Abstract
The relationship between endosomal pH and function is well documented in viral entry, endosomal maturation, receptor recycling, and vesicle targeting within the endocytic pathway. However, specific molecular mechanisms that either sense or regulate luminal pH to mediate these processes have not been identified. Herein we describe the use of novel, compartment-specific pH indicators to demonstrate that yeast Nhx1, an endosomal member of the ubiquitous NHE family of Na+/H+ exchangers, regulates luminal and cytoplasmic pH to control vesicle trafficking out of the endosome. Loss of Nhx1 confers growth sensitivity to low pH stress, and concomitant acidification and trafficking defects, which can be alleviated by weak bases. Conversely, weak acids cause wild-type yeast to present nhx1Δ trafficking phenotypes. Finally, we report that Nhx1 transports K+ in addition to Na+, suggesting that a single mechanism may responsible for both pH and K+-dependent endosomal processes. This presents the newly defined family of eukaryotic endosomal NHE as novel targets for pharmacological inhibition to alleviate pathological states associated with organellar alkalinization.
INTRODUCTION
It is well established that luminal acidification of the endocytic pathway, including the endosome and lysosome/vacuole, is required for associated cellular function (Mellman et al., 1986; Mellman, 1992). Some examples include ligand-receptor dissociation and recycling of surface receptors, lysosome-mediated protein degradation, H+-driven neurotransmitter loading and pH-dependent recycling of synaptic vesicles (Buckley et al., 2000; Nishi and Forgac, 2002). Similarly, viral pathogen entry and propagation is dependent on the pH gradient across the lumen of the endosome (Harley et al., 2001), and the abnormal lysosomal/endosomal morphologies and associated defective trafficking observed in a subset of lysosomal storage disorders are associated with abnormal changes in luminal pH (Futerman and van Meer, 2004). Pioneering experiments performed by Heuser clearly demonstrated that changes in cellular pH alone severely alter organellar morphology and movement (Heuser, 1989). This phenomenon can be explained by net changes in vesicle trafficking between compartments, as luminal pH can direct vesicle trafficking; thus, elevated pH in the endosome promotes endosome to Golgi vesicle movement (van Weert et al., 1995, 1997; also see Nieland et al., 2004). At the molecular level, local increases in pH are believed to be responsible for assembly of vesicle trafficking/sorting machinery in areas of the endosome destined for return to the plasma membrane (Maranda et al., 2001; also see Zeuzem et al., 1992; Aniento et al., 1996). Despite extensive evidence that changes in pH direct trafficking in this pathway, specific molecular mechanisms that control pH itself have not been defined.
The ubiquitous Na+/H+ exchangers of the NHE family are associated with cellular pH regulation (Orlowski and Grinstein, 2004). Recent phylogenetic analysis of the NHE family has revealed two distinct subgroups corresponding to plasma membrane and intracellular transporters (Brett et al., 2005). Although derived from a common ancestral gene, emerging evidence indicates that members of the two subgroups are distinct from one another in ion selectivity, kinetic properties, inhibitor sensitivity, and physiological role. The plasma membrane NHE, represented by the mammalian isoforms NHE1–NHE5, have been extensively characterized and implicated in the regulation of cytoplasmic pH, maintenance of cell volume, Na+ homeostasis, and transepithelial transport of electrolytes. By regulating cytoplasmic pH, these plasma membrane Na+/H+ exchangers are involved in numerous pathophysiological processes including hypertension, epilepsy, postischemic myocardial arrhythmia, and regulation of aqueous humor secretion associated with glaucoma (reviewed by Orlowski and Grinstein, 2004). In contrast, much less is known about the properties of the intracellular subgroup despite the recent discovery of numerous candidate genes from plants, model organisms, and higher vertebrates, including human NHE6–NHE9 (reviewed by Brett et al., 2005). The best-studied ortholog is Nhx1, the endosomal Na+/H+ exchanger of Saccharomyces cerevisiae.
Initially, Nhx1 was shown to mediate vacuolar sequestration of Na+, coupling Na+ movement to the proton gradient established by the vacuolar H+-ATPase and thus contributing to salt and osmotic tolerance (Nass et al., 1997; Nass and Rao, 1999). However, further studies in yeast found that its role in cellular physiology was not limited to ion homeostasis, as nhx1Δ null cells show a “class E” vacuolar protein sorting (VPS) phenotype characterized by enlargement of the late endosomal or prevacuolar compartment and mis-sorting of vacuolar carboxypeptidase Y to the cell surface (Bowers et al., 2000). Supporting evidence for a role in vesicle trafficking came from studies showing that Nhx1 binds to Gyp6, a GTPase-activating protein involved in Ypt6-mediated retrograde traffic to the Golgi (Ali et al., 2004). These studies implicate Nhx1 in vesicular exit from the endosome, although the mechanistic basis for this role remained unclear. In this work, we use novel compartment-specific pH probes in wild-type yeast and null mutants to show that Nhx1 regulates both vacuolar and cytoplasmic pH, in an opposite manner to the V-type H+-ATPase. We demonstrate that pH changes are linked to vesicle trafficking such that manipulation of compartmental pH by weak acids or bases can simulate or ameliorate trafficking defects, respectively. Finally, we show that although both plasma membrane and endosomal Na+/H+ exchangers contribute to ion homeostasis and cytoplasmic pH regulation, Nhx1 uniquely regulates compartmental pH to control traffic. Given the ubiquity of the intracellular NHE and the conservation in vesicle trafficking pathways between yeast and mammalian cells, our findings may be extrapolated to predict a similar role for the intracellular NHE (NHE6–NHE9).
MATERIALS AND METHODS
Yeast Strains, Media, and Growth Conditions
All S. cerevisiae strains used were derivatives of BY4742 (MATα; Invitrogen, Carlsbad, CA). The nhx1Δnha1Δ strain was made by deleting nhx1 in a nha1Δ background as previously described (Nass et al., 1997). Strains were grown at 30°C in APG, a synthetic minimal medium containing 10 mM arginine, 8 mM phosphoric acid, 2% glucose, 2 mM MgSO4, 1 mM KCl, 0.2 mM CaCl2, and trace minerals and vitamins (also see Nass et al., 1997). The pH was adjusted by addition of phosphoric acid to 4.0 or 2.7 or by addition of 10 mM MES and KOH to pH 7.0, as specified. Where indicated, NaCl, KCl, hygromycin B, acetic acid, propionic acid, chloroquine or tetramethylammonium (TMA) was added. Growth assays were performed by inoculating 0.2 ml of APG medium in a 96-well microplate with 1–2 μl of a seed culture grown to saturation. Growth was monitored by measuring A600 nm after culturing for 18–24 h at 30°C.
Plasmid Construction
The gene for pHluorin, a pH-sensitive mutant of GFP, was amplified from a pCI vector containing pHluorin fused to the C-terminus of cellubrevin (a kind gift from Dr. James E. Rothman, Memorial Sloan-Kettering Cancer Center, New York, NY; Miesenbock et al., 1998), using the primer sequences: 5′CGCggatccGCGATGAGTAAAGGAGAACTTTTCACTGGA, 3′ CCGgaattcCGGTTATTTGTATAGTTCATCCATGCC (pHluorin sequences underlined). The resulting 726-base pair fragment, containing the entire pHluorin gene flanked with 5′ BamHI and 3′ EcoRI sites (shown in lower case), was subcloned into pRN69 (a 2μ plasmid containing tandem heat shock promoter sequences in the parent YCplac111 vector; see Nass et al., 1997). The resulting plasmid was named pCB901YpHc.
Measurement of Vacuolar and Cytosolic pH
Vacuolar pH measurements were performed using methods previously described (Ali et al., 2004). Briefly, cells were grown in APG growth medium pH 2.7 for 18 h at 30°C, absorbance readings were taken at 600 nm to measure growth, and cultures were then incubated with 50 μM 2′,7′-bis(2-carboxyethyl)-5,6-carboxyfluorescein-acetoxymethyl ester (BCECF-AM; Molecular Probes, Eugene, OR) at 30°C for 20–30 min, washed, and suspended in APG medium at pH 2.7. Single fluorescence intensity and absorbance readings were taken at 485 and 600 nm, respectively, and normalized background-subtracted fluorescence emission at 485 nm values were calculated (NI485). At least three independent calibration experiments were performed for each strain tested and vacuolar pH values were calculated as described previously (Ali et al., 2004). For cytoplasmic pH measurement, strains were transformed with pCB901YpHc containing the pHluorin gene. Transformants were selected on SC leu- plates and cytoplasmic localization of pHluorin was confirmed by confocal microscopy (see Figure 2B). Seed cultures were grown and washed as described above and then used to inoculate 0.2-ml cultures within a clear-bottomed, black 96-well plate. Growth was monitored by measuring absorbance at 600 nm after culturing for 18 h at 30°C in specified growth media (APG, pH 4.0 and 2.7) and immediately used for fluorescence measurement. Fluorescent intensity and absorbance values were acquired using a BMG FLUOstar Optima multimode plate reader with accompanying BMG FLUOstar Optima Version 1.20 software (BMG Labtechnologies, Durham, NC). Emission fluorescence intensity readings at 405 nm (I405) and 485 nm (I485) excitation wavelengths were acquired and background fluorescence was measured using untransformed, pHluorin-free cultures. At the end of each experiment, a calibration curve of the ratio of fluorescence intensity values versus pH was obtained for each yeast strain as follows. Yeast cultures were incubated in 200 μl of experimental media containing 50 mM MES, 50 mM HEPES, 50 mM KCl, 50 mM NaCl, 0.2 M ammonium acetate, 10 mM NaN3, 10 mM 2-deoxyglucose, 75 μM monensin, and 10 μM nigericin, titrated to eight different pH values within the range of 4.5–8.0 using 1 M NaOH (also see Ali et al., 2004). Experimental I405/I485 values were background-subtracted and then normalized to the ratio value corresponding to pH 7. To estimate cytoplasmic pH, resulting normalized I405/I485 values (NI405/I485) from a given strain were compared with an average calibration curve generated from the four yeast strains tested (see Figure 2B). All experiments were performed at 30°C.
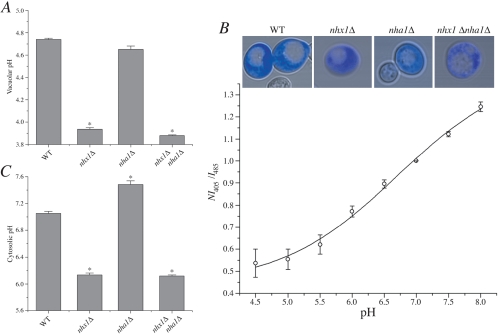
Figure 2.
Nhx1 is important for cellular pH homeostasis. Cultures of isogenic wild-type (WT) or null mutants were grown for 18 h at 30°C in APG medium buffered to pH 2.7 with phosphoric acid. (A) Vacuolar pH in acid-stressed cells. Cultures were loaded with BCECF under conditions that resulted in accumulation of the dye in yeast vacuoles (Ali et al., 2004). Fluorescence was normalized to cell number and vacuolar pH was determined following calibration as described in Materials and Methods. (B) Measuring cytoplasmic pH with pHluorin in yeast. Yeast transformed with the cytoplasmic pH indicator pHluorin were incubated in calibration buffer titrated to 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, or 8.0 as described in Materials and Methods. Fluorescence intensity values (arbitrary units) were collected at 405 and 485 nm and background subtracted, and the ratio was calculated at each pH value. This was normalized to the ratio value corresponding to pH 7 (NI405/I485; data are from 18 to 30 independent experiments for each of four strains tested). Data from all four strains were pooled and mean ratio values are plotted with a fitted nonlinear curve shown as a dark line. Inset: fluorescent confocal micrographs of four different yeast strains transformed with the cytoplasmic pH indicator pHluorin (blue). Scale bar, 5 μm. All images are shown at equal magnification. (C) Cytoplasmic pH in acid-stressed cells. Fluorescence was measured in cultures expressing cytoplasmic pHluorin, and pH values were estimated after calibration (see Figure 2B). Means and calculated SEs are plotted from at least three independent experiments.
FM4–64 Efflux Assay
Using a modified protocol from Wiederkehr et al. (2000), the rate of exocytosis from cells was estimated using the lipophilic styryl dye N-(3-triethylammoniumpropyl)-4-(p-diehtylaminophenyl-hexatrienyl) pyridinium dibromide (FM4–64). In brief, 3-ml cultures of cells were grown in APG medium (pH 4 and 2.7) for 18 h at 30°C, harvested, washed three times with ice cold growth medium, resuspended in ice cold medium at a final concentration of 10 A600 nm/ml and incubated with FM4–64 (0.4 μM; Molecular Probes) on ice for 30 min. After dye loading, cells were then pelleted and resuspended in 1 ml ice-cold growth medium and immediately transferred to a 10.0-mm quartz cuvette equipped with a magnetic stir barrel preheated to 30°C. Fluorescence intensity values were then recorded at 10-s intervals for 2 h using a Aminco Bowman Series 2 Spectrophotometer (Thermo Electron, West Palm Beach, FL) with excitation and emission wavelengths of 515 and 640 nm, respectively.
Ste3-GFP Localization
BY4742 (MATα) strains from the ResGen viable knock collection were transformed with pJLU34, a CEN-URA3 plasmid encoding Ste3-GFP (Urbanowski and Piper, 1999). Transformants were grown in experimental medium at 30°C to logarithmic phase (∼19 h). Each 3-ml culture was briefly centrifuged to pellet the cells and then resuspended in ∼200 μl of the same solution. Cell suspensions of 4 μl were dropped on poly-l-lysine treated coverslips and placed on glass slides. Images were acquired using a Zeiss LSM410 laser confocal microscope (Thornwood, NY) equipped with a Zeiss ×100 oil immersion lens. Digitized images (8-bit) were recorded at 16-times frame averaging, processed using MetaMorph software (Universal Imaging, West Chester, PA), and assembled using Adobe Photoshop software (Adobe Systems, Mountain View, CA). Final images shown are representative of >90% cells observed expressing the indicated Ste3-GFP fusion protein. Each experiment was repeated at least three times.
86Rb Transport Assay
Yeast strains were grown in APG medium to a density 1.5–2 A600 nm units/ml. Cells were harvested by centrifugation, washed once in APG medium, and resuspended in the same medium at a density of 1 × 108 cells/ml. Uptake was initiated by adding 86RbCl (Amersham Biosciences, Piscataway, NJ) at 5 μCi/ml. Samples were incubated at 23°C, and at the indicated times aliquots were withdrawn and rapidly filtered through 0.45-μm HAWP membranes (Millipore, Bedford, MA) and washed twice with 6 ml of wash buffer (10 mM HEPES, Tris, pH 7.4, 150 mM choline chloride). Radioactivity retained on the filters was measured by liquid scintillation counting. For the efflux assay, cells were loaded with 86RbCl exactly as above for a period of 24 h, collected by centrifugation, washed twice in ice-cold Buffer A (10 mM Tris-HCl, pH 6.0, 2 mM MgCl2, 1% glucose, 0.6 M sorbitol), and finally resuspended at 1 × 108 cells/ml in the same buffer at room temperature. At the indicated times, aliquots were withdrawn, filtered, and processed as described above. One-half of the suspension was permeabilized by addition of CuSO4 to a final concentration of 500 μM, whereas the other half received an equal volume of water (Nass et al., 1997). Cupric ions have previously been shown to selectively permeabilize the plasma membranes under these conditions, while leaving the vacuoles intact (Nass et al., 1997). After incubation at 23°C for the indicated times, aliquots were withdrawn, filtered, and processed as described above. To assay 86Rb influx in permeabilized cells, cultures (2 × 108 cells/ml) were preincubated with 500 μM CuSO4 in buffer A for 1 h as above. Uptake was initiated by adding 5 μCi/ml 86RbCl. At the indicated times, aliquots were withdrawn for filtration as above.
Statistical Analysis
Data are reported as mean ± SEM, and statistical comparisons were performed with Student's two-tailed t tests (paired or unpaired, as appropriate); significance was assumed at the 5% level.
RESULTS
Nhx1 Is Required for Cellular pH Homeostasis
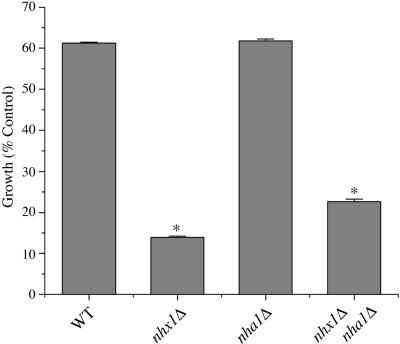
A well-known phenotype of vma mutants, defective in the vacuolar H+ pumping ATPase, is the inability to acidify vacuoles or grow in medium buffered to neutral or alkaline pH (Nelson and Nelson, 1990; Yamashiro et al., 1990). Because Nhx1 is proposed to alkalinize the lumen of the endosome/vacuole by serving as a H+ leak pathway (Nass and Rao, 1998), we hypothesized that excessive vacuolar acidification in the nhx1 null mutant may result in growth sensitivity to low pH. Indeed, we show in Figure 1 that the nhx1Δ mutant shows poor growth in medium buffered to pH 2.7, relative to wild-type. Deletion of the plasma membrane antiporter Nha1 had no effect on low pHo sensitivity, whereas the double mutant nhx1Δnha1Δ behaved similar to the nhx1Δ strain.
Figure 1.
Growth sensitivity to low pH. Cultures of isogenic wild-type (WT) or null mutants were grown for 18 h at 30°C in APG medium buffered to pH 2.7 with phosphoric acid. Absorbance of cultures was measured at 600 nm and values were normalized to growth recorded at under control conditions (APG medium, pH 4.0).
Growth sensitivity to acidic pH in the nhx1Δ mutant could be due to defective pH regulation in the vacuole or cytosol. To assess both these possibilities, we examined the contributions of Nhx1 and Nha1 to vacuolar and cytoplasmic pH using compartment-specific pH-sensitive fluorescent probes. Although the acetoxymethyl ester of the fluorescein based probe BCECF is routinely used to measure cytoplasmic pH in animal cells, the abundance of vacuolar esterases in yeast results in the atypical accumulation of the probe into the vacuole, where it serves as a convenient pH indicator (Ali et al., 2004). In wild-type yeast subjected to low pH stress (medium buffered to pH 2.7), we report a vacuolar pH of nearly 4.8. A further acidification by 0.8–1 pH unit was observed in the nhx1Δ mutant and in the nhx1Δnha1Δ double mutant, whereas vacuolar pH in the nha1Δ mutant was similar to wild-type (Figure 2A). Furthermore, alkaline vacuolar pH values were confirmed in vmaΔ mutants under the same growth conditions (unpublished data). All of these results closely parallel the corresponding changes in cell growth under low pH stress (Figure 1), showing a correlation between low vacuolar pH and poor growth.
We demonstrate, for the first time, use of pHluorin, a pH-sensitive mutant of GFP (Miesenbock et al., 1998), as a probe of cytoplasmic pH in yeast. As described in Materials and Methods, pHluorin localizes to the cytoplasm, and importantly, is excluded from the vacuoles (Figure 2B). Using a dual excitation ratiometric technique, we show that in our experimental system pHluorin has a pKa = 7.14 and can accurately estimate cytoplasmic pH values greater than 8.0 and as low as 5.5. In wild-type cells grown at pH 2.7, cytoplasmic pH was maintained at pH 7. Surprisingly, the nhx1 mutant was found to be significantly more acidic, with a pHc of ∼6.2 (Figure 2C). Cytosolic pH in the nha1 mutant was more alkaline relative to wild-type, as has been reported previously (Sychrova et al., 1999). However, we show that this alkalinization was reversed upon additional deletion of Nhx1, so that cytoplasmic pH in the double mutant resembled that of nhx1Δ. Taken together, our results demonstrate that Nhx1 function is dominant over Nha1 and opposite to the vacuolar H+-ATPase in cellular pH regulation and the response to low pH stress.
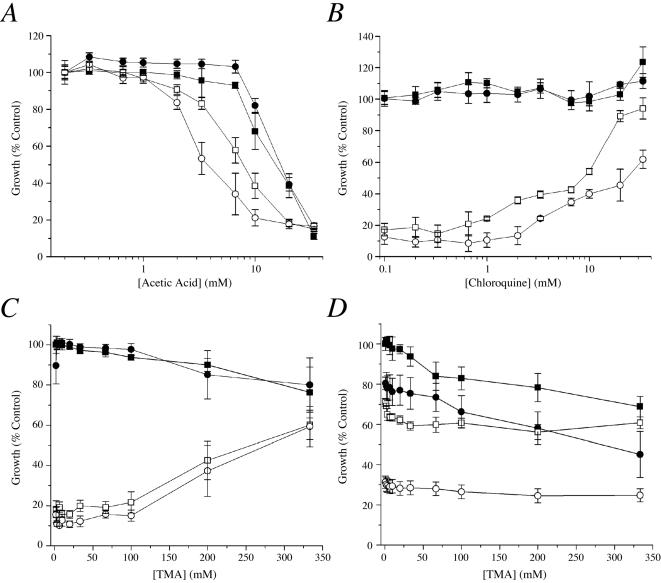
Acidification of vacuolar and cytosolic pH may also be accomplished by adding weak acids to the growth medium. As shown in Figure 3A, growth of nhx1Δ and nhx1Δnha1Δ strains was exacerbated by the addition of weak acids, such as acetic acid or propionic acid (unpublished data), relative to WT and nha1Δ strains. On the other hand, growth of nhx1Δ and nhx1Δnha1Δ strains at low external pH (2.7) could be recovered with the addition of weak bases such as chloroquine (Figure 3B) and tetramethylammonium (TMA; unpublished data). As expected, measurement of pH using the compartment-specific probes described above confirmed that both vacuolar and cytoplasmic pH were acidified and alkalinized by the addition of weak acids and bases, respectively (unpublished data). On the basis of these results, we conclude that the hypersensitivity of nhx1Δ strains to acid stress is due to an inability to compensate for intracellular acidification.
Figure 3.
Effect of weak acids and bases on growth phenotypes. Growth measurements of isogenic wild-type (WT, ▪) or nha1Δ (•), nhx1Δ (□) and nhx1Δ nha1Δ (○) mutants grown for 18 h at 30°C in APG medium adjusted as follows. (A) pH 4.0 in the presence of increasing concentrations of acetic acid. (B) pH 2.7 in the presence of increasing concentrations of the weak base chloroquine. (C) pH 4.0 in the presence of 10 μg/ml Hygromycin B and increasing concentrations of the weak base TMA. (D) pH 4.0 in the presence of 600 mM KCl and increasing concentrations of TMA. Note that growth of mutants in APG pH 4.0 in the absence of weak acid was indistinguishable from wild-type. Mean values normalized to control and calculated SEs are plotted from at least three independent experiments.
Endosomal Vesicle Trafficking Is Regulated by pH
The ability of weak bases to alleviate pH-sensitive growth provided a unique opportunity to ask if defective pH regulation was responsible for other nhx1Δ phenotypes. In earlier work, loss of Nhx1 was shown to confer hypersensitivity to the protein synthesis inhibitor, hygromycin B (Gaxiola et al., 1999). Thus, both nhx1Δ and nhx1Δnha1Δ mutants fail to grow in the presence of low concentrations of hygromycin B (10 μg/ml) that are without effect on wild-type or nha1Δ mutants (Figure 3C). Strikingly, addition of the weak base TMA allows growth recovery of nhx1Δ and nhx1Δ nha1Δ in the presence of hygromycin B to nearly wild-type levels. In contrast, salt sensitivity of nhx1Δ mutants could not be alleviated by weak base. Both plasma membrane and endosomal antiporters were found to be required for tolerance to high concentrations of salt, and addition of TMA did not alleviate growth of any of the mutants in the presence of 800 mM NaCl (unpublished data) or 800 mM KCl (Figure 3D). These results suggest that the mechanism by which Nhx1 confers tolerance to hygromycin B is coupled to cellular pH regulation, whereas salt (NaCl or KCl) tolerance requires monovalent cation exchange by the antiporters themselves.
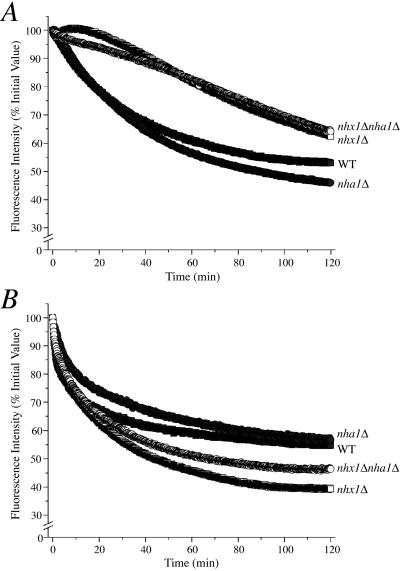
Hygromycin B sensitivity is a shared phenotype of many vesicle-trafficking mutants and appears to be linked to defects in the endocytic and vacuolar biogenesis (VPS) pathways (Conboy and Cyert, 2000; Page et al., 2003). NHX1 itself is allelic to the class E vps44 mutant, which exhibits enlargement of the prevacuolar compartment and defects in vacuolar biogenesis (Bowers et al., 2000). For example, the fluorescent dye FM4–64 intercalates into the plasma membrane and traffics to the vacuole, where it accumulates in wild-type cells. In nhx1Δ cells, FM4–64 was observed to accumulate in the prevacuolar compartment, suggesting that net movement of the dye out of the endosome was impaired (Bowers et al., 2000). Having demonstrated acidification of the endosome/lysosomal compartments in nhx1Δ mutants, we next examined the possibility that the role of Nhx1 in vesicle trafficking was coupled to pH regulation. We used a quantitative, real-time assay previously developed by Riezman and colleagues (Wiederkehr et al., 2000) to monitor rates of FM4–64 efflux from preloaded cells as a measure of dye recycling, and thus exit from endosomal compartments. Before recording dye efflux, it was apparent that nhx1Δ strains accumulated more FM4–64 during the 30-min loading period compared with WT and nha1Δ (unpublished data). Over a 2-h time period, nhx1Δ and nhx1Δ nha1Δ mutants showed significantly reduced rates of FM4–64 efflux relative to WT and nha1Δ strains (Figure 4A). The addition of weak base (333 mM TMA) to the growth medium increased the initial rates of FM4–64 efflux from nhx1Δ and nhx1Δ nha1Δ strains, to be comparable to that of WT (Figure 4B). TMA addition did not have significant effect on loss of fluorescence from WT and nha1Δ, arguing against nonspecific effects of the weak base on FM4–64 fluorescence. These results suggest that pH-compensation of inappropriately acidic compartments in nhx1Δ mutants alleviates defects in endosome-to-surface trafficking, which may be responsible for impaired efflux of the dye.
Figure 4.
pH-dependence of FM4–64 efflux. After dye loading, cell suspensions of isogenic wild-type (WT, ▪) or nhx1Δ (•), nha1Δ (□) and nhx1Δ nha1Δ (○) mutants were placed in a cuvette prewarmed to 30°C at time = 0 and fluorescence was recorded for 2 h in (A) APG pH 4.0 or (B) APG pH 4.0 in the presence of 333 mM TMA. Fluorescence intensity values shown are a percentage of initial I515 values and represent at least three independent experiments.
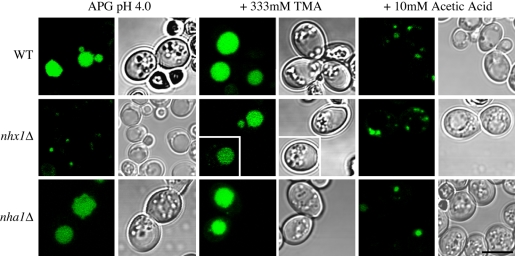
Next, we examined the effect of pH on anterograde traffic to the vacuole from the prevacuolar compartment. Under normal conditions, the G-protein–coupled receptor Ste3-GFP is continuously endocytosed and delivered to the vacuole for degradation. As a result, GFP fluorescence is predominantly located in the vacuole of WT and nha1Δ cells (Figure 5). We show abnormal accumulation of Ste3-GFP in the class E compartment of nhx1Δ mutants, as reported previously (Bowers et al., 2000), suggesting that the late endosome-to-vacuole trafficking pathway is compromised. Addition of TMA (333 mM) resulted in a shift of Ste3-GFP fluorescence from the PVC to a more normal vacuolar distribution in the nhx1Δ strain, whereas the WT and nha1Δ cells remained unchanged (Figure 5, middle column). Conversely, addition of acetic acid (10 mM) resulted in retention of Ste3-GFP in the prevacuolar compartments of WT and nha1Δ mutants, similar to the distribution in nhx1Δ mutant. Under this condition, nhx1Δ cells show Ste3-GFP accumulating in multiple fragmented compartments, suggesting that further acidification causes a more extreme trafficking phenotype that is not tolerated for growth. In summary, the trafficking defects of nhx1Δ may be effectively mimicked by the addition of weak acids or ameliorated by the addition of weak bases. Taken together, we conclude that pH regulation by Nhx1 is critical for trafficking pathways out of the late endosome.
Figure 5.
pH-dependence of Ste3-GFP trafficking. Transmitted light and fluorescent confocal micrographs were acquired from isogenic wild-type (WT, top) or null mutants of Nhx1 (nhx1Δ, middle) and Nha1 (nha1Δ, bottom), transformed with Ste3-GFP (green) grown for 18 h at 30°C in APG pH 4.0 (left), in the presence of 333 mM TMA (middle), or in the presence of 10 mM acetic acid (right). Two nhx1Δ cells grown in the presence of 333 mM TMA are shown from two separate experiments. Scale bar, 5 μm. All fluorescence and transmitted light images are shown at equal magnification. Note the shift in Ste3-GFP fluorescence from punctate endosomes to the vacuolar lumen upon addition of TMA in nhx1Δ cells and conversely, the appearance of Ste3-GFP fluorescence in puncta in WT and nha1Δ cells with addition of 10 mM acetic acid.
Nhx1 Contributes to K+ Sequestration and Homeostasis
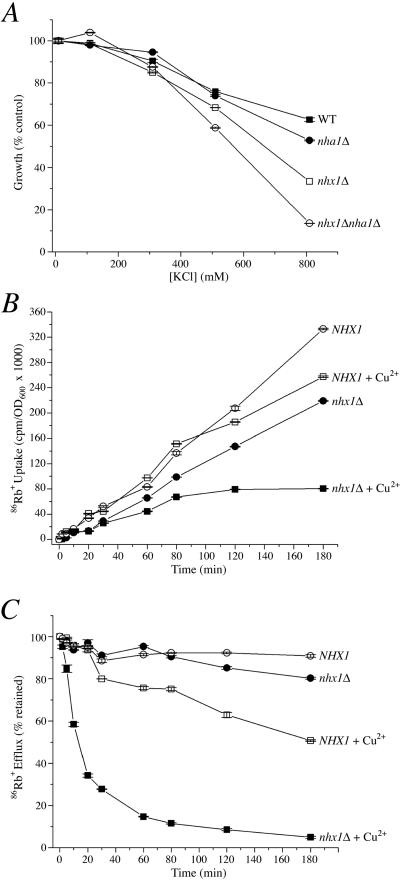
There are well-documented studies showing that Nhx1 and many of its intracellular orthologues in plants confer salt tolerance by sequestering Na+ into the lumen of endocytic compartments (Apse et al., 1999). In addition, there is emerging evidence that cation selectivity of the intracellular NHE extends to K+ as well as Na+ (Numata and Orlowski, 2001; Venema et al., 2002; Nakamura et al., 2004). K+ is the most abundant monovalent cation in the cytoplasm and is therefore a physiologically relevant candidate for monovalent cation exchange by Nhx1, particularly in the context of vesicle trafficking. Thus, we sought to identify a K+-related growth phenotype in yeast. In Figure 6A, we show that the nhx1Δ null mutant is sensitive to high levels of extracellular KCl. Similarly, the mutant lacking the plasma membrane antiporter Nha1 also displays KCl sensitivity, as reported previously (Banuelos et al., 2002). Interestingly, the double null mutant, nhx1Δnha1Δ, is significantly more sensitive than either mutant alone, suggesting that both plasma membrane and intracellular transporters participate in cellular K+ homeostasis
Figure 6.
K+ tolerance correlates with sequestration. (A) Growth sensitivity to extracellular KCl. The isogenic set of wild-type and null mutants was grown in synthetic APG medium supplemented with KCl as indicated. Growth was measured after 18 h and expressed as percent of growth in 1 mM KCl. (B) Uptake of 86Rb into intact and permeabilized cells. The mutant nhx1Δnha1Δ strain was transformed with plasmid expressing wild-type NHX1 (NHX1) or empty vector (Δnhx1) and uptake of 86Rb into intact or Cu2+-treated (plasma membrane permeabilized) cells was quantitated as described in Materials and Methods. (C) Efflux of 86Rb from intact and permeabilized cells. Yeast strains described in B were preloaded with 86Rb, CuSO4 was added to one-half of the preloaded culture (+Cu2+), and at intervals, cells were collected by rapid filtration and the remaining radioactivity determined by liquid scintillation counting of filters. Efflux is presented as percent of cell-associated radioactivity measured immediately before Cu2+ addition. Means and calculated SEs are plotted from three independent experiments.
Next, we used 86Rb as a K+ analogue to determine whether the growth phenotype correlates with K+ transport by Nhx1. Figure 6B shows the time course of 86Rb uptake in the nhx1Δnha1Δ null strain transformed either with a plasmid expressing wild-type Nhx1 or the vector alone. In intact cells, 86Rb uptake was higher in the presence of Nhx1, typically resulting in 1.6-fold greater accumulation at the end of 3 h, relative to nhx1Δ. On selective permeabilization of the plasma membrane by treatment with CuSO4, 86Rb accumulation in cells expressing Nhx1 remained high, indicating that most of the isotope taken up by the cell partitioned into intracellular, Cu2+-impermeable compartments, most likely the endosome and vacuole (Nass et al., 1997). In contrast, sequestration of 86Rb in the absence of Nhx1 is significantly reduced, as evidenced by low uptake into permeabilized cells.
Figure 6C shows the time course of 86Rb efflux from intact, as well as permeabilized cells. Cells were initially loaded with 86Rb over a period of 24 h to maximize cation uptake and sequestration, then washed and resuspended in buffer lacking 86Rb, as described in Materials and Methods. Over a 3-h period, we observed that 86Rb efflux from intact cells was low in both control and nhx1Δ strains. Addition of Cu2+ to permeabilize the plasma membrane elicited an immediate efflux of 86Rb in the nhx1Δ strain, with <5% remaining in 3 h. Thus, in the absence of Nhx1, 86Rb is largely localized to a cytosolic pool that is readily extracted upon permeabilization of the plasma membrane. By contrast, efflux of 86Rb was relatively slow in the control strain even after permeabilization, consistent with sequestration of the cation in a slowly exchanging Cu2+-resistant compartment. These results are strikingly reminiscent of 22Na sequestration by Nhx1, reported in Nass et al. (1997). Taken together, the data show that Nhx1 sequesters K+, presumably into the late endosomal and vacuolar compartments, and that this sequestration is important for K+ tolerance.
DISCUSSION
The Importance of Nhx1 in Cellular pH Regulation
In this study, we use novel compartment-specific pH probes in yeast to demonstrate the importance of Nhx1 in cellular pH homeostasis, based on the following four observations. First, the magnitude of the decrease in vacuolar pH observed in the yeast nhx1 null mutants (∼1 pH unit) upon acid stress affirms a major role for the endosomal NHE in regulating luminal pH. Similar results were observed when arginine was exchanged for ammonium as a nitrogen source for growth (unpublished data), indicating that ammonium buffering has little effect on vacuolar pH under these conditions (Plant et al., 1999). Taken together with a previous observation showing that transposon-induced mutation of a vacuolar NHX1 homolog of Ipomoea nil (Japanese morning glory) changes petal color by acidifying the vacuolar lumen (Fukada-Tanaka et al., 2000), our results implicate a ubiquitous role of the intracellular NHE in organellar pH regulation. Second, the loss of Nhx1 causes concomitant cytoplasmic acidification; an unexpected observation which may reflect an indirect consequence of excessive acidification of the vacuole, and down-regulation of plasma membrane and/or vacuolar H+-ATPases in order to maintain appropriate pH gradients across these membranes. In contrast to Nhx1, loss of the plasma membrane antiporter Nha1 has the expected effect of alkalinizing the cytosol, based on direction of ion transport (Figure 7A). Furthermore, we were surprised to find that the cytosolic alkalinization observed in nha1Δ is reversed in the nha1Δnhx1Δ double mutant, consistent with a dominant role of Nhx1 in cellular pH regulation. Finally, in this study, we make the novel observation that loss of pH homeostasis in the nhx1Δ mutant confers growth sensitivity to acid stress. This is analogous to the well-established method of acid selection of NHE1-deficient cells in culture (Pouyssegur et al., 1984). In mammalian cells, the function of plasma membrane NHE, as exemplified by NHE1, is to clear H+ from the cytosol for cell survival when stressed with acid (Sardet et al., 1989). We propose that the intracellular NHE, including yeast Nhx1, perform an essential cellular function in organellar membranes by alkalinizing the lumen to allow cells to grow in the presence of acid stress.
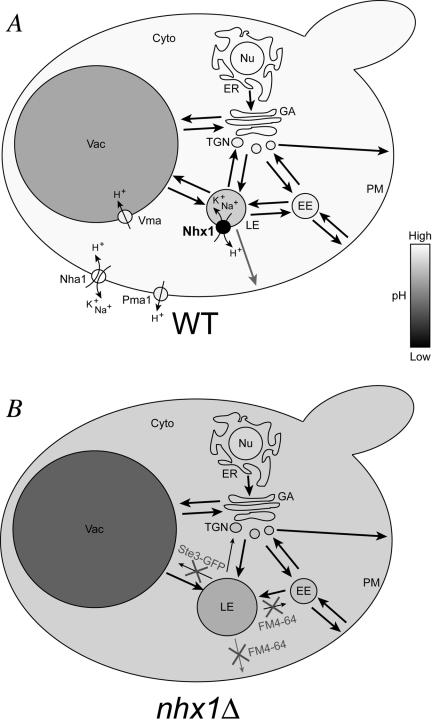
Figure 7.
Summary of nhx1Δ phenotypes. The vacuole (Vac), late endosome (LE), early endosome (EE), plasma membrane (PM), trans-Golgi network (TGN), Golgi apparatus (GA), endoplasmic reticulum (ER), nucleus (Nu) and cytoplasm (Cyto) are indicated for (A) WT and (B) nhx1Δ yeast cells. Compartmental shading is indicative of pH and black arrows between compartments represent vesicle trafficking pathways whereby line weight is representative of rate of trafficking. In A, membrane location and directional ion movement is shown for four transport proteins involved in cellular pH regulation: Nhx1, Nha1, Vma, and Pma1. Note that Vma is also present in all membranes shown with exception of the nucleus and plasma membrane. In B, cellular and growth phenotypes of nhx1Δ are summarized, indicating compartment (late endosome is enlarged) or trafficking pathway (indicated by small arrows) affected. A subset of phenotypes was reported previously by Bowers et al. (2000), Ali et al. (2004), Nass et al. (1997), and Gaxiola et al. (1999).
Thus, Nhx1 serves to alkalinize cellular compartments by transporting H+ in a direction opposite to that of the V-type H+-ATPase and is a major contributor to the H+-leak pathway in endocytic compartments (see Figure 7A). H+-leak is proposed to be essential for development of unique steady state pH gradients across organelles of the secretory and endocytic pathways, in addition to the changing activity and density of the V-ATPase in different compartmental membranes (Grabe and Oster, 2001; Kawasaki-Nishi et al., 2001; Wu et al., 2001; Machen et al., 2003). It has also been proposed that regulation of H+-leak mechanisms may be an important mechanism for acute pH regulation in organelles. Given the well-known regulation of plasma membrane NHE by second-messenger pathways and cytoskeleton interactions (reviewed by Putney et al., 2002; Zachos et al., 2005), it is likely that acute regulation of the intracellular NHE by similar mechanisms will control organellar maturation, movement, morphology, and trafficking (Sun-Wada et al., 2003).
Nhx1 Mediates pH-dependent Vesicle Trafficking Out of the Endosome
In this study, we show that abolishing the effect of nhx1Δ on cellular pH with the addition of a weak base, alleviates a subset of nhx1Δ phenotypes associated with trafficking out of the endosome, including growth sensitivity to hygromycin B, defective FM4–64 efflux and the abnormal accumulation of Ste3-GFP in the late endosome (Figure 7B). It is possible that hygromycin B sensitivity in the nhx1 mutant is in part due to a potential increase in Pma1 activity, which would result in hyperpolarization of the plasma membrane and increased uptake of the weakly cationic drug (Perlin et al., 1989). When also considering additional phenotypes of nhx1Δ such as endosomal enlargement and carboxypeptidase Y (CPY) secretion (characteristics of vps class E mutants) collectively, previous observations taken together with the novel observations made in this study clearly demonstrate that Nhx1 plays an important role in regulating pH to control trafficking out of the endosome.
Having now better defined the role of Nhx1 in trafficking, it is of interest to consider how exchanger activity of Nhx1 might be regulated. It has been shown in mammalian cell cultures that local alkalinization is required to form the Arf6 complex of trafficking machinery required for vesicle exit from the endosome (Maranda et al., 2001). However, the molecular identity of the pH-sensor or regulator has not been identified. We have shown previously that Nhx1 binds and is negatively regulated by Gyp6, a GTPase Activating Protein (GAP) for Ypt6, the Rab GTPase responsible for endosome to Golgi trafficking (Ali et al., 2004). Additional components of this complex can be identified by initially determining whether other trafficking mutants alter endosomal/vacuolar pH and if this change is mediated by Nhx1. Thus, preliminary findings suggest that Nhx1 may participate in pH-mediated assembly of the machinery required for trafficking out of the endosome.
Herein, we also show that the yeast Nhx1 has the ability to transport K+ as well as Na+. In addition, correcting the acidification in nhx1Δ strains did not abolish the growth sensitivity to high concentrations of KCl (or NaCl), indicating that sequestration of these ions into organelles is a property of the transporter itself. Our observations strengthen the conclusion that K+ selectivity is a ubiquitous feature of the intracellular NHE, distinct from the plasma membrane homologues. Similar reports of NHE-mediated K+ transport have been made for plant vacuolar NHE homologues in many plant species, such as Arabadopsis thaliana, as well as for mammalian organellar NHE homologues expressed cell culture (Numata and Orlowski, 2001; Venema et al., 2002; Nakamura et al., 2004). This further illustrates the physiological relevance of utilizing the K+ electrochemical gradient across organellar membranes for counterexchange of H+ required for mediating the role of Nhx1 in vesicle trafficking. This study also shows that under an external KCl stress, Nhx1 and Nha1 contribute equally and independently to clear K+ from the cytoplasm, preventing it from accumulating to toxic concentrations. However, in context of the counterion H+, Nhx1 appears to have a dominant role in cellular pH regulation and an exclusive role in trafficking. Given the wide-spread distribution of Nhx1 orthologues in nearly all eukaryotic species including metazoans and mammals (Brett et al., 2005), where most cells are not exposed to extreme salt stress, it is more likely that the major physiological role of Nhx1 is in pH-mediated vesicle trafficking.
Relevance to Mammalian Physiology
Humans and other mammals have four genes, NHE6–NHE9 that cluster phylogenetically in the intracellular subgroup, including three that are most closely related to yeast Nhx1 (Brett et al., 2005): NHE6 (SLC9A6) found in endosomes (Brett et al., 2002; Nakamura et al., 2004), NHE7 (SLC9A7) located in the trans-Golgi network (Numata and Orlowski, 2001), and NHE9 (SLC9A9), which is most similar in amino acid sequence to yeast Nhx1 and found in the late recycling endosomes (Nakamura et al., 2004) when expressed in mammalian cell culture models. The location of these exchangers within the endomembrane system brings up the possibility that each may regulate pH-mediated trafficking to or from different organelles. By regulating the pH within these compartments, they are likely to influence numerous pH-mediated trafficking events in the endocytic pathway of cells such as linking ligand-receptor dissociation to membrane recycling or lysosomal protein degradation. As preliminary reports show mRNA expression of the three human NHE orthologues in brain tissue (Numata and Orlowski, 2001; Nakamura et al., 2004), we can propose that in neurons these exchangers may act to couple H+-driven neurotransmitter uptake to rate of secretory vesicle recycling (Sudhof, 2004; Wu, 2004). Although the expression of Nhx1 homologues in neutrophils has not been confirmed, human NHE9 shows relatively high mRNA expression in spleen tissue (Nakamura et al., 2004), supporting the idea that an ortholog of Nhx1 may be responsible for pH-dependent K+ influx required for reactive oxygen species induced bacterial killing in vacuoles (Reeves et al., 2002). In context of animal virus particle entry (e.g., herpes simplex, influenza, and West Nile viruses), both the drop in pH in the endosomal lumen and trafficking within the endocytic pathway are required for propagation, (Harley et al., 2001; Khor et al., 2003; Chu and Ng, 2004); both could be potentially influenced by the activity of a mammalian Nhx1 ortholog. Thus, despite the great potential for physiological relevance, the physiological roles of mammalian Nhx1 orthologues are poorly understood and this study justifies and suggests new experimental approaches for future studies.
Acknowledgments
This work was supported by grants from the National Institutes of Diabetes, Digestive and Kidney Diseases to R.R. (R01 DK54214), a predoctoral fellowship from the American Heart Association to C.L.B. and the Johns Hopkins University School of Medicine Cellular and Molecular Physiology and Cellular and Molecular Medicine Graduate Programs.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-11-0999) on January 5, 2005.
Abbreviations used: BCECF, 2′,7′-bis(2-carboxyethyl)-5,6-carboxyfluorescein; FM4–64, N-(3-triethylammoniumpropyl)-4-(p-diethylaminophenyl-hexatrienyl) pyridinium dibromide; GFP, green fluorescent protein; NHE, Na+/H+ exchanger; PVC, prevacuolar compartment; VPS, vacuolar protein sorting; WT, wild type.
References
- Ali, R., Brett, C. L., Mukherjee, S., and Rao, R. (2004). Inhibition of sodium/proton exchange by a Rab-GTPase-activating protein regulates endosomal traffic in yeast. J. Biol. Chem. 279, 4498-4506. [DOI] [PubMed] [Google Scholar]
- Aniento, F., Gu, F., Parton, R. G., and Gruenberg, J. (1996). An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J. Cell Biol. 133, 29-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apse, M. P., Aharon, G. S., Snedden, W. A., and Blumwald, E. (1999). Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285, 1256-1258. [DOI] [PubMed] [Google Scholar]
- Banuelos, M. A., Ruiz, M. C., Jimenez, A., Souciet, J. L., Potier, S., and Ramos, J. (2002). Role of the Nha1 antiporter in regulating K+ influx in Saccharomyces cerevisiae. Yeast 19, 9-15. [DOI] [PubMed] [Google Scholar]
- Bowers, K., Levi, B. P., Patel, F. I., and Stevens, T. H. (2000). The sodium/proton exchanger Nhx1p is required for endosomal protein trafficking in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 11, 4277-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett, C. L., Donowitz, M., and Rao, R. (2005). The evolutionary origins of eukaryotic sodium/proton exchangers. Am. J. Physiol. Cell. Physiol. 288, C223-C239. [DOI] [PubMed] [Google Scholar]
- Brett, C. L., Wei, Y., Donowitz, M., and Rao, R. (2002). Human Na+/H+ exchanger isoform 6 is found in recycling endosomes of cells, not in mitochondria. Am. J. Physiol. Cell. Physiol. 282, C1031-C1041. [DOI] [PubMed] [Google Scholar]
- Buckley, K. M., Melikian, H. E., Provoda, C. J., and Waring, M. T. (2000). Regulation of neuronal function by protein trafficking: a role for the endosomal pathway. J. Physiol. 525, 11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, J.J., and Ng, M. L. (2004). Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J. Virol. 78, 10543-10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy, M. J., and Cyert, M. S. (2000). Luv1p/Rki1p/Tcs3p/Vps54p, a yeast protein that localizes to the late Golgi and early endosome, is required for normal vacuolar morphology. Mol. Biol. Cell 11, 2429-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada-Tanaka, S., Inagaki, Y., Yamaguchi, T., Saito, N., and Iida, S. (2000). Colour-enhancing protein in blue petals. Nature 407, 581. [DOI] [PubMed] [Google Scholar]
- Futerman, A. H., and van Meer, G. (2004). The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell. Biol. 5, 554-565. [DOI] [PubMed] [Google Scholar]
- Gaxiola, R. A., Rao, R., Sherman, A., Grisafi, P., Alper, S. L., and Fink, G. R. (1999). The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc. Natl. Acad. Sci. USA 96, 1480-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabe, M., and Oster, G. (2001). Regulation of organelle acidity. J. Gen. Physiol. 117, 329-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley, C. A., Dasgupta, A., and Wilson, D. W. (2001). Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J. Virol. 75, 1236-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser, J. (1989). Changes in lysosome shape and distribution correlated with changes in cytoplasmic pH. J. Cell Biol. 108, 855-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki-Nishi, S., Bowers, K., Nishi, T., Forgac, M., and Stevens, T. H. (2001). The amino-terminal domain of the vacuolar proton-translocating ATPase a subunit controls targeting and in vivo dissociation, and the carboxyl-terminal domain affects coupling of proton transport and ATP hydrolysis. J. Biol. Chem. 276, 47411-47420. [DOI] [PubMed] [Google Scholar]
- Khor, R., McElroy, L. J., and Whittaker, G. R. (2003). The ubiquitin-vacuolar protein sorting system is selectively required during entry of influenza virus into host cells. Traffic 4, 857-868. [DOI] [PubMed] [Google Scholar]
- Machen, T. E., Leigh, M. J., Taylor, C., Kimura, T., Asano, S., and Moore, H. P. (2003). pH of TGN and recycling endosomes of H+/K+-ATPase-transfected HEK-293 cells: implications for pH regulation in the secretory pathway. Am. J. Physiol. Cell Physiol. 285, C205-C214. [DOI] [PubMed] [Google Scholar]
- Maranda, B., Brown, D., Bourgoin, S., Casanova, J. E., Vinay, P., Ausiello, D. A., and Marshansky, V. (2001). Intra-endosomal pH-sensitive recruitment of the Arf-nucleotide exchange factor ARNO and Arf6 from cytoplasm to proximal tubule endosomes. J. Biol. Chem. 276, 18540-18550. [DOI] [PubMed] [Google Scholar]
- Mellman, I. (1992). The importance of being acid: the role of acidification in intracellular membrane traffic. J. Exp. Biol. 172, 39-45. [DOI] [PubMed] [Google Scholar]
- Mellman, I., Fuchs, R., and Helenius, A. (1986). Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 55, 663-700. [DOI] [PubMed] [Google Scholar]
- Miesenbock, G., De Angelis, D. A., and Rothman, J. E. (1998). Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192-195. [DOI] [PubMed] [Google Scholar]
- Nakamura, N., Tanaka, S., Teko, Y., Mitsui, K., and Kanazawa, H. (2004). Four Na+/H+ exchanger isoforms are distributed to golgi and post-golgi compartments and are involved in organelle pH regulation. J. Biol. Chem. Epub 10.1074/jbc.M410041200. [DOI] [PubMed]
- Nass, R., and Rao, R. (1998). Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. Implications for vacuole biogenesis. J. Biol. Chem. 273, 21054-21060. [DOI] [PubMed] [Google Scholar]
- Nass, R., and Rao, R. (1999). The yeast endosomal Na+/H+ exchanger, Nhx1, confers osmotolerance following acute hypertonic shock. Microbiology 145, 3221-3228. [DOI] [PubMed] [Google Scholar]
- Nass, R., Cunningham, K. W., and Rao, R. (1997). Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+-ATPase. Insights into mechanisms of sodium tolerance. J. Biol. Chem. 272, 26145-26152. [DOI] [PubMed] [Google Scholar]
- Nelson, H., and Nelson, N. (1990). Disruption of genes encoding subunits of yeast vacuolar H+-ATPase causes conditional lethality. Proc. Natl. Acad. Sci. USA 87, 3503-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieland, T. J., Feng, Y., Brown, J. X., Chuang, T. D., Buckett, P. D., Wang, J., Xie, X. S., McGraw, T. E., Kirchhausen, T., and Wessling-Resnick, M. (2004). Chemical genetic screening identifies sulfonamides that raise organellar pH and interfere with membrane traffic. Traffic 5, 478-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi, T., and Forgac, M. (2002). The vacuolar H+-ATPases—nature's most versatile proton pumps. Nat. Rev. Mol. Cell. Biol. 3, 94-103. [DOI] [PubMed] [Google Scholar]
- Numata, M., and Orlowski, J. (2001). Molecular cloning and characterization of a novel (Na+,K+)/H+ exchanger localized to the trans-Golgi network. J. Biol. Chem. 276, 17387-17394. [DOI] [PubMed] [Google Scholar]
- Orlowski, J., and Grinstein, S. (2004). Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pfluegers Arch. 447, 549-565. [DOI] [PubMed] [Google Scholar]
- Page, N. et al. (2003). A Saccharomyces cerevisiae genome-wide mutant screen for altered sensitivity to K1 killer toxin. Genetics 163, 875-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin, D. S., Harris, S. L., Seto-Young, D., and Haber, J. E. (1989). Defective H+-ATPase of hygromycin B-resistant pma1 mutants from Saccharomyces cerevisiae. J. Biol. Chem. 264, 21857-21864. [PubMed] [Google Scholar]
- Plant, P. J., Manolson, M. F., Grinstein, S., and Demaurex, N. (1999). Alternative mechanisms of vacuolar acidification in H+-ATPase-deficient yeast. J. Biol. Chem. 274, 37270-37279. [DOI] [PubMed] [Google Scholar]
- Pouyssegur, J., Sardet, C., Franchi, A., L'Allemain, G., and Paris, S. (1984). A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc. Natl. Acad. Sci. USA 8, 4833-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney, L. K., Denker, S. P., and Barber, D. L. (2002). The changing face of the Na+/H+ exchanger, NHE 1, structure, regulation, and cellular actions. Annu. Rev. Pharmacol. Toxicol. 42, 527-552. [DOI] [PubMed] [Google Scholar]
- Reeves, E. P., Lu, H., Jacobs, H. L., Messina, C. G., Bolsover, S., Gabella, G., Potma, E. O., Warley, A., Roes, J., and Segal, A. W. (2002). Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416, 291-297. [DOI] [PubMed] [Google Scholar]
- Sardet, C., Franchi, A., and Pouyssegur, J. (1989). Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell 56, 271-280. [DOI] [PubMed] [Google Scholar]
- Sudhof, T. C. (2004). The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509-547. [DOI] [PubMed] [Google Scholar]
- Sun-Wada, G.-H., Wada, Y., and Futai, M. (2003). Lysosome and lysosome-related organelles responsible for specialized functions in higher organisms, with special emphasis on vacuolar-type proton ATPase. Cell Struct. Funct. 28, 455-463. [DOI] [PubMed] [Google Scholar]
- Sychrova, H., Ramirez, J., and Pena, A. (1999). Involvement of Nha1 antiporter in regulation of intracellular pH in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 171, 167-172. [DOI] [PubMed] [Google Scholar]
- Urbanowski, J. L., and Piper, R. C. (1999). The iron transporter Fth1p forms a complex with the Fet5 iron oxidase and resides on the vacuolar membrane. J. Biol. Chem. 274, 38061-38070. [DOI] [PubMed] [Google Scholar]
- van Weert, A. W., Dunn, K. W., Gueze, H. J., Maxfield, F. R., and Stoorvogel, W. (1995). Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J. Cell Biol. 130, 821-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weert, A. W., Geuze, H. J., and Stoorvogel, W. (1997). Heterogeneous behavior of cells with respect to induction of retrograde transport from the trans-Golgi network to the Golgi upon inhibition of the vacuolar proton pump. Eur. J. Cell Biol. 74, 417-423. [PubMed] [Google Scholar]
- Venema, K., Quintero, F. J., Pardo, J. M., and Donaire, J.P. (2002). The Arabidopsis Na+/H+ exchanger AtNHX1 catalyzes low affinity Na+ and K+ transport in reconstituted liposomes. J. Biol. Chem. 277, 2413-2418. [DOI] [PubMed] [Google Scholar]
- Wiederkehr, A., Avaro, S., Prescianotto-Baschong, C., Haguenauer-Tsapis, R., and Riezman, H. (2000). The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae. J. Cell Biol. 149, 397-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L. G. (2004). Kinetic regulation of vesicle endocytosis at synapses. Trends Neurosci. 27, 548-554. [DOI] [PubMed] [Google Scholar]
- Wu, M. M., Grabe, M., Adams, S., Tsien, R. Y., Moore, H. P., and Machen, T. E. (2001). Mechanisms of pH regulation in the regulated secretory pathway. J. Biol. Chem. 276, 33027-33035. [DOI] [PubMed] [Google Scholar]
- Yamashiro, C. T., Kane, P. M., Wolczyk, D. F., Preston, R. A., and Stevens, T. H. (1990). Role of vacuolar acidification in protein sorting and zymogen activation: a genetic analysis of the yeast vacuolar proton-translocating ATPase. Mol. Cell. Biol. 10, 3737-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos, N. C., Tse, C. M., and Donowitz, M. (2005). Molecular physiology of intestinal Na+/H+ exchange (NHE). Annu. Rev. Physiol. 67, 18.1-18.33. [DOI] [PubMed] [Google Scholar]
- Zeuzem, S., Zimmermann, P., and Schulz, I. (1992). Association of a 19- and a 21-kDa GTP-binding protein to pancreatic microsomal vesicles is regulated by the intravesicular pH established by a vacuolar-type H+-ATPase. J. Membr. Biol. 125, 231-241.- [DOI] [PubMed] [Google Scholar]