDNF-expressing hematopoietic cells regulate feeding behavior and energy balance by migrating from the bone marrow to the paraventricular nucleus of the hypothalamus.27 Furthermore, it was reported that BDNF is expressed in adipose tissue.28,29 Moreover, humoral factors released by adipose tissue, collectively termed adipokines, and certain metabolites like fatty acids are involved in the regulation of energy expenditure and glucose/lipid metabolism through various systemic actions.30–32 Taken together, these reports suggest the possibility that the BDNF/TrkB axis in adipose tissue may have a role in the regulation of systemic metabolism. To test this hypothesis, we investigated expression of BDNF and TrkB by adipose tissue in a mouse model of dietary obesity and generated various conditional knockout (CKO) mouse models by using the Cre-loxP system. Our findings demonstrated that the adipose tissue BDNF/TrkB axis has a critical role in regulating metabolic homeostasis.
Materials and methods
Animal models
All animal care and experimental procedures were approved by the Chiba University review board. Mice were housed in individual cages in a temperature-controlled facility with a 12-h day/night cycle and free access to water. The mice were fed a normal chow diet (CLEA Rodent diet CE-2; CLEA Japan, Tokyo, Japan) or a high-fat/high-sucrose diet (F2HFHSD, Oriental Yeast, Suita, Japan) from 6 weeks of age. C57BL/6 mice were purchased from Japan SLC (Shizuoka, Japan). Floxed Bdnf mice, Syn1-Cre mice, and Fabp4-Cre mice (with a C57BL/6 background) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Generation and genotyping of floxed Ntrk2 mice (a gift from Luis F Parada, University of Texas Southwestern Medical Center, Dallas, TX, USA) and Adipoq-Cre mice (a gift from Evan D Rosen, Beth Israel Deaconess Medical Center, Boston, MA, USA) have been described previously.33,34
The mice were fasted for 12–14 h before being killed. For fractionation of adipose tissue, freshly isolated epididymal fat pads were dissected, minced in phosphate-buffered saline with 1% bovine serum albumin, and then incubated with 0.05 mg/ml Liberase TM (Roche, Indianapolis, IN, USA) and 50 units/ml DNase I (Sigma-Aldrich, St Louis, MO, USA) for 45 min at 37 °C in an orbital shaker with intermittent pipetting. The digested samples were filtered through a sterile 250-μm nylon mesh, after which the suspension was centrifuged at 1,000 g for 5 min to separate the adipocyte fraction from the stromal vascular fraction.
The genotypes of the mice and the effects of Cre-mediated recombination were assessed by PCR using genomic DNA harvested from the tail tips and various other tissues of mutant mice. To examine tissue-specific deletion of BDNF or TrkB in CKO mice, we analyzed male mice homozygous for the floxed allele with one copy of the Cre recombinase transgene (CKO), littermate mice homozygous for the floxed allele without the Cre recombinase transgene (littermate controls), and wild-type C57BL/6 mice using the following primers: Cre recombinase, 5′-GTTCGCAAGAACCTGATGGACA-3′ and 5′-CTAGAGCCTGTTTTGCACGTTC-3′; wild-type or floxed Bdnf allele, 5'-TGTGATTGTGTTTCTGGTGAC-3′, 5′-GATACATCATGGGCAGTGGA-3′; wild-type or floxed Ntrk2 allele, 5′-ATGTACTCGTTCTACAAATCCTGC-3′, 5′-TCCAGACACATACACGTGCGTGC-3′, and 5′-CAAGAAGTCAGAGACCAGAGAGA-3′.
RNA analysis
Total RNA was extracted from adipose tissue by using an RNeasy Plus Universal kit (Qiagen, Valencia, CA, USA) and was extracted from other tissues with RNA-Bee (Molecular Research Center, Cincinnati, OH, USA). Then reverse transcription was performed by using a QuantiTect reverse transcription kit (Qiagen), and real-time PCR was done with a LightCycler (Roche), the Taqman Universal Probe Library, and Light Cycler Master (Roche) according to the manufacturer’s instructions. For normalization of gene expression, 36B4 mRNA was used as the internal control. Data were analyzed by the 2−1ΔΔCT method. The following primers were used: 36B4, forward 5′-GATGCCCAGGGAAGACAG-3′, reverse 5′-ACAATGAAGCATTTTGGATAATCA-3′; Bdnf, forward 5′-GCCTTTGGAGCCTCCTCTAC-3′, reverse 5′-GCGGCATCCAGGTAATTTT-3′; Ntrk2, forward 5′-CGAACCTGCAGATACCCAAT-3′, reverse 5′-TGCAGGAAAGGGTCACAGA-3′; Ntrk2-tk, forward 5′-TTCTGCCTGCTGGTGATGT-3′, reverse 5′-TCCAGTGGGATCTTATGAAACA-3′, Lep, forward 5′-CAGGATCAATGACATTTCACACA-3′, reverse 5′-GCTGGTGAGGACCTGTTGAT-3′; Adipoq, forward 5′-CAGGCATCCCAGGACATT-3′, reverse 5′-ACCCTTAGGACCAAGAAGACCT-3′; Emr, forward 5′-GGAGGACTTCTCCAAGCCTATT-3′, reverse 5′-AGGCCTCTCAGACTTCTGCTT-3′; Tnf, forward 5′-TGCCTATGTCTCAGCCTCTTC-3′, reverse 5′-GAGGCCATTTGGGAACTTCT-3′.
Measurement of body weight and food intake
Body weight was measured every 2 weeks. Food intake was assessed by housing mice individually. The animals were fed a normal diet or a high-calorie diet ad libitum, and the amount of residual food was weighed for a total of 7 days. In pair-feeding experiments, the same amount of high-calorie diet was given to CKO mice and their littermates each day.
Assessment of locomotor activity
Locomotor activity was assessed by using an implantable intra-abdominal radiofrequency probe and receiver (TA10TA-F20 and RPC-1, Data Sciences International, St Paul, MN, USA). Mice (14–16 weeks old) were placed individually into cages and baseline locomotor activity was monitored. Data were sampled in the continuous mode and analyzed by Dataquest ART2.1.
CT scanning
Adipose tissue was examined by CT (LaTheta, Aloka, Tokyo, Japan) according to the manufacturer’s protocol. We obtained CT scans at 2 mm intervals from diaphragm to the floor of the pelvic cavity.
Laboratory tests
To perform the glucose tolerance test, mice were fasted for 16 h and D-glucose (2 g/kg body weight) was administered by intraperitoneal injection at 1500 h. For the insulin tolerance test, mice were given an intraperitoneal injection of human insulin (1 U/kg body weight) at 1500 h without fasting. In both the tests, blood was collected from the tail vein at 0, 15, 30, 60, and 120 min after administration of glucose or insulin. Then blood glucose concentrations were measured with a Glutest Mint (Sanwa Kagaku Kenkyusho, Nagoya, Japan). Insulin, leptin, and adiponectin were measured by enzyme-linked immunosorbent assay-based immunoassay kits (insulin: Morinaga Institute of Biological Science, Yokohama, Japan; leptin: R&D Systems, Minneapolis, MN, USA; adiponectin: Otsuka Pharmaceutical, Tokyo, Japan) according to the manufacturer’s instruction. Free fatty acid levels were measured by Oriental Yeast.
Statistical analysis
Results are shown as the mean±s.e.m. Differences between groups were examined by Student’s t-test for the comparison of mean values. In all the analyses, P<0.05 was considered statistically significant.
Results
Tissue expression of Bdnf and Ntrk2
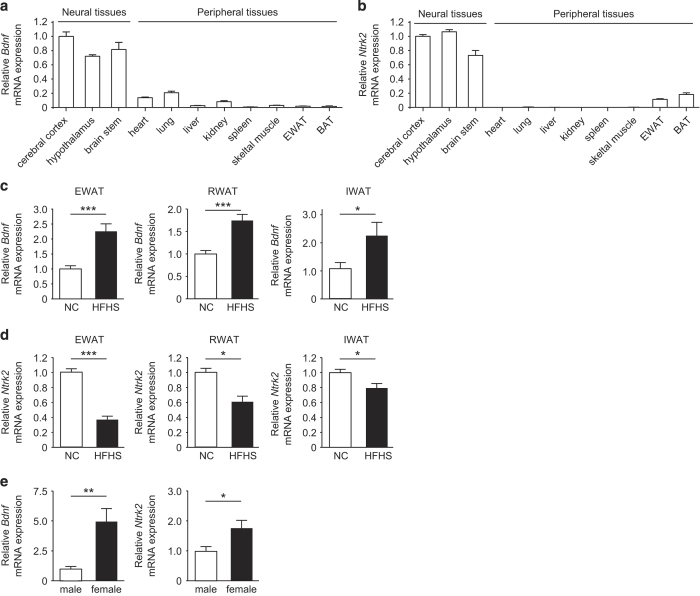
We first examined the expression of Bdnf and Ntrk2 in various tissues of mice fed a normal diet. Bdnf was highly expressed in the brain, whereas intermediate expression was found in the heart, lung, and kidney (Figure 1a). Bdnf was also expressed in adipose tissue, although its expression was much lower than in neural tissues. In addition, Ntrk2 (the gene coding for TrkB) was expressed by adipose tissue at the highest level among the peripheral tissues that we examined (Figure 1b). To investigate the role of the adipose tissue BDNF/TrkB axis in obesity, we examined Bdnf and Ntrk2 expression in mice with dietary obesity owing to feeding with a high-fat/high-sucrose (HFHS) diet. Bdnf expression was markedly upregulated in the adipose tissue of these obese mice (Figure 1c). Unlike Bdnf, expression of Ntrk2 was significantly downregulated in the adipose tissue of obese mice (Figure 1d). No significant changes of Bdnf or Ntkr2 expression were observed in the other peripheral tissues (Supplementary Figure S1A and 1B). We next analyzed gender differences of Bdnf and Ntrk2 expression in adipose tissue, revealing that female mice showed higher expression of both Bdnf and Ntrk2 than male mice (Figure 1e). On the basis of these findings and previous observations,28,29 we speculated that the adipose tissue BDNF/TrkB axis might have a role in regulating metabolic homeostasis, and that its role was influenced by gender.
Figure 1.
Expression of Bdnf and Ntrk2 in peripheral tissues. (a and b) Relative expression of Bdnf (a) and Ntrk2 (b) of mice aged 16 weeks was determined by real-time PCR. Expression in various tissues is shown relative to that in the cerebral cortex; n=5. EWAT, epididymal white adipose tissue; BAT, brown adipose tissue. (c and d) Expression of Bdnf (c) and Ntrk2 (d) in epididymal white adipose tissue (EWAT), perinephric white adipose tissue (RWAT), and inguinal white adipose tissue (IWAT) assessed by real-time PCR in 16-week-old mice fed normal chow (NC) or a high-fat/high-sucrose (HFHS) diet; n=9–10 for EWAT, n=4 for RWAT, and n=4 for IWAT. (e) Real-time PCR analysis of Bdnf and Ntrk2 expression in the epididymal adipose tissue of 21–25-week-old male and female mice fed normal chow; n=9. *P<0.05, **P<0.01, ***P<0.001. Data are shown as the mean±s.e.m.
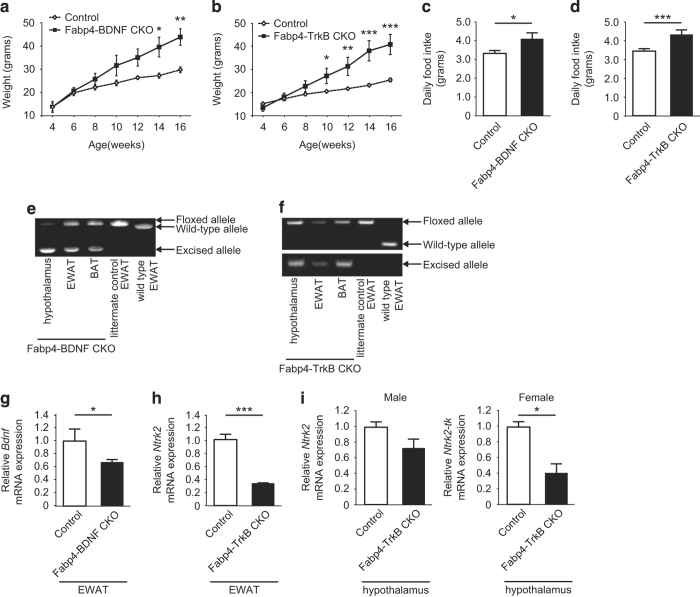
Mice with adipocyte-specific Bdnf or Ntrk2 deficiency exhibit obesity and hyperphagia
To study the role of the BDNF/TrkB axis in adipose tissue, we generated mice lacking Bdnf or Ntrk2 in adipocytes by breeding Bdnfflox/flox or Ntrk2flox/flox mice with Fabp4-Cre mice (Fabp4-BDNF CKO mice and Fabp4-TrkB CKO mice, respectively). Both lines of mice exhibited age-dependent obesity (Figure 2a and b), hyperphagia (Figure 2c and d), and aggressiveness (data not shown). These phenotypic changes suggested that the CKO mice had abnormalities of the central nervous system. Although Fabp4 was originally identified as an adipocyte-specific protein, recent studies have shown that it is also expressed by other types of cells, including neurons.35 As BDNF acts on the hypothalamus to regulate energy homeostasis,5,9,11 we investigated the specificity of Fabp4-Cre-mediated gene recombination. Genomic PCR detected the excised allele of Bdnfflox/flox mice in the hypothalamus as well as in adipose tissue of Fabp4-BDNF CKO mice (Figure 2e). Likewise, the excised allele of Ntrk2flox/flox mice was observed in the hypothalamus of Fabp4-TrkB CKO mice (Figure 2f). Adipose tissue expression of both Bdnf and Ntrk2 was markedly reduced in these CKO mice (Figure 2g and h). Consistent with the genomic PCR data, hypothalamic expression of Ntrk2 was downregulated in Fabp4-TrkB CKO mice (Figure 2i), indicating that Fabp4-Cre-mediated gene recombination in the hypothalamus could affect the metabolic phenotype of Fabp4-TrkB CKO mice.
Figure 2.
Obesity and hyperphagia in mice with adipocyte-specific deficiency of Bdnf or Ntrk2 developed using Fabp4-Cre mice. (a and b) Body weight of Fabp4-BDNF CKO mice (a) and Fabp4-TrkB CKO mice (b) compared with their littermate controls (Control); n=3 for a, n=7–11 for b. *P<0.05, **P<0.01, ***P<0.001. (c and d) Food intake of Fabp4-BDNF CKO mice (c) and Fabp4-TrkB CKO mice (d) compared with their littermate controls (Control) at 8–12 weeks of age; n=4–6 for c, n=6–8 for d. *P<0.05, ***P<0.001. (e and f) PCR analysis of genomic DNA isolated from the hypothalamus, epididymal white adipose tissue (EWAT), and brown adipose tissue (BAT) of Fabp4-BDNF CKO mice (e) and Fabp4-TrkB CKO mice (f), and from EWAT of their littermate controls and wild-type mice. Littermate controls were homozygous for the floxed Bdnf (e) or Ntrk2 (f) allele, but did not carry Cre recombinase. (g) Real-time PCR analysis of Bdnf expression by EWAT in Fabp4-BDNF CKO mice and their littermate controls (Control); n=3. *P<0.05. (h and i) Real-time PCR analysis of Ntrk2 expression in EWAT (h) and the hypothalamus (i) of Fabp4-TrkB CKO mice and their littermate controls (Control); n=3–4. *P<0.05, ***P<0.001. Data are shown as the mean±s.e.m. CKO, conditional knockout.
Neuronal Bdnf deficiency is associated with a similar phenotype to that of Fabp4-BDNF/TrkB CKO mice
To examine whether Fabp4-Cre-mediated recombination in the brain accounted for the obese phenotype of Fabp4-BDNF/TrkB CKO mice, we next generated mice lacking neuronal Bdnf by breeding Bdnfflox/flox mice with Syn1-Cre mice (Syn1-BDNF CKO mice). We confirmed by genomic PCR that Syn1-Cre-mediated recombination was only detected in the nervous system (Supplementary Figure S2A), in agreement with a previous report.36 Bdnf expression was only decreased in the brains of Syn1-BDNF CKO mice (Supplementary Figure S2B). Syn1-BDNF CKO mice exhibited a higher body weight compared with their littermate controls (Supplementary Figure S2C), which was associated with hyperphagia (Supplementary Figure S2D) and increased locomotor activity (Supplementary Figure S2E). These results suggested that Fabp4-Cre-mediated gene recombination in the brain had an influence on the metabolism of Fabp4- BDNF/TrkB CKO mice.
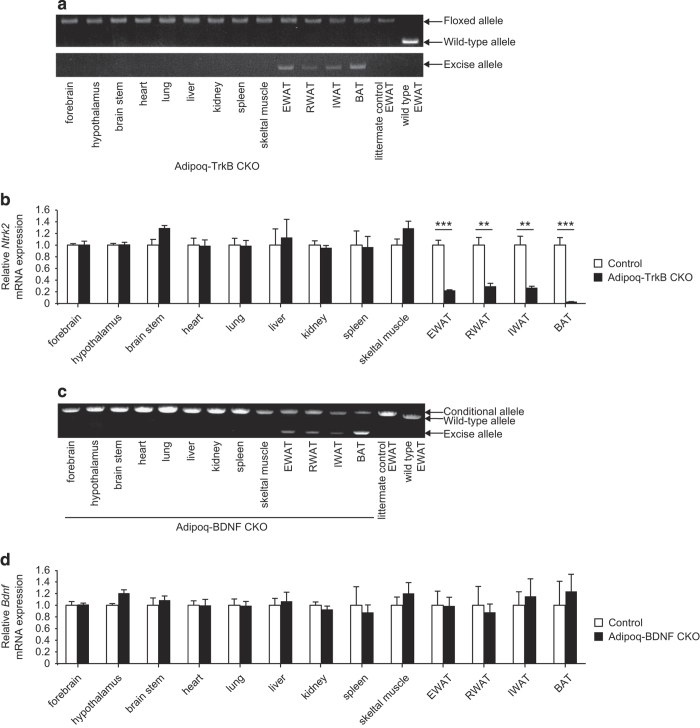
Adipocyte-specific Bdnf/Ntrk2 deletion does not cause obesity in Adipoq-Cre mice
To investigate the possibility of genetic recombination occurring in the brain, we generated a second line of adipocyte-specific Bdnf/Ntrk2 CKO mice by breeding Bdnfflox/flox or Ntrk2flox/flox mice with Adipoq-Cre mice (Adipoq-BDNF CKO mice and Adipoq-TrkB CKO mice, respectively). It has been reported that Cre expression driven by the Adipoq promoter leads to recombination that exclusively affects adipocytes,34,37 and we confirmed that Adipoq-TrkB CKO mice exhibited adipose tissue-specific recombination (Figure 3a) and that Ntrk2 was specifically deleted from adipose tissue (Figure 3b). Although Adipoq-BDNF CKO mice displayed adipose tissue-specific recombination (Figure 3c), Bdnf expression was unexpectedly not suppressed in their adipose tissue (Figure 3d), suggesting that adipocytes do not contribute to the production of BNDF by adipose tissue. In contrast, the pattern of expression in Adipoq-TrkB CKO mice supported the concept that Ntrk2 is predominantly expressed by adipocytes in the adipose tissue. Consistent with these results, real-time PCR analysis of isolated adipocytes and the stromal vascular fraction from adipose tissue revealed that Bdnf expression was elevated in the stromal vascular fraction, while there was higher expression of Ntrk2 in the adipocyte-rich fraction (Supplementary Figure S3).
Figure 3.
Genomic recombination and gene expression in adipocyte-specific Bdnf/Ntrk2 deletion models created using Adipoq-Cre mice. (a) PCR analysis of genomic DNA isolated from various tissues of Adipoq-TrkB CKO mice and from epididymal white adipose tissue (EWAT) of their littermate controls and wild-type mice. Littermate controls were homozygous for the floxed Ntrk2 allele, but did not carry Cre recombinase. (b) Real-time PCR analysis of Ntrk2 expression in various tissues of Adipoq-TrkB CKO mice and their littermate controls (Control); n=5. **P<0.01, ***P<0.001. (c) PCR analysis of genomic DNA isolated from various tissues of Adipoq-BDNF CKO mice and from epididymal white adipose tissue (EWAT) of their littermate controls and wild-type mice. Littermate controls were homozygous for the floxed Bdnf allele, but did not carry Cre recombinase. (d) Real-time PCR analysis of Bdnf expression in various tissues of Adipoq-BDNF CKO mice and their littermate controls (Control); n=5. Data are shown as the mean±s.e.m. CKO, conditional knockout.
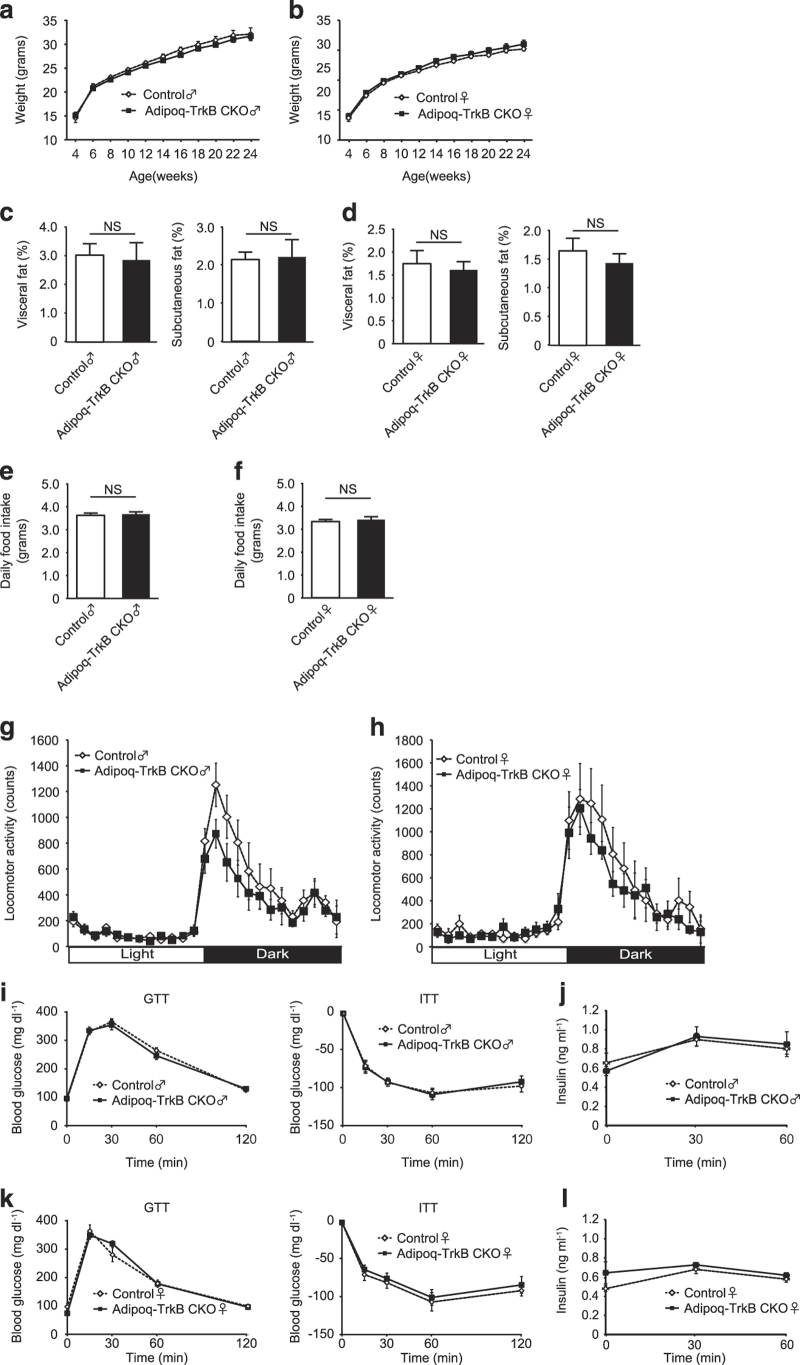
We then focused on TrkB in adipose tissue to investigate the role of the BDNF/TrkB axis in metabolism. As decreased expression of TrkB by adipose tissue was associated with obesity (Figure 1d), we hypothesized that BDNF might positively regulate energy metabolism by acting on adipocyte TrkB. We monitored physiological parameters in male and female mice on a normal diet, revealing that Adipoq-TrkB CKO mice and their littermate controls showed no differences in body weight (Figure 4a and b), fat mass (Figure 4c and d), food intake (Figure 4e and f), or locomotor activity (Figure 4g and h). Consistent with the results for Bdnf expression, Adipoq-Bdnf CKO mice did not display any changes in the body weight, fat mass, food intake, and locomotor activity (data not shown). To assess the role of the adipose tissue BDNF/TrkB axis in glucose homeostasis, we performed glucose tolerance and insulin tolerance tests, and we measured plasma insulin levels during the glucose tolerance test. Normal glucose metabolism was observed in mice of both sexes (Figure 4i–l). Taken together, these results suggest that adipose tissue Ntrk2 only has a minor role in regulating eating behavior and metabolism in mice receiving a normal diet.
Figure 4.
Adipocyte-specific deletion of Ntrk2 does not cause obesity. (a and b) Body weight of Adipoq-TrkB CKO male mice (a) and female mice (b) receiving a normal diet compared with their littermate controls (Control); n=11–12 for a, n=10–11 for b. (c and d) CT analysis of Adipoq-TrkB CKO male mice (c) and female mice (d) receiving a normal diet compared with their littermate controls (Control) at 12–14 weeks of age. The percent fat tissue/body weight ratio is shown for visceral fat and subcutaneous fat; n=6 for c, n=9–10 for d. (e and f) Food intake of Adipoq-TrkB CKO male mice (e) and female mice (f) receiving a normal diet compared with their littermate controls (Control) at 8–12 weeks of age; n=9–10 for e, n=9–10 for f. (g and h) Locomotor activity of Adipoq-TrkB CKO male mice (g) and female mice (h) receiving a normal diet compared with their littermate controls (Control) at 14–16 weeks of age; n=6 for g, n=6 for h. (i) Glucose tolerance test (GTT) and insulin tolerance test (ITT) in Adipoq-TrkB CKO male mice receiving a normal diet compared with their littermate controls (Control) at 12–14 weeks of age; n=9–13 for GTT, n=10–13 for ITT. (j) Plasma insulin level during the GTT shown in Figure 5i; n=9–10. (k) GTT and ITT in Adipoq-TrkB CKO female mice receiving a normal diet compared with their littermate controls (Control) at 12–14 weeks of age; n=15–16 for GTT, n=7–10 for ITT. (l) Plasma insulin level during the GTT shown in Figure 5k; n=7–10. Data are shown as the mean±s.e.m. CKO, conditional knockout.
Adipoq-TrkB CKO female mice on a high-calorie diet show decreased food intake and resistance to obesity
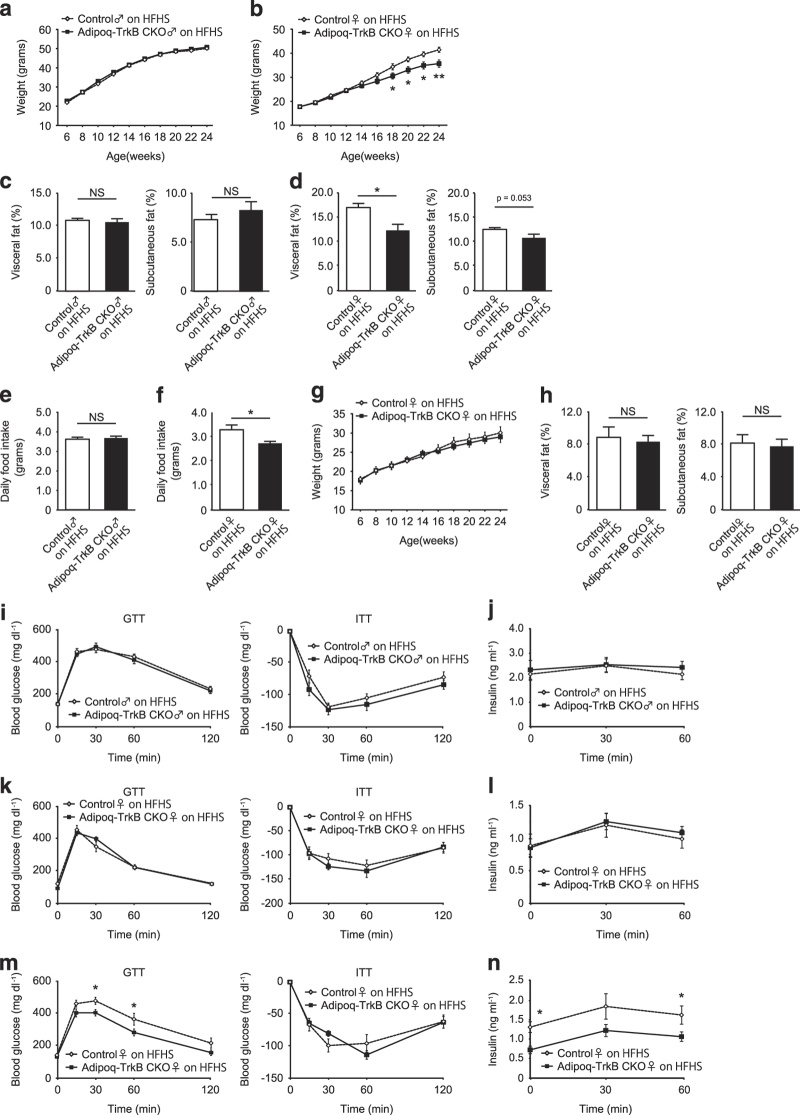
We next examined the effect of a high-calorie diet on the metabolic phenotype of Adipoq-TrkB CKO mice. We found that female, but not male, Adipoq-TrkB CKO mice fed a high-calorie diet had a lower body weight (Figure 5a and b) and less accumulation of adipose tissue (Figure 5c and d) than their littermate controls. Adipoq-TrkB CKO female mice (but not male mice) showed a decrease in food intake (Figure 5e and f), suggesting that TrkB deficiency in adipose tissue leads to weight loss due to lower calorie intake. To test this hypothesis, we next performed a pair-feeding experiment. This showed that providing equal food intake diminished the difference in body weight between female Adipoq-TrkB CKO mice and their littermate controls (Figure 5g), suggesting that TrkB may have a role in regulating food intake. Thus, we assessed glucose metabolism in Adipoq-TrkB CKO female mice at the ages of 12–14 weeks and at 24–26 weeks. There were no differences of fat mass (Figure 5h), glucose tolerance, insulin tolerance, and the plasma insulin level between Adipoq-TrkB CKO mice and their littermate controls at 12–14 weeks (Figure 5i–l). In contrast, Adipoq-TrkB CKO female mice aged 24–26 weeks exhibited improvement of glucose tolerance and had lower insulin levels (Figure 5m and n). HOMA-IR data indicated that Adipoq-TrkB CKO mice had superior insulin sensitivity compared with control mice (14.1±1.7 vs. 6.2±1.2, P<0.01). Although blood glucose levels were significantly lower in Adipoq-TrkB CKO mice than in their littermate controls throughout the ITT (Supplementary Figure S4), there were no differences in the relative decrease of glucose between these two groups (Figure 5m). This suggested that the results of the ITT could have been affected by the lower body weight of Adipoq-TrkB CKO mice.
Figure 5.
Adipoq-TrkB CKO female mice on a high-calorie diet show decreased food intake and resistance to obesity. (a and b) Body weight of Adipoq-TrkB CKO male mice (a) and female mice (b) receiving a high-fat/high-sucrose (HFHS) diet compared with their littermate controls (Control); n=14−16 for a, n=26–33 for b. *P<0.05, **P<0.01. (c and d) CT analysis of Adipoq-TrkB CKO male mice (c) and female mice (d) receiving the HFHS diet compared with their littermate controls (Control) at 24 weeks of age. The percent fat tissue/body weight ratio is shown for visceral fat and subcutaneous fat; n=5 for c, n=7 for d. *P<0.05. (e and f) Food intake of Adipoq-TrkB CKO male mice (f) and female mice (g) receiving the HFHS diet compared with their littermate controls (Control) at 14 weeks of age; n=10–13 for f, n=14–16 for g. *P<0.05. (g) Pair-feeding experiments in Adipoq-TrkB CKO female mice receiving the HFHS diet compared with their littermate controls (Control); n=6. (h) CT analysis of Adipoq-TrkB CKO female mice receiving the HFHS diet compared with their littermate controls (Control) at 12–14 weeks of age. The percent fat tissue/body weight ratio is shown for visceral fat and subcutaneous fat; n=7. (i) Glucose tolerance test (GTT) and insulin tolerance test (ITT) in Adipoq-TrkB CKO male mice receiving the HFHS diet compared with their littermate controls (Control) at 12–14 weeks of age; n=17–18 for GTT, n=15–18 for ITT. (j) Plasma insulin level during the GTT shown in Figure 6i; n=12–15. (k) Glucose tolerance test (GTT) and insulin tolerance test (ITT) in Adipoq-TrkB CKO male mice receiving the HFHS diet compared with their littermate controls (Control) at 12–14 weeks of age; n=10–13 for GTT, n=10 for ITT. (l) Plasma insulin level during the GTT shown in Figure 6k; n=14–15. (m) Glucose tolerance test (GTT) and insulin tolerance test (ITT) in Adipoq-TrkB CKO male mice receiving the HFHS diet compared with their littermate controls (Control) at 24–26 weeks of age; n=14–15 for GTT, n=12–13 for ITT. *P<0.05. (n) Plasma insulin level during the GTT shown in Figure 6m; n=10. *P<0.05. Data are shown as the mean±s.e.m. CKO, conditional knockout.
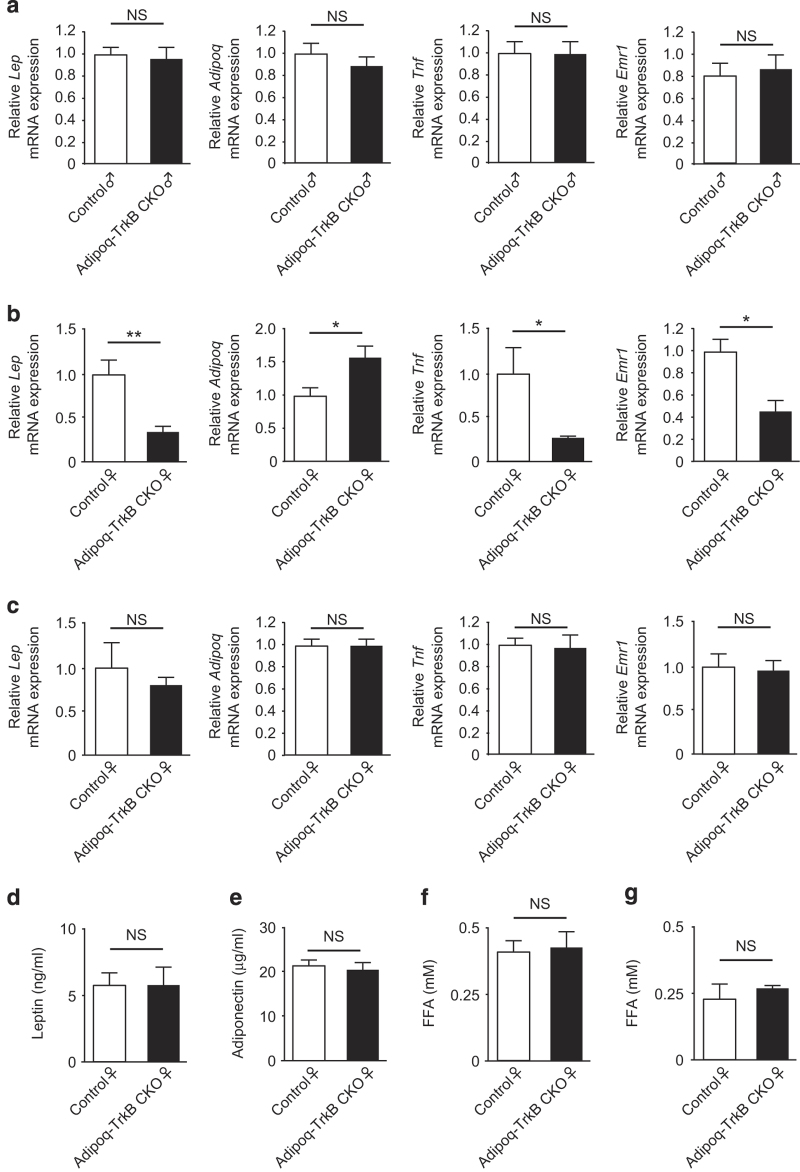
Next, we analyzed adipokine expression. At the age of 24 weeks, female Adipoq-TrkB CKO mice, but not male mice, showed lower expression of Lep (the gene coding for leptin), Tnf, and Emr1 (a marker of macrophages) than their littermate controls (Figure 6a and b). In addition, the expression of adiponectin in adipose tissue was significantly higher in 24-week-old female Adipoq-TrkB CKO mice, but not male mice, compared with their littermate controls (Figure 6a and b). In contrast, there were no significant differences in the expression of these molecules between female Adipoq-TrkB CKO mice and their littermate controls at 12 weeks of age when their body weight did not differ (Figure 6c). These results suggested that reduction of adiposity owing to decreased food intake secondary to deletion of TrkB in adipose tissue led to attenuation of the dysregulated expression of adipokines and adipose tissue inflammation, which could account for the improvement of glucose metabolism in Adipoq-TrkB CKO female mice aged 24–26 weeks. To further address the potential mechanisms underlying the decrease in food intake, we measured circulating levels of leptin, adiponectin, and free fatty acids in Adipoq-TrkB CKO mice and their littermate controls aged 12 weeks. There were no differences between these two groups (Figure 6d–g), suggesting that the adipose tissue BDNF/TrkB axis influences feeding behavior via an unconventional pathway.
Figure 6.
Adipokine expression in adipose tissue of Adipoq-TrkB CKO female mice. (a and b) Real-time PCR analysis of Lep, Adipoq, Tnf, and Emr1 expression in epididymal adipose tissue from Adipoq-TrkB CKO male (a) and female (b) mice and their littermate controls receiving the HFHS diet at 24 weeks of age; n=4–5. (c) Real-time PCR analysis of Lep, Adipoq, Tnf, and Emr1 expression in epididymal adipose tissue from Adipoq-TrkB CKO female mice and their littermate controls receiving the HFHS diet at 12 weeks of age; n=4–5. (d and e) Serum leptin (d) and adiponectin (e) levels in Adipoq-TrkB CKO female mice and their littermate controls receiving the HFHS diet at 12 weeks of age; n=7. (f and g) Serum free fatty acids (FFA) under fasted (f) and fed (g) conditions in Adipoq-TrkB CKO female mice and their littermate controls receiving the HFHS diet at 12 weeks of age; n=4–7. *P<0.05. Data are shown as the mean±s.e.m. CKO, conditional knockout; HFHS, high-fat/high-sucrose.
Discussion
In the present study, we investigated the pathophysiological role of BDNF/TrkB signaling in peripheral tissues. We found significant changes of Bdnf and Ntrk2 expression in the adipose tissue of mice with dietary obesity, with Bdnf being upregulated and Ntrk2 being downregulated. Data from adipocyte-specific CKO models indicated that Ntrk2 is predominantly expressed by adipocytes, while Bdnf is mainly expressed by other cells. Adipocyte-specific deletion of Ntrk2 in female mice, but not male mice, receiving a high-calorie diet led to reduced food intake and less weight gain compared with littermate controls owing to a decrease in fat mass, indicating a potential role of adipose tissue BDNF/TrkB signaling in regulating both appetite and fat storage.
Deletion of TrkB mediated by Adipoq-Cre led to changes in the eating behavior without any alteration of neuronal Ntrk2 expression. As it is known that food intake is regulated by the hypothalamic feeding center,38 our results suggested that the adipose tissue BDNF/TrkB axis remotely regulates feeding behavior in obese mice. It is noteworthy that the hypothalamic BDNF/TrkB axis negatively regulates the appetite, in contrast to positive regulation by the adipose tissue BDNF/TrkB axis. The impact of the adipose tissue BDNF/TrkB axis on feeding behavior appears to be relatively small, as deletion of the hypothalamic and adipose BDNF/TrkB axes by Fabp4-Cre-mediated recombination led to an increase in body weight with hyperphagia. It has been reported that adipose tissue regulates feeding behavior by secreting adipokines like leptin and adiponectin30,39 or by releasing metabolites such as fatty acids.40 However, there were no changes in the circulating levels of these molecules before the onset of obesity in female Adipoq-TrkB CKO mice. Although we observed significant changes of adipokine expression in Adipoq-TrkB CKO mice at 24 weeks of age, these changes were probably secondary to reduced adiposity owing to a decrease in food intake. Thus, metabolic regulation by the adipose tissue BDNF/TrkB axis could involve other adipokines and metabolites, but the molecular mechanisms involved remain to be determined. Future metabolomic analyses could help to identify the molecular pathways regulating communication between adipose tissue and the brain.
It is unclear why deletion of adipose tissue TrkB had different effects on the feeding behavior of male and female mice. Estrogen regulates the expression of BDNF and TrkB and has functions that overlap those of BDNF in the central nervous system.41 In addition, the plasma level of BDNF differs between men and women42,43 and is positively correlated with the estrogen level in female subjects.44 In the present study, we observed higher levels of adipose Bdnf and Ntrk2 expression in female mice than in male mice. These data suggest that there are gender differences in the biological activity of the central and peripheral BDNF/TrkB axes. It is widely accepted that estrogen regulates food intake and adiposity via the estrogen receptor.45 Because adipocytes express the estrogen receptor,46 it may mediate the influence of estrogen on food intake and adiposity.47,48 Our results also raise the possibility that the BDNF/TrkB axis and estrogen may interact in adipocytes, as well as in the brain, for the regulation of appetite and fat accumulation.
It has been reported that BDNF is expressed by adipocytes29 and might influence energy metabolism as one of the adipokines.49 Although we confirmed that Bdnf expression was increased in the adipose tissue of obese mice, we did not detect a significant decrease of Bdnf expression in the adipose tissue of Adipoq-BDNF CKO mice. There is evidence that Cre expression driven by the Fabp4 promoter leads to recombination in macrophages as well as in adipocytes,50 and it has been reported that macrophages express Bdnf,51 suggesting that macrophages may be the main source of BDNF in adipose tissue. In agreement with this idea, we predominantly detected Bdnf expression in the stromal vascular fraction of adipose tissue from obese mice. Accordingly, it is possible that an increase of infiltrating macrophages accounts for upregulation of Bdnf expression in the adipose tissue of obese mice. Moreover, the Fabp4-Cre phenotypic features of hyperphagia and obesity could be at least partly explained by deletion of Bdnf from macrophages, because BDNF-producing hematopoietic cells have been reported to regulate appetite and energy balance by migrating from bone marrow to the hypothalamus.27 Pre-adipocytes are another possible source of BDNF because Bdnf expression was reported to decrease during adipocyte differentiation29 and Adipoq-mediated recombination may be less efficient in pre-adipocytes than in adipocytes. It has been reported that the circulating level of BDNF is affected by various factors in humans,21,42,43 but we could not detect BDNF in mice by immunoassay, presumably owing to a species difference.52 Neurons innervating adipose tissue could be another source of BDNF, but the neuronal contribution appears to be small because adipose tissue Bdnf expression was not downregulated in Syn1-BDNF CKO mice.
In summary, we found that BDNF/TrkB expression in adipose tissue was altered by a high-calorie diet. In contrast to the known role of the BDNF/TrkB axis in the central nervous system, deletion of TrkB from adipocytes led to decreased food intake and reduced accumulation of fat in female mice fed a high-calorie diet, thereby improving various metabolic abnormalities associated with obesity. Thus, inhibition of the adipose tissue BDNF/TrkB axis may be a potential strategy for the treatment of dietary metabolic abnormalities, especially in females.
Acknowledgments
We thank L Parada and E Rosen for providing floxed Ntrk2 mice and Adipoq-Cre mice, respectively. This work was supported by a Grant-in-Aid for Scientific Research (B), a Grant-in-Aid for Scientific Research on Innovative Areas (Stem Cell Aging and Disease), and a Grant-in-Aid for Exploratory Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan and grants from the Ono Medical Research Foundation, the Japan Diabetes Foundation, the Takeda Science Foundation, the Takeda Medical Research Foundation, and the Daiichi-Sankyo Foundation of Life Science (to TM), and a grant from Bourbon (to TM, IS, and YY).
Footnotes
Supplementary Information accompanies the paper on the npj Aging and Mechanisms of Disease website (http://www.nature.com/npjamd)
The authors declare no conflict of interest.
References
- Knusel B , Winslow JW , Rosenthal A , Burton LE , Seid DP , Nikolics K et al. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin 3. Proc Natl Acad Sci USA 1991; 88: 961–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EM , Vogel KS , Davies AM . Neurotrophic factors promote the maturation of developing sensory neurons before they become dependent on these factors for survival. Neuron 1992; 9: 139–150. [DOI] [PubMed] [Google Scholar]
- Cowansage KK , LeDoux JE , Monfils MH . Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol 2010; 3: 12–29. [DOI] [PubMed] [Google Scholar]
- Yoshii A , Constantine-Paton M . Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol 2010; 70: 304–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B , Goulding EH , Zang K , Cepoi D , Cone RD , Jones KR et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci 2003; 6: 736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariohay B , Lebrun B , Moyse E , Jean A . Brain-derived neurotrophic factor plays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology 2005; 146: 5612–5620. [DOI] [PubMed] [Google Scholar]
- Conner JM , Lauterborn JC , Yan Q , Gall CM , Varon S . Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci 1997; 17: 2295–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q , Radeke MJ , Matheson CR , Talvenheimo J , Welcher AA , Feinstein SC . Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J Comp Neurol 1997; 378: 135–157. [PubMed] [Google Scholar]
- Unger TJ , Calderon GA , Bradley LC , Sena-Esteves M , Rios M . Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci 2007; 27: 14265–14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA , Hefti F . BDNF and NGF treatment in lesioned rats: effects on cholinergic function and weight gain. Neuroreport 1992; 3: 405–408. [DOI] [PubMed] [Google Scholar]
- Toriya M , Maekawa F , Maejima Y , Onaka T , Fujiwara K , Nakagawa T et al. Long-term infusion of brain-derived neurotrophic factor reduces food intake and body weight via a corticotrophin-releasing hormone pathway in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol 2010; 22: 987–995. [DOI] [PubMed] [Google Scholar]
- Lyons WE , Mamounas LA , Ricaurte GA , Coppola V , Reid SW , Bora SH et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci USA 1999; 96: 15239–15244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernie SG , Liebl DJ , Parada LF . BDNF regulates eating behavior and locomotor activity in mice. EMBO J 2000; 19: 1290–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M , Fan G , Fekete C , Kelly J , Bates B , Kuehn R et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol 2001; 15: 1748–1757. [DOI] [PubMed] [Google Scholar]
- Thorleifsson G , Walters GB , Gudbjartsson DF , Steinthorsdottir V , Sulem P , Helgadottir A et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 2009; 41: 18–24. [DOI] [PubMed] [Google Scholar]
- Speliotes EK , Willer CJ , Berndt SI , Monda KL , Thorleifsson G , Jackson AU et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010; 42: 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y , Kubo M , Ohmiya H , Takahashi A , Kumasaka N , Hosono N et al. Common variants at CDKAL1 and KLF9 are associated with body mass index in east Asian populations. Nat Genet 2012; 44: 302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W , Cho YS , Zheng W , Dorajoo R , Kato N , Qi L et al. Meta-analysis identifies common variants associated with body mass index in east Asians. Nat Genet 2012; 44: 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JC , Liu QR , Jones M , Levinn RL , Menzie CM , Jefferson-George KS et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med 2008; 359: 918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo GS , Connie Hung CC , Rochford J , Keogh J , Gray J , Sivaramakrishnan S et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci 2004; 7: 1187–1189. [DOI] [PubMed] [Google Scholar]
- Suwa M , Kishimoto H , Nofuji Y , Nakano H , Sasaki H , Radak Z et al. Serum brain-derived neurotrophic factor level is increased and associated with obesity in newly diagnosed female patients with type 2 diabetes mellitus. Metabolism 2006; 55: 852–857. [DOI] [PubMed] [Google Scholar]
- Tonra JR , Ono M , Liu X , Garcia K , Jackson C , Yancopoulos GD et al. Brain-derived neurotrophic factor improves blood glucose control and alleviates fasting hyperglycemia in C57BLKS-Lepr(db)/lepr(db) mice. Diabetes 1999; 48: 588–594. [DOI] [PubMed] [Google Scholar]
- Tsuchida A , Nakagawa T , Itakura Y , Ichihara J , Ogawa W , Kasuga M et al. The effects of brain-derived neurotrophic factor on insulin signal transduction in the liver of diabetic mice. Diabetologia 2001; 44: 555–566. [DOI] [PubMed] [Google Scholar]
- Hanyu O , Yamatani K , Ikarashi T , Soda S , Maruyama S , Kamimura T et al. Brain-derived neurotrophic factor modulates glucagon secretion from pancreatic alpha cells: its contribution to glucose metabolism. Diabetes Obes Metab 2003; 5: 27–37. [DOI] [PubMed] [Google Scholar]
- Teillon S , Calderon GA , Rios M . Diminished diet-induced hyperglycemia and dyslipidemia and enhanced expression of PPARalpha and FGF21 in mice with hepatic ablation of brain-derived neurotropic factor. J Endocrinol 2010; 205: 37–47. [DOI] [PubMed] [Google Scholar]
- Matthews VB , Astrom MB , Chan MH , Bruce CR , Krabbe KS , Prelovsek O et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009; 52: 1409–1418. [DOI] [PubMed] [Google Scholar]
- Urabe H , Kojima H , Chan L , Terashima T , Ogawa N , Katagi M et al. Haematopoietic cells produce BDNF and regulate appetite upon migration to the hypothalamus. Nat Commun 2013; 4: 1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman GJ , Poulos SP , Richardson RL , Barb CR , Andacht T , Kirk HC et al. Secreted proteins and genes in fetal and neonatal pig adipose tissue and stromal-vascular cells. J Anim Sci 2006; 84: 1666–1681. [DOI] [PubMed] [Google Scholar]
- Bernhard F , Landgraf K , Kloting N , Berthold A , Buttner P , Friebe D et al. Functional relevance of genes implicated by obesity genome-wide association study signals for human adipocyte biology. Diabetologia 2013; 56: 311–322. [DOI] [PubMed] [Google Scholar]
- Friedman JM , Halaas JL . Leptin and the regulation of body weight in mammals. Nature 1998; 395: 763–770. [DOI] [PubMed] [Google Scholar]
- Havel PJ . Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes 2004; 53: S143–S151. [DOI] [PubMed] [Google Scholar]
- Yamauchi T , Kamon J , Minokoshi Y , Ito Y , Waki H , Uchida S et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002; 8: 1288–1295. [DOI] [PubMed] [Google Scholar]
- Luikart BW , Nef S , Shipman T , Parada LF . In vivo role of truncated trkb receptors during sensory ganglion neurogenesis. Neuroscience 2003; 117: 847–858. [DOI] [PubMed] [Google Scholar]
- Eguchi J , Wang X , Yu S , Kershaw EE , Chiu PC , Dushay J et al. Transcriptional control of adipose lipid handling by IRF4. Cell Metab 2011; 13: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens K , Bottelbergs A , Baes M . Ectopic recombination in the central and peripheral nervous system by aP2/FABP4-Cre mice: implications for metabolism research. FEBS Lett 2010; 584: 1054–1058. [DOI] [PubMed] [Google Scholar]
- Zhu Y , Romero MI , Ghosh P , Ye Z , Charnay P , Rushing EJ et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev 2001; 15: 859–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY , Russell SJ , Ussar S , Boucher J , Vernochet C , Mori MA et al. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes 2013; 62: 864–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW , Woods SC , Porte D Jr. , Seeley RJ , Baskin DG . Central nervous system control of food intake. Nature 2000; 404: 661–671. [DOI] [PubMed] [Google Scholar]
- Kubota N , Yano W , Kubota T , Yamauchi T , Itoh S , Kumagai H et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 2007; 6: 55–68. [DOI] [PubMed] [Google Scholar]
- Pocai A , Lam TK , Obici S , Gutierrez-Juarez R , Muse ED , Arduini A et al. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest 2006; 116: 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F , Lewis DK . Estrogen-BDNF interactions: implications for neurodegenerative diseases. Front Neuroendocrinol 2006; 27: 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M , Zingler D , Schuhbaeck K , Schloetcke K , Zingler C , Schuff-Werner P et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging 2005; 26: 115–123. [DOI] [PubMed] [Google Scholar]
- Komulainen P , Pedersen M , Hanninen T , Bruunsgaard H , Lakka TA , Kivipelto M et al. BDNF is a novel marker of cognitive function in ageing women: the DR's EXTRA Study. Neurobiol Learn Mem 2008; 90: 596–603. [DOI] [PubMed] [Google Scholar]
- Begliuomini S , Casarosa E , Pluchino N , Lenzi E , Centofanti M , Freschi L et al. Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. Hum Reprod 2007; 22: 995–1002. [DOI] [PubMed] [Google Scholar]
- Brown LM , Clegg DJ . Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol 2010; 122: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes JS , Watson GH . Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev 2004; 5: 197–216. [DOI] [PubMed] [Google Scholar]
- Tanaka M , Nakaya S , Kumai T , Watanabe M , Tateishi T , Shimizu H et al. Effects of estrogen on serum leptin levels and leptin mRNA expression in adipose tissue in rats. Horm Res 2001; 56: 98–104. [DOI] [PubMed] [Google Scholar]
- Davis KE , D Neinast M , Sun K , M Skiles W , D Bills J , A Zehr J et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab 2013; 2: 227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sornelli F , Fiore M , Chaldakov GN , Aloe L . Adipose tissue-derived nerve growth factor and brain-derived neurotrophic factor: results from experimental stress and diabetes. Gen Physiol Biophys 2009; 28: 179–183. [PubMed] [Google Scholar]
- Fu Y , Luo N , Lopes-Virella MF . Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. J Lipid Res 2000; 41: 2017–2023. [PubMed] [Google Scholar]
- Barouch R , Appel E , Kazimirsky G , Brodie C . Macrophages express neurotrophins and neurotrophin receptors. Regulation of nitric oxide production by NT-3. J Neuroimmunol 2001; 112: 72–77. [DOI] [PubMed] [Google Scholar]
- Radka SF , Holst PA , Fritsche M , Altar CA . Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res 1996; 709: 122–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.